Abstract
PURPOSE
To test the hypothesis that, given the current resection eligibility criteria for colorectal liver metastasis (CLM), prior hepatectomy would be associated with improved local tumor control and survival after percutaneous ablation of CLM.
METHODS
This single-institution retrospective study included 82 consecutive patients with 97 CLM treated with ablation (radiofrequency, microwave, or cryoablation) from January 2005 to December 2014. Local tumor progression-free survival (LTPFS), recurrence-free survival at any organ (RFS), and overall survival (OS) were calculated from the time of ablation and compared between patients with (n=49) and without (n=33) prior hepatectomy using the Kaplan-Meier method. Cox regression models were used to identify LTPFS predictors.
RESULTS
Median overall follow-up period was 28 months (range, 4.5–132). The 3-year actuarial LTPFS (patient level: 73% vs 34%, P < 0.001) were significantly higher in patients with than without prior hepatectomy, respectively. Similarly, three-year RFS (23% vs 9.1%, P = 0.026) and OS (78% vs 48%, P = 0.003) were improved in patients with prior hepatectomy. At multivariate analysis, predictors of worse LTPFS were: no prior hepatectomy (hazard ratio [HR] 2.35, 95% confidence interval [CI] 1.02–5.45; P = 0.045), minimal ablation margin < 5mm (HR 2.4, 95% CI 1.18–4.87; P = 0.016), and RAS-mutant tumor (HR 2.65, 95% CI 1.18–5.94; P = 0.019).
CONCLUSION
Prior hepatectomy for CLM is associated with improved local tumor control after percutaneous ablation of post-resection developed CLM.
INTRODUCTION
Up to 50% of patients with colorectal cancer present with or ultimately develop colorectal liver metastases (CLM) during their disease course, which accounts for two-thirds of the colorectal cancer-related deaths (1). Among the local curative therapies for CLM, hepatic resection is considered the modality of choice with most recent series demonstrating 5-years overall survival (OS) of up to 60% (1, 2). Unfortunately, less than 20% of patients with CLM are candidates to resection (3). Percutaneous liver ablation is another local therapy widely utilized for treatment of CLM, traditionally reserved for patients who are not candidates for surgery and for patients with post-hepatectomy recurrences. Most current series demonstrate 5-year OS rates ranging from 21% to 48% after ablation of CLM (4–6).
In patients with CLM, low rates of local recurrence (define as local tumor progression [LTP] for ablation) at the treated CLM are associated with improved OS rates. More recently, tumor biological factors have been recognized as prognosticators for local recurrence and survival in patients undergoing resection and ablation of CLM (7–9). Likewise, the use of adjunctive measures such as neoadjuvant chemotherapy, portal vein embolization, and two-stage hepatic resection, have been linked with improved outcomes after resection of CLM (10–14), which might be attributable to selection of patients with more favorable tumor characteristics during assessment of the response to chemotherapy, growth of the future liver remnant, and recovery after first-stage hepatectomy (15). Despite of such findings, to date it is unclear whether such selection criteria for liver resection would ultimately also affect ablation outcomes of CLM that develop after hepatic resection when compared to ablation of CLM without a prior history of hepatic resection.
Therefore, the aim of this is study is to test the hypothesis that, given the current resection eligibility criteria for colorectal liver metastasis (CLM), prior hepatectomy would be associated with improved local tumor control and survival after ablation of CLM.
