Abstract
Liver disease affects over 25 million people in the United States and, despite advances in medical management resulting in increased survival, a majority of these individuals report multiple co-occurring symptoms that severely impair functioning and quality of life. The purpose of this review is to (1) propose defining these co-occurring symptoms as a symptom cluster of chronic liver disease (CLD), (2) discuss putative underlying biological mechanisms related to CLD, including the liver–gut–brain axis and influence of the microbiome, and (3) discuss the implications for biobehavioral research in this patient population. Biobehavioral research focusing on the interrelated, and possibly synergistic, mechanisms of these symptoms may lead to the development and testing of targeted symptom management interventions for improving function and quality of life in this growing patient population.
Keywords: biobehavioral, liver disease, mechanisms, symptoms
Chronic liver disease (CLD) is common, affecting over 25 million Americans. A vast majority of individuals with CLD report multiple, co-occurring symptoms over the course of the disease that interfere with quality of life and daily functioning. The similarity of the symptom experience among individuals with CLD suggests that there may be common physiologic processes underlying multiple symptoms; however, research in this population has focused on singular symptoms instead of the collective symptom experience. Therefore, the first purpose of this review is to propose defining these co-occurring symptoms as a symptom cluster of CLD. The second purpose is to discuss putative underlying biological mechanisms related to CLD, including the liver–gut–brain axis and influence of the microbiome, a term used to denote the diverse array of microorganisms (microbiota) that inhabit the intestinal tract (Quigley & Monsour, 2013). The third purpose of this review is to posit potential implications of defining the CLD symptom cluster for biobehavioral research in this patient population.
Overview of CLD
CLD encompasses a broad array of liver pathologies including nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH), viral and autoimmune hepatitis, cholestatic diseases (primary biliary cirrhosis [PBC], primary sclerosing cholangitis [PSC]), alcohol-related liver disease, and genetic conditions such as hemochromatosis and alpha-1-antitrypsin deficiency (see Table 1). CLD represents 1 of the 10 leading causes of death in the United States when the subtypes of viral hepatitis, cirrhosis, and liver cancer are combined (Newton & Jones, 2012). Importantly, NAFLD/NASH is now the number one cause of liver disease in Western countries, whereas the prevalence of other CLD subtypes have remained stable or even decreased (World Gastroenterology Organization, 2012). While CLD affects all ages, genders, racial and ethnic groups, and socioeconomic classes (Bell et al., 2008), lower socioeconomic groups and disadvantaged individuals are disproportionately affected, including Hispanic Whites and African Americans (National Institutes of Health, 2009).
Table 1.
Common Etiologies of Chronic Liver Disease (CLD) and Characteristics.
| Etiology | Characteristics |
|---|---|
| Nonalcoholic fatty liver disease (NAFLD) | The most common form of CLD in the United States |
| Associated with obesity, metabolic syndrome, and insulin resistance | |
| Other potential causes include drugs (i.e., methotrexate, glucocorticoids), chemicals (benzene, xylene), growth hormone deficiency, hypothyroidism, nutritional deficiencies, and genetic disorders | |
| Can progress to NASH, a leading cause of cirrhosis in the United States | |
| Infectious hepatitis | Causes inflammation of the liver |
| Over 400 million people worldwide are chronic carriers of hepatitis B and 250 million people are affected by hepatitis C | |
| Viral causes of CLD in humans are hepatitis B, C, and D, Epstein-Barr virus (mononucleosis), adenovirus, and cytomegalovirus | |
| Primary biliary cirrhosis (PBC) | Affects up to 1 in every 4,000 people and has a female-to-male ratio of 9:1 |
| Autoimmune disease causing slow progressive destruction of small bile ducts leading to accumulation of bile (cholestasis) | |
| Tissue damage causes scarring and fibrosis | |
| May lead to cirrhosis | |
| Primary sclerosing cholangitis (PSC) | Autoimmune disease |
| Inflammation, scarring, and blockage of the bile ducts damages liver cells | |
| May lead to cirrhosis | |
| 10–15% lifetime risk of developing cholangiocarcinoma | |
| Alcoholic liver disease | Consumption of large quantities of alcohol over a prolonged period of time |
| Ranges from asymptomatic to fulminant liver failure | |
| Cause of 8% of newly diagnosed cases of CLD and the combination of alcoholic liver disease and hepatitis C accounts for another 22% of cases | |
| Genetic conditions: hemochromatosis and alpha-1-antitrypsin deficiency (A1AD) | Hereditary disorder causing the body to absorb too much iron |
| Symptoms typically develop at age 50–60 years | |
| Can cause damage to the liver, pancreas, and heart | |
| A1AD is an unusual cause of liver disease in children and adults |
Note. CLD = chronic liver disease; NASH = nonalcoholic steatohepatitis; AD = autonomic dysfunction.
