Key Points
Question
What is the most cost-effective strategy for the initial diagnostic evaluation of patients with asymptomatic microscopic hematuria (AMH)?
Findings
In this cost-effectiveness analysis based on inputs from the medical literature, the combination of cystoscopy and renal ultrasound was most cost-effective with an incremental cost of $53 810 per cancer detected.
Meaning
The combination of cystoscopy and ultrasound should be considered first-line in the evaluation of patients with AMH.
This study analyzes cost-effectiveness strategies for the initial diagnostic evaluation of patients with asymptomatic microscopic hematuria.
Abstract
Importance
Asymptomatic microscopic hematuria (AMH) is highly prevalent and may signal occult genitourinary (GU) malignant abnormality. Common diagnostic approaches differ in their costs and effectiveness in detecting cancer. Given the low prevalence of GU malignant abnormality among patients with AMH, it is important to quantify the cost implications of detecting cancer for each approach.
Objective
To estimate the effectiveness, costs, and incremental cost per cancer detected (ICCD) for 4 common diagnostic approaches evaluating AMH.
Design, Setting, and Participants
A decision-analytic model-based cost-effectiveness analysis using inputs from the medical literature. PubMed searches were performed to identify relevant literature for all key model inputs, each of which was derived from the clinical study with the most robust data and greatest applicability. Analysis included adult patients with AMH on routine urinalysis with subgroups of high-risk patients (males, smokers, age ≥50 years) seen in the primary care or urologic referral setting.
Interventions
Four diagnostic approaches were evaluated relative to the reference case of no evaluation: (1) computed tomography (CT) alone; (2) cystoscopy alone; (3) CT and cystoscopy combined; and (4) renal ultrasound and cystoscopy combined.
Main Outcomes and Measures
At termination of the diagnostic period, cancers detected, costs (payer perspective), and ICCD per 10 000 patients evaluated for AMH.
Results
Of the 4 diagnostic approaches analyzed, CT alone was dominated by all other strategies, detecting 221 cancers at a cost of $9 300 000. Ultrasound and cystoscopy detected 245 cancers and was most cost-effective with an ICCD of $53 810. Replacing ultrasound with CT detected just 1 additional cancer at an ICCD of $6 480 484. Ultrasound and cystoscopy remained the most cost-effective approach in subgroup analysis. The model was not sensitive to any inputs within the proposed ranges. Using probabilistic sensitivity analysis, ultrasound and cystoscopy was the dominant strategy in 100% of simulations.
Conclusions and Relevance
The combination of renal ultrasound and cystoscopy is the most cost-effective among 4 diagnostic approaches for the initial evaluation of AMH. The use of ultrasound in lieu of CT as the first-line diagnostic strategy will optimize cancer detection and reduce costs associated with evaluation of AMH. Given our findings, we need to critically evaluate the appropriateness of our current clinical practices, and potentially alter our guidelines to reflect the most effective screening strategies for patients with AMH.
Introduction
Asymptomatic microscopic hematuria (AMH), the presence of 3 or more red blood cells on urinalysis in the absence of genitourinary (GU) symptoms, is highly prevalent, with population-based studies estimating that up to 40.9% of US adults have this finding on urinalysis. Among potential etiologies for AMH, GU malignant abnormality is of particular concern, with studies showing that 0% to 11% of patients with AMH had malignant abnormalities.
The high prevalence of AMH and its role as a potential harbinger of malignant abnormality confers great importance on the diagnostic algorithm for its evaluation. Many clinicians and policy makers, including the American Urological Association (AUA), have advocated for diagnostic protocols that maximally detect occult malignant neoplasms, because delays in treatment of GU cancer may result in patient anxiety, impaired quality of life, and poor clinical outcomes. Others have sought alternative approaches, predominantly driven by the fact that most evaluations for AMH return negative results for malignant abnormality.
The choice of diagnostic protocol for patients with AMH has broad clinical and economic implications. The AUA-recommended protocol, consisting of computed tomographic (CT) urography and cystoscopy, subjects patients to tests that carry considerable risk of morbidity including procedural discomfort, urinary tract infection (UTI), contrast-induced nephropathy (CIN), and radiation exposure, all of which impact quality of life and generate health care costs. These costs must be weighed against the relatively low risk of malignant abnormality. Despite the economic burden of AMH evaluation, there have been few studies evaluating its cost-effectiveness, which have either focused on the role of screening urinary biomarkers or predated modern imaging. We sought to determine the relative cost per cancer detected among 4 diagnostic protocols for the evaluation of AMH, with particular focus on the radiodiagnostic component, because this is the source of greatest morbidity, cost, and controversy. We hypothesized that the replacement or exclusion of CT from diagnostic protocols would considerably reduce costs with minimal compromise on cancer detection.
Methods
Model Overview
We developed a decision-analytic model to simulate cancer detection rates and costs associated with the evaluation of adult patients with AMH. Diagnostic strategies were selected based on international guidelines and alternative approaches endorsed by experts in the literature. Patients entering the model had 3 or more red blood cells on urinalysis, no history of GU malignant abnormality, and concurrent negative urine culture results to exclude UTI. We assumed the presence or absence of GU cancer for each patient on model entry, and this disease status was static throughout. The model horizon was termination of the diagnostic period with either an accurate diagnosis (radiographic or pathologic) or completion of further diagnostic testing for evaluation of false-positive or incidental findings.
Effectiveness was determined by number of cancers detected with each strategy. We used a composite cancer endpoint, which consisted of lower tract (bladder) urothelial, upper tract urothelial (UTUC), and renal cell cancer (RCC). For model purposes, upper and lower tract cancers were considered mutually exclusive. Although these cancers present in tandem, this is an infrequent occurrence owing to the low incidence of UTUC.
The model was programmed in TreeAge Pro (version 2015, TreeAge Software Inc.).
