Abstract
Background
Epilepsy is the most common symptom in patients with supratentorial low-grade gliomas (LGGs), which adversely affects the patient’s quality of life. Poor seizure control with anti-epileptic therapy is an indication for surgery in these patients. Recent studies have sought to identify predictors of postoperative seizure control after surgical resection of LGG; gross total resection was shown to be a significant predictor in this respect. However, the prognostic value of other factors is not clear.
Methods
We performed a systematic review and meta-analysis of 23 studies with a combined study population of 2641 patients with LGG, in order to identify potential factors associated with favorable postoperative seizure control. Data were extracted on age and sex of patient, tumor location, tumor histology, type of seizure, seizure duration, extent of resection, and imaging characteristics.
Results
Patients ≥45 years of age achieved better postoperative seizure control (risk ratio [RR], 0.89; 95% CI, 0.81–0.99). Focal seizures were associated with poor seizure control (RR, 1.32; 95% CI, 1.18–1.49) compared with generalized seizures (RR, 0.77; 95% CI, 0.68–0.87). Prolonged history of seizures (≥1 y) had a negative impact on postoperative seizure control (RR, 1.22; 95% CI, 1.10–1.34). Gross total resection was superior to subtotal resection with respect to postoperative seizure control (RR, 0.68; 95% CI, 0.63–0.73).
Conclusions
This systematic review and meta-analysis identified predictors of postoperative seizure control in patients undergoing surgical resection of LGGs. Our results provide a reference for clinical treatment of LGG-related epilepsy.
Keywords: epilepsy, low-grade glioma, meta-analysis
Low-grade gliomas (LGGs) are well-differentiated, slow-growing, and biologically less aggressive brain tumors. According to the 2016 World Health Organization (WHO) classification of tumors of the CNS,1 LGGs are defined as grade II tumors and are further divided into 2 histological subtypes: astrocytic and oligodendroglial. Seizures are the most common and, sometimes, the only symptom in patients with supratentorial LGGs.2–6 Seizures may lead to motor and language impairment and greatly decrease the patients’ quality of life, thus increasing the economic and psychological burden on individuals, their families, and even the society.7,8 On the contrary, a history of epileptic seizure at diagnosis is a strong favorable prognostic factor for survival, especially for overall survival.9–11 Surgical treatment of LGG has been shown to prolong patient survival, reduce malignant transformation, and improve quality of life as well as seizure control.12–16 Approximately 80% of all patients with preoperative seizures become seizure free (Engel class I)6,17,18; outcomes in this respect tend to vary with the extent of resection (EOR). In addition to the EOR, parameters such as tumor location and histology have been shown to affect the extent of seizure control.19,20 However, the reported outcomes in this respect have tended to vary, ostensibly due to the variability in sample sizes and patient characteristics. Given the significance of different factors on treatment outcomes of LGG, we conducted a systematic review and meta-analysis of relevant studies to assess the potential predictors of seizure control in LGG.
Methods
Search Strategy and Data Extraction
This systematic review and meta-analysis complies with the PRISMA-IPD (Preferred Reporting Items for Systematic Review and Meta-Analyses of Individual Participant Data) guidelines.21 The PubMed database was searched for English language articles published between 1965 and 2016. The MeSH term “epilepsy” or “seizure” and “glioma” or “astrocytoma” or “oligodendroglioma” or “oligoastrocytoma” and the non-MeSH terms “low-grade glioma” and “adult” were used as key words. Animal studies were excluded from the articles retrieved on initial search.
The following exclusion criteria were then applied to narrow down the search: (1) systematic reviews, (2) case reports with <5 adult cases of LGG, (3) words like “children” or “infant” included in the title, (4) studies that refer to only neuronal tumor (ganglioglioma, dysembryoplastic neuroepithelial tumor), (5) seizure outcomes not the main focus of the study, (6) seizure outcomes not presented as Engel class or not directly comparable, and (7) studies of patients with preoperative radiotherapy or chemotherapy. Studies that involved lesions other than LGG were included only if outcomes related to other lesion types could be excluded.
Literature search was performed by a group member and articles were reviewed and selected independently by 2 investigators. Any disagreement between the 2 was resolved by group discussion with the third author.
