Abstract
Objective
To compare complications, disability, and long-term mortality of patients who received direct enteral tube vs nasogastric tube feeding alone after acute stroke.
Methods
We used the Ontario Stroke Registry to identify patients who received direct enteral tubes (DET; gastrostomy or jejunostomy) or temporary nasogastric tubes (NGT) alone during hospital stay after acute ischemic stroke or intracerebral hemorrhage from July 1, 2003, to March 31, 2013. We used propensity matching to compare groups from discharge and evaluated discharge disability, institutionalization, complications, and mortality, with follow-up over 2 years, and with cumulative incidence functions used to account for competing risks.
Results
Among 1,448 patients with DET placement who survived until discharge, 1,421 were successfully matched to patients with NGT alone. Patients with DET had reduced risk of death within 30 days after discharge (9.7% vs 15.3%; hazard ratio [HR] 0.61, 95% confidence interval [CI] 0.49–0.75), but this difference was eliminated after matching on length of stay and discharge disability (HR 0.90, 95% CI 0.70–1.17). Patients with DET had higher rates of severe disability at discharge (modified Rankin Scale score 4–5; 89.6% vs 78.4%), discharge to long-term care (38.0% vs 16.1%), aspiration pneumonia (14.4% vs 5.1%) and other complications, and mortality at 2 years (41.1% vs 35.9%).
Conclusions
Patients with DET placement after acute stroke have more severe disability at discharge compared to those with NGT placement alone, and associated higher rates of institutionalization, medical complications, and long-term mortality. These findings may inform goals of care discussions and decisions regarding long-term tube feeding after acute stroke.
Dysphagia is a common complication after acute stroke, and can affect more than 50% of patients.1,2 People with dysphagia after stroke can experience dehydration, malnutrition, and weight loss,3 and be at risk for pneumonia, severe disability, and death.4 Early dysphagia care often involves the use of nasogastric tubes (NGT) to provide nutrition and hydration where oral intake is limited or unsafe.5 However, patient discomfort and need for frequent replacement limit their long-term use.6
Given the temporary nature of NGT, insertion of a direct enteral tube (DET), typically through percutaneous endoscopic gastrostomy (PEG) or jejunostomy, should be considered when dysphagia is severe or persistent and continued feeding is desired. Advantages include their discrete insertion site and higher tolerability, but PEG placement requires an invasive procedure and is associated with major complications such as infection and hemorrhage, and high mortality in patients with stroke.7,8
Despite the use of DET feeding in up to 10% of patients after stroke,9 remarkably little is known about long-term outcomes such as pneumonia, functional status, and mortality. Recent large observational studies have focused on factors related to the placement of enteral feeding tubes, including hospital volume, socioeconomic status, race, and timing of the procedure.10,11 The Food or Ordinary Diet (FOOD) trial published in 2005 randomized dysphagic stroke patients to receive either NGT or PEG and found no difference in survival at 6 months but an increase of borderline significance in death or severe disability among patients who received PEG.12 An updated Cochrane review found no difference in pneumonia or mortality irrespective of follow-up time.13 However, studies were small, with varying lengths of follow-up, and quality of evidence was deemed low, leaving clinicians, patients, and families with limited information to guide decisions related to artificial feeding after stroke.
We used a large cohort of patients with acute stroke to determine the risk of severe disability, complications, and mortality in those with DET placement compared to a propensity score–matched group of patients who had NGT placement alone.
Methods
Setting
The province of Ontario, Canada, has a population of approximately 13 million people. Residents receive publicly funded coverage for hospital care, physicians' services, and diagnostic tests. Ontario's regional system of stroke care promotes guidelines for early dysphagia detection,14 and approximately 80% of all acute ischemic stroke patients in Ontario are screened for dysphagia within 72 hours poststroke.4
Data sources and study sample
The Ontario Stroke Registry collects detailed clinical information on all consecutive patients with acute stroke seen at regional stroke centers as well as on a population-based sample of patients from every acute care hospital in the province.15 Chart review is completed by trained data abstractors, and chart validation by duplicate chart abstraction has shown excellent agreement for key variables.16 The registry includes data on stroke type and presentation, comorbid conditions, in-hospital procedures, complications, disability at discharge based on the modified Rankin Scale (mRS), and discharge destination.
The registry is housed at the Institute for Clinical Evaluative Sciences (ICES), where it is linked to administrative databases using unique encoded identifiers. We used the registry to provide information on baseline patient characteristics, discharge disability, and destination. We used the Canadian Institute for Health Information–Discharge Abstract Database (CIHI-DAD) and the Canadian Institute for Health Information–National Ambulatory Care Reporting System (CIHI-NACRS) to identify subsequent hospitalizations and emergency department visits for postdischarge complications, the Ontario Registered Persons Database to identify all-cause mortality and to classify patients into different ethnic groups, and the Canada Census to provide information on median neighborhood income quintile. These databases have been validated and are used routinely for health research.17
Patient population and exposure definitions
For this study, we included consecutive patients with ischemic stroke or intracerebral hemorrhage (ICH) who were hospitalized between July 1, 2003, and March 31, 2013, and who received either DET or NGT insertion at any time during admission. We identified those who received NGT placement from the registry, based on chart review by trained abstractors. Data for NGT placement were not available in 2009. To ensure complete ascertainment of all cases, we identified those who received DET placement from both the registry and from CIHI databases using Canadian Classification of Health Interventions (CCI) procedure codes, which have a positive predictive value of 83%.17 Procedure codes for open surgical placement of feeding tubes were excluded (table e-1, links.lww.com/WNL/A136). Although the majority of patients with CCI codes received gastrostomy tubes (80.3%), our sample included both gastrostomy and jejunostomy tubes in order to capture all patients with DET placement. Patients who received NGT followed by DET were included only in the DET group. Patients were excluded if they were younger than 18 years; had an in-hospital stroke; were hospitalized with a stroke more than 72 hours from symptom onset; were not admitted; had a TIA, subarachnoid hemorrhage, or isolated intraventricular hemorrhage; or had DET placement prior to the index stroke.
