Abstract
Background
In 2011, the Advisory Committee for Immunization Practices (ACIP) recommended routine use HPV vaccine for male adolescents.
Methods
We used the 2013 National Immunization Survey-Teen (NIS-Teen) data to assess HPV vaccine uptake (≥1 dose) and series completion (≥3 doses). Multivariable logistic regression analysis and a predictive marginal model were conducted to identify independent predictors of vaccination among adolescent males aged 13–17 years.
Results
HPV vaccination coverage with ≥1 dose was 34.6% while series completion (≥3 doses) was 13.9%. Coverage was significantly higher among non-Hispanic blacks and Hispanics compared with non-Hispanic white males. Multivariable logistic regression showed that characteristics independently associated with a higher likelihood of HPV vaccination (≥1 dose) included: being non-Hispanic black race or Hispanic ethnicity, having mothers who were widowed, divorced, or separated, having 1–3 physician contacts in the past 12 months, a well-child visit at age 11–12 years, having one or two vaccination providers, living in urban or suburban areas, and receiving vaccinations from more than one type of facility (p<0.05). Having mothers with some college or college education, having a higher family income to poverty ratio, living in South or Midwest, and receiving vaccinations from all STD/school/teen clinics or other facilities were independently associated with a lower likelihood of HPV vaccination (p<0.05).
Conclusions
Following recommendations for routine HPV vaccination among male adolescents, uptake in 2013 was low in males. Increased efforts are needed to improve vaccination coverage, especially for those who are least likely to be vaccinated.
Introduction
Human papillomavirus (HPV) is the most common sexually transmitted infection in men and women in the United States (1, 6). More than 50% of sexually active men and women will acquire HPV infection in their lifetime (2). One recent study showed that approximately 79 million persons are currently infected with HPV, and 14 million persons are newly infected each year in the United States (4). Most infections cause no symptoms, but persistent infection may result in disease or cancer. Each year in the United States, an estimated 26,000 new cancers are attributable to HPV, about 17,000 in women and 9,000 in men (4, 5). HPV-related cancers in males include many anal, penile, and oropharyngeal cancers (6–10). HPV-associated oropharyngeal and anal cancers have increased among males (6, 9–11).
Vaccination is an important tool to prevent and control HPV infection and its complications (1). In 2006, quadrivalent HPV vaccine (HPV4) was licensed by the Food and Drug Administration (FDA) for use in females (1). In 2009, the Advisory Committee on Immunization Practices (ACIP) voted and provided guidance that the HPV4 vaccine may be given to males 9 through 26 years, noting that men who have sex with men (MSM) are particularly at risk for conditions associated with HPV infection (12, 13). In 2011, ACIP recommended routine use of HPV4 among males aged 11 or 12 years and recommended HPV4 for males aged 13–21 years who have not been vaccinated previously or who have not completed the 3-dose series (6). Previous study showed that early uptake of HPV vaccination of male adolescents was 1.4% in 2010, and 8.3% in 2011 (14).
We analyzed data from the 2013 National Immunization Survey-Teen (NIS-Teen) to evaluate HPV vaccination uptake, reasons for not receiving vaccination, and to identify factors independently associated with HPV vaccination among male adolescents aged 13–17 years. The results from this study may inform relevant interventions to increase vaccination coverage.
Methods
We analyzed data from the National Immunization Survey-Teen (NIS-Teen), a national, random-digit–dial (RDD) telephone survey sponsored by the Centers for Disease Control and Prevention (CDC). The objective of the NIS-Teen is to provide timely, detailed information regarding vaccination among adolescents aged 13–17 years for vaccines recommended by the ACIP, including HPV vaccine, and to evaluate factors associated with vaccination. Data are collected in the NIS-Teen in two-phases. In the first phase, a RDD household interview is conducted to identify households with age-eligible adolescents and to collect demographic information from the parent or guardian on adolescent, maternal, and household characteristics. Also, the household interview included questions on the teen’s reported vaccination history, reasons for no intent of receiving vaccine among those who have not received HPV vaccination, and access to care. After completing the household interview, consent is requested to contact the immunization provider(s) to collect provider-reported immunization records. If consent is obtained, adolescent’s vaccination providers are mailed a questionnaire to collect provider-reported vaccination histories of each recommended adolescent vaccine as well as any childhood vaccines. Regarding HPV vaccine, date of vaccination and type of HPV vaccines (HPV2, HPV4) from the provider record were collected. Among male adolescents 13–17 years, those who were reported by provider that they received unknown type of vaccine or HPV2 vaccine (0.67%, n=64) were considered receiving HPV4 vaccination since HPV4 is the only HPV vaccine that is recommended for male adolescents (6).
In 2013, the NIS-Teen sampling plan included independent samples of households with a land line and households with a cell phone (15, 16). In 2013, the Council of American Survey Research Organizations (CASRO) landline response rate was 51.1%. A total of 18,246 adolescents with provider-reported vaccination records were included, representing 59.5% of all adolescents from the landline sample with completed household interviews. The cellular-telephone sample CASRO response rate was 23.3%. A total of 12,225 adolescents with provider-reported vaccination records were included, representing 54.5% of all adolescents from the cellular-telephone sample with completed household interviews (15, 16). Our analysis was restricted to male adolescents aged 13–17 years.
Covariates were selected from survey questions to measure associations of HPV vaccination with age group, race/ethnicity, mother’s educational level, mother’s marital status, mother’s age, U.S. born status, poverty level, type of medical insurance, number of physician contacts within past 12 months, provider reported health-care visit at age 11–12 years, number of provider reported vaccination providers, facility type (public, private, hospital, STD/school/teen clinics, mixed [including facilities in more than one category such as private, public, hospital, STD/school/teen clinics], and others [such as military, WIC clinics, and pharmacies]), metropolitan statistical area (MSA), and U.S. region. There were missing values for some of the variables listed above, and the proportions of missing value were small and ranged from 0.1% to 1.3%.
SUDAAN 11.0.1 (Software for the statistical analysis of complex sampling data, Research Triangle Institute, Research Triangle Park, NC) was used to calculate point estimates and 95% confidence intervals (CIs) adjusted for the complex sample design of the NIS-Teen. All analyses account for the complex sampling plan of the NIS-Teen and the survey sampling weights (16). T-tests were used to examine associations with the significance level set at α<0.05. Multivariable logistic regression and predictive marginal models were conducted to derive the adjusted prevalence ratio (PR). The models were checked for multi-collinearity. All variables selected were included in the model. Additionally, Kaplan-Meier survival analysis was conducted to estimate the cumulative proportion of adolescents vaccinated over time by birth cohort. The NIS-Teen was approved by the Centers for Disease Control and Prevention, National Center for Health Statistics Research Ethics Review Board.
Results
The 2013 NIS-Teen included a total of 9,554 male adolescents aged 13–17 years with sufficiently detailed provider data. Table 1 shows the demographic characteristics of the study population. Most were non-Hispanic white (54.3%), had mothers with more than a high school education (61.8%), had mothers who are currently married (66.7%), were born in the U.S. (95.3%), were living in a household with an income >133% of the federal poverty level (66.0%), had one vaccination provider (50.7%), had at least one physician contact within the past year (82.0%), and had all private reported vaccination providers (52.2%).
