Key Points
Question
What is the association of weight gain from early to middle adulthood with health outcomes later in life?
Findings
During a follow-up of 18 years in 92 837 US women and 15 years in 25 303 US men, compared with participants who maintained a stable weight (weight loss ≤2.5 kg or gain <2.5 kg), those who gained a moderate amount of weight (≥2.5-<10.0 kg) had increased incidence of type 2 diabetes (absolute rate difference/100 000 person-years of 98 in women and 111 in men), cardiovascular disease (61 in women), obesity-related cancer (37 in women and 42 in men), and mortality (51 among women who never smoked).
Meaning
Among women and men, moderate weight gain from early to middle adulthood was associated with significantly increased risk of major chronic diseases and mortality.
Abstract
Importance
Data describing the effects of weight gain across adulthood on overall health are important for weight control.
Objective
To examine the association of weight gain from early to middle adulthood with health outcomes later in life.
Design, Setting, and Participants
Cohort analysis of US women from the Nurses’ Health Study (1976-June 30, 2012) and US men from the Health Professionals Follow-Up Study (1986-January 31, 2012) who recalled weight during early adulthood (at age of 18 years in women; 21 years in men), and reported current weight during middle adulthood (at age of 55 years).
Exposures
Weight change from early to middle adulthood (age of 18 or 21 years to age of 55 years).
Main Outcomes and Measures
Beginning at the age of 55 years, participants were followed up to the incident disease outcomes. Cardiovascular disease, cancer, and death were confirmed by medical records or the National Death Index. A composite healthy aging outcome was defined as being free of 11 chronic diseases and major cognitive or physical impairment.
Results
A total of 92 837 women (97% white; mean [SD] weight gain: 12.6 kg [12.3 kg] over 37 years) and 25 303 men (97% white; mean [SD] weight gain: 9.7 kg [9.7 kg] over 34 years) were included in the analysis. For type 2 diabetes, the adjusted incidence per 100 000 person-years was 207 among women who gained a moderate amount of weight (≥2.5 kg to <10 kg) vs 110 among women who maintained a stable weight (weight loss ≤2.5 kg or gain <2.5 kg) (absolute rate difference [ARD] per 100 000 person-years, 98; 95% CI, 72 to 127) and 258 vs 147, respectively, among men (ARD, 111; 95% CI, 58 to 179); hypertension: 3415 vs 2754 among women (ARD, 662; 95% CI, 545 to 782) and 2861 vs 2366 among men (ARD, 495; 95% CI, 281 to 726); cardiovascular disease: 309 vs 248 among women (ARD, 61; 95% CI, 38 to 87) and 383 vs 340 among men (ARD, 43; 95% CI, −14 to 109); obesity-related cancer: 452 vs 415 among women (ARD, 37; 95% CI, 4 to 73) and 208 vs 165 among men (ARD, 42; 95% CI, 0.5 to 94). Among those who gained a moderate amount of weight, 3651 women (24%) and 2405 men (37%) achieved the composite healthy aging outcome. Among those who maintained a stable weight, 1528 women (27%) and 989 men (39%) achieved the composite healthy aging outcome. The multivariable-adjusted odds ratio for the composite healthy aging outcome associated with moderate weight gain was 0.78 (95% CI, 0.72 to 0.84) in women and 0.88 (95% CI, 0.79 to 0.97) in men. Higher amounts of weight gain were associated with greater risks of major chronic diseases and lower likelihood of healthy aging.
Conclusions and Relevance
In these cohorts of health professionals, weight gain during adulthood was associated with significantly increased risk of major chronic diseases and decreased odds of healthy aging. These findings may help counsel patients regarding the risks of weight gain.
This cohort analysis uses Nurses’ Health Study and Health Professionals Follow-Up Study data to examine the association between weight gain in early to middle adulthood and heart disease, cancer, and death later in life.
Introduction
Obesity is a major global health challenge. The worldwide prevalence of obesity (body mass index [calculated as weight in kilograms divided by height in meters squared; BMI] ≥30) was 11% for men and 15% for women in 2014 compared with 5% for men and 8% for women in 1980. Excess adiposity is a well-established risk factor for major chronic diseases, including cardiometabolic disease, certain types of cancer such as postmenopausal breast cancer and colorectum cancer, and overall premature mortality.
Excess adiposity tends to accrue during early and middle adulthood for most people. Among US adults, the mean weight gain is 0.5 to 1.0 kg per year from early to middle adulthood and this modest yearly accumulation of weight eventually leads to obesity over time. Adult weight gain has been associated with an increased risk of type 2 diabetes, coronary heart disease (CHD), hypertension, cholelithiasis, and several types of cancer. However, most prior studies did not consider the total amount of weight change across specific age ranges. It is unclear how weight gain during the transition from early to middle adulthood, when most weight gain occurs, relates to subsequent health consequences. Recommendations for preventing weight gain during adulthood are lacking in public health guidelines. Compared with studies of attained weight or BMI, an investigation of weight change may better capture the effect of excess fat because it factors in individual differences in frame size and lean mass that are difficult to measure in population studies.
This study aimed to systematically examine the association of weight gain from early to middle adulthood (age of 18 or 21 years to age of 55 years) with subsequent risk of major health outcomes, including type 2 diabetes, hypertension, cardiovascular disease, cancer, cholelithiasis, severe osteoarthritis, cataracts, a composite healthy aging outcome, and mortality, in women and men from 2 large, ongoing cohort studies.
Methods
Study Design and Population
A cohort analysis was conducted using the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS). The NHS began in 1976 with the enrollment of 121 701 female registered nurses aged 30 to 55 years, reflecting the racial composition of nurses at that time (97% white). The HPFS began in 1986 with the enrollment of 51 529 male health professionals (including dentists, optometrists, osteopaths, pharmacists, podiatrists, and veterinarians) aged 40 to 75 years. Participants in both cohorts completed an initial questionnaire on medications, lifestyle, and medical history and were followed up biennially via questionnaires that obtained updated information. Follow-up information was available for approximately 90% of each study cohort. The details of both studies were described.
In the recruitment and biennial follow-up questionnaires for both cohorts, participants reported their current weight. The Pearson correlation coefficient between self-reported current weight and the mean of 2 standardized technician-measured weights was 0.97 for both women and men. In the 1982 questionnaire for the NHS and the 1986 questionnaire for the HPFS, participants recalled their weight at 18 years of age for women in the NHS and at 21 years of age for men in the HPFS. In a subgroup of women aged 25 to 42 years in NHS II, the mean difference between measured and recalled weight at the age of 18 years was 1.4 kg, and the correlation was 0.87. Early- to middle-life weight change was defined as the change in weight since early adulthood (ages of 18 years for women and 21 years for men) to middle adulthood (age of 55 years).
The study was approved by the institutional review boards of Brigham and Women’s Hospital and Harvard T. H. Chan School of Public Health, with informed consent indicated by the return of the baseline questionnaire.
Outcomes and Covariates
Potential confounders included age, race, family history of diseases, alcohol consumption and smoking status, physical activity level, use of aspirin, menopausal status and postmenopausal hormone therapy use for women, parity for women, and a history of major chronic conditions reported via the regular biennial questionnaires throughout follow-up. Race/ethnicity was determined by self-selecting 1 of the fixed categories and was considered as a covariate in the models because of the known relevance of race/ethnicity to chronic disease risk. Dietary information was updated using validated semiquantitative food frequency questionnaires in each cohort every 2 to 4 years. Height was reported at the baseline questionnaire for each cohort. Self-reported weight and height were used to calculate each participant’s BMI.
The selected health outcomes included major age-related conditions that could affect the quantity and quality of later life. Every 2 years after baseline of the study, participants were asked to report newly developed health outcomes, including type 2 diabetes, hypertension, cardiovascular disease, cancer, symptomatic gallstones, cholecystectomy, hip replacement, severe osteoarthritis, and cataract extraction during the past 2 years. Participants who self-reported a diagnosis of diabetes were mailed a supplementary questionnaire regarding symptoms, diagnostic tests, and hypoglycemic therapy. The National Diabetes Data Group criteria were used to define cases of type 2 diabetes before 1998 and the American Diabetes Association criteria were used to define cases after 1998.
Self-reported hypertension, type 2 diabetes, cholelithiasis, severe hip osteoarthritis, and cataract extraction were verified in subsamples with high confirmation rates. Cholelithiasis was defined as a composite of unremoved symptomatic gallstones and cholecystectomy. Clinically severe osteoarthritis was defined as total hip replacement, and simultaneously osteoarthritis (or other arthritis). Because cataracts are very common, cataract extraction was used as a clinically significant end point to minimize misclassification and to decrease variation in diagnosis. The diagnoses of cardiovascular disease (a composite of CHD and stroke) and cancer were confirmed by study physicians who reviewed medical records. Obesity-related cancer included the esophagus (adenocarcinoma only), colon and rectum, pancreas, breast (after menopause, women only), endometrium (women only), ovaries (women only), prostate (only advanced and in men), kidney, liver, and gallbladder. Incident cases of cardiovascular disease, type 2 diabetes, overall cancer, and nontraumatic death were included in a composite measure of major chronic diseases. Deaths during follow-up were reported by next of kin, the postal system, and through the records of the National Death Index. The underlying cause of death was assigned according to the eighth and ninth revisions of International Classification of Diseases.
The concept of a “composite healthy aging outcome” included not only disease status but also cognitive and physical functions. Following previous publications, the composite healthy aging outcome was defined using the following 3 domains: (1) no self-reported history of cancer, diabetes, CHD, coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty, congestive heart failure, stroke, kidney failure, chronic obstructive pulmonary disease, Parkinson disease, multiple sclerosis, or amyotrophic lateral sclerosis; (2) no cognitive decline (subjective cognitive decline score = 0 by 6 relevant questions); and (3) no physical limitations (no limitations on moderate activities, and no more than moderate limitations on more demanding physical performance measures of the 36-item Medical Outcomes Study Short-Form Health Survey). Because the cognitive assessments mainly occurred in 2010-2012, participants who were alive in 2010 and had data regarding the 3 domains were included in the analysis for the composite healthy aging outcome.
In the current analysis, the primary outcomes included incident cases of type 2 diabetes, hypertension, cardiovascular disease, obesity-related cancer, cholelithiasis, clinically severe osteoarthritis, cataract extraction, death, the composite healthy aging outcome, and a composite measure of major chronic diseases. The secondary outcomes included incident cases of cardiovascular disease subtypes (ie, CHD and stroke), overall cancer, and mortality caused by cardiovascular disease or cancer. A detailed description of ascertainment and documentation of outcomes appears in the eMethods in the Supplement.
Statistical Analysis
Poisson regression models with robust standard errors were used to estimate incident rates and incident rate ratios (IRRs) and 95% CIs as an indicator of relative risk of incident major health outcomes (from the year when the participant turned 55 years old to 2012). The absolute rate difference (ARD) was estimated by I × (IRR − 1) (I indicates the incidence among the reference category or the study population). For the composite healthy aging outcome, logistic regression models were used to estimate odds ratios and 95% CIs in 2010. Because many outcomes were evaluated simultaneously without adjustment for multiple comparisons, the analyses from this study may be interpreted as exploratory in nature; however, these analyses were based on a priori hypotheses and previous literature.
