Abstract
We hypothesize that aromatase, an enzyme that regulates estrogen production, plays a significant role in the control of intraocular pressure (IOP) and retinal ganglion cells (RGCs). To begin to test our hypothesis, we examined the impact of aromatase absence, which completely eliminates estrogen synthesis, in male and female mice. Studies were performed with adult, age-matched wild type (WT) and aromatase knockout (ArKO) mice. IOP was measured in a masked fashion in both eyes of conscious mice at 12 and 24 weeks of age. Retinas were obtained and processed for RGC counting with a confocal microscope. IOP levels in both 12- and 24-week old female ArKO mice were significantly higher than those of age- and sex-matched WT controls. The mean increase in IOP was 7.9% in the 12-week-, and 19.7% in the 24-week-old mice, respectively. These changes were accompanied by significant 9% and 7% decreases in RGC numbers in the ArKO female mice, relative to controls, at 12- and 24-weeks, respectively. In contrast, aromatase deficiency did not lead to an increased IOP in male mice. There was a significant reduction in RGC counts in the 12-, but not 24-, week-old male ArKO mice, as compared to their age- and sex-matched WT controls. Overall, our findings show that aromatase inhibition in females is associated with elevated IOP and reduced RGC counts.
Introduction
Several researchers have hypothesized that estrogen deficiency predisposes to optic nerve degeneration1–3. This hypothesis is supported by a myriad of observational studies linking estrogen deprivation to an increased risk of open-angle glaucoma4–7. Furthermore, variations in several genes involved in estrogen processing are related to open-angle glaucoma8–10. Conversely, investigators have also reported that estrogen use may decrease the risk of developing glaucoma11, prevent retinal ganglion cell (RGC) death12,13, reduce intraocular pressure (IOP14–17); preserve visual acuity12 and serve as a viable option for treating glaucoma14,18.
However, despite these impressive results, there is no global consensus on the role of estrogens in glaucoma. Indeed, there is controversy. There is no robust evidence indicating an overall sexual predilection for OAG18,19. Tamoxifen, an estrogen receptor blocker that is widely used to treat breast cancer, does not appear to be associated with increased risk of glaucoma20. Abramov and colleagues found no difference in IOP among postmenopausal women with a history of hormone replacement therapy versus women who did not use postmenopausal hormones21. No significant correlation between IOP level and serum estradiol was found among 62 postmenopausal women, 30 of which were using hormone replacement therapy22. Furthermore another study from India did not find relations between female reproductive factors and open-angle glaucoma23.
These discrepant findings may be attributed, at least in part, to variations in experimental design, including differences in the age, sex, and endocrine status of subjects, as well as in the dosage and time course of estrogen therapy, and even in the methods of analysis. Nevertheless, it is extremely important to determine whether estrogen deficiency promotes, and estrogen treatment suppresses, IOP elevation, RGC death and optic neuropathy. The reason is that estrogen dynamics may contribute significantly to the development of primary open-angle glaucoma in women2 and possibly men9. Furthermore, there is an ever-increasing use of aromatase inhibitors for the treatment of breast and ovarian cancer in postmenopausal women. These inhibitors prevent the biosynthesis of estrogens and could possibly enhance the risk and/or severity of glaucoma24.
We used C57BL/6 J - aromatase knockout (ArKO) mice to begin to clarify whether estrogen deprivation is associated with glaucomatous features. The ArKO mice were generated by the targeted disruption of exon IX in the cyp19 gene and lack aromatase activity25. Aromatase is a cytochrome P450 enzyme that catalyzes the formation of estrogens from androgens26–28, and contributes to a number of sex-specific differences throughout the body29–33. In the absence of aromatase, the synthesis of estrogens is completely eliminated34–36. Accordingly, we examined whether female ArKO mice exhibit heightened IOP and RGC loss, as compared to their wildtype (WT) controls. For comparison, we also evaluated male ArKO mice. (Figure 1).
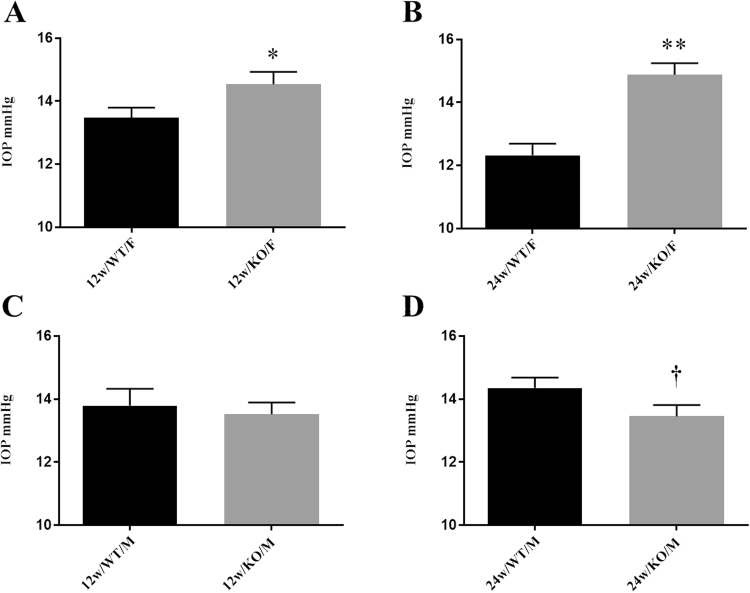
Figure 1.
Influence of complete aromatase absence on the IOP in 12- and 24-week old mice. Columns represent the mean ± SE. Abbreviations: 12w-12 week; 24w-24 week; WT-wild type; KO-knockout; F-female; M-male. Significantly less (p < 0.05; *p < 0.001**) or greater (<0.05)† than WT control.
Results
IOP levels in female and male ArKO mice
To determine whether aromatase absence influences IOP, we measured IOP levels in both eyes of 12- and 24-week old female and male WT and ArKO mice (n = 8/group). Our results demonstrate that IOP levels in both 12- (WT = 13.5 ± 0.3; ArKO = 14.5 ± 0.4, p < 0.05) and 24- (WT = 12.3 ± 0.4; ArKO = 14.9 ± 0.4, p < 0.001) week old female ArKO mice were significantly higher than those of age- and sex-matched WT controls (Fig. 1). The mean increases in IOP ranged from 7.9% (1.1 mmHg) in the 12-week-, to 19.7% (2.6 mmHg) in the 24-week-old mice, respectively.
