Preamble and Transition to ACC/AHA Guidelines to Reduce Cardiovascular Risk
The goals of the American College of Cardiology (ACC) and the American heart Association (AHA) are to prevent cardiovascular diseases (CVD); improve the management of people who have these diseases through professional education and research; and develop guidelines, standards, and policies that promote optimal patient care and cardiovascular health. Toward these objectives, the ACC and AHA have collaborated with the National heart, lung, and blood Institute (NHLBI) and stakeholder and professional organizations to develop clinical practice guidelines for assessment of cardiovascular risk, lifestyle modifications to reduce cardiovascular risk, management of blood cholesterol in adults, and management of overweight and obesity in adults.
In 2008, the NHLBI initiated these guidelines by sponsoring rigorous systematic evidence reviews for each topic by expert panels convened to develop critical questions (CQs), interpret the evidence, and craft recommendations. In response to the 2011 report from the Institute of Medicine on the development of trustworthy clinical guidelines,1 the NHLBI Advisory Council recommended that the NHLBI focus specifically on reviewing the highest-quality evidence and partner with other organizations to develop recommendations.2, 3 Accordingly, in June 2013 the NHLBI initiated collaboration with the ACC and AHA to work with other organizations to complete and publish the 4 guidelines noted above and make them available to the widest possible constituency. Recognizing that the Expert Panels/Work groups did not consider evidence beyond 2011 (except as specified in the methodology), the ACC, AHA, and collaborating societies plan to begin updating these guidelines starting in 2014.
The joint ACC/AHA task Force on Practice Guidelines (Task Force) appointed a subcommittee to shepherd this transition, communicate the rationale and expectations to the writing panels and partnering organizations, and expeditiously publish the documents. The ACC/AHA and partner organizations recruited a limited number of expert reviewers for fiduciary examination of content, recognizing that each document had undergone extensive peer review by representatives of the NHLBI Advisory Council, key federal agencies, and scientific experts. Each writing panel responded to comments from these reviewers. Clarifications were incorporated where appropriate, but there were no substantive changes because the bulk of the content was undisputed.
Although the Task Force led the final development of these prevention guidelines, they differ from other ACC/AHA guidelines. First, as opposed to an extensive compendium of clinical information, these documents are significantly more limited in scope and focus on selected CQs on each topic based on the highest-quality evidence available. Recommendations were derived from randomized trials, meta-analyses, and observational studies evaluated for quality and were not formulated when sufficient evidence was not available. Second, the text accompanying each recommendation is succinct, summarizing the evidence for each question. The Full Panel/Work group reports include more detailed information about the evidence statements (ESs) that serve as the basis for recommendations. Third, the format of the recommendations differs from other ACC/AHA guidelines. Each recommendation has been mapped from the NHLBI grading format to the ACC/AHA Classification of Recommendation/Level of evidence (COR/LOE) construct (Table 1) and is expressed in both formats. Because of the inherent differences in grading systems and the clinical questions driving the recommendations, alignment between the NHLBI and ACC/AHA formats is in some cases imperfect. Explanations of these variations are noted in the recommendation tables, where applicable.
Table 1.
Applying Classification of Recommendation and Level of Evidence
A recommendation with Level of Evidence B or C does not imply the recommendation is weak. Many important clinical questions addressed in the guidelines do not lend themselves to clinical trials. Even when randomized trials are unavailable, there may be a very clear clinical consensus that a particular test or therapy is useful or effective.
Data available from clinical trials or registries about the usefulness/efficacy in different subpopulations, such as sex, age, history of diabetes, history of prior myocardial infarction, history of heart failure, and prior aspirin use.
For comparative-effectiveness recommendations (Class I and IIa; Level of Evidence A and B only), studies that support the use of comparator verbs should involve direct comparisons of the treatments or strategies being evaluated.
In consultation with NHLBI, the policies adopted by the writing panels to manage relationships of authors with industry and other entities (RWI) are outlined in the methods section of each panel report. These policies were in effect when this effort began in 2008 and throughout the writing process and voting on recommendations, until the process was transferred to ACC/AHA in 2013. In the interest of transparency, the ACC/AHA requested that panel authors resubmit RWI disclosures as of July 2013. Relationships relevant to this guideline are disclosed in Appendix 1. None of the ACC/AHA expert reviewers had relevant RWI (Appendix 2). See Appendix 3 for a list of abbreviations used in this guideline.
Systematic evidence reports and accompanying summary tables were developed by the expert panels and NHLBI. The guideline was reviewed by the ACC/AHA Task Force and approved by the ACC board of trustees, the AHA Science Advisory and Coordinating Committee, and The Obesity Society. In addition, ACC/AHA sought endorsement from other stakeholders, including professional organizations. It is the hope of the writing panels, stakeholders, professional organizations, NHLBI, and Task Force that the guidelines will garner the widest possible readership for the benefit of patients, providers, and the public health.
These guidelines are meant to define practices that meet the needs of patients in most circumstances and are not a replacement for clinical judgment. The ultimate decision about care of a particular patient must be made by the healthcare provider and patient in light of the circumstances presented by that patient. As a result, situations might arise in which deviations from these guidelines may be appropriate. These considerations notwithstanding, in caring for most patients, clinicians can employ the recommendations confidently to reduce the risks of atherosclerotic CVD events.
See Tables 2 and 3 for an explanation of the NHLBI recommendation grading methodology.
Table 2.
NHLBI Grading of the Strength of Recommendations
| Grade | Strength of Recommendation* |
|---|---|
| A | Strong recommendation |
| There is high certainty based on evidence that the net benefit† is substantial. | |
| B | Moderate recommendation |
| There is moderate certainty based on evidence that the net benefit is moderate to substantial, or there is high certainty that the net benefit is moderate. | |
| C | Weak recommendation |
| There is at least moderate certainty based on evidence that there is a small net benefit. | |
| D | Recommendation against |
| There is at least moderate certainty based on evidence that there is no net benefit or that risks/harms outweigh benefits. | |
| E | Expert opinion (“There is insufficient evidence or evidence is unclear or conflicting, but this is what the Work Group recommends.”) |
| Net benefit is unclear. Balance of benefits and harms cannot be determined because of no evidence, insufficient evidence, unclear evidence, or conflicting evidence, but the Work Group thought it was important to provide clinical guidance and make a recommendation. Further research is recommended in this area. | |
| N | No recommendation for or against (“There is insufficient evidence or evidence is unclear or conflicting.”) |
| Net benefit is unclear. Balance of benefits and harms cannot be determined because of no evidence, insufficient evidence, unclear evidence, or conflicting evidence, and the Work Group thought no recommendation should be made. Further research is recommended in this area. |
In most cases, the strength of the recommendation should be closely aligned with the quality of the evidence; however, under some circumstances, there may be valid reasons for making recommendations that are not closely aligned with the quality of the evidence (eg, strong recommendation when the evidence quality is moderate, such as smoking cessation to reduce CVD risk or ordering an ECG as part of the initial diagnostic work-up for a patient presenting with possible MI). Those situations should be limited and the rationale explained clearly by the Work Group.
Net benefit is defined as benefits minus risks/harms of the service/intervention.
CVD indicates cardiovascular disease; ECG, electrocardiogram; MI, myocardial infarction; and NHLBI, National Heart, Lung, and Blood Institute.
Table 3.
NHLBI Quality Rating of the Strength of Evidence
| Type of Evidence | Quality Rating* |
|---|---|
|
High |
|
Moderate |
|
Low |
In some cases, other evidence, such as large all-or-none case series (eg, jumping from airplanes or tall structures), can represent high- or moderate-quality evidence. In such cases, the rationale for the evidence rating exception should be explained by the Work Group and clearly justified.
“Well-designed, well-executed” refers to studies that directly address the question; use adequate randomization, blinding, and allocation concealment; are adequately powered; use intention-to-treat analyses; and have high follow-up rates.
Limitations include concerns with the design and execution of a study that result in decreased confidence in the true estimate of the effect. Examples of such limitations include but are not limited to: inadequate randomization, lack of blinding of study participants or outcome assessors, inadequate power, outcomes of interest that are not prespecified for the primary outcomes, low follow-up rates, and findings based on subgroup analyses. Whether the limitations are considered minor or major is based on the number and severity of flaws in design or execution. Rules for determining whether the limitations are considered minor or major and how they will affect rating of the individual studies will be developed collaboratively with the methodology team.
Nonrandomized controlled studies refer to intervention studies where assignment to intervention and comparison groups is not random (eg, quasi-experimental study design).
Observational studies include prospective and retrospective cohort, case-control, and cross-sectional studies.
NHLBI indicates National Heart, Lung, and Blood Institute; and RCT, randomized controlled trial.
1. Introduction/Scope of Guideline
More than 78 million adults in the United States were obese in 2009 and 2010.4 Obesity raises the risk of morbidity from hypertension, dyslipidemia, type 2 diabetes mellitus (diabetes), coronary heart disease (CHD), stroke, gallbladder disease, osteoarthritis, sleep apnea and respiratory problems, and some cancers. Obesity is also associated with increased risk of all-cause and CVD mortality. The biomedical, psychosocial, and economic consequences of obesity have substantial implications for the health and well-being of the US population. According to the 1998 “Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report,”5 overweight is defined as a body mass index (BMI) of 25 kg/m2 to 29.9 kg/m2 and obesity as a BMI of ≥30 kg/m2. Current estimates are that 69% of adults are either overweight or obese, with approximately 35% obese.6 These latest data from the National Health and Nutrition Examination Surveys indicate that for both men and women, obesity estimates for 2009 and 2010 did not differ significantly from estimates for 2003 to 2008 and that increases in the prevalence rates of obesity appear to be slowing down or leveling off.6 Nevertheless, overweight and obesity continue to be highly prevalent, especially in some racial and ethnic minority groups, as well as in those with lower incomes and less education. Overweight and obesity are major contributors to chronic diseases in the united states and present a major public health challenge. Compared with normal-weight individuals, obese patients incur 46% higher inpatient costs, 27% more physician visits and outpatient costs, and 80% higher spending on prescription drugs.7 the medical care costs of obesity in the United States are staggering. In 2008 dollars, these costs totaled about $147 billion.7
The Expert Panel was first convened in September 2008 by the NHLBI in cooperation with the National Institute of Diabetes and Digestive and Kidney Diseases to update the 1998 Clinical Guidelines Report.5 The Expert Panel considered new evidence related to key issues on overweight and obesity evaluation and treatment, particularly in individuals with other risk factors for CVD and diabetes. The key issues identified included the appropriateness of the current BMI and waist circumference cutpoints that are used for determining risk in overweight and obese adults across diverse populations; the impact of weight loss on risk factors for CVD and type 2 diabetes, as well as CVD morbidity and mortality; optimal behavioral, dietary intervention, and other lifestyle treatment approaches for weight loss and weight loss maintenance; and benefits and risks of various bariatric surgical procedures. The Expert Panel’s ultimate goal was to systematically develop ESs and recommendations for 5 CQs to assist clinicians in primary care. The recommendations are based on evidence from a rigorous systematic review and synthesis of recently published medical literature.
This guideline is based on the Full Panel Report, which is provided as an online-only data supplement to the guideline. The Full Panel Report contains background and additional material related to content, methodology, evidence synthesis, rationale, and references and is supported by the NHLBI Systematic Evidence Review, which can be found at http://www.nhlbi.nih.gov/guidelines/obesity/ser/. Refer to the “2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults,” “2013 AHA/ACC guideline on Lifestyle Management to Reduce Cardiovascular Risk,” and “2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk”8–10 for topics outside the scope of the 2013 AHA/ACC/TOS Obesity Guideline.
1.1. Rationale for Updating Obesity Clinical Guidelines
The NHLBI, in cooperation with the National Institute of Diabetes and Digestive and Kidney Diseases, released the 1998 “Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report”11 as a systematic review of the published scientific literature found in MEDLINE from January 1980 to September 1997 on important topics reviewed by the Expert Panel. The published literature was evaluated to determine appropriate treatment strategies that would constitute evidence-based clinical guidelines on overweight and obesity. The San Antonio Cochrane Center assisted in literature abstraction and in organizing the data into evidence tables, and a methodology consultant worked with the Expert Panel to develop ESs and recommendations.
In 2005, the NHLBI initiated the process to update the overweight/obesity guidelines and convened stakeholder groups to provide input on what should be the next-generation guideline development process. The resulting recommendations were used to design the process. To continually improve the quality and impact of the guidelines, the process was updated to assure rigor and minimize bias through the use of strict, evidence-based methodologies to guide the development of ESs and recommendations based on a systematic review of the biomedical literature for a specific period of time.
1.2. CQ-Based Approach
The Expert Panel began its deliberations by developing 23 possible CQs, and after considerable discussion, narrowed the possibilities to 5 targeted CQs. Questions were chosen to aid primary care practitioners (PCPs) and providers who frequently work with obese patients to identify patients at health risk of weight-related comorbidities and to update them on the benefits and risks of weight loss achieved by various approaches. Examples of CQs that were not included for this review included consideration of genetics of obesity, binge-eating disorders, pharmacotherapy, and cost-effectiveness of interventions to manage obesity. For each of the chosen CQs, Expert Panel members reviewed the final list of included and excluded articles, along with the quality ratings, and had the opportunity to raise questions and appeal the ratings to the methodology team. The team then reexamined these articles and presented their rationale for either keeping or changing the quality rating of the articles. Expert Panel members also played a key role in examining the evidence tables and summary tables to be certain the data from each article were accurately displayed.
The body of the present report is organized by CQ and the following information is included for each CQ:
The rationale for its selection is provided, and methods are described.
The body of evidence is summarized, and ESs are presented, which include a rating for quality and a supportive narrative summary.
Recommendations and their strength are accompanied by a narrative summary of how the recommendation was derived from the evidence and a discussion of issues considered by the Expert Panel in formulating the recommendation.
CQ1 and CQ2 were chosen to help providers determine the appropriate criteria to guide a weight loss recommendation. CQ1 addresses the expected health benefits of weight loss as a function of the amount and duration of weight loss. CQ2 addresses the health risks of overweight and obesity and seeks to determine if the current waist circumference cutpoints and the widely accepted BMI cutpoints defining persons as overweight (BMI 25–29.9 kg/m2) and obese (BMI ≥30 kg/m2) are appropriate for population subgroups. Because patients are interested in popular diets that are promoted for weight loss and see the PCP as an authoritative source of information, CQ3 asks which dietary intervention strategies are effective for weight loss efforts. CQ4 seeks to determine the efficacy and effectiveness of a comprehensive lifestyle approach (diet, physical activity, and behavior therapy) to achieve and maintain weight loss. CQ5 seeks to determine the efficacy and safety of bariatric surgical procedures, including benefits and risks. CQ5 also seeks to determine patient and procedural factors that may help guide decisions to enhance the likelihood of maximum benefit from surgery for obesity and related conditions.
1.3. Organization of the Panel
In 2007, the NHLBI sought nominations for panel membership that would ensure adequate representation of key specialties and appropriate expertise. The NHLBI staff reviewed the nominees and selected potential chairs and co-chairs for the panels. A Guidelines Executive Committee was formed, consisting of the chairs from each of the 3 panels (obesity, high blood pressure [BP], and high blood cholesterol) and 3 cross-cutting working groups (lifestyle, risk assessment, and implementation). This committee worked with the NHLBI to select panel members from the list of nominees.
The Obesity Expert Panel comprised 15 members and 3 ex-officio members, including individuals with specific expertise in psychology, nutrition, physical activity, bariatric surgery, epidemiology, internal medicine, and other clinical specialties. The full Obesity Expert Panel met 23 times throughout the years (5 times face-to-face and 18 times via Webinar). Expert Panel chairs asked all members to disclose any conflicts of interest to the full Expert Panel in advance of the deliberations; members with conflicts were asked to recuse themselves from voting on any aspect of the guideline for which a conflict might exist. Each of the 5 CQs had working groups consisting of a leader and various Expert Panel members who met via conference calls to discuss all aspects of the CQ; to review the list of included and excluded articles along with the quality ratings; to review the evidence tables and summary tables; and to develop spreadsheets, ESs, resulting recommendations, and research/evidence gaps. Expert Panel members had the opportunity to raise questions about the included and excluded articles, submit additional articles that were not identified in the original search, appeal the quality ratings on articles, and question articles that were excluded. Each working group presented their findings to the full Expert Panel for all final decisions on ESs and recommendations, including the strength of the evidence.
The evidence-based process followed most of the standards from the Institute of Medicine’s report, Clinical Practice Guidelines We Can Trust.1 The process had support from a methodology contractor and a systematic review and general support contractor and included the following steps:
Constructed CQs relevant to clinical practice.
Identified (a priori) inclusion/exclusion (I/E) criteria for each CQ.
Developed a literature search strategy, based on I/E criteria, for each CQ.
Executed a systematic electronic search of the published literature from relevant bibliographic databases for each CQ. The date range for the overall literature search was from January 1998 to December 2009. Because CQ1 and CQ2 used systematic reviews and meta-analyses, the literature search included those published from January 2000 to October 2011. CQ3 and CQ4 added major randomized controlled trials (RCTs) published after 2009 with >100 people per treatment arm. CQ5 added some major studies published after 2009 that met the I/E criteria.
Screened, by 2 independent reviewers, thousands of abstracts and full-text articles returned from the search to identify relevant original articles, systematic reviews, and meta-analyses. Rigorous validation procedures were applied to ensure that the selected articles met the pre-established detailed I/E criteria before being included in the final review results.
Determined, by 2 independent raters on the methodology team, the quality of each included study (good, fair, and poor).
Abstracted relevant information from the included studies into an electronic central repository database using common templates and types of data elements.
Constructed detailed evidence tables, which organized the data from the abstraction database.
Analyzed the evidence tables and constructed summary tables, which display the evidence in a manageable format to answer specific parts of each CQ.
Used summary tables to develop ESs for each CQ. The quality of evidence for each ES was graded as high, moderate, or low on the basis of scientific methodology, scientific strength, and consistency of results. For CQ1 and CQ2, spreadsheets with relevant data from systematic reviews and meta-analyses were developed rather than summary tables.
Used the graded ESs to write clinical recommendations, and graded the strength of each recommendation. Recommendations were graded as Strong Recommendation (Grade A), Moderate Recommendation (Grade B), Weak Recommendation (Grade C), Recommendation Against (Grade D), Expert Opinion (Grade E), or No Recommendation For or Against (Grade N).
Performed Guideline Implementability Appraisals, planned and coordinated by the NHLBI Implementation Work Group, to identify and address barriers to guideline implementation.