Based on the examination of the distribution of weight change in both cohorts, primary analyses of weight change used the following categories: (1) weight loss greater than 2.5 kg, (2) weight loss between 0 and 2.5 kg or weight gain of less than 2.5 kg (reference category), (3) weight gain between 2.5 and 9.9 kg, (4) weight gain between 10.0 and 19.9 kg, and (5) weight gain of 20.0 kg or more. We chose 10 kg as the cutoff point for moderate weight gain based on the estimated median (10.9 kg in women and 8.8 kg in men) and mean (12.6 kg in women and 9.7 kg in men) weight change of the study participants. The cutoff for the extreme weight gain group (20 kg) was based on selection of the highest 10% to 20% of both the female and male study participants. These weight change cutoff points for categories were comparable with those used in previous studies. For the analysis of a composite measure of major chronic diseases, weight change was further divided, yielding 9 categories (<−2.5 kg, −2.5 to 2.4 kg [reference category], 2.5 to 4.9 kg, 5.0 to 9.9 kg, 10.0 to 14.9 kg, 15.0 to 19.9 kg, 20.0 to 24.9 kg, 25.0 to 29.9 kg, and ≥30.0 kg). The analyses for trend of association across weight change categories were conducted by assigning the median value to each category and modeling this value as a continuous variable.
Several secondary analyses were conducted. We also categorized the study population by quintile cutoffs of weight change in each cohort, and compared the participants who gained more weight (quintiles 2-5) with those who gained the least (quintile 1). In addition, a possible nonlinear relationship between weight change and incidence of disease was tested nonparametrically with restricted cubic splines, and tests for nonlinearity used the likelihood ratio test, comparing the model with only the linear term vs the model with the linear and cubic spline terms. The joint associations of weight change and BMI at the age of 18 or 21 years, and that of BMI at the age 18 or 21 years and at the age of 55 years, with incidence of disease were also estimated.
Multivariable models of Poisson regression and logistic regression were adjusted for age at cohort recruitment (continuous), height (continuous), race (nonwhite or white), pack-years of smoking (never smoker; past smoker with <5, 5-20, or >20 pack-years; or current smoker with <5, 5-20, or >20 pack-years), regular aspirin use (yes or no), status of menopause and hormone therapy (women only: premenopausal, postmenopausal and hormone therapy never use, postmenopausal and current use, or postmenopausal and past use), parity (women only: nulliparous, 1, 2, 3, or ≥4 children), physical activity (in quintiles for women: <2.9, 2.9-7.1, 7.2-13.1, 13.2-23.6, or ≥23.7 metabolic equivalent task [MET]-h/wk; and men: <8.3, 8.3-17.2, 17.3-28.6, 28.7-46.9, or ≥47.0 MET-h/wk), alcohol consumption (women: 0, 0.1-0.4, 0.5-1.9, 2.0-7.0, or ≥8.0 g/d; men: 0-4, 5-9, 10-14, 15-29, or ≥30 g/d), dietary qualify (in quintiles of Alternative Healthy Eating Index), family history, and weight at early adulthood. All covariates were examined at the age of 55 years except for the age at cohort recruitment.
For missing data on physical activity and dietary quality, data were imputed using median values; for missing data on pack-years of smoking and alcohol consumption, participants were assigned to never smoker and nondrinker categories, respectively. Dummy variables were used to indicate missing data for the other covariates. The mean missing data rate for covariates was approximately 5%. Analyses were performed for all participants combined and for subgroups (eg, participants who never smoked). Potential effect modification by smoking status (never vs ever) was evaluated by significance tests of the cross-product interaction terms of smoking status and weight change as a continuous variable using the Wald test.
Based on the multivariable models, several sensitivity analyses were conducted. For the type 2 diabetes analysis, prevalent hypertension and hyperlipidemia and related medication use were further adjusted. For the analyses of cardiovascular disease and mortality, prevalent type 2 diabetes, hypertension, and hyperlipidemia, and medication use for high blood pressure and lipid levels were further adjusted. For obesity-related cancer, history of lower gastrointestinal endoscopy, history of physical examination, mammography (women only), and prostate-specific antigen test (men only) were further adjusted. In additional sensitivity analyses, use of antidepressants and oral steroids was further adjusted among participants with available information.
Cohort-specific analyses were performed to generate IRRs that were pooled together using meta-analysis. Fixed-effects and random-effects models generated similar results. Only results from the random-effects models are presented given heterogeneity across the sexes. Heterogeneity was assessed using the I2 statistic, where an I2 greater than 50% indicated substantial heterogeneity. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc) and R version 3.2.2 (R Foundation). All P values were based on 2-sided tests and were considered statistically significant at P < .05.
Results
Baseline Characteristics and Weight Gain
A total of 92 837 women and 25 303 men were included in the study. Participants were excluded if they died before the age of 55 years; were older than the age of 55 years at cohort recruitment; did not report weight at age of 18 years (for women), age of 21 years (for men), or age of 55 years; or had an extremely low BMI (<17 for women and <18.5 for men) at the age of 55 years (eFigure 1 in the Supplement). From early to middle adulthood, participants gained a mean (SD) of 12.6 kg (12.3 kg) during 37 years (median, 10.9 kg; interquartile range, 4.5-18.9 kg) among women and a mean (SD) of 9.7 kg (9.7 kg) during 34 years (median, 8.8 kg; interquartile range, 3.6-14.8 kg) among men. Characteristics of the study participants across categories of weight change appear in Table 1 for the NHS cohort and in Table 2 for the HPFS cohort. Consistently among women and men, those who gained more weight were more likely to be physically inactive, to be never smokers, to have unhealthy dietary habits, and to have a higher prevalence of chronic diseases at the age of 55 years.
Table 1. Baseline Characteristics of Study Participants at Age of 55 Years in the Nurses’ Health Study (N = 92 837).
| Weight Change Between the Ages of 18 y and 55 ya | ||||||
|---|---|---|---|---|---|---|
| Weight Loss >2.5 kg | Weight Loss ≤2.5 kg or Gain <2.5 kg |
Weight Gain Between ≥2.5 kg and <10.0 kg |
Weight Gain Between ≥10.0 kg and <20.0 kg |
Weight Gain ≥20.0 kg | ||
| No. of participants | 6363 (7) | 10 649 (11) | 26 402 (28) | 28 479 (31) | 20 944 (23) | |
| White race | 6254 (98) | 10 428 (98) | 25 899 (98) | 27 713 (97) | 20 171 (96) | |
| Age at cohort recruitment, mean (SD), y | 42.7 (7.2) | 42.7 (7.3) | 42.7 (7.3) | 42.6 (7.2) | 42.5 (7.3) | |
| Weight at age of 18 y, mean (SD), kg | 66.7 (11.7) | 58.4 (8.1) | 56.2 (7.6) | 55.7 (8.0) | 57.4 (9.0) | |
| Body mass indexb | ||||||
| At age of 18 y, mean (SD) | 24.8 (3.9) | 21.9 (2.6) | 21.0 (2.5) | 20.7 (2.7) | 21.2 (3.1) | |
| <22.5 | 300 (5) | 2019 (19) | 9129 (35) | 11 664 (41) | 7194 (34) | |
| 22.5-25.0 | 1215 (19) | 4224 (40) | 9950 (38) | 9365 (33) | 6562 (31) | |
| >25.0 | 4848 (76) | 4406 (41) | 7323 (28) | 7450 (26) | 7188 (34) | |
| Weight at age of 55 y, mean (SD), kg | 59.1 (9.8) | 58.7 (8.1) | 62.6 (7.8) | 70.2 (8.5) | 87.0 (13.9) | |
| Height, mean (SD), m | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | |
| Physical activity, median (IQR), MET-h/wk | ||||||
| Quintile 1 (<2.9) | 1.3 (0.5-2.3) | 1.4 (0.6-2.3) | 1.4 (0.7-2.3) | 1.5 (0.7-2.3) | 1.4 (0.6-2.2) | |
| Quintile 2 (2.9-7.1) | 4.4 (3.7-5.5) | 4.6 (3.8-5.6) | 4.6 (3.8-5.7) | 4.6 (3.8-5.7) | 4.6 (3.7-5.8) | |
| Quintile 3 (7.2-13.1) | 9.9 (8.4-11.2) | 9.8 (8.2-11.3) | 9.8 (8.3-11.3) | 9.7 (8.3-11.2) | 9.6 (8.1-11.2) | |
| Quintile 4 (13.2-23.6) | 17.6 (15.2-20.4) | 17.8 (15.4-20.4) | 17.5 (15.2-20.4) | 17.4 (15.2-20.2) | 17.1 (15.1-20.1) | |
| Quintile 5 (≥23.7) | 37.6 (29.0-50.9) | 37.5 (28.9-51.1) | 35.9 (28.2-49.0) | 34.8 (27.9-45.4) | 33.9 (27.7-43.2) | |
| Alcohol consumption, g/d | ||||||
| 0 | 1730 (27) | 2600 (24) | 6489 (25) | 7359 (26) | 6570 (31) | |
