Abstract
OBJECTIVE
The human voice is sexually dimorphic in obvious ways, such as differences in fundamental frequency and gross laryngeal anatomy, but also in less apparent ways, such as in the prevalence and types of voice disorders and the manifestation of voice changes in advanced age. Differences between males and females are rarely explored, however, in mechanistic animal studies. The goal of this study was to explore sexual dimorphism in laryngeal function and structure in adult rats by examining ultrasonic vocalization acoustics and muscle fiber size and type in the thyroarytenoid muscle.
STUDY DESIGN
Animal group comparison.
METHODS
Spontaneous ultrasonic vocalizations from 10 male and 10 female adult rats were recorded, classified, and acoustically analyzed. Cross-sections of the thyroarytenoid muscle were stained and imaged for analysis of muscle fiber size and type. Acoustic and muscle parameters were statistically compared between sexes.
RESULTS
Male rats had a lower mean frequency of short ultrasonic vocalizations. Male rats also had a larger mean fiber size in the external division of the thyroarytenoid and larger overall muscle area in both the vocalis and external divisions of the thyroarytenoid. However, muscle fiber type compositions were similar between sexes in both the vocalis and external division of the thyroarytenoid muscles.
CONCLUSION
Functional and structural laryngeal differences exist between adult male and female rats and, therefore, the rat model can be used to further study sexual dimorphism of the voice.
Keywords: larynx, ultrasonic vocalizations, rat, sexual dimorphism, female, thyroarytenoid
Introduction
The human voice is sexually dimorphic in obvious ways, such as the lower habitual speaking fundamental frequency (F0) in men due to longer membranous vocal folds.1,2 Although prominent in adolescence, the voice is sexual dimorphic throughout the lifespan in less apparent ways.3 For example, women are more likely to experience a voice disorder than men.4–6 Also, certain types of voice disorders are more prevalent in one sex or the other.7 Contact ulcers/granulomas, leukoplakia, and polyps are more frequently observed in men, whereas nodules, Reinke’s edema, and pseudocysts are more frequently observed in women.7 Advanced age also differentially impacts the voices of men and women.8–13 For example, women’s average speaking F0 decreases in advanced age while men’s F0 increases.9 Also, vocal fold contact during phonation increases for aged women but decreases for aged men, which likely contributes to vocal quality differences between aged sexes.8,14 Therefore, the human voice is sexual dimorphic in the perception and underlying anatomy of the adult voice, prevalence and type of voice disorders, and the perception and assumed causes of clinically observed laryngeal senescence.
Despite this observed sexual dimorphism,11,14,15 little is known about the sexual dimorphism of the thyroarytenoid (TA), the primary muscle of the vocal fold. In the limb muscles, however, patterns of sexual dimorphism have been identified, such as men having larger muscle fiber cross-sectional areas and a higher ratio of type II (fast twitch) muscle fibers than women.16,17 These sexually dimorphic muscle properties result in differences in muscle performance between sexes.18 Findings from the limb muscles, however, cannot be generalized to the unique intrinsic laryngeal muscles (such as the TA), due to differences between limb and laryngeal muscles in innervation, contractile properties, mitochondrial content, and function.19 Therefore, direct study of intrinsic laryngeal muscles is necessary to understand the neuromuscular mechanisms underlying sexual dimorphism in the voice.
Although the rat larynx is an established model for studying laryngeal neuromuscular mechanisms, there is scant evidence on the extent of sexual dimorphism in the rat larynx.20–22 Only one study has evaluated sexual dimorphism of the rat larynx, finding that female rats have more numerous neuromuscular junction cluster fragments than male rats in the TA muscles.23 Furthermore, this difference was unique to the TA and was not present in the hindlimb muscles or other intrinsic laryngeal muscles.23 However, no study has evaluated the extent of sexual dimorphism within the intrinsic properties (size and muscle fiber composition) of laryngeal muscles. Therefore, the rat model has been underutilized in investigating the neuromuscular mechanisms underlying the differences in laryngeal development and senescence between sexes.
Rat ultrasonic vocalizations (USVs) and human vocalizations are produced using similar laryngeal neuromuscular mechanisms,24,25 allowing the rat larynx to be used to evaluate both functional and structural laryngeal changes associated with aging and disease.22,26 However, the extent of sexual dimorphism in USV acoustic parameters is unknown. The goal of this study was to evaluate the extent of sexual dimorphism in spontaneous USVs and TA muscle fibers of adult rats.
We compared acoustic parameters of spontaneous USVs, muscle fiber size in the TA muscle, and muscle fiber compositions in the TA muscles between adult male and female rats. Based on studies of sexual dimorphism in rat pups, our hypotheses were that adult female rats would produce 1) fewer 50-kHz USVs, 2) with a higher average F0, and 3) similar duration of calls when compared to male rats.27 Based on rat limb literature, our hypotheses were that females would have 1) a smaller overall muscle size, 2) a higher concentration of slow-twitch muscle fibers and 3) smaller individual muscle fiber size diameters compared to male rats.28,29
Materials and Methods
Twenty (10 male, 10 female) Long-Evans adult rats between 10–14 months old were used for this experiment. All rats were singly housed for the duration of the experiment. Methods for this study were approved the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign.