1.4. Document Review and Approval
A formal peer review process was initially completed under the auspices of the NHLBI and included 10 expert reviewers and representatives from multiple federal agencies. This document was also reviewed by 6 expert reviewers nominated by the ACC, AHA, and The Obesity Society after the management of the guideline transitioned to the ACC/AHA. The ACC, AHA, and The Obesity Society reviewers’ RWI information is published in this document (Appendix 2).
This document was approved for publication by the governing bodies of the ACC, the AHA, and The Obesity Society and is endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, American Pharmacists Association, American Society for Nutrition, American Society for Parenteral and Enteral Nutrition, American Society for Preventive Cardiology, American Society of Hypertension, Association of Black Cardiologists, National Lipid Association, Preventive Cardiovascular Nurses Association, The Endocrine Society, and WomenHeart: The National Coalition for Women With Heart disease.
2. Obesity Recommendations and Algorithm
2.1. Summary of Evidence-Based Recommendations
The recommendations in Table 4 serve as a guide for PCPs in making evaluations and treatment decisions for overweight and obese patients. The CQs answered by evidence-based recommendations summarize current literature on the risks of over-weight and obesity and the benefits of weight loss. They also summarize knowledge on the best diets for weight loss, the efficacy and effectiveness of comprehensive lifestyle interventions on weight loss and weight loss maintenance, and the benefits and risks of bariatric surgery. This information will help PCPs decide who should be recommended for weight loss and what health improvements can be expected. The Expert Panel did not choose a CQ that dealt with various aspects of pharmacotherapy for a comprehensive evidence assessment, because at the time the CQs were chosen there was only one approved medication (orlistat) for weight loss. However, CQ1 includes some ESs on the efficacy of orlistat because the effect of pharmacotherapy on weight loss was included in its evidence review.
Table 4.
Summary of Recommendations for Obesity
| Recommendations | NHLBI Grade | NHLBI ES | ACC/AHA COR | ACC/AHA LOE | |
|---|---|---|---|---|---|
| Identifying Patients Who Need to Lose Weight (BMI and Waist Circumference) | |||||
| 1a. | Measure height and weight and calculate BMI at annual visits or more frequently. | E (Expert Opinion) | CQ2 | I | C |
| 1b. | Use the current cutpoints for overweight (BMI 25.0–29.9 kg/m2) and obesity (BMI ≥30 kg/m2) to identify adults who may be at elevated risk of CVD and the current cutpoints for obesity (BMI ≥30 kg/m2) to identify adults who may be at elevated risk of mortality from all causes. | A (Strong) | CQ2 | I | B |
| 1c. | Advise overweight and obese adults that the greater the BMI, the greater the risk of CVD, type 2 diabetes, and all-cause mortality. | A (Strong) | CQ2 | I | B |
| 1d. | Measure waist circumference at annual visits or more frequently in overweight and obese adults. | E (Expert Opinion) | CQ2 | IIa | B |
| Advise adults that the greater the waist circumference, the greater the risk of CVD, type 2 diabetes, and all-cause mortality. The cutpoints currently in common use (from either NIH/NHLBI or WHO/IDF) may continue to be used to identify patients who may be at increased risk until further evidence becomes available. | |||||
| Matching Treatment Benefits With Risk Profiles (Reduction in Body Weight Effect on Risk Factors for CVD, Events, Morbidity and Mortality) | |||||
| 2. | Counsel overweight and obese adults with cardiovascular risk factors (high BP, hyperlipidemia, and hyperglycemia) that lifestyle changes that produce even modest, sustained weight loss of 3%–5% produce clinically meaningful health benefits, and greater weight losses produce greater benefits.
|
A (Strong) | CQ1 | I | A |
| Diets for Weight Loss (Dietary Strategies for Weight Loss) | |||||
| 3a. | Prescribe a diet to achieve reduced calorie intake for obese or overweight individuals who would benefit from weight loss, as part of a comprehensive lifestyle intervention. Any one of the following methods can be used to reduce food and calorie intake:
|
A (Strong) | CQ3 | I | A |
| 3b. | Prescribe a calorie-restricted diet, for obese and overweight individuals who would benefit from weight loss, based on the patient’s preferences and health status, and preferably refer to a nutrition professional* for counseling. A variety of dietary approaches can produce weight loss in overweight and obese adults, as presented in CQ3, ES2. | A (Strong) | CQ3 | I | A |
| Lifestyle Intervention and Counseling (Comprehensive Lifestyle Intervention) | |||||
| 4a. | Advise overweight and obese individuals who would benefit from weight loss to participate for ≥6 months in a comprehensive lifestyle program that assists participants in adhering to a lower-calorie diet and in increasing physical activity through the use of behavioral strategies. | A (Strong) | CQ4 | I | A |
| 4b. | Prescribe on-site, high-intensity (ie, ≥14 sessions in 6 mo) comprehensive weight loss interventions provided in individual or group sessions by a trained interventionist.† | A (Strong) | CQ4 | I | A |
| 4c. | Electronically delivered weight loss programs (including by telephone) that include personalized feedback from a trained interventionist† can be prescribed for weight loss but may result in smaller weight loss than face-to-face interventions. | B (Moderate) | CQ4 | IIa | A |
| 4d. | Some commercial-based programs that provide a comprehensive lifestyle intervention can be prescribed as an option for weight loss, provided there is peer-reviewed published evidence of their safety and efficacy. | B (Moderate) | CQ4 | IIa | A |
| 4e. | Use a very-low-calorie diet (defined as <800 kcal/d) only in limited circumstances and only when provided by trained practitioners in a medical care setting where medical monitoring and high-intensity lifestyle intervention can be provided. Medical supervision is required because of the rapid rate of weight loss and potential for health complications. | A (Strong) | CQ4 | IIa‡ | A |
| 4f. | Advise overweight and obese individuals who have lost weight to participate long term (≥1 year) in a comprehensive weight loss maintenance program. | A (Strong) | CQ4 | I | A |
| 4g. | For weight loss maintenance, prescribe face-to-face or telephone-delivered weight loss maintenance programs that provide regular contact (monthly or more frequently) with a trained interventionist† who helps participants engage in high levels of physical activity (ie, 200–300 min/wk), monitor body weight regularly (ie, weekly or more frequently), and consume a reduced-calorie diet (needed to maintain lower body weight). | A (Strong) | CQ4 | I | A |
| Selecting Patients for Bariatric Surgical Treatment for Obesity (Bariatric Surgical Treatment for Obesity) | |||||
| 5a. | Advise adults with a BMI ≥40 kg/m2 or BMI ≥35 kg/m2 with obesity-related comorbid conditions who are motivated to lose weight and who have not responded to behavioral treatment with or without pharmacotherapy with sufficient weight loss to achieve targeted health outcome goals that bariatric surgery may be an appropriate option to improve health and offer referral to an experienced bariatric surgeon for consultation and evaluation. | A (Strong) | CQ5 | IIa§ | A |
| 5b. | For individuals with a BMI <35 kg/m2, there is insufficient evidence to recommend for or against undergoing bariatric surgical procedures. | N (No Recommendation) | CQ5 | — | — |
| 5c. | Advise patients that choice of a specific bariatric surgical procedure may be affected by patient factors, including age, severity of obesity/BMI, obesity-related comorbid conditions, other operative risk factors, risk of short- and long-term complications, behavioral and psychosocial factors, and patient tolerance for risk, as well as provider factors (surgeon and facility). | E (Expert Opinion) | CQ5 | IIb | C |
Nutrition professional: In the studies that form the evidence base for this recommendation, a registered dietitian usually delivered the dietary guidance; in most cases, the intervention was delivered in university nutrition departments or in hospital medical care settings where access to nutrition professionals was available.
Trained interventionist: In the studies reviewed, trained interventionists included mostly health professionals (eg, registered dietitians, psychologists, exercise specialists, health counselors, or professionals in training) who adhered to formal protocols in weight management. In a few cases, lay persons were used as trained interventionists; they received instruction in weight management protocols (designed by health professionals) in programs that have been validated in high-quality trials published in peer-reviewed journals.
There is strong evidence that if a provider is going to use a very-low-calorie diet, it should be done with high levels of monitoring by experienced personnel; that does not mean that practitioners should prescribe very-low-calorie diets. Because of concern that an ACC/AHA Class I recommendation would be interpreted to mean that the patients should go on a very-low-calorie diet, it was the consensus of the Expert Panel that this maps more closely to an ACC/AHA Class IIa recommendation.
There is strong evidence that the benefits of surgery outweigh the risks for some patients. These patients can be offered a referral to discuss surgery as an option. This does not mean that all patients who meet the criteria should have surgery. This decision-making process is quite complex and is best performed by experts. The ACC/AHA criterion for a Class I recommendation states that the treatment/procedure should be performed/administered. This recommendation as stated does not meet the criterion that the treatment should be performed. Thus, the ACC/AHA classification criteria do not directly map to the NHLBI grade assigned by the Expert Panel.
ACC indicates American College of Cardiology; AHA, American Heart Association; BMI, body mass index; BP, blood pressure; COR, Class of Recommendation; CQ, critical question; CVD, cardiovascular disease; ES, evidence statement; HDL-C, high-density lipoprotein cholesterol; IDF, International Diabetes Federation; LDL-C, low-density lipoprotein cholesterol; LOE, Level of Evidence; NHLBI, National Heart, Lung, and Blood Institute; NIH, National Health Institute; WHO, World Health Organization; and —, not applicable.
2.2. Chronic Disease Management Model for Primary Care of Patients With Overweight and Obesity—Treatment Algorithm
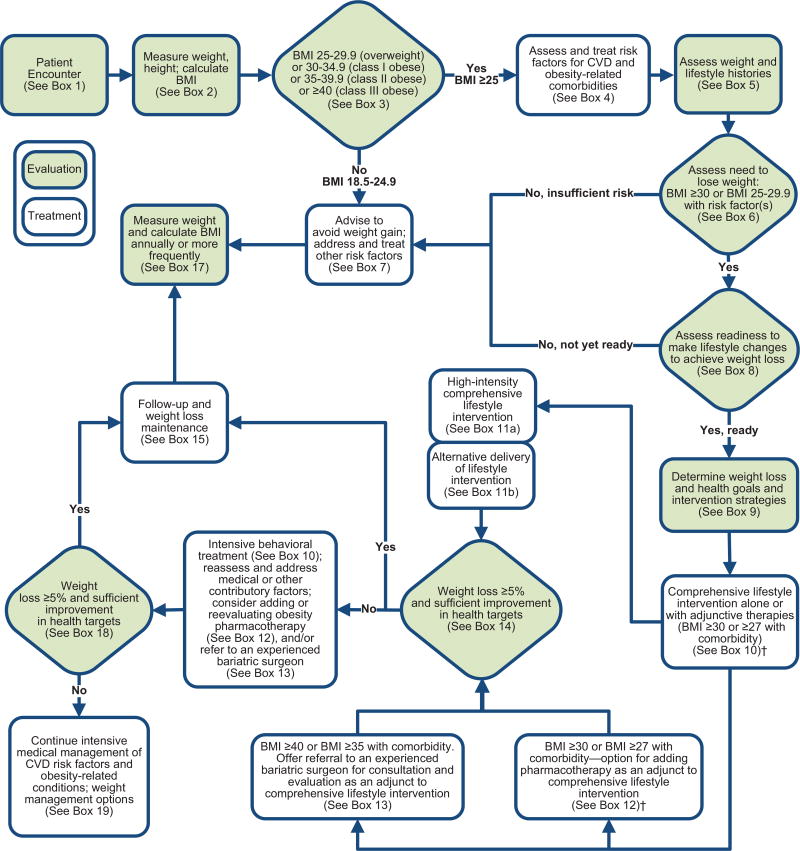
The Expert Panel provides a treatment algorithm, Chronic Disease Management Model for Primary Care of Patients With Overweight and Obesity (Figure 1), to guide PCPs in the evaluation, prevention, and management of excess body weight in their patients. The algorithm incorporates, wherever possible, the recommendations derived from the 5 CQs that yielded ESs and recommendations. However, because the 5 CQs that were considered did not cover the entire scope of evaluation, prevention, and management of overweight/obesity, the panelists provided advice based on other guidelines and expert opinion to give providers a more comprehensive approach to their patients with weight-related issues.
Figure 1.
Treatment Algorithm—Chronic Disease Management Model for Primary Care of Patients With Overweight and Obesity*.
*This algorithm applies to the assessment of overweight and obesity and subsequent decisions based on that assessment. Each step (designated by a box) in this process is reviewed in Section 2.2 and expanded on in subsequent sections. †BMI cutpoint determined by the FDA and listed on the package inserts of FDA-approved obesity medications. BMI indicates body mass index; CVD, cardiovascular disease; and FDA, US Food and Drug Administration.
The algorithm is not intended to supplant initial assessment for cardiovascular risk factors or diseases but rather focuses on the identification of patients with excess body weight and those at risk for obesity-related health problems. Its purpose is to guide weight management decision making.
The algorithm incorporates the recommendations from CQ3 and CQ4 that patients who have sufficient health risk from overweight or obesity receive comprehensive lifestyle intervention. These approaches were all found effective under conditions in which multidisciplinary teams of medical, nutrition, and behavioral experts and other highly trained professionals worked intensively with individuals on weight management. This intervention should be foundational to additional weight management efforts, such as medications or bariatric surgery. It also emphasizes a fundamental principle of chronic disease management—that is, the need to complement a committed patient with informed providers to effectively manage a chronic condition like obesity and its associated cardiovascular risk factors.
3. CQs and Corresponding ESs
Each of the CQs are stated below, together with the number of articles screened against their individual I/E criteria and the number of articles that met the inclusion criteria and were rated as fair or good quality. For CQs that did not have many articles rated fair or good, the articles rated as poor were used (ie, CQ2). The resulting ESs reflect the Expert Panel’s review of the literature. The stated strength of evidence applies to the overall ES, including any bulleted items, unless noted otherwise.
3.1. CQ1: Statement of the Question
Among overweight and obese adults, does achievement of reduction in body weight with lifestyle and pharmacological interventions affect cardiovascular risk factors, CVD events, morbidity, and mortality?
-
1a
Does this effect vary across population subgroups defined by the following demographic and clinical characteristics:
Age
Sex
Race/ethnicity
Baseline BMI
Baseline waist circumference
Presence or absence of comorbid conditions
Presence or absence of cardiovascular risk factors
-
1b
What amount (shown as percent lost, pounds lost, etc.) of weight loss is necessary to achieve benefit with regard to cardiovascular risk factors, morbidity, and mortality?
Are there benefits of cardiovascular risk factors, CVD events, morbidity, and mortality from weight loss?
What are the benefits of more significant weight loss?
-
1c
What is the effect of sustained weight loss for ≥2 years in individuals who are overweight or obese, on cardiovascular risk factors, CVD events, and health and psychological outcomes?
What percent of weight loss needs to be maintained at ≥2 years to be associated with health benefits?
CQ1 was initially intended to be a de novo systematic review of original studies plus systematic reviews and meta-analyses. Because of resource and time constraints, CQ1 was restricted to systematic reviews and meta-analyses published only between January 2000 and October 2011. The titles and abstracts of 1630 publications were screened against the I/E criteria independently by 2 reviewers, which resulted in 669 publications being excluded and 697 publications being retrieved for full-text review to further assess eligibility.* Six hundred ninety-seven full-text publications were independently screened by 2 reviewers, who assessed eligibility by applying the I/E criteria; 669 of these publications were excluded on the basis of ≥1 of the I/E criteria. Of the 697 full-text publications, 42 publications met the criteria and were included. The quality (internal validity) of these 42 publications was assessed using the quality assessment tool developed to assess systematic reviews, meta-analyses, or RCTs. Of these, 14 publications were rated as poor quality. The remaining 28 publications were rated to be of good or fair quality and were included in the evidence base that was used to formulate the ESs and recommendations.12–39 Although the issue of pharmacotherapy was not by itself a CQ, CQ1 was tasked to evaluate this evidence, and several meta-analyses included the effect of orlistat on weight loss and risk factors. None of the systematic reviews or meta-analyses included the Look AHEAD (Action for Health in Diabetes) trial data, which the Expert Panel considered unique in that the number of participants equaled or exceeded the total number of observations in most systematic reviews and meta-analyses. The Look AHEAD papers were included in the database as a critical supplement to the systematic review and meta-analysis information. The ESs were developed from the published literature available as of October 2011 and could not take into account published or unpublished reports of outcomes subsequent to the approval of the statements.
The following ESs reflect the Expert Panel’s review of the literature. See the Full Panel Report supplement for the supportive evidence and spreadsheets.
3.1.1. Weight Loss and Risk of Diabetes
ES1
In overweight and obese adults at risk for type 2 diabetes, average weight losses of 2.5 kg to 5.5 kg at ≥2 years achieved with lifestyle intervention (with or without orlistat) reduce the risk of developing type 2 diabetes by 30% to 60%.
Strength of Evidence: High
ES2
In overweight and obese adults with type 2 diabetes, 2% to 5% weight loss achieved with 1 to 4 years of lifestyle intervention (with or without orlistat) results in modest reductions in fasting plasma glucose concentrations and lowering of hemoglobin A1c by 0.2% to 0.3%.
Strength of Evidence: High
ES3
In overweight and obese adults with type 2 diabetes, those who achieve greater weight loss at 1 year with lifestyle intervention (with or without orlistat) have greater improvements in hemoglobin A1c. Weight loss of 5% to 10% is associated with hemoglobin A1c reductions of 0.6% to 1.0% and reduced need for diabetes medications.
Strength of Evidence: High
ES4
In overweight and obese adults with type 2 diabetes treated for 1 year with lifestyle intervention (with or without orlistat), those who lose more weight achieve greater reductions in fasting plasma glucose concentrations. Those who achieve weight losses of 2% to 5% are more likely to have clinically meaningful (>20 mg/dL) reductions in fasting glucose than those who remain weight stable (defined as gaining ≤2% or losing <2%).
Strength of Evidence: High
ES5
As comprehensive lifestyle treatment of overweight and obese adults with type 2 diabetes continues over 4 years, some weight regain will occur on average; partial weight regain is associated with an increase in hemoglobin A1c, but hemoglobin A1c remains below preintervention levels, and the reduction remains clinically meaningful.23
Strength of Evidence: Moderate
ES6
In observational cohort studies, overweight and obese adults with type 2 diabetes who intentionally lost 9 kg to 13 kg had a 25% decrease in mortality rate compared with weight-stable controls.