| 0.1-0.4 | 257 (4) | 406 (4) | 1082 (4) | 1443 (5) | 1566 (7) | |
| 0.5-1.9 | 1099 (17) | 1812 (17) | 4775 (18) | 5871 (21) | 4877 (23) | |
| 2.0-7.0 | 1358 (21) | 2404 (23) | 6410 (24) | 7087 (25) | 4548 (22) | |
| ≥8.0 | 1919 (30) | 3427 (32) | 7646 (29) | 6719 (24) | 3383 (16) | |
| Smoking status | ||||||
| Never smoker | 1864 (29) | 4077 (38) | 11 335 (43) | 13 034 (46) | 10 152 (48) | |
| Past smoker | ||||||
| <5 pack-years | 568 (9) | 1120 (11) | 3033 (11) | 3274 (12) | 2531 (12) | |
| 5-20 pack-years | 749 (12) | 1468 (14) | 3600 (14) | 3981 (14) | 3013 (14) | |
| >20 pack-years | 682 (11) | 993 (9) | 2581 (10) | 3143 (11) | 2647 (13) | |
| Current smoker | ||||||
| <5 pack-years | 79 (1) | 142 (1) | 311 (1) | 296 (1) | 198 (1) | |
| 5-20 pack-years | 317 (5) | 444 (4) | 922 (3) | 844 (3) | 443 (2) | |
| >20 pack-years | 2104 (33) | 2405 (23) | 4620 (18) | 3907 (14) | 1960 (9) | |
| Alternative Healthy Eating Index, mean (SD)c | ||||||
| Quintile 1 | 30.9 (3.6) | 31 (3.4) | 31.1 (3.5) | 31.2 (3.4) | 31.1 (3.5) | |
| Quintile 2 | 38 (1.4) | 38 (1.4) | 38 (1.4) | 38 (1.4) | 38 (1.4) | |
| Quintile 3 | 42.7 (1.3) | 42.7 (1.3) | 42.7 (1.3) | 42.7 (1.3) | 42.6 (1.3) | |
| Quintile 4 | 47.9 (1.6) | 47.8 (1.7) | 47.7 (1.6) | 47.7 (1.6) | 47.7 (1.6) | |
| Quintile 5 | 57.5 (5.7) | 57.3 (5.4) | 56.8 (5.0) | 56.1 (4.6) | 55.9 (4.4) | |
| Menopause status and postmenopausal hormone use | ||||||
| Premenopausal | 165 (3) | 383 (4) | 1099 (4) | 1143 (4) | 896 (4) | |
| Postmenopausal and never use | 2464 (39) | 3827 (36) | 9110 (35) | 10 040 (35) | 7528 (36) | |
| Postmenopausal and current use | 1813 (28) | 3333 (31) | 8336 (32) | 8818 (31) | 5957 (28) | |
| Postmenopausal and past use | 1124 (18) | 1725 (16) | 4341 (16) | 4494 (16) | 3391 (16) | |
| Missing information | 797 (13) | 1381 (13) | 3516 (13) | 3984 (14) | 3172 (15) | |
| Parity | ||||||
| Nulliparous | 475 (7) | 656 (6) | 1573 (6) | 1548 (5) | 1079 (5) | |
| 1 Child | 567 (9) | 760 (7) | 1855 (7) | 1928 (7) | 1495 (7) | |
| 2 Children | 1797 (28) | 2954 (28) | 7158 (27) | 7817 (27) | 5880 (28) | |
| 3 Children | 1664 (26) | 2908 (27) | 7256 (27) | 7826 (27) | 5656 (27) | |
| ≥4 Children | 1745 (27) | 3175 (30) | 8133 (31) | 8886 (31) | 6400 (31) | |
| Missing information | 115 (2) | 196 (2) | 427 (2) | 474 (2) | 434 (2) | |
| Aspirin use | 2279 (36) | 3794 (36) | 9290 (35) | 10 512 (37) | 8604 (41) | |
| Self-reported conditions | ||||||
| Hypertension | 1270 (20) | 1968 (18) | 6003 (23) | 8832 (31) | 10 278 (49) | |
| Diabetes | 202 (3) | 250 (2) | 689 (3) | 1200 (4) | 2082 (10) | |
| Hypercholesterolemia | 691 (11) | 1225 (12) | 4093 (16) | 5960 (21) | 5389 (26) | |
| Cancer | 211 (3) | 306 (3) | 785 (3) | 965 (3) | 812 (4) | |
| Heart disease | 145 (2) | 180 (2) | 481 (2) | 686 (2) | 730 (3) | |
| Cholelithiasis | 609 (10) | 794 (7) | 2235 (8) | 3431 (12) | 4112 (20) | |
| Severe osteoarthritis | 237 (4) | 366 (3) | 834 (3) | 1088 (4) | 924 (4) | |
| Cataracts | 78 (1) | 95 (1) | 284 (1) | 303 (1) | 331 (2) | |
| Family history | ||||||
| Heart disease | 1700 (27) | 2734 (26) | 6843 (26) | 7587 (27) | 5709 (27) | |
| Cancer | 3448 (54) | 5852 (55) | 14 702 (56) | 15 703 (55) | 11 454 (55) | |
| Diabetes | 1672 (26) | 2683 (25) | 7033 (27) | 8347 (29) | 7136 (34) | |
| Hypertension | 2451 (39) | 4195 (39) | 10 996 (42) | 12 454 (44) | 9544 (46) | |
Abbreviations: IQR, interquartile range; MET, metabolic equivalent task.
Data are expressed as No. (%) unless otherwise indicated.
Calculated as weight in kilograms divided by height in meters squared.
Scores ranged from 0 (nonadherence to Dietary Guidelines for Americans) to 110 (perfect adherence); higher scores reflect a healthier dietary quality.
Table 2. Baseline Characteristics of Study Participants at Age of 55 Years in the Health Professionals Follow-Up Study (N = 25 303).
| Weight Change Between the Ages of 21 y and 55 ya | |||||
|---|---|---|---|---|---|
| Weight Loss >2.5 kg | Weight Loss ≤2.5 kg or Gain <2.5 kg |
Weight Gain Between ≥2.5 kg and <10.0 kg |
Weight Gain Between ≥10.0 kg and <20.0 kg |
Weight Gain ≥20.0 kg | |
| No. of participants | 1764 (7) | 3500 (14) | 9084 (36) | 7697 (30) | 3258 (13) |
| White race | 1732 (98) | 3418 (98) | 8839 (97) | 7477 (97) | 3180 (98) |
| Age at cohort recruitment, mean (SD), y | 46.9 (5.0) | 46.8 (5.0) | 46.8 (5.0) | 46.8 (5.0) | 46.8 (5.0) |
| Weight at age of 21 y, mean (SD), kg | 84.8 (12.3) | 76.5 (9.6) | 73.8 (9.1) | 73.1 (9.9) | 73.5 (11.8) |
| Body mass indexb | |||||
| At age of 21 y, mean (SD) | 26.4 (3.4) | 24.0 (2.5) | 23.2 (2.4) | 22.7 (2.7) | 22.6 (3.5) |
| <22.5 | 94 (5) | 698 (20) | 2949 (32) | 3211 (42) | 1476 (45) |
| 22.5-25.0 | 355 (20) | 1357 (39) | 3518 (39) | 2444 (32) | 796 (24) |
| >25.0 | 1315 (75) | 1445 (41) | 2617 (29) | 2042 (27) | 986 (30) |
| Weight at age of 55 y, mean (SD), kg | 77.6 (10.7) | 77.0 (9.5) | 80.2 (9.2) | 87.3 (10.2) | 100.6 (14.5) |
| Height, mean (SD), m | 1.8 (0.1) | 1.8 (0.1) | 1.8 (0.1) | 1.8 (0.1) | 1.8 (0.1) |
| Physical activity, median (IQR), MET-h/wk | |||||
| Quintile 1 (<8.3) | 4.3 (2.1-6.0) | 4.2 (2.1-6.2) | 4.5 (2.2-6.4) | 4.2 (2.2-6.2) | 3.8 (1.7-6.0) |
| Quintile 2 (8.3-17.2) | 12.6 (10.2-15.0) | 12.4 (10.6-14.9) | 12.7 (10.6-14.9) | 12.6 (10.4-14.9) | 12.4 (10.1-15.0) |
| Quintile 3 (17.3-28.6) | 22.3 (20.1-25.4) | 22.6 (20.1-25.6) | 22.5 (19.9-25.4) | 22.2 (19.8-25.2) | 21.8 (19.7-25.1) |
| Quintile 4 (28.7-46.9) | 36.7 (33.2-41.1) | 36.6 (32.7-41.6) | 36.5 (32.3-41.4) | 35.9 (32.1-40.8) | 35.5 (31.5-40.6) |
| Quintile 5 (≥47.0) | 69.9 (56.3-92.3) | 67.7 (56.1-89.2) | 65.4 (55.1-83.6) | 63.6 (54.0-81.4) | 62.6 (53.0-78.1) |
| Alcohol consumption, g/d | |||||
| 0-4 | 830 (47) | 1608 (46) | 4099 (45) | 3656 (47) | 1690 (52) |
| 5-9 | 285 (16) | 627 (18) | 1530 (17) | 1289 (17) | 469 (14) |
| 10-14 | 228 (13) | 452 (13) | 1180 (13) | 908 (12) | 334 (10) |
| 15-29 | 264 (15) | 513 (15) | 1455 (16) | 1110 (14) | 443 (14) |
| ≥30 | 157 (9) | 300 (9) | 820 (9) | 734 (10) | 322 (10) |
| Smoking status | |||||
| Never smoker | 923 (52) | 2004 (57) | 5054 (56) | 3889 (51) | 1528 (47) |
| Past smoker | |||||
| <5 pack-years | 154 (9) | 344 (10) | 799 (9) | 681 (9) | 308 (9) |
| 5-20 pack-years | 306 (17) | 573 (16) | 1630 (18) | 1386 (18) | 584 (18) |
| >20 pack-years | 218 (12) | 323 (9) | 1011 (11) | 1198 (16) | 649 (20) |
| Current smoker | |||||
| <5 pack-years | 10 (1) | 24 (1) | 45 (1) | 44 (1) | 12 (<1) |
| 5-20 pack-years | 20 (1) | 42 (1) | 81 (1) | 92 (1) | 27 (1) |
| >20 pack-years | 133 (8) | 190 (5) | 464 (5) | 407 (5) | 150 (5) |
| Alternative Healthy Eating Index, mean (SD)c | |||||
| Quintile 1 | 37.7 (4.7) | 38.5 (4.2) | 38.5 (4.2) | 38.2 (4.3) | 37.8 (4.5) |
| Quintile 2 | 46.8 (1.6) | 46.9 (1.7) | 46.8 (1.8) | 46.7 (1.7) | 46.7 (1.7) |
| Quintile 3 | 52.5 (1.6) | 52.4 (1.6) | 52.4 (1.6) | 52.4 (1.6) | 52.3 (1.6) |
| Quintile 4 | 58.3 (1.9) | 58.4 (1.9) | 58.3 (1.9) | 58.2 (1.8) | 58.1 (1.9) |
| Quintile 5 | 68.7 (5.6) | 68.4 (5.2) | 67.6 (4.9) | 67.2 (4.5) | 66.8 (4.3) |
| Aspirin use | 474 (27) | 852 (24) | 2448 (27) | 2264 (29) | 1041 (32) |
| Self-reported conditions | |||||
| Hypertension | 297 (17) | 501 (14) | 1615 (18) | 1804 (23) | 1136 (35) |
| Diabetes | 63 (4) | 111 (3) | 202 (2) | 235 (3) | 209 (6) |
| Hypercholesterolemia | 323 (18) | 803 (23) | 2332 (26) | 2338 (30) | 1064 (33) |
| Cancer | 49 (3) | 120 (3) | 276 (3) | 245 (3) | 99 (3) |
| Heart diseases | 104 (6) | 163 (5) | 384 (4) | 363 (5) | 199 (6) |
| Cholelithiasis | 65 (4) | 105 (3) | 290 (3) | 326 (4) | 198 (6) |
| Severe osteoarthritis | 61 (3) | 133 (4) | 296 (3) | 235 (3) | 106 (3) |
| Cataracts | 26 (1) | 44 (1) | 107 (1) | 124 (2) | 53 (2) |
| Family history | |||||
| Heart disease | 645 (37) | 1141 (33) | 2914 (32) | 2440 (32) | 1016 (31) |
| Cancer | 585 (33) | 1167 (33) | 3109 (34) | 2472 (32) | 1063 (33) |
| Diabetes | 244 (14) | 452 (13) | 1234 (14) | 1122 (15) | 533 (16) |
| Hypertension | 778 (44) | 1387 (40) | 3658 (40) | 3242 (42) | 1397 (43) |
Abbreviations: IQR, interquartile range; MET, metabolic equivalent task.
Data are expressed as No. (%) unless otherwise indicated.
Calculated as weight in kilograms divided by height in meters squared.
Scores ranged from 0 (nonadherence to Dietary Guidelines for Americans) to 110 (perfect adherence); higher scores reflect a healthier dietary quality.