In contrast, aromatase deficiency did not lead to increases in the IOP of 12- (WT = 13.8 ± 0.5; ArKO = 13.5 ± 0.4) or 24- (WT = 14.4 ± 0.3; ArKO = 13.5 ± 0.4) week old male ArKO mice, as compared to WT controls. Rather, the lack of aromatase was associated with a significant decrease in the IOP of 24-week old male mice (p < 0.05; Fig. 1).
Our findings related to the influence of aromatase were the same if we limited IOP comparisons to the same (e.g. right vs. right) or to the opposite (e.g. right vs. left) eyes of sex- and age-matched WT vs ArKO mice (data not shown). There were no significant differences in IOP levels between the left and right eyes of WT mice, or between left and right eyes of ArKO mice. In addition, there was no significant difference between the IOP of 12- vs. 24-week old female or male ArKO mice.
Our IOP data are based upon the analyses of one mean IOP per mouse (i.e. average of 12 IOP values/mouse, originating from 3 values/eye/day, two eyes/mouse, on 2 consecutive days).
RGC counts in female and male ArKO mice
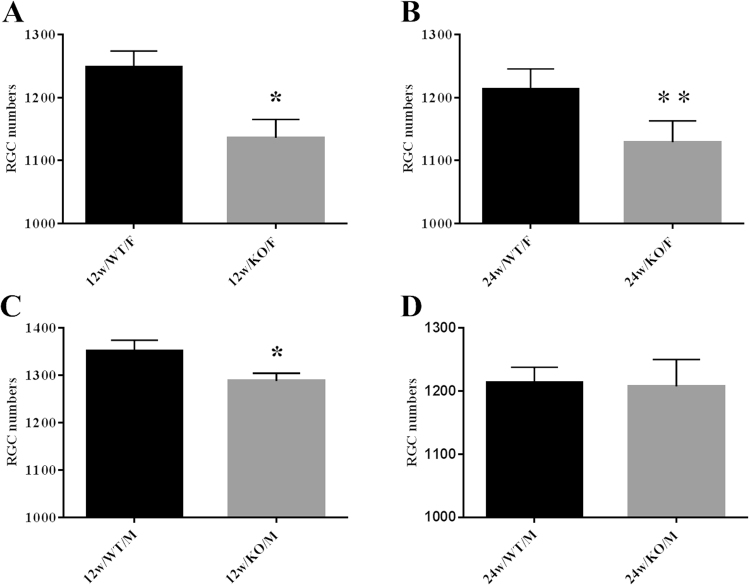
To examine the effect of complete aromatase absence on RGC counts, we analyzed RGC numbers in retinas of 12- and 24-week old female and male WT and ArKO mice (n = 8/group). As shown in Fig. 2, RGC numbers in the ArKO female mice were significantly reduced, as compared to age- and sex-matched WT controls (12 week old, WT = 1,249 ± 25; ArKO = 1,136 ± 29, p = 0.012; 24 week old, WT = 1,213 ± 33; ArKO = 1,129 ± 34, p = 0.049, one tail). Similarly, there was a significant decrease in RGC counts in the 12-week old male ArKO mice, relative to their age- and sex-matched WT controls (WT = 1,351 ± 24; ArKO = 1,288 ± 16, p = 0.047). No significant difference existed between the RGC numbers in 24-week-old male WT and male ArKO mice (WT = 1,214 ± 24; ArKO = 1,208 ± 43). Furthermore, there was no significant difference between the RGC counts of 12- vs. 24-week old female ArKO mice or 12- vs. 24-week old male ArKO mice (Fig. 2).
Figure 2.
Impact of complete aromatase absence on the RGC count in 12- and 24-week old mice. Columns represent the mean ± SE. Abbreviations: 12w-12 week; 24w-24 week; WT-wild type; KO-knockout; F-female; M-male. Significantly less (p < 0.05)* or (p < 0.05, one-tail)** than WT control.
Discussion
Glaucoma is the second leading cause of blindness in the world and is a slowly progressing neurodegenerative disease37,38. It is characterized by a gradual loss of RGCs, which leads to a loss of vision37,39,40. The most common form of glaucoma, occurring in 70 to 90% of patients, is primary open angle glaucoma (POAG)41–43.
In one epidemiological study, women had a significantly lower incidence of POAG, as compared to men, until the age of 80 years44. This sex-related difference has been linked to the extent of lifetime estrogen exposure. Indeed, there is an association between increased estrogen exposure and a reduced POAG risk37. Conversely, studies have shown that a decreased exposure (i.e. early loss of estrogens), due to late menarche, oral contraceptive use, early menopause, and a shorter duration between menarche to menopause, is associated with an increased risk of POAG5–7,37,45. Given this background, we assessed glaucoma features in female ArKO mice.
Our present study demonstrates that aromatase absence is associated with a significant increase in the IOP levels of 12- and 24-week old female ArKO mice. The extent of this IOP difference (i.e. 1.1 to 2.6 mmHg) is comparable to that (i.e. 0.5 to 0.6 mmHg) found between postmenopausal women taking, versus not taking, estrogen replacement therapy as part of a post-hoc analysis of a randomized clinical trial17. In addition, the aromatase deficiency in ArKO mice is associated with a significant decrease in RGC counts. Our findings support the myriad of human data linking estrogen deprivation to the development of glaucoma among women.
In contrast to the responses of ArKO females, aromatase deficiency did not lead to an increased IOP in male mice. There was, though, a significant decrease in RGC counts in the 12-, but not 24-, week-old male ArKO mice, as compared to their age- and sex-matched WT controls. This sexually dimorphic response to complete aromatase absence is not surprising. We have previously found that almost all genes regulated by aromatase in non-retinal ocular tissues (i.e. meibomian and lacrimal glands) are sex-specific, and thus different, in male and female mice46,47. Similar sex-related differences have also been found in the liver, bone, hematopoietic microenvironment, adipose tissue and brain30,48–55. These variations between male and female responses may reflect the loss of estrogens, reduced serum adiponectin56, alterations in neural activity57,58, as well as the heightened serum levels of testosterone, androstenedione, prolactin, follicle-stimulating hormone, luteinizing hormone (LH), insulin-like growth factor 1 (IGF-1) and leptin in ArKO mice25,50,55,56,59–61, some hormone-independent processes62,63, and the influence of Y-linked genes or X inactivation in the ArKO strain64–67. The fact that aromatase deficiency does impact the male eye is consistent with studies demonstrating that aromatase and estrogen actions are very important in other male tissues52–55,68–70.
