Abstract
In this review we describe a series of major concepts introduced during the past 150 years that have contributed to our current understanding about how physiological processes required for well-being and survival are regulated. One can theorize that hierarchical networks involving input-output relationships continuously orchestrate and learn adaptive patterns of observable behaviors, cognition, memory, mood, and autonomic systems. Taken together, these networks function as “good regulators” determining levels of internal variables and act as if there were homeostatic comparators (“homeostats”). The consequences of models with vs. without homeostats remain the same in terms of allostatic load and the eventual switch from stabilizing negative feedback loops to destabilizing, pathogenic positive feedback loops. Understanding this switch seems important for comprehending senescence-related, neurodegenerative disorders that involve the autonomic nervous system. Our general proposal is that disintegration of homeostatic systems causes disorders of regulation in degenerative diseases and that medical cybernetics can inspire and rationalize new approaches to treatment and prevention.
Keywords: Homeostasis, Biocybernetics, Allostasis, Stress, Autonomic
1.1 INTRODUCTION
In this review we describe a series of major concepts introduced during the past 150 years that have contributed to our current understanding about how physiological processes required for well-being and survival are regulated. Some of these concepts, such as homeostasis, stress, and the central autonomic network, are likely to be known to the reader. Others related to biocybernetics, such as good regulators, the law of requisite variety, and allostasis, may not be as familiar.
2.1 The Milieu Intérieur
In the mid-19th century, Claude Bernard expressed most clearly the concept that stability of the internal environment—of what he called the milieu intérieur—is required for survival. He wrote, “The constancy of the internal environment is the condition for free and independent life…All the vital mechanisms, however varied they might be, always have one purpose, that of maintaining the integrity of the conditions of life within the internal environment” (Bernard, 1974). His visionary concept, however, had almost no impact for 50 years, until Walter B. Cannon’s review on homeostasis (Cannon, 1929), in which Cannon acknowledged the importance of Bernard’s statement about the “fixity of the milieu intérieur” and quoted Haldane’s comment, “No more pregnant sentence was ever framed by a physiologist” (Haldane, 1922).
2.2 The Autonomic Nervous System
About the turn of the 20th century, the English physiologist John Newport Langley proposed the term, “Autonomic Nervous System,” to describe the networks of nerves derived from ganglia outside the central nervous system that mediate involuntary body processes. “Autonomic” reflected Langley’s view that the networks function autonomously of the central nervous system (Langley, 1903; Langley, 1921). This notion is incorrect; however, the phrase, “autonomic nervous system,” is by now engrained in the lexicon of medical science.
2.3 Adrenaline, Acetylcholine, and Chemical Neurotransmission
Soon after the discovery by Oliver and Schafer of the striking pressor effect of injected adrenal extract (Oliver et al., 1895), efforts began to identify the active principle (Abel et al., 1897), culminating in Takamine’s identification of adrenaline (Takamine, 1901), (synonymous with Abel’s “epinephrine”). Based on the similarity of the effects of adrenaline to those of sympathetic nerve stimulation, Langley’s student, Thomas R. Elliott, speculated about “a mechanism developed out of the muscle cell, in response to its union with the synapsing sympathetic fibre, the function of which is to receive and transform the nervous impulse. Adrenalin(e) might…be a chemical stimulant liberated on each occasion when the impulse arrives at the periphery” (Elliott, 1904). This statement is widely accepted as the first suggestion of a specific chemical substance being a neurotransmitter.
Chemical neurotransmission was established definitively by Otto Loewi in 1921, when he showed that a substance, “Vagusstoff,” released into the perfusate of an isolated frog heart by stimulating the vagus nerve, slowed the rate of contraction of a second, unconnected frog heart perfused with the effluent perfusate from the first heart (Loewi, 1921). He and a co-worker later identified the Vagusstoff as acetylcholine (Loewi et al., 1926)—the first known neurotransmitter. For this discovery Loewi received a Nobel Prize in 1936.
In his Nobel Prize lecture, Loewi referred to a report that adrenaline in alkaline solution and exposed to oxygen emits green fluorescence upon exposure to ultraviolet light, providing a sensitive, specific test for this compound (Gaddum et al., 1934). Loewi noted that in his frog heart preparation the effluent perfusate showed this reaction after sympathetic nervous stimulation. From this he considered it proven that adrenaline is the neurotransmitter released from sympathetic nerves. At the time it was not appreciated that other catecholamines give the same reaction.
2.4 Homeostasis
Walter B. Cannon introduced “homeostasis,” a word he invented, in 1926 to describe the stability of various constituents in body fluids and of core temperature. The full description of this new concept in his classic 1929 review (Cannon, 1929) was based on the results of his studies and those reported by others in the preceding two decades.
The sole figure in Cannon’s review illustrates diagrammatically how what he called the “sympathico-adrenal” and complementary “vago-insular” systems maintain homeostasis of blood sugar (Figure 1). Increases in blood glucose stimulate pancreatic islet cells to release insulin, which facilitates glucose uptake by organs such as the liver. Decreases in blood glucose reflexively stimulate adrenomedullary cells to release adrenaline, which augments glucose formation from liver glycogen and evokes glucose release into the bloodstream.
Figure 1. (A) Cannon’s concept diagram for homeostasis of blood glucose, and (B) concept diagram depicting homeostasis of a regulated variable by two complementary effectors.
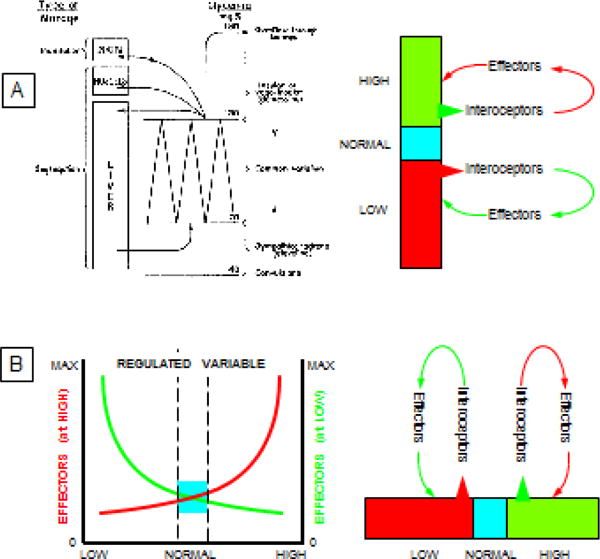
Cannon’s diagrammed how what he called the “sympathico-adrenal” and complementary “vago-insular” systems maintain homeostasis of blood sugar. Increases in blood glucose stimulate pancreatic islet cells to release insulin, which facilitates glucose uptake by organs such as the liver. Decreases in blood glucose reflexively stimulate adrenomedullary cells to release adrenaline, which augments glucose formation from liver glycogen and evokes glucose release into the bloodstream. The double-y graph at the bottom left is a more generalized depiction of Cannon’s schema. Increases in the level of the regulated variable beyond a certain limit (green) evoke effects at multiple levels of the neuraxis that tend to return the level of the regulated variable to within bounds, and decreases in the level of regulated variable (red) evoke effects that also tend to return the level of the regulated variable to within bounds. One effector (e.g., insulin) responds to an increase and one (e.g., the sympathetic adrenergic system) to a decrease in the level of the regulated variable (e.g., serum glucose). The level of the regulated variable is kept within bounds by the input-output relationship, without a homeostatic comparator (e.g., a “glucostat”). Cannon’s diagram is reproduced with permission from the American Physiological Society.
More generally, the level of each internal variable is kept within bounds because of complementary effector output systems when the level increases (green arrows in Figure 1) or decreases (red arrows in Figure 1). Cannon wrote, “When a factor is known which can shift a homeostatic state in one direction it is reasonable to look for automatic control of that factor or for a factor or factors having an opposing effect” (Cannon, 1929).
2.5 Sympathins and Adrenergic Receptors
The fact that adrenaline is excitatory at some sites and inhibitory at others remained unexplained for more than a quarter century. In 1930, when Arturo Rosenblueth joined Cannon at Harvard, their efforts to explain the dual actions of adrenaline led to their proposal that adrenaline is released from sympathetic nerves but is modified in the affected target cells. The chemical messenger was assumed to react with a hypothetical substance, H, to form “sympathin.” Cannon and Rosenblueth hypothesized that there are two types of H, HE and HI, which result in the formation of an excitatory substance, sympathin E, or an inhibitory substance, sympathin I (Cannon et al., 1933).
Because of the acceptance of the notion of sympathins, when Raymond Ahlquist, based on his findings of two different orders of potency of seven sympathomimetic agents in different tissues, postulated that there were two different receptors, alpha and beta, his paper, which challenged the validity of the concept of two kinds of sympathin, was rejected as being speculative (Ahlquist, 1948). The view that adrenaline is the sympathetic neurotransmitter was not discarded until von Euler’s identification of norepinephrine (NE) as the sympathetic neurotransmitter in 1946 (von Euler, 1946), for which he received a Nobel Prize in 1970. Ahlquist’s notion of two receptor types was confirmed by James Black’s development of beta-adrenoceptor blockers, for which Black received a Nobel Prize in 1988.
Cannon believed that the neuronal and hormonal components of the “sympathico-adrenal system” function as a unit. The idea of a unitary sympathoadrenal system persisted for at least 35 years after Cannon’s death (Cryer, 1980). Evidence has accrued, however, that sympathetic noradrenergic and adrenergic responses can be regulated differentially in response to different stressors (Goldstein et al., 2008) or pathophysiologic states (Goldstein et al., 2003).
Homeostasis of internal variables is often taught using the analogy of a thermostat regulating the interior temperature of a house (e.g., (Modell et al., 2015)). Although it may be tempting to postulate that there are multiple internal homeostatic systems, each with its own “homeostat”—a “barostat” for regulating blood pressure (Folkow, 1990), a thermostat for regulating core temperature, a “glucostat” for regulating blood glucose levels (Koeslag et al., 2003), an “osmostat” for regulating serum osmolality (Verbalis et al., 1986), and so forth—no comparator has been identified for any regulated internal variable.
Homeostats are metaphors or models for how the regulation happens—i.e., the body maintains homeostasis as if there were homeostatic comparators. Our concept is of complementary, complex interactions of neuronal networks that function as “stats,” orchestrating a variety of behavioral and physiological responses.
2.6 Negative Feedback Regulation
We propose here that three types of process maintain homeostasis. The first and most well known is error control by negative feedback regulation. The second, which seems not to have been incorporated explicitly previously in concepts of homeostasis, is buffering. The third is feed-forward regulation, which is the most challenging from a theoretical point of view.
Negative feedback regulation is a key—if not the key—mechanism for maintaining physiological homeostasis. Perhaps surprisingly, Cannon did not explicitly include negative feedback regulation in his explanation of homeostasis. Probably the first researcher to emphasize physiological regulation by negative feedback was the Russian physiologist, Pyotr K. Anokhin (1898–1974) in the mid-1930s (Egiazaryan et al., 2007). His “theory of functional systems” involved feedback of two types, the first akin to homeostasis for regulation of internal variables such as glucose levels and the second for regulation of behavioral responses based on feedback from environmental signals.
In a retrospective about Anokhin’s theory, his disciple K.V. Sudakov wrote, “Any deviation of the parameter from the level required for normal life of the organism immediately elicits (through feedback mechanisms or reverse afferentation after Anokhin), a sequence of processes that develop in central and peripheral tissues in order to restore the optimal level of the given result” (Sudakov, 1997). Clearly, “reverse afferentation” was Anokhn’s term for negative feedback.