Relationships Between Weight Change and Incident Rate of Health Outcomes
Separate analyses were conducted for each health outcome after further excluding those with specific prevalent disease at baseline (eFigure 1 in the Supplement). During a mean follow-up of 1 561 919 person-years among women and 343 951 person-years among men, there were 9419 incident cases of type 2 diabetes; 39 585 cases of hypertension; 9399 cases of cardiovascular disease; 9767 cases of obesity-related cancer; 9545 cases of cholelithiasis; 3090 cases of severe osteoarthritis requiring hip replacement; 41 600 cases of cataracts leading to cataract extraction; and 32 422 deaths. A total of 10 919 women (21%) and 6041 men (34%) were classified as having the composite healthy aging outcome in 2010 when the mean age was 76.5 (SD, 7.2) years in women and 70.9 (SD, 4.8) years in men.
In the multivariable models with adjustment for weight at early adulthood and other covariates, weight change from early to middle adulthood was associated with subsequent development of type 2 diabetes, hypertension, cardiovascular disease, cataracts, cholelithiasis, severe osteoarthritis, and mortality (P < .001 for trend for all comparisons except for severe osteoarthritis in men, which had P = .003 for trend; Table 3 and Table 4). Compared with participants who had maintained a stable weight across early and middle life (reference group), those who gained a moderate amount of weight (≥2.5 kg to <10 kg) had higher incidence of type 2 diabetes in women (ARD per 100 000 person-years, 98; IRR, 1.89 [95% CI, 1.66-2.16]) and in men (ARD per 100 000 person-years, 111; IRR, 1.75 [95% CI, 1.39-2.21]); hypertension in women (ARD per 100 000 person-years, 662; IRR, 1.24 [95% CI, 1.20-1.28]) and in men (ARD per 100 000 person-years, 495; IRR, 1.21 [95% CI, 1.12-1.31]); cardiovascular disease in women (ARD per 100 000 person-years, 61; IRR, 1.25 [95% CI, 1.15-1.35]) and in men (ARD per 100 000 person-years, 43; IRR, 1.13 [95% CI, 0.96-1.32]). There were similar results for CHD and stroke (eTable 1 in the Supplement). Moderate weight gain was associated with increased incidence of obesity-related cancer in both women and men (Table 3 and Table 4); such associations were not significant for overall cancer (eTable 1 in the Supplement).
Table 3. Associations of Weight Gain From Early to Middle Adulthood With Major Health Outcomes Later in Life in the Nurses’ Health Study (N = 92 837).
| Weight Loss >2.5 kg | Weight Loss ≤2.5 kg or Gain <2.5 kg |
Weight Gain Between ≥2.5 kg and <10.0 kg |
Weight Gain Between ≥10.0 kg and <20.0 kg |
Weight Gain ≥20.0 kg |
P Value for Trend |
|
|---|---|---|---|---|---|---|
| Type 2 Diabetes | ||||||
| No. of cases | 151 | 268 | 1135 | 2572 | 3681 | |
| Person-years | 121 868 | 214 925 | 516 181 | 497 850 | 283 136 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 80.3 (68.4 to 94.3) | 109.5 (97.2 to 123.3) | 207.2 (195.4 to 219.7) | 493.1 (473.9 to 513.0) | 1150 (1108 to 1193) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −29.2 (−43.7 to −11.5) | 0 | 97.8 (72.1 to 127.1) | 383.7 (325.6 to 449.7) | 1040.8 (905.1 to 1194.6) | |
| Age-adjusted IRR (95% CI)b | 0.99 (0.81 to 1.21) | 1 [Reference] | 1.77 (1.55 to 2.02) | 4.18 (3.68 to 4.74) | 10.61 (9.37 to 12.01) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 0.73 (0.60 to 0.90) | 1 [Reference] | 1.89 (1.66 to 2.16) | 4.50 (3.97 to 5.11) | 10.51 (9.27 to 11.91) | <.001 |
| Hypertension | ||||||
| No. of cases | 2222 | 3890 | 10 099 | 10 601 | 6181 | |
| Person-years | 77 201 | 136 158 | 296 100 | 248 338 | 108 987 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 2486 (2384 to 2592) | 2754 (2672 to 2837) | 3415 (3352 to 3480) | 4364 (4284 to 4445) | 5784 (5639 to 5934) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −267.7 (−388.6 to −140.7) | 0 | 661.5 (545.0 to 782.4) | 1610.3 (1458.9 to 1767.0) | 3031.3 (2804.3 to 3267.3) | |
| Age-adjusted IRR (95% CI)b | 1.01 (0.96 to 1.06) | 1 [Reference] | 1.19 (1.15 to 1.24) | 1.49 (1.44 to 1.54) | 1.98 (1.90 to 2.06) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 0.90 (0.86 to 0.95) | 1 [Reference] | 1.24 (1.20 to 1.28) | 1.58 (1.53 to 1.64) | 2.10 (2.02 to 2.19) | <.001 |
| Cardiovascular Disease | ||||||
| No. of cases | 617 | 815 | 2230 | 2206 | 1779 | |
| Person-years | 122 540 | 216 114 | 523 708 | 529 870 | 342 048 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 267.8 (245.2 to 292.6) | 247.9 (230.7 to 266.5) | 309.2 (295.2 to 323.9) | 334.4 (319.7 to 349.8) | 464.3 (442.4 to 487.3) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | 19.9 (−7.0 to 49.8) | 0 | 61.3 (37.6 to 86.9) | 86.5 (60.5 to 114.7) | 216.4 (178.5 to 257.6) | |
| Age-adjusted IRR (95% CI)b | 1.32 (1.19 to 1.46) | 1 [Reference] | 1.17 (1.08 to 1.26) | 1.23 (1.14 to 1.33) | 1.74 (1.60 to 1.89) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 1.08 (0.97 to 1.20) | 1 [Reference] | 1.25 (1.15 to 1.35) | 1.35 (1.24 to 1.46) | 1.87 (1.72 to 2.04) | <.001 |
| Obesity-Related Cancerc | ||||||
| No. of cases | 500 | 932 | 2445 | 2873 | 2154 | |
| Person-years | 122 744 | 213 041 | 519 500 | 527 937 | 346 115 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 380.7 (346.8 to 418.0) | 415.0 (388.6 to 443.3) | 452.1 (433.9 to 471.0) | 535.1 (515.5 to 555.5) | 629.5 (602.9 to 657.1) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −34.3 (−74.3 to 10.3) | 0 | 37.0 (4.0 to 72.6) | 120.1 (81.3 to 161.9) | 214.4 (166.3 to 266.4) | |
| Age-adjusted IRR (95% CI)b | 0.93 (0.83 to 1.04) | 1 [Reference] | 1.08 (1.00 to 1.16) | 1.26 (1.17 to 1.35) | 1.45 (1.34 to 1.57) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 0.92 (0.82 to 1.02) | 1 [Reference] | 1.09 (1.01 to 1.17) | 1.29 (1.20 to 1.39) | 1.52 (1.40 to 1.64) | <.001 |
| Obesity-Related Cancer Among Never Smokersc | ||||||
| No. of cases | 145 | 343 | 1040 | 1320 | 1086 | |
| Person-years | 38 025 | 85 685 | 231 690 | 250 532 | 173 407 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 344.0 (290.0 to 408.2) | 373.9 (335.5 to 416.6) | 426.4 (400.3 to 454.2) | 517.3 (489.7 to 546.4) | 632.5 (595.6 to 671.8) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −29.8 (−91.5 to 45.3) | 0 | 52.5 (3.4 to 108.0) | 143.4 (84.8 to 209.5) | 258.7 (184.6 to 342.6) | |
| Age-adjusted IRR (95% CI)b | 0.95 (0.78 to 1.16) | 1 [Reference] | 1.13 (1.00 to 1.27) | 1.33 (1.18 to 1.50) | 1.60 (1.42 to 1.81) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 0.92 (0.76 to 1.12) | 1 [Reference] | 1.14 (1.01 to 1.29) | 1.38 (1.23 to 1.56) | 1.69 (1.49 to 1.92) | <.001 |
| Cholelithiasis | ||||||
| No. of cases | 364 | 703 | 2161 | 2964 | 2359 | |
| Person-years | 110 982 | 199 537 | 472 275 | 457 281 | 263 404 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 242.7 (217.6 to 270.8) | 316.4 (293.1 to 341.5) | 436.8 (417.8 to 456.7) | 640.7 (615.9 to 666.5) | 874.1 (834.7 to 915.3) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −73.7 (−103.3 to −39.8) | 0 | 120.4 (84.2 to 160.0) | 324.3 (271.9 to 381.5) | 557.8 (482.9 to 639.7) | |
| Age-adjusted IRR (95% CI)b | 0.93 (0.82 to 1.05) | 1 [Reference] | 1.30 (1.20 to 1.42) | 1.87 (1.72 to 2.03) | 2.62 (2.40 to 2.85) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 0.77 (0.67 to 0.87) | 1 [Reference] | 1.38 (1.27 to 1.51) | 2.03 (1.86 to 2.21) | 2.76 (2.53 to 3.02) | <.001 |
| Severe Osteoarthritis | ||||||
| No. of cases | 204 | 306 | 818 | 814 | 514 | |
| Person-years | 120 651 | 211 517 | 516 058 | 520 985 | 342 129 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 86.4 (73.2 to 100.8) | 92.4 (81.6 to 104.4) | 110.4 (102.0 to 120.0) | 120.0 (111.6 to 129.6) | 128.4 (116.4 to 141.6) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −5.8 (−20.4 to 11.9) | 0 | 18.7 (4.8 to 34.7) | 28.2 (12.9 to 45.8) | 36.8 (18.8 to 57.7) | |
| Age-adjusted IRR (95% CI)b | 1.16 (0.97 to 1.38) | 1 [Reference] | 1.14 (1.00 to 1.30) | 1.21 (1.06 to 1.39) | 1.33 (1.15 to 1.53) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 0.94 (0.78 to 1.13) | 1 [Reference] | 1.20 (1.05 to 1.38) | 1.31 (1.14 to 1.50) | 1.40 (1.20 to 1.62) | <.001 |
| Cataract Extraction | ||||||
| No. of cases | 2607 | 4590 | 11 083 | 11 189 | 7255 | |
| Person-years | 106 238 | 186 004 | 455 982 | 468 244 | 310 332 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 2152 (2081 to 2226) | 2240 (2186 to 2294) | 2255 (2219 to 2291) | 2293 (2257 to 2329) | 2345 (2299 to 2393) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −87.5 (−171.0 to −0.6) | 0 | 15.1 (−46.2 to 78.2) | 53.2 (−10.5 to 118.6) | 105.9 (33.6 to 180.5) | |
| Age-adjusted IRR (95% CI)b | 0.99 (0.95 to 1.02) | 1 [Reference] | 1.00 (0.97 to 1.03) | 1.01 (0.98 to 1.04) | 1.04 (1.01 to 1.07) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 0.96 (0.92 to 1.00) | 1 [Reference] | 1.01 (0.98 to 1.03) | 1.02 (1.00 to 1.05) | 1.05 (1.02 to 1.08) | <.001 |
| Mortality | ||||||
| No. of cases | 2716 | 3701 | 8331 | 8021 | 5740 | |
| Person-years | 126 457 | 221 939 | 540 076 | 546 068 | 354 830 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 1114 (1076 to 1153) | 1050 (1020 to 1080) | 1071 (1049 to 1093) | 1133 (1110 to 1156) | 1411 (1380 to 1443) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | 64.5 (21.5 to 109.2) | 0 | 21.1 (−10.8 to 54.2) | 83.5 (48.7 to 119.4) | 361.6 (314.7 to 410.2) | |
| Age-adjusted IRR (95% CI)b | 1.27 (1.22 to 1.32) | 1 [Reference] | 0.96 (0.93 to 0.99) | 0.99 (0.96 to 1.02) | 1.25 (1.21 to 1.29) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 1.06 (1.02 to 1.10) | 1 [Reference] | 1.02 (0.99 to 1.05) | 1.08 (1.05 to 1.11) | 1.34 (1.30 to 1.39) | <.001 |
| Mortality Among Never Smokers | ||||||
| No. of cases | 528 | 1085 | 2956 | 3243 | 2655 | |
| Person-years | 39 231 | 88 894 | 240 739 | 259 997 | 178 126 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 745.9 (691.4 to 804.6) | 751.0 (711.4 to 792.7) | 801.7 (773.0 to 831.5) | 877.9 (848.4 to 908.4) | 1144 (1104 to 1186) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −5.1 (−66.6 to 61.9) | 0 | 50.8 (6.4 to 97.7) | 126.9 (78.5 to 178.2) | 392.9 (327.5 to 462.4) | |
| Age-adjusted IRR (95% CI)b | 1.12 (1.03 to 1.21) | 1 [Reference] | 1.04 (0.99 to 1.10) | 1.15 (1.08 to 1.21) | 1.56 (1.47 to 1.65) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 0.99 (0.91 to 1.08) | 1 [Reference] | 1.07 (1.01 to 1.13) | 1.17 (1.10 to 1.24) | 1.52 (1.44 to 1.62) | <.001 |
| Composite Healthy Aging Outcomed | ||||||
| Sample size | 2918 | 5764 | 14 910 | 16 329 | 11 019 | |
| No. of participants who achieved outcome | 753 | 1528 | 3651 | 3484 | 1503 | |
| Age-adjusted OR (95% CI)b | 0.97 (0.87 to 1.08) | 1 [Reference] | 0.81 (0.75 to 0.87) | 0.57 (0.53 to 0.61) | 0.26 (0.24 to 0.28) | <.001 |
| Multivariable-adjusted OR (95% CI)a | 1.22 (1.09 to 1.37) | 1 [Reference] | 0.78 (0.72 to 0.84) | 0.56 (0.52 to 0.60) | 0.28 (0.26 to 0.31) | <.001 |
Abbreviations: ARD, absolute rate difference; IRR, incident rate ratio; OR, odds ratio.