Advances in medical management have substantially improved long-term survival in some CLD subtypes by preventing or delaying disease progression (Vernon, Baranova, & Younossi, 2011). Despite these improvements in survival, a majority of individuals living with CLD report multiple distressing symptoms that significantly impair quality of life and functional status (David et al., 2009). The most common symptoms reported by individuals with CLD include fatigue, cognitive impairment (non-encephalopathic), mood disturbances, pain, and sleep disturbances (DeCruz, Espiritu, Zeidler, & Wang, 2012; Elwing, Lustman, Wang, & Clouse, 2006; Jones, 2007; Newton & Jones, 2012). In contrast with other subtype-specific symptoms (pruritis in cholestatic disease or right upper quadrant abdominal pain and depression with hepatitis C virus [HCV]), these co-occurring symptoms develop across the broad array of CLD pathologies (Elliott, Frith, Pairman, Jones, & Newton, 2011; Patanwala et al., 2010). The impact of these symptoms was once thought to be directly related to the pathophysiological consequences of cirrhosis and end-stage liver disease. However, with more people living with CLD, it is now recognized that symptoms can occur throughout the disease course, are not related to disease severity, and frequently do not improve with treatment of the underlying disease process (Newton & Jones, 2012; Swain, 2006).
Co-Occurring Symptoms of CLD as a Symptom Cluster
The hypothesis that co-occurring symptoms develop from shared biological mechanisms was proposed in the oncology literature in 2001 (Dodd et al., 2001). While some researchers define symptom cluster as two or more symptoms that are related, co-occur, and share a common etiology (Miaskowski, Aouizerat, Dodd, & Cooper, 2007), there is still no apparent agreement on the number of symptoms needed and period of time necessary to consider concurrent physiological, psychological, and cognitive symptoms as a symptom cluster (Barsevick, 2007). Nevertheless, symptom clusters have been identified either by clinical observation or statistical analysis of empirical data in a variety of general medical conditions, including congestive heart failure (Jurgens et al., 2009), cancer (Kirkova, Aktas, Walsh, & Davis, 2011), and fibromyalgia (Rutledge, Mouttapa, & Wood, 2009). Symptoms shared among the many subtypes of CLD may represent a symptom cluster common to this patient population and, in that light, may be amenable to shared symptom management strategies.
Putative Mechanisms of the CLD Symptom Cluster
For individuals living with CLD, several biological systems may contribute to the development and persistence of distressing symptoms. These include the immune system, central nervous system (CNS), and the autonomic nervous system, which includes the sympathetic nervous system and parasympathetic nervous system (PNS) divisions as well as the enteric nervous system (ENS). The ENS plays an important role in the bidirectional communication that occurs between the brain and the gut as well as liver–gut–brain signaling, which becomes altered in the presence of CLD (Cryan & Dinan, 2012). The liver–gut–brain axis is a physiological network that orchestrates interactions among the neurological, endocrine, and immune systems and provides the intestinal microbiota and their metabolites with a potential route through which to influence homeostatic functions as well as behavior (Collins, Surette, & Bercik, 2012).
Immune System
Inflammation
Inflammation is a likely candidate for a shared mechanism of commonly experienced symptoms in CLD because it is a fundamental physiological phenomenon involved in the development, initiation, and progression of CLD (Gao, Jeong, & Tian, 2008). The relevance of inflammatory activation in CLD is tied to the importance of the liver in regulating endocrine-immune responses as well as the changes that occur in disease. The liver contains the largest population of fixed macrophages in the body, which represents an important source of the cytokines found in circulation (Tjandra, Sharkey, & Swain, 2000). Elevated circulating cytokine levels have been commonly reported in the setting of CLD (Swain, 2006). Previous studies have linked high circulating levels of proinflammatory cytokines with co-occurring symptoms, including fatigue, depressive symptoms, sleep disturbances, and pain (Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002; Larson & Dunn, 2001). In addition, interferon gamma, a cytokine administered for the treatment of a variety of cancers as well as hepatitis C has also been found to induce fatigue (Raison et al., 2010; Sarkar, Jiang, Evon, Wahed, & Hoofnagle, 2012).
Moreover, chronic low-grade inflammation is a key feature of obesity, a primary cause of the largest growing CLD subtype, NAFLD (World Gastroenterology Organization, 2012). The inflammatory response triggered by obesity involves many components of the classic inflammatory response to pathogens, including systemic increases in circulating inflammatory cytokines and acute phase proteins, recruitment of leukocytes to inflamed tissues, activation of tissue leukocytes, and generation of reparative tissue responses like fibrosis (Sanyal et al., 2001).
Thus, inflammation that accompanies the initial insult to the liver may be an instigating event in the cascade of pathophysiological changes that occur in CLD (Swain, 2006). Alterations in the capacity of the liver to maintain endocrine-immune responses in the setting of CLD may be an important mechanism in explaining shared symptoms.
Liver–gut–brain axis
The liver is one of the largest endocrine glands in the body, second only to the intestinal tract, and receives 80% of its blood supply from the small and large intestines via the portal vein (Seki & Schnabl, 2012). It acts as an organ barrier or filter between the intestinal tract and the rest of the body and provides protection against potentially toxic bacterial products, environmental toxins, and food antigens by launching the innate immune system, an important first line of defense against infection (Gao et al., 2008). Hepatocytes contribute to innate immunity through the production of complement components and secreted pattern recognition receptors (PRRs) that recognize pathogen-associated molecular patterns expressed by invading pathogens, such as lipopolysaccharide and peptidoglycan (Seki & Schnabl, 2012). Normally, the intestinal microbiome, an array of microorganisms predominantly composed of bacteria, is contained within the intestinal lumen by a thin epithelial barrier and does not interfere with liver immune function. Owing to a “core microbiota,” the intestinal microbiome is relatively stable throughout an individual’s life span (Cryan & Dinan, 2012) and both the composition and quantity of the bacteria help sustain homeostasis.