USV Collection
USVs were recorded from each rat in an isolation chamber during the 12-hour dark portion of the light cycle on two randomly selected days. This approach allowed for recording of spontaneous USVs without the influence of an elicitation model, such as a mating encounter. Because the female estrous cycle influences the production of USVs,30 all female rats were recorded only when they were not in estrus, as determined by behavioral signs: rapid darting or spinning, ear wiggling, and lordosis.31
Chambers were acoustically monitored using ultrasonic microphones (CM16/CMPA, Avisoft Bioacoustics, Germany) connected to a USB recording interface (UltraSoundGate 416H, Avisoft Bioacoustics) providing phantom power, pre-amplification, and A/D conversion at a 16-bit, 250-kHz sampling rate. Only acoustic events with an intensity greater than the ambient sound in the chamber triggered recording. All rats were recorded under the same conditions until a minimum of 10 USVs were collected for each rat.
USV Analysis
Although the intensity threshold used during monitoring sessions sufficiently captured USVs, it also recorded some cage noise during activities as eating and locomotion. USVs were automatically identified and separated from these noises using an automatic template matching procedure in XBAT.32 Acoustic parameters of mean F0 and duration of each USV were then automatically measured (SASLab Pro, Avisoft Bioacoustics). The number of USVs per animal was counted and then manually labeled according to the 14-category classification scheme introduced by Wright et al.33 Another trained investigator independently labeling thirty percent of USVs and percent agreement was calculated to assess interrater reliability of labeling.
Muscle Fiber Analysis
After all recordings were completed, rats were euthanized and the whole larynx was excised and cleaned of extrinsic musculature. Tissues were flash-frozen and stored at −80 °C. Using a −20 °C cryostat, 10-micron thick coronal cross-sections containing the mid-belly of the TA were collected and mounted on glass slides. One slide was triple-labeled for myosin heavy chain (MHC) I, MHC IIb and laminin, another slide was triple-labeled for MHC IIa, MHC IIx and laminin, and a third slide was labeled for MHC IIeo, a super-fast extraocular fiber type also found in the larynx and sometime referred to as MHC IIL. 34–36 All primary MHC antibodies were obtained from Developmental Studies Hybridoma Bank (University of Iowa), except for the MHC IIeo antibody which was a gift from Dr. Francisco Andrade at the University of Kentucky. Anti-laminin was obtained from Sigma Aldrich (St. Louis, MO). Appropriate Alexa Fluor secondary antibodies were obtained from Molecular Probes (Eugene, OR). Primary and secondary antibodies and immunohistochemistry procedures are summarized in Table 1. Two different immunohistochemistry protocols were tested using the laryngeal tissues and all antibody combinations.35,37 After running appropriate positive and negative controls, a protocol based on the method described by Greising 37 was used for MHC I, IIb, and laminin, and the protocol of Bloemberg 35 was used for the other two slides.
Table 1.
Antibodies, concentrations, and immunohistochemistry protocols used to label muscle fibers
| Primary antibodies and concentrations | Reactivity | Secondary antibodies and concentrations |
|---|---|---|
| BF-F8 (1:100)* | I | Alexa488 IgG2b, goat/anti-mouse (1:200) |
| BF-F3 (1:100)* | IIb | Alexa Fluor 594, IgG1, goat/anti-mouse (1:200) |
| 6H1 (1:50)* | IIx | Alexa Fluor 594, IgG1, goat/anti-mouse (1:200) |
| SC-71 (1:100)* | IIa | Alexa488 IgG1, goat/anti-mouse (1:200) |
| MHC13 (1:50)*** | IIeo | Alexa488 IgG1, goat/anti-rabbit (1:200) |
| anti-laminin (1:200)** | laminin | Alexa405, goat/anti-rabbit IgG Invitrogen (1:100) |
|
| ||
| Targets | Procedure | Time |
|
| ||
| 1. MHC I and IIb and laminin | Phosphate buffer (PB) wash | 2 × 2 min |
| Cold (4°C) methanol fixation | 10 min | |
| PB wash | 2 × 2 min | |
| Incubate in 10% normal goat serum in PB | 1 hr | |
| PB wash | 2 × 2 min | |
| Incubate in primary antibodies at 4°C | overnight | |
| PB wash | 4 × 2 min | |
| Incubate in secondary antibodies at RT | 30 min | |
| PB wash | 3 × 2 min | |
| Mount coverslips with Prolong Gold antifade reagent | ||
| 1. MHC IIa and IIx and laminin | Air dry | 10 min |
| 2. MHC IIeo | Incubate in 10% normal goat serum in phosphate buffered saline (PBS) | 1 hr |