Eight variables that may affect seizure outcomes were considered in this study. These included age, sex, tumor location, tumor histology, seizure type, seizure duration, EOR, and imaging characteristics. We chose the Engel classification at the time point that the author recommended or at last follow-up (1 mo to 17 y) for the purpose of this analysis, since different studies had different schedules for performing Engel classification (class I, seizure free; class II, rare seizures; class III, worthwhile improvement; and class IV, no seizure improvement or worsening of seizures).22
Evidence Grading
The Oxford Centre for Evidence-Based Medicine–Level of Evidence (2009) (http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/, Accessed 22 August 2017) classifies evidence into 5 levels (classes I to V). class I, the highest evidence level, refers to evidence from homogeneous randomized controlled trials; class V is the lowest level of evidence, which is based on expert opinion. The quality of each included study was assessed based on the Newcastle-Ottawa Scale (NOS) (http://www.ohri.ca/programs/ clinical_epidemiology/oxford.htm, Accessed 22 August 2017), a “star system” developed to assess the quality of nonrandomized studies, especially cohort studies and case-control.23 The number of stars reflects the quality of an article; highest rating is 9 stars (highest quality). Evidence grading and quality assessment was performed independently by 2 members of the study group; any disagreements were resolved by consensus within the group.
After assessment of individual articles, the overall body of evidence was evaluated using the GRADE system proposed by Guyatt and his team24,25 in GRADE profiler (v3.6.1). The GRADE system assigns high, moderate, low, or very low overall quality of evidence based on 5 parameters: risk of bias, inconsistency, indirectness, imprecision, and publication bias.
Meta-Analysis
Risk ratios (RRs) with 95% CIs were calculated using the fixed effects model in Review Manager (v5.3; Cochrane collaboration). Seizure outcomes were evaluated using Engel classification, dichotomized as class I (seizure free) and classes II–IV (not seizure free) for the purpose of statistical analysis. Meta-analysis was performed for comparison of seizure outcomes. Criteria for significance were 95% CI and P < 0.05. Publication bias was evaluated by funnel plot analyses.
Results
Literature Search
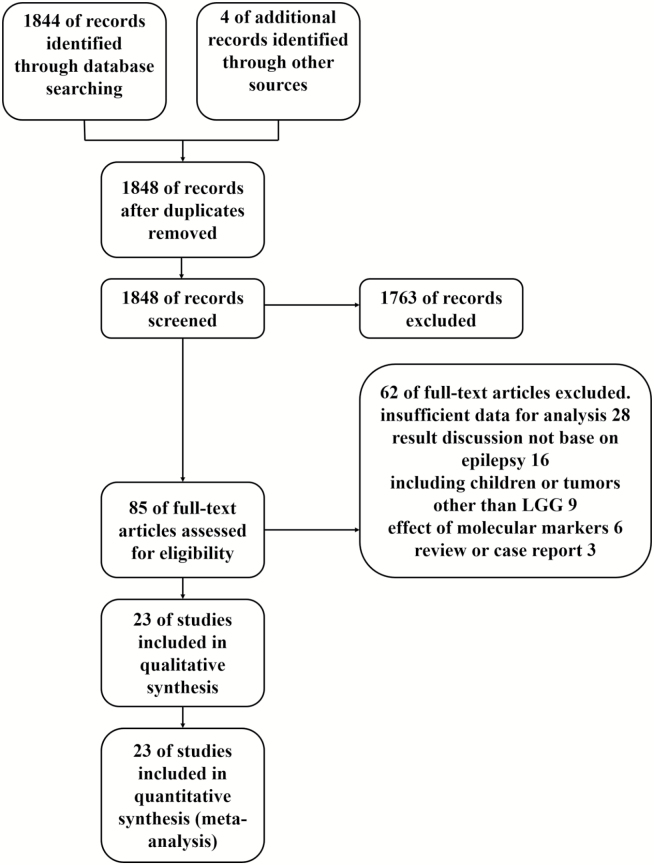
We screened out 1848 nonduplicated citations, of which 1763 studies were excluded after a review of the title and publication type. In line with exclusion criteria, a total of 85 studies qualified for full-text assessment. Finally, 23 studies with a combined study population of 2641 adult patients with LGG were included in the systematic review and the meta-analysis.2,3,9,15,19,20,26–42 Among these, 6 studies were included in the meta-analysis of the effect of age2,3,15,30,31,35; 7 studies for the meta-analysis of sex3,19,20,30,31,39,40; 7 studies for tumor localization9,19,27,29,33,39,40; 10 for tumor histology20,26–28,31,32,37,40–42; 3 for seizure type20,28,32; 5 for seizure duration15,28,32,35,41; 16 for EOR2,3,9,15,19,26,27,30,31,34,36–41; and 3 for the meta-analysis of imaging characteristics.19,28,41 The details of the literature search are presented in Fig. 1.