PATIENTS AND METHODS
Study population
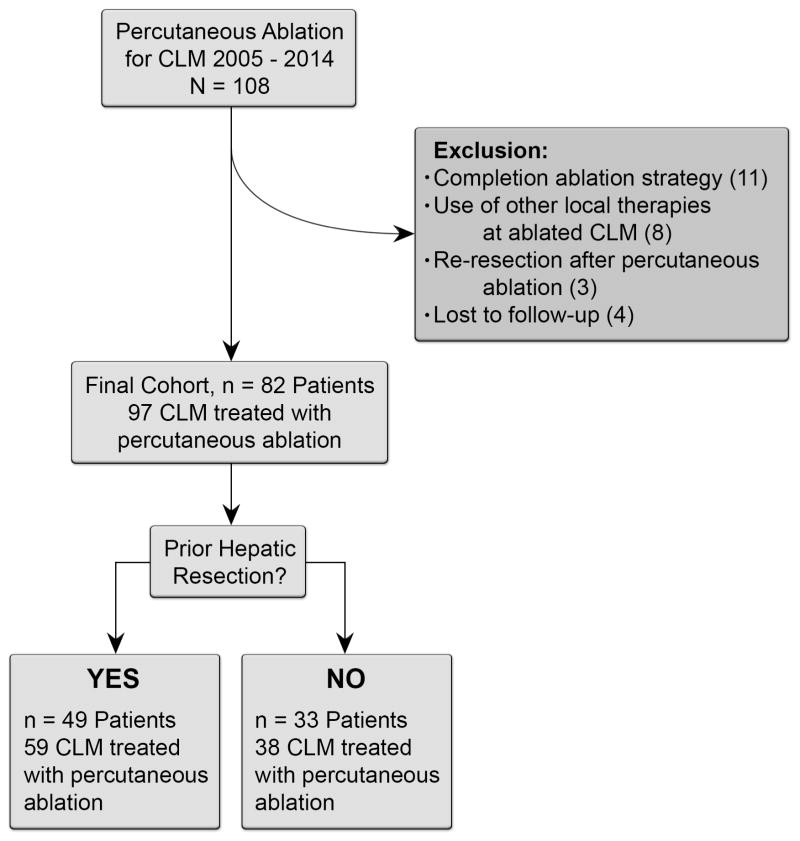
This single-institution retrospective study was compliant with the Health Insurance Portability and Accountability Act and approved by our Institutional Review Board with a waiver of informed consent. Retrospective review of the interventional radiology database identified 108 consecutive patients with CLM who underwent ablation during the January 2005 and December 2014. Of these, 26 patients were excluded from the analysis because ablation was used as a completion treatment strategy to hepatic resection (n = 11), other locoregional therapies were used at the site of the ablated CLM (n = 11), or the patient was lost to follow-up (n = 4), leaving a study population of 82 consecutive patients (54 men and 28 women; median age 59 years [range, 28–92]) with 97 CLM treated with ablation (Figure 1).
Figure 1.
Flowchart diagram of patient selection and exclusion criteria.
Definition of patient cohorts
To permit assessment of the impact of prior hepatic resection on outcomes after ablation of CLM, patients were divided in two cohorts: (i) patients with prior hepatic resection that eradicated all macroscopic CLM that later progressed with new CLM treated with ablation; and (ii) patients without prior hepatic resection who underwent ablation for the treatment of CLMs. Of the 82 patients included in the study, 49 (60%) had prior hepatic resection, and 33 (40%) did not. Patients with prior hepatic resection had a total of 59 CLM ablated, and patients without prior hepatic resection had a total of 38 CLM ablated.
Treatment of CLM strategy
Patients were considered eligible for hepatic resection based on clinical performance status, anatomic, and oncologic criteria. Performance status included Eastern cooperative oncology consortium [ECOG] ≤ 2. Anatomic criteria included being able to perform hepatic resection for eradication of all macroscopic CLM with negative margins and preservation of at least 20% to 30% of the total estimated liver volume, spare two continuous hepatic segments, and maintain vascular inflow and outflow and biliary drainage. Oncologic criteria included absence of clinically significant progression on preoperative systemic therapy. Limited resectable extrahepatic disease was not a contraindication for hepatic resection (15). Adjunctive measures, such as preoperative and adjuvant systemic chemotherapy, portal vein embolization, and two-stage hepatectomy, were performed as per institutional protocol to adequately select patients for hepatic resection. All hepatic resections were performed by one of the five hepato-pancreato biliary surgeons with 7 to 30 years of experience.
Ablation was utilized when patients were ineligible for hepatic resection. Per institutional protocol, patients were eligible for ablation if presenting with fewer than five CLM, measuring ≤ 5 cm each, and if graded as ECOG ≤ 2. No oncologic inclusion criteria was utilized for ablation eligibility. Limited resectable extrahepatic disease was not a contraindication for ablation. All ablations were performed with the intent to completely cover the CLM, but no minimal ablation margin width was established at the time of the present study. All procedures were performed under general anesthesia and computed tomography guidance by one of four interventional radiologists with 7 to 18 years of experience. Ablations were performed with radiofrequency (n = 45 procedures; [Cool-tip ablation system, Covidien, Boulder, CO, USA]), microwave (n = 30 procedures [Certus probe, Certus 140 2·4-GHz ablation system, Neuwave, Madison, WI, USA]), or cryoablation (n = 7 procedures; [Galil Medical Inc., SeedNet MRI cryoablation system, Arden Hills, MN, USA]) systems according to the operator’s choice.
