Abstract.
Globally, approximately 2 billion people lack microbiologically safe drinking water. Boiling is the most prevalent household water treatment method, yet evidence of its health impact is limited. To conduct this systematic review, we searched four online databases with no limitations on language or publication date. Studies were eligible if health outcomes were measured for participants who reported consuming boiled and untreated water. We used reported and calculated odds ratios (ORs) and random-effects meta-analysis to estimate pathogen-specific and pooled effects by organism group and nonspecific diarrhea. Heterogeneity and publication bias were assessed using I2, meta-regression, and funnel plots; study quality was also assessed. Of the 1,998 records identified, 27 met inclusion criteria and reported extractable data. We found evidence of a significant protective effect of boiling for Vibrio cholerae infections (OR = 0.31, 95% confidence interval [CI] = 0.13–0.79, N = 4 studies), Blastocystis (OR = 0.35, 95% CI = 0.17–0.69, N = 3), protozoal infections overall (pooled OR = 0.61, 95% CI = 0.43–0.86, N = 11), viral infections overall (pooled OR = 0.83, 95% CI = 0.7–0.98, N = 4), and nonspecific diarrheal outcomes (OR = 0.58, 95% CI = 0.45–0.77, N = 7). We found no evidence of a protective effect for helminthic infections. Although our study was limited by the use of self-reported boiling and non-experimental designs, the evidence suggests that boiling provides measureable health benefits for pathogens whose transmission routes are primarily water based. Consequently, we believe a randomized controlled trial of boiling adherence and health outcomes is needed.
INTRODUCTION
Across low- and middle-income countries (LMICs), close to 2 billion people lack reliable access to microbiologically safe drinking water, and approximately 500,000 people, mostly children, die annually due to unsafe or insufficient drinking water.1–6 In the most recent (2015) Global Burden of Disease study,7 unsafe water was ranked 14th among global health risks. Point-of-use household water treatment (HWT) technologies are often recommended when reliable access to safe water is limited. Filtration (ceramic, biosand, and micro), chlorination (with/without flocculation), solar disinfection, and ultraviolet (UV) disinfection are the primary HWT technologies currently promoted in LMICs. When used correctly, these HWT technologies effectively improve drinking water quality and can reduce related morbidity and mortality.8–10 However, after decades of extensive promotion efforts, achieving the widespread and sustained adoption of these HWT technologies remains a challenge.11–15
Boiling is the most commonly used reported HWT method globally, with an estimated 1.2 billion users (∼70% of all HWT users).14,16,17 The reported use of boiling is particularly widespread in many Asian nations, including China, where as many as 85% of rural residents report boiling drinking water,16 as well as an estimated 95% in Mongolia and 91% in Indonesia and Vietnam.14 Compared with HWT products such as chlorine or filters, however, relatively few health or water, sanitation, and hygiene (WASH) studies have focused on boiling specifically. Among the boiling-focused studies, most evaluated boiling and water quality outcomes, but not health outcomes. Water-quality-focused studies in Cambodia, Guatemala, India, Indonesia, Peru, and Vietnam all found significant post-boiling reductions of fecal contamination indicators.18–23 Although boiling is straightforward to use and microbiologically effective, as with other HWT methods, its effectiveness depends on correct and consistent use. Boiled water is also susceptible to recontamination, and the fuels used to boil water in LMIC settings often produce household air pollution (HAP).23–26 In addition, there is a potential for injury via skin exposure to hot or boiling water.
The relative paucity of boiling-focused health research has not gone unnoticed. For example, a comprehensive review of point-of-use water treatment technologies and methods for use in emergencies cited a “lack of epidemiological confirmed health impact” for boiling,27 and a recent World Health Organization report noted that there is relatively little research on boiling’s effectiveness for diarrheal reduction.5 Moreover, as noted in the most recent Cochrane Review on interventions to improve drinking water quality, no randomized controlled trials (RCTs) have been conducted to evaluate boiling.28
Similarly, although there are a number of systematic reviews and summary articles on the use of chlorination, filtration, and solar disinfection,3,12,13,29–31 as far as we are aware, there are no such reviews focused on boiling and health outcomes, or on boiling and water quality, specifically (in part because some previous reviews only considered experimental study designs as eligible). Furthermore, these reviews, and most of the WASH studies they are based on, tend to use diarrheal disease as the primary health outcome. Because many pathogens result in diarrheal symptoms, these analyses do little to clarify the relative effectiveness of different HWT methods for exposure to specific pathogens or organism groups.
A clearer understanding of boiling’s impact on water-related disease prevention is needed. We conducted this systematic review and meta-analysis to bring together the evidence on boiling and health outcomes in LMICs. This study is also one of the few such reviews to attempt to estimate pooled effects for specific pathogens and organism groups,32,33 as well as for nonspecific diarrheal disease outcomes.
MATERIALS AND METHODS
Search strategy and selection criteria.
To identify potentially eligible studies, we searched four online databases: PubMed/MEDLINE, EMBASE, Web of Science, and the Cochrane Library. Search terms were selected with the goal of finding all articles that might potentially address health outcomes associated with the boiling of drinking water in LMICs. Four sets of search terms were used to identify all articles focused on drinking water, drinking water treatment (including, but not limited to, boiling), health outcomes known to be associated with the consumption of contaminated drinking water, and the names and alternate names/spellings of all LMICs. Because some search engines retrieve fewer results when truncation is used,34 we included all possible word variants in our lists of search terms (e.g., rather than using “boil*,” we searched for “boils,” “boiled,” and “boiling”). The search terms, sets, and an explanation of the Boolean operators used are provided in Supplemental Table 1.
The final database literature searches were conducted on January 21, 2016 (the complete searches used for each database are provided in Supplemental Tables 2–5). No restrictions were put in place with regard to publication date, type, or language. In addition, a hand-search was conducted by consulting the reference sections of articles already known to discuss boiling and drinking water treatment as well as a targeted search for papers using Google Scholar (grey literature was not included). Following the convention to define eligibility with reference to the population/s, study/intervention, comparisons, and outcomes of interest,35 studies were considered eligible if they included human participants in LMICs; measured infectious health outcomes (disease occurrence) due to pathogens with at least one water-related transmission route; and there was a comparison, or data which could be used to make a comparison, for such outcomes between participants reporting to drink boiled water and those reporting to drink non-boiled/untreated water (any study design with data for such a comparison). We did not include unpublished studies.
After the databases were searched, the results were exported and compiled using the reference management software Endnote (version X7; Thomson Reuters, New York, NY). Duplicates were removed using Endnote’s automated process, followed by a manual search to identify and remove additional duplicates. For the initial record screening step, to avoid inadvertent bias from viewing author name/s, publication type, journal names, and so on, only the record titles and abstracts were reviewed. Titles/abstracts that did not mention boiling but did describe studies focused on drinking water treatment and health outcomes were retained in the hopes that subgroup or control group data related to boiling and health outcomes were reported in the full text. One reviewer (Alasdair Cohen) screened all the titles and abstracts (when available) to determine which were eligible for full-text review. Titles and abstracts from a randomly selected sample of 5% of the initial records were screened by a second reviewer (John M. Colford) and inter-rater reliability was assessed. Similarly, after full-text review (by Alasdair Cohen), 15% of the full-text articles were randomly selected and reviewed for eligibility (by John M. Colford).
Data extraction, calculation, and derivation protocols.
For each eligible study with extractable data associated with the health effects of consuming boiled drinking water, the following summary information was extracted from the full text if available: country where the study was conducted, province/state/region within the country, study population (rural, urban, mixed, etc.), study type and design, year/s the study was conducted, study duration in months, total number of individuals (and/or households) sampled, age/s of participants, whether a random sampling/selection process was used, whether the sampling/method was described, the health outcome/s assessed, whether a protocol for outcome assessment was described, and whether the outcome assessment was direct or based on self-report.
To extract or calculate odds ratios (ORs), such that values < 1.0 would signify a reduction in disease associated with the consumption of boiled drinking water, as well as lower and upper 95% confidence intervals (95% CI) from each study for our meta-analysis, our guiding principle was to use the best available data in all cases. When the data were provided, or could be calculated, we constructed 2 × 2 tables and calculated ORs and 95% CIs. If these values aligned with those reported in the text, we used our calculations. For studies that reported the OR but did not provide sufficient data to construct a 2 × 2 table, we used their reported estimates. When the reported OR reference group was those who did not boil their water, we used the reported upper and lower 95% CI to back-calculate the standard error (SE) of the log(OR) to derive 95% CIs for those who boiled (using the inverse of the reported OR). Similarly, in cases where the authors rounded the 95% CI to one decimal place and the data were available, we back-calculated the SE to derive more precise 95% CIs.
When authors provided adjusted estimates, we recorded them in our dataset and also calculated unadjusted estimates when the data were available, but only used the reported adjusted estimates for the primary analyses presented here. For matched case–control studies, we always used the reported matched odds ratio (MOR) when provided, back-calculating to derive the MOR and 95% CI for the boiling group if needed. If the authors only reported a risk ratio (RR), we treated it as an OR. For additional details, see the Supplemental Dataset 1 (“comments” in the data cells provide the table and/or page number/s where we found the data from each study).
For our analyses of possible publication bias, for those studies where we had to transform and back-calculate 95% CIs and the resulting SEs of the upper and lower 95% CI were not equal, we used the arithmetic mean of the upper and lower values to estimate the boiling SE of the log(OR) (these instances are marked with yellow font in column “AE” of Supplemental Dataset 1). Following data extraction of all eligible studies (by Alasdair Cohen), 30% were randomly selected for data extraction/derivation by a second reviewer (John M. Colford). All extracted data and related calculations were reviewed and discussed by both reviewers.
Data analysis.
We used meta-analysis to estimate pooled effects of boiling drinking water on health outcomes. Because of the differences in pathogenesis for the various disease outcomes assessed in the studies, we chose not to estimate an overall pooled effect for boiling across all disease outcomes. Rather, we created outcome groups by combining studies that assessed bacterial, helminthic, protozoal, and viral infections, as well as diarrheal outcomes with no specified etiology. Because some authors adjusted for covariates and others did not, we used the most adjusted estimates when available. Using only unadjusted outcome effects tended to result in more protective pooled estimates, thus our use of the adjusted estimates when available resulted in more conservative point and pooled estimates overall (unadjusted estimates are provided in Supplemental Dataset 1).
Given our expectation of inter-study variability (due to differences in study design, data collection methods, testing protocols, etc.) and random error, we used meta-analysis with random-effects-based weighting. Because of the known power issues with regard to detecting heterogeneity in meta-analyses generally, and when using subgroups specifically, in addition to using Mantel–Haenszel estimates of heterogeneity, we used the I2 statistic to assess the degree of variation in subgroups which could be attributed to inter-study heterogeneity.36 For studies where the authors provided adjusted effect estimates, we performed meta-analyses using only the adjusted effect estimates.
To further examine heterogeneity and identify potential confounders, we used meta-regression analysis with random effects (controlling for the variance within and between studies) to examine the impact of various study characteristics on the log(OR) for boiling. Specifically, we regressed the log(OR) for boiling on the total number of participants (or households), participant age, whether the study participants lived in rural areas or not, whether the study was an outbreak investigation or not, study duration, whether any type of random selection or sampling method was used to select participants, and lastly, whether the primary health outcome was assessed via self-report or measured directly, meaning infection was confirmed via analysis of stool and/or serum samples (e.g., with enzyme-linked immunosorbent assay, microscopy, direct smear, cell culture, polymerase chain reaction). Because of the relatively small number of studies available for many organism groups, we also estimated adjusted P values using a Monte Carlo permutation test (with 1,000 random permutations). To attempt to evaluate study quality/bias, we scored each study on a variety of criteria and then aggregated the resulting six components into a composite index which we converted to a 10-point scale to assign grades to each study (we adapted the criteria and grading approach from two recent reviews2,37; see Supplemental Table 6). We then incorporated these quality classifications into an additional meta-regression analysis. Because one might expect baseline exposure and boiling adherence to be higher during outbreak events, pooled estimates that included outbreak investigation studies were estimated with and without outbreak data.