Corneille Heymans received a Nobel Prize in 1938 for his studies of chemoreflexes regulating breathing, baroreflexes regulating blood pressure, and interactions between these reflexes (Heymans et al., 1958). The chemoreflex and baroreflex are classic examples of negative feedback regulation. Increasing carbon dioxide tension or decreasing oxygen tension in the carotid arterial blood increases respiration reflexively via chemoreceptor afferents from the carotid body and efferents in the phrenic nerve and evokes blood vessel constriction and increased heart rate via baroreceptor afferents from the carotid sinus and efferents in autonomic nerves. Increased distending pressure in the carotid sinus area reflexively relaxes blood vessels and decreases both heart rate and respiration. It is noteworthy that Cannon’s review, although recognizing that blood pressure was elevated by sympathico-adrenal activation, did not include homeostasis of blood pressure.
Examples of negative feedback regulation mediated by alterations in autonomic outflows abound in autonomic neuroscience. The regulation of blood glucose levels depicted in Figure 1 has been confirmed many times. When glucose levels decrease, effectors such as glucagon and the sympathetic adrenergic system are activated to increase glucose levels (green arrows), and when glucose levels increase, effectors such as insulin-secreting pancreatic islet cells are activated that decrease glucose levels (red arrows).
Another example of homeostasis via negative feedback regulation is the response elicited during performance of the Valsalva maneuver. The increase in thoracic pressure decreases venous return to the heart and consequently decreases cardiac stroke volume and blood pressure; this reflexively increases sympathetic nerve traffic in skeletal muscle (Delius et al., 1972) and augments total peripheral vascular resistance (Korner et al., 1976), lessening the fall in blood pressure. In response to decreased venous return during orthostasis, plasma NE increases rapidly, reflecting reflexive activation of the sympathetic noradrenergic system (Lake et al., 1976).
Intravenous injection of cold saline evokes increases in both sympathetic and adrenal medullary outflows (Goldstein et al., 2001), resulting in cutaneous vasoconstriction that diminishes heat loss and in calorigenesis. The combination effectively attenuates the fall in core temperature.
2.7 Buffering
Buffering is a means of diminishing the intensity of an external disturbance, thereby reducing the required use of reflexive homeostatic mechanisms (Figure 2). Just as insulation in the walls of a house diminishes the requirement of internal adjustment by a furnace in cold weather, many mammals have fur, which creates a layer of motionless air as an insulator above the skin. Other examples of genetically determined insulation based on natural selection in evolution include blubber in whales and closely packed feathers in birds. The barrier to heat loss can be enhanced during more severe cold exposure by reflexive bristling of the hair, mediated by sympathetic nerves (Chaplin et al., 2014); this increases the depth of the layer of motionless air. Behavioral responses to buffer the cold include huddling, seeking shelter, hibernation, and bird migration. Humans can also don appropriate clothing, which is inserting a buffer. Thus, buffering can be energy-dependent or energy-independent. In Figure 3, the + or 0 refers to buffering dependent on or independent of effector activation.
Figure 2. Conceptual model of homeostasis by negative feedback (error control), anticipatory (feed-forward) control, and buffering.
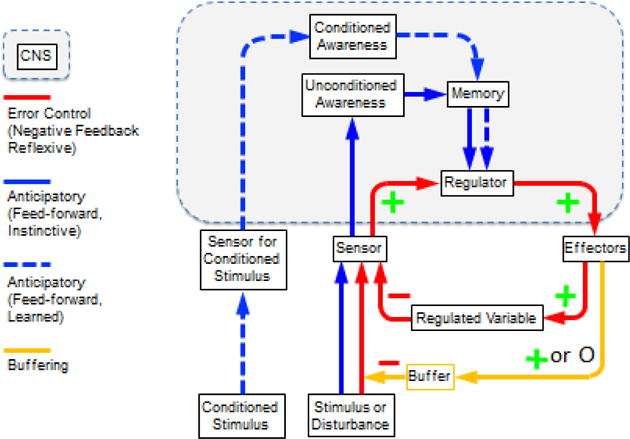
In the model, the effectors are both autonomic and non-autonomic. Effector responses to a disturbance are determined by three forms of regulation. First, effector activities are determined from input-output (afferent-efferent) curves relating sensory input to effector output (error control by negative feedback). Second, via exteroceptive or interoceptive input, effector activities are altered by instinct or imprinting, in advance of a change in the level of the regulated variable. Third, via exteroceptive input, effector activities are altered by associative learning (classical or instrumental conditioning), also in advance of a change in the level of the regulated variable. The extent of sensor activation in response to a disturbance is modulated by buffering. Buffering is a means of diminishing the intensity of an external disturbance, thereby reducing the required use of reflexive homeostatic mechanisms Effector responses can also modify buffering in advance of a disturbance, via instinct or learned behaviors (e.g., piloerection during cold exposure; donning a jacket before entering a cold environment).
Figure 3. Concept diagram showing mechanisms of anticipatory and error-controlled regulation.
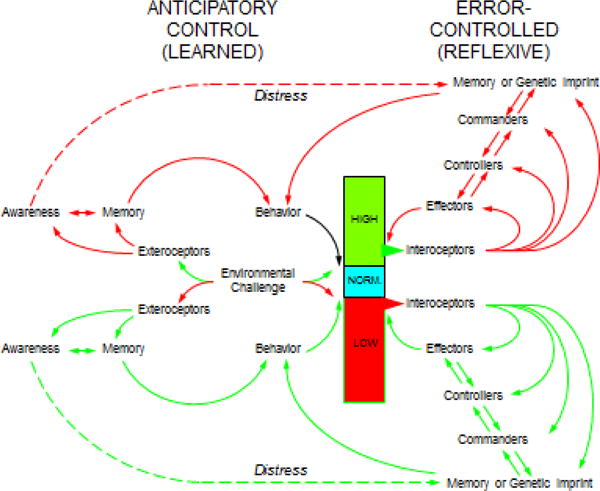
The autonomic responses seem to reflect a hierarchical arrangement, with the lowest level sensory-effector mechanisms in the target cells (e.g., glucose sensing by pancreatic islet cells). Superimposed on these are spinal- and brainstem-mediated reflexes, based on input from interoceptors (here designated “controllers”). The brainstem reflexes in turn are modulated by hypothalamic and limbic centers, also dependent on sensory input (“commanders”). Hypothalamic activity patterns are modulated by the limbic centers, which reflect emotions, habituation, sensitization, imprinting, and classical conditioning. Finally, higher cortical centers are responsible for cognition, instrumentally conditioned learned behaviors, predictions of future events, and interpretations of environmental and social stimuli. Under ordinary circumstances, levels of the regulated variable are kept within bounds by anticipatory (learned) control. When this gives way, error-controlled (reflexive) regulation comes into play. Being reactive, error-controlled regulation is associated with increased variability of the regulated variable. The curved arrows indicate the multiple input-output relationships at ascending strata in the neuraxis, from the target organ (e.g., the pancreatic islet cells) to the autonomic effectors, to lower brainstem “controller” sites mediating reflexes (e.g., the nucleus of the solitary tract, rostral ventrolateral medulla and dorsal motor nucleus of the vagus nerve), to hypothalamic/upper brainstem “commander” sites mediating vigilance, automatic motor behaviors, and patterned instinctive responses (e.g., locus ceruleus, substantia nigra), to limbic sites involving emotional memory and classically conditioned learning (e.g., hippocampus, amygdala), to cortical sites involving social consciousness, restraint of lower centers, instrumentally conditioned learning, and interactions with the environment (e.g., orbitofrontal cortex, anterior cingulate cortex, insular cortex).
In addition to these inherited and acquired forms of buffering, countercurrent heat exchange is a relatively common, efficient mechanism present in animals that limits heat loss through the surfaces of the body exposed to an extremely cold environment (polar regions or ice cold water). The preservation of heat is the result of differences in the temperature in closely adjacent arterial and venous blood vessels. Heat from arterial blood warms the cold venous blood returning from the cold surface, diminishing heat loss from the surfaces exposed to the cold. This arrangement occurs in the feet of penguins standing on ice or birds wading in cold water, the paws of the arctic fox, and the tongue of the whale filtering algae from cold water. The heat transfer between the countercurrent flows depends on the difference in the temperatures and does not require energy other than that required to maintain the flows.
A somewhat more complex countercurrent system in some species has evolved that cools the brain during exposure to high environmental temperatures. This involves two sequential heat transfers. During inhalation, particularly during panting, moisture evaporation cools the nasal mucosa. A second heat transfer occurs when the cooler venous blood from the nasal mucosa flows into the pterygoid plexus surrounding the carotid rete in a sinus at the base of the brain. Here there is a countercurrent transfer of heat from the warm arterial blood, which flows in the direction opposite to the cooler mucosal venous blood. The cooled arterial blood enters the circle of Willis and is delivered to the brain during exposure to a hot environment. This countercurrent exchange mechanism has been described in detail in sheep and cats (Baker, 1972) and has been reported in a number of other species. Although humans do not have a carotid rete, it has been hypothesized that the diversity in human craniofacial features (e.g., nostril width) in different geographical regions reflects survival advantages of mechanisms for selective brain cooling in hot environments (Irmak et al., 2004). Moreover, tonic alterations in sympathetically mediated vasoconstrictor tone may contribute to selective brain cooling (Azzaroni et al., 1995).
2.8 Feed-Forward Regulation
Feed-forward regulation is mediated by anticipatory adjustments in physiological systems based on awareness of a previously experienced or instinctively recognized signal, preceding any change in the level of the regulated variable itself (blue arrows in Figure 2). Ganzel et al. (Ganzel et al., 2010) and Schulkin (Schulkin, 2003) have discussed the difficulty of reconciling classical homeostasis with continual alterations in internal set-points, because regulation by negative feedback is essentially reactive (Ramsay et al., 2014).
Feed-forward regulation is more efficient than negative feedback regulation, because it diminishes or eliminates the need for homeostatic adjustments. An example is the vagal mediation of the “cephalic phase” of insulin release prior to eating, in anticipation of an increase in blood glucose. Another is the sympathetically mediated hemodynamic changes following “central command” in anticipation of exercise. Cannon was referring to feed-forward mechanisms when he noted that internal changes during emotional turmoil prepare the organism for extreme muscular exertion (Cannon, 1939).
Figure 2 shows the relationships of reflexive error control via negative feedback (red), buffering (tan), and anticipatory regulation (blue). The anticipatory control mechanisms can be instinctive (solid lines) or conditioned (dashed lines). A disturbance can arouse anticipatory instinctive responses by pathways involving awareness (conscious or unconscious); and an associated conditioned stimulus can arouse anticipatory responses by pathways involving awareness and conditioned learning. A disturbance is sensed by interoceptors (e.g., gastrointestinal hemorrhage) or exteroceptors (e.g., touching a hot iron), while a conditioned stimulus is sensed by exteroceptors.
Figure 3 depicts in more detail regulation by anticipatory control, which usually is learned and mediated by behavior, and by error control, which is reflexive and mediated by effectors such as components of the autonomic nervous system. Under normal circumstances, in response to anticipation of environmental challenges (e.g., going out into the cold outdoors) levels of regulated variables are kept within bounds mainly by anticipatory behaviors (e.g., donning a jacket), the elicitation of which depend on input from exteroceptors (e.g., visual input), perception of the meaning of the input (awareness, e.g., snow is falling), and memory (when snow falls, the weather is cold). The behavior prevents exposure to the environmental challenge from actually altering levels of the regulated variable, in this case core temperature.
When anticipatory, learned behaviors are insufficient, and an actual change in the level of core temperature occurs, this evokes reflexive increases in sympathetic noradrenergic and adrenergic outflows, which by cutaneous vasoconstriction and calorigenesis maintain core temperature. The reflexive responses may include certain externally observable behaviors, such as shivering, piloerection, and folding of the arms. In distress, awareness and memory result not only in behaviors (e.g., flight) but also in changes in reflexive regulation (dashed lines).