Assessment of response to ablation and clinical outcomes
Post-ablation cross-sectional images (CSI) were consensually assessed by two readers, both with 8 years of experience. All pre-ablation CSI were reviewed to identify the date of diagnosis of each CLM. If a CLM was present on the first CSI study available, the date of this study was considered the date of diagnosis of that particular metastasis. The initial post-ablation CSI study to assess the efficacy of ablation was performed within 4 to 8 weeks.
The minimal radiographic ablation margin was assessed as described previously (16). In short, anatomic liver landmarks adjacent to the pre- and post-ablated CLM were measured and subtracted from each other on the three-dimensional axis. The smallest value was considered to be the minimal margin. Subsequent imaging assessments were performed at 2- to 6-month intervals until patient death or loss to follow-up. Primary and secondary efficacy rates were defined as the number of CLM eradicated after the initial course of ablation and by repeat ablation after documentation of LTP, respectively (17). Residual unablated tumor was defined as irregular peripheral or nodular enhancement within 1 cm of the ablated area on the initial post-ablation CSI study (17). LTP was defined as the appearance of tumor foci within 1 cm of the edge of the ablation zone on CSI after at least one contrast-enhanced post-ablation follow-up study had documented eradication of CLM and absence of viable tissue in the target tumor and surrounding ablation margin (17).
LTP rates were evaluated per patient and per ablated CLM. LTP-free survival (LTPFS) was measured in months from the date of the last ablation session to the date when LTP was detected on CSI. If a patient had two or more CLM treated with ablation, LTP at the site of any ablated CLM was considered to represent LTP in that patient. Finally, recurrence-free survival (RFS) at any site and OS were also measured.
Statistical analysis
Variables extracted from the database or updated by review of electronic medical records for each patient are depicted in detail in Table 1. Continuous data were expressed as median (range). Continuous variables were compared using the Wilcoxon rank-sum test, and categorical variables were compared using the χ2 test. LTPFS and RFS were measured in months from the date of the last image-guided ablation session to the date of detection of recurrence at the ablated CLM or at any organ on CSI or last follow-up, respectively. Univariate and multivariate analyses were performed to identify predictors of LTPFS at 1 and 3 years (primary endpoint) using Cox proportional hazards regression models. OS was measured in months from the date of the last ablation session to the date of death or last follow-up. Survival curves were created by using the Kaplan-Meier method, and differences between the curves of the two patient cohorts were evaluated with the log-rank test. Variables with P < 0.1 in univariate analysis were entered into each multivariate analysis and a P < 0.05 was considered statistically significant in all analyses. All statistical analysis were carried with JMP software (version 12.1.0; SAS Institute Inc, Cary, NC).
Table 1.
Patient, tumor, and treatment characteristics overall and in patients with and without prior hepatic resection*
| Characteristic | Total (n=82) | Prior hepatic resection (n=49) | No prior hepatic resection (n=33) | P value† |
|---|---|---|---|---|
| Sex, M: F | 54: 28 | 32: 17 | 22: 11 | 0.899 |
| Age at CLM ablation, median (range), y | 59 (28–92) | 56 (28–80) | 62 (40–92) | 0.017‡ |
| Primary tumor | ||||
| Location, colon: rectum | 68: 14 | 43: 6 | 25: 8 | 0.157 |
| Midgut origin: hindgut origin | 16: 66 | 7: 42 | 9: 24 | 0.146 |