The intestinal epithelium helps to maintain a symbiotic relationship between the liver and gut microbiome, providing a natural barrier that permits selective entry of specific nutrients and ions and water while preventing entry of harmful molecules, including bacteria (Quigley & Monsour, 2013). Specialized intercellular structures known as tight junctions, along with PRRs, play a large role in regulating immune responses to bacteria and can prompt gut-associated lymphoid tissue when harmful bacteria is detected in the intestinal lumen (Miele et al., 2009; Vajro, Paolella, & Fasano, 2013). In CLD, the gut epithelium can lose its protective qualities due to injury of the tight junctions, which leads to intestinal barrier malfunction or leaky gut (Seki & Schnabl, 2012). Zonulin, a molecule that reversibly modulates the permeability of the tight junctions, is thought to play a key role in intestinal barrier malfunction (Fasano, 2011). Inflammatory mediators activate the promoter region of the zonulin gene, thereby upregulating its production (Moreno-Navarrete, Sabater, Ortega, Ricart, & Fernandez-Real, 2012). In addition, high-fat meal-related hypertriglyceridemia and alcohol consumption can lead to derangement of the tight junctions, thereby causing increased intestinal permeability and plasma endotoxins (Musso, Gambino, & Cassader, 2010).
Intestinal barrier malfunction is associated with increased bacterial translocation, the migration of viable bacteria or bacterial products from the intestinal lumen to mesenteric lymph nodes, and other extraintestinal organs and sites. Increased translocation of bacteria and bacterial products from the intestine promotes NF-κB synthesis by stimulating the production of interleukin (IL)-1β and increases nitric oxide synthesis, leading to ongoing inflammatory activation (Ilan, 2012). As a result, microbial translocation enhances liver inflammation, thus further compromising liver function and contributing to fibrosis (Seki & Schnabl, 2012). Increased barrier permeability can allow bacteria, fungi, parasites, and their toxins to move from the intestinal lumen to the submucosa and bloodstream (Vajro et al., 2013). Ultimately, a disruption of the homeostasis between the gut microbiome and the immune system increases an individual’s disease risk and may alter neurocognitive function (Quigley & Mansour, 2013).
The gut microbiota and its metabolites can access the brain through various routes within the liver–gut–brain axis. These routes include the bloodstream and the area postrema (a structure of the medulla that is outside of the blood-brain barrier and does not contain tight junctions), release of cytokines from mucosal immune cells and gut hormones from enteroendocrine cells, and afferent neural pathways such as the vagus nerve (Collins et al., 2012). This tridirectional communication among the CNS, the peripheral nervous system, and the ENS links emotional and cognitive centers of the brain with peripheral functions (Cryan & O’Mahony, 2011). This axis explains how the microbiota influences CNS function and how the brain can influence gastrointestinal and related immune functions as well as emotional factors such as stress, depression, and anxiety (Collins et al., 2012; Cryan & Dinan, 2012).
In summary, dysregulation of the liver–gut–brain axis is a key feature of CLD and a likely candidate for a shared mechanism of commonly experienced symptoms. As further liver damage occurs, the tight junctions become impaired and the liver can no longer produce the components necessary to mount an adequate innate immune response, resulting in increased vulnerability to bacterial overgrowth and/or translocation. Likewise, intestinal barrier malfunction leads to bacterial translocation and elevated levels of bacterial products in the systemic circulation, causing an ongoing inflammatory response as well as symptom persistence (Schnabl, 2013).
CNS
Altered neurotransmission in the CNS
CLD has been associated with changes in CNS neurotransmission as well as behavior (D’Mello & Swain, 2011). Although altered neurotransmission and neurocognitive changes were traditionally tied only to hepatic encephalopathy, it is now understood that these changes can occur throughout the disease course and can range from minimal to overt (Kappus & Bajaj, 2012). The intestinal microbiota can alter neurochemical communication between the gut and brain because it is a major source of circulating organic acids, tryptophan metabolites, and fermentation products such as lactic and propionic acid (Collins et al., 2012). Although it is unclear how these molecules influence central neurotransmission, it is possible that they can alter the central levels of neurotransmitters in the brain, leading to behavioral changes (Swain, 2006). Support for the premise that the intestinal microbiota can influence behavior comes from studies that have found an association between high levels of self-reported anxiety and elevated fecal concentrations of propionic acid (Tana et al., 2010) as well as the findings that commensal lactobacilli and bifidobacteria produce gamma-aminobutyric acid (Barrett, Ross, O’Toole, Fitzgerald, & Stanton, 2012), an amino acid neurotransmitter with anxiolytic properties. Gut bacteria also produce serotonin (5-HT), dopamine (D), noradrenaline (NA), and acetylcholine (ACh). Alterations in the microbiota–gut–brain signaling and/or availability of substrates required to produce neurotransmitters can alter central neurotransmission and lead to co-occurring symptoms including fatigue, cognitive impairment, and mood and sleep disturbances (D’Mello & Swain, 2011).