2.9 Stress and Distress
Cannon rarely referred to stress; when he did so he always meant a disturbance that imposes a threat to homeostasis. Hans Selye popularized stress as a medical condition (Selye, 1936; Selye, 1950; Selye, 1956; Selye, 1974). Selye defined stress as the non-specific response of the body to any demand imposed upon it (Selye, 1976). He described the non-specific response, which he called the “General Adaptation Syndrome” (Selye, 1956), as occurring in three stages—alarm, mediated by release of hormones from the adrenal cortex and medulla; resistance, in which the body attempts to resist or adapt; and exhaustion, leading to slow recovery or death.
Ambiguity arose when stress was used to refer to a disturbance that threatens homeostasis, the state produced by the disturbance, and the non-specific response to the state. This led to Roberts’s critical observation, “…stress, in addition to being itself and the result of itself, is also the cause of itself” (Roberts, 1950). Subsequently, Selye introduced “stressor” (used for the first time in 1950, according to the Merriam-Webster dictionary), which he defined as that which results in a state of stress (Selye, 1956). The ambiguity did not disappear, however. For example, Chrousos and Gold wrote that stress is a “state of disharmony, or threatened homeostasis” (Chrousos et al., 1992), similar to Selye’s idea of a state, whereas according to McEwen, stress is a “real or interpreted threat to the physiological or psychological integrity of an individual that results in physiological and/or behavioral responses” (McEwen, 2000), similar to Selye’s definition of a stressor. In the remainder of this review, “stressor” is used to denote a disturbance, “stress” a state resulting from the effects of the disturbance, and “stress response” altered activity of one or more effectors attending stress.
According to Selye’s “doctrine of non-specificity,” specific and non-specific responses meet all challenges to homeostasis. It is the shared element, the non-specific response, that reflects stress. It took more than a half century for Selye’s doctrine of non-specificity to be examined critically (Pacak et al., 1998). Pacak et al. showed that without a limiting assumption, the doctrine of non-specificity cannot be disproved, meaning that the doctrine has limited scientific value. Non-specificity becomes testable by assuming that above a certain intensity, the specific components become negligible in the overall response. In this case, non-specificity predicts equal ratios of responses of plasma corticotropin and epinephrine levels between relatively low- and high-intensity stressors, regardless of the stressor; however, this was shown clearly not to be the case for all stressors (e.g., pain vs. hemorrhage). Thus, the experimental data were inconsistent with Selye’s doctrine of non-specificity and argued against the existence of a unitary stress syndrome. An alternative proposal is that each stressor has a “signature,” involving relatively distinctive patterns (“primitive specificity”) of physiological, biochemical, and behavioral responses (Goldstein, 2006; Kvetnansky, 2004; Kvetnansky et al., 1998).
Selye did not view stress as necessarily harmful. He viewed “distress” as damaging or unpleasant stress (Selye, 1974). Selye proposed an immense list of what he called “diseases of adaptation,” from adrenal gland tumors to hypertension, vasculitis, diabetes, allergy, and psychosomatic disorders. Selye’s definition of distress may be misunderstood to indicate that it is solely associated with pathology, although he does include “unpleasant.” To be more precise, we regard distress as a form of stress that is conscious, aversive, generates instinctively communicated signs, and is associated with adrenocortical and adrenomedullary activation (Goldstein, 2006).
2.10 Cybernetics and Biocybernetics
During his stay in Cannon’s laboratory, Arturo Rosenblueth became acquainted with Norbert Wiener and Julian Bigelow, who at the time (wartime in the early 1940s) were working on a servo-mechanism-based rangefinder for anti-aircraft guns. Their machine was “intelligent,” in that it could predict the trajectory of an airplane based on the previous trajectories (“memory”); however, when they tried to correct the machine for friction, it developed uncontrollable swings in motion. Wiener asked Rosenblueth, who had become a neurologist, if there were neurological disorders manifesting similar signs. Rosenblueth described the difficulty of patients with cerebellar injury in drinking a glass of water, due to uncontrolled, oscillatory amplification of voluntary movements. From the similarity of the neurological disorder to malfunction of the rangefinder they realized that both required “a closed loop (negative feedback) allowing the evaluation of the effects of one’s actions and adaptation of future conduct based on past performance” (Rosenblueth et al., 1943). The authors conceived of the idea that control systems in machines could be designed to emulate the nervous system by negative feedback and to self-regulate using the results of earlier activity to improve the likelihood of attaining the goal. Conversely, close examination of the requirements for ideal control might provide directions in research about how the nervous system is designed to gain control of physiological variables via negative feedback and memory.
Rosenblueth, Wiener, and Bigelow classified behavior in terms of (1) active or non-active, then if active (2) purposeful or non-purposeful, then if purposeful (3) feedback-regulated (teleological) or non-feedback-regulated, and then if feedback-regulated (4) predictive (extrapolative) or non-predictive. Teleology was not taken to imply “final causes” and was viewed as synonymous with purpose controlled by feedback. Finally—and provocatively—they argued that the “broad classes of behavior are the same in machines as in living organisms,” regardless of the complexity of the behavior.
Rosenblueth, Wiener, and Bigelow first presented their ideas in 1942 to mathematicians, physiologists and engineers attending a seminar at Princeton’s Institute for Advanced Study. The seminar was extremely successful and induced the Josiah Macy Foundation to sponsor a series of such interdisciplinary meetings. In 1948, Wiener’s book appeared in which he coined the term “cybernetics” (Wiener, 1948). This captured the ideas examined so well that the seminars became known as the Macy Cybernetics Conferences. The participants were attempting to develop a theory of control systems that would be applicable to a wide variety of disciplines, including biology—“biocybernetics” (Wiener, 1965).
Wiener distinguished two forms of biocybernetics, medical biocybernetics and neurocybernetics (Wiener and Schade, 1963). In medical biocybernetics, “homeostasis is the main consideration,” whereas neurocybernetics mainly involves “the pathways of actions via sense-organs, neurons and effectors,” although it was pointed out that “there is no sharp distinction between the two fields.”
The cybernetic approach to understanding brain functions aroused considerable debate. One of the attendees at the Macy Conferences, John von Neumann, gave this opinion, quoted in the thesis of Walter Daniel Hellman (Hellman, 1982): “The difficulties are almost too obvious to mention: They reside in the exceptional complexity of the human nervous system, and indeed of any nervous system…Nothing that we may know or learn about the functioning of the organism can give, without ‘microscopic’ cytological work, any clues regarding the further details of the neural mechanism.”
2.11 Ashby’s Homeostat, Law of Requisite Variety, and Good Regulator Theorem
W. Ross Ashby, one of the founders of the fields of cybernetics and complex systems, introduced a machine he called “the homeostat” in the 1940s. When an initial stable setting was disturbed, this machine was able to select from among a variety of nearly 400,000 randomly presented settings and to self-regulate to reach a new stable state. The multiple possibilities for attaining a stable state led Ashby to describe the homeostat as “ultrastable.” In terms of physiology and behavior, an ultrastable system has the capacity to adapt by trial and error learning.
Ashby’s homeostat, a type of electromagnetic machine, is rather far removed from our concept of the homeostat as in essence a metaphor. We mention Ashby’s homeostat so that the reader is not confused by the use of the same word in the past.
Ashby’s “Law of Requisite Variety” states that for a system to be stable, the number of states of its control mechanism must be greater than or equal to the number of states in the system being controlled, summarized as “variety is necessary to destroy variety” (Ashby, 1956). This law may be viewed as a generalization of concepts intuitively expressed previously by a number of physiologists.
Ashby’s Good Regulator theorem, which Conant and Ashby proved mathematically (Conant et al., 1970), states that a good regulator models well the system it regulates—model meaning that each variable of the regulator corresponds to one and only one of the variables that must be regulated, like the geometry of a good key must complement the geometry of the lock. “Good” means that the regulator is maximally efficient and simple but does not imply that the regulated variable must be kept within bounds (homeostasis).
Ashby and Conant noted corollarily that the living brain, insofar as it is to be successful and efficient as a regulator for survival, must proceed, in learning, by modeling its environment, as described above.
Ashby viewed the occurrence of good regulation in neural control of the internal environment as the product of eons during which natural selection has acted on requisite variety of control systems. Wiener agreed when he wrote that “phylogenetic learning” and “ontogenetic learning” are modes by which the animal can adjust itself to its environment (Wiener, 1948).
2.12 Central Neural Hierarchies
Cannon recognized the key roles of the brain both in coordinating body systems to keep values for internal variables within physiological bounds and in elaborating “emergency” responses. For instance, Cannon and Britton noted that cerebral decortication evoked rage behavior accompanied by hyperglycemia; decorticated adrenalectomized animals exhibited the same behavior but without hyperglycemia (Cannon et al., 1925). These findings were consistent with cortical restraint of primitive emotional behaviors and of emotion-associated adrenomedullary secretion.
Cannon’s student, Philip Bard, used serial brain sectioning in decorticate animals to obtain evidence that physiological concomitants of primitive emotions originate in the hypothalamus (Bard, 1928). The phenomenon of “sham rage” in decorticate animals fit with the view that overall the cerebral cortex exerts inhibitory restraint on the hypothalamic expression of primitive emotional behaviors. On the other hand, stimulation of baroreceptor afferents inhibits sham rage (Bartorelli et al., 1960).
In the 1920s to 1930s W. R. Hess focused on hypothalamic regulation of parasympathetic and sympathetic outflows and their behavioral concomitants. He showed that stimulation of posterior hypothalamic sites that altered functions of internal organs via sympathetic outflows also evoked appropriate behaviors directed towards the environment (“ergotropic” effects). Stimulation of anterior sites evoked signs consistent with generalized parasympathetic activation that also were associated with characteristic behaviors (e.g., postural change associated with defecation), which Hess viewed as protective against overloading (“trophotropic”). The sympathetic-ergotropic and parasympathetic-trophotropic areas seemed to operate in a state of dynamic equilibrium (Hess, 1949). These findings, for which Hess received a Nobel Prize in 1949, demonstrated that autonomic functions depend crucially on the central nervous system and that higher centers modulate autonomic outflows in coordinated neuroendocrine and behavioral patterns.
Hess’s experiments took place before the advent of chemical neuroanatomy. In 1954, Marta Vogt, by noting substantial regional differences in norepinephrine concentrations in the brain (highest in the hypothalamus), provided the first indication that norepinephrine is a central neurotransmitter (Vogt 1954). About 10 years later, a technique was introduced for visualizing catecholaminergic nerve fibers based on fluorescence of catecholamines upon exposure of the tissue to formaldehyde vapor (Falck et al., 1962). With this technique Fuxe and Dahlstrom described catecholaminergic pathways and centers that were distinct from traditional neuroanatomic tracts and nuclei (Dahlstrom et al., 1964; Dahlstrom et al., 1965).
Over the next half century, additional immunohistofluorescence methods enabled detailed description of anatomic and neurochemical pathways participating in autonomic outflows (Bard, 1960; Dahlstrom et al., 1964; Reis et al., 1965; Reis et al., 1988). Adding to the richness of diversity, Hokfelt subsequently reported evidence for co-storage of peptides with catecholamines in brainstem neurons (Hokfelt et al., 1983), and Burnstock developed the concept of purinergic autonomic nerves (Burnstock, 1977). The field of chemical coding based on co-transmission continues to evolve (Burnstock, 2013; Gourine et al., 2009).
Efforts to refine understanding of pathways relating autonomic outflows to particular centers in the brain, including the cerebral cortex, continue to the present day. For instance, Dum et al. recently used transneuronal retrograde transport of a non-pathogenic form of rabies virus to identify cortical areas that communicate through multisynaptic connections with the adrenal medulla (Dum et al., 2016). They identified two general cortical sources of input—a broad network of lateral cortical motor areas that are involved with movement selection, preparation, and execution; and a smaller medial network in multiple cingulate cortical areas that are involved with cognition and emotion.
