Abstract
One in 12 adults suffer with alcohol use disorder (AUD). Studies suggest the younger the age in which alcohol consumption begins the higher the probability of being diagnosed with AUD. Binge/excessive alcohol drinking involves a transition from flexible to inflexible behavior likely involving the dorsal striatum (caudate and putamen nuclei). A major focus of this study was to examine the effect of age of drinking onset on subsequent chronic, voluntary ethanol intake and dorsal striatal circuitry. Data from rhesus monkeys (n = 45) that started drinking as adolescents, young adults or mature adults confirms an age-related risk for heavy drinking. Striatal neuroadaptations were examined using whole-cell patch clamp electrophysiology to record AMPA receptor-mediated miniature excitatory postsynaptic currents (mEPSCs) and GABAA receptor-mediated miniature inhibitory postsynaptic currents (mIPSC) from medium-sized spiny projection neurons located in the caudate or putamen nuclei. In controls, greater GABAergic transmission (mIPSC frequency and amplitude) was observed in the putamen compared to the caudate. With advancing age, in the absence of ethanol, an increase in mIPSC frequency concomitant with changes in mIPSC amplitudes was observed in both regions. Chronic ethanol drinking decreased mIPSC frequency in the putamen regardless of age of onset. In the caudate, an ethanol drinking-induced increase in mIPSC frequency was only observed in monkeys that began drinking as young adults. Glutamatergic transmission did not differ between the dorsal striatal subregions in controls. With chronic ethanol drinking there was a decrease in the postsynaptic characteristics of rise time and area of mEPSCs in the putamen but an increase in mEPSC frequency in the caudate. Together, the observed changes in striatal physiology indicate a combined disinhibition due to youth and ethanol leading to abnormally strong activation of the putamen that could contribute to the increased risk for problem drinking in younger drinkers.
Keywords: caudate, putamen, adolescent drinking, adult drinking, non-human primate, whole-cell patch clamp electrophysiology
Graphical abstract

1. Introduction
Seventeen million or 7% of individuals over the age of 12 in the US have been diagnosed with alcohol use disorder (AUD), a medical term that encompasses a range from risky patterns of drinking to alcoholism and dependence (SAMHSA, 2014). There are several environmental factors that are thought to correlate with the likelihood of being diagnosed with AUD, including the age when ethanol drinking begins. Ethanol use most often begins during late adolescence, with research showing that the average age of first use of alcohol is about 16 years (Degenhardt et al., 2008; SAMHSA, 2014). Individuals that reported their first alcoholic drink before the age of 14 were more likely to develop AUD than those that began drinking at or over the age of 21 (Grant and Dawson, 1998; Merline et al., 2004; SAMHSA, 2014). In fact, risky patterns of drinking that are associated with AUD can manifest as early as adolescence.
The fact that age of onset is a risk factor for AUD suggests a biological basis for the progression from non-harmful to harmful alcohol consumption. The dorsal striatum is implicated in the seeking and taking of drugs of abuse, and in the progression from recreational drug-use to habitual drug seeking and addiction (Gerdeman et al., 2003; Everitt and Robbins, 2005; Vollstädt-Klein et al., 2010). The anterior portion of the primate caudate nucleus, similar to the rodent dorsomedial striatum (DMS), receives afferent input from the association cortices and contributes directly to actions that are sensitive to outcome value (Yin et al. 2005a,b; Haber et al., 2006; Gläscher et al., 2009). The caudoventral portion of the primate putamen nucleus, or dorsolateral striatum (DLS) in rodents, receives afferent input from the sensory and motor cortices and plays a role in inflexible habit learning that leads to behavioral automatization, a process proposed to underlie the maintenance of drug use (Kunzle, 1975; McGeorge and Faull 1989; Yin et al., 2006; Tricomi et al., 2009). Alterations in circuit function and behavioral control that favor sensorimotor striatal control have also been postulated based on studies of instrumental learning and drug use (Yin et al., 2006; Corbit et al., 2012, 2014).
Recent studies indicate that the dorsal striatum of rodents is highly sensitive to the effects of ethanol (Choi et al., 2006; Fanelli et al., 2013; Corbit et al., 2014; Clarke et al., 2015; DePoy et al., 2015; Patton et al., 2015; Wang et al., 2007, 2010, 2015). Ethanol alters the function of striatal circuits in multiple ways that are thought to contribute to acute intoxication, craving, dependence, and withdrawal. In mature adult macaque monkeys (aged 7 – 9.5 years), we found that chronic alcohol self-administration induced a decrease in GABAergic transmission onto MSNs of the putamen, and transmission at these synapses was strongly and negatively correlated with daily alcohol intakes (Cuzon Carlson et al., 2011). We extended these findings to mice chronically consuming alcohol and observed decreased GABAergic transmission in the DLS (Wilcox et al., 2014).
The present study explored the influence of age at the onset of ethanol access on the risk for chronic heavy drinking and related neurotransmission changes in the dorsal striatum. The age range roughly corresponded to adolescence, young adulthood and mature adulthood of male rhesus macaques. A previous report on male monkeys showed that ethanol intake was greatest when drinking began as a young adult or in late adolescence (Helms et al., 2014a). Here, we tested the hypotheses that chronic ethanol will induce changes in dorsal striatal synaptic function that will: (1) differ based on the dorsal striatal subregion, (2) correlate with ethanol intake, and (3) differ with age of onset.
2. Materials and Methods
2.1 Animals
A total of 45 male rhesus macaques (Macaca mulatta) in 4 experimental cohorts were used in this study. Animals were born and reared at the Oregon National Primate Research Center. The monkeys were individually housed indoors with temperature (20-22°C) and humidity (65%) controlled under an 11-hour light cycle with lights on at 7:00 am. Each cohort was housed within the same room, allowing for continuous visual, auditory, and olfactory contact with one another. All cohorts underwent the same standard operating procedures that included a well-defined schedule-induced polydipsia (SIP) protocol as displayed in Fig 1A, conducted according to the Guide for the Care and Use of Laboratory Animals and approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee.
Figure 1. Chronic ethanol drinking model reveals differences in ethanol intake and BEC based on age of onset.
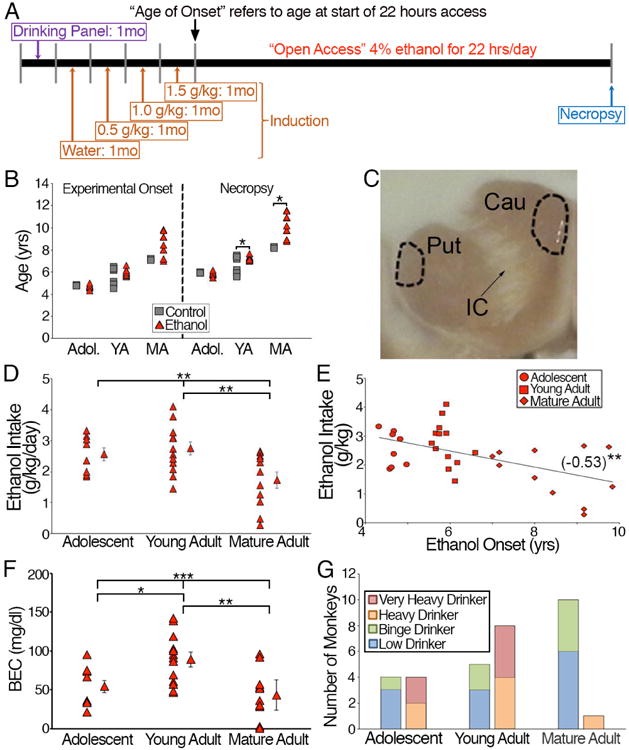
(A) Experimental time line demonstrating the acclimation to the drinking panel, induction period, “open access” period and necropsy during active drinking. (B) Age of onset of the experimental monkeys divided by adolescent, young adult and mature adult. There was no statistical difference between control and ethanol monkeys within the age groups. (C) Image denoting the locations in the putamen (Put) and caudate (Cau) in which MSNs were targeted for whole-cell patch clamp electrophysiology (dashed lines). IC denotes the internal capsule that divides the putamen and caudate. (D) Average daily intake of ethanol is statistically different between the age of onset groups. (E) Ethanol intake negatively correlated with age of onset. Statistics: Pearsons r value noted in parentheses and significance is denoted by asterisks. (F) Average BECs of the experimental monkeys are statistically different between the age of onset groups. (G) Characterization of ethanol-drinking monkeys based on their pattern and total daily intake (categories of low, binge, heavy and very heavy defined in Methods) suggests that monkeys that began drinking during adolescence or young adulthood have a higher proportion of drinkers that developed heavier drinking patterns as classified as very heavy and heavy drinkers than those that began drinking as mature adults.
The monkeys differed in the age at which they were first exposed to ethanol: adolescent (4-5 years, equivalent to ∼15-18 human years; N = 8; INIA cohort 7a), young adulthood (5-6 years, ∼18-24 human years; N = 13; INIA cohorts 5 and 7b) and mature adulthood (7-11 years, ∼25-40 human years; N = 11; INIA cohort 4). Control monkeys were age-matched to their ethanol counterparts, but had no access to ethanol (adolescent: N = 4; young adulthood: N = 7; mature adulthood: N = 2) and the age of experimental onset (Fig. 1B; t-test: adolescent: t10=0.84, p = 0.42; young adult: t18=0.09, p = 0.29; mature adult: t11=1.76, p = 0.11) between control and ethanol-drinking monkeys was not statistically different across the groups. However, the age at necropsy was significantly different between the young adult and mature adult groups but not the adolescent group (Fig. 1B; t-test: adolescent: t10=0.81, p = 0.44; young adult: t18=2.28, p = 0.04; mature adult: t11=2.66, p = 0.02).
2.2 Chronic Oral Ethanol Self-Administration
The chronic ethanol drinking paradigm is shown schematically in Figure 1A. One wall of the home-cage was replaced with an operant panel that was previously described (Helms et al., 2014a) and modified from previous studies (Vivian et al., 2001; Grant et al., 2008). The operant panel consisted of two drinking spouts, a row of three lights (red, white, and green) above each spout that signaled the status of the panel/session (i.e. active or inactive), a central recess containing a dowel pull, a central food cup, and a finger-poke hole with an infrared switch. Monkeys learned to obtain all fluids (water and/or ethanol) and food pellets through the panel.