Diagnostic Strategies
Four diagnostic strategies were evaluated. The first strategy, combination of CT and cystoscopy, is considered the gold standard according to AUA guidelines, employing diagnostic tests with the highest accuracy for detection of upper and lower tract cancers, respectively. The second strategy, combination of renal ultrasound and cystoscopy, meets the recommendations of multiple international guidelines, including the Dutch Guideline on Hematuria and the guideline of the Canadian Urological Association. Furthermore, despite AUA guidelines, many practitioners in the United States use ultrasound in lieu of CT for AMH evaluation. Replacement of CT with ultrasound significantly reduces morbidity through avoidance of radiation and radiographic contrast exposure. Likewise, ultrasound is cheaper and further reduces downstream costs generated by incidental findings on CT, though its sensitivity for cancer detection is inferior.
We compared these strategies to cystoscopy alone and CT alone. Cystoscopy alone has been advocated based on the observation that GU tumors associated with AMH are predominantly located in the lower urinary tract, whereas upper tract tumors comprise just 5% of all urothelial neoplasms. Computed tomographic urography alone has been advocated on the basis of improved sensitivity and specificity of modern CT for detection of lower urinary tract cancers, thereby obviating the need for and sparing the morbidity of cystoscopy. We did not evaluate ultrasound alone owing to the low sensitivity of ultrasound for detection of lower tract malignant abnormalities, which accounts for the majority of GU malignant abnormalities in patients with AMH, thereby rendering ultrasound alone a poor strategy for initial detection of malignant abnormalities in this population.
We did not incorporate voided urine cytology into the above diagnostic strategies because the AUA recommends against its use for evaluation of AMH owing to poor sensitivity and minimal benefit.
All strategies were evaluated relative to the reference case of performing no evaluation.
Clinical Data
Table 1 shows parameter estimates for clinical inputs abstracted from the literature. PubMed searches were performed to identify relevant literature for all key model inputs, including cancer incidence, diagnostic test accuracy, complications, and guideline compliance. For each model input, the clinical study with the most robust data and greatest applicability to the current model was selected as the primary model input. The remaining literature was utilized to generate ranges for sensitivity analysis. Cancer incidence among adult patients with AMH in the United States were obtained from Loo et al, and incidence among high-risk groups (men, smokers, aged ≥50 years) were derived from the same cohort. Ranges for incidence among high-risk subgroups were obtained by inflating the incidence of cancer in the general population using relative risk of cancer among high-risk groups according to recent meta-analyses and population-based studies.
Table 1. Base Case Inputs and Ranges Used in Sensitivity Analysis.
| Variable | Incidence Rates (Range) | References |
|---|---|---|
| Cancer Incidence | ||
| Lower tract | 0.023 (0.000-0.081) | Loo et al, 2013; Golin et al, 1980; Messing et al, 2006 |
| Upper tracta | 0.002 (0.000-0.031) | Loo et al, 2013; Feifer et al, 2010; Lisanti et al, 2014; Lang et al, 2002 |
| Men | ||
| Lower tract | 0.039 (0.000-0.109) | Loo et al, 2013; Jung et al, 2011 |
| Upper tracta | 0.004 (0.000-0.031) | Loo et al, 2013; Jung et al, 2011; Lang et al, 2002 |
| Smokers | ||
| Lower tract | 0.034 (0.000-0.064) | Loo et al, 2013; Cumberbach et al, 2015 |
| Upper tracta | 0.004 (0.000-0.031) | Cumberbach et al, 2015; Lang et al, 2002 |
| Age ≥50 y | ||
| Lower tract | 0.031 (0.000-0.385) | Loo et al, 2013; Jung et al, 2011 |
| Upper tracta | 0.003 (0.000-0.042) | Loo et al, 2013; Jung et al, 2011 |
| Ratio of RCC to UCC | 12:2 | Khadra et al, 2000 |
| Computed Tomography | Helenius et al 2015; Knox et al, 2008; Lisanti et al, 2014; Takeuchi et al, 2015; Razavi et al, 2012; Sadow et al, 2010; Wang et al, 2009; Sudakoff, 2008; Cowan et al, 2007; Helenius et al, 2016; Sadow et al, 2008; Wang et al, 2010 | |
| Lower tract | ||
| Sensitivity | 0.950 (0.590-0.950) | Blick et al, 2012; Sudakoff et al, 2008; Helenius et al, 2016 |
| Specificity | 0.830 (0.830-0.990) | Blick et al, 2012; Helenius et al, 2015 |
| Upper tract | ||
| Sensitivity | 0.960 (0.818-0.970) | Razavi et al, 2012; Sudakoff et al, 2008; Cowan et al, 2007; Chlapoutakis et al, 2010 |
| Specificity | 0.990 (0.930-0.998) | Razavi et al, 2012; Wang et al, 2009; Sudakoff et al, 2008; Chlapoutakis et al, 2010 |
| Cystoscopy | Blick et al, 2012; Helenius et al, 2015; Schmidbauer et al, 2009 | |
| Lower tract | ||
| Sensitivity | 0.980 (0.870-0.980) | Blick et al, 2012; Helenius, et al 2015 |
| Specificity | 0.940 (0.940-1.000) | Blick et al, 2012; Helenius, et al 2015 |
| Renal Ultrasound | Jaffe et al, 2001; Knox et al, 2008; Khadra et al, 2000; Razavi et al, 2012; Datta et al, 2002; Yip et al, 1999; Aslaksen et al, 1990; Unsal et al, 2011 | |
| Upper tract | ||
| Sensitivity | 0.910 (0.560-1.000) | Datta et al, 2002; Aslaksen et al, 1990; Unsal et al, 2011; Speelman et al, 1996 |
| Specificity | 0.990 (0.940-0.990) | Khadra et al, 2000; Aslaksen et al, 1990; Unsal et al, 2011; Speelman et al, 1996 |
| Incidence of CIN following CT | 0.040 (0.000-0.190) | Silver et al, 2015; Marenzi et al, 2004; Golshahi et al, 2014 |
| Incidence of UTI following cystoscopy | 0.019 (0.000-0.030) | Herr, 2015 |
Abbreviations: CIN, contrast-induced nephropathy; RCC, renal cell carcinoma; UCC, urothelial cell carcinoma; UTI, urinary tract infection.
Includes both renal cell carcinoma and urothelial cell carcinoma of ureter and renal pelvis.