| Lymph node metastases | 58 (71) | 36 (73) | 22 (67) | 0.507 |
| Time between primary cancer diagnosis and CLM discovery, median (range), months | 18 (0–295) | 19 (0–199) | 15 (0–295) | 0.166‡ |
| Time between last hepatic resection and ablation, median (range), months | - | 13 (0.9–125) | - | - |
| Pre-ablation chemotherapy | 54 (66) | 28 (57) | 26 (79) | 0.043 |
| ≤6 cycles | 24 (44) | 16 (57) | 8 (31) | 0.051 |
| ≥2 regimens | 13 (24) | 3 (11) | 10 (38) | 0.017 |
| Fluorouracil-based chemotherapy regimen | ||||
| Oxaliplatin | 30 (56) | 14 (50) | 16 (62) | 0.394 |
| Irinotecan | 25 (46) | 14 (50) | 11 (42) | 0.571 |
| Use of bevacizumab | 33 (61) | 15 (54) | 18 (69) | 0.238 |
| Use of anti-EGFR agent | 8 (15) | 5 (18) | 3 (12) | 0.514 |
| Time between last chemotherapy and ablation, median (range), days | 34 (6–3674) | 32 (6–875) | 38 (6–3674) | 0.603‡ |
| Time between CLM discovery and ablation, median (range), days | 139 (4–1397) | 102 (4–828) | 266 (29–1397) | <0.001‡ |
| CEA level at ablation, median (range), ng/mL | 4.3 (0.6–328) | 3.3 (0.6–186) | 5.7 (1.2–328) | 0.085‡ |
| RAS status | ||||
| Wild-type: mutant | 53: 29 | 35: 14 | 18: 15 | 0.117 |
| Clinical risk score¶ | ||||
| 0/1: ≥2 | 53: 29 | 33: 16 | 20: 13 | 0.531 |
| Ablation modality | ||||
| RFA: microwave: cryoablation | 45: 30: 7 | 30: 15: 4 | 15: 15: 3 | 0.350 |
| No. of ablation sessions | ||||
| 1: ≥2 | 63: 19 | 36: 13 | 27: 6 | 0.380 |
| Minimal radiographic ablation margin | ||||
| <5 mm: 5–10 mm: >10 mm | 30: 26: 26 | 16: 15: 18 | 14: 11: 8 | 0.465 |
| Ablated lesion adjacent to major vessel(s)© | 20 (24) | 11 (22) | 9 (27) | 0.618 |
| Liver metastases | ||||
| Synchronous: metachronous | 30: 52 | 14: 35 | 16: 17 | 0.066 |
| Maximum CLM diameter at ablation, median (range), cm | 1.7 (0.6–5.0) | 1.4 (0.6–4.5) | 2.1 (1.0–5.0) | 0.001‡ |
| Tumor number, solitary: multiple | 70: 12 | 40: 9 | 30: 3 | 0.232 |
| Subcapsular lesion | 43 (52) | 24 (49) | 19 (58) | 0.445 |
| Concomitant extrahepatic disease | 19 (23) | 10 (20) | 9 (27) | 0.470 |
| Post-ablation chemotherapy | 43 (52) | 23 (47) | 20 (61) | 0.224 |
| Local tumor progression | 15 (18) | 3 (6.1) | 12 (36) | <0.001 |
Values in table are number of patients (percentage) unless indicated otherwise.
χ2 test unless indicated otherwise.
Wilcoxon rank-sum test.
Clinical risk score was determined by assigning 1 point for each of the following: disease-free interval from detection of primary tumor to detection of liver metastasis <12 months, >1 liver tumor, largest hepatic metastasis > cm, carcinoembryonic antigen level >200 ng/mL, and the presence of extrahepatic disease [Fong et al, Ann Surg, 1999].
A major vessel meant a vessel >3 mm in diameter.
CLM, colorectal liver metastases; EGFR, epidermal growth factor receptor; CEA, carcinoembryonic antigen; RFA, radiofrequency ablation.
RESULTS
Overall primary and secondary ablation efficacy rates were 95% (92 of 97 CLM) and 100%, respectively. Fifteen (18%) of the 82 patients presented with LTP by the end of the analysis period. Ten of these patients had other sites of intrahepatic or extrahepatic metastases in addition to LTP and were treated with systemic therapy. The remaining five patients who had LTP were deemed unsafe for repeat ablation because of tumor size and/or location.
LTP rates in patients with and without prior hepatic resection
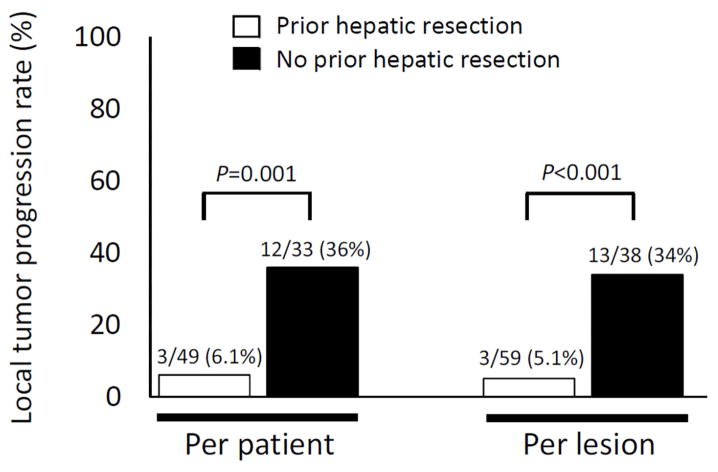
Patients with prior hepatic resection had a significantly lower rate of LTP than patients without prior hepatic resection (6.1% vs 36%, P < 0.001) (Figure 2). Also, when LTP was analyzed per each ablated CLM, LTP rates were significantly lower among CLM in patients with prior hepatic resection than among CLM in patients without prior hepatic resection (5.1% vs 34%, P < 0.001) (Figure 2).