Fig. 1.
Flow diagram of the literature search and the selection of articles.
Evidence Quality
Of the included studies, the quality of evidence from 2 studies was rated as class II and 1 study was rated as class III. For all the other studies, the level of evidence was rated as class IV. None of the included studies was graded as class I. All studies included in this analysis had a retrospective study design. On evaluation based on the NOS, 7 articles were awarded 7 stars and 1 article was awarded 3 stars. The overall body of evidence pertaining to 7 variables was individually evaluated using the GRADE system. The quality of evidence for seizure type was rated as moderate, while that for all other factors was rated as low-grade. The overall grading of evidence related to imaging characteristics was not carried out due to the complexity of MRI characteristics, since MRI data should be further stratified based on presence or absence of enhancement, edema, mass effect, and other characteristics (Tables 1 and 2).
Table 1.
Summary of selected studies
| Study | Adult Patients with LGG (n) | Level of Evidence | Quality Evaluation (Newcastle-Ottawa Scale) | |
|---|---|---|---|---|
| 1 | Tanriverdi, 201636 | 40 | IV | ☆☆☆☆☆☆ |
| 2 | Duffau, 201629 | 16 | II | ☆☆☆☆☆☆☆ |
| 3 | Meguins, 201534 | 65 | IV | ☆☆☆☆☆☆ |
| 4 | Lima, 20152 | 21 | II | ☆☆☆☆☆☆ |
| 5 | Pallud, 20149 | 988 | IV | ☆☆☆☆☆ |
| 6 | Kemerdere, 201415 | 35 | IV | ☆☆☆☆☆ |
| 7 | Ius, 201431 | 52 | IV | ☆☆☆☆☆☆ |
| 8 | Yuan, 201341 | 93 | IV | ☆☆☆☆☆☆ |
| 9 | You, 201339 | 54 | IV | ☆☆☆☆☆☆ |
| 10 | Wilden, 201338 | 7 | IV | ☆☆☆☆☆☆ |
| 11 | Schucht, 201335 | 64 | III | ☆☆☆☆☆☆ |
| 12 | You, Sha, 2012b19 | 508 | IV | ☆☆☆☆☆ |
| 13 | You, Huang, 201240 | 183 | IV | ☆☆☆☆☆ |
| 14 | Ghareeb, 201230 | 15 | IV | ☆☆☆☆☆☆☆ |
| 15 | Chang, 200827 | 332 | IV | ☆☆☆☆☆☆ |
| 16 | Bauer, 200726 | 6 | IV | ☆☆☆☆☆☆ |
| 17 | Choi, 200428 | 16 | IV | ☆☆☆☆☆☆☆ |
| 18 | Zaatreh, 200442 | 43 | IV | ☆☆☆☆☆☆☆ |
| 19 | Luyken, 200320 | 50 | IV | ☆☆☆☆☆☆ |
| 20 | Duffau, 20023 | 11 | IV | ☆☆☆☆☆☆☆ |
| 21 | Wennberg, 199937 | 7 | IV | ☆☆☆☆☆☆☆ |
| 22 | Lombardi, 199733 | 22 | IV | ☆☆☆ |
| 23 | Jooma, 199532 | 14 | IV | ☆☆☆☆☆☆☆ |
Table 2.
Evaluation of the overall body of evidence for 7 factors by GRADE system
| Variables | Quality of Evidence* | |
|---|---|---|
| 1 | Age | ⊕⊕⊝⊝ low |
| 2 | Tumor location | ⊕⊕⊝⊝ low |
| 3 | Histological type | ⊕⊕⊝⊝ low |
| 4 | Seizure type | ⊕⊕⊕⊝ moderate |
| 5 | Seizure duration | ⊕⊕⊝⊝ low |
| 6 | Extent of resection | ⊕⊕⊝⊝ low |
| 7 | KPS score | ⊕⊝⊝⊝ very low |
| 8 | MRI characteristics | — |
*The quality of evidence is divided into 4 levels using GRADEpro system, and the number of symbols “⊕” and “⊝” represents different evidence levels, where “⊕⊕⊕⊕” means high quality and “⊕⊝⊝⊝” means very low quality. Details of the assessment are provided in the GRADE profiler.