TABLE 1.
Sample characteristics of male adolescents aged 13–17 years in the United States, by demographic and access-to-care variables--NIS-Teen 2013
| Characteristic | Sample | %* |
|---|---|---|
| Total | 9,554 | |
| Age (years) | ||
| 13–15 | 5,819 | 61.0 (59.1–62.8) |
| 16–17 | 3,735 | 39.0 (37.2–40.9) |
| Race/ethnicity | ||
| Non-Hispanic White | 6,300 | 54.3 (52.4–56.2) |
| Non-Hispanic Black | 840 | 14.1 (12.8–15.6) |
| Hispanic | 1,461 | 22.8 (21.0–24.7) |
| American Indian/Alaskan Native | 147 | 0.9 (0.7–1.3) |
| Asian | 278 | 3.1 (2.5–3.8) |
| Other | 528 | 4.7 (4.1–5.4) |
| Mother’s educational level | ||
| <High School | 1,054 | 13.9 (12.5–15.5) |
| High School | 1,695 | 24.3 (22.5–26.1) |
| Some college or college graduate | 2,631 | 25.6 (24.0–27.1) |
| >College graduate | 4,174 | 36.2 (34.5–38.0) |
| Mother’s married status | ||
| Married | 6,919 | 66.7 (64.9–68.5) |
| Widowed/divorced/separated | 1,809 | 22.8 (21.2–24.4) |
| Never married | 750 | 10.5 (9.3–11.9) |
| Mother’s age | ||
| ≤34 years | 834 | 9.6 (8.6–10.7) |
| 35–44 years | 4,043 | 45.2 (43.3–47.1) |
| ≥45 years | 4,677 | 45.2 (43.3–47.0) |
| Immigration status | ||
| Born in U.S. | 9,206 | 95.3 (94.1–96.2) |
| Born outside U.S. | 323 | 4.7 (3.8–5.9) |
| Income to poverty ratio | ||
| <1.33% | 2,366 | 34.0 (32.1–36.0) |
| 1.33% – <3.22% | 2,856 | 30.3 (28.6–32.1) |
| 3.22% – <5.03% | 2,118 | 17.1 (15.9–18.4) |
| >5.03% | 2,214 | 18.5 (17.3–19.9) |
| Medical insurance† | ||
| Private insurance only | 4,609 | 42.0 (40.3–43.9) |
| VFC Eligible-Medicaid/IHS/AIAN(All) | 3,827 | 45.3 (43.4–47.2) |
| VFC Eligible-Uninsured | 469 | 6.0 (5.1–7.0) |
| SCHIP(Public) | 269 | 2.9 (2.4–3.5) |
| Military | 314 | 2.9 (2.4–3.5) |
| Other | 66 | 0.9 (0.5–1.8) |
| Physician contacts within past year | ||
| None | 1,468 | 18.0 (16.5–19.6) |
| 1 | 2,777 | 28.6 (26.9–30.3) |
| 2–3 | 3,365 | 34.7 (32.9–36.6) |
| ≥4 | 1,902 | 18.7 (17.3–20.1) |
| Well child visit at age 11–12 years‡ | ||
| Yes | 3,318 | 34.3 (32.5–36.1) |
| No | 1,829 | 16.9 (15.7–18.2) |
| Not licensed§ | 2,182 | 22.7 (21.2–24.3) |
| Don’t know | 2,225 | 26.2 (24.4–28.0) |
| Number of providers | ||
| 1 | 4,757 | 50.7 (48.9–52.6) |
| 2 | 2,842 | 28.9 (27.2–30.6) |
| ≥3 | 1,943 | 20.4 (18.8–22.0) |
| Metropolitan statistical area (MSA) | ||
| Urban area | 3,636 | 38.9 (37.1–40.8) |
| Suburban area | 3,743 | 47.7 (45.8–49.6) |
| Rural area | 2,175 | 13.4 (12.5–14.4) |
| Region | ||
| Northeast | 1,975 | 16.9 (16.0–17.8) |
| Midwest | 2,174 | 21.7 (20.7–22.7) |
| South | 3,132 | 37.5 (36.0–39.0) |
| West | 2,273 | 24.0 (22.3–25.7) |
| Facility type | ||
| All private facilities | 4,470 | 52.2 (50.3–54.1) |
| All public facilities | 1,398 | 14.8 (13.4–16.3) |
| All hospital facilities | 993 | 9.0 (8.0–10.2) |
| All STD/school/teen clinics or other facilities | 160 | 1.8 (1.4–2.3) |
| Mixed|| | 2,343 | 21.0 (19.6–22.5) |
| Other¶ | 141 | 1.2 (0.9–1.6) |
Percentages are weighted.
Insurance categories are mutually exclusive.
Status of health-care visit at age 11–12 years based on provider reported data.
This indicated that the adolescents were ≥13 years of age when HPV vaccination was recommended in 2006.
Mixed indicates that the facility is identified to be in more than one of the facility categories such as private, public, hospital, STD/school/teen clinics.
Includes military, WIC clinics, and pharmacies.
HPV vaccination uptake (≥1 dose)
Overall, HPV vaccination coverage with ≥1 dose was 34.6%, and coverage (≥1 dose) was significantly higher among non-Hispanic blacks (42.2%) and Hispanics (49.6%) compared with non-Hispanic whites (26.7%) (p<0.05) (Table 2).
TABLE 2.