Figure 2.
Local tumor progression rates per patient and per lesion in patients with and without prior hepatic resection.
Survival outcomes in patients with and without prior hepatic resection
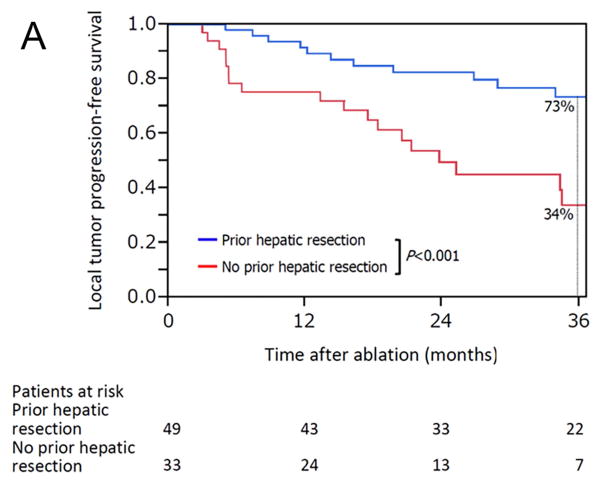
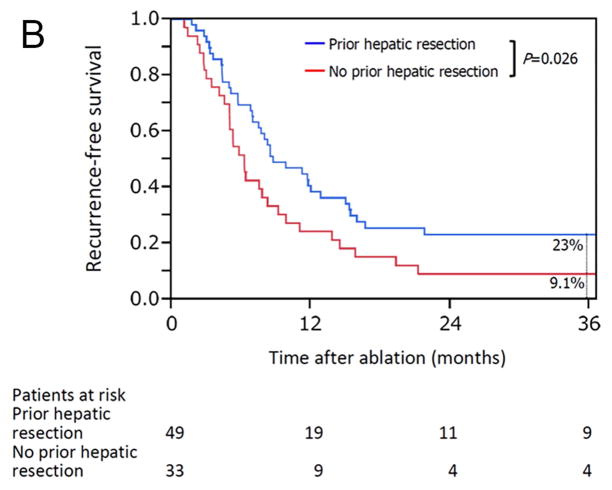
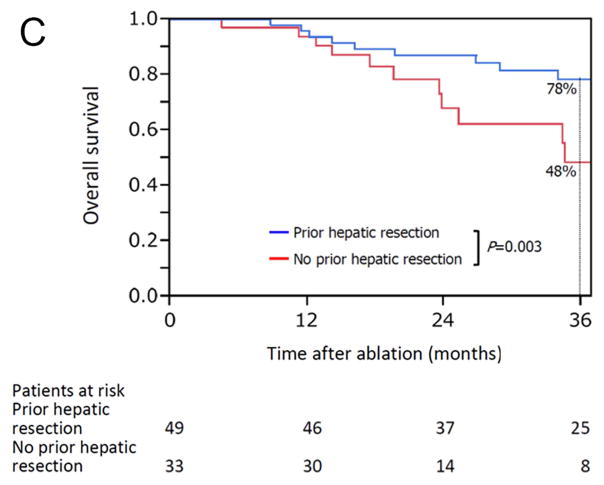
The median overall follow-up period was 28 months (range, 4.5–132), and there was no significant difference in median follow-up period between patients with prior hepatic resection (31 months [range, 4.7–132]) and without prior hepatic resection (24 months [range, 4.5–95]) (P = 0.455). Kaplan-Meier plots for actuarial LTPFS, RFS, and OS in patients with and without prior hepatic resection are presented in Figure 3. The 3-year LTPFS rate was significantly better in patients with than without prior hepatic resection (73% vs 34%; P < 0.001) (Figure 3A). Based on the analysis per each ablated CLM, the 3-year LTPFS rate was also significantly better in CLM with than without prior hepatic resection (73% vs 31%; P < 0.001) (Supplementary Figure 1). Patients with prior hepatic resection also had better outcomes with respect to the 3-year RFS rate at any organ (23% vs 9.1%; P = 0.026) (Figure 3B) and the 3-year OS rate (78% vs 48%; P = 0.003) (Figure 3C).
Figure 3.