Meta-analysis of Individual Factors
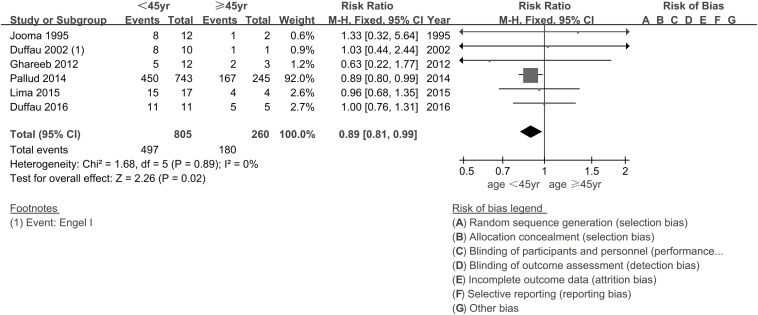
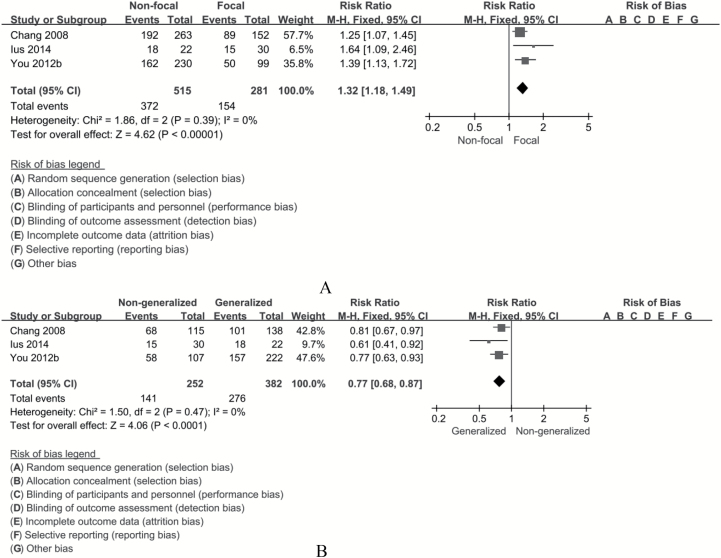
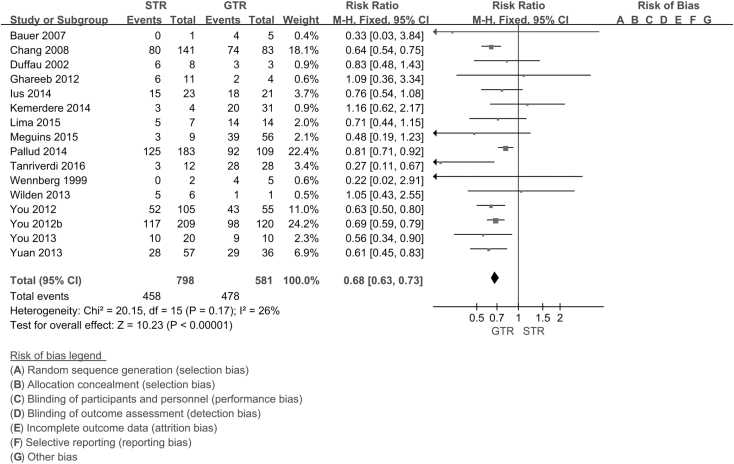
Age of the patient had a strong influence on seizure outcomes. Age ≥45 years was a favorable factor in seizure control (RR, 0.89; 95% CI, 0.81–0.99; P = 0.02) (Fig. 2). Some studies indicated temporal location of tumor as an unfavorable factor for seizure outcomes. Hence, while identifying the effect of tumor location, the author extracted data from 2 aspects—temporal and nontemporal location (as long as the tumor involved the temporal lobe, it was considered in a temporal location). We did not observe any significant difference in postoperative seizure control between tumors located in the temporal lobe and those not located in the temporal lobe (RR, 0.93; 95% CI, 0.86–1.02; P = 0.11). On comparing the astrocyte component of LGGs (oligoastrocytoma is in the astro group), there was no significant difference in seizure control in both groups (RR, 1.00; 95% CI, 0.88–1.13; P = 0.96). In this study, seizure type was disaggregated into focal seizures and generalized seizures according to the new operational classification of seizure type by the International League Against Epilepsy (ILAE) (http://www.ilae.org/Visitors/Centre/Class-Seizure.cfm, Accessed 22 August 2017). Meta-analysis was performed for focal seizure and generalized seizure. Postoperative outcomes in patients with focal seizure were unfavorable (RR, 1.32; 95% CI, 1.18–1.49; P < 0.001; number needed to treat [NNT], 6), while generalized seizure was a favorable factor with respect to seizure control (RR, 0.77; 95% CI, 0.68–0.87; P < 0.001; NNT, 6) (Fig. 3). Patients with seizure duration ≥1 year seemed to have poor seizure control (RR, 1.22; 95% CI, 1.10–1.34; P < 0.001; NNT, 9); however, the meta-analysis was affected by significant heterogeneity which could not be eliminated on sensitivity analysis. Several papers have discussed the influence of EOR on seizure control; however, in the current study, we focused on the effect of gross total resection (GTR) and subtotal resection (STR). GTR was associated with a significantly lower risk of postoperative seizures compared with STR (RR, 0.68; 95% CI, 0.63–0.73; P < 0.001; NNT, 4) (Fig. 4).