Autonomic dysfunction (AD)
AD is common in CLD and manifests as abnormalities in heart rate and blood pressure regulation (Newton et al., 2007). Although AD was once thought to arise only in the setting of cirrhosis, recent studies have reported that it is present in the early precirrhotic stages of liver disease involving NAFLD, PBC, PSC, and HCV (Newton, Wilton, Pairman, Jones, & Day, 2009; Osztovits et al., 2009; Salerno & Cazzaniga, 2009). Both peripheral and central mechanisms can contribute to AD. Peripheral mechanisms involve decreased vascular tone and alterations in the elasticity of the liver, which play a key role in splanchnic buffering (Stevens, Allen, Murray, Jones, & Newton, 2009). Researchers have also described central anatomical and functional effects of CLD that lead to AD, including impaired cerebral autoregulation (Hollingsworth et al., 2009, 2010). One of the most characteristic symptoms of AD is fatigue (Abbas, Jorgensen, & Lindor, 2010; Newton, 2010) that is often accompanied by sleep disturbance and physical inactivity (Newton & Jones, 2012). AD is also associated with mood disorders (Lee, Otgonsuren, Younoszai, Mir, & Younossi, 2013; Weinstein et al., 2011), and cognitive symptoms (Elliott et al., 2011; Newton et al., 2009).
Psychological stress
Perceived psychological stress leads to activation of the hypothalamic–pituitary–adrenocortical (HPA) axis through increased release of cortisol and catecholamines, thereby exerting a facilitative effect on the hepatic inflammatory response. In contrast, the efferent PNS elicits an inhibitory effect on the development of hepatic inflammation (Chida, Sudo, & Kubo, 2006). Patients with CLD have decreased cortisol clearance as well as decreased cortisol binding in plasma resulting in a longer effect of cortisol on the HPA axis (Swain, 2000). Increased psychological stress can induce elevated levels of proinflammatory cytokines in the liver, suggesting a direct link between stress and liver inflammation (Tjandra et al., 2000). Elevated psychosocial stress has also been shown to hasten the course of fibrosis in patients with viral hepatitis by increasing glucocorticoid levels, which, in turn, increases natural killer cell–induced hepatocyte apoptosis (Vere, Streba, Streba, Ionescu, & Sima, 2009).
Within the context of the liver–gut–brain axis, chronic psychological and/or psychosocial stress as well as mood disorders (depression and anxiety in particular) can further contribute to disruption of the intestinal barrier and gut microbiota composition (Soderholm & Perdue, 2001). Psychological stress is associated with increased gut permeability mediated by the release of endogenous glucocorticoids (Swain, 2000). The mechanisms by which stress influences the gut microbial composition may include alterations to the microbial habitat following stress-induced changes in intestinal motility and mucin production (Collins et al., 2012). In addition, the release of catecholamines can alter gene expression in some bacteria, resulting in preferential growth of certain gut bacterial communities (Lyte, Vulchanova, & Brown, 2011). In turn, it has been recently shown that the microbiome influences both brain neurochemistry and function, which can induce alterations in behavior (depression/anxiety) as well as cognition (Clarke et al., 2013; Diaz Heijtz et al., 2011; Neufeld, Kang, Bienenstock, & Foster, 2010).
In summary, common symptoms of CLD may be influenced by activation of the HPA axis that affects the tridirectional relationship among the CNS, ANS, and ENS. Alterations in neurotransmission as well as AD are intricately connected to liver immune responses and may contribute to co-occurring symptoms in CLD.
Implications for Biobehavioral Research
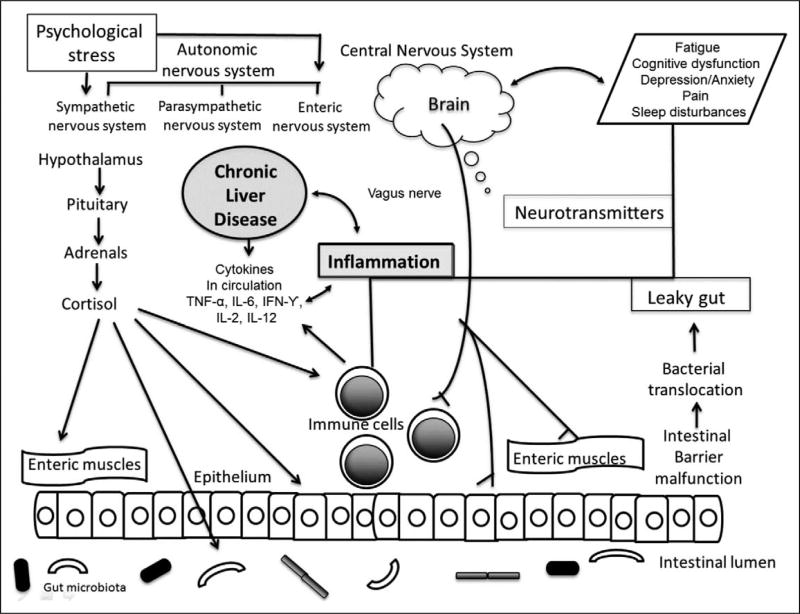
The recognition of shared symptoms among the various etiologies of CLD suggests that there may be common mechanisms contributing to the manifestation of these symptoms over the disease course. Based on this review, we constructed a conceptual framework describing the potential mechanisms underlying the common symptom experience of CLD, which is depicted in Figure 1. Investigations focused on the liver–gut–brain axis suggest a level of system interdependence that may be a crucial factor in identifying the biological substrates of the CLD symptom cluster as well as explaining how they persist over time (Cryan & Dinan, 2012; Schnabl, 2013).