The responses seem to reflect a hierarchical arrangement, with the lowest level sensory-effector mechanisms in the target cells (e.g., glucose sensing by pancreatic islet cells). Superimposed on these are spinal- and brainstem-mediated reflexes, based on input from interoceptors (in Figure 3 these are designated “controllers”). The brainstem reflexes in turn are modulated by hypothalamic and limbic centers, also dependent on sensory input (“commanders”). Consistent with Hess’s view, hypothalamic activity patterns are modulated by the limbic centers, which reflect emotions, habituation, sensitization, imprinting, and classical conditioning. Finally, higher cortical centers are responsible for cognition, instrumentally conditioned learned behaviors, predictions of future events, and interpretations of environmental and social stimuli.
The patterning of stress responses may be instinctive, imprinted, or learned. Instinct is a genetically determined response that is independent of adaptability. An autonomically mediated reflex at the level of the lower brainstem can be considered to be instinctive. Imprinting refers to an environmentally elicited, largely but not exclusively genetically determined behavior (e.g., newly hatched ducklings follow the first moving object they see (Lorenz, 1952)). Pavlovian (classical) conditioning is a form of associative learning and probably requires involvement of limbic structures. Instrumental (operant) conditioning, which affords the greatest amount of adaptability, involves acquisition or extinction of behaviors based on their consequences (reward or positive reinforcement and punishment or negative reinforcement); this form of learning seems to require higher cortical centers.
The notion of central neural hierarchies determining autonomic outflows in emotional states follows the concepts introduced by Anokhin in the 1930s (Sudakov, 1997). Reviews by Craig (Craig, 2013), Critchley (Critchley et al., 2011), Mayer (Mayer, 2011), Damasio (Damasio, 1994; Immordino-Yang et al., 2014) and LeDoux (Rogan et al., 1997) have essentially agreed on involvement of particular clusters of neurons at lower brainstem, hypothalamic, limbic, and frontal and temporal cortex levels.
Combinations of functional magnetic resonance imaging with laboratory psychological challenges in healthy volunteers and in patients with particular brain or autonomic lesions have indicated specific roles of the nodes in this complex network in maintaining homeostasis. For instance, Craig has argued that the parabrachial nucleus (PBN in Figure 4) is the main integration site for all homeostatic afferent activity and that the anterior insular cortex is the site for the subjective image of oneself as a sentient entity—i.e., emotional awareness. The latter is in line with Damasio’s “somatic marker” hypothesis (Damasio, 1994). Critchley has found that expression of conditioning-related neural activity in amygdala and insula depends on both cognitions and representations of bodily states of autonomic arousal. This interpretation provides a neuroanatomic substrate for the proposal by Schachter and Singer of cognitive and physiological determinants of emotional feelings about a half century ago (Schachter et al., 1962). Recent ontogenetic experiments have also supported a role of the central nucleus of the amygdala in predatory behavior (Han et al., 2017).
Figure 4. Systems biologic and integrative physiologic views of homeostatic regulation of blood pressure in response to infusion of a vasoconstrictor.
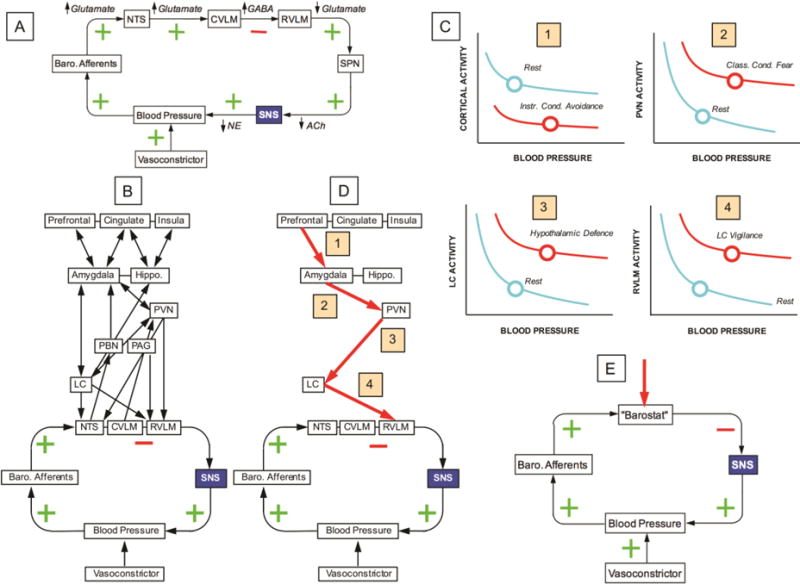
This concept diagram conveys a way neurocybernetics and medical biocybernetics can be linked. Neurocybernetics is focused on the complex systems biologic network, and medical biocybernetics deals with the negative feedback regulation that is necessary for homeostasis. This Figure depicts homeostatic regulation of blood pressure during infusion of a vasoconstrictor. (A) Reflexive responses to a vasoconstrictor involve increased baroreceptor afferent traffic to the nucleus of the solitary tract (NTS), followed by activation of the caudal ventrolateral medulla (CVLM). Release of the inhibitory neurotransmitter GABA from the CVLM terminating in the rostral ventrolateral medulla (RVLM) inhibits RVLM outflow to the sympathetic pre-ganglionic neurons (SPN) in the intermediolateral columns of the spinal cord, resulting in decreased post-ganglionic sympathetic noradrenergic system (SNS) traffic and decreased delivery of norepinephrine (NE) to its receptors on vascular smooth muscle cells. Green positive signs indicate positive relationships, and the red negative sign indicates a negative relationship. Because there is a single negative relationship in the feedback loop, the blood pressure attains a steady-state value lower than that produced by the vasoconstrictor in the absence of the feedback loop. (B) Nodes and relationships in the central autonomic network that mediate modulation of the blood pressure response. (C) Input-output curves at ascending levels of the neuraxis. Locus ceruleus (LC) activation as part of vigilance shifts the relationship between activity of rostral ventrolateral medulla (RVLM) neurons and blood pressure; a defence reaction evoked at the level of the hypothalamus shifts the curve relating LC activity to blood pressure; classically conditioned fear shifts the relationship between paraventricular nucleus (PVN) activity and blood pressure; and instrumentally conditioned avoidance shifts the relationship between cortical activity and blood pressure. (D) Hypothetical pathway by which release from cortical restraint exerts a feed-forward inhibition of baroreflex regulation of blood pressure. (E) The adjustments in the input-output curves have the net effect of resetting a hypothetical “barostat.” Other abbreviations: Class. Cond.=classical conditioned; Instr. Cond.=instrumentally conditioned.
The concept of central neural hierarchies is consistent with Benarroch’s “central autonomic network,” depicted diagrammatically in Figure 4B (Benarroch, 1993). The network includes (in ascending order in the neuraxis) the caudal ventrolateral medulla (CVLM), nucleus of the solitary tract (NTS), dorsal motor nucleus of the vagus, the nucleus ambiguus (NA), rostral ventrolateral medulla (RVLM), raphe nuclei, locus ceruleus (LC), periaqueductal gray matter (PAG), parabrachial nuclear complex (PBN), paraventricular nucleus of the hypothalamus (PVN), amygdala and hippocampus (Hippo.), and insular, anterior cingulate, and retro-orbital or pre-frontal cortex. The recent findings of Dum et al. confirm connections from the cortex to the adrenal medulla (Dum et al., 2016).
Our conception, which is in line with several thought leaders in the field as noted above, dispels the notion that the autonomic nervous system acts largely unconsciously, involuntarily, and independently. Instead, autonomic outflows are altered in concert with conscious, voluntary behaviors and experienced emotions.
2.13 Homeostasis of blood pressure
The concept diagram in Figure 4 conveys a way neurocybernetics and medical biocybernetics can be linked. Neurocybernetics is focused on the complex systems biologic network, and medical biocybernetics deals with the negative feedback regulation that is necessary for homeostasis. The Figure depicts homeostatic regulation of blood pressure during infusion of a vasoconstrictor. Infusion of the vasoconstrictor increases blood pressure, which stimulates baroreceptor afferent traffic to the NTS. Increased activity of NTS glutamatergic neurons stimulates release of GABA in the CVLM, resulting in decreased glutamate release from RVLM neurons to sympathetic pre-ganglionic neurons in the intermediolateral columns of the spinal cord—a homeostatic negative feedback loop (Figure 4A).
This loop is subject to modulation by higher centers that impinge on the medullary NTS, CVLM, RVLM, and NA (Figure 4B). Increased activity in the pontine locus ceruleus (LC) as part of a vigilance reaction (Grant et al., 1988) modifies the relationship between RVLM outflow and blood pressure. At hypothalamic and limbic system levels, there are yet other input-output relationships, for emotional, behavioral, or arousal states. Thus, stimulation of the PVN attenuates responses of neurons of the medullary nucleus of the solitary tract (NTS) that are activated when phenylephrine is injected to increase blood pressure (Duan et al., 1999). Increased activity in limbic system centers such as the amygdala and hippocampus as part of classical fear conditioning alters the relationship between PVN activity and blood pressure. Finally, at higher cortical levels there are yet other input-output relationships for executive functions, psychosocial restraint, operantly conditioned behaviors, and simulations. Release from cortical restraint as part of an instrumentally conditioned avoidance response alters the relationship between cortical activity and blood pressure.
All these processes might contribute to the reflexive response to the vasoconstrictor. Although the pathways and interactions are highly complex (Aston-Jones et al., 1991; Beck et al., 1995; Carrive et al., 2008; Keifer et al., 2015; Radley et al., 2008), and the multi-center pathway depicted in Figure 4D has not been specifically defined, overall the system operates as if there were feed-forward regulation of a “barostat” (red arrow in Figure 4E).
In summary, in panel A of Figure 4 we show the cycle of medullary stations and neurotransmitters in the baroreflex arc that participate in homeostatic responses to a blood pressure perturbation. In panel B we show the hierarchy of centers impinging on the medullary cycle. In panel C we show the concept that at each level of the central autonomic network there are input-output curves that are shifted (allostatic states, discussed below), and because of these shifts the characteristics of the medullary cycle change (panel D) in a manner that gives the impression of a homeostatic comparator—a barostat—as in panel E.
2.14 Homeostatic Mechanisms
Figure 5B depicts a model involving effector sharing by two sensor-effector systems. When two such systems share an effector, a disturbance affecting the level of one regulated variable would be expected to affect the level of another regulated variable, the latter attaining a new steady-state value (Goldstein, 2013). For instance, hyperglycemia can result from adrenaline release attending myocardial infarction, severe injury (Frayn et al., 1985; Oswald et al., 1988), and other stressful situations (Halter et al., 1984). This phenomenon was predicted by Cannon, in that there are many side effects of activation of the sympathico-adrenal system as a common mechanism to maintain homeostasis.
Figure 5. Concept diagrams for negative feedback regulation of internal variables.