Strength of Evidence: Low
ES7
In overweight and obese adults with type 2 diabetes, orlistat with lifestyle intervention results in 2 kg to 3 kg greater weight loss at 1 and 2 years than placebo with lifestyle intervention. The addition of orlistat is associated with greater reductions in fasting blood glucose, averaging 11 mg/dL and 4 mg/dL at 1 and 2 years, as well as an average greater reduction in hemoglobin A1c of 0.4% at 1 year.
Strength of Evidence: High
3.1.2. Weight Loss and Impact on Cholesterol/Lipid Profile
ES1
In overweight or obese adults with or without elevated cardiovascular risk, there is a dose–response relationship between the amount of weight loss achieved by lifestyle intervention and the improvement in lipid profile. The level of weight loss needed to observe these improvements varies by lipid as follows:
At a 3 kg weight loss, a weighted mean reduction in triglycerides of at least 15 mg/dL is observed.
At 5 kg to 8 kg weight loss, low-density lipoprotein cholesterol (LDL-C) reductions of approximately 5 mg/dL and high-density lipoprotein cholesterol (HDL-C) increases of 2 to 3 mg/dL are achieved.
With <3 kg weight loss, more modest and more variable improvements in triglycerides, HDL-C, and LDL-C are observed.
Strength of Evidence: High
ES2
Among overweight and obese adults with type 2 diabetes, 8.0% weight loss at 1 year and 5.3% weight loss over 4 years, compared with usual care control, results in greater average increases (2 mg/dL) in HDL-C and greater average reductions in triglycerides.
Strength of Evidence: Moderate
ES3
A mean 5% weight loss achieved over 4 years by lifestyle intervention in overweight or obese adults with type 2 diabetes is associated with a reduction in newly prescribed lipid-lowering medications compared with controls.
Strength of Evidence: Moderate
ES4
Among overweight and obese adults with type 2 diabetes, there is a dose–response relationship between the amount of weight loss and the increase in HDL-C, which is most pronounced in those who are the least overweight at baseline.
Strength of Evidence: Low
ES5
Compared with placebo, the addition of orlistat to life-style intervention in overweight and obese adults results in an average 3 kg greater weight loss together with an 8 to 12 mg/dL reduction in LDL-C, a 1 mg/dL reduction in HDL-C, and variable changes in triglycerides.
Strength of Evidence: High
3.1.3. Weight Loss and Hypertension Risk
ES1
In overweight or obese adults with elevated cardiovascular risk (including type 2 diabetes and hypertension), there is a dose–response relationship between the amount of weight loss achieved at up to 3 years by lifestyle intervention (alone or with orlistat) and the lowering of BP.
At a 5% weight loss, a weighted mean reduction in systolic and diastolic BP of approximately 3 and 2 mm Hg, respectively, is observed.
At <5% weight loss, there are more modest and more variable reductions in BP.
Strength of Evidence: High
ES2
A 5% mean weight loss difference achieved over 4 years by intensive lifestyle intervention in overweight or obese adults with type 2 diabetes is associated with a lower prevalence of patients who are prescribed antihypertensive medications compared with controls.
Strength of Evidence: Moderate
3.2. CQ2: Statement of the Question
-
2a
Are the current cutpoint values for overweight (BMI 25.0 to 29.9 kg/m2) and obesity (BMI ≥30 kg/m2), compared with BMI 18.5 to 24.9 kg/m2, associated with elevated CVD-related risk (defined below)? Are the waist circumference cutpoints of >102 cm (male) and >88 cm (female) associated with elevated CVD-related risk? How do these cutpoints compare with other cutpoints in terms of elevated CVD-related risk and overall mortality?
Fatal and nonfatal CHD, stroke, and CVD (CHD and stroke)
Overall mortality
Incident type 2 diabetes
Incident dyslipidemia
Incident hypertension
-
2b
Are differences across population subgroups in the relationships of BMI and waist circumference cutpoints with CVD, its risk factors, and overall mortality sufficiently large to warrant different cutpoints? If so, what should they be?
Fatal and nonfatal CHD, stroke, and CVD
Overall mortality
Incident type 2 diabetes
Incident dyslipidemia
-
Incident hypertension
Groups being considered include:
Age
Sex (both male and female)
Race/ethnicity(African American, Hispanic, Native American, Asian, white)
-
2c
What are the associations between weight maintenance and weight gain with elevated CVD-related risk in normal-weight, overweight, and obese adults?
Because of resource limitations, the literature search for CQ2 was limited to studies published between 2000 and 2011, and the evidence review limited to systematic reviews, meta-analyses, and pooled analyses, to limit the number of individual articles to be searched, reviewed, and quality rated. Expert Panel members excluded studies that focused on specific subpopulations with a disease or condition (eg, women with breast cancer, adults on maintenance hemodialysis) and constructed summary evidence tables from the identified articles, and these tables were reviewed and checked by contractor staff for accuracy. Of the 1571 articles initially screened, 15 of the 482 full-text publications met the I/E criteria and were included. The quality (internal validity) of these 15 publications was assessed using the quality assessment tool developed to assess systematic reviews and meta-analyses. Of these, 3 publications were rated as fair40–42; the rest were rated as poor quality but were included in the evidence base because the NHLBI policy indicated that poor studies could be used as part of the evidence base if the majority of included studies were not rated good or fair. The following ESs reflect the Expert Panel's review of the literature.
3.2.1. Current BMI Cutpoints and CVD-Related Risk and All-Cause Mortality
ES1
Among overweight and obese adults, analyses of continuous BMI show that the greater the BMI, the higher the risk of fatal CHD and combined fatal and nonfatal CHD. The current cutpoints for overweight (BMI ≥25.0 kg/m2) and obesity (BMI ≥30 kg/m2) compared with normal weight (BMI 18.5 to <25.0 kg/m2) are associated with elevated risk of combined fatal and nonfatal CHD.
Strength of Evidence: Moderate
ES2
Among overweight and obese adults, analyses of continuous BMI show that the greater the BMI, the higher the risk of fatal CHD and combined fatal and nonfatal CHD in both men and women. The current BMI cutpoints for overweight (BMI ≥25.0 kg/m2) and obesity (BMI ≥30.0 kg/m2) compared with normal weight (BMI 18.5 to <25.0 kg/m2) are associated with elevated risk of fatal CHD in both sexes.
Strength of Evidence: Moderate
ES3
Among overweight or obese adults, analyses of continuous BMI show that the greater the BMI, the higher the risk of fatal stroke overall, as well as ischemic and hemorrhagic stroke. The same relationship holds for combined fatal and nonfatal ischemic stroke but across the entire BMI range, not just in overweight and obese adults. There is no evidence from meta-analyses, pooled analyses, or systematic reviews to change current BMI cutpoints as they relate to risk of stroke.
Strength of Evidence: Moderate
ES4
Among overweight and obese adults, analyses of continuous BMI show that the greater the BMI, the higher the risk of combined fatal and nonfatal CVD. The current cutpoint for obesity (BMI ≥30 kg/m2) compared with normal weight (BMI 18.5 to 24.9 kg/m2) is associated with an elevated risk of fatal CVD in men and women.
Strength of Evidence: Moderate
ES5
In men only, the current BMI cutpoint for overweight (BMI 25.0 to 29.9 kg/m2) compared with normal weight (BMI 18.5 to <25.0 kg/m2) is associated with an elevated risk of fatal CVD. In both men and women, obesity (BMI ≥30.0 kg/m2) compared with normal weight is associated with an elevated risk of fatal CVD.
Strength of Evidence: Low
ES6
With current BMI cutpoints, the relative risk of fatal CVD was higher in obese white women than in obese African-American women compared with normal-weight women. In overweight women, there was no increase in risk of fatal CVD compared with normal-weight women in either race group.
Strength of Evidence: Low
ES7
Analyses of continuous BMI across the entire BMI range show that the greater the BMI, the higher the risk of type 2 diabetes without an indication of a threshold effect.
Strength of Evidence: Moderate
ES8
Among overweight and obese adults, analyses of continuous BMI show that the higher the BMI, the greater the risk of all-cause mortality. The current category for overweight (BMI 25.0 to 29.9 kg/m2) is not associated with elevated risk of all-cause mortality, but a BMI at or above the current cutpoint for obesity (BMI ≥30 kg/m2) is associated with an elevated risk of all-cause mortality, compared with normal weight (18.5 to 24.9 kg/m2).
Strength of Evidence: Moderate
ES9
Sex-specific analyses of continuous BMI among over-weight and obese men and women show that the greater the BMI, the higher the risk of all-cause mortality. The risk of all-cause mortality associated with the current cutpoints of obesity was similar for men and women.
Strength of Evidence: Moderate
3.2.2. Areas of Insufficient Evidence With Regard to Cutpoints for BMI and for Waist Circumference
The Expert Panel was not able to address parts of CQ2 because of the lack of systematic reviews, meta-analyses, and pooled analyses identified in the systematic search. Expert Panel members were aware of a large body of literature from individual studies examining the associations between BMI or waist circumference and hypertension or dyslipidemia, but these studies have not been summarized in meta-analyses, pooled analyses, or systematic reviews that met the criteria. In addition, no studies in the search compared alternative cutpoints with current cutpoints as they relate to risk of CHD, stroke, CVD, overall mortality, and diabetes. No systematic reviews, meta-analyses, or pooled analyses were identified that examined current waist circumference cutpoints as they relate to the risk of all outcomes addressed in CQ2, but the Expert Panel examined meta-analyses of studies that used waist circumference as a continuous variable. There is evidence from systematic reviews, meta-analyses, and pooled analyses that risk factors increase in a continuous manner with waist circumference. Because the Expert Panel was unable to address issues of the adequacy of current waist circumference cutpoints for overweight and obesity in comparison with alternative cutpoints, the choice of cutpoints to apply in patient evaluation is somewhat arbitrary. The Expert Panel was also unable to determine if age-, sex-, or race-specific waist circumference cutpoints for overweight and obesity are warranted to delineate elevated risk of all outcomes examined in CQ2. The absence of evidence from the available systematic reviews, meta-analyses, and pooled analyses for waist circumference cutpoints is not the same as the evidence of absence of usefulness. The Expert Panel acknowledges that this absence does not mean that waist circumference does not provide useful information in certain circumstances. For several of the outcomes, there were no analyses in the studies retrieved that examined current BMI and waist circumference cutpoints stratified by age, sex, and race-ethnicity. Finally, there was a lack of these types of analyses examining the associations between weight maintenance and weight gain with elevated cardiovascular risk in normal-weight, overweight, and obese adults. For this reason, the Expert Panel did not develop ESs addressing questions related to these areas. The methodology team and systematic review team worked closely with Expert Panel members to ensure the accuracy of data and the application of systematic evidence-based methodology.
3.3. CQ3: Statement of the Question
-
3a
In overweight or obese adults, what is the comparative efficacy/effectiveness of diets of differing forms and structures (macronutrient content, carbohydrate and fat quality, nutrient density, amount of energy deficit, and dietary pattern) or other dietary weight loss strategies (eg, meal timing, portion-controlled meal replacements) in achieving or maintaining weight loss?
-
3b
During weight loss or weight maintenance after weight loss, what are the comparative health benefits or harms of the aforementioned diets and other dietary weight loss strategies?
Of the 1422 articles screened against the I/E criteria, 438 full-text articles were retrieved to further assess eligibility. Of the 438 full-text publications, 77 publications met the criteria and were included. A total of 17 trials (23 articles) satisfied the final inclusion criteria for CQ3 and were rated to be of fair or good quality.43–65 the following ESs reflect the Expert Panel’s review of the literature.
3.3.1. Overall Dietary Intervention and Composition—Creating Reduced Dietary Energy Intake
ES1
To achieve weight loss, an energy deficit is required. The techniques for reducing dietary energy intake include the following:
Specification of an energy intake target that is less than that required for energy balance, usually 1200 to 1500 kcal/d for women and 1500 to 1800 kcal/d for men (kilocalorie levels are usually adjusted for the individual’s body weight and physical activity levels);
Estimation of individual energy requirements according to expert guidelines66–68 and prescription of an energy deficit of 500 kcal/d or 750 kcal/d or 30% energy deficit; and
Ad libitum approaches, in which a formal energy deficit target is not prescribed, but lower calorie intake is achieved by restriction or elimination of particular food groups or provision of prescribed foods.
Strength of Evidence: High
ES2
A variety of dietary approaches can produce weight loss in overweight and obese adults. All of the following dietary approaches (listed in alphabetical order) are associated with weight loss if reduction in dietary energy intake is achieved:
A diet from the European Association for the Study of Diabetes Guidelines, which focuses on targeting food groups, rather than formal prescribed energy restriction, while still achieving an energy deficit. Descriptions of the diet can be found in the Full Panel Report supplement.
Higher-protein diet (25% of total calories from protein, 30% of total calories from fat, and 45% of total calories from carbohydrate), with provision of foods that realize an energy deficit.
Higher-protein Zone™-type diet (5 meals/d, each with 40% of total calories from carbohydrate, 30% of total calories from protein, and 30% of total calories from fat) without formal prescribed energy restriction but with a realized energy deficit.
Lacto–ovo–vegetarian–style diet with prescribed energy restriction.
Low-calorie diet with prescribed energy restriction.
Low-carbohydrate diet (initially <20 g/d carbohydrate) without formal prescribed energy restriction but with a realized energy deficit.
Low-fat vegan-style diet (10% to 25% of total calories from fat) without formal prescribed energy restriction but with a realized energy deficit.
Low-fat diet (20% of total calories from fat) without formal prescribed energy restriction but with a realized energy deficit.
Low–glycemic–load diet, either with formal prescribed energy restriction or without formal prescribed energy restriction, but with realized energy deficit.
Lower-fat (≤30% fat), high-dairy (4 servings/d) diets with or without increased fiber and/or low-glycemic-index (low–glycemic-load) foods with prescribed energy restriction.
Macronutrient-targeted diets (15% or 25% of total calories from protein; 20% or 40% of total calories from fat; 35%, 45%, 55%, or 65% of total calories from carbohydrate) with prescribed energy restriction.
Mediterranean-style diet with prescribed energy restriction.
Moderate-protein diet (12% of total calories from protein, 58% of total calories from carbohydrate, and 30% of total calories from fat) with provision of foods that realize an energy deficit.
Provision of high–glycemic-load or low–glycemic-load meals with prescribed energy restriction.
The AHA-style Step 1 diet (prescribed energy restriction of 1500 to 1800 kcal/d, <30% of total calories from fat, <10% of total calories from saturated fat).
Strength of Evidence: High
3.3.2. Overall Dietary Intervention and Composition—Pattern of Weight Loss Over Time With Dietary Intervention
ES3
With dietary intervention in overweight and obese adults, average weight loss is maximal at 6 months, with smaller losses maintained for up to 2 years, while treatment and follow-up tapers. Weight loss achieved by dietary techniques aimed at reducing daily energy intake ranges from 4 kg to 12 kg at 6-month follow-up. Thereafter, slow weight regain is observed, with total weight loss at 1 year of 4 kg to 10 kg and at 2 years of 3 kg to 4 kg.
Strength of Evidence: High
3.3.3. Low-Fat Approaches
ES4a
In overweight and obese adults, there is comparable weight loss at 6 to 12 months with instruction to consume a calorie-restricted (500- to 750-kcal deficit/d) lower-fat diet (<30% of total calories from fat) compared with a higher-fat diet (>40% of total calories from fat). Comprehensive programs of lifestyle change were used in all trials. Comparator diets had ≥40% of total calories from fat, either with a low-carbohydrate or low-glycemic-load diet or one that targets higher fat with either average or low protein.
Strength of Evidence: Moderate
ES4b
With moderate weight loss, lower-fat, higher-carbohydrate diets, compared with higher-fat, lower-carbohydrate diets, have the following differential effects:
Greater reduction in LDL-C,
Lesser reduction in serum triglycerides, and
Lesser increases in HDL-C.
Strength of Evidence: Moderate
ES4c
Evidence is inconsistent with regard to BP differences between lower-fat, higher-carbohydrate diets and higher-fat, lower-carbohydrate diets.
Strength of Evidence: Low
3.3.4. Higher-Protein Approaches (25% to 30% of Energy)
ES5a
In overweight and obese adults, recommendations to increase dietary protein (25% of total calories) as part of a comprehensive weight loss intervention results in weight loss equivalent to that achieved with a typical protein diet (15% of total calories) when both diets are calorie restricted (500- to 750-kcal/d deficit).
Strength of Evidence: High
ES5b
In overweight and obese adults, high-protein diets (25% of total calories) do not result in more beneficial effects on cardiovascular risk factors than typical protein diets (15% of total calories) in the presence of weight loss and other macronutrient changes.
Strength of Evidence: Low
ES5c
On the basis of studies conducted in settings where all food is provided to deliver increased protein (25% of total calories) either as part of caloric restriction or with ad libitum energy consumption, there is insufficient evidence to inform recommendations for weight loss interventions in free-living overweight or obese individuals.
3.3.5. Low-Carbohydrate Approaches (<30 g/d)
ES6a
In overweight and obese adults, there are no differences in weight loss at 6 months with instructions to consume a carbohydrate-restricted diet (20 g/d for up to 3 months, followed by increasing levels of carbohydrate intake up to a point at which weight loss plateaus) in comparison with instruction to consume a calorie-restricted, low-fat diet. The comparator diets on which this statement is based were either a calorie-restricted, higher-carbohydrate, and lower-protein diet (55% of total calories from carbohydrate, 30% of total calories from fat, and 15% of total calories from protein) or a lower-fat European Association for the Study of Diabetes food group dietary pattern (40% of total calories from carbohydrate, 30% of total calories from fat, and 30% of total calories from protein).
Strength of Evidence: Low
ES6b
There is insufficient evidence to comment on the cardiovascular risk factor effects of low-carbohydrate diets.
3.3.6. Complex Versus Simple Carbohydrates
ES7
There is insufficient evidence to comment on the value of substituting either simple or complex carbohydrates for dietary fat in overweight or obese adults for the purpose of weight reduction.
3.3.7. Glycemic Load Dietary Approaches
ES8
In overweight and obese adults, both high– and low–glycemic-load diets produce a comparable weight loss with a similar rate of loss over 6 months.
Strength of Evidence: Low
3.3.8. Dietary Patterns (Mediterranean Style, Vegetarian, and Other Dietary Pattern Approaches)
ES9
In overweight and obese adults, a variety of calorie-restricted dietary patterns (eg, Mediterranean-style diet, lower-fat lacto–ovo-vegetarian or vegan-style diet, or lower-fat diet with high dairy/calcium with added fiber and/or low–glycemic-index [low–glycemic-load] foods) produce weight loss and cardiovascular benefits that are comparable to an energy-restricted, lower-fat dietary pattern (25% to 30% of total calories from fat; Adult treatment Panel III or AHA step 1).