Covariates
Admission stroke severity is documented in the registry using the Canadian Neurological Scale, a validated scale including orientation, level of consciousness, speech, and motor function, and where lower scores indicate greater stroke severity and are associated with mortality at 30 days and 1 year.18,19 We categorized stroke severity a priori as mild (Canadian Neurological Scale ≥8; equivalent to an NIH Stroke Scale [NIHSS] ≤8), moderate (Canadian Neurological Scale 5–7; equivalent to NIHSS 9–13), or severe (Canadian Neurological Scale 0–4; equivalent to NIHSS ≥14) on the basis of previous studies.20–22 Palliative care status is documented in the registry if chart review indicates that a decision (and not just a palliative care consultation) is made to provide a palliative approach to care. Information on ethnicity is collected in the registry, but is missing in over 50% of patients. Therefore, we linked to the Ontario Registered Persons Database and used validated surname algorithms to identify people of Chinese and South Asian descent (the major ethnic groups in Canada).23,24 We imputed socioeconomic status based on median neighborhood income.25 We categorized participating hospitals as regional stroke centers (large institutions with advanced stroke care resources and expertise comparable to comprehensive stroke centers in the United States) or non–regional stroke centers. We obtained rates of mechanical ventilation and tracheostomy from CCI procedure codes (table e-1, links.lww.com/WNL/A136).
Outcomes
We evaluated the following outcomes: (1) all-cause mortality at 30 days and 2 years; (2) severe disability at discharge from acute care, defined as an mRS score of 4–5; (3) discharge to a long-term care or chronic care facility; (4) complications at 2 years, including aspiration pneumonia/pneumonitis (for simplicity referred to as aspiration pneumonia), all-cause pneumonia, pressure ulcer, sepsis, and gastrointestinal hemorrhage. We identified hospitalizations and emergency department visits for postdischarge complications from CIHI-DAD and CIHI-NACRS using ICD-10-CA codes (table e-2, links.lww.com/WNL/A136).
Analysis
SAS Enterprise Guide 9.4 (Cary, NC) was used to conduct all analyses. Since there are likely to be baseline differences between patients who received NGT alone and those who received DET, we used propensity matching to account for confounding due to measured baseline covariates. We matched on the logit of the propensity score using a greedy nearest neighbor algorithm with caliper width equal to 0.2 of the SD of the logit of the propensity score.26 Matching was performed on the following variables: age, sex, Charlson comorbidity score,27 preadmission independence, prior stroke, dementia, atrial fibrillation, diabetes, current smoking, hypertension, hyperlipidemia, arrival from long-term care, stroke severity, admission to stroke unit, stroke type (ischemic vs ICH), palliative care during admission, index period (2003–2008; 2009–2013), and care at regional stroke centers vs other hospital types. In the propensity-matched sample, we used standardized differences to assess the balance of measured baseline covariates between treatment groups. We did not match on pneumonia, as we could not determine whether this complication occurred before or after DET placement during hospitalization.
The registry did not include information on the date of NGT insertion; thus we could not match patients based on the date of procedure. Matching on the date of stroke onset would have introduced immortal time bias, whereby patients with DET would have had guaranteed survival time prior to the exposure.28 Therefore, our main analysis focused on the cohort of patients who survived to discharge, with events counted from the date of discharge. We only matched patients at stroke onset to determine discharge mRS of the entire cohort. We conducted 2 sensitivity analyses. In the first, we removed patients who were managed with a palliative approach during hospitalization to reduce the effect of palliation on early mortality after discharge. In the second, we included length of hospital stay and discharge disability (mRS 4–5) to the propensity match, to account for residual differences between groups at discharge.
There was a significant interaction between time from discharge and hazard ratio (HR) of mortality in patients with DET vs patients with NGT alone (p < 0.001). Therefore, we separated the 2 years after discharge into 5 epochs and used Cox proportional hazard regression models to estimate the effect of DET placement on the hazard of death within each epoch. We then compared the incidence of complications (aspiration pneumonia, all-cause pneumonia, pressure ulcer, sepsis, and gastrointestinal hemorrhage) as a function of time in those with DET vs NGT placement, using cumulative incidence functions to account for the competing risk of death.29 Cox proportional hazard regression models were used to estimate the effect of DET on each complication at 2 years.
Standard protocol approvals, registrations, and patient consents
Data collection for the registry is done without patient consent, since ICES is named as a prescribed entity under provincial privacy legislation. This study was approved by the Sunnybrook Health Sciences Centre Research Ethics Board.
Results
Of 37,870 eligible patients hospitalized with acute stroke, 6,061 had recorded insertion of feeding tubes during their index admission: 4,263 patients with NGT alone and 1,798 with DET.