| Incubate in primary antibodies at RT | 2 hrs | |
| PBS wash | 3 × 5 min | |
| Incubate in secondary antibodies at RT | 1 hr | |
| PBS wash | 3 × 5 min | |
| Mount coverslips with ProlongH Gold antifade reagent | ||
Primary and secondary antibody cocktails were diluted in blocking buffer
Antibodies obtained from (Developmental Studies Hybridoma Bank, Iowa City, IA)
Antibodies obtained from (Sigma-Aldrich, St. Louis, MO)
Anitbodies obrain from (KY)
Antibodies obtained from (Invitrogen: Molecular Probes, Eugene, OR)
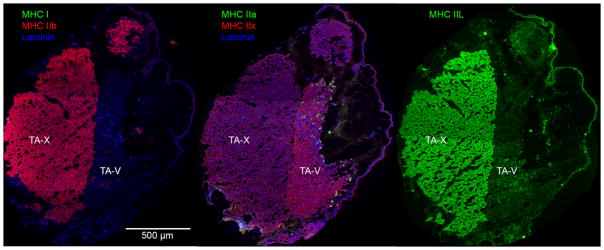
Three-color fluorescent, high-resolution scans of each slide were collected at 20× magnification using a NanoZoomer Digital Pathology System (Hamamatsu Photonics, Japan). Using ImageJ,38 image contrast was enhanced using histogram stretching to improve automatic segmentation of muscle fibers. Each TA image was divided manually into a vocalis division (TA-V) and external division (TA-X) for separate analysis (Figure 1). The total area of each muscle was determined by manually tracing the outline of the entire muscle in ImageJ. The size of the individual muscle fibers was determined using a MATLAB application, Semi-automatic Muscle Analysis using Segmentation of Histology (SMASH), which segmented the muscle fibers based on the laminin staining, removed non-fiber elements from the image, and calculated the minimum feret diameter.39 Minimum feret diameter is a measure of the smallest rectangle width that bounds the borders of the muscle fiber. It is a robust measure of fiber size that is less susceptible to sectioning artifacts than cross-sectional area.39,40 SMASH also automatically calculated the percentage of each MHC isoform positively stained in each sample labeled with anti-laminin. Laminin was not labeled in type IIL samples and, therefore, the percentage of type IIL fibers was determined manually. A second independent rater counted type IIL labeled fibers in 50% of the images to assess interrater reliability using percent agreement.
Fig. 1.
Muscles fibers are stained accordingly: type I green (a), type IIB red (a), type IIA green (b), type IIX red (b), and type IIL green (c). Laminin is outlined in blue (a) (b). Both images (a) and (c) illustrates that the TA-X co-expresses type IIL and IIB, and both fiber types are also present in the TA-V. Image (b) illustrates that type IIX stained most of the TA-V, with type IIA fibers stained in the rostral portion of the TA-V. TA-V = thyroarytenoid vocalis division; TA-X = thyroarytenoid external division.
Statistical Analysis
Each variable was averaged to arrive at a single measure per rat. Then dependent acoustic variables (number, mean frequency, frequency bandwidth, and duration) and muscular variables (muscle fiber size and vocal fold size) were tested for normality using a Shapiro-Wilk test. If data were normal, Welch’s t-test was used to test for significant differences between sexes and non-parametric Mann–Whitney U-test was used non-normal data. The relationships between body weight, fiber size, and mean USV frequency were assessed using linear regression modeling for each sex.
Results
USV Analysis
Overall, the distribution of USV classification was similar between male and female rats, consisting primarily (>85%) of flat and short USVs with a relatively steady-state frequency (Table 2). The inter-rater reliability of classifying USVs was 92%. Because little to none of the USVs were classified into the remaining 12 categories, acoustic parameters of only the flat and short USVs were statistically tested for differences between sexes (Table 3). Of the four acoustic parameters examined, only mean frequency of short vocalizations was significantly lower (p=.04) in males (51 ± 3 kHz) than in females (56 ± 5 kHz).
Table 2.
Raw Number and (Percentage of total) of USVs by Sex
| Category | Female | Male | All |
|---|---|---|---|
| Flat | 276 (67%) | 505 (66%) | 781 (66%) |
| Short | 78 (19%) | 172 (22%) | 250 (21%) |
| Complex | 21 (5%) | 28 (4%) | 49 (4%) |
| Upward ramp | 9 (2%) | 7 (1%) | 16 (1%) |
| Downward ramp | 2 (<1%) | 6 (1%) | 8 (<1%) |
| Flat-trill combo | 0 (0%) | 1 (<1%) | 1 (<1%) |
| Inverted-U | 3 (1%) | 3 (<1%) | 6 (<1%) |
| Step up | 12 (3%) | 18 (2%) | 30 (3%) |
| Step down | 7 (2%) | 12 (2%) | 19 (2%) |
| Multi-step | 5 (1%) | 14 (2%) | 19(2%) |
| Total | 413 (100%) | 766 (100%) | 1179 (100%) |
Table 3.