Figure 1.
Putative mechanisms underlying the symptom experience in chronic liver disease (CLD). Several simultaneously occurring biological mechanisms likely contribute to the development of the CLD symptom cluster. Inflammation is a contributing factor to the development and progression of CLD. The central (CNS), autonomic (ANS), and enteric nervous systems (ENS) respond to stress, whether psychological or physiological (the inflammation). With this response comes the activation of the hypothalamic–pituitary–adrenal (HPA) axis along with further ENS activation with potential for intestinal barrier malfunction and leaky gut. Alterations in the gut microbiota can influence availability of substrates required to produce neurotransmitters in the brain, which is another putative mechanism of co-occurring symptoms. Once developed, the co-occurring symptoms of CLD have a potential bidirectional relationship with the CNS, ANS, and ENS. IFN = interferon; IL = interleukin; TNF = tumor necrosis factor. Adapted from “Mind-altering microorganisms: The impact of the gut microbiota on brain and behavior,” by J. F. Cryan and T. G. Dinan, 2012, Nature Reviews Neuroscience, 13, pp. 701–712. Copyright 2012 by Macmillan Publishers Ltd.
Alterations in the liver–gut–brain axis occur early in CLD, involving inflammatory activation, increased intestinal permeability, microbial translocation, bacterial overgrowth, and changes in the gut microbiota, all of which may contribute to the CLD symptom cluster. Owing to the wide scope of molecular and physiological processes involved in CLD, research to identify the pathogenesis of the CLD symptom cluster will likely require analysis of protein products (proteomics), metabolic profiles (metabolomics), and gene expression (transcriptomics). Although this enterprise may appear daunting, many of the biological samples required to obtain such data are already being collected as a part of the clinical workup of patients with CLD.
Sustained levels of inflammatory mediators locally or in circulation may contribute to the persistence of the CLD symptom cluster. While the liver orchestrates the innate immune response to pathogens, there have been no studies to examine the relationships between the components of innate immunity and the CLD symptom cluster. Cytokine dysregulation and altered levels of circulating immune cell subtypes have been posited as contributors to symptom clusters and can be simultaneously measured in blood samples. Increased intestinal permeability can be evaluated by the pattern of tight junction protein expression and serum level of zonulin (Collins et al., 2012). In addition, small intestine bacterial overflow can be evaluated by measuring levels of breath hydrogen and methane after administration of lactulose or glucose because these gases are produced exclusively by intestinal bacteria (Dupont & Dupont, 2011).
Traditionally, the gut microbiota has been evaluated with stool samples using culture-based techniques, and new developments in “microbial culturomics” now allow identification of previously uncultivated microorganisms from the gut (Lagier et al., 2012). High-throughput DNA sequencing of the gut microbiota allows for more rapid compositional analysis but does not provide direct information on microbial viability or functional potential (Guinane & Cotter, 2013). Regardless of the method used to examine the composition of the gut microbiota, it will also be necessary to examine how the microbiota influences other components of the liver–gut–brain axis.
Biobehavioral research is not only focused on understanding the mechanisms of a disease process and associated symptoms, it is also relevant for developing nursing or nonpharmacologic interventions that may contribute to improved symptom management. Of particular importance to nursing research is the fact that not all patients are vulnerable to experiencing symptom clusters, and the differences between patients are often explained by psychological, social, and/or behavioral factors (Illi et al., 2012; Johansson et al., 2014). Psychological and psychosocial nursing interventions aimed at reducing stress, strengthening self-management skills, and enhancing positive methods of coping have the potential to reduce sympathetic overload and cytokine dysregulation as well as improve quality of life in chronically ill patients. For example, strategies such as mindfulness-based stress reduction meditation programs for cancer survivors have demonstrated decreased levels of stress, reduction in Th1 (pro-inflammatory) cytokines, and enhanced quality of life (Carlson, Speca, Faris, & Patel, 2007). To date, however, there have not been any studies that have examined the influence of such interventions on the symptom experience in individuals with CLD.