Strength of Evidence: Low
3.3.9. Meal Replacement and Adding Foods to Liquid Diets
ES10a
In overweight and obese women, the use of liquid and bar meal replacements is associated with increased weight loss at up to 6 months, in comparison with a balanced deficit diet using only conventional food. Longer-term evidence of continued weight loss advantage is lacking.
Strength of Evidence: Low
ES10b
There is insufficient evidence to comment on the value of adding various types of foods to a low-calorie liquid diet.
3.3.10. Very–Low-Calorie Diet Approaches
ES11a
There is insufficient evidence to comment on the value of liquid protein supplementation after the very–low-calorie diet induction of weight loss as an aid to weight loss maintenance.
ES11b
There is insufficient evidence to comment on strategies to provide more supervision of very–low-calorie diet adherence or to liberalize very–low-calorie diet therapy with the addition of conventional foods as an aid to the induction of weight loss.
3.4. CQ4: Statement of the Question
-
4a
Among overweight and obese adults, what is the efficacy/effectiveness of a comprehensive lifestyle intervention program (ie, comprised of diet, physical activity, and behavior therapy) in facilitating weight loss or maintenance of lost weight?
-
4b
What characteristics of delivering comprehensive lifestyle interventions (eg, frequency and duration of treatment, individual versus group sessions, on site versus telephone/email contact) are associated with greater weight loss or weight loss maintenance?
The wording of the CQ evolved over time, from a comprehensive intervention initially including 2 or more components (dietary prescription, physical activity, or behavioral therapy) to all 3 components being required. Additional exclusion criteria were later put in place to remove trials that included comprehensive lifestyle interventions but were designed principally to compare different dietary interventions. The Expert Panel decided that such trials were more appropriately addressed under CQ3. The titles and abstracts of 2160 publications were screened against the I/E criteria independently by 2 reviewers (ie, independent contractors), which resulted in 1776 publications being excluded and 384 publications being retrieved for full-text review to further assess eligibility. Three hundred eighty-four full-text publications were independently screened by 2 reviewers who assessed eligibility by applying the I/E criteria; 215 of these publications were excluded on the basis of ≥1 of the I/E criteria.
Out of 384 full-text publications, 146 publications met the criteria and were included. The quality (internal validity) of these 146 publications was assessed using the quality assessment tool developed to assess RCts. Of these, 74 publications were excluded because they were rated as poor quality; of those 74 publications, 43 studies were rated poor because of the intention-to-treat and attrition rates. The remaining 51 trials (72 articles) were rated to be of good or fair quality22, 23, 69–138 and were included in the evidence base that was used to formulate the following ESs and recommendations.
3.4.1. Description of the Diet, Physical Activity, and Behavior Therapy Components in High-Intensity, On-Site Lifestyle Interventions
ES1
The principal components of an effective high-intensity, on-site comprehensive lifestyle intervention include 1) prescription of a moderately reduced-calorie diet, 2) a program of increased physical activity, and 3) the use of behavioral strategies to facilitate adherence to diet and activity recommendations. All 3 components should be included:
Reduced-calorie diet: In comprehensive lifestyle interventions, overweight/obese individuals typically are prescribed a diet designed to induce an energy deficit of ≥500 kcal/d. This deficit often is sought by prescribing 1200 to 1500 kcal/d for women and 1500 to 1800 kcal/d for men. Alternatively, dietary energy deficits can be determined by one of the methods described in CQ3.
Increased physical activity: Comprehensive lifestyle intervention programs typically prescribe increased aerobic physical activity (such as brisk walking) for ≥150 min/wk (equal to ≥30 min/d most days of the week). Higher levels of physical activity, approximately 200 to 300 min/wk, are recommended to maintain lost weight or minimize weight regain in the long term (>1 year).
Behavior therapy: Comprehensive lifestyle interventions usually provide a structured behavior change program that includes regular self-monitoring of food intake, physical activity, and weight. These same behaviors are recommended to maintain lost weight, with the addition of frequent (ie, weekly or more often) monitoring of body weight.
Strength of Evidence: High
3.4.2. Comprehensive Interventions Compared With Usual Care, Minimal Care, or No-Treatment Control
ES2a (Short-Term Weight Loss)
In overweight and obese individuals in whom weight loss is indicated and who wish to lose weight, comprehensive lifestyle interventions consisting of diet, physical activity, and behavior therapy (all 3 components) produce average weight losses of up to 8 kg in 6 months of frequent (ie, initially weekly) on-site treatment provided by a trained interventionist† in group or individual sessions. Such losses (which can approximate reductions of 5% to 10% of initial weight) are greater than those produced by usual care (ie, characterized by the limited provision of advice or educational materials). Comparable 6-month weight losses have been observed in treatment-comparison studies of comprehensive lifestyle interventions, which did not include a usual-care control group.
Strength of Evidence: High
ES2b (Intermediate-Term Weight Loss)
Longer-term comprehensive lifestyle interventions, which additionally provide weekly to monthly on-site treatment for another 6 months, produce average weight losses of up to 8 kg at 1 year, losses that are greater than those resulting from usual care. Comparable 1-year weight losses have been observed in treatment-comparison studies of comprehensive lifestyle interventions, which did not include a usual-care control group.
Strength of Evidence: Moderate
ES2c (Long-Term Weight Loss)
Comprehensive lifestyle interventions that, after the first year, continue to provide bimonthly or more frequent intervention contacts, are associated with gradual weight regain of 1 to 2 kg/y (on average) from the weight loss achieved at 6 to 12 months. Long-term (>1 y) weight losses, however, remain larger than those associated with usual care. Comparable findings have been observed in treatment-comparison studies of comprehensive lifestyle interventions, which did not include a usual-care control group.
Strength of Evidence: High
3.4.3. Efficacy/Effectiveness of Electronically Delivered, Comprehensive Interventions in Achieving Weight Loss
ES3
Electronically delivered, comprehensive weight loss interventions developed in academic settings, which include frequent self-monitoring of weight, food intake, and physical activity—as well as personalized feedback from a trained interventionist†—can produce weight loss of up to 5 kg at 6 to 12 months. This loss is greater than that resulting from no or minimal intervention (ie, primarily knowledge based) offered on the Internet or in print.
Strength of Evidence: Moderate
3.4.4. Efficacy/Effectiveness of Comprehensive, Telephone-Delivered Lifestyle Interventions in Achieving Weight Loss
ES4
In comprehensive lifestyle interventions that are delivered by telephone or face-to-face counseling and that also include the use of commercially-prepared prepackaged meals or an interactive Web-based program, the telephone-delivered and face-to-face–delivered interventions produce similar mean net weight losses of approximately 5 kg at 6 months and 24 months, compared with a usual-care control group.
Strength of Evidence: Low
3.4.5. Efficacy/Effectiveness of Comprehensive Weight Loss Programs in Patients Within a Primary Care Practice Setting Compared With Usual Care
ES5
In studies to date, low- to moderate-intensity lifestyle interventions for weight loss provided to overweight or obese adults by primary care practices alone have not been shown to be effective.
Strength of Evidence: High
3.4.6. Efficacy/Effectiveness of Commercial-Based, Comprehensive Lifestyle Interventions in Achieving Weight Loss
ES6
Commercial-based, comprehensive weight loss interventions that are delivered in person have been shown to induce an average weight loss of 4.8 kg to 6.6 kg at 6 months in 2 trials when conventional foods are consumed and 6.6 kg to 10.1 kg at 12 months in 2 trials with provision of prepared food. These losses are greater than those produced by minimal-treatment control interventions.
Strength of Evidence: Low
3.4.7. Efficacy/Effectiveness of Very–Low-Calorie Diets as Used as Part of a Comprehensive Lifestyle Intervention in Achieving Weight Loss
ES7a
Comprehensive, high-intensity, on-site lifestyle interventions that include a medically supervised very–low-calorie diet (often defined as <800 kcal/d), as provided by complete meal replacement products, produce total weight loss of approximately 14.2 kg to 21.0 kg over 11 to 14 weeks, which is larger than that produced by no intervention or usual care (ie, advice and education only).
Strength of Evidence: High
ES7b
After the cessation of a high-intensity lifestyle intervention with a medically supervised very–low-calorie diet of 11 to 14 weeks, weight regain of 3.1 kg to 3.7 kg has been observed during the ensuing 21 to 38 weeks of non-intervention follow-up.
Strength of Evidence: High
ES7c
The prescription of various types (resistance or aerobic training) and doses of moderate-intensity exercise training (eg, brisk walking 135 to 250 min/wk) delivered in conjunction with weight loss maintenance therapy does not reduce the amount of weight regained after the cessation of the very– low-calorie diet, compared with weight loss maintenance therapy alone.
Strength of Evidence: Low
3.4.8. Efficacy/Effectiveness of Comprehensive Lifestyle Interventions in Maintaining Lost Weight
ES8a
After initial weight loss, some weight regain can be expected, on average, with greater regain observed over longer periods of time. Continued provision of a comprehensive weight loss maintenance program (on site or by telephone) for periods of up to 2.5 years after initial weight loss reduces weight regain, as compared with the provision of minimal intervention (ie, usual care). The optimal duration of weight loss maintenance programs has not been determined.
Strength of Evidence: Moderate
ES8b
Of overweight/obese adults who participate in a high-intensity long-term comprehensive lifestyle intervention, 35% to 60% maintain a loss of ≥5% of initial body weight at ≥2 years' follow-up (after randomization).
Strength of Evidence: Moderate
3.4.9. Characteristics of Lifestyle Intervention Delivery That May Affect Weight Loss: Intervention Intensity
ES9a (Moderate-Intensity Interventions)
Moderate-intensity, on-site comprehensive lifestyle interventions, which provide an average of 1 to 2 treatment sessions per month, typically produce mean weight losses of 2 kg to 4 kg in 6 to 12 months. These losses generally are greater than those produced by usual care (ie, minimal-intervention control group).
Strength of Evidence: High
ES9b (Low-Intensity Interventions)
Low-intensity, on-site comprehensive lifestyle interventions, which provide less-than-monthly treatment sessions, do not consistently produce weight loss when compared with usual care.
Strength of Evidence: Moderate
ES9c (Effect of Intervention Intensity)
When weight loss with each intervention intensity (ie, low, moderate, and high) is compared with usual care, high-intensity lifestyle interventions (≥14 sessions in 6 months) typically produce greater net-of-control weight losses than do low-to moderate-intensity interventions.
Strength of Evidence: Moderate
3.4.10. Characteristics of Lifestyle Intervention Delivery That May Affect Weight Loss or Weight Loss Maintenance: Individual Versus Group Treatment
ES10
There do not appear to be substantial differences in the size of the weight losses produced by individual- and group-based sessions in high-intensity, comprehensive lifestyle intervention delivered on site by a trained interventionist.†
Strength of Evidence: Low
3.4.11. Characteristics of Lifestyle Intervention Delivery That May Affect Weight Loss or Weight Loss Maintenance: On-Site Versus Electronically Delivered Interventions
ES11
Weight losses observed in comprehensive lifestyle interventions, which are delivered on site by a trained interventionist† in initially weekly and then biweekly group or individual sessions, are generally greater than weight losses observed in comprehensive interventions that are delivered by Internet or email and that include feedback from a trained interventionist.
Strength of Evidence: Low
3.5. CQ5: Statement of the Question
-
5a
Bariatric Surgery Efficacy. What are the long-term effects of the following surgical procedures on weight loss, weight loss maintenance, cardiovascular risk factors, related comorbidities, and mortality?
Laparoscopic adjustable gastric banding (LAGB)
Laparoscopic Roux-en-Y gastric bypass (RYGB)
Open RYGB
Biliopancreatic diversion (BPD) with and without duodenal switch
-
Sleeve gastrectomy
What are the long-term effects of these surgical procedures in patients with different BMIs and comorbidities?
BMI <35
BMI 35 to <40 with no comorbidities
BMI ≥35 with comorbidities
BMI ≥40 with no comorbidities
-
5b
Predictors. What are the predictors associated with long-term effects of the following surgical procedures on weight loss, weight loss maintenance, cardiovascular risk factors, related comorbidities, and mortality?
LAGB
Laparoscopic RYGB
Open RYGB
BPD with and without duodenal switch
-
Sleeve gastrectomy
What are the predictors associated with long-term effects of these surgical procedures in patients with different BMIs and comorbidities?
BMI <35
BMI 35 to <40 with no comorbidities
BMI ≥35 with comorbidities
BMI ≥40 with no comorbidities
-
5c
Complications: What are the short-term (<30 days) and long-term (≥30 days) complications of the following bariatric surgical procedures? What are the predictors associated with complications?
LAGB
Laparoscopic RYGB
Open RYGB
BPD with and without duodenal switch
-
Sleeve gastrectomy
What are the complications of these surgical procedures in patients with different BMIs and comorbidities?
BMI <35
BMI 35 to <40 with no comorbidities
BMI ≥35 with comorbidities
BMI ≥40 with no comorbidities
Many, if not most, patients with extreme obesity have tried to lose weight numerous times. Some have lost substantial amounts of weight successfully, only to regain it. Although lifestyle intervention is the mainstay of all weight management treatment, there is increasing recognition of the need for adjunctive treatments for patients with obesity who are at high medical risk and who are unable to achieve or maintain sufficient weight loss to improve their health. Bariatric surgery is one treatment option that has been increasingly used in patients with extreme obesity or with lesser degrees of obesity but with obesity-related comorbid conditions. Bariatric surgery is, by definition, invasive and has inherent short-term risks as well as adverse effects that may become apparent only during longer-term follow-up. Incurring these risks may be acceptable if health benefits are sustained over time. Therefore, the Expert Panel believed that evaluation of efficacy endpoints for weight loss and change in cardiovascular risk factors and other health outcomes required studies with a minimum postsurgical follow-up of 2 years and inclusion of a nonsurgical comparator group. Studies evaluating predictors of weight change or medical outcomes, including patient factors (eg, presence or absence of diabetes) or surgical factors (eg, RYGB versus BPD) required studies that directly compared these factors plus a minimum 2-year follow-up. Studies evaluating complications of bariatric surgery required at least 30-day postsurgical follow-up. For observational studies with ≥10 years of follow-up or for studies on BPD or sleeve gastrectomy procedures, sample size ≥100 was required, and for all other observational studies the sample size requirement was ≥500. This sample size requirement was instituted because the most important complications are infrequent (eg, perioperative mortality <1%), such that smaller studies could give inaccurate estimates of complication rates.
The literature search for CQ5 included an electronic search for RCTs, controlled clinical trials, and observational studies published in the literature from January 1998 to December 2009. The search produced 2317 citations, with 9 additional citations identified from nonsearch sources—that is, by Expert Panel members or hand search of systematic reviews and meta-analyses (obtained through the electronic search). Of the 2317 citations identified through the database search, 811 citations were automatically excluded, and the titles and abstracts of the 1515 remaining citations were screened against the I/E criteria for each of the 3 components (efficacy, predictors, and complications) independently by 2 reviewers, which resulted in 1062 publications being excluded. Of the remaining 453 full-text publications, 64 met the I/E criteria, underwent full text review, and were included. The quality (internal validity) of these 64 publications was assessed, and of these, 29 publications were excluded because they were rated as poor quality; 18 studies were rated poor because of the intent-to-treat and/or attrition rates. The remaining 22 studies (35 articles) that met the criteria for at least 1 of the 3 components were rated good or fair quality and included in the evidence base.139–173 For the efficacy, predictors, and complications components, 5 studies (17 articles), 10 studies (12 articles) and 14 studies (15 articles) were rated as good/fair, respectively. A total of 8 articles were used across more than 1 component.141,142,144,148,156,159,168,169
3.5.1 Component 1: Efficacy
A total of 5 studies (17 articles) met the criteria for determining the efficacy of bariatric surgery for weight loss and the impact on obesity-related comorbidities, were rated as good or fair quality, and are included in the summary table. The number of studies meeting inclusion criteria was limited because of the requirement that surgical treatment be compared with a nonsurgical comparator group with a minimum postsurgical follow-up of 2 years.
ES1
In obese adults, bariatric surgery produces greater weight loss and weight loss maintenance than that produced by usual care, conventional medical treatment, lifestyle intervention, or medically supervised weight loss, and weight loss efficacy varies depending on the type of procedure and initial body weight.
Weight loss at 2 to 3 years after a variety of surgical procedures in adults with presurgical BMI ≥30 varies from a mean of 20% to 35% of initial weight and mean difference from nonsurgical comparators of 14% to 37%, depending on procedure.
Strength of Evidence: High
Mean weight loss at 10 years after a variety of bariatric surgical procedures (predominantly vertical banded gastroplasty) is approximately 16% of initial weight, representing a mean weight regain of 7%.
Strength of Evidence: Low
ES2
In obese adults, bariatric surgery generally results in more favorable impact on obesity-related comorbid conditions than that produced by usual care, conventional medical treatment, lifestyle intervention, or medically supervised weight loss.
At 2 to 3 years after a variety of bariatric surgical procedures in adults with BMI ≥30 who achieve mean weight loss of 20% to 35%, fasting glucose and insulin are reduced and incidence of type 2 diabetes is decreased, and there is a greater likelihood of diabetes remission among those with type 2 diabetes at baseline.
Strength of Evidence: High
At 10 years, incidence and prevalence of type 2 diabetes are lower in those who have undergone surgery. However, among those in whom type 2 diabetes remits after surgery, diabetes may recur over time.
Strength of Evidence: Low
At 2 to 3 years after a variety of bariatric surgical procedures in adults with BMI ≥30 who achieve mean weight loss of 20% to 35%, BP or use of BP medication is reduced compared with nonsurgical management. BP tends to increase over time, and at 10 years after surgery, there is no difference in mean systolic BP or the incidence of new cases of hypertension in those who have undergone bariatric surgery compared with those who have not undergone surgery.
Strength of Evidence: Low
Among obese adults with baseline hypertension, a greater percentage are in remission at 2 to 3 years and 10 years after bariatric surgery compared with nonsurgical management.‡
Strength of Evidence: Low
At 2 to 3 years and 10 years after a variety of bariatric surgical procedures in adults with BMI ≥30 who achieve mean weight loss of 20% to 35%, serum triglyceride levels are lower, HDL-C levels are higher, ratio of total cholesterol to HDL-C is lower, and changes in total cholesterol or LDL levels are inconsistent, compared with nonsurgical management.