Mean ± standard deviation and Mann-Whiney U test results for USV acoustic parameters by sex
| Call type | USV parameter | Females | Males | W | p-value |
|---|---|---|---|---|---|
| Flat USVs | Number | 14.32 ± 14.48 | 24.24 ± 24.64 | 37 | 0.55 |
| Frequency (kHz) | 49.61 ± 5.69 | 47.48 ± 4.71 | 34 | 0.4 | |
| Bandwidth (kHz) | 3.96 ± 1.21 | 3.75 ±0.77 | 40 | 0.72 | |
| Duration (ms) | 42.82 ± 17.64 | 44.14 ± 22.23 | 45 | 1 | |
| Short USVs | Number | 4.04 ± 4.0 | 8.13 ± 6.48 | 19 | 0.19 |
| Frequency (kHz) | 55.53 ± 4.56 | 51.28 ± 3.38 | 17 | 0.04* | |
| Bandwidth (kHz) | 2.75 ± 1.21 | 2.29 ± 0.55 | 31 | 0.46 | |
| Duration (ms) | 8.19 ± 0.98 | 8.46 ± 0.33 | 44 | 0.76 |
Muscle Fiber Analysis
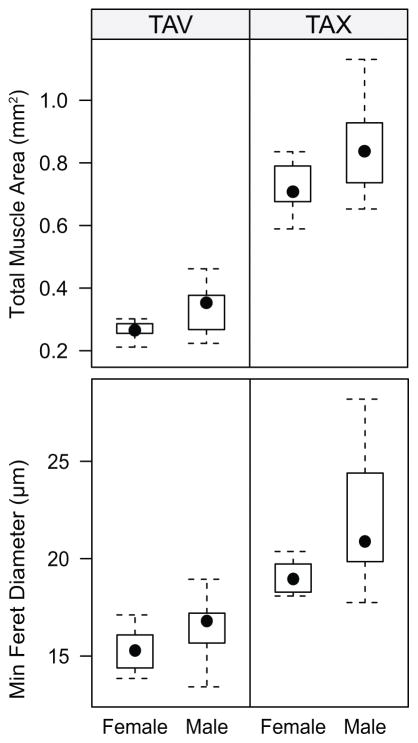
An overall pattern of sexual dimorphism was observed in muscle fiber size. Male rats had larger muscle fibers in both the TA-V and TA-X as well as a larger total muscle area (Figure 3). Compared to female rats, male rats had a significantly larger TA-X muscle fiber size (t(10)=2.46, p=0.03), but a similar TA-V muscle fiber size (t(15)=1.85, p=0.08) (Figure 3). The mean total area of both the TA-V (t(12)=−2.94, p=0.01) and TA-X (t(14)= −2.22, p=0.04) were larger in male rats than female rats (Figure 3).
Fig. 3.
Box-and-whisker plots of the individual muscle fiber sizes of the TA-V (a) and TA-X (b) and the overall muscle sizes of the TA-V (c) and the TA-X (d). A pattern can be noted that male rats typically have larger fiber sizes and muscle area than female rats. TA-V = thyroarytenoid vocalis division; TA-X = thyroarytenoid external division.
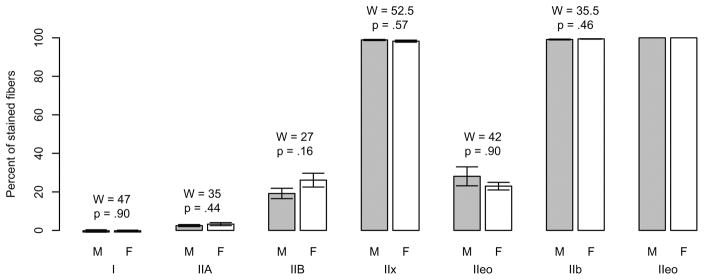
There were no significant differences between sexes in the percentages of MHC compositions in either the TA-X or the TA-V (Figure 2). The overall distribution of muscle fiber types in the TA-X and TA-V were consistent with previous studies of male rats. However, in the TA-V we also found a very small percentage of fibers expressing either type I (~0.1%) or IIA (~3%) in both males and females, which has not been previously reported.41
Fig. 2.
Mean ± standard error and Mann-Whitney U-test results for percentages of muscle fiber types in thyroarytenoid vocalis division and thyroarytenoid external division. Note that fibers can coexpress isoforms; thus, the total percentages exceed 100% within each muscle. F = female; M = male.