There are several specific ways in which estrogen may directly affect vulnerability to glaucoma. One mechanism may involve estrogen binding to saturable, high affinity, and steroid-specific estrogen receptors (ERs) in retinal tissues, especially in RGCs. ERs, which are members of the nuclear receptor superfamily of ligand-inducible transcription factors, mediate most of the actions of estrogens throughout the body71. Estrogens have two nuclear ERs, termed α and β, which are highly homologous in their DNA and ligand binding domains, are often differentially distributed, and which direct different, and sometimes opposite, functions of estrogens71–80. After estrogen binds to the ER, the activated hormone-receptor complex typically associates with estrogen responsive elements in the regulatory region of specific target genes and, in combination with appropriate co-activators and enhancers, modulates gene transcription, protein synthesis and tissue function73. Consistent with this mechanism, we and others have identified ER mRNA and protein in the retina, as well as ERα and ERβ in RGCs81–83. By acting through these nuclear receptors, estrogens might then influence, for example, vascular resistance and the perfusion of the optic nerve, RGCs, and their supporting structures1,84,85.
A second mechanism to explain estrogen effects on the eye may also involve plasma membrane ERs. These pathways, which typically signal within seconds to minutes, involve estrogen interaction with stereospecific membrane ERs and lead to rapid changes in membrane fluidity, the activity of neurotransmitter receptors and/or the control of transcription factors86–92. We have identified membrane ERs in other ocular tissues93. It is also possible that estrogen influence could be modified by the activity of retinal steroidogenic enzymes.
A third mechanism by which estrogens impact glaucoma may be operative in humans, but not mice, and involve the intracrine synthesis of estrogens. Unlike lower mammals (e.g., mice), in which the ovaries and testes are the primary origin of active sex steroids94,95, most biologically active sex steroids in women do not originate from the ovary and do not circulate in the blood. The vast majority of estrogens (i.e. 75% before, and 100% after, menopause), and almost all androgens, are synthesized in peripheral tissues from dehydroepiandrosterone (DHEA)3,94–99. Indeed, humans and primates are unique in possessing adrenal glands that secrete large amounts of DHEA, which is then converted into androgens and estrogens by steroidogenic enzymes in peripheral sites. This hormonal process, termed intracrinology, allows target tissues to adjust the formation and metabolism of sex steroids to local requirements95,97. After intracrine synthesis and local action, sex steroids are metabolized and released into the circulation. The critical steroidogenic enzymes involved in estrogen synthesis and metabolism all exist and are active in the retina3,100–102.
A number of other processes may also be involved in altering the IOP and RGC counts in female ArKO mice. First, the increased serum leptin concentrations in ArKO mice50 may counteract the glaucomatous effect of estrogen deficiency, given that leptin is thought to be a neuroprotective agent103. Such leptin action might contribute to the lack of progressive IOP and RGC changes in female ArKO mice from 12 to 24 weeks of age. Second, changes in adiponectin56, IGF-155 and LH61 levels may affect glaucoma progress104, RGC survival105 and retinal dynamics106, respectively. The obesity107,50 and glucose intolerance108 in these ArKO mice may lead to the development of diabetes109, increased IOP110,111 and glaucoma112. Third, the decreased blood pressure in female ArKO mice113 may promote glaucomatous changes114.
There are limitations in our study. We did not assess the retinal functional consequences in the ArKO mice. We did not extend phenotype observations beyond 24 weeks. Furthermore, we did not explore the mechanism for IOP increase or RGC dropout in female mice, nor the compensatory mechanisms that might have produced minimal or no glaucomatous features in male ArKO mice.
Overall, our results demonstrate that aromatase absence produces a glaucoma phenotype that is more significant in female mice. Our study is a reverse engineering approach to model one aspect of POAG and to support the epidemiological literature suggesting that relative estrogen deficiency increases the risk of POAG, which is a multifactorial disease. Therefore, it is not surprising that the IOP increases and RGC losses were modest and relatively sex-specific. We are not modeling nonspecific end organ optic nerve damage, but rather a complex disease (POAG) by precisely altering one of the many exposures involved in disease pathogenesis. In this regard our findings are very important, because the modest effects on IOP and the RGCs are consistent with epidemiological data showing modest reductions in IOP and in glaucoma risk related to estrogen exposure1,72.
Methods
Mice
We obtained breeding pairs of C57BL/6 J - aromatase knockout (ArKO) heterozygous mice from Dr. Nabil J. Alkayed (Knight Cardiovascular Institute, Oregon Health & Science University, Portland, OR), who, in turn, had procured them from Dr. Orhan Oz (University of Texas Southwestern Medical Center, Dallas, TX). Animals were shipped to Charles River Breeding Laboratories (Wilmington, MA) for initial quarantine, health monitoring and serology, and then forwarded to the Animal Facilities of the Schepens Eye Research Institute (SERI). Mice were housed and bred in constant temperature rooms with fixed light/dark intervals of 12 hours duration. All mice were fed the PicoLab Rodent Diet 20 (#5053; LabDiet, St Louis, MO).
We generated hundreds of WT, ArKO and heterozygous mice for these studies. Twenty one-day-old offspring were genotyped according to modifications of a published protocol25. In brief, genomic DNA was isolated from ear punches by using a GenEluteTM Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich). The DNA was amplified by PCR with a Hybaid OMN-E thermocycler (Thermo Electron Corp) by using exon 9 gene primers (forward: GTGACAGAGACATAAAGATCG; reverse: GTAAATTCATTGGGCTTAGGG) and neo gene primers (forward: ATCAGGATGATCTGGACGAAGA; reverse: CCACAGTCGATGAATCCAGAA). The PCR conditions were 1 cycle (3 minutes at 94 °C), 31 cycles (40 second at 94 °C, 30 second at 55 °C, 45 second at 72 °C) and 1 cycle (5 minutes at 72 °C) and amplicons were evaluated on 2.5% agarose gels. Band sizes were 220 bp from WT mice, 170 bp from ArKO mice, and both fragments from heterozygotes. After mice were sacrificed, we repeated the genotyping to confirm the genetic background.