Sensitivity and specificity of tests for upper and lower tract cancers were obtained from the literature. For strategies using multiple tests, we assumed no synergy between tests evaluating the same portion of the GU tract. For example, the sensitivity and specificity of the strategy combining CT and cystoscopy for detection of lower tract cancer was assumed equal to that of cystoscopy, which demonstrates higher accuracy.
Diagnosis of malignant abnormality was achieved with the first radiographic or pathologic evidence of disease. For radiographic diagnoses, morbidity and costs associated with confirmatory pathological diagnosis were considered beyond the diagnostic window and thus excluded. False-positive findings on initial evaluation resulted in scenario-dependent confirmatory testing. Upper tract cancer on initial evaluation resulted in confirmatory testing consisting of ureteroscopy, CT, or magnetic resonance imaging (MRI) depending on the initial diagnostic modality. False-positive findings for lower tract cancer resulted in confirmatory testing consisting of cystoscopy and biopsy. False-negative findings (ie, undetected cancers) necessarily impacted the effectiveness of each strategy.
Complications and downstream consequences of each diagnostic evaluation were incorporated into the model. Cystoscopic interventions carried a 1.9% risk of febrile UTI, which required repeat urinalysis and urine culture, 1 additional clinic visit, and treatment with oral antibiotics.
Cost Data
Cost inputs are presented in Table 2. All costs were evaluated from the payer perspective and updated to 2016 US dollars ($) using the medical care component of the Consumer Price Index. Discounting was not used because the model time horizon was less than 1 year. National average nonfacility costs associated with diagnostic tests were obtained from the Medicare Physician Fee Schedule (MPFS) and the Medicare Clinical Laboratory Fee Schedule (MCLS). Cost ranges for sensitivity analyses were obtained from the minimum and maximum Medicare reimbursement across all Medicare Administrative Contractor regions. Costs associated with pharmacologic treatment were obtained from the Red Book. Additional costs secondary to incidental findings on CT or complications, such as CIN, were abstracted from the literature.
Table 2. Cost Inputs and Ranges Used in Sensitivity Analysis.
| Variable | CPT/HCPCS Code | Cost (Range), $a | Referencesb |
|---|---|---|---|
| CT | |||
| Urography | 74178 | 356 (270-467) | MPFS |
| Renal | 74176 | 202 (159-257) | MPFS |
| MRI renal protocol | 74183 | 510 (381-678) | MPFS |
| Renal ultrasound | 76770 | 115 (88-150) | MPFS |
| Cystoscopy | 52000 | 208 (166-258) | MPFS |
| Cystoscopy + biopsy | 52204 | 373 (286-480) | MPFS |
| Ureteroscopy | 52351 | 313 (272-418) | MPFS |
| Ureteroscopy + biopsy | 52354 | 430 (375-576) | MPFS |
| Biopsy pathology review | 88305 | 74 (60-94) | MPFS |
| Incidental findings on CT | NA | 409 (51-409) | Morgan et al, 2015; Liu et al, 2005 |
| CIN after CT | NA | 12 975 (6225-16 031) | Subramanian et al, 2007 |
| Clinic visit for UTI | 99212 | 48 (35-55) | MPFS |
| Urinalysis and Urine culture | 81001, 87086 | 15 (9-21) | MCLFS |
| Antibiotics for UTI | NA | 8 (3-19) | Red Book |
Abbreviations: CIN, contrast-induced nephropathy; CPT, common procedural terminology; CT, computed tomography; HCPCS, Healthcare Common Procedure Coding System; MCLFS, Medicare Clinical Laboratory Fee Schedule 2016; MPFS, Medicare Physician Fee Schedule 2016; MRI, magnetic resonance imaging; UTI, urinary tract infection.
All prices reported in 2016 US dollars.
MPFS, Medicare Physician Fee Schedule 2016 at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PFSLookup/index.html?redirect=/pfslookup/; MCLFS, Medicare Clinical Laboratory Fee Schedule 2016 at https://www.cms.gov/medicare/medicare-fee-for-service-payment/clinicallabfeesched/clinlab.html.
Cost-effectiveness Analysis
Owing to the low prevalence of malignant abnormality associated with AMH, cost and effectiveness outcomes were scaled to a rate per 10 000 patients to optimize the possibility of detecting differences between strategies. Incremental analyses were performed by rank ordering strategies with increasing effectiveness relative to the reference strategy. Strategies that were dominated (ie, more costly and less effective) were removed, and an incremental cost per cancer detected (ICCD) was calculated for each strategy. Repeated analyses were performed for each of 3 subpopulations with unique risk of malignant abnormality (men, smokers, and age ≥50 years). We used a willingness-to-pay threshold of $100 000 per cancer detected and performed sensitivity analysis using thresholds of $50 000, $150 000, and $200 000.
Additional sensitivity analyses were performed for all key variables to assess model stability. Probabilistic sensitivity analyses were performed using a triangular distribution with parameters determined by the aforementioned ranges. Additional head-to-head sensitivity analyses were performed for the 2 most optimal strategies according to ICCDs.
National Expenditures
The annual national expenditures secondary to AMH evaluation were estimated for the 2 guideline-endorsed strategies. We used the National Ambulatory Medical Care Survey (NAMCS) to determine the annual number of urologist visits with a diagnosis of AMH in 2012 (485 222). Additional national costs associated with use of CT in lieu of ultrasound were determined by factoring the number of visits by the cost difference between these 2 approaches, assuming 100% compliance with guidelines. Sensitivity analysis was performed using published and abstracted rates of guideline compliance.