Funnel plots were created to visually assess the extent of potential publication bias in combination with the use of Egger’s test.38 Though regressing log(OR)s on corresponding SEs may be prone to false positives, we used Egger’s test (at a 95% CI) to attempt to quantitatively assess the degree of potential publication bias (because we did not have complete 2 × 2 data for all studies, we were limited with regard to the use of other such tests). We analyzed each organism group in isolation and conducted an exploratory analysis stratifying by study design.
All analyses were conducted using STATA (v13.1; StataCorp, College Station, TX). A completed PRISMA39 checklist is provided in Supplemental Table 7.
RESULTS
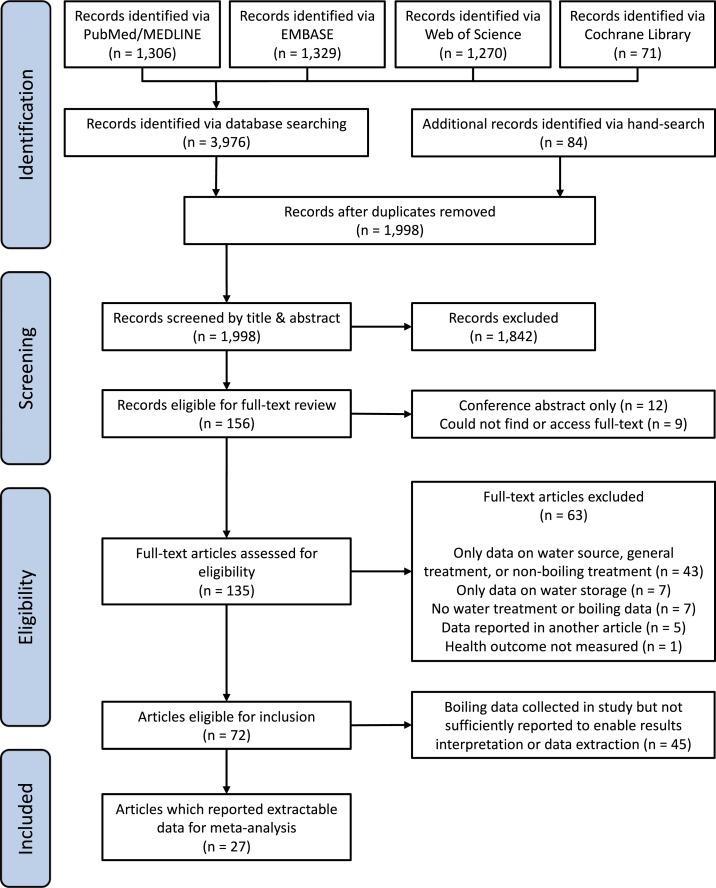
After removing duplicates across the four databases and hand-search results, 1,998 records were identified (see Figure 1). Screening by titles and abstracts resulted in the selection of 156 records for full-text review. For the randomly selected subset of 5% (N = 100) records, there was 93% agreement between the two reviewers (kappa = 0.55), which was considered sufficient given the broad inclusion criteria used for the initial screening. One hundred thirty-five full-text articles were found, published during 1955–2015, with 91% (N = 123) in English, 6% (N = 8) in Spanish, and 3% (N = 4) in Chinese (both reviewers read English and Spanish, and Alasdair Cohen’s Chinese reading ability was sufficient for this review). After full-text review (by Alasdair Cohen), 63 articles were deemed ineligible.40–102 For the randomly selected subset of 15% (N = 23) full-text articles reviewed (by John M. Colford), there was 100% agreement with regard to eligibility (none of these randomly selected articles were published in Chinese). Of the 72 articles eligible for inclusion, 27 reported extractable boiling and health outcome data,103–129 whereas 45 did not report sufficient data for interpretation or extraction.130–174 To check the accuracy of data extraction (by Alasdair Cohen), 30% (N = 8) of these articles were randomly selected and the second reviewer (John M. Colford) performed independent data extraction; this resulted in 100% agreement.
Figure 1.
Flowchart of the systematic review process used to identify eligible studies. This figure appears in color at www.ajtmh.org.
As discussed earlier, the guiding protocol was to use the best available data, and so when presented with a decision we always used the more conservative and/or broadly relevant data. In the interests of consistency and replicability, we only used data provided in the papers, rather than using effect estimates reported elsewhere or non-published data to which we had access (or by contacting authors directly). For example, in Núñez and others,121 we used the verified “Hierve el agua (verificado),” rather than unverified boiling data. Similarly, for our published research on boiling in China,175 since we did not publish the diarrheal RR for all boiling methods, we used the published RR for boiling with metal pots, rather than electric kettle-based boiling (since pot-based boiling is more broadly relevant). In addition, because we could not assume that the water was heated to boiling for all reported boiling cases in all studies, and because pathogen inactivation can occur at temperatures as low as ∼55–60°C, depending on the altitude, pathogens, and boiling durations,176 we considered Iijima and others’115 study on water pasteurization eligible.
The 27 articles from which data were extracted were published over the years 1992–2015, with 81.5% (N = 22) published in English and 18.5% (N = 5) in Spanish. Studies were conducted in countries around the world, with multiples studies in India (N = 4), Malaysia (N = 4), Cuba (N = 3), Peru (N = 3), and China (N = 2). Slightly more than half of the articles (55.6%, N = 15) described results from cross-sectional designs. Of the studies, 40% (N = 11) were conducted with participants from rural areas, 22% (N = 6) urban, and 37% (N = 10) mixed rural and urban. The median number of participants was 283, with a mean of 1,500 (SD = 2,836, N = 25) and the median duration of the study or data collection was 4 months, with a mean of 11.1 months (SD = 18.8, N = 27). Health outcomes were measured directly in 74% of the articles (N = 20), measured and reported in 11% (N = 3), and only reported in 15% (N = 4) (the specific methods used for direct measurement in each study are provided in Supplemental Dataset 1). See Table 1 for a summary of the study characteristics, specific outcomes, and the data sources and methods used to derive effect estimates.
Table 1.
Characteristics of studies included in meta-analysis, organized by organism group
| Specific pathogen or outcome | First author | Published year | Country where study conducted | Year/s study conducted | Study duration (months) | Rural or urban | Number of participants (number of households) | Participant age | Study design | Random selection or sampling used | Outcome measured or reported | OR data source | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Helicobacter pylori | Lee | 2012 | Malaysia | 2002–2008 | 72 | R&U | 161 | A | CC | U | M | OR, C |
| Salmonella typhi | Sharma | 2009 | India | 2005–2006 | 17 | R&U | 246 | M | MCC | Y | M | MOR, R | |
| Vibrio cholerae | Cardenas | 1993 | Colombia | 1991–1992 | 10 | R&U | (209) | M | CS | Y | R | OR, R | |
| V. cholerae | Fredrick | 2015 | India | 2012 | 1 | R&U | 154 | M | MCC | U | M&R | MOR, RT | |
| V. cholerae | Ries | 1992 | Peru | 1991 | 1 | U | 150 | M | MCC | U | M | MOR, R | |
| V. cholerae | Weber | 1994 | Ecuador | 1991 | 1 | U | 189 | C | CC | U | M | OR, C | |
| Helminths | Ascaris | Gunawardena | 2004 | Sri Lanka | 2000 | 6 | R | 176 | M | CS | Y | M | OR, RAT |
| Strongyloides sterocoralis | Herrera | 2006 | Peru | 2003 | 2 | R | 100 | M | CC | U | M | OR, C | |
| Ascaris, Trichuris, hookworm, and multiple | Wordemann | 2006 | Cuba | 2003 & 2004 | 2 | R&U | 1320 | C | CS | Y | M | OR, RT | |
| Multiple | Al-Delaimy | 2014 | Malaysia | 2012 | 4 | R | 498 | C | CS | N | M | OR, C | |
| Protozoa | Blastocystis | Carrero | 2013 | Columbia | – | 1 | R&U | 50 | C | CS | N | M | OR, C |
| Blastocystis | Li | 2007 | China | – | 1 | R | 283 | M | CS | Y | M | OR, RT, & RAT | |
| Blastocystis | Rondon | 2003 | Peru | 1999 | 3 | R&U | 144 | M | CC | U | M | OR, C | |
| Cryptosporidium parvum | Sarkar | 2014a | India | 2008–2013 | 60 | U | 580 | C | NCC | U | M | OR, C, & RA | |
| Giardia | Bello | 2011 | Cuba | 2003 | 6 | R&U | 351 | C | CC | N | M | OR, C, & RAT | |
| Giardia | Choy | 2014 | Malaysia | 2011–2013 | 22 | R | 1330 | M | CS | Y | M | OR, C, & RAT | |
| Giardia | Nunez | 2003 | Cuba | – | 18 | U | 119 | C | L | U | M | OR, C | |
| Entamoeba histolytica, Giardia, and multiple | Wordemann | 2006 | Cuba | 2003 and 2004 | 2 | R and U | 1320 | C | CS | Y | M | OR, RT | |
| Multiple | Marcano | 2013 | Venezuela | 2012 | 2 | U | 324 | M | CS | U | M | OR, C | |
| Viruses | Hepatitis E | Aggarwal | 2002 | India | 1998 | 5 | R and U | 1088 | M | CS | Y | M | RR, R |
| Hepatitis E | Corwin | 1995 | Indonesia | 1993 | 1 | R | 445 | M | CS | U | M | OR, C | |
| Rotavirus | Sarkar | 2014b | Bangladesh | 1993–1997 | 48 | U | 9879 | C | CCh | U | M | OR, C, and RA | |
| Rotavirus | Sarkar | 2014b | Bangladesh | 2008–2012 | 48 | U | 6204 | C | CCh | U | M | OR, C, and RA | |
| Diarrhea | Nonspecific diarrhea | Cardenas | 1993 | Colombia | 1991–1992 | 10 | R and U | (209) | M | CS | Y | R | OR, R |
| Nonspecific diarrhea | Cifuentes | 1998 | Mexico | 1992 | 5 | R | 9435 | M | CS | U | M | OR, C | |
| Nonspecific diarrhea | Cohen | 2015 | China | 2013 | 1 | R | (450) | M | CS | Y | R | RR, R | |
| Nonspecific diarrhea | Iijima | 2001 | Kenya | 1995 | 4 | R | 3420 | M | CS | U | R | OR, C | |
| Nonspecific diarrhea | Kelly | 1997 | Zambia | 1995–1996 | 5 | R and U | 6702 | A | CS | U | M and R | OR, R | |
| Nonspecific diarrhea | Knight | 1992 | Malaysia | 1989 | 2 | R | 196 | C | MCC | Y | M and R | OR, RAT | |
| Nonspecific diarrhea | Psutka | 2013 | Kiribati | 2011 | 1 | R | 153 | C | CS | Y | R | RR, RT |
Rural or urban: R = rural, U = urban; participant age: C = children (age < 18), A = adults (age > 18), M = mixed (all ages), study design: CS = cross-sectional, CC = case–control, MCC = matched case–control, NCC = nested case–control, L = longitudinal, CCh = case-cohort; random selection: Y = yes, N = no, U = unclear; outcome measurement: M = measured directly (details in Supplemental Dataset 1, column CG), R = based on self-report. Outbreak investigations marked in italics (N = 6); OR data source: RR = risk ratio, OR = odds ratio, MOR = matched odds ratio, R = reported, T = transformed, A = adjusted, C = calculated (2 × 2 data).