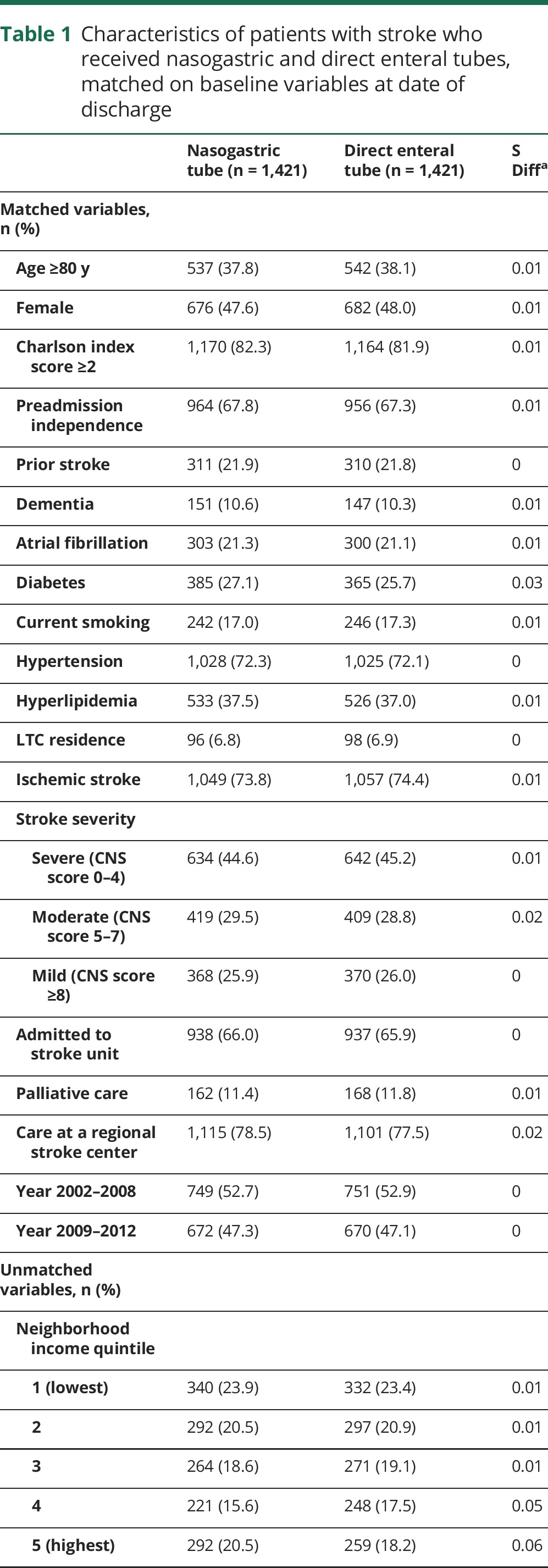
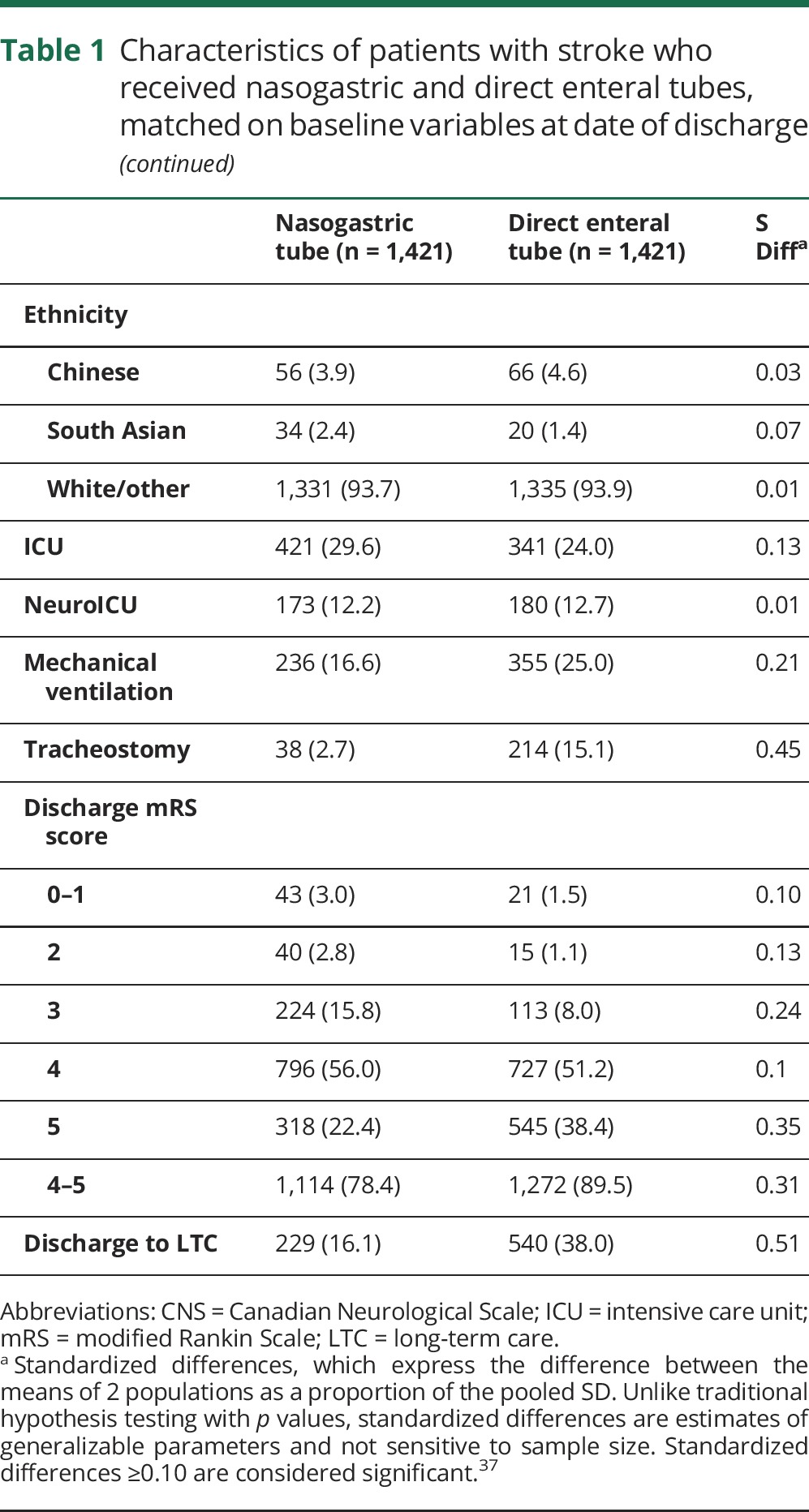
Among 3,984 patients who survived until discharge, 2,536 had NGT alone and 1,448 had DET insertion (unmatched characteristics in table e-3, links.lww.com/WNL/A136). The median time to DET placement was 19 days (interquartile range 12–27). A total of 1,421 patients with DET (98.1%) could be matched to 1,421 patients with NGT alone, with good balance between groups on all matched variables (table 1). Compared to those with NGT, those with DET were overall less likely to receive care in an intensive care unit (ICU) (24.0% vs 29.6%) but more likely to receive mechanical ventilation (25.0% vs 16.6%) and tracheostomy (15.1% vs 2.7%; table 1). From 0 to 29 days after discharge, the hazard