Fig. 2.
Forest plot of risk ratios for age ≥45 years vs age <45 years. Patients aged ≥45 years had better seizure control compared with those aged <45 years (RR, 0.89; 95% CI, 0.81–0.99; P = 0.02).
Fig. 3.
Forest plots of risk ratios for (A) focal seizure vs nonfocal seizure and (B) generalized seizure vs nongeneralized seizure calculated from individual studies. No significant difference is found in postoperative outcomes between patients with focal seizure and those with nonfocal seizure (RR, 1.32; 95% CI, 1.18–1.49; P < 0.001; NNT, 6), while patients with generalized seizures showed significantly better seizure control (RR, 0.77; 95% CI, 0.68–0.87; P < 0.001; NNT, 6).
Fig. 4.
Forest plot of risk ratios for EOR calculated from individual studies. Gross total resection significantly reduced the risk of postoperative seizures compared with subtotal resection (RR, 1.47; 95% CI, 1.37–1.58; P < 0.001; NNT, 4).
Meta-analysis of imaging characteristics revealed no significant association of lesion enhancement, cystic change, edema, and mass effect with the risk of postoperative seizures. Moreover, sex of the patients also did not show any significant association.
Discussion
LGG patients tend to have longer survival and are at a higher risk of seizures compared with patients who have high-grade gliomas. Given the significant impact of epilepsy on a patient’s quality of life, improved control of seizures is an important treatment objective in patients with LGG.43–45 Remission of seizures has a substantial effect on the cognitive and social ability of patients, which is liable to reduce the burden on families and society. To our knowledge, the mechanism of postoperative seizure control is poorly understood; and the results from different studies have been rather inconsistent. Several factors may influence the seizure outcomes, survival, and quality of life of patients. Understanding of these factors is essential to individualize the treatment based on the risk factors.
Surgery is one of the mainstream treatment modalities for gliomas; maximally safe resection of the tumor has been shown to achieve better clinical outcomes and seizure control.35 The vast majority of patients with LGG achieve seizure control. Some of these patients may become seizure free.19,27
In this study, we observed other predictors of seizure outcome in addition to EOR. In agreement with the majority of studies, the current study also confirmed the prognostic value of EOR for postoperative seizure outcomes. Although the precise nature of epileptogenesis is still unclear, tumor may induce epilepsy by virtue of its mass effect, or by the effect of chemicals secreted by glioma cells (such as glutamates).19,34 Compared with STR, GTR improved postoperative seizure control by 47% (P < 0.001), which confirms that maximally safe resection is significantly associated with a favorable prognosis. In the current study, the predictive value of preoperative seizure type for seizure outcomes was also investigated. In previous studies, seizure type was categorized as simple partial seizure, complex partial seizure, and generalized seizure. However, there was a paucity of community acceptance and public understanding of this seizure classification. In the new operational classification of ILAE, the terms “simple partial,” “complex partial,” and “secondarily generalized” have been eliminated, and the term “partial” has been replaced with “focal.” Here we found that in patients with focal seizure, poor postoperative seizure control rate increased by 32% (P < 0.001), while the risk of poor seizure control in generalized seizure was decreased by 23% (P < 0.001). To the best of our knowledge, this is the first systematic review and meta-analysis to investigate the association of preoperative seizure type with seizure outcomes based on the new operational classification. Additionally, we found that patients aged ≥45 years achieved significantly better postoperative seizure control (P = 0.02), which suggests the need to sensitize younger patients in advance with respect to their prospects for postoperative seizure control. Moreover, prolonged history of seizures was associated with an unfavorable prognosis. Compared with patients with seizure duration ≥1 year, more patients with seizure duration <1 year had improved postoperative seizure control (P < 0.001). This could probably be because of the difference in seizure type, education level of the patient, and potential differences in tolerance to epilepsy, which may have influenced patients’ perceptions with respect to seizure duration and seizure outcomes. This underlines the need to detect, diagnose, and treat tumor-related seizures at an early stage. No significant effect of sex, tumor location (temporal vs nontemporal), tumor histology (astro vs non-astro), and imaging characteristics (enhancement, edema, mass effect) on seizure outcomes was observed. As regards tumor location, Pallud et al9 reported that insular location was an independent predictor of uncontrolled epileptic seizures after oncological treatment. However, more studies showed that there was no significant correlation of tumor location with seizure outcome.9,20,39,41 Luyken et al reported that histological subtypes other than astrocytomas were associated with improved seizure outcomes.20 We did not observe any significant difference between the astro and non- astro groups. This discrepancy may have resulted from the non-uniformity in histological diagnostic criteria used in different medical organizations. Moreover, only 3 of the included studies had referred to imaging characteristics which could be further classified and the related articles were insufficient.