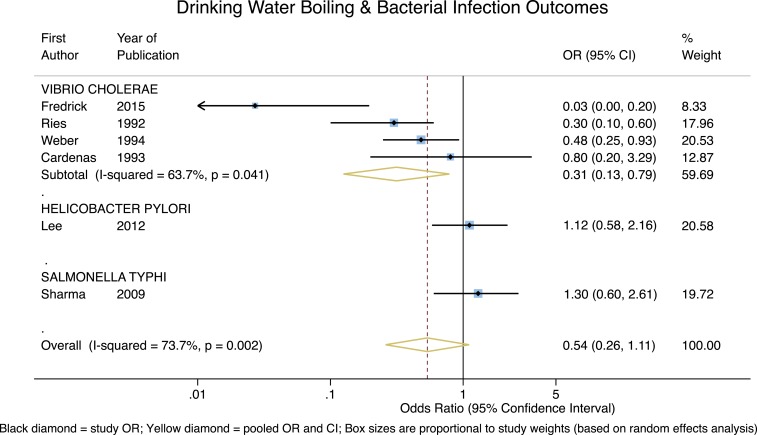
Disease outcomes were organized into bacterial, helminthic, protozoal, and viral groups, as well as nonspecific diarrheal disease outcomes. For bacterial outcomes, as shown in Figure 2, boiling drinking water is associated with a significant and highly protective effect for Vibrio cholerae (OR = 0.31, 95% CI = 0.13–0.79, P = 0.01), though the heterogeneity is somewhat high (I2 = 63.7%). However, effects from the single studies of Helicobacter pylori and Salmonella typhi are neither protective nor significant (P = 0.74 and P = 0.49, respectively). Consequently, although the pooled estimate for these bacterial outcomes is protective, it is not significant (overall OR = 0.54, 95% CI = 0.26–1.11, P = 0.09) and the heterogeneity was high (I2 = 73.7%). In addition, all four V. cholera studies were outbreak investigations; with those studies removed, the pooled estimate for the remaining two bacterial outcomes is neither protective nor significant (overall OR = 1.19, 95% CI = 0.73–1.95, P = 0.48), with essentially zero heterogeneity.
Figure 2.
Forest plot for studies measuring bacterial outcomes. This figure appears in color at www.ajtmh.org.
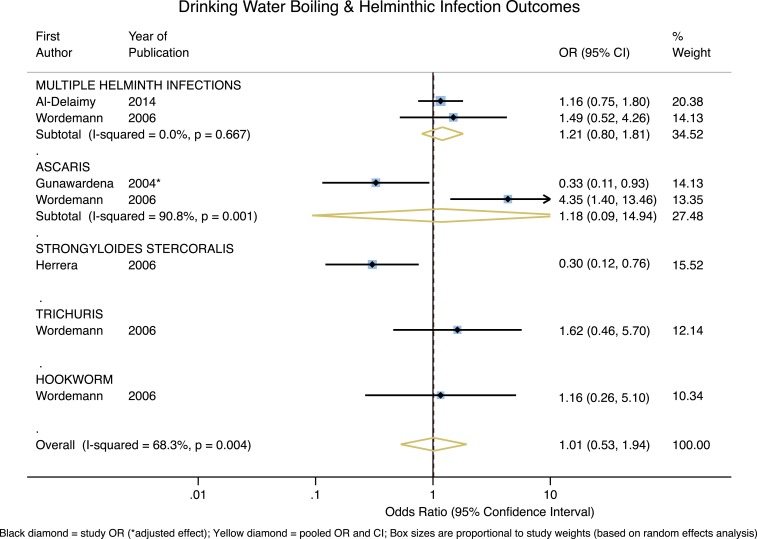
As shown in Figure 3, across helminth infection outcomes, the only significant protective effect associated with boiling is for the single study reporting on Strongyloides stercoralis (OR = 0.30, 95% CI = 0.12–0.76, P = 0.01). The two studies of Ascaris reported significant effects on either side of the null, and across helminthic outcomes the pooled effect estimate is essentially null (overall OR = 1.01, 95% CI = 0.53–1.94, P = 0.97) with high heterogeneity (I2 = 68.3%).
Figure 3.
Forest plot of studies measuring helminthic outcomes. This figure appears in color at www.ajtmh.org.
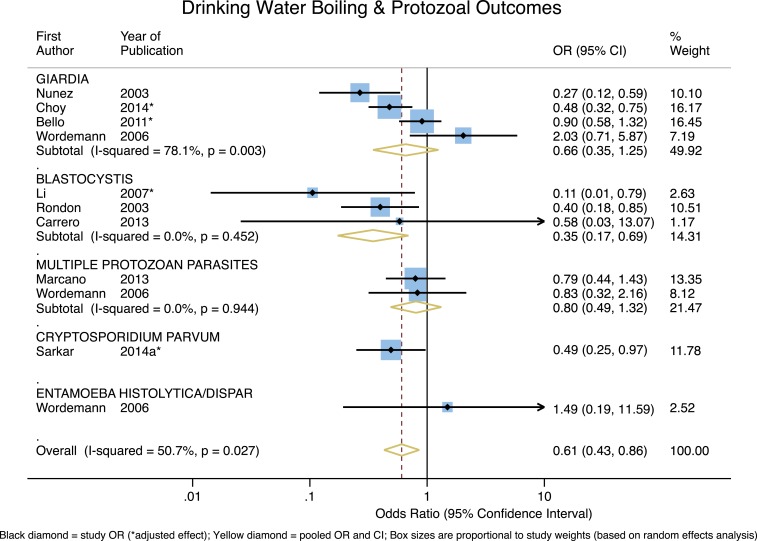
For studies that measured protozoal infections, the pooled effect across the four studies of Giardia suggests that boiling may have a protective effect, but it is not significant (OR = 0.66, 95% CI = 0.35–1.25, P = 0.20) and the heterogeneity is quite high (I2 = 78.1%). Based on the three available studies, boiling is associated with a significant and strong protective effect for Blastocystis (OR = 0.35, 95% CI = 0.17–0.69, P = 0.003), and the variation in the effects does not appear to be attributable to heterogeneity; the heterogeneity statistic also suggests that the underlying effect is relatively constant (P = 0.45). For the two studies that measured the effect of boiling on infection with multiple protozoan parasites, the pooled effect is protective, but not significant (OR = 0.80, 95% CI = 0.49–1.32, P = 0.39) and there is no significant heterogeneity. The one study on Cryptosporidium parvum found a strong and significant protective effect of boiling. The single study on Entamoeba histolytica did not report a protective effect. The overall pooled effect of boiling on protozoan infections was protective and significant (overall OR = 0.61, 95% CI = 0.43–0.86, P = 0.005) with moderate heterogeneity (I2 = 50.7%) (see Figure 4).
Figure 4.
Forest plot of studies measuring protozoal outcomes. This figure appears in color at www.ajtmh.org.
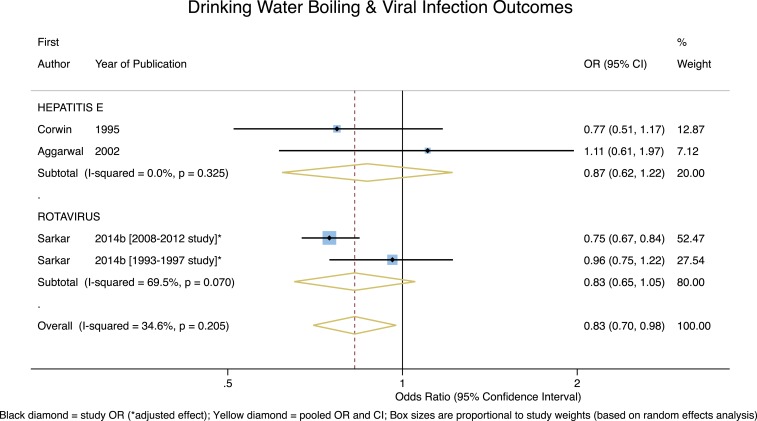
For viral outcomes, as can be seen in Figure 5, though both of the pooled effect estimates for the two studies of Hepatitis E and the two studies of Rotavirus suggested boiling may be protective, neither were significant (P = 0.42 and P = 0.12, respectively). Although the overall pooled estimate for all viral infection outcomes was both protective and significant (overall OR = 0.83, 95% CI = 0.70–0.98, P = 0.02), with low-to-moderate heterogeneity (I2 = 34.6%), this result was due to the large weighting (52.5%) from the Sarkar 2008–2012 study. With the one outbreak investigation (Aggarwal) excluded, the overall pooled estimate for viral infection outcomes remains protective and significant (overall OR = 0.81, 95% CI = 0.68–0.95, P = 0.01), with low-to-moderate heterogeneity (I2 = 39.1%).
Figure 5.
Forest plot of studies measuring viral outcomes. This figure appears in color at www.ajtmh.org.
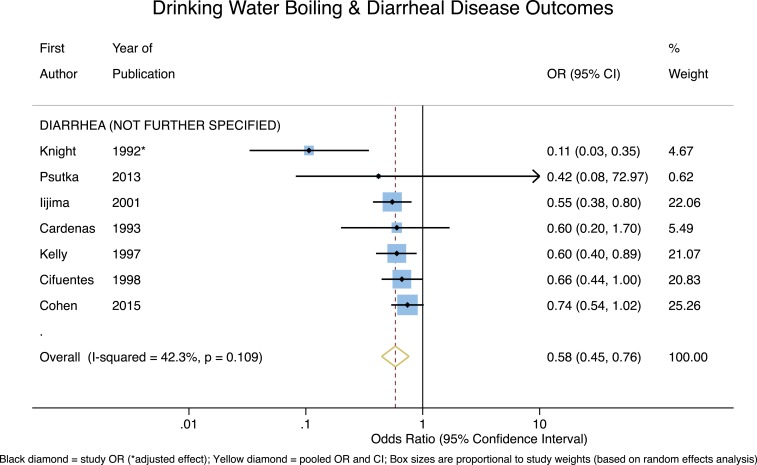
Finally, for the studies with nonspecific diarrheal disease outcomes, shown in Figure 6, the pooled effect estimate indicates that reported boiling of drinking water is significant and strongly protective (OR = 0.58, 95% CI = 0.45–0.77, P < 0.001), and with only moderate heterogeneity (I2 = 42.3%). With the outbreak investigation (Cardenas) removed, the pooled effect estimate remains significant and strongly protective (OR = 0.58, 95% CI = 0.43–0.78, P < 0.001), but with slightly higher heterogeneity (I2 = 51.9%).
Figure 6.
Forest plot of studies measuring non-specific diarrheal outcomes. This figure appears in color at www.ajtmh.org.
Results of the meta-regression analyses for studies with protozoal and diarrheal outcomes indicated that none of the tested variables significantly impacted the effect estimates for boiling (and except for the covariate for total participants in the protozoal outcomes model, none of the Monte Carlo permutation derived P values fell below the 0.05 threshold). Because of the relatively small number of studies in each organism group, there was an insufficient number of observations and/or too much collinearity to estimate covariate coefficients for studies with bacterial, helminthic, and viral outcomes. See Supplemental Tables 8 and 9 for model results.
With regard to possible publication bias, Funnel plots for each outcome group were visually inspected and, aside from nonspecific diarrheal outcomes, none indicated likely publication bias (see Supplemental Figures 1–5). Similarly, Egger’s test did not indicate evidence of a “small study” effect for bacterial outcomes (P = 0.17), nonspecific diarrheal outcomes (P = 0.18), helminthic outcomes (P = 0.96), protozoal outcomes (P = 0.78), or viral outcomes (P = 0.31). In an exploratory effort, we also examined a Funnel plot of all study outcomes (Supplemental Figure 6) which likewise did not indicate publication bias (Egger’s test P = 0.26). After stratifying by study design (Supplemental Figures 7 and 8), there did not appear to be publication bias for the cross-sectional outcomes, though there were indications of publication bias for the other study designs (which were mostly case–controls; Egger’s test P = 0.30 and P = 0.03, respectively).