Monkeys were induced to self-administer ethanol using schedule–induced polydypsia (SIP) as previously described (Vivian et al., 2001; Grant et al., 2008; Fig. 1A). Monkeys were trained to obtain all fluids (ethanol or water) and food using the operant chamber. Banana-flavored pellets were delivered on a fixed-time interval of 300-s until a volume of water or a low concentration of ethanol (4% w/v in water) in increasing volumes equivalent to 0.5, 1.0, and 1.5 g/kg per day was consumed (Fig. 1A). After a 3-hour time out, banana pellets were then delivered on a fixed-ratio of 1 until the daily ration of pellets was consumed. Every cohort had approximately 14 months of daily drinking under these “open access” conditions (Fig. 1A). Throughout the drinking protocol, monkeys complied with awake venipuncture for obtaining blood to measure blood ethanol concentration (BEC) every 5-7 days at 7 hours after session start, as previously described (Grant et al., 2008).
There is strong evidence that this population of monkeys has stable, categorically separate, levels of ethanol intake derived from a Principal Component Analysis (PCA) of daily intakes from >12,000 daily sessions in the open-access condition (i.e., the 14 months of 22 hr/day access to ethanol and water) (Baker et al., 2014, 2017). Very heavy drinkers are defined as having greater than or equal to 10% of open access ethanol drinking days that exceeded 4g/kg as well as an average daily ethanol intake that exceeded 3g/kg. Heavy drinkers are defined as those that had greater or equal to 20% of their open access ethanol drinking days with an intake that exceeded 3g/kg. Binge drinkers are defined as having greater or equal to 55% of their open access days with an intake that exceeded 2g/kg. Low drinkers are defined as those with intake below any of these thresholds. Cohorts of individual monkeys are a random sample of this population and contain unequal distributions of individuals in each category (www.matrr.com). This present design helped address the influence of age on this distribution.
Control monkeys were placed on the same diet, had maltose-dextrose calorically matched to ethanol intakes and had water available ad libitum. They were housed in the same housing rooms and had similar daily routines as that of their ethanol-drinking counterparts. Ethanol drinking data from some of the monkeys (cohorts 4, 5 and 7a) used in this study was previously reported (Helms et al., 2014a) and drinking data for all monkeys are available at www.matrr.com.
2.3 Necropsy and Tissue preparation
Tissue preparation methods were previously published (Daunais et al., 2010; Cuzon Carlson et al., 2011). All monkeys were sent to necropsy on what would have been the next day of drinking, during the 2-hour period in which there was no access to the panels. Briefly, monkeys were anesthetized with ketamine (10mg/kg) and maintained on isoflurane. Monkeys were then perfused with ice-cold monkey perfusion solution containing in mM: NaCl, 124; NaHCO3, 23; NaH2PO4, 3; KCl, 5; MgSO4, 2; d-glucose, 10; CaCl2, 2. A craniotomy was performed and the brain was rapidly removed the brain (∼4 minutes). The brain was placed in a brain matrix (Electron Microscopy Sciences, Hatfield, PA) and a 4mm thick coronal tissue block containing the dorsal striatum and the overlying white matter was isolated (Fig. 1C). The tissue block was sectioned 250μm-thick on a Vibroslicer (Leica, Buffalo Grove, IL) using a ceramic blade in ice-cold, aerated sucrose-based cutting solution containing in mM: sucrose, 194; NaCl, 30; NaHCO3, 26; NaH2PO4, 1.2; KCl, 4.5; MgCl2, 1; d-glucose, 10. Slices were equilibrated in artificial cerebral spinal fluid (ACSF) (NaCl, 124; NaHCO3, 26; NaH2PO4, 1.2; KCl, 4.5; MgCl2, 1; d-glucose, 10; CaCl2, 2) for one hour at 33°C and then transferred to room temperature until experimental use.
2.4 Whole-cell patch clamp electrophysiology
Slices containing the caudate or putamen were transferred to the stage of an upright microscope and continuously perfused with ACSF with or without drugs that isolate GABAergic miniature inhibitory postsynaptic currents (mIPSCs) (NBQX (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide; 5μM), DL-APV (DL-2-amino-5-phosphonopentanoic acid; 50μM), and TTX (1μM) or glutamatergic excitatory miniature postsynaptic currents (mEPSCs) (picrotoxin (100μM) and TTX (tetrodotoxin; 1μM)) maintained at a temperature of 28-32°C with the temperature not varying more than 1°C during a given experiment (Automatic Temperature Controller, Warner Instruments, Hamden, CT). We measured mPSCs as opposed to stimulation-induced synaptic responses to obtain measures of pre- and postsynaptic function that were not biased by the location or source of afferent stimulation.
Individual putative medium spiny projection neurons (MSNs) were observed in real-time under infrared optics using a 40×, 0.8 n.a. water immersion objective with the aid of a monitor, allowing for navigation and placement of the recording pipette. Patch pipettes were pulled from borosilicate glass capillaries (1.5 mm outer diameter, 0.86 mm inner diameter, Sutter Instruments Co., Novato, CA) and filled with a CsCl-based internal solutions containing in mM: CsCl, 150; HEPES, 10; MgCl2, 2; Na-GTP, 0.3; Mg-ATP, 3; BAPTA-4K, 0.2 was used. When filled with internal solution the patch pipettes had a resistance of 2-4 MΩ and all MSNs were held at -60mV during voltage clamp recordings. Recordings were made using a Multiclamp 700B amplifier (Molecular Devices, Foster City, CA). Whole-cell currents were filtered at 2kHz and digitized using Clampex v10.5 at 10kHz. Miniature IPSCs and EPSCs were detected and analyzed offline with MiniAnalysis (Synaptosoft v6.0.7, Decatur, GA). The initial detection of mIPSCs and mEPSCs was performed with amplitude and area as a threshold (10 pA and either 20fc or 50fc for mEPSC or mIPSCs, respectively). This initial amplitude threshold was changed for some recordings dependent on the noise level of the recording. Further visual analysis was then performed and false events rejected (i.e. those that did not show fast rising phases and exponential decay). Series resistance was monitored at regular intervals throughout each experiment (less than 30 MΩ) and data were discarded if resistance changed by more than 15 percent during a given experiment.
2.5 Statistics
At least 3 cells were recorded for mIPSC or mEPSC experiments from each dorsal striatal subregion per animal (n = number of cells; N = number of monkeys). Data are reported as mean + standard error of the mean (SEM). Statistical analysis was performed using Prism 6.0h (GraphPad, La Jolla, CA). Correlation statistics were performed using a Pearson correlation.
Correlation data were reported as average data per animal correlated with either the average daily ethanol intake (g/kg/day) over the 12 months of open access or the age of experimental onset. The Pearson r and p-values are presented.
3.0 Results
3.1 Earlier age of ethanol drinking onset predicts heavy drinking
A detailed analysis of ethanol intake levels in some of the male monkeys from this study (cohort 4, 5, and 7a) has been recently published (Helms et al., 2014a, Baker et al., 2014, 2017), but these data are reported again here with the inclusion of data from cohort 7b. During 22hr/day “open access” to ethanol, monkeys showed a range in their average daily ethanol intake with significant differences between the groups (Fig 1D; one-way ANOVA: F(2,29) = 5.66, p = 0.008). Post hoc analysis revealed a lower ethanol intake in mature adults compared to young adults (Fig 1D; Holm-Sidak's post-hoc test revealed a significant difference between young and mature adults, p<0.01). Average daily ethanol intake over the open access period was negatively correlated with age of onset (Fig. 1E; r = -0.53, p = 0.0018). Average BEC were statistically different between the age groups (Fig. 1F; one-way ANOVA: F(2,29) = 7.28, p = 0.003) and statistically higher in young adults compared to adolescents and mature adults (Fig. 1F; Holm-Sidak's post-hoc test revealed a significant difference between young and adolescent: p < 0.05 as well as young and mature adults: p < 0.01). Together these data suggest that age at the onset of drinking ethanol is a predominant factor that influences subsequent average daily alcohol consumption.
Another way of analyzing the ethanol drinking data is to consider group differences based on categorical levels of ethanol intake (Fig. 1G) as determined by PCA and described by Baker and colleagues (2014, 2017). In the cohort of monkeys that began drinking as adolescents, there were 3 low, 1 binge, 2 heavy and 2 very heavy drinkers (N = 8) (Fig. 1G). In the monkeys whose onset of ethanol drinking began as young adults, there were 3 low, 2 binge, 4 heavy and 4 very heavy drinkers (N = 13) (Fig. 1G). Mature adult-onset drinkers were divided into 6 low, 4 binge, and one very heavy drinkers (N = 11). Baker and colleagues (2017) have previously shown that in 50 rhesus macaques that have undergone the same SIP protocol, half of the population will fall into non-heavy drinkers that includes low drinkers and binge drinker, while the other half of the population will fall into heavy drinkers, that includes those in the heavy drinking and very heavy drinking categories. Due to the small sample sizes in some of the classification of drinkers, we have combined low and binge drinkers (low/binge) as well as heavy and very heavy drinkers (heavy/very heavy). Combining the drinker types suggests that there is an effect of age of onset on the classification of drinkers (χ2 (2, N = 32) = 7.183, p = 0.03; Fig 1G).
3.2 Intrinsic differences in GABAergic neurotransmission measured in MSNs located in the two dorsostriatal subregions
To determine if there were differences in baseline GABAergic transmission between the two dorsal striatal regions, miniature inhibitory postsynaptic currents (mIPSCs) were recorded from MSNs located within the putamen (Fig. 2A) or caudate (Fig. 2B) of control monkeys (dotted areas in Fig. 1C). The mIPSC characteristics of frequency, amplitude, rise time, decay time and area were examined and data was combined regardless of their age of experimental onset (Fig. 2). Miniature inhibitory postsynaptic currents (mIPSCs) were mediated through GABAA receptor channels as addition of a GABAA receptor antagonist (picrotoxin, 100μM) blocked all events (data not shown).
Figure 2. GABAergic transmission onto MSNs differs between the two dorsal striatal subregions in control monkeys.
(A-B) Representative traces of mIPSCs recorded from MSNs located in the putamen (A) and caudate (B) of a young adult ethanol naïve NHP. (C-D) Cumulative probability of mIPSC interevent interval (IEI; C) and amplitude (D) from putamen (black; ncells = 49) and caudate (grey; ncells = 49) MSNs. (E) Average mIPSC rise time and decay time recorded in putamen and caudate MSNs of control monkeys. The mIPSC decay time of caudate MSNs is significantly faster than that of putamen MSNs. (F) Average mIPSC area measured in MSNs located within the putamen and caudate are not statistically different. Statistics: K-S test (panels C and D) and t-test (panels E and F); significance denoted by asterisks (* < 0.05, **** < 0.0001).