Behavioral interventions such as weight loss and exercise can improve physiological aspects of CLD as well as the individual’s level of functioning (Bergasa, Mehlman, & Bir, 2004; Promrat et al., 2010). However, whether or not these interventions affect the symptom experience has yet to be explored. Dietary modifications, including prebiotics and probiotics, can alter the composition of the microbiome with consequential changes in bacterial metabolism (Quigley & Monsour, 2013). These changes can alter the production of short-chain fatty acids and peptidoglycan, which have immune modulatory and regulatory functions, thereby altering the communication between the immune system and brain. Future research should exploit the ability of the microbiota to influence the CLD symptom cluster as well as repair and remodeling of the liver–gut–brain axis (Vajro et al., 2013).
Conclusions
The similarity of the symptom experience among patients with CLD suggests there may be common pathophysiological processes at work, thus raising the possibility of symptom clusters in CLD and the potential for shared management strategies among the various subtypes of the disease. With more individuals living long term with CLD, it is important to understand the biological substrates of the CLD symptom cluster, so that we may begin to develop more targeted and effective symptom management strategies. Recent knowledge gained regarding the liver–gut–brain axis opens new opportunities for nurse-researchers to examine the interdependent and possibly synergistic biological interactions from a systems level. The development of interventions focused on the modulation of the liver–gut–brain axis and the microbiota has potential to positively influence the health of the mind and the body in individuals with CLD as well as other chronic diseases.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the National Institute of Nursing Research (grant #s P30NR011403; R01NR012667 [D. Lyon, PI]; R01NR013932 [A. Starkweather, PI]; NIH/NIAAA R01AA020203; NIH/NIDDK R01DK087913 [J. Bajaj]).
Footnotes
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abbas G, Jorgensen RA, Lindor KD. Fatigue in primary biliary cirrhosis. Nature Reviews Gastroenterology & Hepatology. 2010;7:313–319. doi: 10.1038/nrgastro.2010.62. [DOI] [PubMed] [Google Scholar]
- Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C. γ-aminobuyric acid production by culturable bacteria from the human intestine. Journal of Applied Microbiology. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- Barsevick AM. The elusive concept of the symptom cluster. Oncology Nursing. 2007;34:971–980. doi: 10.1188/07.ONF.971-980. [DOI] [PubMed] [Google Scholar]
- Bell BP, Manos MM, Zaman A, Terrault N, Thomas A, Navarro VJ, Sofair AN. The epidemiology of newly diagnosed chronic liver disease in gastroenterology practices in the United States: Results from population-based surveillance. American Journal of Gastroenterology. 2008;103:2727–2736. doi: 10.1111/j.1572-0241.2008.02071.x. [DOI] [PubMed] [Google Scholar]
- Bergasa NV, Mehlman J, Bir K. Aerobic exercise: A potential therapeutic intervention for patients with liver disease. Medical Hypotheses. 2004;62:935–941. doi: 10.1016/j.mehy.2003.12.041. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Faris P, Patel KD. One year pre–post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction in breast and prostate cancer patients. Brain, Behavior and Immunology. 2007;21:1038–1049. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Chida Y, Sudo N, Kubo C. Does stress exacerbate liver diseases? Journal of Gastroenterology & Hepatology. 2006;21:202–208. doi: 10.1111/j.1440-1746.2006.04110.x. [DOI] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Cryan JF. The microbiome–gut–brain axis during early-life regulates the hippocampal serotonergic system in a gender-dependent manner. Molecular Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nature Review Microbiology. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behavior. Nature Reviews Neuroscience. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterology & Motility. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- David K, Kowdley KV, Unalp A, Kanwal F, Brunt EM, Schwimmer JB NASH CRN Research Group. Quality of life in adults with nonalcoholic fatty liver disease: Baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:1904–1912. doi: 10.1002/hep.22868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCruz S, Espiritu JRD, Zeidler M, Wang TS. Sleep disorders in chronic liver disease. Seminars in Respiratory Critical Care Medicine. 2012;33:26–35. doi: 10.1055/s-0032-1301732. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Science. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello C, Swain MG. Liver-brain inflammation axis. American Journal of Physiology, Gastrointestinal & Liver Physiology. 2011;301:G749–G761. doi: 10.1152/ajpgi.00184.2011. [DOI] [PubMed] [Google Scholar]
- Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, Taylor D. Advancing the science of symptom management. Journal of Advanced Nursing. 2001;33:668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nature Review Gastroenterology and Hepatology. 2011;8:523–531. doi: 10.1038/nrgastro.2011.133. [DOI] [PubMed] [Google Scholar]