Included age at cohort recruitment (continuous), height (continuous), race (nonwhite or white), pack-years of smoking (never smokers; past smoker with <5, 5-20, or >20 pack-years; and current smoker with <5, 5-20, or >20 pack-years), regular aspirin use (yes or no), status of menopause and hormone therapy (premenopausal, postmenopausal and never use; postmenopausal and current use; or postmenopausal and past use), parity (nulliparous, 1, 2, 3, or ≥4 children), physical activity (in quintiles: <2.9, 2.9-7.1, 7.2-13.1, 13.2-23.6, or ≥23.7 metabolic equivalent task h/wk), alcohol consumption (0, 0.1-0.4, 0.5-1.9, 2.0-7.0, or ≥8.0 g/d), dietary qualify (Alternative Healthy Eating Index in quintiles), family history of respective diseases, and weight at age of 18 years.
Included age at cohort recruitment (continuous).
Included the esophagus (adenocarcinoma only), colon and rectum, pancreas, breast (after menopause), endometrium, ovaries, kidney, liver, and gallbladder.
Defined as meeting all of the following criteria: (1) no self-reported history of cancer, diabetes, coronary heart disease, coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty, congestive heart failure, stroke, kidney failure, chronic obstructive pulmonary disease, Parkinson disease, multiple sclerosis, or amyotrophic lateral sclerosis; (2) no cognitive decline; and (3) no physical limitations.
Table 4. Associations of Weight Gain From Early to Middle Adulthood With Major Health Outcomes Later in Life in the Health Professionals Follow-Up Study (N = 25 303).
| Weight Loss >2.5 kg | Weight Loss ≤2.5 kg or Gain <2.5 kg |
Weight Gain Between ≥2.5 kg and <10.0 kg |
Weight Gain Between ≥10.0 kg and <20.0 kg |
Weight Gain ≥20.0 kg |
P Value for Trend |
|
|---|---|---|---|---|---|---|
| Type 2 Diabetes | ||||||
| No. of cases | 56 | 87 | 370 | 608 | 491 | |
| Person-years | 25 145 | 50 920 | 131 069 | 103 693 | 37 569 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 131.4 (99.7 to 173.1) | 147.3 (119.3 to 181.9) | 258.4 (233.0 to 286.6) | 533.0 (490.2 to 579.6) | 1142 (1033 to 1262) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −16.0 (−54.1 to 37.7) | 0 | 111.1 (57.5 to 178.7) | 385.6 (277.9 to 520.5) | 993.9 (757.2 to 1292.7) | |
| Age-adjusted IRR (95% CI)b | 1.30 (0.93 to 1.82) | 1 [Reference] | 1.66 (1.31 to 2.09) | 3.47 (2.77 to 4.34) | 7.78 (6.20 to 9.77) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 0.89 (0.63 to 1.26) | 1 [Reference] | 1.75 (1.39 to 2.21) | 3.62 (2.89 to 4.53) | 7.75 (6.14 to 9.78) | <.001 |
| Hypertension | ||||||
| No. of cases | 396 | 844 | 2370 | 2165 | 817 | |
| Person-years | 16 442 | 34 451 | 81 361 | 55 935 | 16 755 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 2022 (1825 to 2241) | 2366 (2211 to 2532) | 2861 (2746 to 2981) | 3859 (3694 to 4031) | 5009 (4658 to 5387) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −343.6 (−572.0 to −86.1) | 0 | 494.9 (280.8 to 726.4) | 1492.9 (1192.7 to 1818.6) | 2642.8 (2161.2 to 3175.7) | |
| Age-adjusted IRR (95% CI)b | 0.98 (0.87 to 1.11) | 1 [Reference] | 1.19 (1.10 to 1.29) | 1.58 (1.46 to 1.71) | 2.00 (1.81 to 2.20) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 0.85 (0.76 to 0.96) | 1 [Reference] | 1.21 (1.12 to 1.31) | 1.63 (1.50 to 1.77) | 2.12 (1.91 to 2.34) | <.001 |
| Cardiovascular Disease | ||||||
| No. of cases | 107 | 210 | 590 | 581 | 264 | |
| Person-years | 25 868 | 51 631 | 132 748 | 108 068 | 43 279 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 291.6 (238.0 to 357.2) | 339.6 (294.8 to 391.2) | 382.6 (350.8 to 417.3) | 471.8 (432.8 to 514.4) | 545.7 (481.2 to 618.7) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −48.0 (−109.1 to 29.2) | 0 | 43.0 (−13.5 to 109.2) | 132.2 (61.1 to 216.0) | 206.0 (111.6 to 320.2) | |
| Age-adjusted IRR (95% CI)b | 1.02 (0.81 to 1.28) | 1 [Reference] | 1.11 (0.95 to 1.30) | 1.38 (1.18 to 1.62) | 1.62 (1.35 to 1.93) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 0.86 (0.68 to 1.09) | 1 [Reference] | 1.13 (0.96 to 1.32) | 1.39 (1.18 to 1.64) | 1.61 (1.33 to 1.94) | <.001 |
| Obesity-Related Cancerc | ||||||
| No. of cases | 63 | 103 | 331 | 243 | 123 | |
| Person-years | 27 046 | 54 258 | 138 634 | 114 475 | 46 258 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 189.5 (145.9 to 246.2) | 165.3 (135.4 to 201.9) | 207.5 (184.7 to 233.3) | 185.4 (162.4 to 211.7) | 231.8 (192.5 to 279.1) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | 24.2 (−27.4 to 95.1) | 0 | 42.2 (0.5 to 94.4) | 20.1 (−18.9 to 69.4) | 66.4 (10.9 to 139.3) | |
| Age-adjusted IRR (95% CI)b | 1.23 (0.90 to 1.68) | 1 [Reference] | 1.27 (1.02 to 1.59) | 1.16 (0.92 to 1.46) | 1.50 (1.15 to 1.95) | .06 |
| Multivariable-adjusted IRR (95% CI)a | 1.15 (0.83 to 1.58) | 1 [Reference] | 1.26 (1.00 to 1.57) | 1.12 (0.89 to 1.42) | 1.40 (1.07 to 1.84) | .13 |
| Obesity-Related Cancer Among Never Smokersc | ||||||
| No. of cases | 25 | 55 | 153 | 95 | 51 | |
| Person-years | 13 285 | 28 283 | 71 482 | 52 623 | 19 752 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 143.0 (95.2 to 215.0) | 163.7 (124.5 to 215.2) | 184.1 (154.4 to 219.6) | 163.7 (132.6 to 202.1) | 238.9 (179.4 to 318.2) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −20.7 (−74.9 to 66.6) | 0 | 20.4 (−29.2 to 88.3) | 0 (−47.7 to 67.2) | 75.2 (−3.7 to 193.0) | |
| Age-adjusted IRR (95% CI)b | 0.96 (0.60 to 1.54) | 1 [Reference] | 1.11 (0.82 to 1.52) | 0.97 (0.70 to 1.36) | 1.44 (0.98 to 2.10) | .16 |
| Multivariable-adjusted IRR (95% CI)a | 0.87 (0.54 to 1.41) | 1 [Reference] | 1.12 (0.82 to 1.54) | 1.00 (0.71 to 1.41) | 1.46 (0.98 to 2.18) | .08 |
| Cholelithiasis | ||||||
| No. of cases | 60 | 119 | 327 | 328 | 160 | |
| Person-years | 24 964 | 50 097 | 129 188 | 103 912 | 40 330 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 158.7 (120.6 to 209.0) | 192.7 (159.6 to 232.8) | 213.0 (190.1 to 238.8) | 270.7 (240.6 to 304.5) | 339.5 (287.3 to 401.1) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −34.0 (−77.8 to 26.5) | 0 | 20.3 (−20.6 to 70.8) | 77.9 (25.1 to 143.5) | 146.7 (70.9 to 244.3) | |
| Age-adjusted IRR (95% CI)b | 1.01 (0.74 to 1.38) | 1 [Reference] | 1.08 (0.88 to 1.34) | 1.39 (1.12 to 1.71) | 1.81 (1.42 to 2.29) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 0.82 (0.60 to 1.14) | 1 [Reference] | 1.11 (0.89 to 1.37) | 1.40 (1.13 to 1.74) | 1.76 (1.37 to 2.27) | <.001 |
| Severe Osteoarthritis | ||||||
| No. of cases | 31 | 67 | 168 | 114 | 54 | |
| Person-years | 26 880 | 52 867 | 137 294 | 113 780 | 45 901 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 49.2 (33.6 to 73.2) | 81.6 (62.4 to 106.8) | 94.8 (79.2 to 111.6) | 87.6 (72.0 to 106.8) | 114.0 (86.4 to 148.8) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −32.0 (−49.5 to −4.9) | 0 | 12.4 (−10.8 to 43.4) | 6.0 (−17.3 to 37.7) | 31.9 (−2.9 to 82.1) | |
| Age-adjusted IRR (95% CI)b | 0.91 (0.59 to 1.39) | 1 [Reference] | 0.97 (0.73 to 1.29) | 0.81 (0.60 to 1.10) | 0.97 (0.68 to 1.40) | .61 |
| Multivariable-adjusted IRR (95% CI)a | 0.61 (0.39 to 0.94) | 1 [Reference] | 1.15 (0.87 to 1.53) | 1.07 (0.79 to 1.46) | 1.39 (0.96 to 2.01) | .003 |
| Cataract Extraction | ||||||
| No. of cases | 370 | 640 | 1722 | 1512 | 632 | |
| Person-years | 25 405 | 51 122 | 130 969 | 106 938 | 43 238 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 1192 (1077 to 1318) | 1093 (1013 to 1179) | 1165 (1111 to 1221) | 1269 (1207 to 1334) | 1322 (1226 to 1426) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | 98.4 (−37.0 to 251.1) | 0 | 71.3 (−23.8 to 174.9) | 175.9 (68.1 to 293.6) | 229.2 (95.5 to 378.0) | |
| Age-adjusted IRR (95% CI)b | 1.16 (1.03 to 1.31) | 1 [Reference] | 1.07 (0.98 to 1.16) | 1.18 (1.08 to 1.29) | 1.26 (1.13 to 1.39) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 1.09 (0.97 to 1.23) | 1 [Reference] | 1.07 (0.98 to 1.16) | 1.16 (1.06 to 1.27) | 1.21 (1.09 to 1.35) | <.001 |
| Mortality | ||||||
| No. of cases | 309 | 541 | 1305 | 1147 | 611 | |
| Person-years | 26 457 | 52 873 | 136 900 | 112 106 | 44 782 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 746.2 (667.7 to 834.0) | 780.9 (717.9 to 849.3) | 748.5 (707.8 to 791.6) | 804.1 (758.3 to 852.7) | 1082 (1001 to 1170) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −34.6 (−125.5 to 68.9) | 0 | −32.4 (−99.2 to 41.1) | 23.3 (−51.0 to 105.2) | 301.3 (187.5 to 428.5) | |