The cumulative probability of the interevent interval of mIPSCs recorded from putamen MSNs was rightward shifted compared to that obtained from mIPSCs from caudate MSN mIPSCs, suggesting a higher probability of shorter interevent intervals (nputamen = 49; ncaudate = 49; K-S test; D = 0.52, p < 0.0001; Fig. 2C). There was no statistical difference between the cumulative probability of mIPSC amplitudes from putamen MSNs and caudate MSNs (nputamen = 49; ncaudate = 49; K-S test; D = 0.07, p = 0.61; Fig. 2D), suggesting that the distribution of amplitude sizes is not different between the two dorsal striatal regions.
We also observed a significant difference in the average decay time of mIPSCs, with putamen MSNs displaying a greater decay time constant compared to caudate MSNs (t-test; t96 = 1.99; p < 0.05; Fig. 2E). However, no statistical differences were observed in mIPSC rise time (t-test; t96 = 0.74; p = 0.46; Fig. 2E) and area (t-test; t96 = 1.81; p = 0.07; Fig. 2F) between MSNs of the two dorsal striatal subregions. These data suggest greater GABA release or synapse number in the putamen compared to caudate as evidenced by the increased in mIPSC frequency. The difference in mIPSC amplitude as well as longer decay time suggests that there are postsynaptic differences either in GABAA receptor number or the expression of different subunits between MSNs located in the putamen and caudate.
3.3 Age-related alterations in GABAergic transmission onto MSNs of control monkey putamen and caudate
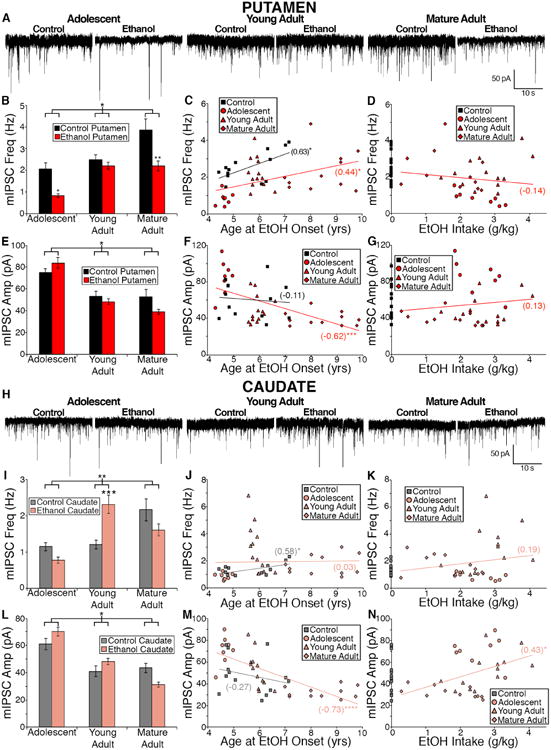
We next examined age-related differences GABAergic transmission in the putamen and caudate within control monkeys. The control monkeys were divided into groups based on their age when the experiment began, as they were age matched to the ethanol counterparts of their respective cohorts (Fig. 1B) and representative traces from each age group are displayed (Fig 3A, H). Examination of mIPSC frequency measured in control putamen MSNs (one-way ANOVA, F(2,46) = 4.91, p = 0.01, nadolescent = 11, nyoung adult = 30, nmature adult = 8; Fig. 3B) and caudate MSNs (one-way ANOVA, F(2,46) = 7.22, p = 0.002, nadolescent = 11, nyoung adult = 31, nmature adult = 7; Fig. 3I) revealed a significant difference based on age such that there is a developmental increase in mIPSC frequency regardless of dorsal striatum subregion. To examine the correlation between the age of onset and mIPSC frequency, we averaged data obtained from individual animals (number cells per animal = 2-6). In agreement with the above average data there was a positive correlation between the age of experimental onset and the individual average mIPSC frequency measured in MSNs of the putamen (r = 0.63, p = 0.02; N = 13, Fig. 3C) and the caudate (r = 0.58, p = 0.04; N = 13, Fig. 3J) of control monkeys.
Figure 3. Differential effects of age and chronic ethanol exposure on GABAergic transmission onto putamen and caudate MSNs.
(A) Representative traces of mIPSCs recorded in the putamen of control and ethanol drinking male monkeys. (B) Average mIPSC frequency measured in putamen MSNs (Control: adolescent: ncells = 11; young adult: ncells = 30; mature adult: ncells = 8;. Ethanol: adolescent: ncells = 23; young adult: ncells = 50; mature adult: ncells = 29). (C-D) Average mIPSC frequency measured in the putamen of individual monkeys correlated with age of onset (C) or daily average ethanol intake (D); Control: Nmonkey = 13; Ethanol: Nmonkey = 32. Statistics: Pearsons r value noted in parentheses and significance is denoted by asterisks. (E) Average mIPSC amplitude measured in putamen MSNs. (F-G) Average mIPSC amplitude measured in the putamen of individual monkeys correlated with age of onset (F) or daily average ethanol intake (G). Statistics: Pearsons r value noted in parentheses and significance is denoted by asterisks. (H) Representative traces of mIPSCs recorded in the caudate of control and ethanol drinking monkeys that began drinking as adolescents, young adults, and mature adults. (I) Average mIPSC frequency measured in caudate MSNs (Control: adolescent: ncells = 11; young adult: ncells = 31; mature adult: ncells = 7). Ethanol: adolescent: ncells = 22; young adult: ncells = 48; mature adult: ncells = 30). (J-K) Average mIPSC frequency measured in the caudate of individual monkeys correlated with age of onset (J) or daily average ethanol intake (K). Statistics: Pearsons r value noted in parentheses and significance is denoted by asterisk. (L) Average mIPSC amplitude measured in caudate MSNs. (M-N) Average mIPSC amplitude measured in the caudate of individual monkeys correlated with age of onset (M) or daily average ethanol intake (N). Statistics: Pearsons r value noted in parentheses and significance is denoted by asterisks (* < 0.05, ** < 0.01, *** < 0.005, **** < 0.0001).
Similarly, there was an age-related decrease in mIPSC amplitude regardless of dorsal striatum region in control monkeys (one-way ANOVA, putamen: F(2,46) = 4.48, p = 0.02; Fig. 3E; caudate: F(2,46) = 4.86, p = 0.01; Fig. 3L). However, we failed to observe a correlation between age of experimental onset and the average of mIPSC amplitude measured in cells from each individual control monkey recorded from the putamen (r = -0.11, p = 0.73, Fig. 3F) or caudate (r = -0.27, p = 0.37, Fig. 3M). An age-related decrease in mIPSC decay time in putamen MSNs (F(2,46) = 3.31; p = 0.045) and in caudate MSNs, mIPSC decay time (F(2,46) = 4.63; p = 0.01) and area (F(2,46) = 8.68; p = 0.0006) suggests a potential age related change in the functional expression of GABAA receptor subunits.
3.4 GABAergic transmission onto putamen MSNs is depressed in chronic ethanol drinking monkeys
We next examined whether the two independent variables of age of experimental onset and ethanol-drinking alter GABAergic transmission in the dorsal striatal subregions. In putamen slices, there was a statistically significant interaction between the age of experimental onset and treatment on mIPSC frequency (F(2, 145) = 3.29, p = 0.04; Fig 3B). There was also a main effect of age of onset (F(2, 145) = 16.54, p < 0.0001, Fig 3B) and a main effect of treatment (F(1, 145) = 22.04, p < 0.0001) suggesting increased mIPSC frequency in the putamen with increased age of experimental onset, as well as, ethanol-drinkers displaying a statistically decreased mIPSC frequency compared to controls. Post hoc analysis indicates a significant ethanol-induced decrease in mIPSC frequency in adolescent and mature adult groups. When we averaged the data per animal (number of cells per animal = 2 - 6), the ethanol-drinkers displayed an age-dependent increase in putamen mIPSC frequency similar to that seen in controls (r=0.44, p=0.01; Fig 3C). Although the mIPSC frequency measured in ethanol-drinkers was statistically lower than controls (Fig. 3B), we did not observe a correlation between this mIPSC characteristic and average daily ethanol intake (r=-0.14, p=0.43, Fig 3D).
With regards to the mIPSC amplitude measured from putamen MSNs, there was a significant interaction between age of experimental onset and treatment (F(2,145) = 3.21, p = 0.04; Fig 3E). There was also a main effect of age of onset (F(2,145) = 31.72, p < 0.0001, Fig 3E), suggesting decreased mIPSC amplitude with increased age of experimental onset. However we did not observe a main effect of chronic ethanol-drinking (F(1,145) = 0.86, p = 0.36, Fig. 3E), suggesting that ethanol-drinking alone did not account for changes in mIPSC amplitude measured in putamen MSNs. Although we did not observe an age-related correlation in mIPSC amplitude in control monkeys, there was a significant correlation of age and mIPSC amplitude in the ethanol-drinking monkeys (r=-0.62, p=0.0002, Fig 3F). Similar to mIPSC frequency, there was no correlation between an individual's average mIPSC amplitude measured in putamen MSNs and average daily ethanol intake (r = 0.13, p = 0.48; Fig. 3G).
We did not observe a statistical interaction between the age of experimental onset and treatment group on mIPSC rise time, decay time, or area in putamen MSNs (data not shown). Although we did not observe a statistical interaction between age of onset and ethanol drinking (F(2,145) = 1.38, p = 0.25), a main effect of age of onset (F(2, 145) = 9.63, p = 0.0001) and treatment (F(1, 145) = 9.09, p = 0.003) was found on mIPSC decay time measured from putamen MSNs, suggesting that there is a developmental decrease in decay time regardless of treatment group and that chronic ethanol drinking increases the decay time. We also observed a main effect of age of experimental onset on mIPSC area measured in putamen MSNs (F(2, 145) = 4.60, p = 0.01) but no such effect of ethanol treatment (F(1, 145) = 0.91, p = 0.35) and no significant interaction between the two factors (F(2, 145) = 0.08, p = 0.92), suggesting decreased area of putamen mIPSCs with increasing age regardless of ethanol exposure.