- Elliott C, Frith J, Pairman J, Jones DEJ, Newton JL. Reduction in functional ability is significant postliver transplantation compared with matched liver disease and community dwelling controls. Transplant International. 2011;24:588–595. doi: 10.1111/j.1432-2277.2011.01240.x. [DOI] [PubMed] [Google Scholar]
- Elwing JE, Lustman PJ, Wang HL, Clouse RE. Depression, anxiety, and nonalcoholic steatohepatitis. Psychosomatic Medicine. 2006;68:563–569. doi: 10.1097/01.psy.0000221276.17823.df. [DOI] [PubMed] [Google Scholar]
- Fasano A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity and cancer. Physiology Reviews. 2011;91:151–175. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- Gao B, Jeong W, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Therapeutic Advances in Gastroenterology. 2013;6:295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth KG, Jones DEJ, Aribisala BS, Thelwall PE, Taylor R, Newton JL, Blamire AM. Globus pallidus magnetisation transfer ratio, T1 and T2 in primary biliary cirrhosis: Relationship with disease stage and age. Journal of Magnetic Resonance Imaging. 2009;29:780–784. doi: 10.1002/jmri.21555. [DOI] [PubMed] [Google Scholar]
- Hollingsworth KG, Jones DEJ, Taylor R, Frith J, Blamire AM, Newton JL. Impaired cerebral autoregulation in primary biliary cirrhosis—Implications for the pathogenesis of cognitive decline. Liver International. 2010;30:878–885. doi: 10.1111/j.1478-3231.2010.02259.x. [DOI] [PubMed] [Google Scholar]
- Ilan Y. Leaky gut and the liver: A role for bacterial translocation in nonalcoholic steatohepatitis. World Journal of Gastroenterology. 2012;18:2609–2618. doi: 10.3748/wjg.v18.i21.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illi J, Miaskowski C, Cooper B, Levine JD, Dunn L, West C, Aouizerat BE. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance and depression. Cytokine. 2012;58:437–447. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson P, Riegel B, Svensson E, Brostrom A, Alehagen U, Dahlstrom U, Jaarsma T. Sickness behavior in community-dwelling elderly associations with impaired cardiac function and inflammation. Biological Research for Nursing. 2014;16:105–113. doi: 10.1177/1099800412466170. [DOI] [PubMed] [Google Scholar]
- Jones DEJ. Fatigue in cholestatic liver disease: Is it all in the mind? Journal of Hepatology. 2007;46:992–994. doi: 10.1016/j.jhep.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Jurgens CY, Moser DK, Armola R, Carlson B, Sethares K, Riegel B. Symptom clusters of heart failure. Research in Nursing and Health. 2009;32:551–560. doi: 10.1002/nur.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappus M, Bajaj J. Covert hepatic encephalopathy: Not as minimal as you might think. Clinical Gastroenterology & Hepatology. 2012;10:1208–1219. doi: 10.1016/j.cgh.2012.05.026. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology and psychosomatic medicine: Back to the future. Psychosomatic Medicine. 2002;64:15–28. doi: 10.1097/00006842-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Kirkova J, Aktas A, Walsh D, Davis MP. Cancer symptom clusters: Clinical and research methodology. Journal of Palliative Medicine. 2011;14:1149–1165. doi: 10.1089/jpm.2010.0507. [DOI] [PubMed] [Google Scholar]
- Lagier J, Armougom F, Million M, Hugon P, Pagnier I, Robert C, Raoult D. Microbial culturomics: Paradigm shift in the human gut microbiome study. Clinical Microbiology and Infection. 2012;18:1185–1193. doi: 10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- Larson SJ, Dunn AJ. Behavioral effects of cytokines. Brain, Behavior & Immunity. 2001;15:371–387. doi: 10.1006/brbi.2001.0643. [DOI] [PubMed] [Google Scholar]
- Lee K, Otgonsuren M, Younoszai Z, Mir HM, Younossi ZM. Association of chronic liver disease with depression: A population-based study. Psychosomatics. 2013;54:52–59. doi: 10.1016/j.psym.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: Catecholamines and mucosa–bacteria interactions. Cell & Tissue Research. 2011;2431:23–32. doi: 10.1007/s00441-010-1050-0. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Aouizerat BE, Dodd M, Cooper B. Conceptual issues in symptom cluster research and their implications for quality-of-life assessment in patients with cancer. Journal of the National Cancer Institute Monographs. 2007;37:39–46. doi: 10.1093/jncimonographs/lgm003. [DOI] [PubMed] [Google Scholar]
- Miele L, Valenza V, LaTorre G, Montalto M, Cammarota G, Ricci R, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernandez-Real J. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS One. 2012;7:e37160. doi: 10.1371/journal.pone.0037160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G, Gambino R, Cassader M. Gut microbiota as a regulator of energy homeostasis and ectopic fat deposition: Mechanisms and implications for metabolic disorders. Current Opinion in Lipidology. 2010;21:76–83. doi: 10.1097/MOL.0b013e3283347ebb. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Action plan for liver disease research: A report of the Liver disease subcommittee of the digestive diseases interagency coordinating committee. Bethesda, MD: Author; 2009. [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology and Motility. 2010;23:255–264. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- Newton JL. Systemic symptoms in non-alcoholic fatty liver disease. Digestive Disorders. 2010;28:214–219. doi: 10.1159/000282089. [DOI] [PubMed] [Google Scholar]