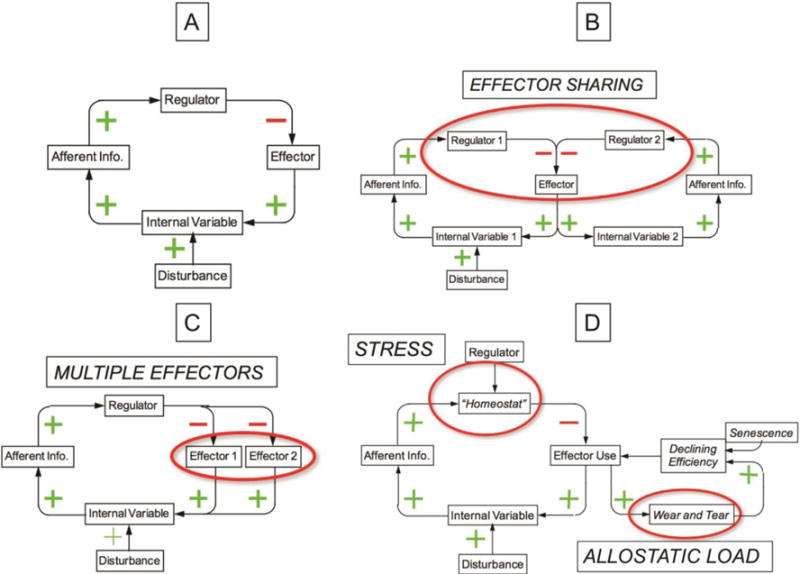
(A) Simple negative feedback loop. In response to a constant disturbance, the level of the internal variable attains a steady state value. (B) Diagram of multiple effectors. Having multiple effectors extends the range of control, enables compensatory activation when one effector fails, and provides a basis for patterning of stress responses. (C) Diagram of effector sharing. Effector sharing can account for phenomena such as hyperglycemia in shock and hyponatremia in decompensated congestive heart failure. (D) Homeostatic definitions of stress and allostatic load. Stress is a condition in which the internal variable remains out of bounds despite allostatic adjustments.
The complexity provided by the incorporation of multiple effectors (Figure 5C) extends the range of control, allows at least some regulation of the internal variable if the primary effector fails, by compensatory activation of supplementary effectors. Having multiple effectors also provides the requisite variety that enables evolution of specific, adaptive effector patterns (Goldstein, 2006; Goldstein, 2013). For instance, alterations in metabolic activity in brain centers involved with heart rate and electrodermal responses can occur in related but distinctive patterns (Eisenbarth et al., 2016).
Some clinical situations involve challenges to homeostasis of multiple regulated variables simultaneously. For instance, pulmonary edema from heart failure is associated with baroreflex-cardiovagal failure and consequently tachycardia (Figure 6), unloading of cardiopulmonary baroreceptors restraining SNS outflows and consequently neurogenic hypertension, intrapulmonary shunting of blood and consequently hypoxemia, activation of the renin-angiotensin-aldosterone system and consequently sodium retention and increased extracellular fluid volume, and vasopressin release and consequently water retention and hyponatremia.
Figure 6. Hypothetical allostatic adjustments in compensated heart failure.
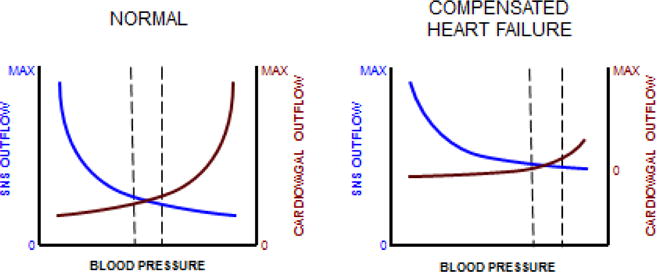
Normally blood pressure is kept within bounds (between the vertical dashed lines) by increases in SNS outflow when the blood pressure falls and increases in cardiovagal outflow when the blood pressure rises. In compensated heart failure the curves are shifted, such that blood pressure and SNS outflow are higher and cardiovagal outflow lower. In this setting, when the blood pressure is increased (e.g., by injection of a vasoconstrictor), there is little or no increase in cardiovagal outflow.
2.15 Allostasis and Allostatic Load
Even before Cannon conceptualized homeostasis he recognized that states such as pain, fear, hunger, and rage are attended by altered levels of many physiological and biochemical variables (Cannon, 1919). Regarding this apparent paradox Cannon later wrote, “The changes which occur in emotional turmoil might, at first glance, be regarded as a gross disturbance of homeostasis. So they would be, by themselves; but they can be explained, I believe, as preparatory for extreme muscular exertion. If that takes place, the changes in the fluid matrix at once become useful and are promptly counteracted by the effects of the exertion itself” (Cannon, 1939).
The concept of allostasis incorporates alterations in the tolerated steady state level— “stability through change” (Sterling et al., 1988). For example, a respiratory viral infection can be associated with a new steady state involving low grade fever, elevated heart rate, and malaise. The allostatic adjustments keep the regulated variables at altered stable state levels. Allostasis corresponds to movement to different sensory input-effector output curves.
Although allostatic adjustments are both compensatory and adaptive, they can come at a cost—allostatic load—corresponding to long-term “wear and tear” (Figure 5D). Many examples of allostatic load in experimental animals as well as in humans involve the effects of repeated episodes or chronic duration of stress in which brain activation of neuroendocrine functions plays a key role. (McEwen, 2016).
2.16 Homeostasis and Adaptation to Hypobaric Hypoxia
Homeostatic mechanisms are necessary to ensure an adequate supply of atmospheric oxygen to support metabolism required to meet the energy requirements of bodily functions. As described above, Heymans demonstrated that chemoreceptors in the carotid sinus initiate reflexes mediating immediate requirements of oxygen by adjustments of pulmonary and cardiovascular function. At the time of these discoveries it was already known that acclimatization to the lower oxygen partial pressure in the atmosphere at high altitudes involves a gradual increase in the levels of hemoglobin and red blood cells carrying oxygen from the lungs to the rest of the body (Haldane, 1924). The changes that occur in acclimatization are reversible on return to the lower altitudes.
It was not until the late 1960s that systematic studies were initiated about adaptation (which refers to irreversible developmental or genetic changes) of humans living at high altitudes with attending exposure to hypobaric hypoxia. Studies by Frisancho in the Peruvian Andes. showed that exposure to high altitude during the period of growth and development at high altitude results in the attainment of a greater capacity for pulmonary ventilation and larger residual lung volume than at sea level (Frisancho, 1975). Developmental differences, however, could account for only about 30% of the difference between high altitude and sea level residents; the remainder, he speculated, were due to genetic differences.
Further studies of Andeans living at high altitude found that they had elevated hemoglobin levels, as one would expect; however, when it was reported that Tibetans living at even higher altitudes do not have elevated hemoglobin levels, it became clear that the two populations differ in mechanisms of adaptation to the same environmental challenge. Tibetans have accelerated ventilatory responses to additional hypoxia, higher levels of nitric oxide (resulting in less pulmonary vasoconstriction), and more rapid blood flow to larger capillary beds in skeletal muscle (Beall, 2007).
The discovery of HIF-1α and HIF-2α opened a new approach to solving the issue of adaptation to hypoxia. HIF proteins are formed rapidly, and with normal O2 levels two of their proline residues are hydroxylated, making them degradable via the ubiquitin pathway. With hypoxia, however, they are not degraded and form heterodimers with another protein (HIF-1β) and are translocated to the nucleus. There the complex binds to a response elements on DNA, altering expression of many different genes related to erythropoiesis, nitrogen oxide formation, angiogenesis, energy metabolism, vascular remodeling, vasomotor responses, and cell proliferation and viability. Beall led an international team of over 2 dozen other scientists to seek candidate genes that might be related to Tibetan adaptation to living at a high altitude. In Tibetans living at high altitude, over two dozen single nucleotide polymorphisms were located near the gene encoding hemoglobin production (EPASI) and correlated with the low hemoglobin level in the Tibetans (Beall et al., 2010). Other studies have strongly implicated EGLN1, the gene that encodes the enzyme that hydroxylates proline residues on HIF-1α (Simonson et al., 2015.
It is beyond the scope of this review to describe the findings of many studies seeking gene expression changes that fully account for the adaptation to high altitude hypoxia by the different populations and animals. These studies have led to new and better understanding of genetics and evolutionary processes by admixture, modes of natural selection (e.g. successful reproduction and infant survival), convergent evolution, adaptive introgression, etc. Nevertheless, they have not fully explained all the various mechanisms of adaptation to high altitude.
2.17 Pleiotropy, Senescence, and Homeostatic Capacity
Both Bernard and Cannon emphasized that the capacity to maintain the constancy of the inner world of the body decreases with aging (Cannon, 1939). They paid little attention to diseases and none to chronic, multi-system disorders of senescence; however, modern medicine is increasingly concerned with the management of aging-related disorders of regulation. By allowing larger fluctuations of key internal variables there is more allostatic load and a greater likelihood of induction of positive feedback loops.
In their book, Why We Get Sick, Nesse and Williams ask, “If senescence so devastates our fitness, why hasn’t natural selection eliminated it?…Our bodies do have some capacity to repair damage and replace worn-out parts; it is just that this capacity is limited. The body can’t maintain itself indefinitely. Why not?” (Nesse et al., 1994).
They answer with an application of Williams’s pleiotropic theory. Pleiotropy is a situation where one gene produces more than one phenotypic trait. In the context of senescence, the pleiotropic theory states that genes that give a benefit in youth impose a cost with age. In Williams’s words, “…senescence results from genes that increase youthful vigor at the price of vigor later on…” (Williams, 1957).
Pleiotropy seems somewhat analogous to gene sharing, which has been defined as a situation in which the same protein fulfils at least two markedly different functions; that is, under the presumption that a particular gene encodes a particular protein, different functions share the same gene.
The susceptibility of some Andean natives to chronic mountain sickness (Appenzeller et al., 2003) may provide an example of this form of pleiotropy. Compared to other Peruvians living at high altitude, those with chronic mountain sickness have relatively increased gene expression for hypoxia inducible factor-1α and vascular endothelial growth factor-121 (Appenzeller et al., 2003). The ability of a fetus to increase expression of these genes in response to hypoxemia might enhance the likelihood of the individual surviving to birth, but this survival advantage could come at the cost of increased risk of chronic mountain sickness in adulthood. In terms of homeostatic systems, the allostatic adjustments increase allostatic load.
Analysis of genotype data for the Nepalese Sherpa has revealed that Tibetans are a mixture of ancestral populations related to the Sherpa and Han Chinese (Jeong et al., 2014). Migrants from low altitude acquired adaptive alleles for hemoglobin saturation traits from the highlanders by introgression, which refers to the movement of a gene or genes from one gene pool to another. The admixture of genes increases the variety necessary to enhance adaptation further.
This adaptive introgression resulted in evolution over a relatively brief period of time because of enhanced survival of newborns with the more adaptive trait (Beall et al., 2004). By this mechanism, evolution can take place without genotypic changes such as mutations. Nevertheless, the greater ability to adapt in this setting is the result of increased variety upon which natural selection can act, in keeping with Ashby’s law of requisite variety.
According to our definition, stress is an allostatic state in which a regulated internal variable is outside the acceptable range. Thus, if oxygen delivery remained within bounds, the condition would not be a stress, although there could be secondary potentially pathologic consequences (allostatic load).
In summary, Williams’s pleiotropy concept applies not so much to aging per se as to diseases associated with aging. Briefly, these conditions exist because of an evolutionary tradeoff—enhanced survival in the young reproducers at the expense of degeneration of homeostatic systems in the elderly.
2.18 Allostatic Load from Compensatory Activation: Heart Failure
An example of allostatic load from compensatory activation is found in the natural history of congestive heart failure. When intrinsic pumping capacity declines, cardiac output is maintained via a negative feedback loop involving enhanced cardiac sympathetic neuronal activity, increasing delivery of norepinephrine (NE) to the myocardial cells. The curve relating NE outflow to cardiac output is shifted—an allostatic adjustment. Analogous adjustments in sympathetic activity and NE delivery in the heart (Esler et al., 1995) and in the body as a whole (Seals et al., 2000) are typical of “normal” aging. So is decreased cardiovagal outflow (Jones et al., 2003). As illustrated in Figure 6, in “compensated” heart failure there are allostatic shifts in the curves relating complementary sympathetic and cardiovagal activity to blood pressure. These shifts give the appearance of blood pressure being regulated by a barostat to attain a new set-point.