3.5 Caudate MSNs of ethanol-drinking monkeys display altered GABAergic transmission
In caudate slices, there was a statistically significant interaction between the age of experimental onset and treatment on mIPSC frequency (F(2, 143) = 5.24, p = 0.006; Fig 3I). There was also a main effect of age of onset (F(2, 143) = 6.30, p = 0.002, Fig 3I) suggesting increased mIPSC frequency with increased age of experimental onset. However, we did not observe an effect of ethanol-drinking (F(1, 143) = 0.07, p = 0.80). Post hoc analysis indicates a significant ethanol-induced increase in mIPSC frequency in only those animals that began the experiment as young adults. There were no correlations between the average mIPSC frequency measured in caudate MSNs of individual ethanol-drinking monkeys and either their age of onset (r = 0.03, p = 0.87; Fig 3J) or their average daily intake (r = 0.19, p = 0.30; Fig 3K).
Similar to the putamen, there was a statistically significant interaction between age of experimental onset and treatment on mIPSC amplitude measured in caudate MSNs (F(2,143) = 4.44, p = 0.01; Fig 3L). There was also a main effect of age of onset (F(2,143) = 27.64, p < 0.0001, Fig 3L), suggesting decreased mIPSC amplitude with increased age of experimental onset. Similar to the putamen, we did not observe a main effect of chronic ethanol-drinking (F(1,143) = 0.19, p = 0.66, Fig. 3L), suggesting that ethanol-drinking alone did not contribute to changes in mIPSC amplitude measured in caudate MSNs. We observed a strong negative correlation between mIPSC amplitude and age of ethanol-drinking onset in MSNs recorded in the caudate in ethanol drinking monkeys (r = -0.73, p < 0.0001; Fig 3M), but no such correlation with age at onset in recordings from control monkey MSNs (r = -0.27, p = 0.37; Fig 3M). Caudate mIPSC amplitude positively correlated with ethanol intake (r = 0.43, p = 0.01, Fig 3N). Taken together, both the caudate and putamen show decreased mIPSC amplitude in mature adult ethanol drinking monkeys.
We did not observe a significant interaction between the age of experimental onset and treatment group on mIPSC rise time, decay time, or area in caudate MSNs. Although we did not observe a statistical interaction, we did observe a main effect of age of onset and treatment on mIPSC rise time (main effect age: F(2,143) = 5.34, p = 0.006; main effect of treatment: F(1,143) = 4.92, p = 0.03), decay time (main effect age: F(2,143) = 3.82, p = 0.02; main effect of treatment: F(1,143) = 8.66, p = 0.004) and area (main effect age: F(2,143) = 36.58, p < 0.0001; main effect of treatment: F(1,143) = 9.03, p = 0.003) measured from caudate MSNs.
3.6 No subregion- or age-related differences in glutamatergic transmission in control monkey MSNs
To determine if there were differences in baseline fast glutamatergic transmission between the two dorsal striatal region, miniature excitatory postsynaptic currents (mEPSCs) were recorded from MSNs located within the putamen or caudate (Fig. 1C) of control monkeys that began the experiment as adolescents (cohort 7a, N = 4) and young adults (cohort 7b, N = 4). The mEPSC characteristics of frequency, amplitude, rise time, decay time and area were examined (Table 1). Unlike what was observed for GABAergic transmission, we did not observe any statistical differences in any of the mEPSC characteristics in MSNs recorded from the control monkey putamen versus the caudate (Table 1).
Table 1. mEPSC characteristics were similar in MSNs recorded from the putamen and caudate of control monkeys.
Data shown are mean mEPSC characteristics (± SEM, ncells = 14-15).
| Control Putamen | Control Caudate | p value | |
|---|---|---|---|
| mEPSC Frequency | 0.53 ± 0.11 | 0.43 ± 0.07 | 0.46 |
| mEPSC Amplitude | 35.62 ± 2.64 | 39.75 ± 5.61 | 0.52 |
| mEPSC Rise Time | 5.09 ± 0.67 | 4.70 ± 0.53 | 0.65 |
| mEPSC Decay Time | 7.48 ± 0.69 | 7.62 ± 0.77 | 0.89 |
| mEPSC Area | 310.9 ± 28.09 | 270.1 ± 41.00 | 0.43 |
3.7 Glutamatergic transmission onto MSNs of the control monkey putamen, but not caudate, changes with increasing age
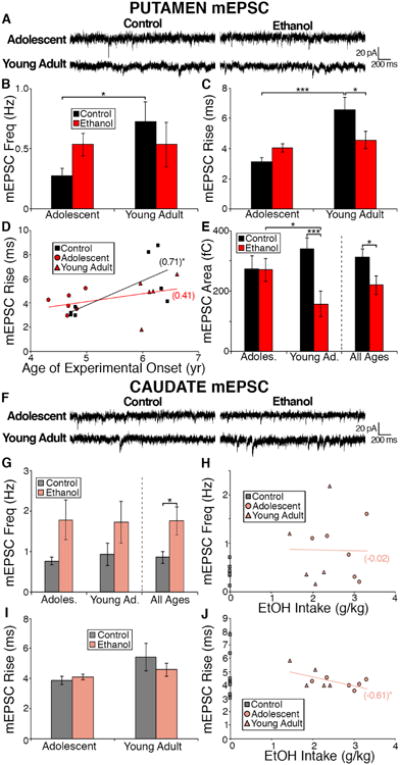
We next analyzed whether there was a developmental alteration in glutamatergic transmission in the two dorsal striatal subregions of the control monkeys. In MSNs located in the control putamen, we observed a significant increase in mEPSC frequency (t12 = 2.24, p = 0.04; Fig 4B) and rise time (t12 = 3.44, p = 0.005; Fig. 4C) with increasing age of onset. We also observed a significant positive correlation between mEPSC rise time and age of onset in the control putamen (r = 0.71, p = 0.048, Fig. 4D). We did not observe any age-related changes in the mEPSC characteristics of amplitude (t12 = 1.62, p = 0.13; data not shown), decay time (t12 = 1.28, p = 0.22; data not shown), or area (t12 = 1.17, p = 0.26; Fig. 4E) in the control putamen.
Figure 4. Chronic ethanol drinking alters glutamatergic transmission onto putamen MSNs through postsynaptic mechanisms and via presynaptic release onto caudate MSNs.
(A) Representative traces of mEPSCs recorded in the putamen of control and ethanol drinking monkeys. (B-C) Average mEPSC frequency (B) and amplitude (C) measured in putamen MSNs is not significantly altered by chronic ethanol exposure (Control: adolescent: ncells = 6; young adult: ncells = 8; Ethanol: adolescent: ncells = 11; young adult: ncells = 9). (D) Average mEPSC rise time is decreased in ethanol drinkers that began drinking as young adults and not as adolescents. (E) Average mEPSC area is decreased in ethanol drinks compared to controls that is driven bby young adult drinkers. (F) Representative traces of mEPSCs recorded in the caudate of control and ethanol drinking monkeys that began drinking as adolescents and young adults. (G) Average mEPSC frequency measured in caudate MSNs is increased in ethanol drinkers compared to controls. (Control: adolescent: ncells = 7; young adult: ncells = 8; Ethanol: adolescent: ncells = 10; young adult: ncells = 10) (H) Average mEPSC frequency measured in the caudate of individual monkeys correlated with daily average ethanol intake. (I) Average mEPSC amplitude measured in caudate MSNs. (J) Average mEPSC amplitude measured in the caudate of individual monkeys correlated with daily average ethanol intake. Data are expressed as mean ± SEM. (H, I) Statistics: Pearsons r value noted in parentheses and significance is denoted by asterisk. significance is denoted by asterisks (* < 0.05, *** < 0.005).
Unlike the putamen, in the caudate MSNs of control monkeys, we did not observe an effect of age on any of the measured mEPSC characteristics (frequency: t13 = 0.54, p = 0.60; amplitude: t13 = 0.72, p = 0.48; rise: t13 = 1.52, p = 0.15; decay: t13 = 0.31, p = 0.76; area: t13 = 1.21, p = 0.25). These data indicate a differential effect of late brain development resulting in an increase in mEPSC frequency and rise time recorded in putamen MSNs. These findings also suggest a putamen-specific increase in glutamate release/synapses as well as the composition of the postsynaptic, presumably AMPA, glutamate receptors, or other factors that affect postsynaptic responsiveness, as the putamen matures.
3.8 Chronic ethanol exposure has inconsistent effects on glutamatergic transmission in the monkey dorsal striatum
The effect of ethanol drinking on glutamatergic transmission onto MSNs of the putamen and caudate was examined in slices from monkeys that began consuming ethanol as adolescents or young adults (adolescent (cohort 7a): control N=4, ethanol N=6; young adult (cohort 7b): control N=4, ethanol N=5). No differences were observed between ethanol-drinkers and controls in mEPSC frequency (t32 = 0.03, p = 0.98; Fig 4B), amplitude (t32 = 0.14, p = 0.89; data not shown), or decay time (t32 = 0.12, p = 0.91; data not shown) measured in putamen MSNs across the age groups. However, rise time of mEPSCs recorded from putamen MSNs, did exhibit a significant main effect of age of onset (F(1,30) = 10.95, p = 0.002) and a significant interaction between age of onset and treatment (F(1,30) = 9.07, p = 0.005), but no significant effect of ethanol drinking (F(1,30) = 0.44, p = 0.51; Fig. 4C). Post hoc analysis revealed a decrease in mEPSC rise time in the putamen of ethanol-drinkers that began consuming ethanol as young adults compared to their age-matched controls (t30 = 2.66, p = 0.01; Fig. 4C). In regards to the mEPSC area measured in putamen MSNs, we observed a significant main effect of ethanol-drinking (F(1,30) = 5.00, p = 0.03; Fig 4E) and a significant interaction between age of onset and treatment (F(1,30) = 4.70, p = 0.004), but no significant effect of age of experimental onset (F(1,30) = 0.34, p = 0.56; Fig. 4E). Post hoc analysis revealed a significant decrease in area between adolescent ethanol-drinkers and young adult ethanol drinkers (t30 = 2.15, p = 0.04; Fig. 4E) and between young adult controls and young adult ethanol drinkers (t30 = 3.18, p = 0.003; Fig. 4E). The decrease in rise time and area of mEPSCs recorded in putamen MSNs of ethanol drinking young adult monkeys suggest an ethanol-induced change in the postsynaptic, presumably, AMPA-type, glutamate receptor.