Kaplan-Meier curves for (A) local tumor progression-free survival (P < 0.001), (B) recurrence-free survival (freedom from recurrence at any site) (P = 0.026), and (C) overall survival (P = 0.003) in patients with (blue line) and without (red line) prior hepatic resection.
Predictors of LTPFS at 1 year and 3 years
The Cox proportional hazards regression models showed that the independent predictors of a higher risk of LTP were absence of prior hepatic resection (hazard ratio [HR] 2·35, 95% confidence interval [CI] 1·02–5·45; P = 0·045), minimal radiographic ablation margin < 5 mm (HR 2·40, 95% CI 1·18–4.87; P = 0·016), and RAS mutation (HR 2·65, 95% CI 1·18–5·94; P = 0·019) (Table 2).
Table 2.
Univariate and multivariate analyses for local tumor progression-free survival
| N | Local tumor progression-free survival rate, %* | Univariate P value† | Hazard ratio (95% CI) | Multivariate P value‡ | ||
|---|---|---|---|---|---|---|
| 1 year | 3 years | |||||
| All patients | 82 | 85 | 58 | - | - | - |
| Sex§ | ||||||
| F | 28 | 75 | 42 | 0.053 | 1.18 (0.52–2.60) | 0.693 |
| M | 54 | 90 | 67 | |||
| Age, years | ||||||
| ≥60 | 39 | 85 | 58 | 0.851 | - | - |
| <60 | 43 | 85 | 57 | |||
| Primary tumor location | ||||||
| Colon | 68 | 82 | 60 | 0.550 | - | - |
| Rectum | 14 | 100 | 51 | |||
| Primary tumor origin | ||||||
| Midgut | 16 | 59 | 37 | 0.249 | - | - |
| Hindgut | 66 | 91 | 63 | |||
| Primary tumor lymph node metastasis | ||||||
| Yes | 58 | 88 | 64 | 0.320 | - | - |
| No | 24 | 79 | 47 | |||
| History of hepatic resection§ | ||||||
| No | 33 | 75 | 34 | <0.001 | 2.35 (1.02–5.45) | 0.045 |
| Yes | 49 | 92 | 73 | |||
| Pre-ablation chemotherapy | ||||||
| Yes | 54 | 85 | 49 | 0.157 | - | - |
| No | 28 | 86 | 78 | |||
| Pre-ablation chemotherapy cycles§ | ||||||
| >6 | 30 | 79 | 49 | 0.073 | 1.78 (0.81–3.89) | 0.150 |
| ≤6 or no chemotherapy | 52 | 88 | 63 | |||
| Fluorouracil-based chemotherapy regimen | ||||||
| Oxaliplatin | ||||||
| Yes | 30 | 86 | 46 | 0.250 | - | - |
| No | 52 | 84 | 68 | |||
| Irinotecan | ||||||
| Yes | 25 | 83 | 46 | 0.237 | - | - |
| No | 57 | 86 | 63 | |||
| Use of bevacizumab | ||||||
| Yes | 33 | 81 | 43 | 0.159 | - | - |
| No | 49 | 87 | 70 | |||
| Use of anti-EGFR agent | ||||||
| Yes | 8 | 75 | 75 | 0.229 | - | - |
| No | 74 | 86 | 57 | |||
| Time between CLM discovery and ablation, days§ | ||||||
| >120 | 46 | 85 | 51 | 0.074 | 1.35 (0.57–3.05) | 0.490 |
| ≤120 | 36 | 85 | 66 | |||
| Ablation modality | ||||||
| RFA | 45 | 89 | 61 | 0.320 | - | - |
| Microwave or cryoablation | 37 | 80 | 54 | |||
| Minimal radiographic ablation margin, mm§ | ||||||
| <5 | 30 | 80 | 38 | 0.028 | 2.40 (1.18–4.87) | 0.016 |
| ≥5 | 52 | 88 | 69 | |||
| CLM location | ||||||
| Subcapsular | 43 | 79 | 53 | 0.240 | - | - |
| Non-subcapsular | 39 | 92 | 64 | |||
| CEA level at ablation, ng/mL | ||||||
| ≥5 | 36 | 78 | 55 | 0.472 | - | - |
| <5 | 46 | 93 | 60 | |||
| Maximum CLM diameter at ablation, cm§ | ||||||
| ≥2 | 32 | 81 | 47 | 0.009 | 1.90 (0.94–3.91) | 0.072 |
| <2 | 50 | 88 | 66 | |||
| Number of CLM | ||||||
| Solitary | 70 | 88 | 60 | 0.376 | - | - |
| Multiple | 12 | 67 | 46 | |||
| Concomitant extrahepatic metastases | ||||||
| Presence | 19 | 95 | 42 | 0.119 | - | - |
| Absence | 63 | 82 | 65 | |||
| RAS status § | ||||||
| Mutant | 29 | 67 | 26 | <0.001 | 2.65 (1.18–5.94) | 0.019 |
| Wild-type | 53 | 94 | 74 | |||
| Post-ablation chemotherapy | ||||||
| Yes | 43 | 83 | 57 | 0.701 | - | - |
| No | 39 | 87 | 60 | |||
Kaplan-Meier analysis.