Male rats weighed almost twice as much as female rats (608 ± 80g vs. 341 ± 32g, respectively). Despite this, linear regressions revealed that body weight did not predict fiber size of the TA-X or the TA-V, nor was weight related to the mean frequency of flat or short USVs. Therefore, although weight was sexually dimorphic in and of itself, it did not account for the observed sexual dimorphism in fiber size or mean frequency of short USVs. Furthermore, the regressions using TA muscle fiber size to predict mean frequency were not significant. Thus, neither weight nor TA fiber size were related to mean frequency of USVs with adjusted r-square values ranging from 0 to 0.2.
Discussion
We found evidence of sexual dimorphism in the function and structure of the adult rat larynx. Specifically, the mean frequency of short USVs was higher in female rats and muscle fiber size of the TA muscle was smaller. These two sexually dimorphic features, however, appear to be independent of each other. There was no direct relationship between USV frequency and TA muscle size.
USVs
Female rats had a significantly higher mean frequency than male rats in short USVs. This higher mean frequency may be attributed to either an anatomical or physiological difference in USV production between sexes, or perhaps to a difference in social function. Because the USV is created using a constriction within the larynx,25 the female rat larynx may be smaller in size creating a smaller laryngeal constriction and raising frequency. This anatomical difference, however, would likely raise the frequency of all USVs. We only found a difference in the short category of USVs, not the flat USVs. Despite no difference in mean frequency of flat USVs, female rats did produce flat USV in the 75-kHz range, which was not observed of the male flat USVs (Figure 4). The higher frequency may also serve a social function. Higher frequency USVs are more easily localized than lower frequency USVs; therefore, the higher mean frequency of female short USVs might aid male rats in locating the female for mating encounters.30,42
Fig. 4.
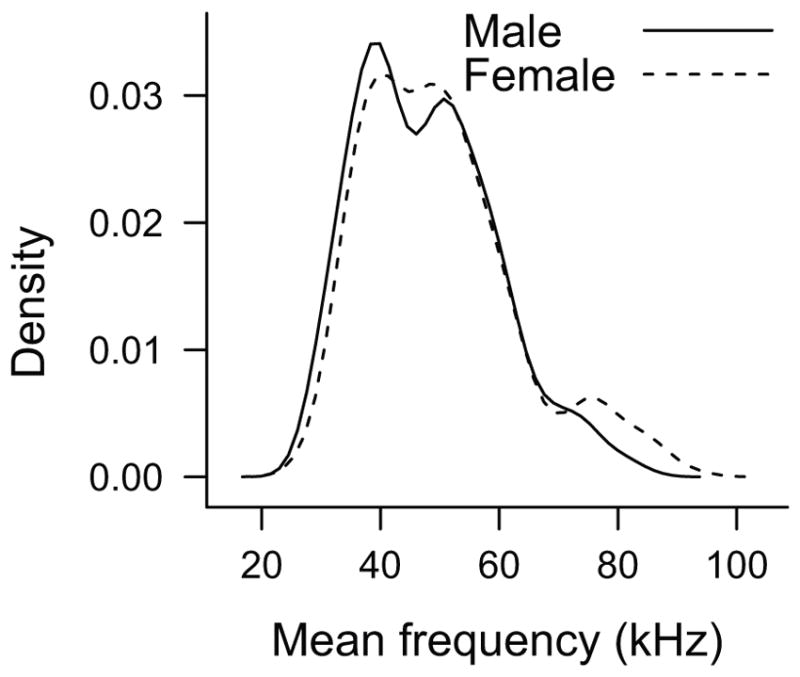
Density plot of the mean frequency of all flat USVs produced by male (solid line) and female (dashed line) rats. Male and female rats have a similar binary distribution of flat USVs in the 40- and 55-kHz range, whereas female rats have another small peak in the distribution of flat USVs centered around 75-kHz range. USV = ultrasonic vocalizations.
Most vocalizations for both male and female rats in isolation were unmodulated (flat or short) USVs. This finding indicated that in social isolation both sexes produce fewer complex vocalizations than in social or reward situations such as mating, rough-and-tumble play, or food/drug reward models.43 Also, the total number of vocalizations for both sexes was relatively low when compared to social situations, which is consistent with previous findings that indicate that social interaction is associated with 50-kHz vocalizations.44–47
The relatively few vocalizations produced in social isolation was a limitation to evaluating sex differences in rat USVs. Social isolation was useful in removing the influence of social interactions that elicit vocalizations in rats; however, by controlling the social environment, we recorded fewer USVs from the animals. Furthermore, female rats were recorded during menestrus or diestrus when females produce fewer vocalizations than during high hormonal surges of proestrus and estrus.30,48 Although male and female rats produced similar numbers of vocalizations in this study, adult female rats may produce more vocalizations when accounting for all stages of the estrous cycle.