Results
Base Case
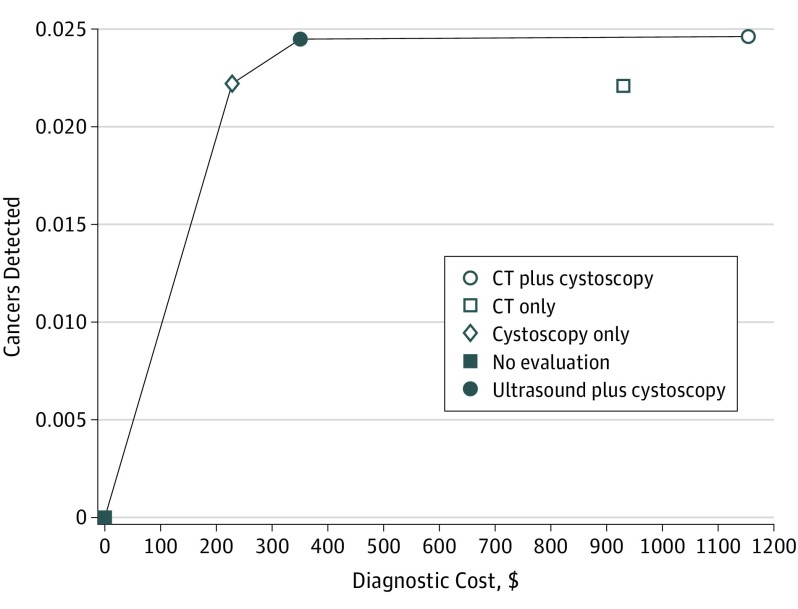
Compared with no evaluation, CT alone detected the fewest additional cancers, 221 per 10 000 patients (Table 3). At a cost of $9 300 000 per 10 000 patients, the CT-alone strategy was dominated by all others (Figure). Cystoscopy alone detected 222 cancers at an ICCD of $10 287 compared with no evaluation. Addition of ultrasound resulted in the detection of 23 additional cancers at an ICCD of $53 810 compared with cystoscopy alone. Replacing ultrasound with CT detected just 1 additional cancer at an ICCD of $6 480 484, far exceeding the willingness-to-pay threshold.
Table 3. Incremental Cost per Cancer Detected (ICCD) for Diagnostic Strategies.
| Strategy | Cancers Detecteda | Cost, $a | Δ Cancers | Δ Cost, $ | ICCD, $ |
|---|---|---|---|---|---|
| No evaluation | 0 | 0 | NA | NA | NA |
| CT only | 221 | 9 300 000 | 221 | 9 300 000 | Dominated |
| Cystoscopy only | 222 | 2 284 000 | 222 | 2 284 000 | 10 287 |
| Renal ultrasound + cystoscopy | 245 | 3 504 400 | 23 | 1 220 400 | 53 810 |
| CT + cystoscopy | 246 | 11 540 200 | 1 | 8 035 800 | 6 480 484 |
Abbreviations: CT, computed tomography; ICCD, Incremental cost per cancer detected; NA, not applicable.
Cancers detected and costs are reported as rate per 10 000 patients. Costs are reported in 2016 US dollars.
Figure. Cancers Detected and Costs of Diagnostic Protocols for Evaluation of Asymptomatic Microscopic Hematuria.
CT indicates computed tomography.
On 1-way sensitivity analysis, the model was stable with variation of all inputs across the proposed ranges. A tornado diagram of inputs to which the ICCD was most sensitive in a head-to-head comparison of ultrasound and cystoscopy vs CT and cystoscopy is presented in the eFigure in the Supplement. Ultrasound and cystoscopy was the optimal strategy across all 1-way sensitivity analyses.
Our results were stable throughout a probabilistic sensitivity analysis using 1000 Monte Carlo simulations. The strategy of ultrasound and cystoscopy was optimal in 100% of simulations.
Subgroup Analysis
Among high-risk groups, all strategies detected a greater number of cancers compared with the reference case (eTable in the Supplement). For men, smokers, and those aged 50 years or older, CT alone detected 382, 332, and 302 cancers per 10 000 patients, respectively. However, owing to high diagnostic costs, CT alone was dominated across all 3 groups. Cystoscopy alone detected 384, 334, and 303 cancers at ICCDs of $6047, $6918, and $7594, respectively, compared with no evaluation. The addition of ultrasound resulted in the detection of 39, 34, and 31 additional cancers for each high-risk group compared with cystoscopy alone. Replacing ultrasound with CT detected just 2 additional cancers in each group. The ICCDs for this strategy were more favorable (lower) than that of the base case but remained well above the willingness-to-pay threshold at $3 720 417, $4 297 326, and $4 727 059, respectively.
Our results were stable throughout a probabilistic sensitivity analysis using 1000 Monte Carlo simulations. The strategy of ultrasound and cystoscopy was optimal in 100% of simulations.
National Expenditures
In 2012, the diagnosis of microscopic hematuria was associated with 2.69% of patient visits to urologists, accounting for 485 222 visits. Assuming 100% urologist compliance with guidelines, use of CT instead of ultrasound would detect 60.2 additional cancers nationally at an incremental cost of $389 914 648 (Table 4). In the setting of imperfect compliance, use of CT instead of ultrasound would detect anywhere from 3 to 38 additional cancers nationally at an incremental cost ranging from $19 495 732 to $245 646 228.
Table 4. Projected Annual Additional National Costs and Cancers Detected With Use of Computed Tomography vs Ultrasound for Evaluation of Asymptomatic Microscopic Hematuria According to Rate of Compliance With Recommended Evaluation.
| Compliance, % | Additional Costs, $ | Additional Cancers Detected | Cohort | Reference |
|---|---|---|---|---|
| 100 | 389 914 648 | 60 | Theoretical | NA |
| 63 | 245 646 228 | 38 | Patients who underwent CT evaluation | Loo et al, 2013 |
| 49 | 191 058 177 | 30 | Patients who underwent any imaging | Buteau et al, 2014 |
| 36 | 140 369 273 | 22 | Patients who underwent upper and lower tract evaluation | Shinagare et al, 2014 |
| 5 | 19 495 732 | 3 | Patients who underwent upper and lower tract evaluation | Buteau et al, 2014 |
Abbreviations: CT, computed tomography; NA, not applicable.