Human papillomavirus (HPV) vaccination coverage (≥ 1 dose) among male adolescents aged 13–17 years in the United States, by demographic and access-to-care characteristics--NIS-Teen 2013
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
|
| ||||
| Vaccination coverage (≥ 1 dose) | Vaccination with ≥1 dose, Prevalence Ratio (PR) | Vaccination coverage (≥ 1 dose) | Vaccination with ≥1 dose, Prevalence Ratio (PR) | |
|
| ||||
| Characteristic | % (95% CI) | PR (95% CI) | % (95% CI) | PR (95% CI) |
| Total | 34.6 (32.7–36.5) | |||
| Age (years) | ||||
| 13–15* | 34.9 (32.5–37.5) | Ref | 33.2 (30.7–35.8) | Ref |
| 16–17 | 34.1 (31.3–37.0) | 0.98 (0.87–1.09) | 37.1 (33.6–40.7) | 1.12 (0.98–1.28) |
| Race/ethnicity | ||||
| Non-Hispanic White* | 26.7 (24.9–28.6) | Ref | 29.3 (27.1–31.6) | Ref |
| Non-Hispanic Black | 42.2 (36.8–47.8)† | 1.58 (1.36–1.83)† | 41.4 (35.9–47.1)† | 1.41 (1.21–1.65)† |
| Hispanic | 49.6 (44.5–54.8)† | 1.86 (1.64–2.11)† | 43.9 (39.2–48.8)† | 1.50 (1.30–1.72)† |
| American Indian/Alaskan Native | 38.6 (25.8–53.2) | 1.44 (1.00–2.09) | 41.2 (25.7–58.7) | 1.41 (0.92–2.15) |
| Asian | 26.3 (18.5–36.1) | 0.99 (0.70–1.39) | 24.6 (17.0–34.3) | 0.84 (0.59–1.20) |
| Other | 34.4 (27.8–41.7)† | 1.29 (1.04–1.60)† | 34.1 (27.6–41.3) | 1.16 (0.94–1.44) |
| Mother’s educational level | ||||
| <High School* | 47.0 (41.1–53.0) | Ref | 36.1 (30.8–41.8) | Ref |
| High School | 36.7 (32.3–41.4)† | 0.78 (0.65–0.93)† | 33.9 (30.1–37.9) | 0.94 (0.78–1.13) |
| Some college or college graduate | 27.9 (24.7–31.3)† | 0.59 (0.50–0.71)† | 29.4 (26.3–32.7)† | 0.81 (0.67–0.99)† |
| >College graduate | 33.1 (30.5–35.8)† | 0.70 (0.61–0.82)† | 38.5 (35.2–41.8) | 1.06 (0.88–1.29) |
| Mother’s married status | ||||
| Married* | 31.4 (29.3–33.7) | Ref | 33.0 (30.7–35.4) | Ref |
| Widowed/divorced/separated | 38.8 (34.8–43.0)† | 1.24 (1.09–1.40)† | 38.7 (34.9–42.7)† | 1.17 (1.04–1.33)† |
| Never married | 44.3 (37.9–51.0)† | 1.41 (1.20–1.66)† | 36.1 (30.2–42.4) | 1.09 (0.90–1.32) |
| Mother’s age | ||||
| ≤34 years* | 40.4 (35.0–46.0) | Ref | 38.4 (33.1–44.0) | Ref |
| 35–44 years | 35.3 (32.3–38.3) | 0.87 (0.74–1.03) | 34.6 (31.9–37.3) | 0.90 (0.77–1.06) |
| ≥45 years | 32.7 (30.1–35.5)† | 0.81 (0.69–0.95)† | 34.0 (31.3–36.9) | 0.89 (0.75–1.05) |
| Immigration status | ||||
| Born in U.S.* | 33.9 (32.0–35.8) | Ref | 34.2 (32.3–36.2) | Ref |
| Born outside U.S. | 48.6 (37.5–59.9)† | 1.43 (1.13–1.82)† | 43.5 (34.2–53.3) | 1.27 (1.01–1.60) |
| Income to poverty ratio | ||||
| <133%* | 44.2 (40.5–48.0) | Ref | 41.5 (37.3–45.7) | Ref |
| 133% – <322% | 29.8 (26.4–33.4)† | 0.67 (0.58–0.78)† | 31.0 (27.8–34.3)† | 0.75 (0.64–0.87)† |
| 322% – <503% | 25.9 (22.8–29.3)† | 0.59 (0.50–0.68)† | 28.2 (24.5–32.2)† | 0.68 (0.56–0.82)† |
| >503% | 32.8 (29.4–36.5)† | 0.74 (0.65–0.85)† | 33.9 (29.9–38.1)† | 0.82 (0.68–0.98)† |
| Medical insurance | ||||
| Private insurance only* | 29.6 (27.2–32.2) | Ref | 33.9 (30.9–37.1) | Ref |
| VFC Eligible-Medicaid/IHS/AIAN(All) | 39.5 (36.4–42.6)† | 1.33 (1.19–1.50)† | 35.8 (33.0–38.8) | 1.06 (0.93–1.20) |
| VFC Eligible-Uninsured | 32.8 (25.2–41.4) | 1.11 (0.85–1.44) | 31.7 (24.5–39.9) | 0.94 (0.72–1.22) |
| SCHIP(Public) | 42.0 (32.5–52.1)† | 1.42 (1.10–1.82)† | 40.4 (31.3–50.2) | 1.19 (0.92–1.53) |
| Military | 28.6 (20.9–37.8) | 0.96 (0.71–1.31) | 33.4 (25.0–42.9) | 0.98 (0.74–1.30) |
| Other | --‡ | --‡ | --‡ | --‡ |
| Physician contacts within past year | ||||
| None* | 28.7 (24.3–33.6) | Ref | 28.0 (24.0–32.4) | Ref |
| 1 | 33.9 (30.6–37.4) | 1.18 (0.98–1.43) | 34.1 (31.0–37.4)† | 1.22 (1.02–1.45)† |
| 2–3 | 39.6 (36.2–43.0)† | 1.38 (1.15–1.65)† | 39.7 (36.7–42.8)† | 1.42 (1.20–1.68)† |
| ≥4 | 32.5 (29.0–36.2) | 1.13 (0.93–1.38) | 32.7 (29.1–36.5) | 1.17 (0.97–1.41) |
| Well-child visit at age 11–12 years | ||||
| Yes | 39.9 (36.6–43.2)† | 1.55 (1.32–1.83)† | 39.8 (36.6–43.2)† | 1.40 (1.20–1.64)† |
| No* | 25.7 (22.2–29.5) | Ref | 28.4 (24.6–32.6) | Ref |
| Not Liciensed§ | 32.4 (28.9–36.1)† | 1.26 (1.05–1.51)† | 31.4 (27.1–36.0) | 1.10 (0.90–1.36) |
| Don’t know | 35.4 (31.3–39.7)† | 1.38 (1.15–1.66)† | 34.7 (31.0–38.6)† | 1.22 (1.03–1.45)† |
| Number of providers | ||||
| 1 | 35.6 (33.0–38.3)† | 1.19 (1.01–1.39)† | 36.4 (33.7–39.2)† | 1.23 (1.04–1.45)† |
| 2 | 36.2 (32.6–39.8)† | 1.20 (1.01–1.43)† | 35.2 (32.1–38.5)† | 1.18 (1.01–1.39)† |
| ≥3* | 30.0 (26.0–34.4) | Ref | 29.7 (25.7–34.1) | Ref |
| Metropolitan statistical area (MSA) | ||||
| Urban area | 40.1 (37.0–43.2)† | 1.73 (1.49–2.02)† | 37.9 (35.1–40.8)† | 1.40 (1.20–1.62)† |
| Suburban area | 33.4 (30.5–36.3)† | 1.44 (1.23–1.69)† | 33.9 (31.1–36.9)† | 1.25 (1.08–1.45)† |
| Rural area* | 23.1 (20.2–26.3) | Ref | 27.2 (23.7–30.9) | Ref |
| Region | ||||
| Northeast | 42.2 (38.8–45.6) | 1.00 (0.86–1.17) | 42.2 (38.8–45.8) | 1.06 (0.91–1.22) |
| Midwest | 27.8 (25.1–30.6)† | 0.66 (0.56–0.78)† | 31.1 (28.2–34.2)† | 0.78 (0.67–0.90)† |
| South | 30.4 (27.8–33.2)† | 0.72 (0.62–0.85)† | 29.8 (27.2–32.5)† | 0.75 (0.64–0.86)† |
| West* | 42.0 (36.7–47.5) | Ref | 40.0 (35.3–44.8) | Ref |
| Facility type | ||||
| All private facilities* | 34.4 (31.9–37.1) | Ref | 33.4 (30.8–36.0) | Ref |
| All public facilities | 33.1 (27.9–38.8) | 0.96 (0.80–1.15) | 31.5 (26.7–36.7) | 0.94 (0.79–1.13) |
| All hospital facilities | 38.5 (32.3–45.1) | 1.12 (0.93–1.34) | 37.3 (31.6–43.3) | 1.12 (0.94–1.32) |
| All STD/school/teen clinics or other facilities | 15.8 (9.6–25.1)† | 0.46 (0.28–0.75)† | 13.6 (7.8–22.7)† | 0.41 (0.24–0.70)† |
| Mixed|| | 36.9 (33.0–41.0) | 1.07 (0.94–1.22) | 41.3 (37.2–45.5)† | 1.24 (1.09–1.41)† |
| Other¶ | 28.4 (16.6–44.0) | 0.82 (0.50–1.35) | 43.0 (27.1–60.4) | 1.29 (0.86–1.94) |
Reference level.