All experiments with these mice were approved by the SERI Institutional Animal Care and Use Committee (IACUC) and adhere to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
IOP measurements
Measurements of IOP were performed on conscious mice. We developed procedures to measure IOP (n = 6 consecutive measurements per IOP value, 3 values/eye/day, 2 consecutive days) at the central cornea of awake mice with a TonoLab tonometer (Icare, Tampere, Finland). We secured mice in DecapiCone bags, which calms the animals and also prevents head movement. Measurements of the IOP were performed in a masked fashion between 10 and 11 AM. We did not use anesthesia, because such exposure is known to significantly alter IOP115.
We tested our procedures on 5 mice for three consecutive days to determine whether adaptation to the apparatus and examination process was required. Our results showed that an adaptation period is not necessary. IOP measurements in the right and left eyes remained consistent in a given mouse, and typically varied little from day to day. The mean IOP variation in right and left eyes of the WT and ArKO mice from the first to second days of IOP measurements is shown in Table 1.
Table 1.
Variation in mean IOP levels in right and left eyes of mice from days 1 to 2 of IOP measurements.
| Age (weeks) | Genotype | Sex | Right eye (mmHg) | Left eye (mmHg) |
|---|---|---|---|---|
| 12 | WT | F | −0.1 | −0.3 |
| 12 | KO | F | −0.3 | −0.7 |
| 12 | WT | M | +0.8 | +0.6 |
| 12 | KO | M | −0.8 | −1.5 |
| 24 | WT | F | −0.7 | +0.3 |
| 24 | KO | F | +1.0 | +0.5 |
| 24 | WT | M | −1.3 | −1.7 |
| 24 | KO | M | +0.6 | +1.1 |
IOP levels (n = 6 consecutive measurements per IOP value, 3 values/eye/day, 2 consecutive days) were recorded at the central cornea of awake mice. The difference between the mean IOP values on Days 1 and 2 in the right and left eyes of female and male WT and ArKO mice (n = 8/mice group) at 12 and 24 weeks of age are shown. The “−” symbol stands for “negative” (i.e. the group mean IOP value decreased from Day 1 to 2 by that amount). The “+” symbol stands for “positive” (i.e. the group mean IOP value increased from Day 1 to 2 by that amount).
RGC counting
At the termination of experiments, mice were sacrificed by CO2 inhalation, tissue was obtained to confirm genotype, and the vasculature was irrigated with phosphate-buffered saline (PBS). Eyes were removed, fixed in 4% paraformaldehyde for 2 hours, and then processed for the preparation of retinal flat mounts (cut into 4 quadrants116,117). Retinal samples were permeated with 0.5% Triton X-100 for 15 minutes at −80 °C, exposed to mouse anti-mouse Brn3a antibody (Millipore; diluted 1:200 in blocking buffer [2% bovine serum albumin and 2% Triton]) overnight at 4 °C, then incubated with donkey anti-mouse IgG antibody (Millipore; diluted 1:200 in blocking buffer) for 2 hours at room temperature. The use of the mouse anti-mouse Brn3a antibody for the identification of RGCs in mouse retinal sections has previously been described118,119. Samples were then mounted and RGCs were imaged with a confocal microscope (Leica TCS SP5 CLSM, zoom = 800 fold). RGCs were counted manually in 3 random areas along the centerline of each quadrant (total = 3 counts/quadrant, 12 counts/retina). More specifically, we used the optic nerve head as the origin with 3 standard regions distributed at 1-mm intervals along the central line of each quadrant. The area of each region at 800x magnification equaled 0.037 mm2. This analytical approach permitted us to account for retinal eccentricity. Overall, we optimized the techniques for the identification of mouse RGCs (Fig. 3).
Figure 3.
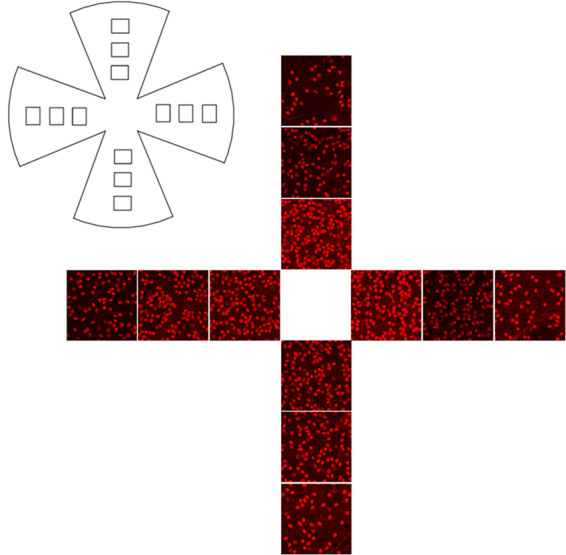
Immunohistochemical identification of mouse RGCs. Retinal samples were mounted and RGCs imaged with a confocal microscope. Cells were counted manually in 3 random areas along the centerline of each quadrant (total = 3 counts/quadrant, 12 counts/retina). RGCs were clearly visible by using a primary anti-Brn3a antibody and a secondary anti-IgG antibody.
Statistical analyses
Normality of the data was confirmed by using the Shapiro-Wilk and Kolmogorov-Smirnov tests. Unless otherwise noted, IOP and RGC comparisons were performed with two-tailed Student’s t-tests. A P < 0.05 was considered significant. Data are provided as the mean and standard error. All statistical analyses were performed with SPSS (IBM, Armonk, New York) and/or Prism 7 (GraphPad Software, Inc., La Jolla, CA).
Data availability statement
All data generated or analyzed during this study are included in this published article.
Acknowledgements
The authors express their appreciation to Nabil J. Alkayed, Nicole Libal, Molly Malone (Portland, OR), Orhan Oz (Dallas, TX), Evan Simpson (Clayton, Victoria, Australia), Sara J. M. Tellefsen (Solbergelva, Norway), and Xi Han, Huatao Xie, Jessica Hoadley, Michele Mammolenti, Jerome Mauris, Sandra Michaud, Jie Ma, Yiqing Li, Donald Pottle, Candace Beiler, and Kathleen Gallagher (Boston, MA, USA) for their assistance. The China Scholarship Council, the Harvard Glaucoma Center of Excellence, the Glaucoma Research Foundation (San Francisco, CA), the Margaret S. Sinon Scholar in Ocular Surface Research fund, the Yong Zhang Research Fund, and the Guoxing Yao Research Fund.
