Abstract
Background
Hepatitis C virus (HCV) infection is common among kidney transplant (KTx) recipients. However, the impact of HCV infection on long-term graft and recipient survival after KTx from the large-scale data remains to be determined.
Methods
We used the Organ Procurement and Transplantation Network (OPTN) database to identify all adults undergoing KTx in 2004–2006 in the United States. A propensity score (PS) was created to match each HCV-positive recipient with a HCV-negative control for unbiased comparisons. Survival analysis was conducted to evaluate recipient and death-censored graft survival.
Results
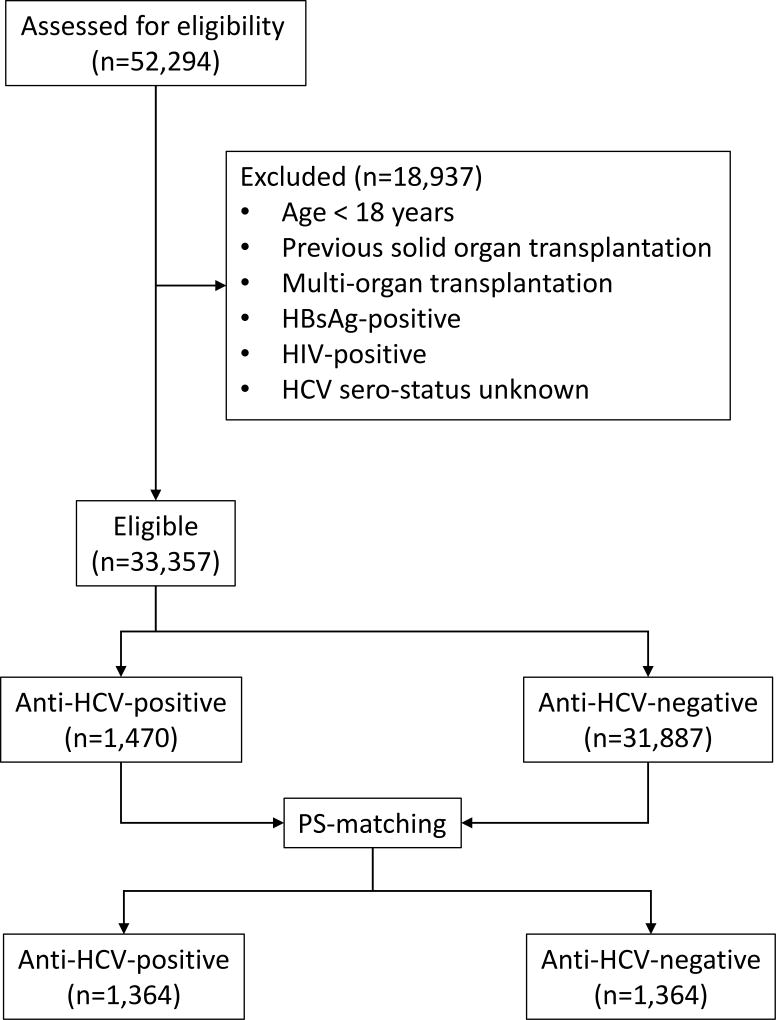
Out of 33,357 adult primary KTx recipients, 1470 (4.4%) were HCV-positive. 1,364 HCV-positive and -negative pairs were selected based on PS-matching. Based on the multivariable regression models, HCV is associated with a higher risk of death (hazard ratio [HR]=1.50, 95% confidence interval [95% CI=1.28–1.75) and graft failure (HR=1.26, 95% CI=1.08–1.47). Infection was a more common cause of death in HCV-positive patients than in HCV-negative recipients (HR=1.64, 95% CI=1.12–2.42). The incidence of death due to liver failure was 0.23% per year among HCV-positive recipients, whereas no HCV-negative recipients died from liver failure. Graft failure due to recurrent disease was higher in HCV-positive than in HCV-negative recipients (HR=2.00; 95% CI=1.06–3.78).
Conclusion
HCV infection is associated with decreased long-term recipient and graft survival. Future studies are needed to examine whether recently available, safe and effective antiviral therapy improves the long-term clinical outcome in these patients.
Introduction
Hepatitis C virus (HCV), known as the most common chronic blood-borne infection in the United States, not only causes liver related morbidity and mortality but also extrahepatic complications, including mixed cryoglobulinemia and various glomerulopathy.1 Further, patients with chronic kidney disease (CKD) receiving maintenance hemodialysis (HD) are at an increased risk for acquisition of HCV. According to a report based on data from 2002, 7.8% of HD patients in the US have HCV infection.2 Many of these patients eventually undergo kidney transplantation (KTx).
HCV infection has an important impact on the outcome in KTx recipients. Calcineurin inhibitors interrupt transcriptional pathways to activate cytokines needed for immune activation, mostly affecting T-helper cells. As activated T-cells are the main cell types in the immune response to HCV, the use of calcineurin inhibitors impair immune control of HCV which is already inadequate in patients with chronic HCV infection.3 In addition, glucocorticoid therapy may independently promote HCV replication.4 The end results of immunosuppression are not only more aggressive progression of liver disease but also recurrence or de novo occurrence of HCV-related renal lesions in KTx recipients.
Until very recently, management of patients with severe CKD and KTx recipients presented a very difficult challenge to nephrologists and hepatologists caring for these patients. This was mainly because interferon-alfa, the mainstay for therapy against HCV for nearly two decades, has immune modulating effects and precipitates graft rejection in KTx recipients. Antiviral therapy attempted prior to KTx to avoid this concern was also fraught with difficulties because tolerability of interferon and ribavirin, the latter a necessary adjunct to interferon. Furthermore, interferon and ribavirin combination had poor effectiveness, which was even worse in patients receiving hemodialysis.