Concerning estimated study quality/bias, four studies (11%) received a low grade, 10 (29%) a medium grade, and 21 (60%) a high grade (see Supplemental Table 10). For none of the pathogen-specific outcomes were there more than two studies with significant pooled ORs which also fell into different quality/bias classifications (see Supplemental Table 11). For diarrheal outcomes, the pooled ORs for the studies assessed to be of low and medium quality were protective and significant, but approximately equal (though the CI was tighter for the medium-quality studies: low-quality studies OR = 0.60, 95% CI = 0.40–0.89, N = 2; medium-quality studies OR = 0.60, 95% CI = 0.50–0.78, N = 3); the pooled OR for the high-quality diarrheal studies was the lowest, but not significant (high-quality studies OR = 0.31, 95% CI = 0.05–2.03, N = 2).
DISCUSSION
The results of our systematic review and meta-analyses suggest that boiling’s protective effect is stronger for some pathogens and organism groups than for others. These findings appear to align with current understandings of transmission pathways for different pathogens and the role of drinking water treatment,177 such that for those pathogens with primarily water-related transmission routes, reported boiling appears to be protective.
One potential complication with regard to understanding boiling’s differential effect on specific pathogens is related to whether water is actually boiled, or merely heated.176 Although boiling water at 100°C (at sea level) should inactivate all known pathogenic organisms in the water, at temperatures less than 100°C rates of pathogen inactivation vary by temperature, duration, and the organism in question (as altitude increases the boiling point decreases).176,178 For example, at sea level, a one log reduction in the concentration of S. typhi can be achieved in ∼77 seconds at 55°C, or approximately 4 seconds at 60°C, whereas for pathogenic Escherichia coli (O157:H7) a one log reduction is achieved in ∼223 seconds at 55°C, or ∼67 seconds at 60°C.179 Inactivation levels for a protozoa, such as C. parvum, also vary considerably based on the temperature and exposure duration.180
When boiling is promoted, generally or in the context of boiling advisories, the usual recommendation is to bring water to a rolling boil since this treatment endpoint can be easily observed.178 If we assume that most study participants who reported boiling did bring their water to a rolling boil, then—putting aside for the moment issues of safe storage, secondary contamination, and consistent adherence—full pathogen inactivation is to be expected.176 In this respect, boiling is superior to other HWT methods wherein the susceptibility of pathogens in drinking water varies based on the method of treatment, water turbidity, and the pathogen in question.177 There is also considerable variation in inactivation effects for different pathogens depending on which specific variant of given HWT is used (e.g., the variable effectiveness of different forms of chlorine on E. coli).181
Looking to our results for bacterial outcomes, V. cholerae bacteria are transmitted via the fecal–oral route with contaminated drinking water serving as the most common transmission pathway182; it is, therefore, not surprising that boiling appears to provide such a strong preventative effect. For H. pylori, on the other hand, the global prevalence is relatively high and quite varied geographically, infection is often asymptomatic, and though transmission remains poorly understood, the oral–oral route is suspected to be the primary method of transmission183,184; as such, the lack of evidence for boiling’s preventative effect is perhaps not surprising. Salmonella typhi, on the other hand, is also spread via the fecal–oral route, and foodborne transmission appears to be more common than water-related transmission,185 hence boiling alone would not be expected to reliably prevent infection.
This same logic may be applied to pathogens in the helminthic, protozoal, and viral outcome groups. Broadly speaking, helminth infections are usually the result of consuming foods contaminated with feces or soils that contain helminth eggs, or via contact with exposed skin.58,177 That water is not the primary transmission route for helminthic infections is consistent with our overall null findings for the impact of boiling on various helminthic pathogens (aside from the significant protective effect associated with S. stercoralis, based on one study). Though water is not the only transmission route for protozoal infections, reported boiling appears to be broadly protective across specific protozoa. Boiling’s highly protective effect for C. parvum, though based only on one study, is noteworthy given C. parvum’s extreme resistance to chlorine inactivation.186 The apparent effectiveness of boiling on viral outcomes may also be of interest, given that enteric viruses are primarily transmitted through the fecal–oral route via contaminated food or water, though airborne transmission has also been reported.187,188 The possible protective effect of boiling for rotavirus is noteworthy given the relative challenge of inactivating rotavirus with non-boiling HWT (as compared with other viral pathogens).177
Our results also show that reported boiling has a strong, and highly significant, protective effect for nonspecific diarrheal disease outcomes. To better contextualize these findings, in Table 2 we provide a comparison of the pooled OR for diarrheal outcomes associated with reported boiling and the pooled effect estimates from previous systematic reviews on diarrheal outcomes and other HWT methods (as mentioned earlier, most HWT health studies use nonspecific diarrhea as the primary outcome, so we cannot create similar tables to compare pathogen-specific outcomes). An important caveat, however, is that in contrast to most of these other systematic reviews, we did not restrict our inclusion criteria to include only experimental designs (i.e., those using randomized or quasi-randomized assignment and control groups), because there are no published reports of such studies for boiling. Therefore, it is likely that the effect estimates in Table 2 have lower likelihoods of bias as compared with our pooled estimate for reported boiling and diarrheal outcomes. In addition, the pooled estimate from our study does not control for safe post-boiling water storage (with consistent boiling adherence and safe storage, the protective effect might be stronger). With these caveats in mind, we see that the pooled effects associated with filtration are the strongest, followed closely by the pooled estimate for reported boiling from our study (based on data from seven studies). With regard to HWT methods and their impact on diarrheal outcomes, this side-by-side comparison suggests that boiling is at least as effective as the other primary methods of HWT, and perhaps more effective than some.
Table 2.
Pooled effect estimates of HWT methods on diarrheal outcomes from other systematic review and meta analysis studies
| HWT method | Pooled estimate | 95% CI | Studies | Source |
|---|---|---|---|---|
| Boiling | OR = 0.58 | 0.45–0.77 | 7 | This study |
| Chlorine | RR = 0.71 | 0.58–0.87 | 10 | 13 |
| Chlorine | OR = 0.77 | 0.58–1.02 | 3 | 30 |
| Chlorine | RR = 0.77 | 0.65–0.91 | 14 | 28 |
| Filtration | OR = 0.37 | 0.27–0.49 | 2 | 30 |
| Filtration | RR = 0.48 | 0.38–0.59 | 18 | 28 |
| Filtration | RR = 0.53* | 0.41–0.67 | (∼14)† | 3 |
| Filtration: adjusted for non-blinding | RR = 0.66* | 0.47–0.92 | (∼14)† | 3 |
| Flocculant and disinfection | RR = 0.69 | 0.58–0.82 | 4 | 28 |
| Flocculant and disinfection | OR = 0.77 | 0.65–0.90 | 2 | 30 |
| Solar disinfection | RR = 0.62 | 0.42–0.94 | 4 | 28 |
| Solar disinfection | OR = 0.69 | 0.63–0.74 | 2 | 30 |
| Chlorine or solar disinfection‡ | RR = 0.82* | 0.69–0.96 | (∼22)† | 3 |
| Chlorine or solar disinfection: adjusted‡ | RR = 0.99* | 0.76–1.27 | (∼22)† | 3 |
| Various HWT | RR = 0.65 | 0.48–0.88 | 12 | 29 |
| Various HWT | OR = 0.65 | 0.56–0.76 | 10 | 30 |
| Various HWT | ES = 0.56§ | 0.48–0.65 | 28 | 12 |
CI = confidence interval; HWT = household water treatment; ES = effect size; OR = odds ratio; RR = risk ratio.
The presented pooled effects from Wolf and others (2014) do not include studies/estimates with safe-storage.
It was unclear from the text (or supplementary information) how many studies were used to derive these pooled estimates.
The authors explained their decision to calculate the RR for chlorination and solar disinfection as follows: “The results for chlorine and solar interventions were very similar and so, for convenience, they were combined in all analyses” [p935].3
Waddington and others (2009) transformed study effect estimates into a “common metric” ES.
Our study had a number of limitations. The primary limitations were 2-fold: none of the included studies were based on experimental designs, and boiling was assessed via self-report in almost all studies, meaning there was likely substantial heterogeneity in boiling consistency and adherence. Indeed, there is likely substantial heterogeneity between (and within) studies due to differences in boiling methods, frequencies, durations, consistency of use, and methods for storing boiled water and associated risks of secondary contamination.23,25,26 Though the results we present here do not control for post-boiling safe storage (due to a lack of data), if we assume that many or most of the households from which data were collected did not practice safe post-boiling storage, boiling combined with safe storage would likely result in an even more preventative net effect for water-related infectious disease outcomes. For example, in Wolf and others’ systematic review,3 when the authors controlled for the use of safe storage, the pooled effect estimates for filtration and chlorine/solar disinfection were more protective (with and without adjustment for non-blinding).
Our study had other limitations as well. Among the 156 studies identified for full-text review, we were unable to retrieve the full-text for nine records, meaning potentially eligible data may not be included in our meta-analyses. Another limitation of our study (common to many such systematic reviews) is the treatment of reported RRs as ORs, because in cases where outcomes are not rare, ORs tend to be larger than RRs. In addition, as may be apparent from our assessment of study bias/quality, for a number of studies there were nontrivial differences in the apparent methodological rigor underlying data collection and analysis. In addition, six of the studies included in this meta-analysis were outbreak-motivated studies, meaning the effect associated with boiling might have been less pronounced during non-outbreak periods when the disease incidence and associated risks were lower. However, the potential bias associated with these outbreak investigations only had the potential to change our conclusions for the interpretation of reported boiling’s impact on bacterial outcomes (since four of the six outbreak studies focused on V. cholera, which we controlled for [see Figure 2]). Finally, the comparatively limited number of studies identified for some of the pathogen-specific outcomes makes it challenging to interpret many of the results, or to speak to the generalizability of our findings with regard to other populations and regions.
With regard to broader limitations, the current global estimates of boiling prevalence are mostly based on self-report, may be overreported in some instances, and do not provide sufficient data on differences in the consistency of boiling or on the use of safe or unsafe post-boiling storage. In addition, although many of the HWT RCT studies we identified and reviewed did mention the use of boiling in study control groups, none provided health outcome data for participants who practiced boiling (in the main text or online supplementary information). Similarly, in many of these and similar HWT-focused papers, baseline water treatment practices in the control group, such as boiling or filtration, are often aggregated into a catch-all category “water treatment.” Consequently, we were unable to extract data from many of the studies we identified as otherwise eligible (a point we sought to highlight in Figure 1). In the interests of improved reporting, replication, and facilitating systematic reviews, we therefore recommend that, when feasible, more comprehensive results and/or data from WASH RCTs should be provided in supplementary information and/or data repositories.
As mentioned earlier, the use of boiling in LMIC settings itself has a number of limitations: boiled water is susceptible to recontamination, boiling does not remove chemical or metal contaminants, the fuels needed for boiling can be relatively costly, and many of the fuels currently used to boil drinking water produce HAP. The first two limitations are, however, not unique to boiling. Solar and UV disinfection, as well as filtration, provide no residual disinfectant (and therefore require safe storage),25 and aside from flocculants and relatively expensive filters, none of the primary HWT methods adequately remove chemical or metal contaminants. In many LMIC settings, fuel costs may be a significant barrier to the adoption of boiling, and HAP is especially problematic in rural areas where households use wood, agricultural refuse, coal, or other solid-fuels to boil their water, as well as for cooking and heating. HAP exposure causes a number of cardiovascular and respiratory diseases, and is ranked eighth among global health risks.7 HAP exposure is also one of the primary environmental causes of premature death, with 3.9 million attributable deaths in 2010.24
As discussed earlier, unlike the variable effectiveness of other HWT methods, if drinking water is heated to boil, full pathogen inactivation should be achieved regardless of the organism groups, specific pathogens, or water turbidity. In light of the evidence of reported boiling’s impact on health outcomes presented here, and taking into consideration its widespread use globally and the well-documented challenges promoting retail HWT products,11,12,15 it may be worthwhile to evaluate the potential health gains that could be realized by building upon existing preferences for boiled water to promote safer and more reliable methods or technologies for water boiling. Such an effort would also require a clearer understanding of the sociocultural factors underlying preferences for boiling, as well as would-be barriers to adoption. In conclusion, we believe the evidence presented here highlights the need for a more proportionate focus on boiling in the WASH policy, practitioner, and research communities, and that a definitive boiling-focused RCT is justified.