Discussion
While routine urinalysis for screening of GU malignant abnormalities is not presently recommended by any major health organization, hundreds of thousands of patients annually undergo urinalysis for various indications and are found to have microscopic hematuria prompting further evaluation. We found that the combination of renal ultrasound and cystoscopy was the most cost-effective approach for the evaluation of AMH. The superiority of this approach over the use of CT and cystoscopy is driven primarily by higher costs of CT and its associated complications, albeit rare. These costs were accompanied by minimal gains in cancer detection because ultrasound technology nearly reaches the sensitivity of CT for the detection of upper tract malignant abnormalities. Given the low prevalence of upper tract malignant abnormalities in patients with AMH, the small advantage in the sensitivity of CT imaging modalities does not compensate for the significant additional costs. Likewise, CT and cystoscopy was not a cost-effective first-line approach among patients with higher risk of malignant abnormality, because the absolute risk of malignant abnormality in this group remains low.
While ultrasound should be considered first-line, we urge clinicians to incorporate individualized patient-care and shared decision-making in the pursuit of follow-up CT or MRI. Though guidelines target optimal population-wide policy, patient preferences and risk factors must be considered on an individualized basis. Guidelines for the diagnosis and treatment of other GU malignant abnormalities, most notably prostate cancer, include shared decision-making as a central tenet. Evaluation of AMH should follow this paradigm, because risk tolerance for CT-associated complications or uncertainty with regard to occult malignant abnormalities may vary. Furthermore, each clinical scenario may entail unique considerations or risk factors that have not been incorporated into the current model, such as family history of malignant abnormality, high number of RBCs on urinalysis, or presence of multiple risk factors.
In the wake of the Affordable Care Act, the landscape of US health care has changed dramatically. Accountable care organizations (ACOs), along with other policy initiatives, continue to emphasize high-value and patient-centered care across all medical disciplines. In particular, diagnostic radiology has been recognized as an area ripe for transformation through stewardship and paradigm shifts. Likewise, while surgeons and surgical care have been largely excluded from initial ACO models, recent authors have recognized that the integration of surgical care is paramount. The prevalent condition of AMH sits at the crossroads of these 2 disciplines and offers an opportunity for the provision of high-value, individualized patient care.
Implementation of ultrasound-based guidelines will substantially reduce national expenditures associated with AMH evaluation by up to $390 million. Although these reductions are rough estimates and do not account for the costs associated with delayed diagnosis of cancers that would have otherwise been detected by CT, they do represent a potential for large economic savings. In addition, the recommendation of ultrasound in lieu of CT may have the unintended but desirable consequence of improving compliance with hematuria evaluation. Studies have demonstrated poor rates of urologic referral and compliance with hematuria evaluation among patients presenting to primary care physicians (PCPs). Prior authors have speculated that these low rates result from the unwillingness of PCPs to subject their patients to morbid evaluations, and the replacement of CT with ultrasound could therefore improve referral rates. Likewise, inclusion of PCPs in the development of future guidelines may help to ensure higher compliance, ultimately resulting in greater cancer detection.
Strengths and Limitations
Our study has a number of strengths. This is the first study to comprehensively model the effectiveness and costs of AMH evaluation in the era of modern imaging. Our findings are strengthened by the robust data inputs derived from extensive literature surrounding the incidence of cancer and accuracy of CT in AMH evaluation. Furthermore, stability of the model across all clinically determined ranges reinforces the findings and provides strong evidence for changing clinical practice.
However, our results must be interpreted in the context of the study design. First, the study is limited by the short model horizon, which prevented modeling the downstream effect of missed cancers on stage at presentation, life expectancy, quality of life, and costs. We chose the diagnostic period based on the lack of data on delayed diagnosis of bladder cancer, UTUC, and RCC. Second, owing to the heterogeneity of GU malignant abnormalities and the paucity of literature examining the impact of early detection on quality-adjusted life-years (QALY), we used the primary outcome of cancers detected in lieu of QALY as a measure of effectiveness. Although it is optimal for contextualizing population-based effects of AMH evaluation, this approach did not differentiate effectiveness and costs among patients with distinct types of GU cancers, nor did it model downstream costs of delayed cancer diagnosis. Whereas some studies have demonstrated poor outcomes among patients with delayed diagnosis of urothelial cancer, others have found that those with asymptomatic and symptomatic presentations had equivalent oncologic prognoses. Third, limited data existed on accuracy of ultrasound for UTUC diagnosis. Though sensitivity analyses compensated for these data, further studies are needed to better determine the utility of ultrasound in this setting. Fourth, owing to limitations of NAMCS, estimates of national expenditures presume evaluation of all patients with microscopic hematuria, including symptomatic patients, which may inflate this estimate. Furthermore, we included only visits to urologists, which likely excluded a considerable number of patients who may undergo AMH evaluation by other providers (eg, gynecologists). This portion of the analysis is intended as an estimate for illustrative purposes and may not precisely capture national cost savings associated with distinct diagnostic strategies, which would require more robust analysis with cost-driven data inputs. Fifth, the time horizon for our analysis could not account for the potential costs and morbidity associated with radiation from CT. On a population level over time, the incidence of secondary malignant abnormalities owing to radiation exposure would be substantial. As such, an ultrasound-based protocol would not only prove less costly in the long term, it would likely reduce overall morbidity secondary to radiation, thereby strengthening the case for ultrasound-based protocols from a policy perspective. Sixth, incidence of CIN was abstracted from a review evaluating percutaneous coronary intervention, which likely overestimates incidence for CT owing to lower contrast requirement. However, this estimation was accounted for in sensitivity analysis and did not change model outcomes. Finally, MR urography was not evaluated as an initial diagnostic evaluation owing to high costs and inadequate access. However, MRI offers the advantage of high sensitivity without radiation exposure and may be optimal for specific patients.