Compensatory activation of the SNS effector, however, increases risk—allostatic load. NE promotes myocardial hypertrophy (Patel et al., 1989), which increases the demand for oxygen and other metabolic fuels delivered by coronary perfusion. SNS activation also reduces thresholds for arrhythmias (Lukas et al., 1989; Meredith et al., 1991).
Heart failure becomes “decompensated” when cardiac pump function declines to below a tolerable level despite maximal SNS stimulation. The patient has pulmonary edema and dyspnea and as part of a distress response experiences the classic “feeling of impending doom” that has been associated from time immemorial with massive adrenomedullary release of EPI. Rather than augmenting left ventricular myocardial contractility, too much EPI is toxic to myocardial cells (Kubota et al., 1990; Rona, 1985). Myocardial contractility decreases further, “stress cardiopathy” develops, and the pulmonary edema worsens. Within a sometimes surprisingly short interval from the onset of decompensated heart failure, the patient dies—within minutes because of a catecholamine-evoked ventricular arrhythmia, hours because of intractable pulmonary edema, or days because of critically decreased perfusion of body organs such as the kidneys.
In several ways, physiologic negative feedback loops in this scenario have given way to pathophysiologic positive feedback loops. The transition from a negative feedback loop to a positive feedback loop is the transition from a stable to an unstable internal environment and a harbinger of the end of homeostasis.
2.19 Allostatic Load and Parkinson’s Disease
The situation in Parkinson’s disease (PD) might also exemplify pathogenic consequences of allostatic load. The catecholamine “autotoxicity” theory is based on inherent cytotoxicity of products of enzymatic or spontaneous oxidation of catecholamines in the cells in which the catecholamines are produced (Goldstein et al., 2014). Individuals with relatively efficient central catecholamine systems might have survival advantages during their reproductive years, in terms of being able to increase rapidly and massively the delivery of catecholamines to their receptors as part of initiating behaviors, experiencing emotions, learning to respond to classically conditioned stimuli, acquiring and retaining appetitive or avoidance behaviors, or compensating for loss of catecholaminergic nerve terminals by increasing pathway traffic to the residual terminals.
The advantages associated with these allostatic adjustments, however, could come at the cost of accelerated senescence of the catecholaminergic neurons. Most of released catecholamine is recycled by neuronal reuptake. Stressors that evoke release of catecholamines in effect shift intra-neuronal catecholamines from vesicular to cytoplasmic pools. Accordingly, repeated episodes of stress could cause neuronal injury via autotoxicity, and the more substantial the catecholamine release, the greater the amount of autotoxicity and the more likely the manifestations of aging-related catecholaminergic neurodegeneration.
The results of a recent study fit with this notion. In rats, chronic restraint, which evokes activation of catecholaminergic neurons inside and outside the brain (Kvetnansky et al., 1992a; Kvetnansky et al., 1992b; Pacak et al., 1992; Pacak et al., 1993), reduces the numbers of substantia nigra dopaminergic and locus ceruleus noradrenergic neurons (Sugama et al., 2016), as in PD (Zarow et al., 2003).
2.20 Cybernetic Medicine (Scientific Integrative Medicine)
Cybernetic medicine (also called scientific integrative medicine (Goldstein, 2013)) relates regulation of internal variables by negative feedback, buffering, and anticipatory control to medical disorders. Multi-system diseases and disorders reflect disturbances of regulation that lead to adverse effects of compensatory activation or of declining efficiency of homeostatic negative feedback loops. Factors such as stress, maladaptation, allostatic load, and diminished resilience may alter not only the manifestations of but also determine the outcomes of acute and chronic disorders.
In higher organisms, such as humans, the brain has a major role in regulation of internal variables, via hierarchies of central neural networks. The brain controls levels of many internal regulated variables simultaneously.
Neuronal plasticity enables modifications in the efficiency of neuronal transmission necessary to regulate organ and systemic processes. Such plasticity provides the variability needed to satisfy the law of requisite variety and acquire good regulators.
Major goals of cybernetic medicine are therapeutic interventions to compensate for or retard failures of homeostasis and to detect early or prevent the development of catastrophic positive feedback loops.
Three types of current experimental therapeutic approaches for disorders of homeostasis can be viewed as applications of biocybernetics. Carotid sinus stimulation or renal sympathetic radiofrequency ablation may attenuate refractory hypertension (Krum et al., 2009; Lohmeier et al., 2011). Intravenous infusion of norepinephrine or an automated abdominal binder may mitigate neurogenic orthostatic hypotension (Goldstein et al., 2012; Okamoto et al., 2016; Polinsky et al., 1983; Zekeridou et al., 2015); and vagus nerve stimulation, by directly or reflexively evoking effects on elaboration of cytokines, may be useful to treat conditions involving auto-immunity such as rheumatoid arthritis (Koopman et al., 2016).
3.0 Summary and Commentary
The concepts of homeostasis and regulation by negative feedback, buffering, and feed-forward mechanisms are fundamental in autonomic neuroscience. Maintaining the stability of the milieu intérieur is necessary for survival and reproduction. These three mechanisms—each involving autonomic effectors—evolved to meet environmental challenges associated with continually changing ecological niches.
The effectors are not regulated in isolation. Instead, central networks determine associative processing (cognition, awareness, memory, interpretation, anticipation, executive functions), movement (skeletal muscle behaviors), emotional and motivational states, and activities of components of the autonomic nervous system, in coordinated patterns.
One can theorize that hierarchies of input-output relationships continuously orchestrate and learn adaptive patterns of observable behaviors, cognition, memory, mood, and autonomic systems. Taken together, these hierarchies function as good regulators determining levels of internal variables and act as if there were homeostatic comparators.
The concepts of stress, allostasis, and allostatic load are valuable for constructing computer models that predict effects of manipulations of regulated variables in the setting of multiple effectors and effector sharing (Goldstein, 2013) and can help understand the development of chronic degenerative conditions such as heart failure and PD.
The consequences of models with vs. without metaphorical homeostats remain the same in terms of allostatic load and the eventual switch from homeostatic negative feedback loops to destabilizing, pathogenic positive feedback loops. Understanding this switch seems important for comprehending senescence-related, neurodegenerative disorders that involve the autonomic nervous system and that are becoming an increasing public health burden.
According Wiener and Ashby, the principles of cybernetics apply to the brain just as well as to all control systems that maintain stability by goal-directed, feedback-regulated behaviors. Cybernetics and system control theory were formal, mathematical, logical generalizations that applied to machines—enhanced later by the complexity and rapidity of computers—what physiologists dating back to about 100 years earlier had expressed intuitively about the mechanisms determining the stability of internal variables essential for life. Mental phenomena such as consciousness, cognition, and mood seem beyond computer models of neuronal networks. To explain and mimic in computers what has evolved over eons and involves about 200 billion widely branching neurons seems still to be a major if not impossible task.
Current systems biology is essentially an extension of biocybernetics. It is descriptive, whereas integrative physiology is predictive. The human genome project was also descriptive, and at the time one could have argued about the added value of identifying genotypic bases of inherited diseases; however, advances in prenatal diagnosis, risk assignments, and gene therapy emerged. At this point, we do not see how biocybernetics as applied to medicine adds to what is known or how it can predict future discoveries. Medical cybernetics does offer the potential to identify disease-associated characteristic patterns by molecular and neuroimaging “fingerprinting,” and several experimental applications are undergoing development to treat disorders of homeostasis.
We envision further evolution of homeostatic concepts to encompass integration of autonomic nervous with behavioral, endocrine, autocrine-paracrine, and cytokine effectors. Our general proposal is that disintegration of homeostatic systems causes disorders of regulation in degenerative diseases and that medical cybernetics can inspire and rationalize new approaches to treatment and prevention.
Acknowledgments
The research reported here was supported (in part) by the Division of Intramural Research, NINDS.
Glossary
- Adaptive introgression
the movement of a segment of DNA from one species into the gene pool of another, resulting in greater ability to adapt as a result of increased variety upon which natural selection can act.
- Allostasis
An adaptive state that involves adjusted physiological input-output relationships—“stability through change.”
- Allostatic Load
Increased risk resulting from an allostatic state.
- Autonomic nervous system (Langley)
The networks of nerves derived from ganglia outside the central nervous system that mediate involuntary body processes.
- Biocybernetics (Wiener)
The study of control mechanisms in biological science. Neurocybernetics mainly involves the descriptions of pathways of actions via sense-organs, neurons and effectors and corresponds to a complex systems biologic network. In medical biocybernetics the main considerations are disease states and therapeutic applications.
- Buffering
A means to mitigate the effects of a disturbance.
- Central autonomic network (Benarroch)
The hierarchy of pathways and relationships by which central neural processes regulate levels of regulated variables via the autonomic nervous system.
- Compensatory activation (Goldstein)
A consequence of multiple effectors in a negative feedback loop, where disabling one effector increases activity of an another effector.
- Cybernetics (Wiener)
The science of automatic communication and control systems.
- Distress (Goldstein)
A form of stress that is conscious, aversive, generates instinctively communicated signs, and is associated with adrenocortical and adrenomedullary activation
- Effector sharing (Goldstein)
A consequence of multiple homeostatic negative feedback loops, in which two homeostatic systems share the same effector.
- Good regulator (Ashby)
A good regulator models well the system it regulates. Corollarily, the brain as a good regulator learns to form a model (or models) of its environment.
- Homeostasis (Cannon)
The stability of the body’s inner world, maintained by coordinated systems that keep values for key internal variables within bounds.
- Homeostat (Ashby)
A machine that demonstrated stability by negative feedback and requisite variety.
- Homeostat (Goldstein & Kopin)
A metaphorical comparator that senses a discrepancy between perceptions about the level of a regulated variable and a set-point for responding.
- Imprinting (Lorenz)
Learning early in life that is rapid and apparently independent of the consequences of behavior.
- Law of Requisite Variety (Ashby)
For a system to be stable, the number of states of its control mechanism must be greater than or equal to the number of states in the system being controlled.
- Medical cybernetics (Wiener)
The aspect of cybernetics that emphasizes negative feedback regulation in understanding mechanisms and treatment of clinical disorders.
- Model
A system in which a variable of a regulator corresponds to one and only one of the variables that are being regulated.
- Milieu Intérieur (Bernard)
The fluid environment bathing all the cells of the body.
- Neurocybernetics (Wiener)
The aspect of biocybernetics that mainly involves the pathways of actions via sense-organs, neurons and effectors and corresponds to a complex systems biologic network.
- Phylogenetic learning (Wiener)
A means of adaptation in evolution based on survival advantages of inherited behaviors.
- Pleiotropy (Williams)
In genetics, a situation where one gene influences two or more seemingly unrelated phenotypic traits.
- Scientific integrative medicine (Goldstein)
A way of medical thinking that links systems biology with integrative physiology and disorders of regulation.
- Stress (Selye)
The non-specific response of the body to any demand imposed upon it.
- Stress (Goldstein & Kopin)
A condition in which there is a perceived discrepancy between the desired and sensed state, such that alterations in effectors tend to reduce the discrepancy.
- Teleology (Rosenblueth, Wiener, and Bigelow)
The study of purpose or goals. According to Rosenblueth et al., purposeful behavior is behavior that is not random, and teleology is purpose controlled by feed-back.
- Ultrastability (Ashby)
In physiology and behavior, having the capacity to adapt by trial and error learning. Ashby defined ultrastability as follows: “Two systems of continuous variables…interact, so that a primary feedback… exists between them. Another feedback, working intermittently and at a much slower order of speed, goes from the environment to certain continuous variables which in their turn affect some step-mechanisms, the effect being that the step-mechanisms change value when and only when these variables pass outside given limits. The step-mechanisms affect the reacting part; by acting as parameters to it, they determine how it shall react to the environment.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None.