CI = confidence interval; OR = odds ratio; R = rural; U = urban; k = 1,000. Cells with “–” indicate instances where there was too much collinearity with the associated covariate. Cells with “–” indicate instances where there were an insufficient number of observations available.
CI = confidence interval; OR = odds ratio; R = rural; U = urban; k = 1,000. Cells with “–” indicate instances where there was too much collinearity with the associated covariate. Cells with “–” indicate instances where there were an insufficient number of observations available
Supplementary Material
Supplemental Figure and Table.
Note: Supplemental figures, tables and dataset appear at www.ajtmh.org.
REFERENCES
- 1.WHO/UNICEF , 2014. Progress on Drinking Water and Sanitation: 2014 Update. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.Bain R, Cronk R, Wright J, Yang H, Slaymaker T, Bartram J, 2014. Fecal contamination of drinking-water in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med 11: e1001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf J, et al., 2014. Systematic review: assessing the impact of drinking water and sanitation on diarrhoeal disease in low- and middle-income settings: systematic review and meta-regression. Trop Med Int Health 19: 928–942. [DOI] [PubMed] [Google Scholar]
- 4.Prüss-Ustün A, et al., 2014. Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: a retrospective analysis of data from 145 countries. Trop Med Int Health 19: 894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO , 2014. Preventing Diarrhoea Through Better Water, Sanitation and Hygiene: Exposures and Impacts in Low- and Middle-Income Countries. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 6.WHO , 2016. Results of Round I of the WHO International Scheme to Evaluate Household Water Treatment Technologies. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 7.Lim SS, et al., 2016. Measuring the health-related Sustainable Development Goals in 188 countries: a baseline analysis from the Global Burden of Disease Study 2015. Lancet 388: 1813–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lantagne D, Quick R, Mintz E, 2007. Household water treatment and safe storage options in developing countries: a review of current implementation practices. Parker M, Williams A, Youngblood C, eds. Water Stories: Expanding Opportunities in Small-scale Water and Sanitation Projects. Washington, DC: Woodrow Wilson International Center for Scholars (Environmental Change and Security Program).
- 9.Zwane AP, Kremer M, 2007. What works in fighting diarrheal diseases in developing countries? A critical review. World Bank Res Obs 22: 1–24. [Google Scholar]
- 10.Hunter PR, 2009. Household water treatment in developing countries: comparing different intervention types using meta-regression. Environ Sci Technol 43: 8991–8997. [DOI] [PubMed] [Google Scholar]
- 11.Figueroa ME, Kincaid DL, 2010. Social, Cultural and Behavioral Correlates of Household Water Treatment and Storage. Health Communication Insights. Baltimore, MD: Johns Hopkins Bloomberg School of Public Health and USAID. [Google Scholar]
- 12.Waddington H, Snilstveit B, White H, Fewtrell L, 2009. Water, Sanitation and Hygiene Interventions to Combat Childhood Diarrhoea in Developing Countries. Systematic review and meta-analysis. 3ie Synthetic Reviews. Washington and London: The International Initiative for Impact Evaluation (3ie).
- 13.Arnold B, Colford J, 2007. Treating water with chlorine at point-of-use to improve water quality and reduce child diarrhea in developing countries: a systematic review and meta-analysis. Am J Trop Med Hyg 76: 354–364. [PubMed] [Google Scholar]
- 14.Rosa G, Clasen T, 2010. Estimating the scope of household water treatment in low- and medium-income countries. Am J Trop Med Hyg 82: 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amrose S, Burt Z, Ray I, 2015. Safe drinking water for low-income regions. Annu Rev Environ Resour 40: 203–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao Y, 2009. China rural drinking water and environmental health survey [in Chinese]. Chinese J Environ Health 26: 1–2. [Google Scholar]
- 17.Yang H, Wright J, Gundry SW, 2012. Household water treatment in China. Am J Trop Med Hyg 86: 554–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clasen T, McLaughlin C, Nayaar N, Boisson S, Gupta R, Desai D, Shah N, 2008. Microbiological effectiveness and cost of disinfecting water by boiling in semi-urban India. Am J Trop Med Hyg 79: 407–413. [PubMed] [Google Scholar]
- 19.Clasen T, Thao DH, Boisson S, Shipin O, 2008. Microbiological effectiveness and cost of boiling to disinfect drinking water in rural Vietnam. Environ Sci Technol 42: 4255–4260. [DOI] [PubMed] [Google Scholar]
- 20.Rosa G, Miller L, Clasen T, 2010. Microbiological effectiveness of disinfecting water by boiling in rural Guatemala. Am J Trop Med Hyg 82: 473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sodha S, Menon M, Trivedi K, Ati A, Figueroa ME, Ainslie R, Wannemuehler K, Quick RE, 2011. Microbiologic effectiveness of boiling and safe water storage in south Sulawesi, Indonesia. J Water Health 9: 577–585. [DOI] [PubMed] [Google Scholar]
- 22.Brown J, Sobsey MD, 2012. Boiling as household water treatment in Cambodia: a longitudinal study of boiling practice and microbiological effectiveness. Am J Trop Med Hyg 87: 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosa G, Huaylinos ML, Gil A, Lanata C, Clasen T, 2014. Assessing the consistency and microbiological effectiveness of household water treatment practices by urban and rural populations claiming to treat their water at home: a case study in Peru. PLoS One 9: e114997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith KR, et al., 2014. Millions dead: how do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu Rev Public Health 35: 185–206. [DOI] [PubMed] [Google Scholar]
- 25.Wright J, Gundry S, Conroy R, 2004. Household drinking water in developing countries: a systematic review of microbiological contamination between source and point-of-use. Trop Med Int Health 9: 106–117. [DOI] [PubMed] [Google Scholar]
- 26.Clasen T, 2015. Household water treatment and safe storage to prevent diarrheal disease in developing countries. Curr Environ Health Rep 2: 69–74. [DOI] [PubMed] [Google Scholar]
- 27.Loo S, Fane A, Krantz W, Lim T, 2012. Emergency water supply: a review of potential technologies and selection criteria. Water Res 46: 3125. [DOI] [PubMed] [Google Scholar]
- 28.Clasen T, Alexander K, Sinclair D, Boisson S, Peletz R, Chang H, Majorin F, Cairncross S, 2015. Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst Rev 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JJM, 2005. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis 5: 42–52. [DOI] [PubMed] [Google Scholar]
- 30.Clasen T, Schmidt W-P, Rabie T, Roberts I, Cairncross S, 2007. Interventions to improve water quality for preventing diarrhoea: systematic review and meta-analysis. BMJ 334: 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clasen TF, Alexander KT, Sinclair D, Boisson S, Peletz R, Chang HH, Majorin F, Cairncross S, 2015. Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst Rev 10: CD004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speich B, Croll D, Fürst T, Utzinger J, Keiser J, 2016. Effect of sanitation and water treatment on intestinal protozoa infection: a systematic review and meta-analysis. Lancet Infect Dis 16: 87–99. [DOI] [PubMed] [Google Scholar]
- 33.Taylor DL, Kahawita TM, Cairncross S, Ensink JH, 2015. The impact of water, sanitation and hygiene interventions to control cholera: a systematic review. PLoS One 10: e0135676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volpato ESN, Betini M, El Dib R, 2014. Testing search strategies for systematic reviews in the Medline literature database through PubMed. J Eval Clin Pract 20: 117–120. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JPT, Green C, 2011. Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0) The Cochrane Collaboration. Available at: http://www.cochrane-handbook.org/. Accessed December 1, 2015.
- 36.Higgins JPT, Thompson SG, Deeks JJ, Altman DG, 2003. Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uribe-Leitz T, Jaramillo J, Maurer L, Fu R, Esquivel MM, Gawande AA, Haynes AB, Weiser TG, 2016. Variability in mortality following caesarean delivery, appendectomy, and groin hernia repair in low-income and middle-income countries: a systematic review and analysis of published data. Lancet Glob Health 4: e165–e174. [DOI] [PubMed] [Google Scholar]
- 38.Egger M, Smith GD, Schneider M, Minder C, 1997. Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moher D, Liberati A, Tetzlaff J, Altman DG, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Ann Intern Med 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 40.Al-Shamiri A, Al-Zubairy A, Al-Mamari R, 2010. The prevalence of Cryptosporidium spp. in children, Taiz District, Yemen. Iran J Parasitol 5: 26–32. [PMC free article] [PubMed] [Google Scholar]
- 41.Bailey IW, Archer L, 2004. The impact of the introduction of treated water on aspects of community health in a rural community in Kwazulu-Natal, South Africa. Water Sci Technol 51: 105–110. [PubMed] [Google Scholar]
- 42.Balen J, et al, 2011. Risk factors for helminth infections in a rural and a peri-urban setting of the Dongting Lake area, People’s Republic of China. Int J Parasitol 41: 1165–1173. [DOI] [PubMed] [Google Scholar]
- 43.Breiman RF, Shultz A, Omollo JO, Burke H, Qassim M, Ochieng JB, Weinberg M, Feikin DR, 2009. Cholera outbreak in Kenyan refugee camp: risk factors for illness and importance of sanitation. Am J Trop Med Hyg 80: 640–645. [PubMed] [Google Scholar]
- 44.Cabada MM, Goodrich MR, Graham B, Villanueva-Meyer PG, Lopez M, Argue E, White AC, Jr, 2014. Fascioliasis and eosinophilia in the highlands of Cuzco, Peru and their association with water and socioeconomic factors. Am J Trop Med Hyg 91: 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ceran N, Yuksel Kocdogan F, Mert D, Erdem I, Dede B, Adaleti R, Ozyurek S, Karagul E, Goktas P, 2012. Hepatitis A seroprevalence in children and young adults in Istanbul, Turkey: seroprevalence change and associated factors. J Viral Hepat 19: 72–76. [DOI] [PubMed] [Google Scholar]
- 46.Chiller TM, Mendoza CE, Lopez MB, Alvarez M, Hoekstra RM, Keswick BH, Luby SP, 2006. Reducing diarrhoea in Guatemalan children: randomized controlled trial of flocculant-disinfectant for drinking-water. Bull World Health Organ 84: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clasen T, et al., 2014. Effectiveness of a rural sanitation programme on diarrhoea, soil-transmitted helminth infection, and child malnutrition in Odisha, India: a cluster-randomised trial. Lancet Glob Health 2: e645–e653. [DOI] [PubMed] [Google Scholar]
- 48.Conroy RM, Elmore-Meegan M, Joyce T, McGuigan KG, Barnes J, 1996. Solar disinfection of drinking water and diarrhoea in Maasai children: a controlled field trial. Lancet 348: 1695–1697. [DOI] [PubMed] [Google Scholar]
- 49.Conroy RM, Meegan ME, Joyce T, McGuigan K, Barnes J, 2001. Solar disinfection of drinking water protects against cholera in children under 6 years of age. Arch Dis Child 85: 293–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corwin AL, et al, 1999. The unique riverine ecology of hepatitis E virus transmission in southeast Asia. Trans R Soc Trop Med Hyg 93: 255–260. [DOI] [PubMed] [Google Scholar]
- 51.Crump JA, et al., 2007. Comparing serologic response against enteric pathogens with reported diarrhea to assess the impact of improved household drinking water quality. Am J Trop Med Hyg 77: 136–141. [PubMed] [Google Scholar]
- 52.Doni NY, Gurses G, Simsek Z, Zeyrek FY, 2015. Prevalence and associated risk factors of intestinal parasites among children of farm workers in the southeastern Anatolian region of Turkey. Ann Agric Environ Med 22: 438–442. [DOI] [PubMed] [Google Scholar]
- 53.Doocy S, Burnham G, 2006. Point-of-use water treatment and diarrhoea reduction in the emergency context: an effectiveness trial in Liberia. Trop Med Int Health 11: 1542–1552. [DOI] [PubMed] [Google Scholar]
- 54.du Preez M, Conroy RM, Ligondo S, Hennessy J, Elmore-Meegan M, Soita A, McGuigan KG, 2011. Randomized intervention study of solar disinfection of drinking water in the prevention of dysentery in Kenyan children aged under 5 years. Environ Sci Technol 45: 9315–9323. [DOI] [PubMed] [Google Scholar]
- 55.Du Preez M, Conroy RM, Wright JA, Moyo S, Potgieter N, Gundry SW, 2008. Short report: use of ceramic water filtration in the prevention of diarrheal disease: a randomized controlled trial in rural South Africa and Zimbabwe. Am J Trop Med Hyg 79: 696–701. [PubMed] [Google Scholar]
- 56.du Preez M, Conroy RM, Wright JA, Moyo S, Potgieter N, Gundry SW, 2008. Use of ceramic water filtration in the prevention of diarrheal disease: a randomized controlled trial in rural South Africa and Zimbabwe. Am J Trop Med Hyg 79: 696–701. [PubMed] [Google Scholar]
- 57.Du Preez M, McGuigan KG, Conroy RM, 2010. Solar disinfection of drinking water in the prevention of dysentery in South African children aged under 5 years: the role of participant motivation. Environ Sci Technol 44: 8744–8749. [DOI] [PubMed] [Google Scholar]
- 58.Echazu A, et al., 2015. Effect of poor access to water and sanitation as risk factors for soil-transmitted helminth infection: selectiveness by the infective route. PLoS Negl Trop Dis 9: e0004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ercumen A, Arnold BF, Kumpel E, Burt Z, Ray I, Nelson K, Colford JM, Jr, 2015. Upgrading a piped water supply from intermittent to continuous delivery and association with waterborne illness: a matched cohort study in urban India. PLoS Med 12: e1001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farooqui A, Khan A, Kazmi SU, 2009. Investigation of a community outbreak of typhoid fever associated with drinking water. BMC Public Health 9: 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferdous F, Ahmed S, Das SK, Farzana FD, Latham JR, Chisti MJ, Faruque AS, 2014. Aetiology and clinical features of dysentery in children aged < 5 years in rural Bangladesh. Epidemiol Infect 142: 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Folarin TB, Oloruntoba EO, Ayede AI, 2013. Water quality and risk of diarrhoeal infections among children under five in Ibadan, Nigeria. African Journal of Biomedical Research 16: 67–76. [Google Scholar]
- 63.Franco-Aguirre JQ, Cardona-Tapias AA, Cardona-Arias JA, 2015. Health related quality of life and associated factors in patients with spondyloarthropathies, Medellin, Colombia, 2014. Archivos de Medicina 11: 9.