p value < 0.05 by t test for comparisons within each variable with the indicated reference level.
Estimate may not be reliable due to relative standard error > 30% or sample size < 30.
This indicated that the adolescents were ≥13 years of age when HPV vaccination was recommended in 2006.
Mixed indicates that the facility is identified to be in more than one of the facility categories such as private, public, hospital, STD/school/teen clinics.
Includes military, WIC clinics, and pharmacies.
In bivariable analyses, among male adolescents, other characteristics that were significantly associated with a higher level of HPV vaccination coverage compared with the referent group included: mother of adolescent being never married, widowed, divorced, or separated, being born outside the United States, being VFC eligible but not uninsured or with SCHIP insurance (the state children’s health insurance), having 2 or 3 physician contacts in the past 12 months, having a well-child visit at age 11–12 years, having one or two vaccination providers, and living in urban or suburban areas (p<0.05). Those whose mothers had high school or more than high school education, those whose mother’s age was ≥45 years, those with an income to poverty ratio >133%, those living in the Midwest or South, and those receiving vaccinations from all STD/school/teen clinics or other facilities were characteristics associated with a lower level of HPV vaccination (Table 2).
HPV vaccination uptake (≥3 doses)
Overall, HPV vaccination uptake (≥3 doses) was 13.9%. Coverage (≥3 doses) was significantly higher among non-Hispanic blacks (15.7%) and Hispanics (20.3%) compared with non-Hispanic whites (11.1%) (p<0.05) (Table 3).
TABLE 3.
Human papillomavirus (HPV) vaccination coverage (≥3 dose) among male adolescents aged 13–17 years in the United States, by demographic and access-to-care characteristics--NIS-Teen 2013
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
|
| ||||
| Vaccination coverage (≥3 dose) | Vaccination with ≥3 dose, Prevalence Ratio (PR) | Vaccination coverage (≥ 3 dose) | Vaccination with ≥3 dose, Prevalence Ratio (PR) | |
|
| ||||
| Characteristic | % (95% CI) | PR (95% CI) | % (95% CI) | PR (95% CI) |
| Total | 13.9 (12.5–15.3) | |||
| Age (years) | ||||
| 13–15* | 13.5 (11.8–15.5) | Ref | 13.3 (11.5–15.5) | Ref |
| 16–17 | 14.4 (12.3–16.7) | 1.06 (0.86–1.30) | 15.1 (12.4–18.2) | 1.13 (0.86–1.48) |
| Race/ethnicity | ||||
| Non-Hispanic White* | 11.1 (9.9–12.6) | Ref | 11.9 (10.4–13.5) | Ref |
| Non-Hispanic Black | 15.7 (12.3–19.9)† | 1.41 (1.08–1.85)† | 15.9 (12.3–20.4)† | 1.34 (1.01–1.77)† |
| Hispanic | 20.3 (16.2–25.1)† | 1.82 (1.41–2.34)† | 18.5 (14.8–22.8)† | 1.56 (1.19–2.04)† |
| American Indian/Alaskan Native | --‡ | --‡ | --‡ | --‡ |
| Asian | 9.1 (5.5–14.7) | 0.82 (0.49–1.36) | 7.7 (4.6–12.5) | 0.65 (0.39–1.08) |
| Other | 12.2 (8.6–16.8) | 1.09 (0.77–1.56) | 12.9 (9.2–17.7) | 1.08 (0.76–1.55) |
| Mother’s educational level | ||||
| <High School* | 20.9 (16.3–26.2) | Ref | 17.6 (13.4–22.8) | Ref |
| High School | 13.8 (10.7–17.5)† | 0.66 (0.47–0.93)† | 14.4 (11.3–18.1) | 0.81 (0.58–1.15) |
| Some college or college graduate | 11.1 (8.8–14.0)† | 0.53 (0.38–0.74)† | 12.3 (9.8–15.4) | 0.70 (0.48–1.01) |
| >College graduate | 13.2 (11.5–15.0)† | 0.63 (0.48–0.83)† | 13.3 (11.2–15.6) | 0.75 (0.53–1.06) |
| Mother’s married status | ||||
| Married* | 13.3 (11.7–15.1) | Ref | 13.8 (12.1–15.7) | Ref |
| Widowed/divorced/separated | 15.5 (12.4–19.2) | 1.16 (0.90–1.50) | 15.6 (12.5–19.4) | 1.13 (0.87–1.47) |
| Never married | 14.2 (10.8–18.4) | 1.06 (0.79–1.42) | 11.9 (8.8–15.8) | 0.86 (0.62–1.20) |
| Mother’s age | ||||
| ≤34 years* | 14.2 (10.7–18.7) | Ref | 13.7 (10.3–17.9) | Ref |
| 35–44 years | 12.6 (10.7–14.7) | 0.88 (0.64–1.22) | 12.5 (10.8–14.5) | 0.91 (0.67–1.24) |
| ≥45 years | 15.1 (13.0–17.4) | 1.06 (0.77–1.46) | 15.6 (13.3–18.3) | 1.14 (0.83–1.57) |
| Immigration status | ||||
| Born in U.S.* | 13.4 (12.1–14.9) | Ref | 13.6 (12.3–15.1) | Ref |
| Born outside U.S. | 22.2 (13.5–34.3) | 1.65 (1.02–2.68) | 21.7 (13.4–33.3) | 1.60 (1.00–2.56) |
| Income to poverty ratio | ||||
| <133%* | 17.5 (14.7–20.6) | Ref | 16.6 (13.1–20.8) | Ref |
| 133% – <322% | 11.1 (8.8–13.9)† | 0.64 (0.48–0.84)† | 12.1 (9.7–15.0) | 0.73 (0.51–1.03) |
| 322% – <503% | 10.2 (8.4–12.5)† | 0.59 (0.45–0.76)† | 10.7 (8.4–13.5)† | 0.64 (0.44–0.95)† |
| >503% | 15.1 (12.6–18.1) | 0.87 (0.68–1.11) | 14.9 (11.9–18.7) | 0.90 (0.61–1.32) |
| Medical insurance | ||||
| Private insurance only* | 13.2 (11.3–15.3) | Ref | 15.2 (12.4–18.4) | Ref |
| VFC Eligible-Medicaid/IHS/AIAN(All) | 15.3 (13.1–17.9) | 1.16 (0.94–1.44) | 14.0 (11.8–16.5) | 0.92 (0.69–1.23) |
| VFC Eligible-Uninsured | 6.3 (4.0–9.9)† | 0.48 (0.29–0.77)† | 5.5 (3.3–9.3)† | 0.37 (0.20–0.66)† |
| SCHIP(Public) | 19.6 (12.6–29.1) | 1.49 (0.95–2.32) | 19.2 (12.8–27.9) | 1.27 (0.82–1.96) |