of death was lower in those with DET than those with NGT alone (9.7% vs 15.3%; HR 0.61, 95% confidence interval [CI] 0.49–0.75). Patients with NGT alone and early mortality are compared to those with DET in table e-4.
Table 1.
Characteristics of patients with stroke who received nasogastric and direct enteral tubes, matched on baseline variables at date of discharge
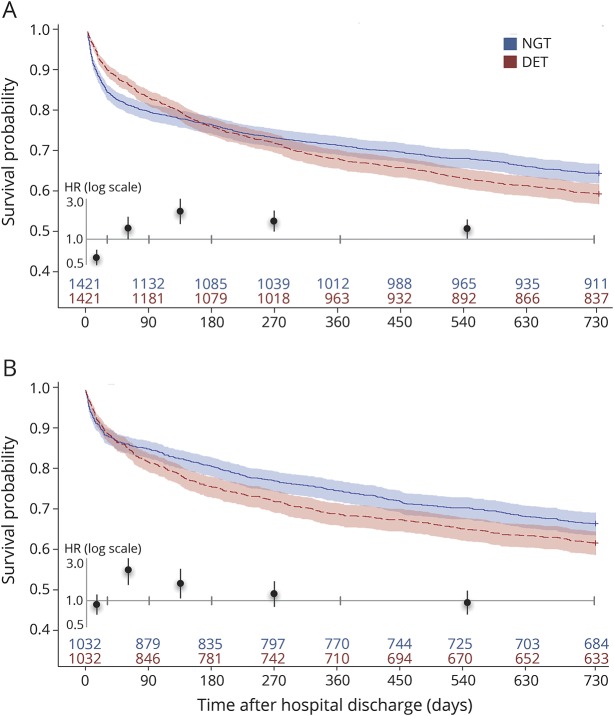
There was a higher hazard of death with DET from 30 to 89 days after discharge (HR 1.35, 95% CI 1.0–1.84), 90–179 days (HR 2.23, 95% CI 1.51–3.0), 180–365 days (HR 1.64, 95% CI 1.23–2.12), and 366–730 days (HR 1.32; 95% CI 1.02–1.71; table 2 and figure 1A). Two-year mortality was higher in those with DET than those with NGT (41.1% vs 35.9%, p = 0.004).
Table 2.