- 64.Freeman MC, Clasen T, 2011. Assessing the impact of a school-based safe water intervention on household adoption of point-of-use water treatment practices in southern India. Am J Trop Med Hyg 84: 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garrett V, Ogutu P, Mabonga P, Ombeki S, Mwaki A, Aluoch G, Phelan M, Quick RE, 2008. Diarrhoea prevention in a high-risk rural Kenyan population through point-of-use chlorination, safe water storage, sanitation, and rainwater harvesting. Epidemiol Infect 136: 1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gundry SW, Wright JA, Conroy RM, Du Preez M, Genthe B, Moyo S, Mutisi C, Potgieter N, 2009. Child dysentery in the Limpopo Valley: a cohort study of water, sanitation and hygiene risk factors. J Water Health 7: 259–266. [DOI] [PubMed] [Google Scholar]
- 67.Guthmann JP, et al., 2006. A large outbreak of hepatitis E among a displaced population in Darfur, Sudan, 2004: yhe role of water treatment methods. Clin Infect Dis 42: 1685–1691. [DOI] [PubMed] [Google Scholar]
- 68.Guthmann JP, Mercer AJ, Gandubert C, Morin F, 1996. Guinea worm disease in Ayod, Upper Nile Province, southern Sudan: a cross-sectional study. Trop Med Int Health 1: 117–123. [DOI] [PubMed] [Google Scholar]
- 69.Han J, et al., 2015. A newly discovered epidemic area of Echinococcus multilocularis in west Gansu Province in China. PLoS One 10: e0132731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haque QM, et al., 1996. Epidemiological study on contamination of water and diarrheal diseases in a rural community in northeast Thailand. Environ Health Prev Med 1: 20–27. [DOI] [PubMed] [Google Scholar]
- 71.Hartinger S, Commodore A, Hattendorf J, Lanata C, Gil A, Verastegui H, Aguilar-Villalobos M, Mäusezahl D, Naeher L, 2013. Chimney stoves modestly improved indoor air quality measurements compared with traditional open fire stoves: results from a small-scale intervention study in rural Peru. Indoor Air 23: 342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hartinger SM, Lanata CF, Hattendorf J, Gil AI, Verastegui H, Ochoa T, Maeusezahl D, 2011. A community randomised controlled trial evaluating a home-based environmental intervention package of improved stoves, solar water disinfection and kitchen sinks in rural Peru: Rationale, trial design and baseline findings. Contemp Clin Trials 32: 864–873. [DOI] [PubMed] [Google Scholar]
- 73.Hatch DL, Waldman RJ, Lungu GW, Piri C, 1994. Epidemic cholera during refugee resettlement in Malawi. Int J Epidemiol 23: 1292–1299. [DOI] [PubMed] [Google Scholar]
- 74.Hughes JM, Boyce JM, Levine RJ, Khan M, Aziz KM, Huq MI, Curlin GT, 1982. Epidemiology of eltor cholera in rural Bangladesh: importance of surface water in transmission. Bull World Health Organ 60: 395–404. [PMC free article] [PubMed] [Google Scholar]
- 75.Issa M, McHenry M, Issa AA, Blackwood RA, 2015. Access to safe water and personal hygiene practices in the Kulandia refugee camp (Jerusalem). Infect Dis Rep 7: 6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jain S, Sahanoon OK, Blanton E, Schmitz A, Wannemuehler KA, Hoekstra RM, Quick RE, 2010. Sodium dichloroisocyanurate tablets for routine treatment of household drinking water in periurban Ghana: a randomized controlled trial. Am J Trop Med Hyg 82: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jinadu MK, Olusi SO, Agun JI, Fabiyi AK, 1991. Childhood diarrhea in rural Nigeria: dtudies on prevalence, mortality and socio-environmental factors. J Diarrhoeal Dis Res 9: 323–327. [PubMed] [Google Scholar]
- 78.Kakakhel ZM, et al., 2011. Assessment of frequency of diarrhoea in relation to drinking water among residents of Nurpur Shahan, Pakistan. J Pak Med Assoc 61: 934–937. [PubMed] [Google Scholar]
- 79.Luby SP, Agboatwalla M, Painter J, Altaf A, Billhimer W, Keswick B, Hoekstra RM, 2006. Combining drinking water treatment and hand washing for diarrhoea prevention, a cluster randomised controlled trial. Trop Med Int Health 11: 479–489. [DOI] [PubMed] [Google Scholar]
- 80.Machdar E, van der Steen NP, Raschid-Sally L, Lens PN, 2013. Application of quantitative microbial risk assessment to analyze the public health risk from poor drinking water quality in a low income area in Accra, Ghana. Sci Total Environ 449: 134–142. [DOI] [PubMed] [Google Scholar]
- 81.Maeusezahl D, et al., 2009. Solar drinking water disinfection (SODIS) to reduce childhood diarrhoea in rural Bolivia: a cluster-randomized, controlled trial. PLoS Med 6: e1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mellor JE, Smith JA, Learmonth GP, Netshandama VO, Dillingham RA, 2012. Modeling the complexities of water, hygiene, and health in Limpopo Province, South Africa. Environ Sci Technol 46: 13512–13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moe CL, Sobsey MD, Samsa GP, Mesolo V, 1991. Bacterial indicators of risk of diarrheal disease from drinking-water in the Philippines. Bull World Health Organ 69: 305–317. [PMC free article] [PubMed] [Google Scholar]
- 84.Omar MS, Mahfouz AA, Abdel Moneim M, 1995. The relationship of water sources and other determinants to prevalence of intestinal protozoal infections in a rural community of Saudi Arabia. J Community Health 20: 433–440. [DOI] [PubMed] [Google Scholar]
- 85.Pereira M, Atwill ER, Barbosa AP, Silva SAE, Garcia-Zapata MTA, 2002. Intra-familial and extra-familial risk factors associated with Cryptosporidium parvum infection among children hospitalized for diarrhea in Goiania, Goias, Brazil. Am J Trop Med Hyg 66: 787–793. [DOI] [PubMed] [Google Scholar]
- 86.Prieto PA, Martin JAC, Marie GC, 2000.. Drinking water quality and diarrheal diseases in Cuba1996–1997 [in Spanish]. Rev Panam Salud Publica 7: 313–318.10893971 [Google Scholar]
- 87.Rai B, Pal R, Kar S, Tsering DC, 2010. Solar disinfection improves drinking water quality to prevent diarrhea in under-five children in Sikkim, India. J Glob Infect Dis 2: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reller ME, Mendoza CE, Lopez MB, Alvarez M, Hoekstra RM, Olson CA, Baier KG, Keswick BH, Luby SP, 2003. A randomized controlled trial of household-based flocculant-disinfectant drinking water treatment for diarrhea prevention in rural Guatemala. Am J Trop Med Hyg 69: 411–419. [PubMed] [Google Scholar]
- 89.Sady H, Al-Mekhlafi HM, Mahdy MA, Lim YA, Mahmud R, Surin J, 2013. Prevalence and associated factors of schistosomiasis among children in Yemen: implications for an effective control programme. PLoS Negl Trop Dis 7: e2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sarkar R, Sivarathinaswamy P, Thangaraj B, Sindhu KN, Ajjampur SS, Muliyil J, Balraj V, Naumova EN, Ward H, Kang G, 2013. Burden of childhood diseases and malnutrition in a semi-urban slum in southern India. BMC Public Health 13: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sobel J, Gomes TAT, Ramos RTS, Hoekstra M, Rodrigue D, Rassi V, Griffin PM, 2004. Pathogen-specific risk factors and protective factors for acute diarrheal illness in children aged 12–59 months in Sao Paulo, Brazil. Clin Infect Dis 38: 1545–1551. [DOI] [PubMed] [Google Scholar]
- 92.Stauber CE, Kominek B, Liang KR, Osman MK, Sobsey MD, 2012. Evaluation of the impact of the plastic BioSand filter on health and drinking water quality in rural Tamale, Ghana. Int J Environ Res Public Health 9: 3806–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stigler-Granados P, Quintana PJE, Gersberg R, Zuniga ML, Novotny T, 2014. Comparing health outcomes and point-of-use water quality in two rural indigenous communities of Baja California, Mexico before and after receiving new potable water infrastructure. J Water Sanit Hyg Dev 4: 672–680. [Google Scholar]
- 94.Swerdlow DL, Malenga G, Begkoyian G, Nyangulu D, Toole M, Waldman RJ, Puhr DN, Tauxe RV, 1997. Epidemic cholera among refugees in Malawi, Africa: treatment and transmission. Epidemiol Infect 118: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swerdlow DL, et al. 1992. Waterborne transmission of epidemic cholera in Trujillo, Peru: lessons for a continent at risk. Lancet 340: 28–33. [DOI] [PubMed] [Google Scholar]
- 96.Tian L, et al., 2012. Survey on Blastocystis hominis infection in HIV positive individuals in Fuyang City, Anhui Province [in Chinese]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 24: 303, 310. [PubMed] [Google Scholar]
- 97.Tran HH, Bjune G, Nguyen BM, Rottingen JA, Grais RF, Guerin PJ, 2005. Risk factors associated with typhoid fever in Son La province, northern Vietnam. Trans R Soc Trop Med Hyg 99: 819–826. [DOI] [PubMed] [Google Scholar]
- 98.Tuttle J, Ries AA, Chimha RM, Perera CU, Bean NH, Griffin PM, 1995. Antimicrobial-resistant epidemic Shigella-dysenteriae type-1 in Zambia: modes of transmission. J Infect Dis 171: 371–375. [DOI] [PubMed] [Google Scholar]
- 99.Wellin E, 1955. Water boiling in a Peruvian town. Paul BD, ed. Health, Culture and Community: Case Studies of Public Reactions to Health Programs. New York, NY: Russell Sage Foundation. [Google Scholar]
- 100.Xiao S, Lin C, Chen K, 1997. Evaluation of effectiveness of comprehensive control for diarrhea diseases in rural areas of east Fujian and analysis of its cost-benefit [in Chinese] Zhonghua Yu Fang Yi Xue Za Zhi 31: 40–41. [PubMed] [Google Scholar]
- 101.Yassin MM, Abu Amr SS, Al-Najar HM, 2006. Assessment of microbiological water quality and its relation to human health in Gaza Governorate, Gaza Strip. Public Health 120: 1177–1187. [DOI] [PubMed] [Google Scholar]
- 102.Zhu Y, et al., 2009. Analysis on the epidemiological characteristics of Escherichia coli O157:H7 infection in Xuzhou, Jiangsu, China, 1999. J Nanjing Medical Univ 23: 20–24. [Google Scholar]
- 103.Aggarwal R, Kumar R, Pal R, Naik S, Semwal SN, Naik SR, 2002. Role of travel as a risk factor for hepatitis E virus infection in a disease-endemic area. Indian J Gastroenterol 21: 14–18. [PubMed] [Google Scholar]