Author Contributions
D.A.S., L.R.P. and W.R.K. designed the strategy and experimentation. X.C. and Y.L. standardized and performed the mice breeding, genotyping, IOP measurement and RGC staining. X.C., Y.L. and Y.Z. contributed to the data acquisition. X.C., Y.L. and D.A.S. analyzed the data including statistics. X.C., Y.L., L.R.P. and D.A.S. wrote the manuscript. W.K. critiqued the manuscript. All authors have approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vajaranant TS, Pasquale LR. Estrogen deficiency accelerates aging of the optic nerve. Menopause. 2012;19:942–947. doi: 10.1097/gme.0b013e3182443137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei X, et al. Is low dose of estrogen beneficial for prevention of glaucoma? Medical hypotheses. 2012;79:377–380. doi: 10.1016/j.mehy.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Cascio C, Deidda I, Russo D, Guarneri P. The estrogenic retina: The potential contribution to healthy aging and age-related neurodegenerative diseases of the retina. Steroids. 2015;103:31–41. doi: 10.1016/j.steroids.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Vajaranant TS, et al. Risk of glaucoma after early bilateral oophorectomy. Menopause. 2014;21:391–398. doi: 10.1097/GME.0b013e31829fd081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquale LR, Rosner BA, Hankinson SE, Kang JH. Attributes of female reproductive aging and their relation to primary open-angle glaucoma: a prospective study. Journal of glaucoma. 2007;16:598–605. doi: 10.1097/IJG.0b013e318064c82d. [DOI] [PubMed] [Google Scholar]
- 6.Lee AJ, Mitchell P, Rochtchina E, Healey PR, Blue Mountains Eye S. Female reproductive factors and open angle glaucoma: the Blue Mountains Eye Study. The British journal of ophthalmology. 2003;87:1324–1328. doi: 10.1136/bjo.87.11.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hulsman CA, et al. Is open-angle glaucoma associated with early menopause? The Rotterdam Study. American journal of epidemiology. 2001;154:138–144. doi: 10.1093/aje/154.2.138. [DOI] [PubMed] [Google Scholar]
- 8.Pasquale LR, et al. Estrogen pathway polymorphisms in relation to primary open angle glaucoma: an analysis accounting for gender from the United States. Molecular vision. 2013;19:1471–1481. [PMC free article] [PubMed] [Google Scholar]
- 9.Mabuchi, F. et al. Estrogen receptor beta gene polymorphism and intraocular pressure elevation in female patients with primary open-angle glaucoma. American journal of ophthalmology149, 826–830 e821–822, 10.1016/j.ajo.2009.12.030 (2010). [DOI] [PubMed]
- 10.de Voogd S, et al. Estrogen receptors alpha and beta and the risk of open-angle glaucoma: the Rotterdam Study. Archives of ophthalmology. 2008;126:110–114. doi: 10.1001/archopht.126.1.110. [DOI] [PubMed] [Google Scholar]
- 11.Newman-Casey PA, et al. The potential association between postmenopausal hormone use and primary open-angle glaucoma. JAMA ophthalmology. 2014;132:298–303. doi: 10.1001/jamaophthalmol.2013.7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prokai-Tatrai K, et al. 17beta-estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Molecular pharmaceutics. 2013;10:3253–3261. doi: 10.1021/mp400313u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou X, et al. Retinal ganglion cell protection by 17-beta-estradiol in a mouse model of inherited glaucoma. Developmental neurobiology. 2007;67:603–616. doi: 10.1002/dneu.20373. [DOI] [PubMed] [Google Scholar]
- 14.Tint NL, et al. Hormone therapy and intraocular pressure in nonglaucomatous eyes. Menopause. 2010;17:157–160. doi: 10.1097/gme.0b013e3181b82fb4. [DOI] [PubMed] [Google Scholar]
- 15.Russo R, et al. 17Beta-estradiol prevents retinal ganglion cell loss induced by acute rise of intraocular pressure in rat. Progress in brain research. 2008;173:583–590. doi: 10.1016/S0079-6123(08)01144-8. [DOI] [PubMed] [Google Scholar]
- 16.Sator MO, et al. Reduction of intraocular pressure in a glaucoma patient undergoing hormone replacement therapy. Maturitas. 1998;29:93–95. doi: 10.1016/S0378-5122(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 17.Vajaranant TS, et al. Effects of Hormone Therapy on Intraocular Pressure: The Women’s Health Initiative-Sight Exam Study. American journal of ophthalmology. 2016;165:115–124. doi: 10.1016/j.ajo.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nixon E, Simpkins JW. Neuroprotective effects of nonfeminizing estrogens in retinal photoreceptor neurons. Investigative ophthalmology & visual science. 2012;53:4739–4747. doi: 10.1167/iovs.12-9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vajaranant TS, Nayak S, Wilensky JT, Joslin CE. Gender and glaucoma: what we know and what we need to know. Current opinion in ophthalmology. 2010;21:91–99. doi: 10.1097/ICU.0b013e3283360b7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paganini-Hill A, Clark LJ. Eye problems in breast cancer patients treated with tamoxifen. Breast cancer research and treatment. 2000;60:167–172. doi: 10.1023/A:1006342300291. [DOI] [PubMed] [Google Scholar]
- 21.Abramov Y, et al. Does postmenopausal hormone replacement therapy affect intraocular pressure? Journal of glaucoma. 2005;14:271–275. doi: 10.1097/01.ijg.0000169390.17427.b7. [DOI] [PubMed] [Google Scholar]
- 22.Toker E, Yenice O, Temel A. Influence of serum levels of sex hormones on intraocular pressure in menopausal women. Journal of glaucoma. 2003;12:436–440. doi: 10.1097/00061198-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Nirmalan PK, et al. Female reproductive factors and eye disease in a rural South Indian population: the Aravind Comprehensive Eye Survey. Investigative ophthalmology & visual science. 2004;45:4273–4276. doi: 10.1167/iovs.04-0285. [DOI] [PubMed] [Google Scholar]
- 24.Fabian CJ. The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. International journal of clinical practice. 2007;61:2051–2063. doi: 10.1111/j.1742-1241.2007.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones ME, Boon WC, Proietto J, Simpson ER. Of mice and men: the evolving phenotype of aromatase deficiency. Trends in endocrinology and metabolism: TEM. 2006;17:55–64. doi: 10.1016/j.tem.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocrine reviews. 2009;30:343–375. doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]
- 28.Czajka-Oraniec I, Simpson ER. Aromatase research and its clinical significance. Endokrynologia Polska. 2010;61:126–134. [PubMed] [Google Scholar]