Muscles
Although TA muscle fibers are larger in male rats, similar to hindlimb muscles, TA muscle fiber type composition is similar between the sexes. In contrast, sexual dimorphism of muscle fiber type composition has been reported in the hindlimb muscles.28,29 These findings further support that results from the hindlimb literature cannot be generalized to the intrinsic muscles of the larynx.49 Hindlimb muscle properties such as fiber size and fiber type composition may be more sexually dimorphic than the faster twitch muscles, such as those found in the TA muscles.
Nevertheless, although difference in fiber type composition may not exist between adult male and female rats’ TA muscles, there may be a sexual dimorphic response of the TA muscle to advanced age. Previous literature suggests that the number of muscle fibers decreases in the TA and, depending on which fibers are more susceptible to denervation, this may reveal differences between male and female rat TA muscles in old age.50 Fiber type composition warrants further study in an aged rat model to test this hypothesis.
Conclusion
Understanding sex differences in acoustic parameters of USV production and the intrinsic properties of the TA muscles is critical for modeling sexual dimorphism in humans. The most salient finding of this study was the sexual dimorphism of fiber size in the TA-V and TA-X, which cannot be explained by the body weight difference of sexes. By establishing differences and similarities between sexes in acoustic parameters and muscular properties of the TA muscle, this study allows future investigations to evaluate sexual dimorphism in acoustic parameters in social contexts and determine if acoustic and neuromuscular differences are differentially impacted by age between sexes.
Acknowledgments
Thank you to Colleen McMullen and Dr. Francisco Andrade for providing the MHC IIeo antibody as well as members of the University of Illinois Urbana-Champaign Voice Laboratory who contributed to analysis and data collection: Emily Itoku, Anna Fischer, Medha Sataluri, and Adam Hillgren. Images were collected at the Core Facilities at the Carl R. Woese Institute for Genomic Biology. The monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA.
Footnotes
Level of Evidence: NA, Animal studies and basic research
The authors have no conflicts of interest or financial disclosures related relevant to the subject of this manuscript.
Contributor Information
Charles Lenell, Department of Communicative Sciences and Disorders, New York University, New York, NY, USA
Aaron M. Johnson, NYU Voice Center, Department of Otolaryngology – Head and Neck Surgery, New York University School of Medicine, New York, NY, USA
References
- 1.Kahane JC. A morphological study of the human prepubertal and pubertal larynx. Am J Anat. 1978 Jan;151(1):11–19. doi: 10.1002/aja.1001510103. [DOI] [PubMed] [Google Scholar]
- 2.Titze IR. Physiologic and Acoustic Differences between Male and Female Voices. J Acoust Soc Am. 1989 Apr;85(4):1699–1707. doi: 10.1121/1.397959. [DOI] [PubMed] [Google Scholar]
- 3.Abitbol J, Abitbol P, Abitbol B. Sex hormones and the female voice. J Voice. 1999 Sep;13(3):424–446. doi: 10.1016/s0892-1997(99)80048-4. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya N. The prevalence of voice problems among adults in the United States. Laryngoscope. 2014 Oct;124(10):2359–2362. doi: 10.1002/lary.24740. [DOI] [PubMed] [Google Scholar]
- 5.Simberg S, Udd H, Santtila P. Gender Differences in the Prevalence of Vocal Symptoms in Smokers. J Voice. 2015 Sep;29(5):588–591. doi: 10.1016/j.jvoice.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Roy N, Merrill RM, Thibeault S, Parsa RA, Gray SD, Smith EM. Prevalence of voice disorders in teachers and the general population. J Speech Lang Hear Res. 2004 Apr;47(2):281–293. doi: 10.1044/1092-4388(2004/023). [DOI] [PubMed] [Google Scholar]
- 7.Zhukhovitskaya A, Battaglia D, Khosla SM, Murry T, Sulica L. Gender and age in benign vocal fold lesions. Laryngoscope. 2015 Jan;125(1):191–196. doi: 10.1002/lary.24911. [DOI] [PubMed] [Google Scholar]
- 8.Linville SE. Source characteristics of aged voice assessed from long-term average spectra. J Voice. 2002 Dec;16(4):472–479. doi: 10.1016/s0892-1997(02)00122-4. [DOI] [PubMed] [Google Scholar]
- 9.Brown WS, Morris RJ, Hollien H, Howell E. Speaking Fundamental-Frequency Characteristics as a Function of Age and Professional Singing. J Voice. 1991 Dec;5(4):310–315. [Google Scholar]