- 104.Al-Delaimy AK, Al-Mekhlafi HM, Nasr NA, Sady H, Atroosh WM, Nashiry M, Anuar TS, Moktar N, Lim YA, Mahmud R, 2014. Epidemiology of intestinal polyparasitism among Orang Asli school children in rural Malaysia. PLoS Negl Trop Dis 8: e3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bello J, Núñez FA, González OM, Fernández R, Almirall P, Escobedo AA, 2011. Risk factors for Giardia infection among hospitalized children in Cuba. Ann Trop Med Parasitol 105: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cardenas V, Saad C, Varona M, Linero M, 1993. Waterborne cholera in Riohacha, Colombia, 1992. Bull Pan Am Health Organ 27: 313–330. [PubMed] [Google Scholar]
- 107.Carrero SHS, 2013. Prevalence of intestinal parasites and risk factors in schoolchildren in Chicamocha Kennedy I school in the Municipality of Tuta, Boyacá—Colombia [in Spanish] Universidad y Salud 15: 218–224. [Google Scholar]
- 108.Choy SH, Al-Mekhlafi HM, Mahdy MA, Nasr NN, Sulaiman M, Lim YA, Surin J, 2014. Prevalence and associated risk factors of Giardia infection among indigenous communities in rural Malaysia. Sci Rep 4: 6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cifuentes E, 1998. The epidemiology of enteric infections in agricultural communities exposed to wastewater irrigation: perspectives for risk control. Int J Environ Health Res 8: 203–213. [Google Scholar]
- 110.Cohen A, Tao Y, Luo Q, Zhong G, Romm J, Colford JM, Jr, Ray I, 2015. Microbiological evaluation of household drinking water treatment in rural China shows benefits of electric kettles: a cross-sectional study. PLoS One 10: e0138451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Corwin A, et al. 1995. Two years’ investigation of epidemic hepatitis E virus transmission in West Kalimantan (Borneo), Indonesia. Trans R Soc Trop Med Hyg 89: 262–265. [DOI] [PubMed] [Google Scholar]
- 112.Fredrick T, Ponnaiah M, Murhekar MV, Jayaraman Y, David JK, Vadivoo S, Joshua V, 2015. Cholera outbreak linked with lack of safe water supply following a tropical cyclone in Pondicherry, India, 2012. J Health Popul Nutr 33: 31–38. [PMC free article] [PubMed] [Google Scholar]
- 113.Gunawardena GSA, Karunaweera ND, Ismail MM, 2004. Socio-economic and behavioural factors affecting the prevalence of Ascaris infection in a low-country tea plantation in Sri Lanka. Ann Trop Med Parasitol 98: 615–621. [DOI] [PubMed] [Google Scholar]
- 114.Herrera J, Marcos L, Terashima A, Alvarez H, Samalvides F, Gotuzzo E, 2006. Factors associated with Strongyloides stercoralis infection in an endemic area in Peru [in Spanish] Rev Gastroenterol Peru 26: 357–362. [PubMed] [Google Scholar]
- 115.Iijima Y, Karama M, Oundo JO, Honda T, 2001. Prevention of bacterial diarrhea by pasteurization of drinking water in Kenya. Microbiol Immunol 45: 413–416. [DOI] [PubMed] [Google Scholar]
- 116.Kelly P, Baboo KS, Ndubani P, Nchito M, Okeowo NP, Luo NP, Feldman RA, Farthing MJ, 1997. Cryptosporidiosis in adults in Lusaka, Zambia, and its relationship to oocyst contamination of drinking water. J Infect Dis 176: 1120–1123. [DOI] [PubMed] [Google Scholar]
- 117.Knight SM, Toodayan W, Caique WC, Kyi W, Barnes A, Desmarchelier P, 1992. Risk factors for the transmission of diarrhoea in children: a case-control study in rural Malaysia. Int J Epidemiol 21: 812–818. [DOI] [PubMed] [Google Scholar]
- 118.Lee YY, et al., 2012. Sociocultural and dietary practices among Malay subjects in the northeastern region of Peninsular Malaysia: a region of low prevalence of Helicobacter pylori infection. Helicobacter 17: 54–61. [DOI] [PubMed] [Google Scholar]
- 119.Li L-H, et al., 2007. Molecular epidemiology of human Blastocystis in a village in Yunnan province, China. Parasitol Int 56: 281–286. [DOI] [PubMed] [Google Scholar]
- 120.Marcano Y, Suarez B, Gonzalez M, Gallego L, Hernandez T, Naranjo M, 2013. Epidemiological characterization of intestinal parasitic diseases in the community 18 de Mayo, Santa Rita, Aragua state, Venezuela, 2012. Bol Malariol Salud Ambient 53: 135–145. [Google Scholar]
- 121.Núñez FA, López JL, de la Cruz AM, Finlay CM, 2003. Factores de riesgo de la infección por Giardia lamblia en niños de guarderías infantiles de Ciudad de La Habana, Cuba. Cad Saude Publica 19: 677–682. [DOI] [PubMed] [Google Scholar]
- 122.Psutka R, Priest P, Davies T, Rakunuea T, Iddings S, Reiffer A, 2013. Assessing the demographic, behavioural and environmental characteristics and the potential effectiveness of a household water filter in the Republic of Kiribati. J Water Sanit Hyg Dev 3: 530–540. [Google Scholar]
- 123.Ries AA, et al. 1992. Cholera in Piura, Peru: a modern urban epidemic. J Infect Dis 166: 1429–1433. [DOI] [PubMed] [Google Scholar]
- 124.Rondón B, Vargas M, Velarde N, Terashima I, Tello R, 2003. Human blastocystosis: Prospective study symptomatology and associated epidemiological factors [in Spanish] Rev Gastroenterol Peru 23: 29–35. [PubMed] [Google Scholar]
- 125.Sarkar R, et al., 2014. Risk factors for cryptosporidiosis among children in a semi urban slum in southern India: a nested case-control study. Am J Trop Med Hyg 91: 1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sarker MH, et al., 2014. Changing characteristics of rotavirus diarrhea in children younger than five years in urban Bangladesh. PLoS One 9: e105978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sharma PK, Ramakrishnan R, Hutin Y, Manickam P, Gupte MD, 2009. Risk factors for typhoid in Darjeeling, West Bengal, India: evidence for practical action. Trop Med Int Health 14: 696–702. [DOI] [PubMed] [Google Scholar]
- 128.Weber JT, et al. 1994. Epidemic cholera in Ecuador: multidrug-resistance and transmission by water and seafood. Epidemiol Infect 112: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wördemann M, et al., 2006. Prevalence and risk factors of intestinal parasites in Cuban children. Trop Med Int Health 11: 1813–1820. [DOI] [PubMed] [Google Scholar]
- 130.Abebe LS, Smith JA, Narkiewicz S, Oyanedel-Craver V, Conaway M, Singo A, Amidou S, Mojapelo P, Brant J, Dillingham R, 2014. Ceramic water filters impregnated with silver nanoparticles as a point-of-use water-treatment intervention for HIV-positive individuals in Limpopo Province, South Africa: a pilot study of technological performance and human health benefits. J Water Health 12: 288–300. [DOI] [PubMed] [Google Scholar]
- 131.Alam AY, Adil MM, Qureshi AA, 2008. Knowledge, attitude and practices survey on hygiene and there impact on health. Rawal Med J 33: 67–70. [Google Scholar]
- 132.Almeida LM, Werneck GL, Cairncross S, Coeli CM, Costa MC, Coletty PE, 2001. The epidemiology of hepatitis A in Rio de Janeiro: environmental and domestic risk factors. Epidemiol Infect 127: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bhandari D, Tandukar S, Parajuli H, Thapa P, Chaudhary P, Shrestha D, Shah PK, Sherchan JB, Sherchand JB, 2015. Cyclospora infection among school children in Kathmandu, Nepal: prevalence and associated risk factors. Trop Med Health 43: 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boisson S, Schmidt W-P, Berhanu T, Gezahegn H, Clasen T, 2009. Randomized controlled trial in rural Ethiopia to assess a portable water treatment device. Environ Sci Technol 43: 5934–5939. [DOI] [PubMed] [Google Scholar]
- 135.Boisson S, Stevenson M, Shapiro L, Kumar V, Singh LP, Ward D, Clasen T, 2013. Effect of household-based drinking water chlorination on diarrhoea among children under five in Orissa, India: a double-blind randomised placebo-controlled trial. PLoS Med 10: e1001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brown J, Sobsey MD, Loomis D, 2008. Local drinking water filters reduce diarrheal disease in Cambodia: a randomized, controlled trial of the ceramic water purifier. Am J Trop Med Hyg 79: 394–400. [PubMed] [Google Scholar]
- 137.Brown JM, Proum S, Sobsey MD, 2008. Escherichia coli in household drinking water and diarrheal disease risk: evidence from Cambodia. Water Sci Technol 58: 757–763. [DOI] [PubMed] [Google Scholar]
- 138.Chen H, et al, 2012. Change of water consumption and its potential influential factors in Shanghai: a cross-sectional study. BMC Public Health 12: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Clasen T, Garcia Parra G, Boisson S, Collin S, 2005. Household-based ceramic water filters for the prevention of diarrhea: a randomized, controlled trial of a pilot program in Colombia. Am J Trop Med Hyg 73: 790–795. [PubMed] [Google Scholar]
- 140.Clasen TF, Brown J, Collin S, Suntura O, Cairncross S, 2004. Reducing diarrhea through the use of household-based ceramic water filters: a randomized, controlled trial in rural Bolivia. Am J Trop Med Hyg 70: 651–657. [PubMed] [Google Scholar]
- 141.Clasen TF, Brown J, Collin SM, 2006. Preventing diarrhoea with household ceramic water filters: assessment of a pilot project in Bolivia. Int J Environ Health Res 16: 231–239. [DOI] [PubMed] [Google Scholar]
- 142.Corwin AL, et al., 1996. A waterborne outbreak of hepatitis E virus transmission in southwestern Vietnam. Am J Trop Med Hyg 54: 559–562. [DOI] [PubMed] [Google Scholar]