| Age-adjusted IRR (95% CI)b | 1.14 (1.00 to 1.30) | 1 [Reference] | 0.95 (0.86 to 1.04) | 1.06 (0.96 to 1.17) | 1.47 (1.33 to 1.64) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 0.96 (0.84 to 1.09) | 1 [Reference] | 0.96 (0.87 to 1.05) | 1.03 (0.93 to 1.13) | 1.39 (1.24 to 1.55) | <.001 |
| Mortality Among Never Smokers | ||||||
| No. of cases | 114 | 228 | 547 | 420 | 221 | |
| Person-years | 13 008 | 27 964 | 70 938 | 51 971 | 19 186 | |
| Multivariable-adjusted incidence/100 000 person-yearsa | 538.8 (446.4 to 650.3) | 607.6 (533.1 to 692.5) | 598.3 (547.4 to 653.8) | 666.3 (604.5 to 734.5) | 952.2 (837.2 to 1083.0) | |
| Multivariable-adjusted ARD/100 000 person-yearsa | −68.8 (−172.7 to 60.0) | 0 | −9.3 (−90.5 to 84.6) | 58.7 (−36.3 to 169.5) | 344.7 (189.7 to 529.7) | |
| Age-adjusted IRR (95% CI)b | 1.07 (0.87 to 1.31) | 1 [Reference] | 0.96 (0.83 to 1.11) | 1.07 (0.92 to 1.24) | 1.60 (1.35 to 1.90) | <.001 |
| Multivariable-adjusted IRR (95% CI)a | 0.89 (0.72 to 1.10) | 1 [Reference] | 0.98 (0.85 to 1.14) | 1.10 (0.94 to 1.28) | 1.57 (1.31 to 1.87) | <.001 |
| Composite Healthy Aging Outcomed | ||||||
| Sample size | 1266 | 2538 | 6564 | 5377 | 1989 | |
| No. of participants who achieved outcome | 471 | 989 | 2405 | 1684 | 492 | |
| Age-adjusted OR (95% CI)b | 0.96 (0.83 to 1.11) | 1 [Reference] | 0.87 (0.79 to 0.96) | 0.63 (0.57 to 0.70) | 0.42 (0.37 to 0.48) | <.001 |
| Multivariable-adjusted OR (95% CI)a | 1.14 (0.98 to 1.33) | 1 [Reference] | 0.88 (0.79 to 0.97) | 0.66 (0.59 to 0.73) | 0.45 (0.39 to 0.52) | <.001 |
Abbreviations: ARD, absolute rate difference; IRR, incident rate ratio; OR, odds ratio.
Included age at cohort recruitment (continuous), height (continuous), race (nonwhite or white), pack-years of smoking (never smokers; past smoker with <5, 5-20, or >20 pack-years; and current smoker with <5, 5-20, or >20 pack-years), regular aspirin use (yes or no), physical activity (in quintiles: <8.3, 8.3-17.2, 17.3-28.6, 28.7-46.9, or ≥47.0 metabolic equivalent task h/wk), alcohol consumption (0-4, 5-9, 10-14, 15-29, or ≥30 g/d), dietary qualify (Alternative Healthy Eating Index in quintiles), family history of respective diseases, and weight at age of 21 years.
Included age at cohort recruitment (continuous).
Included the esophagus (adenocarcinoma only), colon and rectum, pancreas, prostate (advanced only), kidney, liver, and gallbladder.
Defined as meeting all of the following criteria: (1) no self-reported history of cancer, diabetes, coronary heart disease, coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty, congestive heart failure, stroke, kidney failure, chronic obstructive pulmonary disease, Parkinson disease, multiple sclerosis, or amyotrophic lateral sclerosis; (2) no cognitive decline; and (3) no physical limitations.
Moderate weight gain was associated with increased incidence of cholelithiasis and severe osteoarthritis in women only (Table 3). Moderate weight gain was not associated with incidence of cataracts leading to cataract extraction in either sex. Nevertheless, more than moderate weight gain was generally associated with increased incidence of these diseases in both women and men. Moderate weight gain was associated with increased mortality only in women who never smoked (ARD per 100 000 person-years, 51; IRR, 1.07 [95% CI, 1.01-1.13]), whereas an extreme weight gain of more than 20 kg was associated with 393 (in women who never smoked) and 345 (in men who never smoked) additional deaths per 100 000 person-years. Weight loss was not associated with higher mortality in participants who never smoked (Table 3 and Table 4). The association of weight change with mortality was more pronounced among women who never smoked than that among those who ever smoked (P < .001 for interaction), but not among men. The magnitude of the association with cardiovascular mortality was larger than with cancer mortality, and both associations were statistically significant (eTable 2 in the Supplement). Further adjustment of prevalence of hypertension, hypercholesterolemia, diabetes, the use of antihypertensive and cholesterol-lowering medications, and cancer screening (eTable 3 in the Supplement), or use of antidepressants and oral steroids (eTable 4 in the Supplement) for selected outcomes did not materially change the results.
A total of 3651 women (24%) and 2405 men (37%) who gained a moderate amount of weight achieved the composite healthy aging outcome, whereas 1528 women (27%) and 989 men (39%) who maintained a stable weight achieved the composite healthy aging outcome. The multivariable-adjusted odds ratio for the composite healthy aging outcome associated with moderate weight gain was 0.78 (95% CI, 0.72-0.84) in women and 0.88 (95% CI, 0.79-0.97) in men</quizref> (Table 3 and Table 4).
The quintile analyses showed similar results for major health outcomes (eTables 5 and 6 in the Supplement). The estimated increased incidence for participants with moderate weight gain (quintile 2) and extreme weight gain (quintile 5) compared with those who lost weight or maintained weight (quintile 1) was comparable with that in main analyses for participants who gained a moderate amount of weight (2.5-10.0 kg) and those who gained an extreme amount of weight (>20 kg) compared with those who maintained a stable weight (weight loss ≤2.5 kg or gain <2.5 kg).
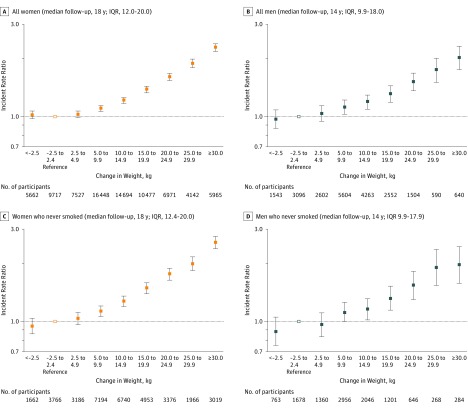
A monotonic increase in the risk of a composite measure of major chronic diseases was observed throughout the range of weight gain categories, and this increased risk started to be statistically significant with increasing weight gain beginning with the participants who gained 5.0 to 9.9 kg (IRR, 1.11 [95% CI, 1.07-1.14] in women; IRR, 1.12 [95% CI, 1.03-1.21] in men; Figure 1A and Figure 1B). Weight loss was not significantly associated with the incidence rate of a composite measure of major chronic diseases (Figure 1). Between the ages of 55 and 75 years, 45.5% of women and 41.4% of men who gained more than 30 kg developed at least 1 major chronic health outcome compared with 26.8% of women and men who maintained their weight (eFigure 2 in the Supplement).
Figure 1. Associations of Weight Gain From Early to Middle Adulthood With the Risk of a Composite Outcome Measure of Major Chronic Diseases.
Assessed from the age of 55 years to 2012. The composite outcome measure of major chronic diseases was composed of incident cases of type 2 diabetes, cardiovascular disease, cancer, and nontraumatic death. Among the 81 603 women, 35 148 developed at least 1 of the major chronic diseases, including 14 709 cases of cancer, 6888 cases of type 2 diabetes, 4317 cases of cardiovascular disease, and 9234 nontraumatic deaths. Among the 22 394 men, 6831 developed at least 1 of the major chronic diseases, including 3608 cases of cancer, 1302 cases of type 2 diabetes, 953 cases of cardiovascular disease, and 968 nontraumatic deaths. After adjustment for age at cohort recruitment (continuous), height (continuous), race (nonwhite or white), pack-years of smoking (never smokers; past smoker with <5, 5-20, or >20 pack-years; and current smoker with <5, 5-20, or >20 pack-years), regular aspirin use (yes or no), status of menopause and hormone therapy (women only: premenopausal, postmenopausal and hormone therapy never use, postmenopausal and current use, or postmenopausal and past use), parity (women only: nulliparous, 1, 2, 3, or ≥4 children), physical activity (in quintiles for women: <2.9, 2.9-7.1, 7.2-13.1, 13.2-23.6, or ≥23.7 metabolic equivalent task [MET]-h/wk; and men: <8.3, 8.3-17.2, 17.3-28.6, 28.7-46.9, or ≥47.0 MET-h/wk), alcohol consumption (women: 0, 0.1-0.4, 0.5-1.9, 2.0-7.0, or ≥8.0 g/d; men: 0-4, 5-9, 10-14, 15-29, or ≥30 g/d), dietary qualify (Alternative Healthy Eating Index in quintiles), family history of diabetes, cardiovascular disease, cancer, and weight at age of 18 years in women and at age of 21 years in men. IQR indicates interquartile range; error bars, 95% CIs.