Studies with small sample size were not excluded from this study, since meta-analysis produces a weighted average of each included study and eliminates the effect of small sample size. The distribution of the weight can ensure the accuracy and credibility of the result, and the comprehensiveness of included data can also be guaranteed.
To our knowledge, this study is the largest systematic review and meta-analysis of prognostic factors for postoperative seizure control in patients with LGG. However, some limitations of our study need to be acknowledged. First, based on the status quo in the research field of tumor-related epilepsy, the number of included studies is relatively insufficient; moreover, the overall quality of the body of evidence was moderate to low. Future studies are warranted to enrich the research field. Second, funnel plots were constructed for tumor histology and EOR, which suggested that the meta-analysis of EOR may have been affected by publication bias because of the overwhelming number of studies that supported favorable outcomes of GTR compared with those of STR on seizure control. The third limitation concerns the heterogeneity with respect to seizure duration (fixed model, I2 = 60%); five of the included studies were subjected to sensitivity analysis. Re-analysis after sequential exclusion of one study at a time did not eliminate the effect of heterogeneity. We believe that it is not feasible to eliminate all potential confounding factors due to the effect of recall bias, differences in the social construct of epilepsy, and varied tolerance levels among patients for epilepsy. The small number of included studies may have also contributed to the heterogeneity. Lastly, due to the restricted scope of the present study, this article did not discuss the influence of tumor volume, tumor side, and the usage of anti-epileptic drugs (AEDs). One of the included studies showed that when tumor volume was less than 30 cm3, seizure outcome was better.31 However, the studies related to lesion volume and seizure outcome are insufficient and can hardly get a uniform data standard. To our knowledge, so far there is not a universally accepted guideline about the prophylactic use or postoperative use of AEDs, and some studies included in our meta-analysis did not provide the details, including the choice of AED, time of withdrawal, etc. These aspects need to be evaluated in future studies.
Conclusion
This systematic review suggested that GTR was associated with more favorable postoperative seizure control than STR. Other factors, such as generalized seizure, seizure duration <1 year, and age ≥45 years, were associated with improved seizure outcomes. We recommend early detection of tumor-related epilepsy and timely decision to perform maximally safe resection in order to achieve superior seizure control and improve quality of life.
Funding
This work was supported by funds from the National Key Research and Development Plan (2016YFC0902500), the National High Technology Research and Development Program (2015CB755500), and the National Natural Science Foundation of China (No. 81601452).