- 143.Crump JA, Otieno PO, Slutsker L, Keswick BH, Rosen DH, Hoekstra RM, Vulule JM, Luby SP, 2005. Household based treatment of drinking water with flocculant-disinfectant for preventing diarrhoea in areas with turbid source water in rural western Kenya: cluster randomised controlled trial. BMJ 331: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Aceituno AMF, Stauber CE, Walters AR, Sanchez REM, Sobsey MD, 2012. A randomized controlled trial of the plastic-housing BioSand filter and its impact on diarrheal disease in Copan, Honduras. Am J Trop Med Hyg 86: 913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dunkle SE, et al., 2010. Epidemic cholera in a crowded urban environment, Port-au-Prince, Haiti. Emerg Infect Dis 17: 2143–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ercumen A, Naser AM, Unicomb L, Arnold BF, Colford JM, Luby SP, 2015. Effects of source-versus household contamination of tubewell water on child diarrhea in Rural Bangladesh: a randomized controlled trial. PLoS One 10: e0121907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Graf J, Meierhofer R, Wegelin M, Mosler H-J, 2008. Water disinfection and hygiene behaviour in an urban slum in Kenya: impact on childhood diarrhoea and influence of beliefs. Int J Environ Health Res 18: 335–355. [DOI] [PubMed] [Google Scholar]
- 148.Graf J, Togouet SZ, Kemka N, Niyitegeka D, Meierhofer R, Pieboji JG, 2010. Health gains from solar water disinfection (SODIS): evaluation of a water quality intervention in Yaounde, Cameroon. J Water Health 8: 779–796. [DOI] [PubMed] [Google Scholar]
- 149.Gruber JS, Reygadas F, Arnold BF, Ray I, Nelson K, Colford JM, 2013. A stepped wedge, cluster-randomized trial of a household UV-disinfection and safe storage drinking water intervention in rural Baja California Sur, Mexico. Am J Trop Med Hyg 89: 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gungoren B, Latipov R, Regallet G, Musabaev E, 2007. Effect of hygiene promotion on the risk of reinfection rate of intestinal parasites in children in rural Uzbekistan. Trans R Soc Trop Med Hyg 101: 564–569. [DOI] [PubMed] [Google Scholar]
- 151.Guo Z, Li Y, Xu Z, Ji F, Wang L, Chen K, 2002. A case-control study on risk factors of helicobacter pylori infection in out-patients with stomach diseases. Zhonghua yu fang yi xue za zhi. Chin J Prev Med 36: 187–190. [PubMed] [Google Scholar]
- 152.Ilechukwu GC, Ilechukwu CG, Ozumba AN, Ojinnaka NC, Ibe BC, Onwasigwe CN, 2010. Some behavioural risk factors for intestinal helminthiasis in nursery and primary school children in Enugu, south eastern Nigeria. Niger J Clin Pract 13: 288–293. [PubMed] [Google Scholar]
- 153.Imanishi M, Kweza PF, Slayton RB, Urayai T, Ziro O, Mushayi W, Francis-Chizororo M, Kuonza LR, Ayers T, Freeman MM, 2014. Household water treatment uptake during a public health response to a large typhoid fever outbreak in Harare, Zimbabwe. Am J Trop Med Hyg 90: 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kinuthia GK, Gicheru MM, Ngure PK, Kabiru EW, 2012. Lifestyles and practices that enhance malaria and typhoid fever in Njoro District, Kenya. J Community Health 37: 224–233. [DOI] [PubMed] [Google Scholar]
- 155.Lindquist ED, George CM, Perin J, Neiswender De Calani KJ, Norman WR, Davis TP, Jr, Perry H, 2014. A cluster randomized controlled trial to reduce childhood diarrhea using hollow fiber water filter and/or hygiene-sanitation educational interventions. Am J Trop Med Hyg 91: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nguyen VD, Sreenivasan N, Lam E, Ayers T, Kargbo D, Dafae F, Jambai A, Alemu W, Kamara A, Islam MS, 2014. Cholera epidemic associated with consumption of unsafe drinking water and street-vended water—eastern Freetown, Sierra Leone, 2012. Am J Trop Med Hyg 90: 518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Novoa Reyes I, et al., 2014. Recurrence rate of Helicobacter pylori infection two years after successful eradication in Peruvian patients presenting with postprandial distress syndrome [in Spanish] Rev Gastroenterol Peru 34: 15–21. [PubMed] [Google Scholar]
- 158.Opryszko MC, Majeed SW, Hansen PM, Myers JA, Baba D, Thompson RE, Burnham G, 2010. Water and hygiene interventions to reduce diarrhoea in rural Afghanistan: a randomized controlled study. J Water Health 8: 687–702. [DOI] [PubMed] [Google Scholar]
- 159.Quick RE, Kimura A, Thevos A, Tembo M, Shamputa I, Hutwagner L, Mintz E, 2002. Diarrhea prevention through household-level water disinfection and safe storage in Zambia. Am J Trop Med Hyg 66: 584–589. [DOI] [PubMed] [Google Scholar]
- 160.Quick RE, et al., 1999. Diarrhoea prevention in Bolivia through point-of-use water treatment and safe storage: a promising new strategy. Epidemiol Infect 122: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Qureshi H, Shahid A, Mujeeb SA, 2000. Exposer rate of hepatitis E (IgG) in a selected population of children and adults in Karachi. J Pak Med Assoc 50: 352–354. [PubMed] [Google Scholar]
- 162.Romano N, Lim YAL, Amir NFH, Nissapatorn V, Mahmud R, 2011. Seroprevalence and sources of toxoplasmosis among Orang Asli (Indigenous) communities in Peninsular Malaysia. Am J Trop Med Hyg 85: 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sanchez-Perez HJ, Vargas-Morales MG, Mendez-Sanchez JD, 2000. Bacteriological quality of drinking water in areas of high levels of poverty in Chiapas, Mexico. Salud Publica Mex 42: 397–406. [DOI] [PubMed] [Google Scholar]
- 164.Siqueira AA, et al., 2010. Outbreak of acute gastroenteritis in young children with death due to rotavirus genotype G9 in Rio Branco, Brazilian Amazon region, 2005. Int J Infect Dis 14: e898–e903. [DOI] [PubMed] [Google Scholar]
- 165.Stauber CE, Ortiz GM, Loomis DP, Sobsey MD, 2009. A randomized controlled trial of the concrete biosand filter and its impact on diarrheal disease in Bonao, Dominican Republic. Am J Trop Med Hyg 80: 286–293. [PubMed] [Google Scholar]
- 166.Stauber CE, Printy ER, McCarty FA, Liang KR, Sobsey MD, 2012. Cluster randomized controlled trial of the plastic BioSand water filter in Cambodia. Environ Sci Technol 46: 722–728. [DOI] [PubMed] [Google Scholar]
- 167.Tarekegn M, Enquselassie F, 2012. A case control study on determinants of diarrheal morbidity among under-five children in Wolaita Soddo Town, southern Ethiopia. Ethiop J Health Dev 26: 78–85. [Google Scholar]
- 168.Tiwari SS, Schmidt WP, Darby J, Kariuki ZG, Jenkins MW, 2009. Intermittent slow sand filtration for preventing diarrhoea among children in Kenyan households using unimproved water sources: randomized controlled trial. Trop Med Int Health 14: 1374–1382. [DOI] [PubMed] [Google Scholar]
- 169.Vasquez WF, Aksan A-M, 2015. Water, sanitation, and diarrhea incidence among children: evidence from Guatemala. Water Policy 17: 932–945. [Google Scholar]
- 170.Ventura RJ, Muhi E, de los Reyes VC, Sucaldito MN, Tayag E, 2015. A community-based gastroenteritis outbreak after Typhoon Haiyan, Leyte, Philippines, 2013. Western Pac Surveill Response J 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vollaard AM, Ali S, van Asten H, Widjaja S, Visser LG, Surjadi C, van Dissel JT, 2004. Risk factors for typhoid and paratyphoid fever in Jakarta, Indonesia. JAMA 291: 2607–2615. [DOI] [PubMed] [Google Scholar]
- 172.Wanyiri JW, et al., 2013. Infectious diarrhoea in antiretroviral therapy-naïve HIV/AIDS patients in Kenya. Trans R Soc Trop Med Hyg 107: 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Watson L, Shibata T, Ansariadi S, Maidin A, Nikitin I, Wilson J, 2015. Understanding modifiable risk factors associated with childhood diarrhea in an eastern Indonesian urban setting. Int J Health Promot Educ 53: 42–54. [Google Scholar]
- 174.Zhou ZF, Zhang YS, Wang YM, 1997. Seroprevalence of Helicobacter pylori infection among Yi and Han nationalities in Yunxian County, Yunnan Province. Zhonghua Liu Xing Bing Xue Za Zhi 18: 18–21. [PubMed] [Google Scholar]
- 175.Cohen A, Tao Y, Luo Q, Zhong G, Romm J, Colford JM, Jr, Ray I, 2015. Microbiological evaluation of household drinking water treatment in rural China shows benefits of electric kettles: a cross-sectional study. PLoS One 10: e0138451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.WHO , 2015. Technical Brief: Boil Water. Geneva, Switzerland: The World Health Organization. [Google Scholar]
- 177.WHO , 2004. Guidelines for Drinking-Water Quality, 3rd edition. Vol. 1: Recommendations. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 178.WHO , 2011. Guidelines for Drinking-Water Quality, 4th edition. Malta/Geneva, Switzerland: World Health Organization. [Google Scholar]
- 179.Spinks AT, Dunstan RH, Harrison T, Coombes P, Kuczera G, 2006. Thermal inactivation of water-borne pathogenic and indicator bacteria at sub-boiling temperatures. Water Res 40: 1326–1332. [DOI] [PubMed] [Google Scholar]
- 180.Fayer R, 1994. Effect of high temperature on infectivity of Cryptosporidium parvum oocysts in water. Appl Environ Microbiol 60: 2732–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cho M, Kim J, Kim JY, Yoon J, Kim J-H, 2010. Mechanisms of Escherichia coli inactivation by several disinfectants. Water Res 44: 3410–3418. [DOI] [PubMed] [Google Scholar]
- 182.Nelson EJ, Harris JB, Morris JG, Calderwood SB, Camilli A, 2009. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Microbiol 7: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Brown LM, 2000. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev 22: 283–297. [DOI] [PubMed] [Google Scholar]
- 184.Dunn BE, Cohen H, Blaser MJ, 1997. Helicobacter pylori. Clin Microbiol Rev 10: 720–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Newell DG, et al., 2010. Food-borne diseases—the challenges of 20 years ago still persist while new ones continue to emerge. Int J Food Microbiol 139 (Suppl): S3–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Korich DG, Mead JR, Madore MS, Sinclair NA, Sterling CR, 1990. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl Environ Microbiol 56: 1423–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Abad FX, Pintó RM, Bosch A, 1994. Survival of enteric viruses on environmental fomites. Appl Environ Microbiol 60: 3704–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ijaz MK, Zargar B, Wright KE, Rubino JR, Sattar SA, 2016. Generic aspects of the airborne spread of human pathogens indoors and emerging air decontamination technologies. Am J Infect Control 44: S109–S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure and Table.