Conclusions
The combination of ultrasound and cystoscopy is the most cost-effective among 4 diagnostic approaches for the initial evaluation of AMH. The use of ultrasound in lieu of CT as the first-line diagnostic strategy will reduce the cost, morbidity, and national expenditures associated with evaluation of AMH. Clinicians and policy makers should consider changing future guidelines in accordance with this finding.
eTable. Incremental Cost per Cancer Detected (ICCD) For Diagnostic Strategies Among Subgroups
eFigure. One-Way Sensitivity Analysis
References
- 1.Mohr DN, Offord KP, Melton LJ III. Isolated asymptomatic microhematuria: a cross-sectional analysis of test-positive and test-negative patients. J Gen Intern Med. 1987;2(5):318-324. [DOI] [PubMed] [Google Scholar]
- 2.Loo RK, Lieberman SF, Slezak JM, et al. Stratifying risk of urinary tract malignant tumors in patients with asymptomatic microscopic hematuria. Mayo Clin Proc. 2013;88(2):129-138. [DOI] [PubMed] [Google Scholar]
- 3.Mohr DN, Offord KP, Owen RA, Melton LJ III. Asymptomatic microhematuria and urologic disease: a population-based study. JAMA. 1986;256(2):224-229. [PubMed] [Google Scholar]
- 4.Carson CC III, Segura JW, Greene LF. Clinical importance of microhematuria. JAMA. 1979;241(2):149-150. [PubMed] [Google Scholar]
- 5.Golin AL, Howard RS. Asymptomatic microscopic hematuria. J Urol. 1980;124(3):389-391. [DOI] [PubMed] [Google Scholar]
- 6.Bard RH. The significance of asymptomatic microhematuria in women and its economic implications: a ten-year study. Arch Intern Med. 1988;148(12):2629-2632. [PubMed] [Google Scholar]
- 7.Bourgade V, Drouin SJ, Yates DR, et al. Impact of the length of time between diagnosis and surgical removal of urologic neoplasms on survival. World J Urol. 2014;32(2):475-479. doi: 10.1007/s00345-013-1045-z [DOI] [PubMed] [Google Scholar]
- 8.Hollenbeck BK, Dunn RL, Ye Z, et al. Delays in diagnosis and bladder cancer mortality. Cancer. 2010;116(22):5235-5242. doi: 10.1002/cncr.25310 [DOI] [PubMed] [Google Scholar]
- 9.Nielsen M, Qaseem A; High Value Care Task Force of the American College of Physicians . Hematuria as a marker of occult urinary tract cancer: advice for high-value care from the American College of Physicians. Ann Intern Med. 2016;164(7):488-497. [DOI] [PubMed] [Google Scholar]
- 10.Seklehner S, Remzi M, Fajkovic H, et al. Prospective multi-institutional study analyzing pain perception of flexible and rigid cystoscopy in men. Urology. 2015;85(4):737-741. [DOI] [PubMed] [Google Scholar]
- 11.Herr HW. The risk of urinary tract infection after flexible cystoscopy in patients with bladder tumor who did not receive prophylactic antibiotics. J Urol. 2015;193(2):548-551. [DOI] [PubMed] [Google Scholar]
- 12.Silver SA, Shah PM, Chertow GM, Harel S, Wald R, Harel Z. Risk prediction models for contrast induced nephropathy: systematic review. BMJ. 2015;351:h4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian S, Tumlin J, Bapat B, Zyczynski T. Economic burden of contrast-induced nephropathy: implications for prevention strategies. J Med Econ. 2007;10(2):119-134. [DOI] [PubMed] [Google Scholar]
- 14.Morgan AE, Berland LL, Ananyev SS, Lockhart ME, Kolettis PN. Extraurinary incidental findings on CT for hematuria: the radiologist’s role and downstream cost analysis. AJR Am J Roentgenol. 2015;204(6):1160-1167. [DOI] [PubMed] [Google Scholar]
- 15.Heller MT, Tublin ME. In search of a consensus: evaluation of the patient with hematuria in an era of cost containment. AJR Am J Roentgenol. 2014;202(6):1179-1186. [DOI] [PubMed] [Google Scholar]
- 16.Jung H, Gleason JM, Loo RK, Patel HS, Slezak JM, Jacobsen SJ. Association of hematuria on microscopic urinalysis and risk of urinary tract cancer. J Urol. 2011;185(5):1698-1703. [DOI] [PubMed] [Google Scholar]
- 17.Lotan Y, Svatek RS, Sagalowsky AI. Should we screen for bladder cancer in a high-risk population? a cost per life-year saved analysis. Cancer. 2006;107(5):982-990. [DOI] [PubMed] [Google Scholar]
- 18.Novicki DE, Stern JA, Nemec R, Lidner TK. Cost-effective evaluation of indeterminate urinary cytology. J Urol. 1998;160(3 pt 1):734-736. [DOI] [PubMed] [Google Scholar]
- 19.Rodgers M, Nixon J, Hempel S, et al. Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation. Health Technol Assess. 2006;10(18):iii-iv, xi-259. [DOI] [PubMed] [Google Scholar]
- 20.Feifer AH, Steinberg J, Tanguay S, Aprikian AG, Brimo F, Kassouf W. Utility of urine cytology in the workup of asymptomatic microscopic hematuria in low-risk patients. Urology. 2010;75(6):1278-1282. [DOI] [PubMed] [Google Scholar]
- 21.Mowatt G, Zhu S, Kilonzo M, et al. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol Assess. 2010;14(4):1-331, iii-iv. [DOI] [PubMed] [Google Scholar]
- 22.Corwin HL, Silverstein MD. The diagnosis of neoplasia in patients with asymptomatic microscopic hematuria: a decision analysis. J Urol. 1988;139(5):1002-1006. [DOI] [PubMed] [Google Scholar]
- 23.Davis R, Jones JS, Barocas DA, et al. ; American Urological Association . Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. 2012;188(6)(suppl):2473-2481. [DOI] [PubMed] [Google Scholar]
- 24.Blick CG, Nazir SA, Mallett S, et al. Evaluation of diagnostic strategies for bladder cancer using computed tomography (CT) urography, flexible cystoscopy and voided urine cytology: results for 778 patients from a hospital haematuria clinic. BJU Int. 2012;110(1):84-94. [DOI] [PubMed] [Google Scholar]