- 29.Takeda K, et al. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. The Journal of endocrinology. 2003;176:237–246. doi: 10.1677/joe.0.1760237. [DOI] [PubMed] [Google Scholar]
- 30.Hewitt KN, Boon WC, Murata Y, Jones ME, Simpson ER. The aromatase knockout mouse presents with a sexually dimorphic disruption to cholesterol homeostasis. Endocrinology. 2003;144:3895–3903. doi: 10.1210/en.2003-0244. [DOI] [PubMed] [Google Scholar]
- 31.Murata Y, Robertson KM, Jones ME, Simpson ER. Effect of estrogen deficiency in the male: the ArKO mouse model. Molecular and cellular endocrinology. 2002;193:7–12. doi: 10.1016/S0303-7207(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 32.Dieudonne MN, et al. Sex steroids and leptin regulate 11beta-hydroxysteroid dehydrogenase I and P450 aromatase expressions in human preadipocytes: Sex specificities. The Journal of steroid biochemistry and molecular biology. 2006;99:189–196. doi: 10.1016/j.jsbmb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Fester L, et al. Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. The Journal of steroid biochemistry and molecular biology. 2012;131:24–29. doi: 10.1016/j.jsbmb.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal VR, et al. Upregulation of estrogen receptors in the forebrain of aromatase knockout (ArKO) mice. Molecular and cellular endocrinology. 2000;162:9–16. doi: 10.1016/S0303-7207(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 35.Simpson ER. Models of aromatase insufficiency. Seminars in reproductive medicine. 2004;22:25–30. doi: 10.1055/s-2004-823024. [DOI] [PubMed] [Google Scholar]
- 36.Yan M, et al. Functional modification of pituitary somatotropes in the aromatase knockout mouse and the effect of estrogen replacement. Endocrinology. 2004;145:604–612. doi: 10.1210/en.2003-0646. [DOI] [PubMed] [Google Scholar]
- 37.Dewundara SS, Wiggs JL, Sullivan DA, Pasquale LR. Is Estrogen a Therapeutic Target for Glaucoma? Seminars in ophthalmology. 2016;31:140–146. doi: 10.3109/08820538.2015.1114845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tham YC, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt KG, Bergert H, Funk RH. Neurodegenerative diseases of the retina and potential for protection and recovery. Current neuropharmacology. 2008;6:164–178. doi: 10.2174/157015908784533851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Progress in retinal and eye research. 2003;22:465–481. doi: 10.1016/S1350-9462(03)00026-0. [DOI] [PubMed] [Google Scholar]
- 41.Glaucomafoundation. PRIMARY OPEN-ANGLE GLAUCOMA (POAG), https://www.glaucomafoundation.org/Primary_Open-Angle_Glaucoma.htm, (accessed 17.07.07).
- 42.Fingeret, M. Optometric Clinical Practice Guideline Care Of The Patient With Open Angle Glaucoma. http://www.aoa.org/documents/optometrists/CPG-9.pdf (accessed 17.07.07) (2010).
- 43.BrightFocusFoundation. Types of Glaucoma. http://www.brightfocus.org/glaucoma/types(accessed 17.03.03), (accessed 17.03.03).
- 44.Mukesh BN, McCarty CA, Rait JL, Taylor HR. Five-year incidence of open-angle glaucoma: the visual impairment project. Ophthalmology. 2002;109:1047–1051. doi: 10.1016/S0161-6420(02)01040-0. [DOI] [PubMed] [Google Scholar]
- 45.Pasquale LR, Kang JH. Female reproductive factors and primary open-angle glaucoma in the Nurses’ Health Study. Eye. 2011;25:633–641. doi: 10.1038/eye.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darabad RR, et al. Influence of aromatase absence on the gene expression and histology of the mouse meibomian gland. Investigative ophthalmology & visual science. 2013;54:987–998. doi: 10.1167/iovs.12-10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahimi Darabad R, et al. Does estrogen deficiency cause lacrimal gland inflammation and aqueous-deficient dry eye in mice? Experimental eye research. 2014;127:153–160. doi: 10.1016/j.exer.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simpson ER, et al. Estrogen–the good, the bad, and the unexpected. Endocrine reviews. 2005;26:322–330. doi: 10.1210/er.2004-0020. [DOI] [PubMed] [Google Scholar]
- 49.Chavez C, Gogos A, Jones ME, van den Buuse M. Psychotropic drug-induced locomotor hyperactivity and prepulse inhibition regulation in male and female aromatase knockout (ArKO) mice: role of dopamine D1 and D2 receptors and dopamine transporters. Psychopharmacology. 2009;206:267–279. doi: 10.1007/s00213-009-1604-6. [DOI] [PubMed] [Google Scholar]
- 50.Jones ME, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hewitt KN, Pratis K, Jones ME, Simpson ER. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology. 2004;145:1842–1848. doi: 10.1210/en.2003-1369. [DOI] [PubMed] [Google Scholar]
- 52.Hill RA, Pompolo S, Jones ME, Simpson ER, Boon WC. Estrogen deficiency leads to apoptosis in dopaminergic neurons in the medial preoptic area and arcuate nucleus of male mice. Molecular and cellular neurosciences. 2004;27:466–476. doi: 10.1016/j.mcn.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 53.Xu Y, et al. Study of Sex Differences in Duloxetine Efficacy for Depression in Transgenic Mouse Models. Frontiers in cellular neuroscience. 2017;11:344. doi: 10.3389/fncel.2017.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:11748–11755. doi: 10.1523/JNEUROSCI.1887-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oz OK, et al. Bone phenotype of the aromatase deficient mouse. The Journal of steroid biochemistry and molecular biology. 2001;79:49–59. doi: 10.1016/S0960-0760(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 56.Van Sinderen ML, et al. Hepatic glucose intolerance precedes hepatic steatosis in the male aromatase knockout (ArKO) mouse. PloS one. 2014;9:e87230. doi: 10.1371/journal.pone.0087230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dalla C, Antoniou K, Papadopoulou-Daifoti Z, Balthazart J, Bakker J. Oestrogen-deficient female aromatase knockout (ArKO) mice exhibit depressive-like symptomatology. The European journal of neuroscience. 2004;20:217–228. doi: 10.1111/j.1460-9568.2004.03443.x. [DOI] [PubMed] [Google Scholar]
- 58.Costagliola C, Parmeggiani F, Sebastiani A. SSRIs and intraocular pressure modifications: evidence, therapeutic implications and possible mechanisms. CNS drugs. 2004;18:475–484. doi: 10.2165/00023210-200418080-00001. [DOI] [PubMed] [Google Scholar]
- 59.Britt KL, et al. The ovarian phenotype of the aromatase knockout (ArKO) mouse. The Journal of steroid biochemistry and molecular biology. 2001;79:181–185. doi: 10.1016/S0960-0760(01)00158-3. [DOI] [PubMed] [Google Scholar]