Strength of Evidence: Low
Most measures of health-related quality of life are improved at 2 and 10 years after bariatric surgery.
Strength of Evidence: Moderate
Total mortality is decreased compared with nonsurgical management at mean follow-up of 11 years after undergoing a variety of bariatric surgical procedures (predominantly vertical banded gastroplasty) in patients with mean BMI >40 who achieve a mean long-term weight loss of 16%.
Strength of Evidence: Low
ES3
There are insufficient data on the efficacy of bariatric surgical procedures for weight loss and maintenance or risk factors for CVD ≥2 years after surgery in patients with a BMI <35.
3.5.2. Component 2: Predictors
A total of 10 studies (12 articles) met the inclusion criteria, were rated as good or fair quality, and are included in the summary table.141,142,144,148,151,155,156,159,161,168,169,172 The studies were required to have a comparator group but not necessarily a nonsurgical comparator, as well as outcomes of specific bariatric operative procedures.
ES4
Weight loss after bariatric surgery expressed as percentage of total body weight loss varies by procedure.
In direct comparative studies at 2 to 3 years after surgery:
Weight loss after gastric bypass exceeds that achieved after LAGB.
Strength of Evidence: Moderate
Weight losses after BPD, gastric bypass, and sleeve gastrectomy are similar.
Strength of Evidence: Low
In direct comparative studies at 5 to 10 years after surgery:
Weight loss after gastric bypass exceeds that achieved after LAGB.
Strength of Evidence: Low
ES5
The remission of obesity-related comorbidities varies by procedure.
Type 2 diabetes remission or improved glycemic control occurs with increasing frequency according to procedure as follows: LAGB, gastric bypass, BPD.
Strength of Evidence: Low
Reduction in the prevalence of hypertension is more frequent after gastric bypass than after LAGB.
Strength of the Evidence: Low
The prevalence of dyslipidemia is lower after gastric bypass than after LAGB.
Strength of Evidence: Low
3.5.3. Component 3: Complications
Fourteen studies met the inclusion criteria for complications. The complication evidence base included those studies from the efficacy and predictors searches that included complication data,141,156 as well as those studies that met the expanded search criteria.139,143,145,146,152,153,160,170,171
3.5.3.1. Laparoscopic Adjustable Gastric Banding
ES6
Perioperative (≤30 day) and longer-term (>30 days) complications after bariatric surgery vary by procedure and patient-derived risk factors. When LAGB is performed by an experienced surgeon:
Perioperative complications are infrequent and do not tend to be life-threatening: major adverse outcomes (1%), such as deep venous thrombosis and reoperations, and minor complications (3%), such as wound infection.
Strength of Evidence: Moderate
Longer-term complications continue to occur over time and may require operative correction: misplacement of band, approximately 3% to 4%; erosion of gastric wall, approximately 1%; and port complication, 5% to 11%.
Strength of Evidence: Moderate
The rate of longer-term LAGB failure leading to removal of the band with or without conversion to another bariatric procedure varies from 2% to 34%. Inadequate weight loss is the most often reported basis for removal of band.
Strength of Evidence: Moderate
3.5.3.2. Roux-en-Y Gastric Bypass
ES6 (continued)
Perioperative (≤ 30 days) and longer-term (>30 days) complications after bariatric surgery vary by procedure and patient-derived risk factors. When gastric bypass is performed by an experienced surgeon:
Perioperative complications consist of a major adverse outcome in approximately 4% to 5% of patients, including mortality (0.2%), deep vein thrombosis and/or pulmonary embolism (0.4%), and a need for reoperation (3% to 5%). The rate of any complication, major or minor, is 2% to 18%.
Strength of Evidence: Moderate
Perioperative complications are less frequent for the laparoscopic approach than for open incision.
Strength of Evidence: Moderate
When open gastric bypass is performed by an experienced surgeon:
Perioperative complications consist of a major adverse outcome in approximately 8% of patients, including mortality (2%), deep vein thrombosis or pulmonary embolism (1%), and a need for reoperation (5%).
Strength of Evidence: Low
Perioperative complications are associated with extremely high BMI, inability to walk 200 feet, history of deep vein thrombosis or pulmonary embolism, and history of obstructive sleep apnea.
Strength of the Evidence: Low
3.5.3.3. Biliopancreatic Diversion
ES6 (continued)
Perioperative (≤ 30 days) and longer-term (>30 days) complications after bariatric surgery vary by procedure and patient-derived risk factors. The mortality rate for BPD was reported by 2 of the 3 included studies. When BPD is performed by an experienced surgeon:
Perioperative complications occur in 2% to 8% of cases and include mortality (<1%) and deep vein thrombosis or pulmonary embolism (0.4%). The frequency of anastomotic leak, hemorrhage, and wound complication is variable.
Strength of the Evidence: Low
One- to three-year complications include: anemia (13% to 20%); deficiency of protein (0.3% to 3.0%), iron (17%), or zinc (6%); and neuropathy (0.4%). Deficiency of vitamin d and elevated parathyroid hormone may exceed 40%.
When BPD is performed by open incision, the rate of ventral hernia can be as high as 72%.
Strength of the Evidence: Low
3.5.3.4. Laparoscopic Sleeve Gastrectomy
ES6 (continued)
Perioperative (≤ 30 days) and longer-term (>30 days) complications after bariatric surgery vary by procedure and patient-derived risk factors. When laparoscopic sleeve gastrectomy is performed by an experienced surgeon:
There is insufficient evidence to establish the incidence of perioperative and longer-term complications.
4. Gaps in Evidence and Future Research Needs
The Expert Panel identified gaps in evidence supporting the 5 chosen CQs. For each CQ, the Expert Panel summarized recommendations for future research. See the Full Panel Report Supplement for a more detailed and comprehensive discussion.
4.1. CQ1. (Benefits of Weight Loss)
The literature available in systematic reviews and meta-analyses did not specifically address whether age, sex, race, or baseline BMI or waist circumference modifies the beneficial effects of weight loss on cardiovascular risk factors. Likewise, the systematic reviews and meta-analyses did not specifically address the issue of how baseline comorbid conditions and cardiovascular risk factors modify the response to weight loss. Nevertheless, high-quality literature that addresses these issues could exist. Given that caveat and the present evidence review, future research in this area should address the following issues:
Do the observed improvements in cardiovascular risk factors, need for medications, and improved quality of life associated with weight loss differ by age, sex, race, or BMI or waist circumference?
What is the cost-effectiveness of modest weight loss as a preventive strategy for those at risk of developing type 2 diabetes?
What is the best approach to identify and engage those who can benefit from weight loss?
4.2. CQ2. (Risks of Overweight and Obesity)
Because evidence-based methods to identify patients with elevated risk for CVD, its risk factors, and all-cause mortality are essential for healthcare practitioners, more systematic reviews, meta-analyses, and pooled analyses are needed to inform future guidelines in the following areas:
-
Studies are needed that compare current BMI and waist circumference cutpoints with alternative cutpoints for predicting risk to optimize the specificity of cutpoints.
-
–
Studies should examine the independent and combined effects of BMI and waist circumference to determine if both in combination are better at predicting elevated risk than either alone.
-
–
Such studies should explicate the methods and logical framework that guides the choice of optimal cutpoints.
-
–
Studies comparing the predictive ability of BMI and waist circumference with more objective measures of percent body fat, such as dual-energy x-ray absorptiometry or magnetic resonance imaging, may enhance risk prediction of cutpoints and/or combinations of BMI and waist circumference.
-
–
-
Similar studies are needed to assess whether overall cutpoints are appropriate for population subgroups stratified by age, sex, and race/ethnicity.
-
–
Studies that compare risk across different age groups should report absolute risk estimates. This is especially important when examining age.
-
–
Studies are needed on racial-ethnic differences in risk within Western countries, particularly in Asian Americans and Hispanic Americans.
-
–
Longitudinal studies are needed that assess the risks associated with weight change (accounting for intentionality) in normal-weight, overweight, and obese adults to determine the role of weight change trajectory in risk assessment.
4.3. CQ3. (Dietary Interventions for Weight Loss)
More research is needed to inform future guidelines about dietary interventions for weight loss.
Because long-term dietary adherence is problematic in weight management, to determine the best dietary approach to sustain weight loss over the long term, studies are needed that:
Test the impact of tailoring choice of dietary interventions on the individual’s ability to adhere in the long term.
Test pragmatic approaches to diet intervention delivery in free-living individuals for at least 2 years duration.
Evaluate the physiological and biological adaptations to weight loss, so as to refine methods of caloric restriction during weight reduction and maintenance.
4.4. CQ4. (Lifestyle Interventions for Weight Loss)
More research is needed to inform future guidelines focusing on improvements in efficiency and efficacy, optimizing delivery and dissemination, and targeting special populations. The research is needed in the following areas:
-
On-site (face-to-face), comprehensive, high-intensity lifestyle interventions (14 or more contacts in first 6 months) represent the standard for behavioral weight loss interventions. Further research can help improve efficiency of these interventions with studies that:
-
–
Evaluate optimal frequency (and duration) of contact.
-
–
Evaluate characteristics of those who lose less weight in response to a standard, comprehensive behavioral intervention, and develop alternative approaches for their treatment.
-
–
Evaluate effective methods of delivering lifestyle interventions remotely (eg, Internet, mobile phone, text messaging, telephone, DVDs, or some combination of these) to achieve and maintain clinically meaningful weight loss.
-
–
Because of changing demographics, there is a need for further research to understand the most appropriate strategies and prescriptions for weight loss for some key populations, including older adults and racial/ethnic groups.
-
Because the efficacy of on-site (face-to-face), comprehensive, high-intensity lifestyle intervention has been established in academic settings, translational studies are needed that:
-
–
Evaluate programs that can be delivered in community, work-site, and other settings (including commercial programs).
-
–
Determine the personal characteristics, skills, and training required of a lifestyle interventionist.
-
–
Identify the optimal role for PCPs to play in the management of obesity by lifestyle modification.
-
–
Evaluate head-to-head comparisons of the relative effectiveness and associated costs of delivering interventions on site (face-to-face), remotely, or by a combination of approaches (ie, hybrid delivery).
-
–
-
Because maintenance of lost weight over the long term has been challenging, studies are needed that:
-
–
Evaluate strategies to promote additional weight loss beyond the first 6 months, the time at which weight loss plateaus in most individuals.
-
–
Evaluate novel methods of improving the maintenance of lost weight.
-
–
Further study is needed on the effect of weight loss treatment on healthcare utilization and cost.
4.5. CQ5. (Surgical Procedures for Weight Loss)
More research is needed to inform future guidelines in the following areas:
Because bariatric surgery offers the potential for prevention or remission of diabetes, better control of cardiovascular risk factors, improvement in quality of life and possibly decreased mortality, there is a need for research to better characterize those patients who are most likely to benefit from and least likely to suffer adverse consequences of bariatric surgical procedures.
-
Large and well-designed experimental, quasi-experimental, and observational studies with long-term follow-up are needed to determine whether the risks and benefits of bariatric surgery are sustained over time. Studies are needed that:
-
–
Evaluate which surgical procedures are best applied to different populations, on the basis of factors such as presence and duration of comorbid conditions, age, sex, race/ethnicity, degree and duration of obesity, underlying genetic etiologies, and psychosocial or behavioral characteristics.
-
–
Evaluate the implementation of bariatric surgery in nonacademic settings, which may be more reflective of real-world clinical practice.
-
–
Supplementary Material
Box 1: Patient Encounter for Obesity Prevention and Management.
A patient encounter for obesity prevention and management is defined as an interaction with a PCP who assesses a patient's weight status to determine presence of overweight or obesity and need for further assessment and treatment.
Box 2: Measure Weight and Height; Calculate BMI.
With the patient wearing light clothing or an examination gown and no shoes, weight and height are measured and the BMI calculated. BMI can be calculated manually (weight in kg/[height in meters]2) or electronically by using the electronic medical record or other resources. The BMI should be documented in the patient medical record.
Box 3: BMI 25–29.9 (overweight), BMI 30–34.9 (class I obese), BMI 35–39.9 (class II obese), or BMI ≥40 (class III obese [extreme obesity]).
These BMI cutpoints define overweight and class I to III obese individuals and identify adults who may be at increased risk for CVD and other obesity-related conditions. Within these categories, additional personal risk assessment is needed because degree of risk can vary (Box 4 and CQ2).
Box 4: Assess and Treat Cardiovascular Risk Factors and Obesity-Related Comorbidities.
Assess risk of CVD and/or presence of obesity-related comorbidities. Risk assessment for CVD and diabetes in a person with overweight or class I to III obesity includes history; physical examination; and clinical and laboratory assessments, including BP, fasting blood glucose, and fasting lipid panel (expert opinion). A waist circumference measurement is recommended for individuals with BMI 25–34.9 kg/m2 to provide additional information on risk. It is unnecessary to measure waist circumference in patients with BMI ≥35 kg/m2 because the waist circumference will likely be elevated and will add no additional risk information. The Expert Panel recommends, by expert opinion, using the current cutpoints (>88 cm [>35 in] for women and >102 cm [>40 in] for men) as indicative of increased cardiometabolic risk.
Because obesity is associated with increased risk of hypertension, dyslipidemia, diabetes, and a host of other comorbidities, the clinician should assess for associated conditions. The Expert Panel recommends, by expert opinion, that intensive management of cardiovascular risk factors (hypertension, dyslipidemia, prediabetes, or diabetes) or other obesity-related medical conditions (eg, sleep apnea) be instituted if they are found, regardless of weight loss efforts.
Box 5: Assess Weight and Lifestyle Histories.
The Expert Panel recommends, by expert opinion, that the clinician assess weight and lifestyle histories and determine other potential contributory factors: Ask questions about history of weight gain and loss over time, details of previous weight loss attempts, dietary habits, physical activity, family history of obesity, and other medical conditions or medications that may affect weight. Answers to these questions may provide useful information about the origins of or maintaining factors for overweight and obesity, including success and difficulties with previous weight loss or maintenance efforts. This information can help the clinician determine any adjustments to the patient's medical regimen that can assist weight management efforts and provide appropriate advice on lifestyle change. The information may also impact recommendations for treatment.
Box 6: Assess Need to Lose Weight.
YES: BMI ≥30 or BMI 25–29.9 with additional risk factor(s)
Weight loss treatment is indicated for ≥ 1) obese individuals and 2) overweight individuals with 1 indicators of increased cardiovascular risk (eg, diabetes, prediabetes, hypertension, dyslipidemia, elevated waist circumference) or other obesity-related comorbidities.
NO: BMI <25 or BMI 25–29.9 without additional risk
Normal-weight patients (BMI 18.5–24.9 kg/m2 should be advised to avoid weight gain (Box 7).
Patients who are overweight (BMI 25–29.9 kg/m2) who do not have indicators of increased cardiovascular risk (eg, diabetes, prediabetes, hypertension, dyslipidemia, elevated waist circumference) or other obesity-related comorbidities should be advised to avoid additional weight gain (Box 7).
Box 7: Advise to Avoid Weight Gain and Address Other Risk Factors.
A. Normal weight
Individuals who are normal weight (BMI 18.5–24.9 kg/m2) and do not have a history of overweight or obesity should be counseled on the desirability of avoiding weight gain to prevent the health risks of increased body weight.
B. Overweight without additional risk factors or normal weight with a history of overweight or obesity
For individuals who are overweight (BMI 25–29.9 kg/m2) and who do not have indicators of increased cardiovascular risk (eg, diabetes, prediabetes, hypertension, dyslipidemia, elevated waist circumference) or other obesity-related comorbidities, and for individuals who have a history of overweight and are now normal weight with risk factors at acceptable levels, advise patients to frequently measure their own weight and to avoid weight gain by adjusting their food intake if they start to gain more than a few pounds. Also, advise patients that engaging in regular physical activity will help them avoid weight gain.
C. Overweight or obese individuals who would benefit from weight loss but who are not currently prepared or able to lose weight
Periodically assess the patient's interest in and readiness for weight loss as shown in Box 8, and counsel the patient on the desirability of avoiding additional weight gain to prevent greater health risk. Regardless of patient's interest in or readiness for weight loss intervention, any cardiovascular risk factors and obesity-related health conditions should be evaluated and treated.
Box 8: Assess Readiness to Make Lifestyle Changes to Achieve Weight Loss and Identify Barriers to Success.
The Expert Panel advises (expert opinion) that the clinician and patient agree on whether weight loss is appropriate. The clinician, together with the patient, should assess whether the patient is prepared and ready to undertake the measures necessary to succeed at weight loss before beginning comprehensive counseling efforts. The clinician can ask, "How prepared are you to make changes in your diet, to be more physically active, and to use behavior change strategies such as recording your weight and food intake?" These are the components of a comprehensive lifestyle intervention.
The decision to undertake weight loss efforts must be made in the context of competing priorities (eg, smoking cessation may supersede a weight loss effort; life events may make the effort at weight reduction futile until a future time). If the patient is not prepared to undertake these changes, attempts to counsel the patient on how to make lifestyle changes are likely to be counterproductive.
Box 9: Determine Weight Loss and Health Goals and Intervention Strategies.
Clinician and patient devise weight loss and health goals and comprehensive lifestyle treatment strategies to achieve these goals.
Recommended goals for weight loss
A realistic and meaningful weight loss goal is an important first step. Although sustained weight loss of as little as 3%–5% of body weight may lead to clinically meaningful reductions in some cardiovascular risk factors, larger weight losses produce greater benefits. The Expert Panel recommends as an initial goal the loss of 5%–10% of baseline weight within 6 months.
Recommended methods for weight loss
Weight loss requires creating an energy deficit through caloric restriction, physical activity, or both. An energy deficit of ≥500 kcal/d typically may be achieved with dietary intake of 1200–1500 kcal/d for women and 1500–1800 kcal/d for men. The choice of calorie-restricted diet can be individualized to the patient's preferences and health status (CQ3). Very-low-calorie diets (<800 kcal/d) should be used only in limited circumstances in a medical care setting where medical supervision and a highintensity lifestyle intervention can be provided. If a specialized diet for CVD risk reduction, diabetes, or other medical conditions is also prescribed, referral to a nutrition professional* is recommended (CQ3).
Recommendations for management of medical conditions during weight loss
While weight loss treatment is ongoing, manage risk factors such as hypertension, dyslipidemia, and other obesity-related conditions. This includes monitoring the patient's requirements for medication change as weight loss progresses, particularly for antihypertensive medications and diabetes medications that can cause hypoglycemia.