Conflict of interest statement. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Louis DN, Perry A, Reifenberger G et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Lima GL, Duffau H. Is there a risk of seizures in “preventive” awake surgery for incidental diffuse low-grade gliomas?J Neurosurg. 2015;122(6):1397–1405. [DOI] [PubMed] [Google Scholar]
- 3. Duffau H, Capelle L, Lopes M, Bitar A, Sichez JP, van Effenterre R. Medically intractable epilepsy from insular low-grade gliomas: improvement after an extended lesionectomy. Acta Neurochir (Wien). 2002;144(6):563–572; discussion 572. [DOI] [PubMed] [Google Scholar]
- 4. Bartolomei JC, Christopher S, Vives K, Spencer DD, Piepmeier JM. Low-grade gliomas of chronic epilepsy: a distinct clinical and pathological entity. J Neurooncol. 1997;34(1):79–84. [DOI] [PubMed] [Google Scholar]
- 5. Berger MS, Ghatan S, Haglund MM, Dobbins J, Ojemann GA. Low-grade gliomas associated with intractable epilepsy: seizure outcome utilizing electrocorticography during tumor resection. J Neurosurg. 1993;79(1):62–69. [DOI] [PubMed] [Google Scholar]
- 6. Britton JW, Cascino GD, Sharbrough FW, Kelly PJ. Low-grade glial neoplasms and intractable partial epilepsy: efficacy of surgical treatment. Epilepsia. 1994;35(6):1130–1135. [DOI] [PubMed] [Google Scholar]
- 7. Aaronson NK, Taphoorn MJ, Heimans JJ et al. Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol. 2011;29(33):4430–4435. [DOI] [PubMed] [Google Scholar]
- 8. Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159–168. [DOI] [PubMed] [Google Scholar]
- 9. Pallud J, Audureau E, Blonski M et al. Epileptic seizures in diffuse low-grade gliomas in adults. Brain. 2014;137(Pt 2):449–462. [DOI] [PubMed] [Google Scholar]
- 10. Schaller B, Rüegg SJ. Brain tumor and seizures: pathophysiology and its implications for treatment revisited. Epilepsia. 2003;44(9):1223–1232. [DOI] [PubMed] [Google Scholar]
- 11. Lote K, Stenwig AE, Skullerud K, Hirschberg H. Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer. 1998;34(1):98–102. [DOI] [PubMed] [Google Scholar]
- 12. Smith JS, Chang EF, Lamborn KR et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. [DOI] [PubMed] [Google Scholar]
- 13. Englot DJ, Han SJ, Berger MS, Barbaro NM, Chang EF. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery. 2012;70(4):921–928; discussion 928. [DOI] [PubMed] [Google Scholar]
- 14. Phi JH, Kim SK, Cho BK et al. Long-term surgical outcomes of temporal lobe epilepsy associated with low-grade brain tumors. Cancer. 2009;115(24):5771–5779. [DOI] [PubMed] [Google Scholar]
- 15. Kemerdere R, Yuksel O, Kacira T et al. Low-grade temporal gliomas: surgical strategy and long-term seizure outcome. Clin Neurol Neurosurg. 2014;126:196–200. [DOI] [PubMed] [Google Scholar]
- 16. Ruda R, Bello L, Duffau H, Soffietti R. Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol. 2012;14(Suppl 4):iv55–iv64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Franceschetti S, Binelli S, Casazza M et al. Influence of surgery and antiepileptic drugs on seizures symptomatic of cerebral tumours. Acta Neurochir (Wien). 1990;103(1-2):47–51. [DOI] [PubMed] [Google Scholar]
- 18. Villemure JG, de Tribolet N. Epilepsy in patients with central nervous system tumors. Curr Opin Neurol. 1996;9(6):424–428. [DOI] [PubMed] [Google Scholar]
- 19. You G, Sha ZY, Yan W et al. Seizure characteristics and outcomes in 508 Chinese adult patients undergoing primary resection of low-grade gliomas: a clinicopathological study. Neuro Oncol. 2012;14(2): 230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luyken C, Blümcke I, Fimmers R et al. The spectrum of long-term epilepsy-associated tumors: long-term seizure and tumor outcome and neurosurgical aspects. Epilepsia. 2003;44(6):822–830. [DOI] [PubMed] [Google Scholar]
- 21. Stewart LA, Clarke M, Rovers M et al. ; PRISMA-IPD Development Group. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313(16):1657–1665. [DOI] [PubMed] [Google Scholar]
- 22. Engel J. Outcome with respect to epileptic seizures. Surgical Treatment of the Epilepsies. 1993:609–621. [Google Scholar]
- 23. Jiang J, Tang Q, Feng J et al. Association between SLCO1B1 -521T>C and -388A>G polymorphisms and risk of statin-induced adverse drug reactions: a meta-analysis. Springerplus. 2016;5(1):1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guyatt GH, Oxman AD, Vist GE et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guyatt GH, Oxman AD, Sultan S et al. ; GRADE Working Group. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–1316. [DOI] [PubMed] [Google Scholar]