(*) Dr. Kopin died August 1, 2017. This essay is dedicated to his memory.---DSG
References
- Abel JJ, Crawford AC. On the blood-pressure-raising constituent of the suprarenal capsule. Bull Johns Hopkins Hosp. 1897;8:151–157. [Google Scholar]
- Ahlquist RP. A study of adrenotropic receptors. Am J Physiol. 1948;153:586–600. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- Appenzeller O, Minko T, Pozharov V, Bonfichi M, Malcovati L, Gamboa J, Bernardi L. Gene expression in the Andes; relevance to neurology at sea level. J Neurol Sci. 2003;207:37–41. doi: 10.1016/s0022-510x(02)00356-8. [DOI] [PubMed] [Google Scholar]
- Ashby WR. An Introduction to Cybernetics. Chapman & Hall; London, UK: 1956. [Google Scholar]
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- Azzaroni A, Parmeggiani PL. Postural and sympathetic influences on brain cooling during the ultradian wake-sleep cycle. Brain Res. 1995;671:78–82. doi: 10.1016/0006-8993(94)01323-a. [DOI] [PubMed] [Google Scholar]
- Baker MA. Influence of the carotid rete on brain temperature in cats exposed to hot environments. J Physiol. 1972;220:711–728. doi: 10.1113/jphysiol.1972.sp009731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard P. A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. Am J Physiol. 1928:490–516. [Google Scholar]
- Bard P. Anatomical organization of the central nervous system in relation to control of the heart and blood vessels. Physiol Rev. 1960;(Suppl. 4):3–26. [PubMed] [Google Scholar]
- Bartorelli C, Bizzi E, Libretti A, Zanchetti A. Inhibitory control of sinocarotid pressoceptive afferents on hypothalamic autonomic activity and sham rage behavior. Arch Ital Biol. 1960;98:308–326. [Google Scholar]
- Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci U S A. 2007;104(Suppl 1):8655–8660. doi: 10.1073/pnas.0701985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui P, Weale ME, Xu Y, Xu Z, Yang L, Zaman MJ, Zeng C, Zhang L, Zhang X, Zhaxi P, Zheng YT. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA. 2010;107:11459–11464. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Song K, Elston RC, Goldstein MC. Higher offspring survival among Tibetan women with high oxygen saturation genotypes residing at 4,000 m. Proc Natl Acad Sci U S A. 2004;101:14300–14304. doi: 10.1073/pnas.0405949101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- Bernard C. Lectures on the Phenomena of Life Common to Animals and Vegetables. Charles C Thomas; Springfield, IL: 1974. [Google Scholar]
- Burnstock G. The purinergic nerve hypothesis. Ciba Found Symp. 1977:295–314. doi: 10.1002/9780470720301.ch17. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Cotransmission in the autonomic nervous system. Handb Clin Neurol. 2013;117:23–35. doi: 10.1016/B978-0-444-53491-0.00003-1. [DOI] [PubMed] [Google Scholar]
- Cannon WB. Bodily Changes in Pain, Hunger, Fear and Rage. D. Appleton & Co.; New York: 1919. [Google Scholar]
- Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9:399–431. [Google Scholar]
- Cannon WB. The Wisdom of the Body. W.W. Norton; New York: 1939. [Google Scholar]
- Cannon WB, Britton SW. Studies on the conditions of activity in endocrine glands. XV. Pseudoaffective medulliadrenal secretion. Am J Physiol. 1925;72:283–294. [Google Scholar]
- Cannon WB, Rosenblueth A. Studies on conditions of activity in endocrine organs. XXIX. Sympathin E and Sympathin I. Am J Physiol. 1933;104:557–574. [Google Scholar]
- Carrive P, Gorissen M. Premotor sympathetic neurons of conditioned fear in the rat. Eur J Neurosci. 2008;28:428–446. doi: 10.1111/j.1460-9568.2008.06351.x. [DOI] [PubMed] [Google Scholar]
- Chaplin G, Jablonski NG, Sussman RW, Kelley EA. The role of piloerection in primate thermoregulation. Folia Primatol (Basel) 2014;85:1–17. doi: 10.1159/000355007. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. J Am Med Assoc. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Conant RC, Ashby WR. Every good regulator of a system must be a model of that system. Int J Systems Sci. 1970;1:89–97. [Google Scholar]
- Craig AD. Cooling, pain, and other feelings from the body in relation to the autonomic nervous system. Handb Clin Neurol. 2013;117:103–109. doi: 10.1016/B978-0-444-53491-0.00009-2. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Nagai Y, Gray MA, Mathias CJ. Dissecting axes of autonomic control in humans: Insights from neuroimaging. Auton Neurosci. 2011;161:34–42. doi: 10.1016/j.autneu.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Physiology and pathophysiology of the human sympathoadrenal neuroendocrine system. N Engl J Med. 1980;303:436–444. doi: 10.1056/NEJM198008213030806. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl. 1964;232:1–55. [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. II. Experimetnally induced changes in the intraneuronal amine levels of bulbospinal neuron systems. Acta Physiol Scand Suppl. 1965;247:1–36. [PubMed] [Google Scholar]
- Damasio A. Descartes’ Error Emotion, Reason, and the Human Brain. Avon Books Inc.; New York: 1994. [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand. 1972;84:82–94. doi: 10.1111/j.1748-1716.1972.tb05157.x. [DOI] [PubMed] [Google Scholar]
- Duan YF, Kopin IJ, Goldstein DS. Stimulation of the paraventricular nucleus modulates firing of neurons in the nucleus of the solitary tract. Am J Physiol. 1999;277:R403–R411. doi: 10.1152/ajpregu.1999.277.2.R403. [DOI] [PubMed] [Google Scholar]
- Dum RP, Levinthal DJ, Strick PL. Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla. Proc Natl Acad Sci USA. 2016;113:9922–9927. doi: 10.1073/pnas.1605044113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egiazaryan GG, Sudakov KV. Theory of functional systems in the scientific school of P.K. Anokhin. J Hist Neurosci. 2007;16:194–205. doi: 10.1080/09647040600602805. [DOI] [PubMed] [Google Scholar]
- Eisenbarth H, Chang LJ, Wager TD. Multivariate brain prediction of heart rate and skin conductance responses to social threat. J Neurosci. 2016;36:11987–11998. doi: 10.1523/JNEUROSCI.3672-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott TR. On the action of adrenalin. J Physiol. 1904;31:xx–xxi. doi: 10.1113/jphysiol.1905.sp001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler MD, Turner AG, Kaye DM, Thompson JM, Kingwell BA, Morris M, Lambert GW, Jennings GL, Cox HS, Seals DR. Aging effects on human sympathetic neuronal function. Am J Physiol. 1995;268:R278–R285. doi: 10.1152/ajpregu.1995.268.1.R278. [DOI] [PubMed] [Google Scholar]
- Falck B, Hillarp NA, Thieme G, Torp A. Fluorescence of catecholamines and related compounds condensed with formaldehyde. J Histochem Cytochem. 1962;10:348–354. [Google Scholar]
- Folkow B. Structural factor” in primary and secondary hypertension. Hypertension. 1990;16:89–101. doi: 10.1161/01.hyp.16.1.89. [DOI] [PubMed] [Google Scholar]
- Frayn KN, Little RA, Maycock PF, Stoner HB. The relationship of plasma catecholamines to acute metabolic and hormonal responses to injury in man. Circ Shock. 1985;16:229–240. [PubMed] [Google Scholar]
- Frisancho AR. Functional adaptation to high altitude hypoxia. Science. 1975;187:313–319. doi: 10.1126/science.1089311. [DOI] [PubMed] [Google Scholar]
- Gaddum JH, Schild H. Depressor substances in extracts of intestine. J Physiol. 1934;83:1–14. doi: 10.1113/jphysiol.1934.sp003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychol Rev. 2010;117:134–174. doi: 10.1037/a0017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS. Adrenaline and the Inner World: An Introduction to Scientific Integrative Medicine. The Johns Hopkins University Press; Baltimore, MD: 2006. [Google Scholar]
- Goldstein DS. Concepts of scientific integrative medicine applied to the physiology and pathophysiology of catecholamine systems. Compr Physiol. 2013;3:1569–1610. doi: 10.1002/cphy.c130006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Frank SM. The wisdom of the body revisited: The adrenomedullary response to mild core hypothermia in humans. Endocrine Regul. 2001;35:3–7. [PubMed] [Google Scholar]
- Goldstein DS, Holmes C, Frank SM, Naqibuddin M, Dendi R, Snader S, Calkins H. Sympathoadrenal imbalance before neurocardiogenic syncope. Am J Cardiol. 2003;91:53–58. doi: 10.1016/s0002-9149(02)02997-1. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: A meta-analysis. Endo Regul. 2008;42:111–119. [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ, Sharabi Y. Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol Ther. 2014;144:268–282. doi: 10.1016/j.pharmthera.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sewell L, Holmes C, Pechnik S, Diedrich A, Robertson D. Temporary elimination of orthostatic hypotension by norepinephrine infusion. Clin Auton Res. 2012;22:303–306. doi: 10.1007/s10286-012-0176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Wood JD, Burnstock G. Purinergic signalling in autonomic control. Trends Neurosci. 2009;32:241–248. doi: 10.1016/j.tins.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Grant SJ, Aston-Jones G, Redmond DE., Jr Responses of primate locus coeruleus neurons to simple and complex sensory stimuli. Brain Res Bull. 1988;21:401–410. doi: 10.1016/0361-9230(88)90152-9. [DOI] [PubMed] [Google Scholar]
- Haldane JBS. Respiration. Yale University Press; New Haven, CT: 1922. [Google Scholar]
- Haldane JS. A lecture on acclimatization to high altitudes. Br Med J. 1924;2:885–890. doi: 10.1136/bmj.2.3333.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halter JB, Beard JC, Porte D., Jr Islet function and stress hyperglycemia: plasma glucose and epinephrine interaction. Am J Physiol. 1984;247:E47–52. doi: 10.1152/ajpendo.1984.247.1.E47. [DOI] [PubMed] [Google Scholar]
- Han W, Tellez LA, Rangel MJ, Jr, Motta SC, Zhang X, Perez IO, Canteras NS, Shammah-Lagnado SJ, van den Pol AN, de Araujo IE. Integrated control of predatory hunting by the central nucleus of the amygdala. Cell. 2017;168:311–324 e318. doi: 10.1016/j.cell.2016.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess WR. Das Zwischenhirn. B Schwabe; Basel: 1949. [Google Scholar]
- Heymans C, Neil E. Reflexogenic Areas of the Cardiovascular System. Little, Brown & Co.; Boston: 1958. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Lundberg JM, Tatemoto K, Mutt V, Terenius L, Polak J, Bloom S, Sasek C, Elde R, Goldstein M. Neuropeptide Y (NPY)- and FMRFamide neuropeptide-like immunoreactivities in catecholamine neurons of the rat medulla oblongata. Acta Physiol Scand. 1983;117:315–318. doi: 10.1111/j.1748-1716.1983.tb07214.x. [DOI] [PubMed] [Google Scholar]
- Immordino-Yang MH, Yang XF, Damasio H. Correlations between social-emotional feelings and anterior insula activity are independent from visceral states but influenced by culture. Front Hum Neurosci. 2014;8:728. doi: 10.3389/fnhum.2014.00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmak MK, Korkmaz A, Erogul O. Selective brain cooling seems to be a mechanism leading to human craniofacial diversity observed in different geographical regions. Med Hypotheses. 2004;63:974–979. doi: 10.1016/j.mehy.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Jeong C, Alkorta-Aranburu G, Basnyat B, Neupane M, Witonsky DB, Pritchard JK, Beall CM, Di Rienzo A. Admixture facilitates genetic adaptations to high altitude in Tibet. Nat Commun. 2014;5:3281. doi: 10.1038/ncomms4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PP, Christou DD, Jordan J, Seals DR. Baroreflex buffering is reduced with age in healthy men. Circulation. 2003;107:1770–1774. doi: 10.1161/01.CIR.0000057811.86187.88. [DOI] [PubMed] [Google Scholar]