Box 10: Weight Loss Option—Comprehensive Lifestyle Intervention Alone or With Adjunctive Therapies‡.
All patients for whom weight loss is recommended should be offered or referred for comprehensive lifestyle intervention (Box 11a and 11b). Comprehensive lifestyle intervention, preferably with a trained interventionist† or nutrition professional*, is foundational to weight loss (Box 11a) regardless of augmentation by medications or bariatric surgery.
By expert opinion, if the weight and lifestyle history indicates that the patient has never participated in a comprehensive lifestyle intervention program as defined in CQ4 and in Box 11a, it is recommended that he or she be encouraged to undertake such a program before the addition of adjunctive therapies since a substantial proportion of patients will lose sufficient weight to improve health with comprehensive lifestyle treatment alone. This recommendation may be modified by the availability of comprehensive lifestyle intervention or by patient factors, such as medical conditions that warrant earlier initiation of more intensive treatment.
If the patient has been unable to lose weight or sustain weight loss with comprehensive lifestyle intervention and he or she has a BMI ≥30 kg/m2 or BMI ≥27 kg/m2 with comorbidity, adjunctive therapies may be considered.
Patients who are otherwise appropriate candidates for obesity drug treatment or bariatric surgery, whose weight and lifestyle history indicate a history of inability to achieve or sustain weight loss and who have previously participated in a comprehensive lifestyle intervention, may be offered the option to add pharmacotherapy at the time of initiation of a lifestyle intervention program (BMI ≥30 kg/m2 or BMI ≥27 kg/m2 with comorbidity) or to be referred for evaluation for bariatric surgery (BMI ≥40 kg/m2 or BMI ≥35 kg/m2 with comorbidity) (expert opinion).‡
Box 11a. Offer or Refer for High-Intensity Comprehensive Lifestyle Intervention.
The most effective behavioral weight loss treatment is an in-person, high-intensity (ie, ≥14 sessions in 6 months) comprehensive weight loss intervention provided in individual or group sessions by a trained interventionist† (CQ4). The principal components of an effective highintensity, on-site comprehensive lifestyle intervention include 1) prescription of a moderately reduced-calorie diet, 2) a program of increased physical activity, and 3) the use of behavioral strategies to facilitate adherence to diet and activity recommendations. As shown in CQ4, comprehensive lifestyle intervention consisting of diet, physical activity, and behavior therapy produces average weight losses of approximately 8 kg in a 6-month period of frequent, in-person treatment. This approximates losses of 5%–10% of initial weight. The observed average weight loss of approximately 8 kg includes people who have variable weight loss (ie, some more and some less than average), so accurate prediction of individual weight loss is not possible. After 6 months, most patients will equilibrate (caloric intake balancing energy expenditure) and will require adjustment of energy balance if they are to lose additional weight. As demonstrated in CQ4, continued intervention contact after initial weight loss treatment is associated with better maintenance of lost weight (Box 15).
Box 11b. Options for Alternative Modes of Delivery of Lifestyle Intervention.
In primary care offices where frequent, in-person individual or group sessions led by a trained interventionist† or a nutrition professional* are not possible or available by referral, the physician may consider alternative modes of delivery. As found in CQ4, emerging evidence supports the efficacy, albeit with less weight loss, of electronically delivered interventions (eg, by Internet or telephone) that provide personalized feedback by a trained interventionist† and of some commercial programs that provide counseling (face-to-face or telephonic) with or without prepackaged meals. The Expert Panel recommends, by expert opinion, that physicians may refer to these alternative sources provided their outcomes are supported by scientific evidence of safety and efficacy. An additional option if a high-intensity comprehensive lifestyle intervention program is not available or feasible is referral to a nutrition professional* for dietary counseling.
Box 12. Option for Adding Pharmacotherapy as an Adjunct to Comprehensive Lifestyle Intervention‡.
The Expert Panel did not review comprehensive evidence for pharmacotherapy for weight loss. On the basis of expert opinion, the panelists recommend that for individuals with BMI ≥30 kg/m2 or BMI ≥27 kg/m2 with ≥1 obesity-associated comorbid condition(s) who are motivated to lose weight, pharmacotherapy can be considered as an adjunct to comprehensive lifestyle intervention to help achieve targeted weight loss and health goals. Medications should be FDA approved, and clinicians should be knowledgeable about the product label. The provider should weigh the potential risks of the medication being considered against the potential benefits of successful weight loss for the individual patient. The rationale for use of medications is to help patients adhere to a lower-calorie diet more consistently to achieve sufficient weight loss and health improvements when combined with increased physical activity. The available medications work through effects on appetite or fat absorption. Medications work to reinforce lifestyle change and should be prescribed as an adjunct to lifestyle interventions as defined in Boxes 11a and 11b.
Box 13. Offer Referral to an Experienced Bariatric Surgeon for Consultation and Evaluation.
For adults with a BMI ≥40 kg/m2 or BMI ≥35 kg/m2 with obesity-related comorbid conditions who are motivated to lose weight and who have not responded to behavioral treatment (with or without pharmacotherapy) with sufficient weight loss to achieve targeted health outcome goals, advise that bariatric surgery may be an appropriate option to improve health and offer referral to an experienced bariatric surgeon for consultation and evaluation (CQ5 for additional information). Because bariatric surgery leads to improvements in both weight-related outcomes and many obesity-related comorbid conditions, the benefit-to-risk ratio may be favorable inappropriately selected patients at high risk for obesity-related morbidity and mortality. In the absence of RCTs to identify the optimal duration and weight loss outcomes of nonsurgical treatment before bariatric surgery is recommended, the decision to proceed to surgery should be based on multiple factors: patient motivation, treatment adherence, operative risk, and optimization of comorbid conditions, among others. Bariatric surgery should be considered an adjunct to lifestyle treatment: behavioral treatment, appropriate dietary modification and physical activity.
Box 14. Weight Loss ≥5% of Initial Body Weight and Sufficient Improvement in Health Targets?
Achieving the goals noted in Box 9 of approximately 5%–10% of initial weight with a comprehensive lifestyle intervention should be considered successful weight reduction that leads to decreased risk for development of or amelioration of obesity-related medical conditions and cardiovascular risk factors for many patients. Some patients will require additional weight loss to achieve targeted health outcome goals.
If the patient achieves the weight loss and health outcome goals previously identified by clinician and patient, the clinician should consider the weight loss maintenance strategies described in Box 15 using the disease management model of obesity treatment. If these weight loss or health outcome goals are not achieved with current treatment, the clinician can consider intensification of behavioral treatment (Box 16), and/or the addition or reevaluation of obesity pharmacotherapy (Box 12), or referral for evaluation for bariatric surgery (Box 13) in patients otherwise meeting BMI and comorbidity criteria.
Box 15: Weight Loss Maintenance.
Typically, obesity is a chronic condition that develops over an individual's lifetime. The prevalence of obesity has greatly increased over the past 30 years, most likely because of environmental changes that promote increased consumption of high-calorie palatable foods, decreased physical activity, and more sedentary behavior. In this environment, it is difficult to maintain a healthy weight and prevent weight gain. Long-term research has shown that continuing weight loss maintenance interventions produce better long-term results than limited term intervention programs. Clinicians must acknowledge the lifelong challenge that patients experience with obesity, provide support and encouragement, be prepared to assist patients with addressing small weight gains before they become larger ones, and reinstitute weight management efforts as early as possible in the course of regain.
The usual pattern of weight loss in patients undergoing a lifestyle intervention is that maximum weight loss is achieved at 6 months, followed by plateau and gradual regain over time. This is also true for medication-assisted weight loss, although weight regain may be slower with continued medication use. For bariatric surgery patients, it may take much longer for weight to plateau (CQ3, CQ4, and CQ5).
The strategies for weight maintenance after successful loss differ from the strategies for achieving weight loss. Flexibility and willingness to try different approaches are recommended. Patients should be advised that participation in a long-term (≥1 y) comprehensive weight loss maintenance program with monthly or more frequent contact, in person or by telephone, can improve successful weight maintenance. Strategies such as frequent self-weighing (at least weekly), consumption of a reduced-calorie diet, and high levels of physical activity (>200 min/wk) are associated with better weight maintenance over time.
Box 16: Unable to Lose Enough Weight With Current Treatment to Meet Weight or Targeted Health Goals.
By expert opinion, if patients are unable to lose enough weight to meet weight or targeted health outcome goals with their current treatment, consider offering or referring for more intensive behavioral treatment than is currently being attempted, an alternative diet including options for meal replacement, referral to a nutrition professional*, addition of obesity pharmacotherapy, or referral for evaluation for bariatric surgery if otherwise appropriate. The clinician should also assess the patient's medication regimen for drugs that may contribute to weight gain and consider adjustments if medically appropriate. If the patient is currently taking an obesity medication but has not lost at least 5% of initial body weight after 12 weeks on a maximal dose of the medication, the provider should reassess the risk-to-benefit ratio of that medication for the patient and consider discontinuation of that drug.
Box 17: Measure Weight and Calculate BMI Annually or More Frequently.
Weight should be measured and BMI calculated and documented by the clinician at least annually in all patients. For those who have never been overweight or who are weight stable, a 1-year interval is appropriate for the reassessment of BMI. For overweight or obese individuals or those of normal weight with a history of overweight, more frequent monitoring may be appropriate. Although these follow-up intervals are not evidence based, they are a reasonable compromise between the need to identify weight gain at an early stage and the need to limit the time, effort, and cost of repeated measurements.
Box 18. Weight Loss ≥5% of Initial Body Weight and Sufficient Improvement in Health Targets?
Determine if the intensified treatment strategies instituted in Box 16 have led to both successful weight loss and sufficient risk factor/comorbidity reduction to achieve the health goals determined by patient and clinician.
Box 19. Continue Intensive Medical Management of Cardiovascular Risk Factors and Obesity-Related Conditions and Periodic Assessment of Weight Management Options.
Actively and intensively manage cardiovascular risk factors and obesity-related conditions, regardless of the patient's ability to achieve or sustain weight loss. Periodically reassess and address medical or other contributory factors and the potential to institute or reinstitute additional weight management options as shown in Box 16.
Acknowledgments
The CQ2 Working group of the obesity Expert Panel acknowledges the valuable contributions of Eva Erber, MS, Graduate Research Assistant at the university of North Carolina at Chapel Hill.
Presidents and Staff
American College of Cardiology
John Gordon Harold, MD, MACC, President
Shalom Jacobovitz, Chief Executive Officer
William J. Oetgen, MD, MBA, FACC, Executive Vice President, Science, Education & Quality
Charlene May, Senior Director, Science and Clinical Policy
American College of Cardiology/American Heart Association
Lisa Bradfield, CAE, Director, Science and Clinical Policy
Ezaldeen Ramadhan III, Team Leader, Science and Clinical Policy
American Heart Association
Mariell Jessup, MD, FACC, FAHA, President
Nancy Brown, Chief Executive Officer
Rose Marie Robertson, MD, FAHA, Chief Science Officer
Gayle R. Whitman, PhD, RN, FAHA, FAAN, Senior Vice President, Office of Science Operations
Marco Di Buono, PhD, Vice President of Science and Research
Jody Hundley, Production Manager, Scientific Publications, Office of Science Operations
National Heart, Lung, and Blood Institute
Glen Bennett, MPH
Melinda Kelley, PhD
Melissa McGowan, MHS, CHES
Kathryn Y. McMurry, MS
Denise Simons-Morton, MD, PhD
Appendix 1
Author Relationships With Industry and Other Entities (Relevant)—2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults
| Committee Member | Employment | Consultant | Speaker’s Bureau |
Ownership/ Partnership/ Principal |
Personal Research |
Expert Witness |
|---|---|---|---|---|---|---|
| Michael D. Jensen, Co-Chair | Mayo Clinic | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None |
| Endocrine Research Unit— Professor of Medicine, Endocrinology, Metabolism, Diabetes, Nutrition, and Internal Medicine Division | 2013
|
2013 None | 2013 None | 2013 None | 2013 None | |
| Donna H. Ryan, Co-Chair | Pennington Biomedical Research Center—Associate Executive Director for Clinical Research | 2008–2012
|
2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None |
2013
|
2013 None | 2013
|
2013 None | 2013 None | ||
| Caroline M. Apovian | Boston Medical Center—Professor of Medicine and Pediatrics; Center for Nutrition and Weight Management— Director | 2008–2012
|
2008–2012 None | 2008–2012 None | 2008–2012
|
2008–2012 None |
2013
|
2013 None | 2013 None | 2013 | 2013 None | ||
| Jamy D. Ard | Wake Forest University— Assistant Professor of Epidemiology and Prevention; Weight Management Center—Co-Director | 2008–2012
|
2008–2012 None | 2008–2012 None | 2008–2012
|
2008–2012 None |
2013
|
2013 None | 2013 None | 2013 None | 2013 None | ||
| Anthony G. Comuzzie | Southwest Foundation for Biomedical Research—Scientist, Department of Genetics | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None |
| 2013 None | 2013 None | 2013 None | 2013 None | 2013 None | ||
| Karen A. Donato | NHLBI—Acting Director, Division for the Application of Research Discoveries | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None |
| 2013 None | 2013 None | 2013 None | 2013 None | 2013 None | ||
| Frank B. Hu | Harvard University School of Public Health—Professor, Nutrition and Epidemiology | 2008–2012
|
2008–2012 None | 2008–2012 None | 2008–2012
|
2008–2012 None |
2013
|
2013 None | 2013 None | 2013
|
2013 None | ||
| Van S. Hubbard, Ex-Officio | NIDDK—Director, NIH Division of Nutrition Research Coordination | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None |
| 2013 None | 2013 None | 2013 None | 2013 None | 2013 None | ||
| John M. Jakicic | University of Pittsburgh—Professor and Chair, Physical Activity and Weight Management Research Center | 2008–2012
|
2008–2012 None | 2008–2012 None | 2008–2012
|
2008–2012 None |
2013
|
2013 None | 2013 None | 2013
|
2013 None | ||
| Robert F. Kushner | Northwestern University Feinberg School of Medicine—Professor, Division of General Internal Medicine | 2008–2012
|
2008–2012 None | 2008–2012 None | 2008–2012
|
2008–2012 None |
| 2013 None | 2013 None | 2013 None | 2013
|
2013 None | ||
| Catherine M. Loria, Ex-Officio | NHLBI—Nutritional Epidemiologist | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None |
| 2013 None | 2013 None | 2013 None | 2013 None | 2013 None | ||
| Barbara E. Millen | Boston Nutrition Foundation— Chairman; Millennium Prevention—President | 2008–2012 None | 2008–2012 None | 2008–2012 | 2008–2012 None | 2008–2012 None |
| 2013 None | 2013 None | 2013 | 2013 None | 2013 None | ||
| Cathy A. Nonas | NYC Dept of Health and Mental Hygiene—Senior Advisor, Bureau for Chronic Disease Prevention and Tobacco Control | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None |
| 2013 None | 2013 None | 2013 None | 2013 None | 2013 None | ||
| F. Xavier Pi-Sunyer | Columbia University—Professor of Medicine, College of Physicians and Surgeons | 2008–2012
|
2008–2012 None | 2008–2012 None | 2008–2012
|
2008–2012 None |
2013
|
2013 None | 2013 None | 2013
|
2013 None | ||
| June Stevens | University of North Carolina at Chapel Hill—Chair, Department of Nutrition; Department of Epidemiology Schools of Public Health and Medicine—Professor | 2008–2012
|
2008–2012 None | 2008–2012 None | 2008–2012
|
2008–2012 None |
| 2013 None | 2013 None | 2013 None | 2013
|
2013 None | ||
| Victor J. Stevens | Kaiser Permanente Center for HealthResearch—Assistant Director, Epidemiology and Disease Prevention | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None |
| 2013 None | 2013 None | 2013 None | 2013 None | 2013 None | ||
| Thomas A. Wadden | Perelman School of Medicine at the University of Pennsylvania—Professor of Psychology in Psychiatry; Center for Weight and Eating Disorders—Director | 2008–2012
|
2008–2012 None | 2008–2012 None | 2008–2012
|
2008–2012 None |
2013
|
2013 None | 2013 None | 2013 None | 2013 None | ||
| Bruce M. Wolfe | Oregon Health and Science University—Professor of Surgery | 2008–2012
|
2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None |
2013
|
2013 None | 2013 None | 2013 None | 2013 None | ||
| Susan Z. Yanovski, Ex-Officio | NIDDK—Co-Director, Office of Obesity Research, Division of Digestive Diseases and Nutrition | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None | 2008–2012 None |
| 2013 None | 2013 None | 2013 None | 2013 None | 2013 None |
This table reflects the relevant healthcare-related relationships of authors with industry and other entities provided by the Expert Panel during the document development process (2008–2012). Both compensated and uncompensated relationships are reported. These relationships were reviewed and updated in conjunction with all meetings and conference calls of the Expert Panel during the document development process. Authors with relevant relationships during the document development process recused themselves from voting on recommendations relevant to their relationships. In the spirit of full transparency, the ACC and AHA asked Expert Panel members to provide updates and approve the final version of this table, which includes current relevant relationships (2013). To review the NHLBI and ACC/AHA’s current comprehensive policies for managing relationships with industry and other entities, please refer to http://www.nhlbi.nih.gov/guidelines/cvd_adult/coi-rwi_policy.htm and http://www.cardiosource.org/Science-And-Quality/Practice-Guidelines-and-Quality-Standards/Relationships-With-Industry-Policy.aspx.
Per ACC/AHA policyv: A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity, or ownership of ≥$10 000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted.
Significant relationship.
No financial benefit.
ACC indicates American College of Cardiology; AHA, American Heart Association; NHLBI, National Heart, Lung, and Blood Institute; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; NIH, National Health Institute; PI, principal investigator; and TOS, The Obesity Society.