| Military | 10.4 (6.4–16.4) | 0.79 (0.48–1.29) | 13.0 (7.8–20.8) | 0.85 (0.51–1.42) |
| Other | --‡ | --‡ | --‡ | --‡ |
| Physician contacts within past year | ||||
| None* | 10.9 (8.0–14.9) | Ref | 11.3 (8.6–14.7) | Ref |
| 1 | 13.9 (11.4–16.9) | 1.27 (0.88–1.84) | 14.1 (11.7–16.9) | 1.25 (0.90–1.72) |
| 2–3 | 15.4 (13.0–18.1) | 1.40 (0.98–2.01) | 15.4 (13.1–18.1) | 1.36 (1.00–1.85) |
| ≥4 | 14.0 (11.8–16.5) | 1.28 (0.89–1.83) | 13.8 (11.5–16.4) | 1.22 (0.88–1.67) |
| Well-child visit at age 11–12 years | ||||
| Yes | 16.8 (14.3–19.5)† | 2.50 (1.82–3.44)† | 17.0 (14.5–19.8)† | 2.18 (1.58–3.02)† |
| No* | 6.7 (5.1–8.8) | Ref | 7.8 (5.8–10.3) | Ref |
| Not Liciensed§ | 14.3 (11.9–17.1)† | 2.14 (1.53–2.98)† | 13.4 (10.4–17.0)† | 1.72 (1.16–2.54)† |
| Don’t know | 14.3 (11.3–17.9)† | 2.13 (1.48–3.06)† | 14.2 (11.3–17.8)† | 1.83 (1.28–2.60)† |
| Number of providers | ||||
| 1 | 14.5 (12.6–16.7) | 1.26 (0.97–1.63) | 14.5 (12.5–16.7) | 1.17 (0.87–1.57) |
| 2 | 14.3 (11.8–17.3) | 1.24 (0.93–1.66) | 14.3 (11.8–17.1) | 1.15 (0.86–1.54) |
| ≥3* | 11.6 (9.3–14.3) | Ref | 12.4 (9.7–15.7) | Ref |
| MSA | ||||
| Urban area | 16.9 (14.7–19.4)† | 2.13 (1.68–2.71)† | 16.2 (14.2–18.5)† | 1.62 (1.27–2.08)† |
| Suburban area | 13.0 (11.0–15.4)† | 1.64 (1.26–2.12)† | 13.1 (11.0–15.5)† | 1.31 (1.01–1.71)† |
| Rural area* | 8.0 (6.5–9.6) | Ref | 10.0 (8.0–12.4) | Ref |
| Region | ||||
| Northeast | 18.5 (15.9–21.3) | 1.27 (0.91–1.76) | 17.5 (14.9–20.4) | 1.29 (0.92–1.81) |
| Midwest | 11.7 (9.8–13.9) | 0.80 (0.57–1.13) | 13.3 (11.1–15.8) | 0.98 (0.71–1.34) |
| South | 12.6 (10.8–14.7) | 0.87 (0.62–1.21) | 13.0 (11.1–15.0) | 0.96 (0.71–1.30) |
| West* | 14.6 (10.8–19.4) | Ref | 13.6 (10.2–17.8) | Ref |
| Facility type | ||||
| All private facilities* | 14.6 (12.6–16.8) | Ref | 13.6 (11.7–15.8) | Ref |
| All public facilities | 12.2 (8.8–16.8) | 0.84 (0.59–1.20) | 12.5 (9.2–16.9) | 0.92 (0.65–1.31) |
| All hospital facilities | 15.7 (12.0–20.3) | 1.08 (0.80–1.46) | 15.8 (12.0–20.4) | 1.16 (0.86–1.56) |
| All STD/school/teen clinics or other facilities | --‡ | --‡ | --‡ | --‡ |
| Mixed|| | 13.6 (11.3–16.2) | 0.93 (0.74–1.17) | 16.4 (13.2–20.1) | 1.20 (0.92–1.56) |
| Other¶ | --‡ | --‡ | --‡ | --‡ |
Reference level.
p value < 0.05 by t test for comparisons within each variable with the indicated reference level.
Estimate may not be reliable due to relative standard error >30% or sample size <30.
This indicated that the adolescents were ≥13 years of age when HPV vaccination was recommended in 2006.
Mixed indicates that the facility is identified to be in more than one of the facility categories such as private, public, hospital, STD/school/teen clinics.
Includes military, WIC clinics, and pharmacies.
In bivariable analyses, among male adolescents, other characteristics that were significantly associated with a higher level of HPV vaccination coverage (≥3 doses) compared with the referent group included: having a well-child visit at age 11–12 years, and living in urban or suburban areas (p<0.05). A mother with a high school or more than high school education, income to poverty ratio between 133% and 503%, and being VFC eligible but not insured were characteristics associated with a lower level of HPV vaccination (≥3 doses) (p<0.05) (Table 3).
Factors associated with HPV vaccination based on multivariable logistic regression models
Multivariable logistic regression showed that characteristics independently associated with a higher likelihood of HPV vaccination (≥1 dose) included: being non-Hispanic black race or Hispanic ethnicity, having mothers who were widowed, divorced, or separated, having 1 to 3 physician contacts in the past 12 months, having a well-child visit at age 11–12 years, having one or two vaccination providers, living in urban or suburban areas, and receiving vaccinations from mix of facility types (p<0.05). Having mothers with some college or college education, those with a higher family income to poverty ratio, those living in South or Midwest, and those receiving vaccinations from all STD/school/teen clinics or other facilities were characteristics independently associated with a lower likelihood of HPV vaccination (p<0.05) (Table 2). Statistical tests found that multi-collinearity was not identified among all variables assessed in this multivariable logistic model.
Multivariable logistic regression showed that characteristics independently associated with a higher likelihood of HPV vaccination (≥3 dose) included: being non-Hispanic black race or Hispanic ethnicity, having a well-child visit at age 11–12 years, and living in urban or suburban areas (p<0.05). Income to poverty ratio between 322% and 503%, and being VFC eligible but not insured were characteristics that were associated with a lower level of HPV vaccination (≥3 doses) (p<0.05) (Table 3). Statistical tests found that multi-collinearity was not identified among all variables assessed in this multivariable logistic model.