Log-rank test.
Cox regression model.
Variables entered into the Cox regression model.
EGFR, epidermal growth factor receptor; CLM, colorectal liver metastases; RFA, radiofrequency ablation; CEA, carcinoembryonic antigen.
DISCUSSION
The present study demonstrates that rates of LTP in patients undergoing ablation of CLM was significantly lower in patients with prior hepatic resection than in patients without prior hepatic resection. Such lower rates of LTP were translated on significant improved 3-year LTPFS rates of patients with prior history of hepatic resection when compared to patients without prior hepatic resection at a patient level (73% vs 34%, respectively, P < 0.001), as well on per each ablated CLM (73% vs 31%; P < 0.001). The multivariate analysis of predictors of LTPFS confirmed that, in addition to minimal radiographic ablation margins < 5 mm and mutant RAS, absence of prior hepatic resection was an independent predictor of worse LTPFS. As expected, patients with prior hepatic resection also had significantly better 3-year RFS and OS rates than patients without prior hepatic resection.
Several adjunctive strategies are routinely utilized in the current clinical practice to facilitate resection and reduce perioperative morbidity and mortality. Surgery is typically reserved for patients with CLM who are judged to have a higher chance of sustained benefit as demonstrated by optimal morphological response to systemic chemotherapy, lack of oncological progression following preoperative systemic chemotherapy and first-stage hepatectomy, and adequate growth of future liver-remnant after portal vein embolization (15, 18, 19). The present study findings suggest that the process of selecting patients for hepatic resection might have also translated into a significantly lower rate of LTP following CLM ablation that developed after hepatic resection.
Recently, biological factors such as mutant RAS status and midgut origin of the colorectal cancer have been linked to worse outcomes after ablation (7, 8). This present study did not show any significant differences between patients with and without prior hepatic resection with respect to those biological factors. Nevertheless, other still undiscovered biological factors and comutations might be associated with a less aggressive tumor behavior among surgical patients. For instance, BRAF mutation, which is associated with worse prognosis and occurs in approximately 5% to 10% of patients with colorectal cancer, is only seen in less than 2% of patients with CLM undergoing hepatic resection. This finding highlights the importance of better understanding the impact of tumor biology on outcomes of patients with CLM treated with local therapies.
The comparison of patients with and without prior hepatic resection did demonstrate some differences in patient characteristics between those two cohorts. As anticipated, patients with prior hepatic resection were younger, less likely to receive pre-ablation chemotherapy, received fewer chemotherapy regimens, had their CLM ablated sooner after discovery, and had smaller CLM treated with ablation. Such inherent differences are a consequence of the currently employed surgical inclusion criteria and suggest that patients with prior hepatic resection were healthier and had less aggressive tumors or tumors that presented earlier. Despite of that, multivariate analysis did not show that any of those variables were associated with improved LTPFS. Such findings emphasize that comparison of local and overall outcomes of patients undergoing hepatic resection and patients undergoing ablation are problematic given the fundamental differences between those two patient populations. Moreover, as demonstrated by this current analysis, patients with prior hepatic resection who underwent ablation of CLM that developed after hepatic resection have LTP rates similar to the recurrence rates after surgical resection of CLM in the most recent series (20).
At present, management of CLM that develop after hepatic resection is open to debate; questions regarding the use of ablation, repeat resection, and preoperative chemotherapy remain unanswered. Patients undergoing ablation of CLM that developed after hepatic resection had a 3-year OS rate of 78% with OS measured from the time of CLM ablation, a rate similar to the 3-year OS rate after first and second hepatectomy for CLM in current series (3, 9). Such similar OS rates can be regarded as an argument for the use of percutaneous ablation as an effective therapy for patients who present with newly developed CLM after hepatic resection.