In the caudate, we observed a main effect of ethanol-drinking (F(1,31) = 4.36, p = 0.045) on mEPSC frequency, but no effect of age of experimental onset (F(1,31) = 0.02, p = 0.90) or an interaction between the two (F(1,31) = 0.07, p = 0.80) (Fig. 4G). There was also no significant correlation between the average daily ethanol intake and average mEPSC frequency in individual ethanol drinkers in caudate MSNs (r = -0.02, p = 0.95; Fig. 4H). In contrast, the mEPSC amplitude recorded in caudate MSNs, was not affected by ethanol drinking (F(1,31) = 2.41, p = 0.13), age of onset (F(1,31) = 1.94, p = 0.17), or the interaction between ethanol and the age of onset (F(1,31) = 0.02, p = 0.89) (data not shown). There was also no correlation observed between mEPSC amplitude and average daily ethanol intake (r = 0.57, p = 0.07; data not shown). The mEPSC characteristics of rise, decay or area recorded from caudate MSNs did not reveal a main effect of ethanol drinking (rise: F(1,31) = 0.34, p = 0.57, Fig 4I; decay: F(1,31) = 0.027, p = 0.87; area: F(1,31) = 0.024, p = 0.88), main effect of age of onset (rise: F(1,31) = 3.91, p = 0.06, Fig 4I; decay: F(1,31) = 0.0001, p = 0.99; area: F(1,31) = 1.66, p = 0.24), or an interaction between the two (rise: F(1,31) = 1.06, p = 0.31, Fig 4I; decay: F(1,31) = 0.22, p = 0.64; area: F(1,31) = 0.43, p = 0.52). However, we did observe a negative correlation between average daily intake of ethanol over the 12 months of open access and the average mEPSC rise time of each individual (r = -0.61, p = 0.047; Fig 4J), suggesting that this characteristic might predict heavy drinking. Therefore, our data suggest that chronic ethanol drinking selectively alters glutamate release or number of glutamatergic synapses in the caudate but has no effect on release in the putamen.
3.9 Dorsal striatal GABAergic transmission is particularly sensitive to the age of ethanol onset as well as pattern of ethanol intake
For GABAergic transmission onto MSNs of the putamen and caudate, we examined the two independent variables of age of experimental onset and pattern of ethanol drinking comparing low/binge to heavy/very heavy drinkers as defined by Baker and colleagues (2014), as predictors of mIPSC characteristics. This examination allows for the determination of characteristics of transmission that are associated not only alcohol itself but with specific patterns of ethanol intake and therefore related to ethanol intake levels that on average were below 2g/kg (low/binge drinkers) or greater than 3g/kg (heavy/very heavy drinkers) (Baker et al., 2014).
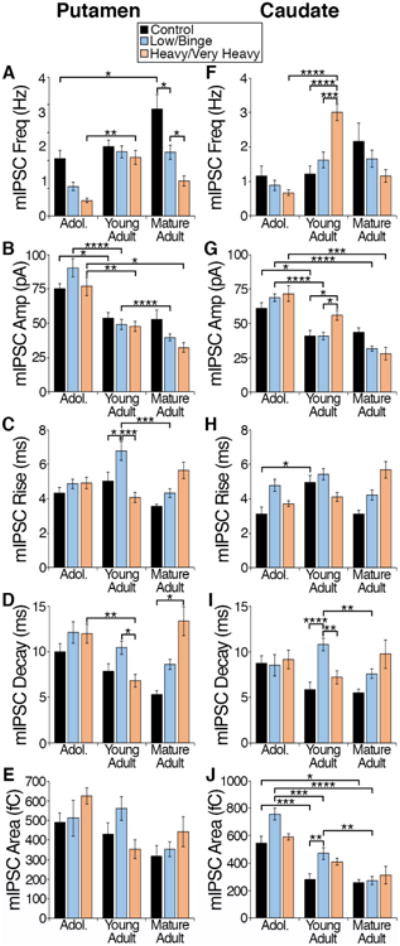
This analysis revealed characteristics of GABAergic transmission that displayed a statistically significant interaction between the age of experimental onset and pattern of intake. In putamen MSNs, there was a significant interaction between the age of experimental onset and the pattern of intake in mIPSC frequency (F(4, 142) = 2.45, p = 0.049; Fig 5A), rise time (F(4, 142) = 2.99, p = 0.02; Fig 5C) and decay time (F(4, 142) = 5.33, p = 0.0005; Fig 5D). The mIPSC characteristics of mIPSC frequency and decay also displayed a main effect of the pattern of ethanol drinking (frequency: F(2, 142) = 13.24, p < 0.0001; Fig 5A; decay: F(2, 142) = 6.62, p = 0.002; Fig 5D) and a main effect of age of experimental onset (frequency: F(2, 142) = 10.74, p < 0.0001; Fig 5A; decay: F(2, 142) = 5.96, p = 0.003; Fig 5D), suggesting alterations in these characteristics with increase age of experimental onset and due to the pattern of ethanol intake.
Figure 5. Examination of the effect of chronic ethanol on GABAergic transmission onto putamen and caudate MSNs due to the age of experimental onset and pattern of intake (no ethanol, low/binge drinkers, and heavy/very heavy drinkers).
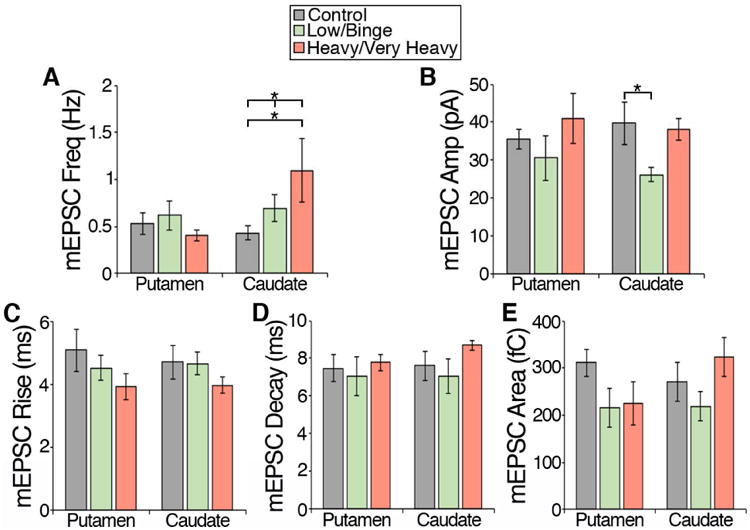
Monkeys were grouped based on their age of experimental onset (adolescent, young adult, and mature adult) and pattern of intake (control, low/binge, and heavy/very heavy). The mIPSC characteristics of frequency, amplitude, rise time, decay time and area were examined in MSNs recorded in the putamen (A-E; control: ncells = 49; low/binge: ncells = 63; heavy/very heavy: ncells = 39) and caudate (F-J; control: ncells = 49; low/binge: ncells = 62; heavy/very heavy: ncells = 38). Data are expressed as mean + SEM. Statistics: Two-way ANOVA, Tukey's multiple comparisons test, significance is denoted by asterisks (* < 0.05, ** <0.01, *** < 0.005, **** <0.001).
GABAergic transmission onto caudate MSNs suggests that there was a statistically significant interaction between the age of experimental onset and ethanol drinking pattern in the mIPSC characteristics of frequency (F(4, 140) = 5.00, p = 0.0008; Fig 5F), amplitude (F(4, 140) = 3.10, p = 0.02; Fig 5G), rise (F(4, 140) = 3.64, p = 0.008; Fig 5H) and decay (F(4, 140) = 3.67, p = 0.007; Fig 5I). A main effect of drinking pattern was found in mIPSC rise (F(2, 140) = 3.69=8, p = 0.03; Fig 5H), decay: F(2, 140) = 5.29, p = 0.006; Fig 5I) and area (F(2, 140) = 5.22, p = 0.007; Fig 5J) of caudate MSNs suggesting that these characteristics are particularly sensitive to the pattern of ethanol intake.
Post hoc analysis using Tukey's multiple comparisons test, revealed that GABAergic transmission is particularly sensitive to the pattern of ethanol intake in monkeys that began the drinking as young adults. Our analysis did not reveal mIPSC characteristics measured in the adolescent putamen or caudate that were sensitive to the pattern of ethanol intake. However, in young adults, the characteristics of transmission that appeared to be uniquely sensitive to heavy/very heavy doses or patterns of chronic ethanol drinking include mIPSC frequency and amplitude measured in the caudate (Fig. 5F-G). Our analysis also revealed characteristics of GABAergic transmission in the young adult onset group that may predict future ethanol consumption or that are sensitive to low/binge levels of chronic ethanol consumption such as mIPSC rise and decay measured in putamen MSNs (Fig. 5C-D) as well as mIPSC decay and area measured in caudate MSNs (Fig. 5 I-J). In monkeys that began drinking as mature adults, mIPSC frequency measured in putamen MSNs is sensitive to the dose/pattern of ethanol intake as mIPSC frequency decreases with increasing dose/pattern of intake (Fig. 5A).
3.10 Glutamatergic transmission in the caudate and not the putamen is sensitive to the pattern of ethanol intake
Due to the collection of mEPSCs in only cohorts 7a and 7b we were unable to examine the effects of age of onset and ethanol-drinking pattern on mEPSC characteristics measured in MSNs of the putamen and caudate. Therefore, we examined the effects of the pattern of chronic ethanol drinking, comparing low/binge to heavy/very heavy drinkers as defined by Baker and colleagues (2014), on glutamatergic transmission onto MSNs located within the putamen and caudate.
In putamen MSNs, mEPSC characteristics of frequency (F(2,31) = 0.61, p = 0.55), amplitude (F(2,31) = 1.02, p = 0.37), rise (F(2,31) = 0.99, p = 0.38), decay (F(2,31) = 0.13, p = 0.88) and area (F(2,31) = 2.17, p = 0.13) were not statistically different between control, low/binge drinkers, and heavy/very heavy drinkers (Fig. 6).
Figure 6. Effect of chronic ethanol on glutamatergic transmission onto putamen and caudate MSNs due to the pattern of intake (no ethanol, low/binge drinkers, and heavy/very heavy drinkers).