Trends of HPV vaccination among male adolescents
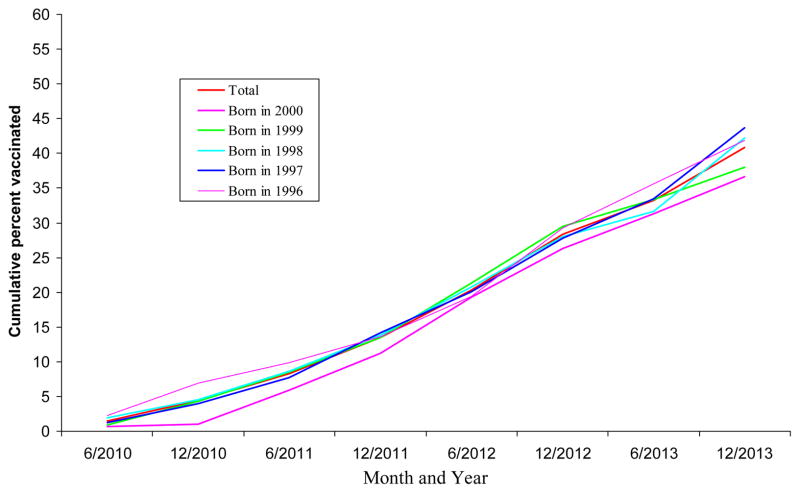
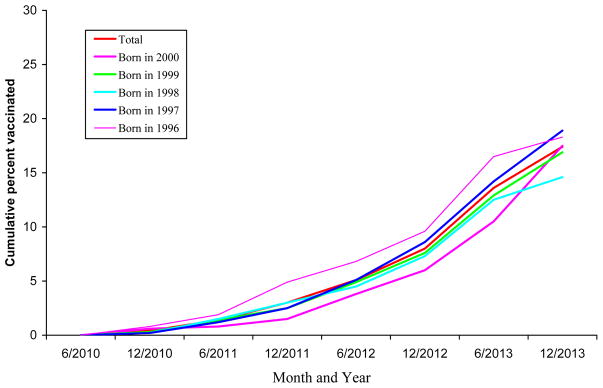
Overall, HPV vaccination coverage among male adolescents significantly increased from 2010 to 2013 (test for trend, p<0.05). In addition, among each birth cohort, the cumulative percent receiving HPV vaccination among male adolescents increased slowly before June 2011 and increased considerably after June 2011, particularly, after December 2011 (Figure 1, Figure 2).
Figure 1.
Cumulative percent of HPV vaccination (≥1 dose) uptake over time by birth cohort among male adolescents 13–17 years, NIS-Teen, 2013
Total, born in 2000, born in 1999, born in 1998, born in 1997, born in 1996
Figure 2.
Cumulative percent of HPV vaccination (≥3 dose) uptake over time by birth cohort among male adolescents 13–17 years, NIS-Teen, 2013
Total, born in 2000, born in 1999, born in 1998, born in 1997, born in 1996
Parent-reported reasons for non-receipt of HPV vaccination among male adolescents
The most common reason reported by parents for having no intent for their boys to receive HPV vaccination among those who have not received any HPV vaccinations was that the respondent thought their provider did not recommend it (24.0%). Other reasons included that it was not needed or not necessary (18.9%), lack of knowledge (16.4%), the adolescent was not sexually active (8.1%), and safety concern/side effects (7.3%) (Table 4). Percentages reported among males aged 13–15 years were similar with those aged 16–17 years (Table 4).
Table 4.
Main reasons for not receiving HPV vaccination* among male non-vaccinees aged 13–17 years, --NIS-Teen 2013
| 13–17 years | 13–15 years | 16–17 years | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Main reason | Sample | % | Sample | % | Sample | % |
| Not recommended | 861 | 24.0 (21.8–26.4) | 506 | 22.9 (20.1–25.9) | 355 | 25.8 (22.2–29.6) |
| Not needed or not necessary | 621 | 18.9 (16.8–21.2) | 385 | 19.5 (16.8–22.5) | 236 | 18.1 (14.8–21.9) |
| Lack of knowledge | 566 | 16.4 (14.5–18.5) | 329 | 16.3 (13.8–19.2) | 237 | 16.5 (13.7–19.8) |
| Not sexually active | 309 | 8.1 (6.8–9.7) | 196 | 8.2 (6.4–10.3) | 113 | 8.0 (6.0–10.6) |
| Safety concern/side effects | 248 | 7.3 (5.9–9.0) | 151 | 7.7 (5.8–10.0) | 97 | 6.8 (4.9–9.3) |
| Not appropriate age | 135 | 3.2 (2.5–4.2) | 105 | 3.8 (2.9–5.0) | 30 | 2.3 (1.2–4.4) |
| Not a school requirement | 134 | 3.5 (2.7–4.5) | 80 | 4.2 (3.0–5.8) | 54 | 2.5 (1.7–3.5) |
| Child is male | 119 | 4.2 (3.1–5.7) | 67 | 4.6 (3.1–6.8) | 52 | 3.6 (2.2–5.7) |
| Family/parental decision | 113 | 3.0 (2.2–4.1) | 64 | 2.8 (1.9–4.2) | 49 | 3.2 (1.8–5.6) |
| Costs/uninsured/insurance doesn’t fully cover shots | 68 | 1.5 (1.0–2.1) | 38 | 1.3 (0.8–2.2) | 30 | 1.8 (1.1–2.8) |
| Child fearful/shot could be painful | 50 | 1.7 (0.9–3.2) | 15 | 1.4 (0.4–4.7) | 35 | 2.2 (1.5–3.3) |
| Need more info/new vaccine | 41 | 0.9 (0.5–1.4) | 23 | 0.6 (0.3–1.2) | 18 | 1.2 (0.6–2.4) |
| intend to complete but have not yet/already planned | 32 | 1.0 (0.6–1.7) | 16 | 0.7 (0.4–1.4) | 16 | 1.5 (0.7–3.2) |
| Other reason | 73 | 2.0 (1.4–2.8) | 38 | 2.1 (1.3–3.3) | 35 | 1.9 (1.2–2.9) |
Percentage of main reasons for no intent of receiving HPV vaccination in the next 12 months among male non-vaccinees aged 13–17 years.
Discussion
Findings from our analysis show that HPV vaccine coverage (≥1 dose) among male adolescents aged 13–17 years was 34.6% and ≥3 HPV doses was 13.9%, approximately 1–2 years after the quadrivalent HPV vaccine was routinely recommended by ACIP for male adolescents (6). Coverage increased steadily after the ACIP recommendation, but remained low, as about two-thirds of male adolescents were still unvaccinated with HPV in 2013.