- 60.McPherson SJ, et al. Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology. 2001;142:2458–2467. doi: 10.1210/endo.142.6.8079. [DOI] [PubMed] [Google Scholar]
- 61.Robertson KM, et al. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7986–7991. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rinn JL, Snyder M. Sexual dimorphism in mammalian gene expression. Trends in genetics: TIG. 2005;21:298–305. doi: 10.1016/j.tig.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Isensee J, Ruiz Noppinger P. Sexually dimorphic gene expression in mammalian somatic tissue. Gender medicine. 2007;4(Suppl B):S75–95. doi: 10.1016/S1550-8579(07)80049-0. [DOI] [PubMed] [Google Scholar]
- 64.Ostrer H. Invited review: sex-based differences in gene expression. Journal of applied physiology. 2001;91:2384–2388. doi: 10.1152/jappl.2001.91.5.2384. [DOI] [PubMed] [Google Scholar]
- 65.Disteche, C. M., Filippova, G. N. & Tsuchiya, K. D. Escape from X inactivation. Cytogenetic and genome research99, 36–43, doi:71572 (2002). [DOI] [PubMed]
- 66.Xu J, Disteche CM. Sex differences in brain expression of X- and Y-linked genes. Brain research. 2006;1126:50–55. doi: 10.1016/j.brainres.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 67.Migeon BR. The role of X inactivation and cellular mosaicism in women’s health and sex-specific diseases. Jama. 2006;295:1428–1433. doi: 10.1001/jama.295.12.1428. [DOI] [PubMed] [Google Scholar]
- 68.MacGillivray MH, Morishima A, Conte F, Grumbach M, Smith EP. Pediatric endocrinology update: an overview. The essential roles of estrogens in pubertal growth, epiphyseal fusion and bone turnover: lessons from mutations in the genes for aromatase and the estrogen receptor. Hormone research. 1998;49(Suppl 1):2–8. doi: 10.1159/000053061. [DOI] [PubMed] [Google Scholar]
- 69.Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. The Journal of clinical endocrinology and metabolism. 1999;84:4677–4694. doi: 10.1210/jcem.84.12.6290. [DOI] [PubMed] [Google Scholar]
- 70.Plumari L, et al. Changes in the arginine-vasopressin immunoreactive systems in male mice lacking a functional aromatase gene. Journal of neuroendocrinology. 2002;14:971–978. doi: 10.1046/j.1365-2826.2002.00866.x. [DOI] [PubMed] [Google Scholar]
- 71.Hamilton KJ, Arao Y, Korach KS. Estrogen hormone physiology: reproductive findings from estrogen receptor mutant mice. Reproductive biology. 2014;14:3–8. doi: 10.1016/j.repbio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clinical reviews in allergy & immunology. 2011;40:66–73. doi: 10.1007/s12016-010-8203-5. [DOI] [PubMed] [Google Scholar]
- 73.Kuiper GG, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 74.Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. Journal of immunology. 1998;161:27–34. [PubMed] [Google Scholar]
- 75.Lubahn DB, et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Korach KS. Insights from the study of animals lacking functional estrogen receptor. Science. 1994;266:1524–1527. doi: 10.1126/science.7985022. [DOI] [PubMed] [Google Scholar]
- 78.Couse JF, et al. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Molecular endocrinology. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 79.Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I. The distribution of estrogen receptor-beta mRNA in forebrain regions of the estrogen receptor-alpha knockout mouse. Endocrinology. 1997;138:5649–5652. doi: 10.1210/endo.138.12.5712. [DOI] [PubMed] [Google Scholar]
- 80.Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 81.Wickham LA, et al. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta ophthalmologica Scandinavica. 2000;78:146–153. doi: 10.1034/j.1600-0420.2000.078002146.x. [DOI] [PubMed] [Google Scholar]
- 82.Kobayashi K, Kobayashi H, Ueda M, Honda Y. Estrogen receptor expression in bovine and rat retinas. Investigative ophthalmology & visual science. 1998;39:2105–2110. [PubMed] [Google Scholar]
- 83.Munaut C, et al. Presence of oestrogen receptor type beta in human retina. The British journal of ophthalmology. 2001;85:877–882. doi: 10.1136/bjo.85.7.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kang JH, et al. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with sex and postmenopausal hormone use. Investigative ophthalmology & visual science. 2010;51:971–979. doi: 10.1167/iovs.09-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ohmichi M, et al. Rapid changes of flow-mediated dilatation after surgical menopause. Maturitas. 2003;44:125–131. doi: 10.1016/S0378-5122(02)00320-1. [DOI] [PubMed] [Google Scholar]
- 86.Berthois Y, et al. Estradiol membrane binding sites on human breast cancer cell lines. Use of a fluorescent estradiol conjugate to demonstrate plasma membrane binding systems. Journal of steroid biochemistry. 1986;25:963–972. doi: 10.1016/0022-4731(86)90330-4. [DOI] [PubMed] [Google Scholar]
- 87.Davis TL, Whitesell JD, Cantlon JD, Clay CM, Nett TM. Does a nonclassical signaling mechanism underlie an increase of estradiol-mediated gonadotropin-releasing hormone receptor binding in ovine pituitary cells? Biology of reproduction. 2011;85:770–778. doi: 10.1095/biolreprod.111.091926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morales A, Gonzalez M, Marin R, Diaz M, Alonso R. Estrogen inhibition of norepinephrine responsiveness is initiated at the plasma membrane of GnRH-producing GT1-7 cells. The Journal of endocrinology. 2007;194:193–200. doi: 10.1677/JOE-06-0001. [DOI] [PubMed] [Google Scholar]
- 89.Hewitt SC, Deroo BJ, Korach KS. Signal transduction. A new mediator for an old hormone? Science. 2005;307:1572–1573. doi: 10.1126/science.1110345. [DOI] [PubMed] [Google Scholar]
- 90.Micevych P, Kuo J, Christensen A. Physiology of membrane oestrogen receptor signalling in reproduction. Journal of neuroendocrinology. 2009;21:249–256. doi: 10.1111/j.1365-2826.2009.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hammes SR, Levin ER. Minireview: Recent advances in extranuclear steroid receptor actions. Endocrinology. 2011;152:4489–4495. doi: 10.1210/en.2011-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soltysik K, Czekaj P. Membrane estrogen receptors - is it an alternative way of estrogen action? Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. 2013;64:129–142. [PubMed] [Google Scholar]
- 93.Kam, W. R., Rahimi Darabad, R. & Sullivan, D. A. Membrane steroid receptors are expressed by human meibomian gland epithelial cells. ARVO eabstract17 (2014).