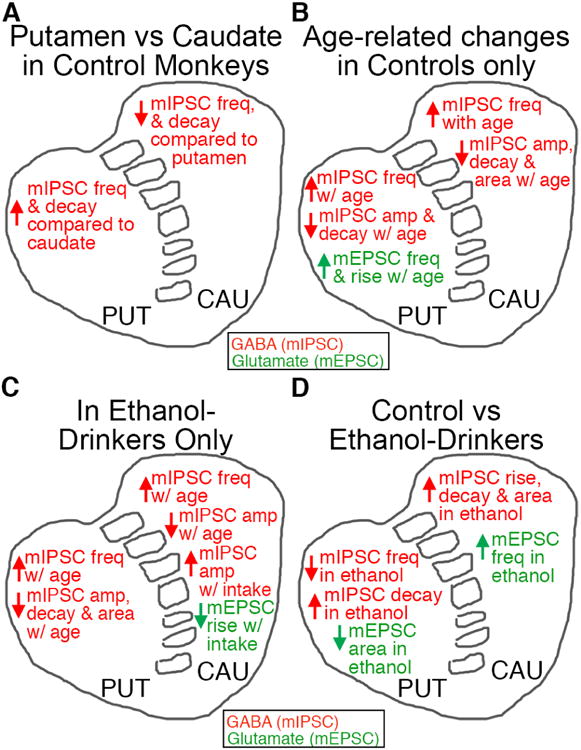
The mEPSC characteristics of frequency (A), amplitude (B), rise time (C), decay time (D) and area (E) were examined in MSNs recorded in the putamen (control: ncells = 14; low/binge: ncells = 12; heavy/very heavy: ncells = 8) and caudate (control: ncells = 15; low/binge: ncells = 11; heavy/very heavy: ncells = 9). Data are expressed as mean + SEM. Statistics: One-way ANOVA, Holm-Sidak's multiple comparisons test, significance is denoted by asterisks (* < 0.05).
In caudate MSNs, the characteristic of mEPSC frequency appeared to be sensitive to dose/pattern of chronic ethanol drinking. Frequency of mEPSCs recorded in caudate MSNs from ethanol naïve monkeys (F(2,32) = 3.50, p = 0.04; post hoc analysis: control vs heavy/very heavy: t32 = 2.65, p = 0.01; Fig. 6A) were significantly decreased compared to heavy/very heavy drinkers but not compared to low/binge drinkers, suggesting either an ethanol dose-dependent increase in mEPSC frequency or that this aspect of transmission is sensitive to higher levels of alcohol intake. Our analysis also suggested that mEPSC amplitude measured in caudate MSNs may predict future ethanol consumption. The mEPSC amplitude measured in caudate MSNs (Fig. 6B) showed a significant difference between control and low/binge groups (t32 = 2.21, p = 0.03), without a significant difference between control and heavy/very heavy drinkers (t32 = 0.25, p = 0.81), or between low/binge and heavy/very heavy drinkers (t32 = 1.72, p = 0.10), suggesting that mEPSC amplitude may be alcohol sensitive or specific to low/binge drinking, or it may suggest that these characteristics predict low/binge alcohol consumption.
4.0 Discussion
In this model of long-term chronic ethanol self-administration in rhesus macaque monkeys, the monkeys that began drinking as late adolescents or young adults drank more on average that older adults. Monkeys that began drinking alcohol as young adults attained the highest BECs and this age group also had a higher proportion of drinkers with categorically higher daily ethanol drinking (binge drinkers, heavy drinkers, and very heavy drinkers) compared to the other age groups. Because the criteria demarcating very heavy, heavy and binge drinkers include the percentage of days of intakes over 4.0 g/kg and 3.0 g/kg, rather than just average daily intake, patterns of intakes that lead to heavy and very heavy drinking days are an integral feature of these categories. Thus, although the average daily intake was similar between adolescent and young adult drinkers, the young adults' pattern of intake leading to a high proportion of days with very heavy drinking (> 4.0 g/kg) also lead to higher BECs. Overall, the results confirm and expand our previous report of age-related risk for heavy drinking in male rhesus monkeys (Helms et al., 2014a). For the macaque monkey, without cultural, legal or economic constraints, there appears to be a biological basis for increased risk of heavy drinking days in individuals that began to drink as late adolescents or young adults compared to fully mature adults. Thus, differences between the mature adult brain and the maturing adolescent and young adult brain may help to explain why many young drinkers consume much larger amounts of alcohol than middle-aged and older adults (Merline et al., 2004; Wang et al., 2010).
Psychological stress is also known to activate the putamen (Wang et al., 2005), and the induction of polydipsia under conditions of intermittent food delivery is known to activate the hypothalamic-pituitary-adrenal axis, a neuroendocrine system that controls the stress response (Mittleman et al., 1988; López-Grancha et al., 2006). In a single cohort of male cynomolgus monkeys under the same induction protocol as in these studies, adrenocorticotropic hormone (ACTH) but not cortisol levels were increased and fell when the induction schedule was terminated (Helms et al., 2013). In the current male rhesus cohorts, the adolescent and young adult monkeys (cohorts 5 and 7a) had higher baseline ACTH and cortisol concentrations than the mature adult monkeys (cohort 4) (adolescent: ACTH 93.9 ± 19.2, cort 28.1 ± 2.2; young adult: ACTH 54.6 ± 4.9, cort 28.4 ± 1.2; mature adult: ACTH 32.8 ± 5.1, cort 16.7 ± 1.5) that decreased to similar levels during SIP induction (adolescent: ACTH 51.4 ± 5.5, cort 21.1 ± 1.8; young adult: ACTH 51.2 ± 7.7, cort 20.3 ± 1.5; mature adult: ACTH 16.8 ± 2.1, cort 14.1 ± 1.9). Thus, it is possible that the younger animals may have a differential response to induction of ethanol mediated by a heightened stress response both at baseline and under schedule induction that contributed to the increased ethanol self-administration.
The current study focused on brain function within two subregions of the dorsal striatum that have been implicated in the transition from hedonic drug use to use that is habitual and inflexible (Everitt and Robbins, 2005; Hogarth et al., 2013a; reviewed in Chen et al., 2011; Hogarth et al., 2013b; Barker and Taylor, 2014; O'Tousa and Grahame, 2014). Both the caudate and the putamen were examined for alterations in both GABAergic IPSCs and glutamatergic EPSCs across age in tissue taken without imposing alcohol abstinence, providing a snapshot of the dorsal striatum on what would have been another day of drinking. The data are summarized in Figure 7. In agreement with previous findings in rodent (Wilcox et al., 2014) and monkey (Cuzon Carlson et al., 2011) that underwent chronic ethanol exposure, there was a consistent depression of mIPSC frequency in putamen MSNs. Similar to our previous study in the mouse, we also confirmed a lack of a consistent effects on mIPSC frequency measured in caudate MSNs from ethanol drinkers (Wilcox et al., 2014). Overall, our findings indicate that the output of the putamen is disinhibited following chronic ethanol drinking, while the caudate output is less affected, or even inhibited (e.g. in the young adult group). This synaptic alteration enhances the putamen/sensoriomotor influence that could foster habitual ethanol seeking and drinking.
Figure 7.
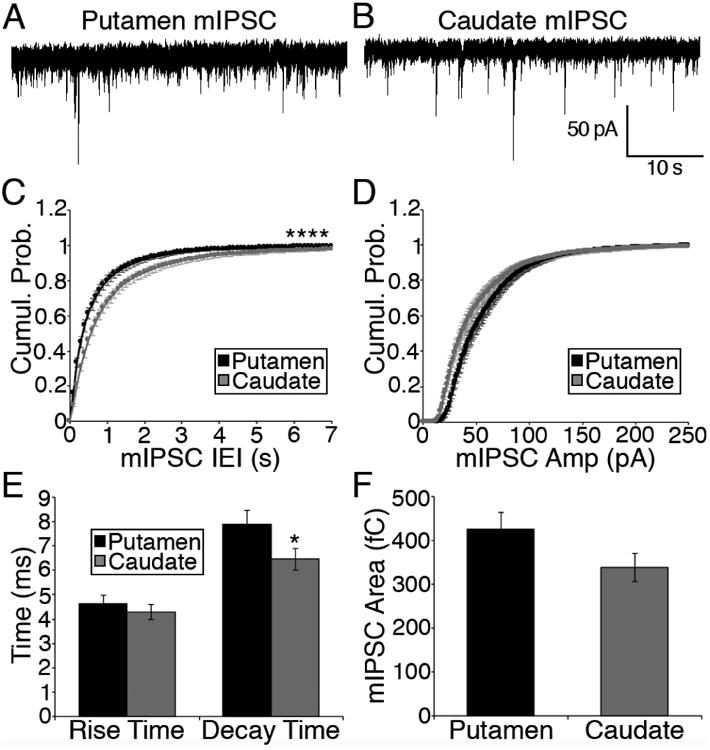
Overview of data obtained highlighting differences observed between the putamen and caudate of control monkeys (A), age-related changes in control monkeys (B), age- or intake-related changes in ethanol monkeys (C), and between control and ethanol-drinkers (D). GABAergic transmission changes are in red. Glutamatergic transmission changes are in green.
We have now extended these data to show that the chronic ethanol drinking-induced decrease in mIPSC frequency in putamen is independent of age of onset. Although we observed average age of onset group differences in mIPSC amplitude measured in ethanol-naïve caudate and putamen MSNs we failed to observe a correlation between the average mIPSC amplitude in individual monkeys and age of experimental onset. However, a strong negative correlation between mIPSC average daily intakes was observed in ethanol drinking monkeys, suggesting that mIPSC amplitude is a risk factor for future heavy ethanol consumption.
It must be noted that the putamen of young adult ethanol drinking monkeys is affected by two factors that decrease synaptic inhibition. The mIPSC frequency is lower in the adolescent and young adult putamen than in mature adults. However, this increase in mIPSC frequency with age may be balanced by age-related changes in postsynaptic responsiveness. This is true regardless of drinking status. The addition of ethanol drinking leads to a further decrease in frequency that is greater in drinkers that begin as adolescents (∼60% decrease) compared to young adult (∼20%) or mature adult drinkers (∼40%). Thus, the combined disinhibition due to youth and ethanol may lead to abnormally strong activation of the putamen, and drive habitual ethanol drinking resulting in high levels of intoxication.