Early uptake of HPV vaccine among male adolescents is similar to early uptake of other vaccines recommended for adolescents. Meningococcal conjugate vaccine (MCV4), Tetanus, diphtheria and acellular pertussis vaccine (Tdap), and HPV vaccine (female), three new vaccines recommended for adolescents in 2005, 2005, and 2006, had reported vaccination coverage levels of 10.8%, 11.7%, and 25.1% (approximately one year after ACIP’s recommendations), respectively, for receipt of ≥ 1 dose (17–20). MCV4, Tdap, and HPV vaccination (female) coverage were 30.4%, 32.4%, and 37.2%, respectively, approximately two years after ACIP’s recommendations (17–20). Although HPV vaccination uptake in boys was similar to uptake in girls 1–2 years after ACIP recommendations were made, one might have expected uptake in boys to be higher in the same amount of time since providers already had the vaccine on stock (so no delays in obtaining supply) and they had experience with administering the vaccine.
Our analysis showed that HPV vaccination coverage among males significantly increased from 2010 to 2013 (test for trend, p<0.05). One previous study also indicated that early uptake of HPV vaccination (≥1 dose) of male adolescents increased from 1.4% in 2010 to 8.3% in 2011 (14). Another study showed that HPV vaccination (≥1 dose) of Hispanic males 13–17 years increased from 2.8% in 2010 to 31.7% in 2012 (21), and our study showed that the coverage (≥1 dose) among Hispanic males 13–17 years further increased to 49.6% in 2013. The results from previous research and our study reflect the adoption of ACIP recommendations during this period. Studies have found that HPV vaccine awareness was lower among male adolescents and their parents (22) which may impact uptake of this vaccine. Continued monitoring will determine if HPV vaccination coverage continues to increase or levels off as has been observed for HPV vaccination of females (15, 23).
Racial and ethnic disparities were noted, with non-Hispanic black male adolescents having significantly higher HPV vaccine coverage (≥1 dose) compared with non-Hispanic white males. Disparities were also noted with Hispanics having significantly higher HPV vaccine coverage (both ≥ 1 dose and ≥3 doses) compared with non-Hispanic white males. This disparity remained after controlling for other factors. Racial and ethnic disparities in HPV vaccination were similar to those among female adolescents (22). HPV vaccination coverage was generally higher among teens living in poverty, which might reflect the VFC program’s (24) effectiveness at reaching these young persons. Further research is needed to better understand the factors associated with racial/ethnic differences in HPV vaccination coverage among male adolescents.
Several other characteristics, such as having more physician contacts in the past 12 months, and having a well-child visit at age 11–12 years were independently associated with HPV vaccination initiation, consistent with other vaccination studies among adolescents (25–28). Physician recommendations for vaccination are associated with a patient’s decision to get vaccinated (25–28). Persons who have more physician contacts may have more opportunities to discuss their vaccination status and receive vaccination.
Some barriers may hinder vaccine uptake. Our analysis indicated that the most common primary reasons for not receiving HPV vaccination among male adolescents 13–17 years were that their provider did not recommend HPV vaccination (24.0%), that the vaccine was not needed or not necessary (18.9%), lack of knowledge (16.4%), the adolescent was not sexually active (8.1%), and safety concern/side effects” (7.3%). These stated reasons for not receiving HPV vaccine indicate that parents may have limited knowledge regarding HPV vaccine and ACIP recommendations. To help facilitate discussions between clinicians and parents, CDC has developed several resources, including a tips sheet with suggested language for talking with parents (29).
The Guide to Community Preventive Services has identified evidence based strategies to improve vaccination coverage, including standing orders programs, reminder/recall systems to notify clinicians and parents when a vaccine is due, and use of Immunization Information Systems (30). While these interventions may be helpful for adolescents who need to receive their second or third dose of HPV vaccine, it is not clear that they will be sufficient for improving initiation of the series. Additional efforts will be needed to improve provider practices and recommendation of this vaccine at age of 11–12 years to all adolescent boys and girls.
The findings in this study are subject to several limitations. First, household response rates were 51.1% (landline households) and 23.3% (cellular phone households), respectively. Only 59.5% (landline) and 54.5% (cellular telephone) of completed household interviews also had adequate provider-verified vaccination data. Some bias may remain after weighting adjustments designed to mitigate potential bias from incomplete data from the sample frame and non-response (31, 32). Second, some provider-confirmed vaccination histories might not include all vaccinations received (e.g., vaccinations administered in nontraditional settings such as emergency departments) and might have underestimated vaccination coverage.
One to two years after the vaccine was recommended for male adolescents, only 34.6% had initiated HPV vaccination (≥1 dose), and 13.9% had ≥3 HPV doses. Coverage increased steadily after the ACIP recommendation for routine vaccination of males, but remained low, as about two-thirds of male adolescents were still unvaccinated with HPV in 2013. To further increase HPV vaccination coverage, comprehensive strategies included: 1) providers should routinely assess the HPV vaccination status of their patients, especially when they have a well-child visit, to ensure that the adolescent is fully vaccinated and recommending catch-up vaccination and administering all age-appropriate vaccines during the same clinical encounter; 2) it is important to ensure all vaccine providers are knowledgeable about HPV vaccine recommendations for males and females, with provision for current information on efficacy and safety of vaccines; 3) reducing parental out-of-pocket expenses; 4) using media promotions and educational programs for parents to improve HPV awareness; and 5) efforts should be directed to provide comprehensive, accessible, and appropriate communication messages on HPV and HPV vaccine directed to male adolescent and parents (29, 30, 33). Additionally, the Affordable Care Act may help improve vaccination coverage since the expanded enrollment in public and private insurance programs expected from provisions of the Affordable Care Act is likely to improve access to health care services, including vaccination (34). Continued monitoring of HPV vaccination uptake among males is useful for evaluating vaccination campaigns and for planning and implementing strategies to increase vaccination coverage. Increased efforts are needed to improve HPV vaccination coverage for all adolescents to reduce HPV infections and related cancers.
What’s know?
HPV is the most common sexually transmitted infection in the United States. More than 50% of sexually active men and women will acquire HPV infection in their lifetime. In 2011, HPV was recommended for routine use among male adolescents.
What’s added?
This is the first study to evaluate HPV vaccination coverage among male adolescents in the United States using provider reported vaccination data. HPV coverage was low among male adolescents, leaving a large population susceptible to HPV infection maturing into adulthood.
ABBREVIATIONS
- HPV
Human papillomavirus
- ACIP
Advisory Committee on Immunization Practices
- FDA
Food and Drug Administration
- PR
Prevalence ratio
- 95% CI
95% confidence interval
- MSM
Men who have sex with men
- RDD
Random-digit–dial
- CDC
Centers for Disease Control and Prevention
- STD
Sexual transmitted diseases
- WIC
Women, Infants, and Children
- MSA
Metropolitan statistical area
- SCHIP
State Children’s Health Insurance Program
- NIS-Teen
National Immunization Survey-Teen
- VFC
Vaccines for Children
Footnotes
Financial disclosure: The authors have indicated they have no financial relationships relevant to this article to disclose
Potential conflicts of interest: The authors have indicated they have no potential conflicts of interest to disclose
Clinical Trial Registration: This is not a clinical trial study
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Contributor statement:
Peng-jun Lu: Dr. Lu conceptualized and designed the study, and drafted the initial manuscript.