- 25.Wollin T, Laroche B, Psooy K. Canadian guidelines for the management of asymptomatic microscopic hematuria in adults. Can Urol Assoc J. 2009;3(1):77-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaffe JS, Ginsberg PC, Gill R, Harkaway RC. A new diagnostic algorithm for the evaluation of microscopic hematuria. Urology. 2001;57(5):889-894. [DOI] [PubMed] [Google Scholar]
- 27.Edwards TJ, Dickinson AJ, Natale S, Gosling J, McGrath JS. A prospective analysis of the diagnostic yield resulting from the attendance of 4020 patients at a protocol-driven haematuria clinic. BJU Int. 2006;97(2):301-305. [DOI] [PubMed] [Google Scholar]
- 28.Genega EM, Porter CR. Urothelial neoplasms of the kidney and ureter: an epidemiologic, pathologic, and clinical review. Am J Clin Pathol. 2002;117(suppl):S36-S48. [DOI] [PubMed] [Google Scholar]
- 29.Hong SK, Ahn C, Kim HH. The value of cystoscopy as an initial diagnostic modality for asymptomatic microscopic hematuria. J Korean Med Sci. 2001;16(3):309-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldstein MS, Hentz JG, Gillett MD, Novicki DE. Should the upper tracts be imaged for microscopic haematuria? BJU Int. 2005;96(4):612-617. [DOI] [PubMed] [Google Scholar]
- 31.Helenius M, Brekkan E, Dahlman P, Lönnemark M, Magnusson A. Bladder cancer detection in patients with gross haematuria: computed tomography urography with enhancement-triggered scan versus flexible cystoscopy. Scand J Urol. 2015;49(5):377-381. [DOI] [PubMed] [Google Scholar]
- 32.Capalbo E, Kluzer A, Peli M, et al. Bladder cancer diagnosis: the role of CT urography. Tumori. 2015;101(4):412-417. [DOI] [PubMed] [Google Scholar]
- 33.Knox MK, Cowan NC, Rivers-Bowerman MD, Turney BW. Evaluation of multidetector computed tomography urography and ultrasonography for diagnosing bladder cancer. Clin Radiol. 2008;63(12):1317-1325. [DOI] [PubMed] [Google Scholar]
- 34.Rhéaume-Lanoie J, Lepanto L, Fradet V, Billiard JS, Tang A. Diagnostic performance of ultrasound for macroscopic hematuria in the era of multidetector computed tomography urography. Can Assoc Radiol J. 2014;65(3):253-259. [DOI] [PubMed] [Google Scholar]
- 35.Messing EM, Madeb R, Young T, et al. Long-term outcome of hematuria home screening for bladder cancer in men. Cancer. 2006;107(9):2173-2179. [DOI] [PubMed] [Google Scholar]
- 36.Lisanti CJ, Toffoli TJ, Stringer MT, DeWitt RM, Schwope RB. CT evaluation of the upper urinary tract in adults younger than 50 years with asymptomatic microscopic hematuria: is IV contrast enhancement needed? AJR Am J Roentgenol. 2014;203(3):615-619. [DOI] [PubMed] [Google Scholar]
- 37.Lang EK, Macchia RJ, Thomas R, et al. Computerized tomography tailored for the assessment of microscopic hematuria. J Urol. 2002;167(2 pt 1):547-554. [DOI] [PubMed] [Google Scholar]
- 38.Cumberbatch MG, Rota M, Catto JW, La Vecchia C. The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol. 2016;70(3):458-466. [DOI] [PubMed] [Google Scholar]
- 39.Khadra MH, Pickard RS, Charlton M, Powell PH, Neal DE. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol. 2000;163(2):524-527. [PubMed] [Google Scholar]
- 40.Takeuchi M, Konrad AJ, Kawashima A, Boorjian SA, Takahashi N. CT urography for diagnosis of upper urinary tract urothelial carcinoma: are both nephrographic and excretory phases necessary? AJR Am J Roentgenol. 2015;205(3):W320-W327. [DOI] [PubMed] [Google Scholar]
- 41.Razavi SA, Sadigh G, Kelly AM, Cronin P. Comparative effectiveness of imaging modalities for the diagnosis of upper and lower urinary tract malignancy: a critically appraised topic. Acad Radiol. 2012;19(9):1134-1140. [DOI] [PubMed] [Google Scholar]
- 42.Sadow CA, Wheeler SC, Kim J, Ohno-Machado L, Silverman SG. Positive predictive value of CT urography in the evaluation of upper tract urothelial cancer. AJR Am J Roentgenol. 2010;195(5):W337-43. [DOI] [PubMed] [Google Scholar]
- 43.Wang LJ, Wong YC, Chuang CK, Huang CC, Pang ST. Diagnostic accuracy of transitional cell carcinoma on multidetector computerized tomography urography in patients with gross hematuria. J Urol. 2009;181(2):524-531. [DOI] [PubMed] [Google Scholar]
- 44.Sudakoff GS, Dunn DP, Guralnick ML, Hellman RS, Eastwood D, See WA. Multidetector computerized tomography urography as the primary imaging modality for detecting urinary tract neoplasms in patients with asymptomatic hematuria. J Urol. 2008;179(3):862-867. [DOI] [PubMed] [Google Scholar]
- 45.Cowan NC, Turney BW, Taylor NJ, McCarthy CL, Crew JP. Multidetector computed tomography urography for diagnosing upper urinary tract urothelial tumour. BJU Int. 2007;99(6):1363-1370. [DOI] [PubMed] [Google Scholar]
- 46.Helenius M, Dahlman P, Lonnemark M, Brekkan E, Wernroth L, Magnusson A. Comparison of post contrast CT urography phases in bladder cancer detection. Eur Radiol. 2016;26(2):585-591. [DOI] [PubMed] [Google Scholar]
- 47.Sadow CA, Silverman SG, O’Leary MP, Signorovitch JE. Bladder cancer detection with CT urography in an Academic Medical Center. Radiology. 2008;249(1):195-202. [DOI] [PubMed] [Google Scholar]
- 48.Wang LJ, Wong YC, Huang CC, et al. Multidetector computerized tomography urography is more accurate than excretory urography for diagnosing transitional cell carcinoma of the upper urinary tract in adults with hematuria. J Urol. 2010;183(1):48-55. [DOI] [PubMed] [Google Scholar]