Here we also document baseline differences in GABAergic synaptic transmission but not glutamatergic transmission in control brains between MSNs recorded from the two dorsostriatal subregions (Fig. 7A). MSNs located within the putamen displayed increased mIPSC frequency, amplitude and decay time compared to caudate MSNs. The net effect of the difference in GABA release (suggested by difference in mIPSC frequency) and altered postsynaptic receptor expression/function (suggested by the difference in mIPSC amplitude and decay time) between these subregions may be a greater GABAergic input/transmission in the putamen compared to the caudate. When the data were analyzed based on the age of experimental onset in control monkeys, we observed an age-related increase in GABA release in both subregions that was driven by the changes in mature adults (Fig. 7B). We also obtained evidence of an increase in glutamate release (suggested by an increase in mEPSC frequency) and rise time, only in putamen MSNs (Fig. 7B). However, it is possible that our lack of analysis of glutamatergic transmission in the mature adult might have precluded observation of any age-related difference in the caudate. The observed developmental change in striatal circuitry mirrors human and rodent studies demonstrating that corticostriatal connections develop heavily during adolescence (Alexander et al., 1986; Ghetti and Bunge, 2012), as well as developmental alterations in GABAergic transmission (Fritschy et al., 1994; Santhakumar et al., 2010). Because the age at which drinking and synaptic measurements were made differ for the different age-of-onset groups (i.e. slices were taken at a young final age in younger age of onset subjects), the group differences that we observe reflect the status of the brain at the time when measurements are made, superimposed upon the age at which drinking began.
We also observed an ethanol-induced increase in mEPSC frequency in caudate, but not putamen MSNs (Fig. 7D). This is in contrast to our previous report of increased glutamatergic transmission in MSNs of the putamen of monkeys (Cuzon Carlson et al., 2011). This apparent discrepancy may be due to the differences in the drinking protocol in the current study versus the study performed in 2011 that include the length of drinking history (current study was 1 versus 3 years, respectively), imposed abstinence prior to necropsy (current study was not abstinence versus three 28 day periods of imposed abstinence, respectively) and the drinking state that the brains were obtained (current study was during ethanol access i.e. would have been another day of drinking versus abstinence, respectively). Increased glutamatergic drive in the putamen may develop only after prolonged cycles of intoxication and abstinence.
Rodent studies have provided strong evidence that GABAergic and glutamatergic transmission within the striatum are modulated by ethanol in a cell-type specific manner. For example, chronic ethanol potentiates glutamatergic transmission onto D1-expressing MSNs, resulting in an overall increase in the activity of D1-expressing MSNs (Jeanes et al., 2014; Wang et al., 2015; Cheng et al., 2017; Renteria et al., 2017). A potentiation of glutamatergic transmission onto dMSNs with ethanol exposure was found to be concomitant with an increase in GABAergic transmission onto iMSNs (Cheng et al., 2017), potentially due to an ethanol-induced increase in GABAergic interneuron connectivity onto D2-MSNs (Gittis et al., 2011). Since D1-MSNs and D2-MSNs are thought to have opposing effects on basal ganglia output, understanding the neurotransmission effects of chronic ethanol on the different MSN subtypes may elucidate their roles in AUD. Unfortunately, we do not have the tools to easily separate D1-and D2-MSNs in NHP that are available in rodents. Thus, we cannot resolve this issue in the present study.
The striatum also contains cholinergic interneurons that influence striatal output by modulating excitability, neurotransmitter release and synaptic plasticity, in addition to facilitating acute ethanol effects in the striatum (Adermark et al., 2011; Blomeley et al., 2011). Chronic ethanol exposure alters striatal cholinergic transmission by decreasing cholinergic acetyltransferase (Pelham et al., 1980) and decreasing muscarinic binding sites that negatively correlated with voluntary ethanol intake in rodents (Syvälahti et al., 1988; Wahlström and Nordberg, 1988, 1991). Therefore, it is possible that an ethanol-induced decrease in cholinergic transmission may play a role in decreasing GABAergic transmission onto MSNs through decreased activation of nicotinic receptors on GABAergic interneurons (Koós and Tepper, 2002). Similar to analysis of ethanol effects on MSN subtypes, understanding the role of the cholinergic system in the effects of ethanol intake in the NHP is an important subject for future research.
We exclusively measured miniature events in this study and therefore we cannot distinguish if the observed changes in frequency are due to alterations in synaptic numbers or transmitter release. Future experiments measuring electrically-evoked response may be able to provide additional information about the roles of these two factors (e.g. with analysis of responses to paired-pulse stimulation and analysis of AMPA and NMDA contributions to transmission). However, these studies would have to be performed in such a fashion that electrically-evoked release can be compared across animals and ages independent of developmental and inter-individual differences in striatal morphology.
The glutamatergic and GABAergic data examined in the two onset age groups in which both data sets were collected (adolescent and young adult) may suggest chronic ethanol-induced imbalance between excitation and inhibition. In chronic ethanol drinkers that began drinking alcohol during adolescence, the balance of excitation/inhibition may be biased towards excitation in the putamen due to the observed decrease in GABAergic transmission (observed as a decrease in mIPSC frequency), and suggestions of either no change or a slight increase in glutamatergic transmission. This bias towards excitation in the putamen of adolescent drinkers may be less pronounced in individuals that began consuming alcohol as young adults as at this time point there no observed ethanol-induced impairments in GABAergic transmission, combined with a decrease in measures of postsynaptic glutamatergic function.
In the caudate of drinkers that began consuming as adolescents, there was no ethanol-induced alteration in GABAergic transmission, yet a potential increase in glutamatergic transmission, suggesting a bias towards excitation. In ethanol-drinkers that began drinking as young adults, the balance of excitation/inhibition appears to be biased towards greater inhibition as there is a significant ethanol-induced increase in mIPSC frequency and in postsynaptic characteristics of decay time and area that would suggest an increase in GABAergic transmission measured in caudate MSNs. This coincides with a small increase in glutamate release.
The relationship between striatal synaptic transmission and age of onset or subsequent ethanol intake is not as clear cut as the relationship between age or onset and intake. For example, chronic ethanol increased mIPSC frequency in the caudate of monkeys that began drinking as young adults but not those that began drinking as adolescents or mature adults (Fig. 3I). This apparent resilience to neuroadaptation changes appears to conflict with the notion that adolescence is a time period of vulnerability to mental disease including addiction. One explanation may be due to incomplete development of striatal neurons or circuits, particularly those that are alcohol-sensitive at the adolescent stage. Similarly, a lack of ethanol-induced alterations in the mIPSC frequency measured in the caudate of the mature adult may reflect a ceiling effect as GABAergic synapses are already at their maximum number and efficacy.
Alcohol use in humans is often initiated during adolescence, a stage in which the brain is still developing and when one is more likely to engage in harmful and risky behaviors that include drug seeking and abuse (Grunbaum et al., 2004; Degenhardt et al., 2008; SAMHSA, 2014). In fact, individuals that begin drug use (including ethanol use) during adolescence are more likely to display drug-use disorders within their lifetime (Grant and Dawson, 1998; Merline et al., 2004; SAMHSA, 2014). Our findings indicate that adolescent onset leads to higher intake in a primate species that shares many features with humans. These observations indicate that biological and local environmental variables contribute to the increased drinking risk with adolescent onset and that higher risk may not be attributable solely to unique aspects of human culture. The work on this non-human primate model has now identified several behavioral, hormonal, and neurobiological factors related to excessive ethanol intake (Cuzon Carlson et al., 2011; Asquith et al., 2014; Helms et al., 2012a, 2012b, 2013, 2014b; Beattie et al., 2015; Pleil et al., 2015; Cervera-Juanes et al., 2016; Siciliano et al., 2015, 2016a, 2016b; Turecek et al., 2016), and future studies should provide information as to how these factors predict and shape ethanol drinking behavior and accompanying neurobiological changes.
Highlights.
- Ethanol drinking and neurotransmission data was obtained from 45 rhesus macaques.
- Ethanol drinking depresses GABAergic transmission in the putamen but not caudate.
- Striatal GABAergic currents are more sensitive to drinking pattern in young adults.
- Glutamatergic currents in caudate, but not putamen, are sensitive to drinking pattern.
- Abnormal putamen activation may contribute to problem drinking in younger drinkers.
Acknowledgments
Work supported by Division of Intramural Clinical and Biological Research NIAAA (ZIA AA 000407; PI: David Lovinger); Integrative Neuroscience Initiative on Alcoholism (U01 AA13510; PI: Kathleen Grant); INIA Administrative Core (U24 AA13641; PI: Kathleen Grant); Support for National Primate Research Center (P51 OD 011092; PI: Joseph Robertson).