David Yankey, Jenny Jeyarajah, Alissa O’Halloran: Mr. Yankey and Mrs. Jeyarajah and O’Halloran carried out the initial analyses, interpretation of the data, and reviewed and revised the manuscript.
Laurie D. Elam-Evans, Philip J. Smith, Shannon Stokley, James A. Singleton, Eileen F. Dunne: Drs. Elam-Evans, Smith, Stokley, Singleton, and Dunne revised and critically reviewed the manuscript.
Funding sources: None
References
- 1.Centers for Disease Control and Prevention (CDC) Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 2.Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3–8. doi: 10.1016/s0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 3.Dunne EF, Markowitz LE, Saraiya M, Stokley S, Middleman A, Unger ER, et al. CDC grand rounds: Reducing the burden of HPV-associated cancer and disease. MMWR. 2014;63(4):69–72. [PMC free article] [PubMed] [Google Scholar]
- 4.Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC, et al. Sexually transmitted infections among U.S. women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187–93. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Human papillomavirus (HPV)-associated cancers. Atlanta, GA: US Department of Health and Human Services, CDC; 2013. [Accessed March 26, 2014]. Available at: http://www.cdc.gov/cancer/hpv/statistics/cases.htm. [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Recommendations on the Use of Quadrivalent Human Papillomavirus Vaccine in Males — Advisory Committee on Immunization Practices (ACIP), 2011. MMWR. 2011;60(50):1705–1708. [PubMed] [Google Scholar]
- 7.Joseph DA, Miller JW, Wu X, et al. Understanding the burden of human papillomavirus-associated anal cancers in the U. S Cancer. 2008;113(10 Suppl):2892–900. doi: 10.1002/cncr.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillison ML, Chaturvedi AK, Lowy DR. HPV Prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113(10 Suppl):3036–46. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saraiya M. Burden of HPV-associated cancers in the United States. Presentation before the Advisory Committee on Immunization Practices (ACIP); February 24, 2011; Atlanta, GA: US Department of Health and Human Services, CDC; 2011. [Accessed March 26 2014]. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb11/11-2-hpv-rela-cancer.pdf. [Google Scholar]
- 11.Jemal A, Simard EP, Dorell C, Noone AM, Markowitz LE, Kohler B, et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) FDA Licensure of Quadrivalent Human Papillomavirus Vaccine (HPV4, Gardasil) for Use in Males and Guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR. 2010;59(20):630–632. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) The Advisory Committee on Immunization Practices (ACIP): Summary Report. Atlanta, Georgia: Oct 27–28, 2010. [Accessed May, 2012]. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/min-archive/min-oct10.pdf. [Google Scholar]
- 14.Reiter PL, Gilkey MB, Brewer NT. HPV vaccination among adolescent males. Vaccine. 2013;31(26):2816–2821. doi: 10.1016/j.vaccine.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC) National and state vaccination coverage among adolescents aged 13–17 years – United States, 2013. MMWR. 2014;63(29):625–633.25. [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) [Accessed August 18, 2015];National Immunization Survey-Teen. Available at: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NIS/NISTEENPUF13_DUG.pdf.
- 17.Centers for Disease Control and Prevention (CDC) National vaccination coverage among adolescents aged 13–17 years. MMWR. 2007;56(34):885–888. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC) National, State, and Local Area Vaccination Coverage Among Adolescents Aged 13--17 Years --- United States, 2008. MMWR. 2009;58(36):997–1001. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Vaccination coverage among adolescents aged 13–17 years. MMWR. 2008;57(40):1100–1103. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) [Accessed January 28 2014];Vaccine and immunization. Available at: http://www.cdc.gov/vaccines/vpd-vac/hpv/default.htm.
- 21.Reiter PL, Brewer NT, Gilkey MB, Katz ML, Paskett ED, Smith JS. Early adoption of the human papillomavirus vaccine among Hispanic adolescent males in the United States. Cancer. 2014;120(20):3200–3207. doi: 10.1002/cncr.28871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatta MP, Phillips L. Human Papillomavirus Vaccine Awareness, Uptake, and Parental and Health Care Provider Communication Among 11- to 18-Year-Old Adolescents in a Rural Appalachian Ohio County in the United States. J Rural Health. 2014 Jul 8; doi: 10.1111/jrh.12079. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) National and state vaccination coverage among adolescents aged 13–17 years – United States, 2012. MMWR. 2013;62(34):685–693. [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) [Accessed May 22, 2014];Immunization Grant Program (Section 317) Available at: at http://www.cdc.gov/vaccines/imz-managers/guides-pubs/ipom/
- 25.Dorell CG, Yankey D, Byrd KK, Murphy TV. Hepatitis a vaccination coverage among adolescents in the United States. Pediatrics. 2012 Feb;129(2):213–21. doi: 10.1542/peds.2011-2197. [DOI] [PubMed] [Google Scholar]
- 26.Jain N, Hennessey K. Hepatitis B vaccination coverage among U.S. adolescents, National Immunization Survey-Teen, 2006. J Adolesc Health. 2009 Jun;44(6):561–7. doi: 10.1016/j.jadohealth.2008.10.143. [DOI] [PubMed] [Google Scholar]
- 27.Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics. 2011 Nov;128(5):830–9. doi: 10.1542/peds.2011-0950. [DOI] [PubMed] [Google Scholar]
- 28.Lu PJ, Jain N, Cohn AC. Meningococcal conjugate vaccination among adolescents aged 13–17 years, United States, 2007. Vaccine. 2010;28:2350–55. doi: 10.1016/j.vaccine.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC) [Accessed March 27, 2015];Preteen and teen vaccines. Available at http://www.cdc.gov/vaccines/who/teens/for-hcp/hpv-resources.html.
- 30.Centers for Disease Control and Prevention (CDC) The community guide. Atlanta, GA: 2013. [Accessed November 26, 2014]. The guide to community preventive services. Available at: http://www.thecommunityguide.org/index.html. [Google Scholar]
- 31.Dolson D. [Accessed November 25, 2014];Errors of Non-Observation: Dwelling Nonresponse and Coverage Error in Traditional Censuses. Available at: http://www.amstat.org/sections/srms/proceedings/y2012/files/303670_71713.pdf.
- 32.Proceedings of the Survey Research Methods Section. American Statistical Association; 2012. [Accessed February 4, 2015]. Available at http://www.amstat.org/sections/srms/proceedings/allyears.html. [Google Scholar]
- 33.Hughes J, Cates JR, Liddon N, Smith JS, Gottlieb SL, Brewer NT. Disparities in how parents are learning about the human papillomavirus vaccine. Cancer Epidemiol Biomarkers Prev. 2009;18(2):363–72. doi: 10.1158/1055-9965.EPI-08-0418. [DOI] [PubMed] [Google Scholar]
- 34. [Accessed August 5, 2015];The Affordable Care Act. Available at: http://www.healthcare.gov/law/full/index.html.