- 10.Morsomme D, Jamart J, Boucquey D, Remade M. Presbyphonia: voice differences between the sexes in the elderly. Comparison by Maximum Phonation Time, Phonation Quotient and Spectral Analysis. Logopedics, phoniatrics, vocology. 1997;22(1):9–14. [Google Scholar]
- 11.Pontes P, Yamasaki R, Behlau M. Morphological and functional aspects of the senile larynx. Folia Phoniatr Logop. 2006;58(3):151–158. doi: 10.1159/000091729. [DOI] [PubMed] [Google Scholar]
- 12.Hammond TH, Gray SD, Butler J, Zhou R, Hammond E. Age- and gender-related elastin distribution changes in human vocal folds. Otolaryngol Head Neck Surg. 1998 Oct;119(4):314–322. doi: 10.1016/S0194-5998(98)70071-3. [DOI] [PubMed] [Google Scholar]
- 13.Hammond TH, Gray SD, Butler JE. Age- and gender-related collagen distribution in human vocal folds. Ann Otol Rhinol Laryngol. 2000 Oct;109(10 Pt 1):913–920. doi: 10.1177/000348940010901004. [DOI] [PubMed] [Google Scholar]
- 14.Ma EP, Love AL. Electroglottographic evaluation of age and gender effects during sustained phonation and connected speech. J Voice. 2010 Mar;24(2):146–152. doi: 10.1016/j.jvoice.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Martins RHG, Benito Pessin AB, Nassib DJ, Branco A, Rodrigues SA, Matheus SMM. Aging voice and the laryngeal muscle atrophy. Laryngoscope. 2015;125(11):2518–2521. doi: 10.1002/lary.25398. [DOI] [PubMed] [Google Scholar]
- 16.Welle S, Tawil R, Thornton CA. Sex-related differences in gene expression in human skeletal muscle. PLoS One. 2008;3(1):e1385. doi: 10.1371/journal.pone.0001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol. 1989 Oct;257(4 Pt 1):E567–572. doi: 10.1152/ajpendo.1989.257.4.E567. [DOI] [PubMed] [Google Scholar]
- 18.Wust RC, Morse CI, de Haan A, Jones DA, Degens H. Sex differences in contractile properties and fatigue resistance of human skeletal muscle. Exp Physiol. 2008 Jul;93(7):843–850. doi: 10.1113/expphysiol.2007.041764. [DOI] [PubMed] [Google Scholar]
- 19.Thomas L, Harrison A, Stemple J. Aging thyroarytenoid and limb skeletal muscle: lessons in contrast. J Voice. 2008;22(4):430–450. doi: 10.1016/j.jvoice.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Inagi K, Schultz E, Ford CN. An anatomic study of the rat larynx: establishing the rat model for neuromuscular function. Otolaryngol Head Neck Surg. 1998 Jan;118(1):74–81. doi: 10.1016/S0194-5998(98)70378-X. [DOI] [PubMed] [Google Scholar]
- 21.Riede T. Stereotypic laryngeal and respiratory motor patterns generate different call types in rat ultrasound vocalization. J Exp Zool A Ecol Genet Physiol. 2013 Apr;319(4):213–224. doi: 10.1002/jez.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connor NP, Suzuki T, Sewall GK, Lee K, Heisey DM. Neuromuscular Changes in Aged Rat Thyroidarytenoid Muscle. Ann Otol Rhinol Laryngol. 2002/07;111(7) doi: 10.1177/000348940211100703. [DOI] [PubMed] [Google Scholar]
- 23.Feng X, Zhang T, Ralston E, Ludlow CL. Differences in neuromuscular junctions of laryngeal and limb muscles in rats. Laryngoscope. 2012 May;122(5):1093–1098. doi: 10.1002/lary.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riede T. Subglottal pressure, tracheal airflow, and intrinsic laryngeal muscle activity during rat ultrasound vocalization. J Neurophysiol. 2011 Nov;106(5):2580–2592. doi: 10.1152/jn.00478.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson AM, Ciucci MR, Russell JA, Hammer MJ, Connor NP. Ultrasonic output from the excised rat larynx. J Acoust Soc Am. 2010 Aug;128(2):EL75–79. doi: 10.1121/1.3462234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basken JN, Connor NP, Ciucci MR. Effect of aging on ultrasonic vocalizations and laryngeal sensorimotor neurons in rats. Exp Brain Res. 2012 Jun;219(3):351–361. doi: 10.1007/s00221-012-3096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowers JM, Perez-Pouchoulen M, Edwards NS, McCarthy MM. Foxp2 mediates sex differences in ultrasonic vocalization by rat pups and directs order of maternal retrieval. J Neurosci. 2013 Feb 20;33(8):3276–3283. doi: 10.1523/JNEUROSCI.0425-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drzymala-Celichowska H, Karolczak J, Redowicz MJ, Bukowska D. The content of myosin heavy chains in hindlimb muscles of female and male rats. J Physiol Pharmacol. 2012 Apr;63(2):187–193. [PubMed] [Google Scholar]