Footnotes
Financial Disclosures: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adermark L, Clarke B, Soderpalm B, Ericson M. Ethanol-induced modulation of synaptic output from the dorsolateral striatum in rat is regulated by cholinergic interneurons. Neurochem Int. 2011;58:696–699. doi: 10.1016/j.neuint.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Asquith M, Pasala S, Engelmann F, Haberthur K, Meyer C, Park B, et al. Chronic ethanol consumption modulates growth factor release, mucosal cytokine production, and microRNA expression in nonhuman primates. Alcohol Clin Exp Res. 2014;38:980–993. doi: 10.1111/acer.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EJ, Farro J, Gonzales S, Helms C, Grant KA. Chronic alcohol self-administration in monkeys shows long-term quantity/frequency categorical stability. Alcohol Clin Exp Res. 2014;38(11):2835–2843. doi: 10.1111/acer.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EJ, Walter NA, Salo A, Rivas Perea P, Moore S, Gonzales S, et al. Identifying future drinkers: behavioral analysis of monkeys initiating drinking to intoxication is predictive of future drinking classification. Alcohol Clin Exp Res. 2017;41:626–636. doi: 10.1111/acer.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Taylor JR. Habitual alcohol seeking: modeling the transition from casual drinking to addiction. Neurosci Biobehav Rev. 2014;47:281–294. doi: 10.1016/j.neubiorev.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie MC, Maldonado-Devincci AM, Porcu P, O'Buckley TK, Daunais JB, Grant KA, et al. Voluntary ethanol consumption reduces GABAergic neuroactive steroid (3a,5a)3-hydroxypregnan-20-one (3a-5a-THP) in the amygdala of the cynomolgus monkey. Addict Biol. 2015 doi: 10.1111/abd.12326.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeley CP, Cains S, Smith R, Bracci E. Ethanol affects striatal interneurons directly and projection neurons through a reduction in cholinergic tone. Neuropsychopharm. 2011;36:1033–1046. doi: 10.1038/npp.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera-Juanes R, Wilhem LJ, Park B, Lee R, Locke J, Helms C, et al. MAOA expression predicts vulnerability for alcohol use. Mol Psychiatry. 2016;21:472–479. doi: 10.1038/mp.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Cuzon Carlson VC, Wang J, Beck A, Heinz A, Ron D, et al. Striatal involvement in human alcoholism and alcohol consumption, and withdrawal in animal models. Alcohol Clin Exp Res. 2011;35:1739–1748. doi: 10.1111/j.1530-0277.2011.01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Huang CCY, Ma T, Wei X, Wang X, Lu J, Wang J. Distinct synaptic strengthening of the striatal direct and indirect pathways drives alcohol consumption. Biol Psych. 2017;81:918–929. doi: 10.1016/j.biopsych.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Kim KJ, Cho HS, Kim SY, Cho YJ, Hahn SJ, et al. Acute inhibition of corticostriatal synaptic transmission in the rat dorsal striatum by ethanol. Alcohol. 2006;40:95–101. doi: 10.1016/j.alcohol.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Clarke RB, Soderpalm B, Lotfi A, Ericson M, Adermark L. Involvement of inhibitory receptors in modulating dopamine signaling and synaptic activity following acute exposure in striatal subregions. Alcohol Clin Exp Res. 2015;39:2364–2374. doi: 10.1111/acer.12895. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol Psych. 2012;72:389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual responding for alcohol depends upon both AMPA and D2 receptor signaling in the dorsolateral striatum. Front Behav Neurosci. 2014;8:301. doi: 10.3389/fnbeh.2014.00301. doi:00.3389/fnbeh.2014.00301. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, et al. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharm. 2011;36:2513–2528. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, Kraft RA, Davenport AT, Burnett EJ, Maxey VM, Szeliga KT, et al. MRI- guided dissection of the nonhuman primate brain: a case study. Methods. 2010;50:199–204. doi: 10.1016/j.ymeth.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5:e141. doi: 10.1371/journal.pmed.0050141.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Wright T, Camp M, Crowley N, Noronha B, et al. Chronic alcohol alters rewarded behaviors and striatal plasticity. Addict Bio. 2015;20:345–348. doi: 10.1111/adb.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1491. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fanelli RR, Klein JT, Reese RM, Robinson DL. Dorsomedial and dorsolateral striatum exhibit distinct phasic neuronal activity during alcohol self-administration in rats. Eur J Neurosci. 2013;38:2637–2648. doi: 10.1111/ejn.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA- receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. TINS. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Bunge SA. Neural changes underlying the development of episodic memory during middle childhood. Dev Cogn Neurosci. 2012;2:381–395. doi: 10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Hang GB, LaDow ES, Shoenfeld LR, Atallah BV, Finkbeiner S, et al. Rapid target-specific remodeling of fast-spiking inhibitory circuits after loss of dopamine. Neuron. 2011;71:858–868. doi: 10.1016/j.neuron.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Hampton AM, O'Doherty JP. Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cereb Cortex. 2009;19:483–495. doi: 10.1093/cercor/bhn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of drug use and its association with DSM-IV drug abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1998;10:163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szelica KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunbaum JA, Kann L, Kinchen S, Ross J, Hawkins J, Lowry R, et al. Youth risk behavior surveillance – United States 2003. MMWR Surveill Summ. 2004;53:1–96. [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Valzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connection, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Messaoudi I, Jeng S, Freeman WM, Vrana KE, Grant KA. A longitudinal analysis of circulating stress-related proteins and chronic ethanol self-administration in cynomolgus macaques. Alcohol Clin Exp Res. 2012a;36:995–1003. doi: 10.1111/j.1530-0277.2011.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, McClintick MN, Grant KA. Social rank, chronic ethanol self-administration, and diurnal pituitary-adrenal activity in cynomolgus monkeys. Psychopharm. 2012b;224:133–143. doi: 10.1007/s00213-012-2707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Gonzales SW, Green HL, Szeliga KT, Rogers LS, Grant KA. Diurnal pituitary-adrenal activity during schedule-induced polydipsia of water and ethanol in cynomolgus monkeys (Macaca fascicularis) Psychopharm. 2013;228:541–549. doi: 10.1007/s00213-013-3052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Rau A, Shaw J, Stull C, Gonzales SW, Grant KA. The effects of age at the onset of drinking to intoxication and chronic ethanol self-administration in male rhesus macaques. Psychopharm. 2014a;231:1853–1861. doi: 10.1007/s00213-013-3417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Park B, Grant KA. Adrenal steroid hormones and ethanol self-administration in male rhesus macaques. Psychopharm. 2014b;231:3425–3436. doi: 10.1007/s00213-014-3590-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L, Field M, Rose AK. Phasic transition from goal-directed to habitual control over drug-seeking produced by conflicting reinforce expectancy. Addict Biol. 2013a;18:88–97. doi: 10.1111/adb.12009. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Balleine BW, Corbit LH, Killcross S. Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Ann NY Acad Sci. 2013b;1282:12–24. doi: 10.1111/j.1749-6632.2012.06768.x. [DOI] [PubMed] [Google Scholar]
- Jeanes ZM, Buske TR, Morrisett RA. Cell type-specific encoding of ethanol exposure in the nucleus accumbens shell. Neuroscience. 2014;277:184–195. doi: 10.1016/j.neuroscience.2014.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koós T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975;88:195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- López-Grancha M, López-Crespo G, Venero C, Cañadas F, Sánchez-Santed F, Sandi C, et al. Differences in corticosterone level due to inter-food interval length: implications for schedule-induced polydipsia. Horm Behav. 2006;49:166–172. doi: 10.1016/j.yhbeh.2005.05.019. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Merline AC, O'Malley PM, Schulenberg JE, Bachman JG, Johnston LD. Substance use among adults 35 years of age: prevalence, adulthood predictors, and impact of adolescent substance use. Am J Public Health. 2004;94:96–102. doi: 10.2105/ajph.94.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittleman G, Jones GH, Robbins TW. The relationship between schedule-induced polydipsia and pituitary-adrenal activity: pharmacological and behavioral manipulations. Behav Brain Res. 1988;28:315–324. doi: 10.1016/0166-4328(88)90134-9. [DOI] [PubMed] [Google Scholar]
- O'Tousa D, Grahame N. Habit formation: implications for alcoholism research. Alcohol. 2014;48:327–335. doi: 10.1016/j.alcohol.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton MH, Roberts BM, Lovinger DM, Mathur BN. Ethanol disinhibits dorsolateral striatal medium spiny neurons through activation of a presynaptic delta opioid receptor. Neuropsychopharm. 2015;41:1831–1840. doi: 10.1038/npp.2015.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham RW, Marquis JK, Kugelmann K, Munsat TL. Prolonged ethanol consumption produces persistent alterations of cholinergic function in rat brain. Alcohol Clin Exp Res. 1980;4:282–287. doi: 10.1111/j.1530-0277.1980.tb04815.x. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Helms CM, Sobus JR, Daunais JB, Grant KA, Kash TL. Effects of chronic alcohol consumption on neuronal function in the non-human primate BNST. Addict Biol. 2015 doi: 10.111/adb.12289.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Buske TR, Morrisett RA. Long-term subregion-specific encoding of enhanced ethanol intake by D1DR medium spiny neurons of the nucleus accumbens. Addict Biol. 2017 doi: 10.1111/adb.12526.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Jones RT, Mody I. Developmental regulation and neuroprotective effects of striatal tonic GABAA currents. Neuroscience. 2010;167:644–655. doi: 10.1016/j.neuroscience.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Cuzon Carlson VC, Helms CM, Lovinger DM, Grant KA, et al. Voluntary ethanol intake predicts k-opioid receptor supersensitivity and regionally distinct dopaminergic adaptations in macaques. J Neurosci. 2015;35:5959–5968. doi: 10.1523/JNEUROSCI.4820-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Yorgason JT, Lovinger DM, Mateo Y, Jimenez VA, et al. Increased presynaptic regulation of dopamine neurotransmission in the nucleus accumbens core following chronic ethanol self-administration in female macaques. Psychopharm. 2016a;233:1435–1443. doi: 10.1007/s00213-016-4239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Yorgason JT, Mateo Y, Helms CM, Lovinger DM, et al. Chronic ethanol self-administration in macaques shifts dopamine feedback inhibition to predominantly D2 receptors in nucleus accumbens core. Drug Alcohol Depend. 2016b;158:159–163. doi: 10.1016/j.drugalcdep.2015.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Pub No SMA 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- Syvälahti EK, Hietala J, Röyttä M, Grönroos J. Decrease in the number of rat brain dopamine and muscarinic receptors after chronic alcohol intake. Pharmacol Toxicol. 1988;62:210–212. doi: 10.1111/j.1600-0773.1988.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Balleine BW, O'Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci. 2009;29:2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecek J, Han VZ, Cuzon Carlson VC, Grant KA, Welsh JP. Electrical coupling and synchronized subthreshold oscillations in the inferior olive of the rhesus macaque. J Neurosci. 2016;36:6497–6502. doi: 10.1523/JNEUROSCI.4495-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majersky LS, Thomas BW, Shively CA, et al. Induction and maintenance of alcohol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, et al. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Wahlström G, Nordberg A. Relation between voluntary ethanol intake in rats and changes in striatal muscarinic binding sites seen after induction of stable ethanol intake by an intermittent ethanol treatment. Brain Res. 1988;474:189–191. doi: 10.1016/0006-8993(88)90683-x. [DOI] [PubMed] [Google Scholar]
- Wahlström G, Nordberg A. Changes in drinking behavior and cholinergic binding sites induced by intermittent long-term ethanol treatment in the male rat. Alcohol Alcohol. 1991;26:575–584. doi: 10.1093/oxfordjournals.alcalc.a045161. [DOI] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furian PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. PNAS. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, et al. Ethanol induces long-term facilitation of NH2B-MNDA receptor activity in the dorsal striatum implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cheng Y, Wang X, Roltsch Hellard E, Ma T, Gil H, et al. Alcohol elicits functional and structural plasticity selectively in dopamine D1 receptor-expressing neurons of the dorsomedial striatum. J Neurosci. 2015;35:11634–11643. doi: 10.1523/JNEUROSCI.0003-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox MV, Cuzon Carlson VC, Sherazee N, Sprow GM, Bock R, Thiele TE, et al. Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharm. 2014;39:579–594. doi: 10.1038/npp.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Blockade of NMDA receptors in the dorsomedial striatum prevents action-outcome learning in instrumental conditioning. Eur J Neurosci. 2005a;22:505–512. doi: 10.1111/j.1460-9568.2005.04219.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005b;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleing BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res. 2006;166:189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]