Postdischarge mortality and complications in patients with stroke who received nasogastric and direct enteral tubes, matched on baseline variables at date of discharge
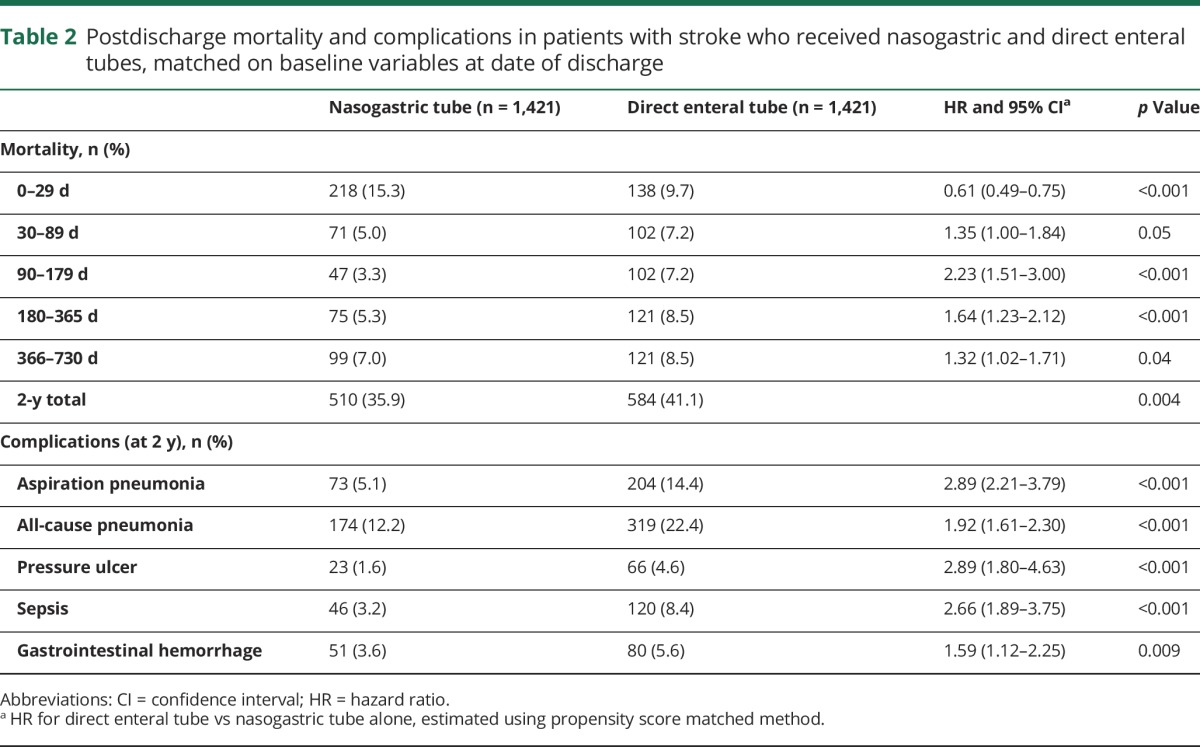
Figure 1. Survival probability from acute discharge in patients who received direct enteral tubes versus nasogastric tubes alone.
Survival probability with 95% confidence intervals calculated from Cox proportional hazard models in patients with direct enteral tubes (DET; red) versus nasogastric tubes alone (NGT; blue), from discharge date, with propensity matching on (A) baseline variables, and (B) baseline variables plus length of stay and modified Rankin Scale score. Number of patients alive is shown for all time points. Mortality hazard ratios (HR) for DET vs. NGT are shown for all 5 epochs (inset, tic marks showing intervals; see table 2 and table e-8 for values).
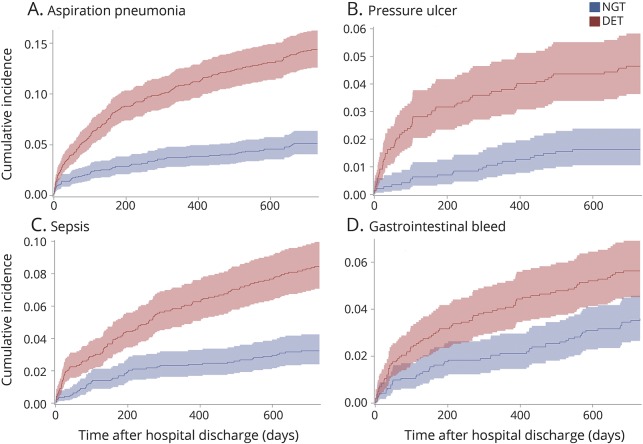
Compared to those who received NGT alone, patients with DET were more likely to be severely disabled at discharge (mRS 4–5; 89.5% vs 78.4%) due to a higher rate of patients with mRS 5 (38.4% vs 22.4%; table 1 and figure 2A), and to be discharged to a long-term or chronic care facility (38.0% vs 16.1%). Results for disability at discharge were similar when all patients were matched from time of stroke onset (figure 2B; table e-5, links.lww.com/WNL/A136). The incidence of complications was higher in the DET than in the NGT group, with a 2-year risk of postdischarge aspiration pneumonia of 14.4% vs 5.1% (HR 2.89, 95% CI 2.21–3.79), for all-cause pneumonia of 22.4% vs 12.2% (HR 1.92, 95% CI 1.61–2.30), for pressure ulcer of 4.6% vs 1.6% (HR 2.89, 95% CI 1.80–4.63), for sepsis of 8.4% vs 3.2% (HR 2.66, 95% CI 1.89–3.75), and for gastrointestinal hemorrhage of 5.6% vs 3.6% (HR 1.59, 95% CI 1.12–2.25; table 2 and figure 3).
Figure 2. Modified Rankin Scale (mRS) score distributions at discharge in patients who received direct enteral tubes and nasogastric tubes alone.