Appendix 2
Expert Reviewer Relationships With Industry and Other Entities—2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults
| Reviewer | Representation | Employment | Consultant | Speaker’s Bureau |
Ownership/ Partnership/ Principal |
Personal Research |
Institutional, Organizational, or Other Financial Benefit |
Expert Witness |
|---|---|---|---|---|---|---|---|---|
| William H. Dietz | ACC/AHA | Centers for Disease Control and Prevention— Director, Division of Nutrition and Physical Activity | None | None | None | None | None | None |
| Penny Gordon-Larsen | TOS | University of North Carolina, Gillings School of Global Public Health—Professor, Department of Nutrition | None | None | None | None | None | None |
| Lee M. Kaplan | TOS | Massachusetts General Hospital—Director, Weight Center | None | None |
|
None | None | |
| Paul Poirier | ACC/AHA | Laval University, Institut Universitaire de Cardiologie et Pneumologie, Hôpital Laval—Faculty of Pharmacy |
|
None | None | None | None | None |
| Susan J. Pressler | ACC/AHA Task Force on Practice Guidelines | University of Michigan School of Nursing—Professor | None | None | None | None |
|
None |
| Rena R. Wing | TOS | Brown University— Professor, Psychiatry & Human Behavior | None | None | None | None | None | None |
This table represents the relationships of reviewers with industry and other entities that were self-disclosed at the time of peer review. It does not necessarily reflect relationships with industry at the time of publication. To review the NHLBI and ACC/AHA’s current comprehensive policies for managing relationships with industry and other entities, please refer to http://www.nhlbi.nih.gov/guidelines/cvd_adult/coi-rwi_policy.htm and http://www.cardiosource.org/Science-And-Quality/Practice-Guidelines-and-Quality-Standards/Relationships-With-Industry-Policy.aspx.
No financial benefit.
Significant relationship.
ACC indicates American College of Cardiology; AHA, American Heart Association; and TOS, The Obesity Society.
Appendix 3
Abbreviations
- BMI
body mass index
- BP
blood pressure
- BPD
biliopancreatic diversion
- CHD
coronary heart disease
- CVD
cardiovascular disease
- COR
Class of Recommendation
- CQ
critical question
- ES
evidence statement
- HDL-C
high-density lipoprotein cholesterol
- I/E
inclusion/exclusion
- LAGB
laparoscopic adjustable gastric banding
- LDL-C
low-density lipoprotein cholesterol
- LOE
Level of Evidence
- NHLBI
National Heart, Lung, and Blood Institute
- PCP
primary care practitioner
- RWI
relationships of authors with industry and other entities
- RYGB
laparoscopic Roux-en-Y gastric bypass
Footnotes
This document was approved by the American Heart Association Science Advisory and Coordinating Committee, the American College of Cardiology Board of Trustees, and The Obesity Society Board of Trustees in November 2013. The Academy of Nutrition and Dietetics affirms the value of this guideline.
The online-only Data Supplement is available with this article at http://circ.AHAjournals.org/lookup/suppl/doi:10.1161/01.cir.0000437739.71477.ee/-/DC1.
Nutrition professional: In the studies that form the evidence base for this recommendation, a registered dietitian usually delivered the dietary guidance; in most cases, the intervention was delivered in university nutrition departments or in hospital medical care settings where access to nutrition professionals was available.
Trained interventionist: In the studies reviewed, trained interventionists included mostly health professionals (eg, registered dietitians, psychologists, exercise specialists, health counselors, or professionals in training) who adhered to formal protocols in weight management. In a few cases, lay persons were used as trained interventionists; they received instruction in weight management protocols (designed by health professionals) in programs that have been validated in high-quality trials published in peer-reviewed journals.
BMI cutpoint determined by the FDA and listed on the package inserts of FDA-approved obesity medications. BMI indicates body mass index; BP, blood pressure; CQ, critical question; CVD, cardiovascular disease; FDA, US Food and Drug Administration; PCP, primary care practitioner; and RCT, randomized controlled trial.
Some papers were not appropriate for inclusion for reasons other than the criteria, ie, they did not address the question.
Remission was defined variously depending on the study.
References
- 1.Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 2.Gibbons GH, Harold JG, Jessup M, et al. The next steps in developing clinical practice guidelines for prevention. Circulation. 2013;128:1716–7. doi: 10.1161/CIRCULATIONAHA.113.005548. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons GH, Shurin SB, Mensah GA, et al. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2013;62:1396–8. doi: 10.1016/j.jacc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 5.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein EA, Trogdon JG, Cohen JW, et al. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–31. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 8.Goff DC, Jr, Lloyd-Jones DM, D’Agostino RB, Sr, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 9.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S76–S99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 10.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 11.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. WMJ. 1998;97:20–1. 24–5, 27–37. [PubMed] [Google Scholar]
- 12.Aucott L, Poobalan A, Smith WCS, et al. Effects of weight loss in overweight/ obese individuals and long-term hypertension outcomes: a systematic review. Hypertension. 2005;45:1035–41. doi: 10.1161/01.HYP.0000165680.59733.d4. [DOI] [PubMed] [Google Scholar]
- 13.Aucott L, Poobalan A, Smith WCS, et al. Weight loss in obese diabetic and non-diabetic individuals and long-term diabetes outcomes–a systematic review. Diabetes Obes Metab. 2004;6:85–94. doi: 10.1111/j.1462-8902.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 14.Avenell A, Broom J, Brown TJ, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess. 2004;8:iii–iv. 1–182. doi: 10.3310/hta8210. [DOI] [PubMed] [Google Scholar]
- 15.Avenell A, Brown TJ, McGee MA, et al. What interventions should we add to weight reducing diets in adults with obesity? A systematic review of randomized controlled trials of adding drug therapy, exercise, behaviour therapy or combinations of these interventions. J Hum Nutr Diet. 2004;17:293–316. doi: 10.1111/j.1365-277X.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 16.Avenell A, Brown TJ, McGee MA, et al. What are the long-term benefits of weight reducing diets in adults? A systematic review of randomized controlled trials. J Hum Nutr Diet. 2004;17:317–35. doi: 10.1111/j.1365-277X.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 17.Douketis JD, Macie C, Thabane L, et al. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond) 2005;29:1153–67. doi: 10.1038/sj.ijo.0802982. [DOI] [PubMed] [Google Scholar]
- 18.Galani C, Schneider H. Prevention and treatment of obesity with lifestyle interventions: review and meta-analysis. Int J Public Health. 2007;52:348–59. doi: 10.1007/s00038-007-7015-8. [DOI] [PubMed] [Google Scholar]
- 19.Horvath K, Jeitler K, Siering U, et al. Long-term effects of weight-reducing interventions in hypertensive patients: systematic review and meta-analysis. Arch Intern Med. 2008;168:571–80. doi: 10.1001/archinte.168.6.571. [DOI] [PubMed] [Google Scholar]
- 20.Hutton B, Fergusson D. Changes in body weight and serum lipid profile in obese patients treated with orlistat in addition to a hypocaloric diet: a systematic review of randomized clinical trials. Am J Clin Nutr. 2004;80:1461–8. doi: 10.1093/ajcn/80.6.1461. [DOI] [PubMed] [Google Scholar]
- 21.Johansson K, Sundström J, Neovius K, et al. Long-term changes in blood pressure following orlistat and sibutramine treatment: a meta-analysis. Obes Rev. 2010;11:777–91. doi: 10.1111/j.1467-789X.2009.00693.x. [DOI] [PubMed] [Google Scholar]
- 22.Look AHEAD Research Group. Pi-Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Look AHEAD Research Group. Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–75. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norris SL, Zhang X, Avenell A, et al. Long-term non-pharmacological weight loss interventions for adults with prediabetes. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD005270. CD005270. [DOI] [PubMed] [Google Scholar]
- 25.Norris SL, Zhang X, Avenell A, et al. Long-term non-pharmacologic weight loss interventions for adults with type 2 diabetes. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD004095.pub2. CD004095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norris SL, Zhang X, Avenell A, et al. Pharmacotherapy for weight loss in adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD004096.pub2. CD004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norris SL, Zhang X, Avenell A, et al. Long-term effectiveness of lifestyle and behavioral weight loss interventions in adults with type 2 diabetes: a meta-analysis. Am J Med. 2004;117:762–74. doi: 10.1016/j.amjmed.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 28.Padwal R, Li SK, Lau DCW. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD004094.pub2. CD004094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pirozzo S, Summerbell C, Cameron C, et al. Should we recommend low-fat diets for obesity? Obes Rev. 2003;4:83–90. doi: 10.1046/j.1467-789x.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 30.Poobalan AS, Aucott LS, Smith WCS, et al. Long-term weight loss effects on all cause mortality in overweight/obese populations. Obes Rev. 2007;8:503–13. doi: 10.1111/j.1467-789X.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 31.Poobalan A, Aucott L, Smith WCS, et al. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes–a systematic review. Obes Rev. 2004;5:43–50. doi: 10.1111/j.1467-789x.2004.00127.x. [DOI] [PubMed] [Google Scholar]
- 32.Rucker D, Padwal R, Li SK, et al. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194–9. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw K, Gennat H, O'Rourke P, et al. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD003817.pub3. CD003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siebenhofer A, Horvath K, Jeitler K, et al. Long-term effects of weight-reducing drugs in hypertensive patients. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD007654.pub2. CD007654. [DOI] [PubMed] [Google Scholar]
- 35.Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD005105.pub2. CD005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuah NA, Amiel C, Qureshi S, et al. Transtheoretical model for dietary and physical exercise modification in weight loss management for overweight and obese adults. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD008066.pub2. CD008066. [DOI] [PubMed] [Google Scholar]
- 37.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–6. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witham MD, Avenell A. Interventions to achieve long-term weight loss in obese older people: a systematic review and meta-analysis. Age Ageing. 2010;39:176–84. doi: 10.1093/ageing/afp251. [DOI] [PubMed] [Google Scholar]
- 39.Padwal R, Li SK, Lau DCW. Long-term pharmacotherapy for overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Int J Obes Relat Metab Disord. 2003;27:1437–46. doi: 10.1038/sj.ijo.0802475. [DOI] [PubMed] [Google Scholar]
- 40.Lenz M, Richter T, Mühlhauser I. The morbidity and mortality associated with overweight and obesity in adulthood: a systematic review. Dtsch Arztebl Int. 2009;106:641–8. doi: 10.3238/arztebl.2009.0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emerging Risk Factors Collaboration. Wormser D, Kaptoge S, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–95. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prospective Studies Collaboration. Whitlock G, Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skov AR, Toubro S, Bülow J, et al. Changes in renal function during weight loss induced by high vs low-protein low-fat diets in overweight subjects. Int J Obes Relat Metab Disord. 1999;23:1170–7. doi: 10.1038/sj.ijo.0801048. [DOI] [PubMed] [Google Scholar]
- 44.Skov AR, Toubro S, Rønn B, et al. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord. 1999;23:528–36. doi: 10.1038/sj.ijo.0800867. [DOI] [PubMed] [Google Scholar]
- 45.Skov AR, Haulrik N, Toubro S, et al. Effect of protein intake on bone mineralization during weight loss: a 6-month trial. Obes Res. 2002;10:432–8. doi: 10.1038/oby.2002.60. [DOI] [PubMed] [Google Scholar]
- 46.Torgerson JS, Agren L, Sjöström L. Effects on body weight of strict or liberal adherence to an initial period of VLCD treatment. A randomised, one-year clinical trial of obese subjects. Int J Obes Relat Metab Disord. 1999;23:190–7. doi: 10.1038/sj.ijo.0800816. [DOI] [PubMed] [Google Scholar]
- 47.Ashley JM, St Jeor ST, Perumean-Chaney S, et al. Meal replacements in weight intervention. Obes Res. 2001;9(Suppl 4):312S–20S. doi: 10.1038/oby.2001.136. [DOI] [PubMed] [Google Scholar]
- 48.Poppitt SD, Keogh GF, Prentice AM, et al. Long-term effects of ad libitum low-fat, high-carbohydrate diets on body weight and serum lipids in overweight subjects with metabolic syndrome. Am J Clin Nutr. 2002;75:11–20. doi: 10.1093/ajcn/75.1.11. [DOI] [PubMed] [Google Scholar]
- 49.Wien MA, Sabaté JM, Iklé DN, et al. Almonds vs complex carbohydrates in a weight reduction program. Int J Obes Relat Metab Disord. 2003;27:1365–72. doi: 10.1038/sj.ijo.0802411. [DOI] [PubMed] [Google Scholar]
- 50.Due A, Toubro S, Skov AR, et al. Effect of normal-fat diets, either medium or high in protein, on body weight in overweight subjects: a randomised 1-year trial. Int J Obes Relat Metab Disord. 2004;28:1283–90. doi: 10.1038/sj.ijo.0802767. [DOI] [PubMed] [Google Scholar]
- 51.Turner-McGrievy GM, Barnard ND, Scialli AR, et al. Effects of a low-fat vegan diet and a Step II diet on macro- and micronutrient intakes in overweight postmenopausal women. Nutrition. 2004;20:738–46. doi: 10.1016/j.nut.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Wadden TA, Foster GD, Sarwer DB, et al. Dieting and the development of eating disorders in obese women: results of a randomized controlled trial. Am J Clin Nutr. 2004;80:560–8. doi: 10.1093/ajcn/80.3.560. [DOI] [PubMed] [Google Scholar]
- 53.Lejeune MPGM, Kovacs EMR, Westerterp-Plantenga MS. Additional protein intake limits weight regain after weight loss in humans. Br J Nutr. 2005;93:281–9. doi: 10.1079/bjn20041305. [DOI] [PubMed] [Google Scholar]
- 54.McAuley KA, Hopkins CM, Smith KJ, et al. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin-resistant obese women. Diabetologia. 2005;48:8–16. doi: 10.1007/s00125-004-1603-4. [DOI] [PubMed] [Google Scholar]
- 55.Thompson WG, Rostad Holdman N, Janzow DJ, et al. Effect of energy-reduced diets high in dairy products and fiber on weight loss in obese adults. Obes Res. 2005;13:1344–53. doi: 10.1038/oby.2005.163. [DOI] [PubMed] [Google Scholar]
- 56.Burke LE, Styn MA, Steenkiste AR, et al. A randomized clinical trial testing treatment preference and two dietary options in behavioral weight management: preliminary results of the impact of diet at 6 months—PREFER study. Obesity (Silver Spring) 2006;14:2007–17. doi: 10.1038/oby.2006.235. [DOI] [PubMed] [Google Scholar]
- 57.Pittas AG, Roberts SB, Das SK, et al. The effects of the dietary glycemic load on type 2 diabetes risk factors during weight loss. Obesity (Silver Spring) 2006;14:2200–9. doi: 10.1038/oby.2006.258. [DOI] [PubMed] [Google Scholar]
- 58.Das SK, Gilhooly CH, Golden JK, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85:1023–30. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 59.Ebbeling CB, Leidig MM, Feldman HA, et al. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297:2092–102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 60.Turner-McGrievy GM, Barnard ND, Scialli AR. A two-year randomized weight loss trial comparing a vegan diet to a more moderate low-fat diet. Obesity (Silver Spring) 2007;15:2276–81. doi: 10.1038/oby.2007.270. [DOI] [PubMed] [Google Scholar]
- 61.Esposito K, Maiorino MI, Ciotola M, et al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med. 2009;151:306–14. doi: 10.7326/0003-4819-151-5-200909010-00004. [DOI] [PubMed] [Google Scholar]
- 62.Frisch S, Zittermann A, Berthold HK, et al. A randomized controlled trial on the efficacy of carbohydrate-reduced or fat-reduced diets in patients attending a telemedically guided weight loss program. Cardiovasc Diabetol. 2009;8:36. doi: 10.1186/1475-2840-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153:147–57. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burke LE, Hudson AG, Warziski MT, et al. Effects of a vegetarian diet and treatment preference on biochemical and dietary variables in overweight and obese adults: a randomized clinical trial. Am J Clin Nutr. 2007;86:588–96. doi: 10.1093/ajcn/86.3.588. [DOI] [PubMed] [Google Scholar]
- 66.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Carnegie Institute of Washington publication 279. Washington, DC: Carnegie Institution of Washington; 1919. [Google Scholar]
- 67.Mifflin MD, St Jeor ST, Hill LA, et al. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–7. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 68.Joint FAO/WHO/UNU Expert Consultation on Energy and Protein Requirements. Energy and Protein Requirements: report of Joint FAO/WHO/UNU Expert Consultation. Technical Report Series No. 724. Geneva: World Health Organization; 1985. pp. 1–206. [PubMed] [Google Scholar]
- 69.Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360:481–90. doi: 10.1056/NEJMoa0806375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–48. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 71.Tate DF, Jackvony EH, Wing RR. Effects of Internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: a randomized trial. JAMA. 2003;289:1833–6. doi: 10.1001/jama.289.14.1833. [DOI] [PubMed] [Google Scholar]
- 72.Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an Internet weight loss program. Arch Intern Med. 2006;166:1620–5. doi: 10.1001/archinte.166.15.1620. [DOI] [PubMed] [Google Scholar]
- 73.Tate DF, Jeffery RW, Sherwood NE, et al. Long-term weight losses associated with prescription of higher physical activity goals. Are higher levels of physical activity protective against weight regain? Am J Clin Nutr. 2007;85:954–9. doi: 10.1093/ajcn/85.4.954. [DOI] [PubMed] [Google Scholar]
- 74.Teixeira PJ, Silva MN, Coutinho SR, et al. Mediators of weight loss and weight loss maintenance in middle-aged women. Obesity (Silver Spring) 2010;18:725–35. doi: 10.1038/oby.2009.281. [DOI] [PubMed] [Google Scholar]
- 75.ter Bogt NCW, Bemelmans WJE, Beltman FW, et al. Preventing weight gain: one-year results of a randomized lifestyle intervention. Am J Prev Med. 2009;37:270–7. doi: 10.1016/j.amepre.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 76.Truby H, Baic S, deLooy A, et al. Randomised controlled trial of four commercial weight loss programmes in the UK: initial findings from the BBC “diet trials”. BMJ. 2006;332:1309–14. doi: 10.1136/bmj.38833.411204.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 78.Uusitupa M, Louheranta A, Lindström J, et al. The Finnish Diabetes Prevention Study. Br J Nutr. 2000;83(Suppl 1):S137–42. doi: 10.1017/s0007114500001070. [DOI] [PubMed] [Google Scholar]