This study has some limitations. First, the retrospective nature of the current analysis based on relatively small number of patients might have created a selection bias. Further validation of this study including a larger number of patients is needed; Second, the study covers a 10-year period where several different systemic therapies were utilized and no information in respect to the impact of each individual systemic therapies can be established. Finally, the minimal follow-up period after ablation of CLM was short, although in line with follow-up periods in other studies of LTP after ablation of CLM (6).
In conclusion, patients who undergo ablation of CLM that develop after hepatic resection have significantly lower rates of LTP and consequent improved 3-years LTPFS than patients who undergo ablation of CLM without prior hepatic resection. These findings suggest that patients undergoing percutaneous ablation for CLM that develop after hepatic resection experience sustained benefit from both hepatic resection and post-resection ablation therapies, supporting both therapeutic approaches. Finally, care should be taken in comparing local tumor control outcomes after ablation in patients with CLM with and without prior hepatectomy since such patients might harbor fundamentally different CLM.
Supplementary Material
Acknowledgments
Financial support: Supported in part by the NIH/NCI under award number P30CA016672.
Footnotes
Conflicts of interested: none
Not presented at SIR meeting
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–83. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wieser M, Sauerland S, Arnold D, Schmiegel W, Reinacher-Schick A. Peri-operative chemotherapy for the treatment of resectable liver metastases from colorectal cancer: A systematic review and meta-analysis of randomized trials. BMC Cancer. 2010;10:309. doi: 10.1186/1471-2407-10-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239(6):818–25. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson AB, 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, et al. Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11(2):141–52. doi: 10.6004/jnccn.2013.0022. [DOI] [PubMed] [Google Scholar]
- 5.Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes--A 10-year experience at a single center. Radiology. 2016;278(2):601–11. doi: 10.1148/radiol.2015142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–68. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 7.Odisio BC, Yamashita S, Huang SY, Harmoush S, Kopetz SE, Ahrar K, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg. 2017 doi: 10.1002/bjs.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamashita S, Odisio BC, Huang SY, Kopetz SE, Ahrar K, Chun YS, et al. Embryonic origin of primary colon cancer predicts survival in patients undergoing ablation for colorectal liver metastases. Eur J Surg Oncol. 2017 doi: 10.1016/j.ejso.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Denbo JW, Yamashita S, Passot G, Egger M, Chun YS, Kopetz SE, et al. RAS Mutation Is Associated with Decreased Survival in Patients Undergoing Repeat Hepatectomy for Colorectal Liver Metastases. J Gastrointest Surg. 2017;21(1):68–77. doi: 10.1007/s11605-016-3189-9. [DOI] [PubMed] [Google Scholar]
- 10.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–15. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 11.Mitry E, Fields AL, Bleiberg H, Labianca R, Portier G, Tu D, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26(30):4906–11. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 12.Khatri VP, Chee KG, Petrelli NJ. Modern multimodality approach to hepatic colorectal metastases: solutions and controversies. Surg Oncol. 2007;16(1):71–83. doi: 10.1016/j.suronc.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Karanjia ND, Lordan JT, Fawcett WJ, Quiney N, Worthington TR. Survival and recurrence after neo-adjuvant chemotherapy and liver resection for colorectal metastases: a ten year study. Eur J Surg Oncol. 2009;35(8):838–43. doi: 10.1016/j.ejso.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst. 2017;109(9) doi: 10.1093/jnci/djx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun YS, Vauthey J-N. Local Therapy for Colorectal Liver Metastases: Establishing Today’s Level of Evidence and Defining Tomorrow’s Roadmap. JNCI: Journal of the National Cancer Institute. 2017;109(9) doi: 10.1093/jnci/djx018. djx018-djx. [DOI] [PubMed] [Google Scholar]
- 16.Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes-A 10-year Experience at a Single Center. Radiology. 2016;278(2):601–11. doi: 10.1148/radiol.2015142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol. 2014;25(11):1691–705. e4. doi: 10.1016/j.jvir.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blazer DG, 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26(33):5344–51. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 19.Shindoh J, Vauthey JN, Zimmitti G, Curley SA, Huang SY, Mahvash A, et al. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg. 2013;217(1):126–33. doi: 10.1016/j.jamcollsurg.2013.03.004. discussion 33–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vigano L, Procopio F, Cimino MM, Donadon M, Gatti A, Costa G, et al. Is Tumor Detachment from Vascular Structures Equivalent to R0 Resection in Surgery for Colorectal Liver Metastases? An Observational Cohort. Ann Surg Oncol. 2016;23(4):1352–60. doi: 10.1245/s10434-015-5009-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.