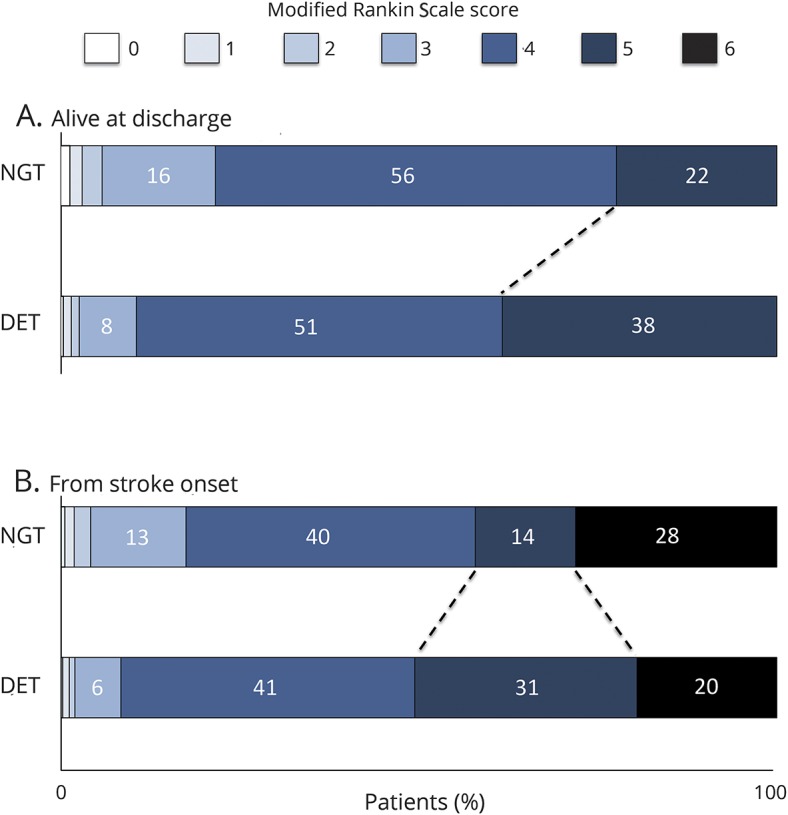
mRS score distributions at discharge in patients with direct enteral tubes (DET) and nasogastric tubes (NGT) alone, matched at (A) discharge date and (B) stroke onset. mRS score 0 indicates no symptoms, 1 indicates no clinically significant disability, 2 indicates slight disability, 3 indicates moderate disability, 4 indicates moderately severe disability, 5 indicates severe disability, and 6 indicates death. Among those with DET, there is a higher proportion of patients within mRS 5 (bedridden, incontinent, and requiring constant nursing care and attention).
Figure 3. Postdischarge complications in patients who received direct enteral tubes (DET) vs nasogastric tubes (NGT) alone.
Cumulative incidence of postdischarge complications with 95% confidence intervals in DET (red) and NGT (blue) groups, including (A) aspiration pneumonia, (B) pressure ulcer, (C) sepsis, and (D) gastrointestinal hemorrhage. Patients with DET had higher rates of all complications at all time points from discharge until 2 years (p < 0.01 for difference between patients with DET and NGT, using the Gray test for equality of cumulative incidence functions).
When patients treated with a palliative approach were removed from the analyses, the difference in early survival was attenuated but remained significant, and other findings were similar (table e-6, links.lww.com/WNL/A136). When length of stay and mRS at discharge were included in the propensity matching, there was no longer a difference in the hazard of death within 30 days for those with DET vs NGT (HR 0.90, 95% CI 0.70–1.17), but there was still a higher hazard of death from days 30–89 (HR 2.22, 95% CI 1.50–3.27) and 90–179 (HR 1.56, 95% CI 1.06–2.28), and a higher rate of death at 2 years (38.7% vs 33.7%, p = 0.02; figure 1B and tables e-7 and e-8). Complication rates were similar to those observed in the primary analyses (table e-8).
Discussion
In this study of patients undergoing feeding tube placement after stroke, we found that those who received a DET (gastrostomy or jejunostomy) had lower mortality within 30 days after discharge compared to those who received temporary NGT alone, but this difference was not sustained after matching on length of stay and functional status at discharge. Patients with DET had significantly higher rates of severe disability, long-term care placement, pneumonia and other complications, and mortality at 2 years than those with NGT alone.
The decision to undergo direct feeding tube placement can be ethically challenging, given that the vast majority of stroke survivors with DET are dependent on caregivers. There are significant levels of depression among patients with PEG tubes, and high levels of stress experienced by relatives of patients.30 Physicians also perceive pressures from family or other health care professionals in arriving at a recommendation for placement.31 Guidelines generally advocate the use of DET at 2-4 weeks after stroke for patients who are projected to require long-term enteral feeding.32–34 However, lack of data on outcomes limits informed discussions and appropriate patient selection in the clinical setting.
The FOOD trial was the largest randomized controlled trial on artificial feeding after stroke, allocating patients to NGT or PEG within days of admission. The trial found no difference in survival at 6 months, but an increase of borderline significance in absolute risk of death or poor outcome (mRS 4–5) with PEG.12 Our findings are consistent with those of the FOOD trial, with small differences attributable to study design (observational study vs randomized trial), ascertainment of outcomes (from hospital discharge vs time of randomization), patient crossover in the FOOD trial (28% of patients randomized to NGT later received PEG), and longer follow-up time in our study.
Our primary analysis showed higher early mortality after discharge in those who received NGT compared to DET. However, the subgroup of patients with NGT and early mortality had a shorter length of stay and were much more likely to be severely disabled and to be treated with a palliative approach at discharge compared to those who received DET, implying that many within this subgroup may have been discharged early for the purposes of palliation. Consistent with this, the difference in early mortality between those with NGT and DET was eliminated when the groups were matched for length of stay and disability at discharge.
The overall higher rate of severe disability in those with DET insertion likely contributed to their increased risk of late complications and mortality compared to patients with NGT. A Cochrane review showed no significant difference in pneumonia between NGT and PEG, although studies were small and quality of evidence was low.13 In our study, patients with DET feeding had higher odds of pneumonia, pressure ulcer, sepsis, and gastrointestinal hemorrhage over 2 years compared to those with temporary NGT insertion alone. These associations were generally maintained even after matching on discharge disability. Dysphagia alone has been associated with higher odds of pneumonia, disability, and mortality,4 and likely contributed to the higher rate of these outcomes among patients with DET.