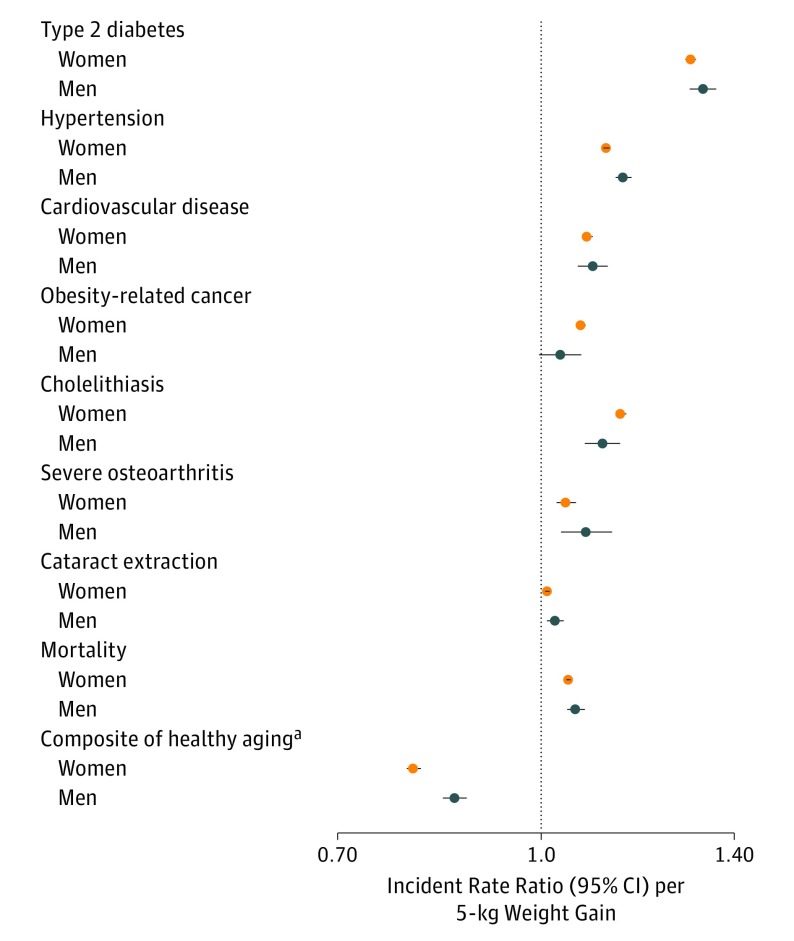
The associations of per 5-kg weight gain with an increased incident rate of individual health outcomes and decreased odds of achieving the composite healthy aging outcome appear in Figure 2. In multivariable-adjusted models, each 5-kg weight gain was associated with increased risk of type 2 diabetes in women (ARD per 100 000 person-years, 142.6 [95% CI, 136.7 to 148.6]) and in men (ARD per 100 000 person-years, 151 [95% CI, 136.9 to 165.5]); hypertension in women (ARD per 100 000 person-years, 458.8 [95% CI, 436.9 to 480.8]) and in men (ARD per 100 000 person-years, 495.7 [95% CI, 443.0 to 549.2]); cardiovascular disease in women (ARD per 100 000 person-years, 36.9 [95% CI, 32.2 to 41.5]) and in men (ARD per 100 000 person-years, 45.5 [95% CI, 32.2 to 59.2]); obesity-related cancer in women (ARD per 100 000 person-years, 36.9 [95% CI, 32.0 to 41.8]) and in men (ARD per 100 000 person-years, 7.6 [95% CI, −0.9 to 16.3]); and mortality in women (ARD per 100 000 person-years, 76.7 [95% CI, 69.6 to 83.9]) and in men (ARD per 100 000 person-years, 65.1 [95% CI, 47.7 to 82.8]).
Figure 2. Associations of Weight Gain From Early to Middle Adulthood With Risk of Individual Health Outcomes.
After adjustment for age at cohort recruitment (continuous), height (continuous), race (nonwhite or white), pack-years of smoking (never smokers; past smoker with <5, 5-20, or >20 pack-years; and current smoker with <5, 5-20, or >20 pack-years), regular aspirin use (yes or no), status of menopause and hormone therapy (women only: premenopausal, postmenopausal and never use, postmenopausal and current use, or postmenopausal and past use), parity (women only: nulliparous, 1, 2, 3, or ≥4 children), physical activity (in quintiles for women: <2.9, 2.9-7.1, 7.2-13.1, 13.2-23.6, or ≥23.7 metabolic equivalent task [MET]-h/wk; and men: <8.3, 8.3-17.2, 17.3-28.6, 28.7-46.9, or ≥47.0 MET-h/wk), alcohol consumption (women: 0, 0.1-0.4, 0.5-1.9, 2-7, or ≥8 g/d; men: 0-4, 5-9, 10-14, 15-29, or ≥30 g/d), dietary qualify (Alternative Healthy Eating Index in quintiles), family history of respective diseases and weight at age of 18 years in women and at age of 21 years in men. Obesity-related cancer includes the esophagus (adenocarcinoma only), colon and rectum, pancreas, breast (after menopause, women only), endometrium (women only), ovaries (women only), prostate (advanced only, men only), kidney, liver, and gallbladder.
aA composite healthy aging outcome was defined as being free of 11 chronic diseases and major cognitive or physical impairment. Expressed as odds ratio (95% CI) per 5-kg weight gain.
In a meta-analysis of women and men, the pooled IRR per 5-kg weight gain was 1.31 (95% CI, 1.28-1.33) for type 2 diabetes (I2 = 59%); 1.14 (95% CI, 1.10-1.17) for hypertension (I2 = 93%); 1.08 (95% CI, 1.08-1.09) for cardiovascular disease (I2 = 0%); 1.06 (95% CI, 1.02-1.09) for obesity-related cancer (I2 = 72%); 1.05 (95% CI, 1.04-1.07 for mortality (I2 = 60%); and 1.08 (95% CI, 1.07-1.08) for mortality among never smokers (I2 = 0%). The pooled odds ratio per 5-kg weight gain was 0.83 (95% CI, 0.77-0.89) for the composite healthy aging outcome (I2 = 97%). The cubic splines of weight change with selected individual health outcomes, including type 2 diabetes, cardiovascular disease, cancer, and mortality appear in eFigure 3 in the Supplement. The increased risk for developing type 2 diabetes was more pronounced with higher levels of weight gain (eg, >10 kg) (P < .001 for nonlinearity).
In the joint analyses of early adulthood BMI and weight change since early adulthood, the highest incident rate for chronic diseases was observed in the group with the highest BMI levels at early adulthood and the largest amount of weight gain in both women and men (eFigure 4 in the Supplement). These associations were independent of each other for incident rate of type 2 diabetes and cardiovascular disease (P < .001 for main effect), as well as for mortality among participants who never smoked (P < .001 for main effect). The joint association of BMIs at young and middle adulthood was also examined (eFigure 5 in the Supplement). Per unit increase of BMI at middle adulthood compared with that at early adulthood was associated with a higher incident rate of type 2 diabetes among both women and men (P < .001 for interaction between BMIs at young and middle adulthood).
Discussion
In 2 large, long-term cohorts, weight gain from early to middle adulthood was associated with increased risk of morbidity and mortality, and decreased odds of achieving the composite healthy aging outcome among women and men, independent of weight at early adulthood. Weight gain as little as 5 kg was associated with significantly elevated incidence of a composite measure of major chronic diseases, consisting of type 2 diabetes, cardiovascular disease, cancer, and nontraumatic death.
The adverse metabolic effects of excess adiposity are known to increase the risk of cardiometabolic disease. The proposed mechanisms include the development of dyslipidemia and insulin resistance, enhanced secretion of proinflammatory cytokines, and overactivation of the sympathetic nervous system. Weight gain is associated with most metabolic conditions. In the current study, the magnitude of associated risk varied for specific disorders, and the strongest direct association was seen for type 2 diabetes, followed by cholelithiasis and hypertension.
The associations with adiposity may vary according to characteristics of cancer; breast, endometrium, colon and rectum, kidney, pancreas, esophagus, gallbladder, ovaries, thyroid, and possibly prostate are classified as types of obesity-related cancer. In the current study, most women were postmenopausal and not receiving hormonal therapy, and a direct association of adulthood weight change with cancer and obesity-related cancer (in which breast cancer was the major contribution) was observed in women (especially those who never smoked). In men, the association with risk of cancer (in which colorectal cancer and advanced prostate cancer were the main components) was less clear, possibly in part due to a smaller sample size. For colorectal cancer, the association with early adulthood adiposity was particularly important, and a substantial increase in risk was seen in men who were overweight during early adulthood and subsequently gained the most weight (IRR, 3.5; 95% CI, 1.5-8.3). This finding is consistent with previous reports of weight trajectories and weight gain on obesity-related cancer.
Worldwide, osteoarthritis is one of the leading causes of disability and cataracts are the principal cause of blindness and visual impairment. The current study indicated that weight gain from early to middle adulthood was associated with increased risk of severe hip osteoarthritis and clinically significant cataracts leading to extraction. To the best of our knowledge, this is the first cohort study on early- to middle-life weight change and risk of clinically significant cataracts leading to extraction.
In the current cohorts of women and men, adulthood weight gain was associated with lower odds of the composite healthy aging outcome later in life, defined by a full spectrum of morbidity and functional outcomes. These findings confirm previous results from smaller studies of weight change and a decreased probability of achieving the composite healthy aging outcome, and those reporting adiposity was associated with individual components such as cognitive decline, physical limitations, and impaired health-related quality of life. Such data provide strong evidence that maintaining a healthy weight throughout early and middle adulthood is associated with overall health in those who survive to older ages.
Compared with the current study linking weight gain with mortality, many previous studies have reported no effect of weight gain on mortality, or linked weight loss to a higher risk of death. Confounding by smoking and reverse causation (ie, weight loss due to chronic conditions) may have contributed to those findings. By assessing weight change from the ages of 18 or 21 years to the age of 55 years, the current study attempted to capture the changes in fat mass during adulthood before the major age-related loss of muscle mass starts, and to minimize reverse causation due to ill health. In the current study, the analyses were repeated among never smokers only, in which the monotonic direct association of adulthood weight change with the risk of death was strengthened in women (P < .001 for interaction). Adulthood weight loss was not related to a higher mortality rate. In addition, a higher BMI at early adulthood and subsequent weight gain were independently associated with increased mortality later in life.