- 94.Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. The Journal of clinical endocrinology and metabolism. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- 95.Labrie F. Intracrinology. Molecular and cellular endocrinology. 1991;78:C113–118. doi: 10.1016/0303-7207(91)90116-A. [DOI] [PubMed] [Google Scholar]
- 96.Labrie F, Belanger A, Simard J, Van L-T, Labrie C. DHEA and peripheral androgen and estrogen formation: intracinology. Annals of the New York Academy of Sciences. 1995;774:16–28. doi: 10.1111/j.1749-6632.1995.tb17369.x. [DOI] [PubMed] [Google Scholar]
- 97.Labrie F, et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–328. doi: 10.1016/S0039-128X(98)00007-5. [DOI] [PubMed] [Google Scholar]
- 98.Labrie F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. The Journal of steroid biochemistry and molecular biology. 2015;145:133–138. doi: 10.1016/j.jsbmb.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 99.Luu-The V, Labrie F. The intracrine sex steroid biosynthesis pathways. Progress in brain research. 2010;181:177–192. doi: 10.1016/S0079-6123(08)81010-2. [DOI] [PubMed] [Google Scholar]
- 100.Guarneri P, et al. Neurosteroidogenesis in rat retinas. Journal of neurochemistry. 1994;63:86–96. doi: 10.1046/j.1471-4159.1994.63010086.x. [DOI] [PubMed] [Google Scholar]
- 101.Guarneri P. Neurosteroids in retina: synthesis and neuronal function. In Genazzani AR, Petraglia F., Purdy RH, editors. The brain: source and target for sex steroid hormones. New York, The Parthenon. Publishing Group, 1996, pp. 63–81.
- 102.Cascio C, et al. 17beta-estradiol synthesis in the adult male rat retina. Experimental eye research. 2007;85:166–172. doi: 10.1016/j.exer.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 103.Gupta A. Leptin as a neuroprotective agent in glaucoma. Medical hypotheses. 2013;81:797–802. doi: 10.1016/j.mehy.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 104.Zhou G, Liu B. Single nucleotide polymorphisms of metabolic syndrome-related genes in primary open angle glaucoma. International journal of ophthalmology. 2010;3:36–42. doi: 10.3980/j.issn.2222-3959.2010.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ma J, et al. Transplantation of Human Neural Progenitor Cells Expressing IGF-1 Enhances Retinal Ganglion Cell Survival. PloS one. 2015;10:e0125695. doi: 10.1371/journal.pone.0125695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lei ZM, Rao CV. Neural actions of luteinizing hormone and human chorionic gonadotropin. Seminars in reproductive medicine. 2001;19:103–109. doi: 10.1055/s-2001-13917. [DOI] [PubMed] [Google Scholar]
- 107.Misso ML, et al. Cellular and molecular characterization of the adipose phenotype of the aromatase-deficient mouse. Endocrinology. 2003;144:1474–1480. doi: 10.1210/en.2002-221123. [DOI] [PubMed] [Google Scholar]
- 108.Van Sinderen M, et al. Sexual dimorphism in the glucose homeostasis phenotype of the Aromatase Knockout (ArKO) mice. The Journal of steroid biochemistry and molecular biology. 2017;170:39–48. doi: 10.1016/j.jsbmb.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 109.Le May C, et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramdas WD, et al. Lifestyle and risk of developing open-angle glaucoma: the Rotterdam study. Archives of ophthalmology. 2011;129:767–772. doi: 10.1001/archophthalmol.2010.373. [DOI] [PubMed] [Google Scholar]
- 111.Soto I, et al. DBA/2 J mice are susceptible to diabetic nephropathy and diabetic exacerbation of IOP elevation. PloS one. 2014;9:e107291. doi: 10.1371/journal.pone.0107291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Costa L, Cunha JP, Amado D, Pinto LA, Ferreira J. Diabetes Mellitus as a Risk Factor in Glaucoma’s Physiopathology and Surgical Survival Time: A Literature Review. Journal of current glaucoma practice. 2015;9:81–85. doi: 10.5005/jp-journals-10008-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Head GA, Obeyesekere VR, Jones ME, Simpson ER, Krozowski ZS. Aromatase-deficient (ArKO) mice have reduced blood pressure and baroreflex sensitivity. Endocrinology. 2004;145:4286–4291. doi: 10.1210/en.2004-0421. [DOI] [PubMed] [Google Scholar]
- 114.Levine RM, Yang A, Brahma V, Martone JF. Management of Blood Pressure in Patients with Glaucoma. Current cardiology reports. 2017;19:109. doi: 10.1007/s11886-017-0927-x. [DOI] [PubMed] [Google Scholar]
- 115.Ding C, Wang P, Tian N. Effect of general anesthetics on IOP in elevated IOP mouse model. Experimental eye research. 2011;92:512–520. doi: 10.1016/j.exer.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chan-Ling TG. vascular, and neuronal cytogenesis in whole-mounted cat retina. Microscopy research and technique. 1997;36:1–16. doi: 10.1002/(SICI)1097-0029(19970101)36:1<1::AID-JEMT1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 117.Mead B, et al. Comparative evaluation of methods for estimating retinal ganglion cell loss in retinal sections and wholemounts. PloS one. 2014;9:e110612. doi: 10.1371/journal.pone.0110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De la Huerta I, Kim IJ, Voinescu PE, Sanes JR. Direction-selective retinal ganglion cells arise from molecularly specified multipotential progenitors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17663–17668. doi: 10.1073/pnas.1215806109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Caprara C, et al. HIF1A is essential for the development of the intermediate plexus of the retinal vasculature. Investigative ophthalmology & visual science. 2011;52:2109–2117. doi: 10.1167/iovs.10-6222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.