Our study has some limitations that deserve mention. First, a randomized controlled trial is ideal when comparing 2 interventions. However, given ethical and logistical challenges, additional randomized trials on enteral feeding are unlikely to be performed after the large FOOD trial. Although we performed propensity matching to optimize balance between groups, we cannot rule out residual confounding. Our findings of differential ICU, intubation, and tracheostomy use in those receiving and not receiving DET suggest that unmeasured differences in patient characteristics remained even after matching. We did not have information on other factors potentially associated with outcomes, such as dysphagia severity, stroke location, timing of NGT insertion, and duration of tube feeding. Second, we had no information on patient and family preferences, goals of care, and discussions leading to decisions regarding feeding tube placement. In addition, we could not identify situations where DET was considered but not pursued, or reasons for foregoing DET placement, which may range from improvement of swallowing function to pursuing palliative care. Indeed, a recent study found that over 50% of patients hospitalized with serious illness viewed relying on a feeding tube as living in a state equal to or worse than death.35 Third, some cases of NGT were likely missed by chart review, given the bedside nature of the procedure and potential for lack of documentation. However, due to our propensity-matched design, where 98% of patients with DET were well-matched in the main analysis, we do not think this would have significantly affected the results. We were also unable to identify complications which did not result in a hospital visit, and may have underestimated complications such as pressure ulcers where coding may be inconsistent. Fourth, a significant proportion (about 1 in 5 patients) in our cohort receiving DET had jejunostomy rather than gastrostomy tubes, and we do not know if this was done with the goal of reducing aspiration, or addressing specific indications such as obstruction or gastroparesis.36 The inclusion of jejunostomies in this study should be kept in mind when generalizing to centers that exclusively use gastrostomy. Despite these limitations, our findings using carefully matched comparison groups provide useful information on long-term outcomes after feeding tube insertion in a real-world setting.
We found that patients with DET feeding after acute stroke, compared to those with NGT alone, had greater disability, long-term care placement, complications, and long-term mortality. Our findings may be useful in the development of contemporary clinical guidelines and to inform discussions among health care practitioners, patients, and family members with regards to direct enteral feeding after stroke.
Glossary
- CCI
Canadian Classification of Health Interventions
- CI
confidence interval
- CIHI-DAD
Canadian Institute for Health Information–Discharge Abstract Database
- CIHI-NACRS
Canadian Institute for Health Information–National Ambulatory Care Reporting System
- DET
direct enteral tube
- FOOD
Food or Ordinary Diet
- HR
hazard ratio
- ICD-10-CA
International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada
- ICES
Institute for Clinical Evaluative Sciences
- ICH
intracerebral hemorrhage
- ICU
intensive care unit
- mRS
modified Rankin Scale
- NGT
nasogastric tube
- NIHSS
NIH Stroke Scale
- PEG
percutaneous endoscopic gastrostomy
Footnotes
Editorial, page 305
Author contributions
Raed Joundi: study concept and design, data interpretation, writing manuscript. Gustavo Saposnik: study design, critical revision of manuscript. Rosemary Martino: study design, critical revision of manuscript. Jiming Fang: data analysis and interpretation. Joan Porter: study design, critical revision of manuscript. Moira Kapral: study design, data interpretation, critical revision of manuscript, study supervision.
Study funding
This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed in the material are those of the authors, and not necessarily those of CIHI. M.K.K. and G.S. are supported by Mid-Career Investigator Awards from the Heart and Stroke Foundation of Canada. R.M. is supported by a Canada Research Chair (Tier II) in Swallowing Disorders.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Perry L, Love CP. Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia 2001;16:7–18. [DOI] [PubMed] [Google Scholar]
- 2.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005;36:2756–2763. [DOI] [PubMed] [Google Scholar]
- 3.Tippett DC. Clinical challenges in the evaluation and treatment of individuals with poststroke dysphagia. Top Stroke Rehabil 2011;18:120–133. [DOI] [PubMed] [Google Scholar]
- 4.Joundi RA, Martino R, Saposnik G, Giannakeas V, Fang J, Kapral MK. Predictors and outcomes of dysphagia screening after acute ischemic stroke. Stroke 2017;48:900–906. [DOI] [PubMed] [Google Scholar]
- 5.Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. J Cereb Circ 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 6.Geeganage C, Beavan J, Ellender S, Bath PMW. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database Syst Rev 2012:CD000323. [DOI] [PubMed] [Google Scholar]
- 7.Wijdicks EF, McMahon MM. Percutaneous endoscopic gastrostomy after acute stroke: complications and outcome. Cerebrovasc. Dis 1999;9:109–111. [DOI] [PubMed] [Google Scholar]