- 49.Chlapoutakis K, Theocharopoulos N, Yarmenitis S, Damilakis J. Performance of computed tomographic urography in diagnosis of upper urinary tract urothelial carcinoma, in patients presenting with hematuria: systematic review and meta-analysis. Eur J Radiol. 2010;73(2):334-338. [DOI] [PubMed] [Google Scholar]
- 50.Schmidbauer J, Remzi M, Klatte T, et al. Fluorescence cystoscopy with high-resolution optical coherence tomography imaging as an adjunct reduces false-positive findings in the diagnosis of urothelial carcinoma of the bladder. Eur Urol. 2009;56(6):914-919. [DOI] [PubMed] [Google Scholar]
- 51.Datta SN, Allen GM, Evans R, Vaughton KC, Lucas MG. Urinary tract ultrasonography in the evaluation of haematuria–a report of over 1,000 cases. Ann R Coll Surg Engl. 2002;84(3):203-205. [PMC free article] [PubMed] [Google Scholar]
- 52.Yip SK, Peh WC, Tam PC, Li JH, Lam CH. Role of ultrasonography in screening for urological malignancies in patients presenting with painless haematuria. Ann Acad Med Singapore. 1999;28(2):174-177. [PubMed] [Google Scholar]
- 53.Aslaksen A, Halvorsen OJ, Göthlin JH. Detection of renal and renal pelvic tumours with urography and ultrasonography. Eur J Radiol. 1990;11(1):54-58. [DOI] [PubMed] [Google Scholar]
- 54.Unsal A, Calişkan EK, Erol H, Karaman CZ. The diagnostic efficiency of ultrasound guided imaging algorithm in evaluation of patients with hematuria. Eur J Radiol. 2011;79(1):7-11. [DOI] [PubMed] [Google Scholar]
- 55.Speelman HR, Kessels AG, Bongaerts AH, et al. Haematuria: intravenous urography, ultrasound or both? Rofo. 1996;165(6):524-528. [DOI] [PubMed] [Google Scholar]
- 56.Marenzi G, Lauri G, Assanelli E, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44(9):1780-1785. [DOI] [PubMed] [Google Scholar]
- 57.Golshahi J, Nasri H, Gharipour M. Contrast-induced nephropathy: a literature review. J Nephropathol. 2014;3(2):51-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu W, Mortelé KJ, Silverman SG. Incidental extraurinary findings at MDCT urography in patients with hematuria: prevalence and impact on imaging costs. AJR Am J Roentgenol. 2005;185(4):1051-1056. [DOI] [PubMed] [Google Scholar]
- 59.Red Book Online. Truven Health Analytics, Inc. 2016. http://micromedex.com/products/product-suites/clinical-knowledge/redbook. Accessed March 29, 2016.
- 60.Consumer Price Index Tables. http://www.bls.gov/cpi/cpid1602.pdf2016. Accessed March 29. 2016.
- 61.Medicare Physician Fee Schedule. https://http://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed November 20, 2015.
- 62.Centers for Medicare and Medicaid Services Medicare Clinical Laboratory Fee Schedule 2016. https://www.cms.gov/medicare/medicare-fee-for-service-payment/clinicallabfeesched/. Accessed March 18, 2016.
- 63.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796-797. [DOI] [PubMed] [Google Scholar]
- 64.Buteau A, Seideman CA, Svatek RS, et al. What is evaluation of hematuria by primary care physicians? Use of electronic medical records to assess practice patterns with intermediate follow-up. Urol Oncol. 2014;32(2):128-134. [DOI] [PubMed] [Google Scholar]
- 65.Shinagare AB, Silverman SG, Gershanik EF, Chang SL, Khorasani R. Evaluating hematuria: impact of guideline adherence on urologic cancer diagnosis. Am J Med. 2014;127(7):625-632. [DOI] [PubMed] [Google Scholar]
- 66.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Durand DJ, Lewin JS, Berkowitz SA. Medical-imaging stewardship in the accountable care era. N Engl J Med. 2015;373(18):1691-1693. [DOI] [PubMed] [Google Scholar]
- 68.Seltzer SE, Lee TH. The transformation of diagnostic radiology in the ACO era. JAMA. 2014;312(3):227-228. [DOI] [PubMed] [Google Scholar]
- 69.Mukherji SK. The potential impact of accountable care organizations with respect to cost and quality with special attention to imaging. J Am Coll Radiol. 2014;11(4):391-396. [DOI] [PubMed] [Google Scholar]
- 70.Dupree JM, Patel K, Singer SJ, et al. Attention to surgeons and surgical care is largely missing from early Medicare accountable care organizations. Health Aff (Millwood). 2014;33(6):972-979. [DOI] [PubMed] [Google Scholar]
- 71.McCarthy M. Physicians show strong leadership in US accountable care organizations but surgeons are largely left out. BMJ. 2014;348:g3939. [DOI] [PubMed] [Google Scholar]
- 72.Raman JD, Shariat SF, Karakiewicz PI, et al. ; Upper-Tract Urothelial Carcinoma Collaborative Group . Does preoperative symptom classification impact prognosis in patients with clinically localized upper-tract urothelial carcinoma managed by radical nephroureterectomy? Urol Oncol. 2011;29(6):716-723. [DOI] [PubMed] [Google Scholar]
- 73.Ramirez D, Gupta A, Canter D, et al. Microscopic haematuria at time of diagnosis is associated with lower disease stage in patients with newly diagnosed bladder cancer. BJU Int. 2016;117(5):783-786 [DOI] [PubMed] [Google Scholar]
- 74.Chang ML, Hou JK. Cancer risk related to gastrointestinal diagnostic radiation exposure. Curr Gastroenterol Rep. 2011;13(5):449-457. [DOI] [PubMed] [Google Scholar]
- 75.Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175-184. [DOI] [PubMed] [Google Scholar]
- 76.Lee KS, Zeikus E, DeWolf WC, Rofsky NM, Pedrosa I. MR urography versus retrograde pyelography/ureteroscopy for the exclusion of upper urinary tract malignancy. Clin Radiol. 2010;65(3):185-192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Incremental Cost per Cancer Detected (ICCD) For Diagnostic Strategies Among Subgroups
eFigure. One-Way Sensitivity Analysis