- 26. Bauer R, Dobesberger J, Unterhofer C et al. Outcome of adult patients with temporal lobe tumours and medically refractory focal epilepsy. Acta Neurochir (Wien). 2007;149(12):1211–1216; discussion 1216. [DOI] [PubMed] [Google Scholar]
- 27. Chang EF, Potts MB, Keles GE et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108(2):227–235. [DOI] [PubMed] [Google Scholar]
- 28. Choi JY, Chang JW, Park YG, Kim TS, Lee BI, Chung SS. A retrospective study of the clinical outcomes and significant variables in the surgical treatment of temporal lobe tumor associated with intractable seizures. Stereotact Funct Neurosurg. 2004;82(1):35–42. [DOI] [PubMed] [Google Scholar]
- 29. Duffau H. Long-term outcomes after supratotal resection of diffuse low-grade gliomas: a consecutive series with 11-year follow-up. Acta Neurochir (Wien). 2016;158(1):51–58. [DOI] [PubMed] [Google Scholar]
- 30. Ghareeb F, Duffau H. Intractable epilepsy in paralimbic World Health Organization Grade II gliomas: should the hippocampus be resected when not invaded by the tumor?J Neurosurg. 2012;116(6):1226–1234. [DOI] [PubMed] [Google Scholar]
- 31. Ius T, Pauletto G, Isola M et al. Surgery for insular low-grade glioma: predictors of postoperative seizure outcome. J Neurosurg. 2014;120(1):12–23. [DOI] [PubMed] [Google Scholar]
- 32. Jooma R, Yeh HS, Privitera MD, Gartner M. Lesionectomy versus electrophysiologically guided resection for temporal lobe tumors manifesting with complex partial seizures. J Neurosurg. 1995;83(2):231–236. [DOI] [PubMed] [Google Scholar]
- 33. Lombardi D, Marsh R, de Tribolet N. Low grade glioma in intractable epilepsy: lesionectomy versus epilepsy surgery. Acta Neurochir Suppl. 1997;68:70–74. [DOI] [PubMed] [Google Scholar]
- 34. Meguins LC, Adry RA, Silva Júnior SC et al. Gross-total resection of temporal low grade gliomas is a critically important factor in achieving seizure-freedom. Arq Neuropsiquiatr. 2015;73(11):924–928. [DOI] [PubMed] [Google Scholar]
- 35. Schucht P, Ghareeb F, Duffau H. Surgery for low-grade glioma infiltrating the central cerebral region: location as a predictive factor for neurological deficit, epileptological outcome, and quality of life. J Neurosurg. 2013;119(2):318–323. [DOI] [PubMed] [Google Scholar]
- 36. Tanriverdi T, Kemerdere R, Baran O et al. Long-term surgical and seizure outcomes of frontal low-grade gliomas. Int J Surg. 2016;33(Pt A):60–64. [DOI] [PubMed] [Google Scholar]
- 37. Wennberg R, Quesney LF, Lozano A, Olivier A, Rasmussen T. Role of electrocorticography at surgery for lesion-related frontal lobe epilepsy. Can J Neurol Sci. 1999;26(1):33–39. [PubMed] [Google Scholar]
- 38. Wilden JA, Voorhies J, Mosier KM, O’Neill DP, Cohen-Gadol AA. Strategies to maximize resection of complex, or high surgical risk, low-grade gliomas. Neurosurg Focus. 2013;34(2):E5. [DOI] [PubMed] [Google Scholar]
- 39. You G, Feng L, Yan W et al. BCL2A1 is a potential biomarker for postoperative seizure control in patients with low-grade gliomas. CNS Neurosci Ther. 2013;19(11):882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. You G, Huang L, Yang P et al. Clinical and molecular genetic factors affecting postoperative seizure control of 183 Chinese adult patients with low-grade gliomas. Eur J Neurol. 2012;19(2):298–306. [DOI] [PubMed] [Google Scholar]
- 41. Yuan Y, Xiang W, Yanhui L et al. Ki-67 overexpression in WHO grade II gliomas is associated with poor postoperative seizure control. Seizure. 2013;22(10):877–881. [DOI] [PubMed] [Google Scholar]
- 42. Zaatreh MM, Firlik KS, Spencer DD, Spencer SS. Temporal lobe tumoral epilepsy: characteristics and predictors of surgical outcome. Neurology. 2003;61(5):636–641. [DOI] [PubMed] [Google Scholar]
- 43. Klein M. Health-related quality of life aspects in patients with low-grade glioma. Adv Tech Stand Neurosurg. 2010;35:213–235. [DOI] [PubMed] [Google Scholar]
- 44. Duffau H. Surgery of low-grade gliomas: towards a ‘functional neurooncology’. Curr Opin Oncol. 2009;21(6):543–549. [DOI] [PubMed] [Google Scholar]
- 45. Gunnarsson T, Olafsson E, Sighvatsson V, Hannesson B. Surgical treatment of patients with low-grade astrocytomas and medically intractable seizures. Acta Neurol Scand. 2002;105(4):289–292. [DOI] [PubMed] [Google Scholar]