- 8.Wilmskoetter J, Simpson KN, Bonilha HS. Hospital Readmissions of Stroke Patients with Percutaneous Endoscopic Gastrostomy Feeding Tubes. J Stroke Cerebrovasc. Dis. 2016;25:2535–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilmskoetter J, Simpson AN, Simpson KN, Bonilha HS. Practice patterns of percutaneous endoscopic gastrostomy tube placement in acute stroke: are the guidelines achievable? J Stroke Cerebrovasc Dis 2016;25:2694–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George BP, Kelly AG, Schneider EB, Holloway RG. Current practices in feeding tube placement for US acute ischemic stroke inpatients. Neurology 2014;83:874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George BP, Kelly AG, Albert GP, Hwang DY, Holloway RG. Timing of percutaneous endoscopic gastrostomy for acute ischemic stroke: an observational study from the US nationwide inpatient sample. Stroke 2016;48:420–427. [DOI] [PubMed] [Google Scholar]
- 12.Dennis MS, Lewis SC, Warlow C; FOOD Trial Collaboration. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet 2005;365:764–772. [DOI] [PubMed] [Google Scholar]
- 13.Gomes CA, Andriolo RB, Bennett C, et al. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database Syst Rev 2015:CD008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hebert D, Lindsay MP, McIntyre A, et al. Canadian stroke best practice recommendations: stroke rehabilitation practice guidelines, update 2015. Int J Stroke 2016;11:459–484. [DOI] [PubMed] [Google Scholar]
- 15.Cadilhac DA, Kim J, Lannin NA, et al. National stroke registries for monitoring and improving the quality of hospital care: a systematic review. Int J Stroke 2016;11:28–40. [DOI] [PubMed] [Google Scholar]
- 16.Kapral MK, Hall RE, Stamplecoski M, et al. Registry of the Canadian Stroke Network: Report on the 2008/09 Ontario Stroke Audit. Toronto: Institute for Clinical Evaluative Sciences; 2011. Available at: ices.on.ca/∼/media/Files/Atlases-Reports/2011/RCSN-2008-09-Ontario-stroke-audit/Full%20report.ashx. Accessed May 22, 2017. [Google Scholar]
- 17.Juurlink DN, Preyra C, Croxford R, et al. Canadian Institute for Health Information Discharge Abstract Database: A Validation Study. Toronto: Institute for Clinical Evaluative Sciences; 2006. Available at: ices.on.ca/Publications/Atlases-and-Reports/2006/Canadian-Institute-for-Health-Information. Accessed May 22, 2017. [Google Scholar]
- 18.Côté R, Battista RN, Wolfson C, Boucher J, Adam J, Hachinski V. The Canadian Neurological Scale: validation and reliability assessment. Neurology 1989;39:638–643. [DOI] [PubMed] [Google Scholar]
- 19.Stavem K, Lossius M, Rønning OM. Reliability and validity of the Canadian Neurological Scale in retrospective assessment of initial stroke severity. Cerebrovasc Dis 2003;16:286–291. [DOI] [PubMed] [Google Scholar]
- 20.Saposnik G, Fang J, O'Donnell M, Hachinski V, Kapral MK, Hill MD. Escalating levels of access to in-hospital care and stroke mortality. Stroke 2008;39:2522–2530. [DOI] [PubMed] [Google Scholar]
- 21.Saposnik G, Kapral MK, Liu Y, et al. IScore: a risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation 2011;123:739–749. [DOI] [PubMed] [Google Scholar]
- 22.Bushnell CD, Johnston DC, Goldstein LB. Retrospective assessment of initial stroke severity: comparison of the NIH Stroke Scale and the Canadian Neurological Scale. Stroke 2001;32:656–660. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Wang F, Schopflocher D, et al. Development and validation of a surname list to define Chinese ethnicity. Med Care 2006;44:328–333. [DOI] [PubMed] [Google Scholar]
- 24.Cummins C, Winter H, Cheng KK, et al. An assessment of the Nam Pehchan computer program for the identification of names of south Asian ethnic origin. J Public Health Med 1999;21:401–406. [DOI] [PubMed] [Google Scholar]
- 25.Kapral MK, Fang J, Chan C, et al. Neighborhood income and stroke care and outcomes. Neurology 2012;79:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med 2014;33:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 28.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 2008;167:492–499. [DOI] [PubMed] [Google Scholar]
- 29.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 30.Rickman J. Percutaneous endoscopic gastrostomy: psychological effects. Br J Nurs 1998;7:723–729. [DOI] [PubMed] [Google Scholar]
- 31.Callahan CM, Haag KM, Buchanan NN, Nisi R. Decision-making for percutaneous endoscopic gastrostomy among older adults in a community setting. J Am Geriatr Soc 1999;47:1105–1109. [DOI] [PubMed] [Google Scholar]
- 32.Gomes F, Hookway C, Weekes CE. Royal College of Physicians Intercollegiate Stroke Working Party evidence-based guidelines for the nutritional support of patients who have had a stroke. J Hum Nutr Diet 2014;27:107–121. [DOI] [PubMed] [Google Scholar]
- 33.Wirth R, Smoliner C, Jäger M, Warnecke T, Leischker AH, Dziewas R. Guideline clinical nutrition in patients with stroke. Exp Transl Stroke Med 2013;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stroud M, Duncan H, Nightingale J; British Society of Gastroenterology. Guidelines for enteral feeding in adult hospital patients. Gut 2003;52(suppl 7):vii1–vii12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin EB, Buehler AE, Halpern SD. States worse than death among hospitalized patients with serious illnesses. JAMA Intern Med 2016;176:1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Itkin M, DeLegge MH, Fang JC, et al. Multidisciplinary practical guidelines for gastrointestinal access for enteral nutrition and decompression from the Society of Interventional Radiology and American Gastroenterological Association (AGA) Institute, with endorsement by Canadian Interventional Radiological Association (CIRA) and Cardiovascular and Interventional Radiological Society of Europe (CIRSE). Gastroenterology 2011;141:742–765. [DOI] [PubMed] [Google Scholar]
- 37.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Comm Statist Simulation Comput 2009;38:1228–1234. [Google Scholar]