- 79.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011;19:1987–98. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365:1969–79. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.West DS, Gorin AA, Subak LL, et al. A motivation-focused weight loss maintenance program is an effective alternative to a skill-based approach. Int J Obes (Lond) 2011;35:259–69. doi: 10.1038/ijo.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.West DS, Elaine Prewitt T, Bursac Z, et al. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring) 2008;16:1413–20. doi: 10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]
- 83.West DS, DiLillo V, Bursac Z, et al. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30:1081–7. doi: 10.2337/dc06-1966. [DOI] [PubMed] [Google Scholar]
- 84.Whelton PK, Appel LJ, Espeland MA, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279:839–46. doi: 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]
- 85.Wing RR, Tate DF, Gorin AA, et al. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563–71. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 86.Wolf AM, Conaway MR, Crowther JQ, et al. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570–6. doi: 10.2337/diacare.27.7.1570. [DOI] [PubMed] [Google Scholar]
- 87.Womble LG, Wadden TA, McGuckin BG, et al. A randomized controlled trial of a commercial internet weight loss program. Obes Res. 2004;12:1011–8. doi: 10.1038/oby.2004.124. [DOI] [PubMed] [Google Scholar]
- 88.Andersen RE, Wadden TA, Bartlett SJ, et al. Effects of lifestyle activity vs structured aerobic exercise in obese women: a randomized trial. JAMA. 1999;281:335–40. doi: 10.1001/jama.281.4.335. [DOI] [PubMed] [Google Scholar]
- 89.Appel LJ, Clark JM, Yeh H-C, et al. Comparative effectiveness of weightloss interventions in clinical practice. N Engl J Med. 2011;365:1959–68. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blumenthal JA, Sherwood A, Gullette EC, et al. Exercise and weight loss reduce blood pressure in men and women with mild hypertension: effects on cardiovascular, metabolic, and hemodynamic functioning. Arch Intern Med. 2000;160:1947–58. doi: 10.1001/archinte.160.13.1947. [DOI] [PubMed] [Google Scholar]
- 91.Borg P, Kukkonen-Harjula K, Fogelholm M, et al. Effects of walking or resistance training on weight loss maintenance in obese, middle-aged men: a randomized trial. Int J Obes Relat Metab Disord. 2002;26:676–83. doi: 10.1038/sj.ijo.0801962. [DOI] [PubMed] [Google Scholar]
- 92.Byrne NM, Meerkin JD, Laukkanen R, et al. Weight loss strategies for obese adults: personalized weight management program vs. standard care. Obesity (Silver Spring) 2006;14:1777–88. doi: 10.1038/oby.2006.205. [DOI] [PubMed] [Google Scholar]
- 93.Christian JG, Bessesen DH, Byers TE, et al. Clinic-based support to help overweight patients with type 2 diabetes increase physical activity and lose weight. Arch Intern Med. 2008;168:141–6. doi: 10.1001/archinternmed.2007.13. [DOI] [PubMed] [Google Scholar]
- 94.Cussler EC, Teixeira PJ, Going SB, et al. Maintenance of weight loss in overweight middle-aged women through the Internet. Obesity (Silver Spring) 2008;16:1052–60. doi: 10.1038/oby.2008.19. [DOI] [PubMed] [Google Scholar]
- 95.Dale KS, Mann JI, McAuley KA, et al. Sustainability of lifestyle changes following an intensive lifestyle intervention in insulin resistant adults: Follow-up at 2-years. Asia Pac J Clin Nutr. 2009;18:114–20. [PubMed] [Google Scholar]
- 96.Diabetes Prevention Program Research Group. Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eriksson J, Lindström J, Valle T, et al. Prevention of Type II diabetes in subjects with impaired glucose tolerance: the Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia. 1999;42:793–801. doi: 10.1007/s001250051229. [DOI] [PubMed] [Google Scholar]
- 98.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 99.Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291:2978–84. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 100.Fitzgibbon ML, Stolley MR, Schiffer L, et al. Obesity reduction black intervention trial (ORBIT): 18-month results. Obesity (Silver Spring) 2010;18:2317–25. doi: 10.1038/oby.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fogelholm M, Kukkonen-Harjula K, Nenonen A, et al. Effects of walking training on weight maintenance after a very-low-energy diet in premenopausal obese women: a randomized controlled trial. Arch Intern Med. 2000;160:2177–84. doi: 10.1001/archinte.160.14.2177. [DOI] [PubMed] [Google Scholar]
- 102.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619–26. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gold BC, Burke S, Pintauro S, et al. Weight loss on the web: A pilot study comparing a structured behavioral intervention to a commercial program. Obesity (Silver Spring) 2007;15:155–64. doi: 10.1038/oby.2007.520. [DOI] [PubMed] [Google Scholar]
- 104.Greaves CJ, Middlebrooke A, O'Loughlin L, et al. Motivational interviewing for modifying diabetes risk: a randomised controlled trial. Br J Gen Pract. 2008;58:535–40. doi: 10.3399/bjgp08X319648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haapala I, Barengo NC, Biggs S, et al. Weight loss by mobile phone: a 1-year effectiveness study. Public Health Nutr. 2009;12:2382–91. doi: 10.1017/S1368980009005230. [DOI] [PubMed] [Google Scholar]
- 106.Harvey-Berino J, Pintauro S, Buzzell P, et al. Effect of internet support on the long-term maintenance of weight loss. Obes Res. 2004;12:320–9. doi: 10.1038/oby.2004.40. [DOI] [PubMed] [Google Scholar]
- 107.Harvey-Berino J, West D, Krukowski R, et al. Internet delivered behavioral obesity treatment. Prev Med. 2010;51:123–8. doi: 10.1016/j.ypmed.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heshka S, Greenway F, Anderson JW, et al. Self-help weight loss versus a structured commercial program after 26 weeks: a randomized controlled study. Am J Med. 2000;109:282–7. doi: 10.1016/s0002-9343(00)00494-0. [DOI] [PubMed] [Google Scholar]
- 109.Heshka S, Anderson JW, Atkinson RL, et al. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA. 2003;289:1792–8. doi: 10.1001/jama.289.14.1792. [DOI] [PubMed] [Google Scholar]
- 110.Hunter CM, Peterson AL, Alvarez LM, et al. Weight management using the internet a randomized controlled trial. Am J Prev Med. 2008;34:119–26. doi: 10.1016/j.amepre.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 111.Jakicic JM, Winters C, Lang W, et al. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: a randomized trial. JAMA. 1999;282:1554–60. doi: 10.1001/jama.282.16.1554. [DOI] [PubMed] [Google Scholar]
- 112.Jakicic JM, Marcus BH, Gallagher KI, et al. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. JAMA. 2003;290:1323–30. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- 113.Jakicic JM, Marcus BH, Lang W, et al. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168:1550–1559. doi: 10.1001/archinte.168.14.1550. discussion 1559–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaukua J, Pekkarinen T, Sane T, et al. Health-related quality of life in WHO class II-III obese men losing weight with very-low-energy diet and behaviour modification: a randomised clinical trial. Int J Obes Relat Metab Disord. 2002;26:487–95. doi: 10.1038/sj.ijo.0801953. [DOI] [PubMed] [Google Scholar]
- 115.Kaukua J, Pekkarinen T, Sane T, et al. Sex hormones and sexual function in obese men losing weight. Obes Res. 2003;11:689–94. doi: 10.1038/oby.2003.98. [DOI] [PubMed] [Google Scholar]
- 116.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kulzer B, Hermanns N, Gorges D, et al. Prevention of diabetes self-management program (PREDIAS): effects on weight, metabolic risk factors, and behavioral outcomes. Diabetes Care. 2009;32:1143–6. doi: 10.2337/dc08-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kumanyika SK, Espeland MA, Bahnson JL, et al. Ethnic comparison of weight loss in the Trial of Nonpharmacologic Interventions in the Elderly. Obes Res. 2002;10:96–106. doi: 10.1038/oby.2002.16. [DOI] [PubMed] [Google Scholar]
- 119.Leermakers EA, Perri MG, Shigaki CL, et al. Effects of exercisefocused versus weight-focused maintenance programs on the management of obesity. Addict Behav. 1999;24:219–27. doi: 10.1016/s0306-4603(98)00090-2. [DOI] [PubMed] [Google Scholar]
- 120.Lindström J, Eriksson JG, Valle TT, et al. Prevention of diabetes mellitus in subjects with impaired glucose tolerance in the Finnish Diabetes Prevention Study: results from a randomized clinical trial. J Am Soc Nephrol. 2003;14:S108–13. doi: 10.1097/01.asn.0000070157.96264.13. [DOI] [PubMed] [Google Scholar]
- 121.Lindström J, Louheranta A, Mannelin M, et al. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26:3230–6. doi: 10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- 122.Logue E, Sutton K, Jarjoura D, et al. Transtheoretical model-chronic disease care for obesity in primary care: a randomized trial. Obes Res. 2005;13:917–27. doi: 10.1038/oby.2005.106. [DOI] [PubMed] [Google Scholar]
- 123.Look AHEAD Research Group. Wadden TA, West DS, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–52. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–10. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 125.Miller GD, Nicklas BJ, Davis CC, et al. Is serum leptin related to physical function and is it modifiable through weight loss and exercise in older adults with knee osteoarthritis? Int J Obes Relat Metab Disord. 2004;28:1383–90. doi: 10.1038/sj.ijo.0802737. [DOI] [PubMed] [Google Scholar]
- 126.Morgan LM, Griffin BA, Millward DJ, et al. Comparison of the effects of four commercially available weight-loss programmes on lipid-based cardiovascular risk factors. Public Health Nutr. 2009;12:799–807. doi: 10.1017/S1368980008003236. [DOI] [PubMed] [Google Scholar]
- 127.Nicklas BJ, Ambrosius W, Messier SP, et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79:544–51. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 128.Perri MG, Nezu AM, McKelvey WF, et al. Relapse prevention training and problem-solving therapy in the long-term management of obesity. J Consult Clin Psychol. 2001;69:722–6. [PubMed] [Google Scholar]
- 129.Perri MG, Limacher MC, Durning PE, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS ) randomized trial. Arch Intern Med. 2008;168:2347–54. doi: 10.1001/archinte.168.21.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rejeski WJ, Focht BC, Messier SP, et al. Obese, older adults with knee osteoarthritis: weight loss, exercise, and quality of life. Health Psychol. 2002;21:419–26. doi: 10.1037//0278-6133.21.5.419. [DOI] [PubMed] [Google Scholar]
- 131.Rock CL, Flatt SW, Sherwood NE, et al. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: a randomized controlled trial. JAMA. 2010;304:1803–10. doi: 10.1001/jama.2010.1503. [DOI] [PubMed] [Google Scholar]
- 132.Rock CL, Pakiz B, Flatt SW, et al. Randomized trial of a multifaceted commercial weight loss program. Obesity (Silver Spring) 2007;15:939–49. doi: 10.1038/oby.2007.614. [DOI] [PubMed] [Google Scholar]
- 133.Stenius-Aarniala B, Poussa T, Kvarnström J, et al. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ. 2000;320:827–32. doi: 10.1136/bmj.320.7238.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 135.Stolley MR, Fitzgibbon ML, Schiffer L, et al. Obesity reduction black intervention trial (ORBIT): six-month results. Obesity (Silver Spring) 2009;17:100–6. doi: 10.1038/oby.2008.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jeffery RW, Wing RR, Sherwood NE, et al. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;78:684–9. doi: 10.1093/ajcn/78.4.684. [DOI] [PubMed] [Google Scholar]
- 137.Miller GD, Rejeski WJ, Williamson JD, et al. The Arthritis, Diet and Activity Promotion Trial (ADAPT): design, rationale, and baseline results. Control Clin Trials. 2003;24:462–80. doi: 10.1016/s0197-2456(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 138.Wadden TA, West DS, Neiberg RH, et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009;17:713–22. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Adami GF, Papadia F, Carlini F, et al. Effect of biliopancreatic diversion on hypertension in severely obese patients. Hypertens Res. 2005;28:119–23. doi: 10.1291/hypres.28.119. [DOI] [PubMed] [Google Scholar]
- 140.Agaba EA, Shamseddeen H, Gentles CV, et al. Laparoscopic vs open gastric bypass in the management of morbid obesity: a 7-year retrospective study of 1,364 patients from a single center. Obes Surg. 2008;18:1359–63. doi: 10.1007/s11695-008-9455-5. [DOI] [PubMed] [Google Scholar]
- 141.Angrisani L, Lorenzo M, Borrelli V. Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 5-year results of a prospective randomized trial. Surg Obes Relat Dis. 2007;3:127–32. doi: 10.1016/j.soard.2006.12.005. discussion 132–3. [DOI] [PubMed] [Google Scholar]
- 142.Bessler M, Daud A, Kim T, et al. Prospective randomized trial of banded versus nonbanded gastric bypass for the super obese: early results. Surg Obes Relat Dis. 2007;3:480–4. doi: 10.1016/j.soard.2007.01.010. discussion 484–5. [DOI] [PubMed] [Google Scholar]
- 143.Biertho L, Steffen R, Branson R, et al. Management of failed adjustable gastric banding. Surgery. 2005;137:33–41. doi: 10.1016/j.surg.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 144.Dixon JB, O'Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 145.Favretti F, Cadière GB, Segato G, et al. Laparoscopic banding: selection and technique in 830 patients. Obes Surg. 2002;12:385–90. doi: 10.1381/096089202321087922. [DOI] [PubMed] [Google Scholar]
- 146.Favretti F, Segato G, Ashton D, et al. Laparoscopic adjustable gastric banding in 1791 consecutive obese patients: 12-year results. Obes Surg. 2007;17:168–75. doi: 10.1007/s11695-007-9043-0. [DOI] [PubMed] [Google Scholar]
- 147.Grunstein RR, Stenlöf K, Hedner JA, et al. Two year reduction in sleep apnea symptoms and associated diabetes incidence after weight loss in severe obesity. Sleep. 2007;30:703–10. doi: 10.1093/sleep/30.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ikonomidis I, Mazarakis A, Papadopoulos C, et al. Weight loss after bariatric surgery improves aortic elastic properties and left ventricular function in individuals with morbid obesity: a 3-year follow-up study. J Hypertens. 2007;25:439–47. doi: 10.1097/HJH.0b013e3280115bfb. [DOI] [PubMed] [Google Scholar]
- 149.Karlsson J, Sjöström L, Sullivan M. Swedish obese subjects (SOS)—an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord. 1998;22:113–26. doi: 10.1038/sj.ijo.0800553. [DOI] [PubMed] [Google Scholar]
- 150.Karlsson J, Taft C, Rydén A, et al. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes (Lond) 2007;31:1248–61. doi: 10.1038/sj.ijo.0803573. [DOI] [PubMed] [Google Scholar]
- 151.Kehagias I, Karamanakos SN, Argentou M, et al. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI< 50 kg/ m2. Obes Surg. 2011;21:1650–6. doi: 10.1007/s11695-011-0479-x. [DOI] [PubMed] [Google Scholar]
- 152.Larrad-Jiménez A, Díaz-Guerra CS-C, de Cuadros Borrajo P, et al. Short-, mid- and long-term results of Larrad biliopancreatic diversion. Obes Surg. 2007;17:202–10. doi: 10.1007/s11695-007-9035-0. [DOI] [PubMed] [Google Scholar]
- 153.Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Flum DR, Belle SH, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lopez-Jimenez F, Bhatia S, Collazo-Clavell ML, et al. Safety and efficacy of bariatric surgery in patients with coronary artery disease. Mayo Clin Proc. 2005;80:1157–62. doi: 10.4065/80.9.1157. [DOI] [PubMed] [Google Scholar]
- 155.Marinari GM, Papadia FS, Briatore L, et al. Type 2 diabetes and weight loss following biliopancreatic diversion for obesity. Obes Surg. 2006;16:1440–4. doi: 10.1381/096089206778870085. [DOI] [PubMed] [Google Scholar]
- 156.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–85. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 157.Narbro K, Agren G, Jonsson E, et al. Sick leave and disability pension before and after treatment for obesity: a report from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord. 1999;23:619–24. doi: 10.1038/sj.ijo.0800890. [DOI] [PubMed] [Google Scholar]
- 158.Naslund I. Lessons from the Swedish Obese Subjects Study: The effects of surgically induced weight loss on obesity comorbidity. Surg Obes Relat Dis. 2005;1:140–4. doi: 10.1016/j.soard.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 159.O'Brien PE, Dixon JB, Laurie C, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med. 2006;144:625–33. doi: 10.7326/0003-4819-144-9-200605020-00005. [DOI] [PubMed] [Google Scholar]
- 160.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sekhar N, Torquati A, Youssef Y, et al. A comparison of 399 open and 568 laparoscopic gastric bypasses performed during a 4-year period. Surg Endosc. 2007;21:665–8. doi: 10.1007/s00464-006-9151-2. [DOI] [PubMed] [Google Scholar]
- 162.Sjöström CD. Surgery as an intervention for obesity. Results from the Swedish obese subjects study. Growth Horm IGF Res. 2003;13(Suppl A):S22–6. doi: 10.1016/s1096-6374(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 163.Sjöström CD, Lissner L, Wedel H, et al. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999;7:477–84. doi: 10.1002/j.1550-8528.1999.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 164.Sjöström CD, Peltonen M, Sjöström L. Blood pressure and pulse pressure during long-term weight loss in the obese: the Swedish Obese Subjects (SOS) Intervention Study. Obes Res. 2001;9:188–95. doi: 10.1038/oby.2001.20. [DOI] [PubMed] [Google Scholar]
- 165.Sjostrom CD, Peltonen M, Wedel H, et al. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension. 2000;36:20–5. doi: 10.1161/01.hyp.36.1.20. [DOI] [PubMed] [Google Scholar]
- 166.Sjöström L. Bariatric surgery and reduction in morbidity and mortality: experiences from the SOS study. Int J Obes (Lond) 2008;32(Suppl 7):S93–7. doi: 10.1038/ijo.2008.244. [DOI] [PubMed] [Google Scholar]
- 167.Sjöström L, Gummesson A, Sjöström CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–62. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 168.Sjöström L, Lindroos A-K, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 169.Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 170.Steffen R, Biertho L, Ricklin T, et al. Laparoscopic Swedish adjustable gastric banding: a five-year prospective study. Obes Surg. 2003;13:404–11. doi: 10.1381/096089203765887741. [DOI] [PubMed] [Google Scholar]
- 171.Wölnerhanssen BK, Peters T, Kern B, et al. Predictors of outcome in treatment of morbid obesity by laparoscopic adjustable gastric banding: results of a prospective study of 380 patients. Surg Obes Relat Dis. 2008;4:500–6. doi: 10.1016/j.soard.2008.03.252. [DOI] [PubMed] [Google Scholar]
- 172.Weber M, Müller MK, Bucher T, et al. Laparoscopic gastric bypass is superior to laparoscopic gastric banding for treatment of morbid obesity. Ann Surg. 2004;240:975–82. doi: 10.1097/01.sla.0000145924.64932.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Weiner RA, Weiner S, Pomhoff I, et al. Laparoscopic sleeve gastrectomy—influence of sleeve size and resected gastric volume. Obes Surg. 2007;17:1297–305. doi: 10.1007/s11695-007-9232-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.