- 29.Mierzejewska-Krzyzowska B, Drzymala-Celichowska H, Bukowska D, Celichowski J. Gender differences in morphometric properties of muscle fibres measured on cross-sections of rat hindlimb muscles. Anat Histol Embryol. 2012 Apr;41(2):122–129. doi: 10.1111/j.1439-0264.2011.01111.x. [DOI] [PubMed] [Google Scholar]
- 30.Thomas DA, Barfield RJ. Ultrasonic Vocalization of the Female Rat (Rattus-Norvegicus) during Mating. Anim Behav. 1985 Aug;33:720–725. [Google Scholar]
- 31.Suckow MA, WSH, Franklin CL. The Laboratory Rat. Amsterdam: Elsevier; 2006. Reproduction and Breeding. [Google Scholar]
- 32.Barker DJ, Herrera C, West MO. Automated detection of 50-kHz ultrasonic vocalizations using template matching in XBAT. J Neurosci Methods. 2014 Oct 30;236:68–75. doi: 10.1016/j.jneumeth.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright JM, Gourdon JC, Clarke PB. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology (Berl) 2010 Jul;211(1):1–13. doi: 10.1007/s00213-010-1859-y. [DOI] [PubMed] [Google Scholar]
- 34.Zou K, De Lisio M, Huntsman HD, et al. Laminin-111 improves skeletal muscle stem cell quantity and function following eccentric exercise. Stem Cells Transl Med. 2014 Sep;3(9):1013–1022. doi: 10.5966/sctm.2014-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One. 2012;7(4):e35273. doi: 10.1371/journal.pone.0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiotani A, Flint PW. Expression of extraocular-superfast-myosin heavy chain in rat laryngeal muscles. Neuroreport. 1998 Nov 16;9(16):3639–3642. doi: 10.1097/00001756-199811160-00015. [DOI] [PubMed] [Google Scholar]
- 37.Greising S, Call J, Lund T, Blazar B, Tolar J, Lowe D. Skeletal muscle contractile function and neuromuscular performance in Zmpste24 −/− mice, a murine model of human progeria. Age. 2012;34(4):805–819. doi: 10.1007/s11357-011-9281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11(7):36–42. [Google Scholar]
- 39.Smith LR, Barton ER. SMASH - semi-automatic muscle analysis using segmentation of histology: a MATLAB application. Skelet Muscle. 2014;4:21. doi: 10.1186/2044-5040-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briguet A, Courdier-Fruh I, Foster M, Meier T, Magyar JP. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul Disord. 2004 Oct;14(10):675–682. doi: 10.1016/j.nmd.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Rhee HS, Lucas CA, Hoh JFY. Fiber types in rat laryngeal muscles and their transformations after denervation and reinnervation. J Histochem Cytochem. 2004 May;52(5):581–590. doi: 10.1177/002215540405200503. [DOI] [PubMed] [Google Scholar]
- 42.Nyby J, Whitney G. Ultrasonic Communication of Adult Myomorph Rodents. Neurosci Biobehav Rev. 1978 Spring;2(1):1–14. [Google Scholar]
- 43.Burgdorf J, Panksepp J, Moskal JR. Frequency-modulated 50 kHz ultrasonic vocalizations: a tool for uncovering the molecular substrates of positive affect. Neurosci Biobehav Rev. 2011 Oct;35(9):1831–1836. doi: 10.1016/j.neubiorev.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 44.Barfield RJ, Auerbach P, Geyer LA, Mcintosh TK. Ultrasonic Vocalizations in Rat Sexual-Behavior. Am Zool. 1979;19(2):469–480. [Google Scholar]
- 45.Wohr M, Schwarting RK. Affective communication in rodents: ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res. 2013 Oct;354(1):81–97. doi: 10.1007/s00441-013-1607-9. [DOI] [PubMed] [Google Scholar]
- 46.Wohr M, Houx B, Schwarting RK, Spruijt B. Effects of experience and context on 50-kHz vocalizations in rats. Physiol Behav. 2008 Mar 18;93(4–5):766–776. doi: 10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 47.Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol. 1998 Mar;112(1):65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- 48.Huang HH, Steger RW, Bruni JF, Meites J. Patterns of sex steroid and gonadotropin secretion in aging female rats. Endocrinology. 1978 Nov;103(5):1855–1859. doi: 10.1210/endo-103-5-1855. [DOI] [PubMed] [Google Scholar]
- 49.Thomas LB, Harrison AL, Stemple JC. Aging thyroarytenoid and limb skeletal muscle: lessons in contrast. J Voice. 2008 Jul;22(4):430–450. doi: 10.1016/j.jvoice.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Nishida N, Taguchi A, Motoyoshi K, Hyodo M, Gyo K, Desaki J. Age-related changes in rat intrinsic laryngeal muscles: analysis of muscle fibers, muscle fiber proteins, and subneural apparatuses. Eur Arch Otorhinolaryngol. 2013 Mar;270(3):975–984. doi: 10.1007/s00405-012-2231-0. [DOI] [PubMed] [Google Scholar]