- Keifer OP, Jr, Hurt RC, Ressler KJ, Marvar PJ. The Physiology of Fear: Reconceptualizing the Role of the Central Amygdala in Fear Learning. Physiology (Bethesda) 2015;30:389–401. doi: 10.1152/physiol.00058.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeslag JH, Saunders PT, Terblanche E. A reappraisal of the blood glucose homeostat which comprehensively explains the type 2 diabetes mellitus–syndrome X complex. J Physiol. 2003;549:333–346. doi: 10.1113/jphysiol.2002.037895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, Tak PP. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2016;119:8284–8289. doi: 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner PI, Tonkin AM, Uther JB. Reflex and mechanical circulatory effects of graded Valsalva maneuvers in normal man. J Appl Physiol. 1976;40:434–440. doi: 10.1152/jappl.1976.40.3.434. [DOI] [PubMed] [Google Scholar]
- Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- Kubota T, Yamazaki N, Sudo J, Monma Y, Kaku T, Okuyama T, Tanabe T. Protective effects of adrenoceptor-blocking agents on myocardial injury induced by epinephrine in mice. The Journal of toxicological sciences. 1990;15:1–13. doi: 10.2131/jts.15.1. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R. Stressor specificity and effect of prior experience on catecholamine biosynthetic enzyme phenylethanolamine N-methyltransferase. Ann N Y Acad Sci. 2004;1032:117–129. doi: 10.1196/annals.1314.009. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Armando I, Weise VK, Holmes C, Fukuhara K, Deka-Starosta A, Kopin IJ, Goldstein DS. Plasma dopa responses during stress: dependence on sympathoneural activity and tyrosine hydroxylation. J Pharmacol Exp Ther. 1992a;261:899–909. [PubMed] [Google Scholar]
- Kvetnansky R, Goldstein DS, Weise VK, Holmes C, Szemeredi K, Bagdy G, Kopin IJ. Effects of handling or immobilization on plasma levels of 3,4-dihydroxyphenylalanine, catecholamines, and metabolites in rats. J Neurochem. 1992b;58:2296–2302. doi: 10.1111/j.1471-4159.1992.tb10977.x. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Pacak K, Sabban EL, Kopin IJ, Goldstein DS. Stressor specificity of peripheral catecholaminergic activation. Adv Pharmacol. 1998;42:556–560. doi: 10.1016/s1054-3589(08)60811-x. [DOI] [PubMed] [Google Scholar]
- Lake CR, Ziegler MG, Kopin IJ. Use of plasma norepinephrine for evaluation of sympathetic neuronal function in man. Life Sci. 1976;18:1315–1325. doi: 10.1016/0024-3205(76)90210-1. [DOI] [PubMed] [Google Scholar]
- Langley JN. The autonomic nervous system. Brain. 1903;26:1–26. [Google Scholar]
- Langley JN. The Autonomic Nervous System W. Heffer and Sons Ltd.; Cambridge, England: 1921. [Google Scholar]
- Loewi O. Uber humorale Ubertragbarkeit der Herzenvenwirkung. Pflugers Arch. 1921;189:239–242. [Google Scholar]
- Loewi O, Navratil E. Uber humorale Ubertragbarkeit der Herzenvenwirkung. X. Mitteilung Uber das Schicksal des Vagusstoffs. Pflugers Arch. 1926;214:678–688. [Google Scholar]
- Lohmeier TE, Iliescu R. Chronic lowering of blood pressure by carotid baroreflex activation: mechanisms and potential for hypertension therapy. Hypertension. 2011;57:880–886. doi: 10.1161/HYPERTENSIONAHA.108.119859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K. King Solomon’s Ring. Thomas Y. Crowell Co.; New York: 1952. [Google Scholar]
- Lukas A, Ferrier GR. Arrhythmic effects of norepinephrine in a model of cardiac ischemia and reperfusion. Can J Physiol Pharmacol. 1989;67:765–771. doi: 10.1139/y89-122. [DOI] [PubMed] [Google Scholar]
- Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. Stress, definition and concepts of. Academic Press; San Diego: 2000. pp. 508–509. [Google Scholar]
- Meredith IT, Broughton A, Jennings GL, Esler MD. Evidence of a selective increase in cardiac sympathetic activity in patients with sustained ventricular arrhythmias. N Engl J Med. 1991;325:618–624. doi: 10.1056/NEJM199108293250905. [DOI] [PubMed] [Google Scholar]
- Modell H, Cliff W, Michael J, McFarland J, Wenderoth MP, Wright A. A physiologist’s view of homeostasis. Adv Physiol Educ. 2015;39:259–266. doi: 10.1152/advan.00107.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM, Williams GC. Why We Get Sick The New Science of Darwinian Medicine. Times Books; New York: 1994. [Google Scholar]
- Okamoto LE, Diedrich A, Baudenbacher FJ, Harder R, Whitfield JS, Iqbal F, Gamboa A, Shibao CA, Black BK, Raj SR, Robertson D, Biaggioni I. Efficacy of servo-controlled splanchnic venous compression in the treatment of orthostatic hypotension: A randomized comparison with midodrine. Hypertension. 2016;68:418–426. doi: 10.1161/HYPERTENSIONAHA.116.07199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Schafer EA. On the physiological action of extract of the suprarenal capsules. J Physiol. 1895;17:ix–xiv. doi: 10.1113/jphysiol.1895.sp000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald GA, Smith CC, Delamothe AP, Betteridge DJ, Yudkin JS. Raised concentrations of glucose and adrenaline and increased in vivo platelet activation after myocardial infarction. Br Heart J. 1988;59:663–671. doi: 10.1136/hrt.59.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, Armando I, Fukuhara K, Kvetnansky R, Palkovits M, Kopin IJ, Goldstein DS. Noradrenergic activation in the paraventricular nucleus during acute and chronic immobilization stress in rats: An in vivo microdialysis study. Brain Res. 1992;589:91–96. doi: 10.1016/0006-8993(92)91165-b. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Kvetnansky R, Fukuhara K, Armando I, Kopin IJ, Goldstein DS. Effects of single or repeated immobilization on release of norepinephrine and its metabolites in the central nucleus of the amygdala in conscious rats. Neuroendocrinology. 1993;57:626–633. doi: 10.1159/000126417. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. Heterogeneous neurochemical responses to different stressors: A test of Selye’s doctrine of nonspecificity. Am J Physiol. 1998;275:R1247–R1255. doi: 10.1152/ajpregu.1998.275.4.R1247. [DOI] [PubMed] [Google Scholar]
- Patel MB, Loud AV, King BD, Anversa P, Sack D, Hintze TH. Global myocardial hypertrophy in conscious dogs with chronic elevation of plasma norepinephrine levels. J Mol Cell Cardiol. 1989;21(Suppl 5):49–61. doi: 10.1016/0022-2828(89)90771-2. [DOI] [PubMed] [Google Scholar]
- Polinsky RJ, Samaras GM, Kopin IJ. Sympathetic neural prosthesis for managing orthostatic hypotension. Lancet. 1983;1:901–904. doi: 10.1016/s0140-6736(83)91329-6. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Williams B, Sawchenko PE. Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J Neurosci. 2008;28:5806–5816. doi: 10.1523/JNEUROSCI.0552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay DS, Woods SC. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol Rev. 2014;121:225–247. doi: 10.1037/a0035942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis DJ, Gunne LM. Brain catecholamines: relation to the defense reaction evoked by brain stimulation in the cat. Science. 1965;156:1768–1770. doi: 10.1126/science.156.3783.1768. [DOI] [PubMed] [Google Scholar]
- Reis DJ, Morrison S, Ruggiero DA. The C1 area of the brainstem in tonic and reflex control of blood pressure. State of the art lecture. Hypertension. 1988;11(Suppl. I):I-8–I-13. doi: 10.1161/01.hyp.11.2_pt_2.i8. [DOI] [PubMed] [Google Scholar]
- Roberts FF. Stress and the General Adaptation Syndrome. Br Med J. 1950:104–105. doi: 10.1136/bmj.1.4667.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Rona G. Catecholamine cardiotoxicity. Journal of molecular and cellular cardiology. 1985;17:291–306. doi: 10.1016/s0022-2828(85)80130-9. [DOI] [PubMed] [Google Scholar]
- Rosenblueth A, Wiener N, Bigelow J. Behavior, purpose and teleology. Philosophy of Science. 1943;10:18–24. [Google Scholar]
- Schachter S, Singer J. Cognitive, social, and physiological determinants of emotional state. Psychol Rev. 1962;69:379–399. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Schulkin J. Rethinking Homeostasis: Allostatic Regulation in Physiology and Pathophysiology. The MIT Press; Cambridge, MA: 2003. [Google Scholar]
- Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528:407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Selye H. A Treatise Based on the Concepts of the General-Adaptation Syndrome and the Diseses of Adaptation. Acta, Inc.; Montreal, Canada: 1950. The Physiology and Pathology of Exposure to Stress. [Google Scholar]
- Selye H. The Stress of Life. McGraw-Hill; New York: 1956. [Google Scholar]
- Selye H. Stress without Distress. New American Library; New York: 1974. [Google Scholar]
- Selye H. Stress without Distress. Springer US; New York, NY: 1976. [Google Scholar]
- Simonson TS, Huff CD, Witherspoon DJ, Prchal JT, Jorde LB. Adaptive genetic changes related to haemoglobin concentration in native high-altitude Tibetans. Exp Physiol. 2015;100:1263–1268. doi: 10.1113/EP085035. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher J, Reason J, editors. Handbook of Life Stress, Cognition, and Health. Johns Wiley & Sons Inc.; New York: 1988. pp. 629–649. [Google Scholar]
- Sudakov K. The theory of functional systems: General postulates and principles of dynamic organization. Integ Physiol Behav Sci. 1997;32:392–414. doi: 10.1007/BF02688634. [DOI] [PubMed] [Google Scholar]
- Sugama S, Sekiyama K, Kodama T, Takamatsu Y, Takenouchi T, Hashimoto M, Bruno C, Kakinuma K. Chronic restraint stress triggers dopaminergic and noradrenergic: Possible role of chronic stress in the onset of Parkinson’s disease. Brain Behav Imm. 2016;51:39–46. doi: 10.1016/j.bbi.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamine J. The isolation of the active principle of the suprarenal gland. J Physiol. 1901;27:30P–39P. [Google Scholar]
- Verbalis JG, Baldwin EF, Robinson AG. Osmotic regulation of plasma vasopressin and oxytocin after sustained hyponatremia. Am J Physiol. 1986;250:R444–451. doi: 10.1152/ajpregu.1986.250.3.R444. [DOI] [PubMed] [Google Scholar]
- von Euler US. A specific sympathomimetic ergone in adrenergic nerve fibres (sympathin) and its relations to adrenaline and nor-adrenaline. Acta Physiol Scand. 1946;12:73–96. [Google Scholar]
- Wiener N. Cybernetics: or Control and Communication in the Animal and the Machine. The M.I.T. Press; Cambridge, MA: 1948. [Google Scholar]
- Wiener N. Perspectives in cybernetics. Prog Brain Res. 1965;17:399–408. doi: 10.1016/s0079-6123(08)60174-0. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- Zekeridou A, Michel P, Medlin F, Hayoz D, Lalive PH, Kuntzer T. Successful long-term ambulatory norepinephrine infusions in a patient with pure autonomic failure. Clin Auton Res. 2015;25:251–253. doi: 10.1007/s10286-015-0290-1. [DOI] [PubMed] [Google Scholar]