- Newton JL, Hudson M, Tachtatzis P, Sutcliffe K, Pairman J, Burt JA, Jones DE. Population prevalence and symptom associations of autonomic dysfunction in primary biliary cirrhosis. Hepatology. 2007;45:1496–1505. doi: 10.1002/hep.21609. [DOI] [PubMed] [Google Scholar]
- Newton JL, Jones DEJ. Managing systemic symptoms in chronic liver disease. Journal of Hepatology. 2012;56:S46–S55. doi: 10.1016/S0168-8278(12)60006-3. [DOI] [PubMed] [Google Scholar]
- Newton JL, Wilton K, Pairman J, Jones DEJ, Day CP. Fatigue and autonomic dysfunction in non-alcoholic fatty liver disease. Clinical Autonomic Research. 2009;19:319–326. doi: 10.1007/s10286-009-0031-4. [DOI] [PubMed] [Google Scholar]
- Osztovits J, Horvarth T, Abonyi M, Toth T, Visneyi Z, Beko G, Szalay F. Chronic hepatitis C virus infection associated with autonomic dysfunction. Liver International. 2009;29:1473–1478. doi: 10.1111/j.1478-3231.2009.02075.x. [DOI] [PubMed] [Google Scholar]
- Patanwala I, Newton JL, Elliot C, Frith J, Ghazala C, Pairman J, Jones DE. Functional capacity is significantly impaired in primary biliary cirrhosis and related to orthostatic symptoms. Gut. 2010;59:A19–A20. doi: 10.1097/MEG.0b013e3283470256. [DOI] [PubMed] [Google Scholar]
- Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley EMM, Monsour HP. Targeting the microbiota in the management of gastrointestinal and liver disease. Reviews in Gastroenterology Peru. 2013;33:139–144. [PubMed] [Google Scholar]
- Raison CL, Rye DB, Woolwine BJ, Vogt GJ, Bautista BM, Spivey JR, Miller AH. Chronic interforeon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: Association with fatigue, motor slowing, and increased evening cortisol. Biological Psychiatry. 2010;68:942–949. doi: 10.1016/j.biopsych.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge DN, Mouttapa M, Wood PB. Symptom cluster in fibromyalgia: Potential utility in patient assessment and treatment evaluation. Nursing Research. 2009;58:359–367. doi: 10.1097/NNR.0b013e3181b499d2. [DOI] [PubMed] [Google Scholar]
- Salerno F, Cazzaniga M. Autonomic dysfunction: Often present but usually ignored in patients with liver disease. Liver International. 2009;29:1451–1453. doi: 10.1111/j.1478-3231.2009.02141.x. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Clore JN. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Jiang Z, Evon DM, Wahed AS, Hoofnagle JH. Fatigue before, during and after antiviral therapy of chronic hepatitis C: Results from the Virahep-C study. Journal of Hepatology. 2012;57:946–952. doi: 10.1016/j.jhep.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabl B. Linking intestinal homeostasis and liver disease. Current Opinion in Gastroenterology. 2013;29:264–270. doi: 10.1097/MOG.0b013e32835ff948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: Crosstalk between the liver and gut. Journal of Physiology. 2012;590:447–458. doi: 10.1113/jphysiol.2011.219691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderholm JD, Perdue MH. Stress and the gastrointestinal tract II: Stress and intestinal barrier function. American Journal of Physiology, Gastrointestinal & Liver Physiology. 2001;280:G7–G13. doi: 10.1152/ajpgi.2001.280.1.G7. [DOI] [PubMed] [Google Scholar]
- Stevens S, Allen J, Murray A, Jones DEJ, Newton JL. Microvascular optical assessment confirms the presence of peripheral autonomic dysfunction in primary biliary cirrhosis. Liver International. 2009;29:1467–1472. doi: 10.1111/j.1478-3231.2009.02079.x. [DOI] [PubMed] [Google Scholar]
- Swain M. Stress and the gastrointestinal tract I: Stress and hepatic inflammation. American Journal of Physiology, Gastrointestinal & Liver Physiology. 2000;279:G1135–G1138. doi: 10.1152/ajpgi.2000.279.6.G1135. [DOI] [PubMed] [Google Scholar]
- Swain M. Fatigue in liver disease: Pathophysiology and clinical management. Canadian Journal of Gastroenterology. 2006;20:181–188. doi: 10.1155/2006/624832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tana C, Umesake Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterology & Motility. 2010;22:512–519. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- Tjandra K, Sharkey KA, Swain MG. Progressive development of Th1-type hepatic cytokine profile in rats with experimental cholangitis. Hepatology. 2000;31:280–290. doi: 10.1002/hep.510310204. [DOI] [PubMed] [Google Scholar]
- Vajro P, Paolella G, Fasano A. Microbiota and gut-liver axis: Their influences on obesity and obesity-related liver disease. Journal of Pediatric Gastroenterology and Nutrition. 2013;56:461–468. doi: 10.1097/MPG.0b013e318284abb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vere CC, Streba CT, Streba LM, Ionescu AG, Sima F. Psychosocial stress and liver disease status. World Journal of Gastroenterology. 2009;15:2980–2986. doi: 10.3748/wjg.15.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Alimentary Pharmacology & Therapeutics. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- Weinstein AA, Kallman Price J, Stepanova M, Poms LW, Fang Y, Moon J, Younossi ZM. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127–132. doi: 10.1016/j.psym.2010.12.019. [DOI] [PubMed] [Google Scholar]
- World Gastroenterology Organization. World gastroenterology organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Milwaukee, WI: Author; 2012. [DOI] [PubMed] [Google Scholar]