This study has several strengths. The large sample size, regular follow-up of study participants, and detailed repeated assessment of lifestyle factors provided high statistical power to examine various major health outcomes. The advantage of focusing on weight gain throughout adult life is that it primarily reflects the accumulation of excess adiposity from early to middle adulthood, which is often ignored by individuals and their physicians because the consequences of modest weight accumulation may not yet be apparent. Both the NHS and the HPFS have collected detailed, validated, and repeated assessments of self-reported weight and lifestyle factors, allowing careful control for potential confounding factors. Also, a subgroup analysis was conducted among participants who never smoked when analyzing cancer and mortality to avoid confounding by smoking status.
This study also has several limitations. First, weight at early adulthood was recalled at a later age. Although validation studies suggested good validity (correlation coefficients between recalled and measured weight were 0.87 in women and 0.80 in men, and the difference in means were 1.4 kg in women and 1.3 kg in men), some misclassifications of weight change were inevitable. Because the weight data were collected before the study outcomes, such misclassification was likely nondifferential and thus, the true associations might have been underestimated. Furthermore, the magnitude of weight gain from early to middle adulthood observed in our cohorts is comparable with that in the general US population (eg, the weight gains during the corresponding life course in women and men in the National Health and Nutrition Examination Survey were both about 10 kg). Nevertheless, the potential for systematic error in the assessment of weight change needs to be considered, especially considering that individuals tend to underreport their weight. It is possible that our findings might have exaggerated the true risks associated with the measured weight change from early to middle adulthood.
Second, the participants were all health professionals and were mostly white. The homogeneity of health care access and socioeconomic status helps to minimize confounding and enhances the internal validity, but the results might not be generalizable to all populations. Third, the risk of chronic diseases and mortality was not assessed in relation to changes in waist circumference, a marker of abdominal adiposity. Fourth, given that multiple outcomes were analyzed in this study, a chance finding could not be completely ruled out. Fifth, 2 of the calculated I2 values for outcomes (93% for hypertension and 97% for the composite healthy aging outcome) were at very high levels, indicating that pooled analyses may have been statistically inappropriate and thus the results should be interpreted for men and women separately.
Conclusions
In these cohorts of health professionals, weight gain during adulthood was associated with significantly increased risk of major chronic diseases and decreased odds of healthy aging. These findings may help counsel patients regarding the risks of weight gain.
eMethods
eReferences
eFigure 1. Analysis population and exclusion criteria of specific health outcomes
eFigure 2. Association of early to middle adulthood weight gain with the risk of composite major chronic disease from age of 55 to 75 years
eFigure 3. Non-parametric restricted cubic splines of early to middle adulthood weight change (per kg) and major health outcomes
eFigure 4. Joint association of early to middle adulthood weight change and body mass index at early adulthood with risk of individual major health outcomes in women from the Nurses’ Health Study (A, C, E, G) and men from the Health Professionals Follow-up Study (B, D, F, H)
eFigure 5. Joint association of body mass index at early adulthood and that at middle adulthood with risk of major health outcomes in women from the Nurses’ Health Study (A, C, E, G) and men from the Health Professionals Follow-up Study (B, D, F, H)
eTable 1. Association between early to middle adulthood weight gain and secondary health outcomes in the Nurses’ Health Study and the Health Professionals Follow-Up Study
eTable 2. Association between middle life weight gain and overall and cause-specific mortality in the Nurses' Health Study and Health Professionals Follow-Up Study
eTable 3. Association between middle life weight gain and major health outcomes in the Nurses' Health Study and Health Professionals Follow-Up Study with further adjustment of disease status, health screening and medication use
eTable 4. Association between middle life weight gain and major health outcomes in the Nurses' Health Study and Health Professionals Follow-Up Study with further adjustment of use of antidepressants and oral steroids
eTable 5. Association between quintiles of early/middle adulthood weight change and major health outcomes in the Nurses' Health Study
eTable 6. Association between quintiles of early to middle adulthood weight gain and health outcomes in the Health Professionals Follow-Up Study
References
- 1.World Health Organization Obesity. http://www.who.int/gho/ncd/risk_factors/obesity_text/en/. Accessed August 14, 2016.
- 2.World Health Organization Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed August 6, 2016.
- 3.Hruby A, Manson JE, Qi L, et al. Determinants and consequences of obesity. Am J Public Health. 2016;106(9):1656-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutfless S, Maruthur NM, Wilson RF, et al. Strategies to Prevent Weight Gain Among Adults. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 5.Hu FB, ed. Obesity Epidemiology. New York, NY: Oxford University Press; 2008. [Google Scholar]
- 6.Maclure KM, Hayes KC, Colditz GA, et al. Weight, diet, and the risk of symptomatic gallstones in middle-aged women. N Engl J Med. 1989;321(9):563-569. [DOI] [PubMed] [Google Scholar]
- 7.Song M, Hu FB, Spiegelman D, et al. Adulthood weight change and risk of colorectal cancer in the Nurses’ Health Study and Health Professionals Follow-up Study. Cancer Prev Res (Phila). 2015;8(7):620-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheehan TJ, DuBrava S, DeChello LM, Fang Z. Rates of weight change for black and white Americans over a twenty year period. Int J Obes Relat Metab Disord. 2003;27(4):498-504. [DOI] [PubMed] [Google Scholar]
- 9.US Departments of Health and Human Services and Agriculture Dietary Guidelines for Americans: 2015-2020. https://health.gov/dietaryguidelines/2015/guidelines/. Accessed June 19, 2017.
- 10.Bao Y, Bertoia ML, Lenart EB, et al. Origin, methods, and evolution of the three Nurses’ Health studies. Am J Public Health. 2016;106(9):1573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464-468. [DOI] [PubMed] [Google Scholar]
- 12.Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466-473. [DOI] [PubMed] [Google Scholar]
- 13.Troy LM, Hunter DJ, Manson JE, et al. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19(8):570-572. [PubMed] [Google Scholar]
- 14.Willett WC, ed. Nutritional Epidemiology. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 15.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894-900. [DOI] [PubMed] [Google Scholar]
- 16.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790-797. [DOI] [PubMed] [Google Scholar]
- 17.Leitzmann MF, Rimm EB, Willett WC, et al. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med. 1999;341(11):777-784. [DOI] [PubMed] [Google Scholar]
- 18.Karlson EW, Mandl LA, Aweh GN, et al. Total hip replacement due to osteoarthritis. Am J Med. 2003;114(2):93-98. [DOI] [PubMed] [Google Scholar]
- 19.Weintraub JM, Willett WC, Rosner B, et al. A prospective study of the relationship between body mass index and cataract extraction among US women and men. Int J Obes Relat Metab Disord. 2002;26(12):1588-1595. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute Obesity and cancer. https://www.cancer.gov/cancertopics/factsheet/Risk/obesity. Accessed August 24, 2016.
- 21.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37(4):433-440. [DOI] [PubMed] [Google Scholar]
- 23.Sun Q, Townsend MK, Okereke OI, et al. Adiposity and weight change in mid-life in relation to healthy survival after age 70 in women. BMJ. 2009;339:b3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eliassen AH, Colditz GA, Rosner B, et al. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296(2):193-201. [DOI] [PubMed] [Google Scholar]
- 25.Ahn J, Schatzkin A, Lacey JV Jr, et al. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med. 2007;167(19):2091-2102. [DOI] [PubMed] [Google Scholar]
- 26.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551-561. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canale MP, Manca di Villahermosa S, Martino G, et al. Obesity-related metabolic syndrome. Int J Endocrinol. 2013;2013:865965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song M, Willett WC, Hu FB, et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138(10):2383-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keum N, Greenwood DC, Lee DH, et al. Adult weight gain and adiposity-related cancers. J Natl Cancer Inst. 2015;107(2):djv088. [DOI] [PubMed] [Google Scholar]
- 31.Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis. Ann Rheum Dis. 2014;73(7):1323-1330. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention Common eye disorders. https://www.cdc.gov/visionhealth/basics/ced/index.html. Accessed September 1, 2016.
- 33.Prickett C, Brennan L, Stolwyk R. Examining the relationship between obesity and cognitive function. Obes Res Clin Pract. 2015;9(2):93-113. [DOI] [PubMed] [Google Scholar]
- 34.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988-2004. JAMA. 2007;298(17):2020-2027. [DOI] [PubMed] [Google Scholar]
- 35.Pan A, Kawachi I, Luo N, et al. Changes in body weight and health-related quality of life: 2 cohorts of US women. Am J Epidemiol. 2014;180(3):254-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allman-Farinelli M. Body mass index and mortality. Am J Epidemiol. 2014;179(2):145-146. [DOI] [PubMed] [Google Scholar]
- 37.Strand BH, Wills AK, Langballe EM, et al. Weight change in midlife and risk of mortality from dementia up to 35 years later. J Gerontol A Biol Sci Med Sci. 2017;72(6):855-860. [DOI] [PubMed] [Google Scholar]
- 38.Rhoads GG, Kagan A. The relation of coronary disease, stroke, and mortality to weight in youth and in middle age. Lancet. 1983;1(8323):492-495. [DOI] [PubMed] [Google Scholar]
- 39.McDowell MA, Fryar CD, Ogden CL, Flegal KM. Anthropometric reference data for children and adults. Natl Health Stat Report. 2008;10(10):1-48. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eReferences
eFigure 1. Analysis population and exclusion criteria of specific health outcomes
eFigure 2. Association of early to middle adulthood weight gain with the risk of composite major chronic disease from age of 55 to 75 years
eFigure 3. Non-parametric restricted cubic splines of early to middle adulthood weight change (per kg) and major health outcomes
eFigure 4. Joint association of early to middle adulthood weight change and body mass index at early adulthood with risk of individual major health outcomes in women from the Nurses’ Health Study (A, C, E, G) and men from the Health Professionals Follow-up Study (B, D, F, H)
eFigure 5. Joint association of body mass index at early adulthood and that at middle adulthood with risk of major health outcomes in women from the Nurses’ Health Study (A, C, E, G) and men from the Health Professionals Follow-up Study (B, D, F, H)
eTable 1. Association between early to middle adulthood weight gain and secondary health outcomes in the Nurses’ Health Study and the Health Professionals Follow-Up Study
eTable 2. Association between middle life weight gain and overall and cause-specific mortality in the Nurses' Health Study and Health Professionals Follow-Up Study
eTable 3. Association between middle life weight gain and major health outcomes in the Nurses' Health Study and Health Professionals Follow-Up Study with further adjustment of disease status, health screening and medication use
eTable 4. Association between middle life weight gain and major health outcomes in the Nurses' Health Study and Health Professionals Follow-Up Study with further adjustment of use of antidepressants and oral steroids
eTable 5. Association between quintiles of early/middle adulthood weight change and major health outcomes in the Nurses' Health Study
eTable 6. Association between quintiles of early to middle adulthood weight gain and health outcomes in the Health Professionals Follow-Up Study