Large scale, epidemiological data to address the long term outcome of KTx recipients with HCV infection are sparse.5,6 Moreover, unbiased comparison between HCV-positive and -negative patients is difficult in population-based studies, because patients with HCV infection may be systematically different from those without HCV in terms of socioeconomic and behavioral risk factors linked to the acquisition of their infection or extrahepatic comorbidities (eg, diabetes) that affect the outcome of KTx. In this work, we use data reported to the Organ Procurement and Transplantation Network (OPTN) to determine the impact of HCV infection on long term graft and recipient survival after KTx, taking precautions to address the inherent differences between patients with and without HCV infection.
Materials and Methods
Study Design and Subjects
This is a retrospective cohort study in which HCV-positive KTx recipients and propensity score-matched HCV-negative recipients are compared to each other with regard to recipient and graft survival. The data were obtained from OPTN in the form of a Standard Transplant Analysis and Research (STAR) file, updated as of November 2014.
All adult (> 18 years), primary KTx recipients between January 2004 and December 2006 were identified. This time frame was chosen to fulfill the goal of analyzing long term post-KTx results with up to 10 years of follow-up prior to the introduction of the modern, “direct acting” anti-HCV therapy. HCV infection of the recipient was defined by a positive anti-HCV result at KTx, as HCV RNA was not recorded in the data. Subjects were excluded if they had any previous solid organ transplantation or received multi-organ transplantation. Patients with concomitant hepatitis B virus infection (defined by positive hepatitis B surface antigen, HBsAg), human immunodeficiency virus (HIV) infection, or unknown serologic status of HCV were also excluded.
Data Elements
From the STAR file, data necessary for the planned analyses were extracted. Recipient factors included age, gender, body mass index (BMI), race/ethnicity, diabetes mellitus, hypertension, length of time on dialysis, peak class I and II panel reactive antibody (PRA, divided into <10% versus ≥10%), and the primary kidney disease etiology. The primary kidney disease etiology was categorized into (a) hypertensive nephropathy, (b) diabetic nephropathy, (c) glomerulonephropathy, (d) polycystic kidney disease, (e) other and (f) unknown. Donor factors consisted of age, gender, diabetes and hypertension, as well as living versus deceased donor. Our primary analytic plan was to include all donors in the main analysis and to conduct a sensitivity analysis restricted to live donor KTx. Transplant factors considered included the number of human leukocyte antigen (HLA) mismatches (categorized into 0–1, 2–4 and 5–6) and cold ischemia time (grouped into 24 hours or less versus longer than 24 hours). Majority of these variables had very few missing data.
Recipient and graft survival times up to 10 years from KTx were determined. Recipient survival was defined as the interval between KTx and recipient death or last follow-up. Graft survival was calculated by the time from KTx to graft failure or last follow-up. Causes of death were divided into cardiovascular disease, infection, malignancy, graft failure, liver failure, and others. Graft failure was categorized into primary graft failure, acute rejection, chronic rejection, recurrent disease, glomerular pathology, BK virus infection, graft thrombosis, infection, noncompliance, and others.
Statistical Analysis
Propensity score (PS) matching was performed between HCV-positive and -negative recipients using a greedy algorithm with 8 to 1-digit match of propensity scores. The covariates for PS matching included all of the recipient, donor and transplant variables shown in Table 1. Differences between HCV-positive and -negative recipients in the entire cohort were tested using the Student’s t-test or the Wilcoxon rank-sum test for continuous variables and the chi-squared test for categorical variables. In the PS-matched cohort, differences were tested using paired t-test or the Wilcoxon signed-rank test for continuous variables and McNemar's test for categorical variables. The standardized difference between HCV-positive and -negative subjects was calculated for continuous variables as and for binary variables as , where Mean1 and Mean2 are the means in HCV-positive and HCV-negative groups, respectively, and SD1 and SD2 the standard deviations in HCV-positive and HCV-negative groups, respectively. A standardized difference < 0.1 was considered negligible, ie, satisfactory matching.
Table 1.
Recipient, donor and transplant characteristics of primary adult kidney transplant recipients in the entire and propensity score matched cohorts
| Entire cohort | Propensity score matched cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| HCV+ (N=1,470) |
HCV− (N=31,887) |
p | HCV+ (N=1,364) |
HCV− (N=1,364) |
Standardized difference |
p | ||
| Recipient factor | ||||||||
| Age (year)a | 51.2 ± 10.1 | 49.9 ± 13.7 | 0.07 | 51.2 ± 10.0 | 50.6 ± 13.5 | 0.043 | 0.25 | |
| Male | 73.4% | 60.0% | <0.01 | 73.5% | 72.9% | 0.013 | 0.69 | |
| BMI (kg/m2)a | 26.8 ± 5.1 | 27.6 ± 5.4 | <0.01 | 26.8 ± 5.1 | 26.6 ± 4.9 | 0.029 | 0.43 | |
| African American | 51.1% | 23.4% | <0.01 | 50.5% | 50.4% | 0.003 | 0.88 | |
| Diabetes mellitus | 35.2% | 32.0% | 0.01 | 34.8% | 33.8% | 0.02 | 0.61 | |
| Hypertension | 88.1% | 85.3% | <0.01 | 88.6% | 88.0% | 0.016 | 0.68 | |
| Time on HD (years)b | 2.7 (1.1–5.0) | 1.8 (0.4–3.8) | <0.01 | 2.8 (1.2–5.0) | 3.0 (1.2–5.1) | 0.012 | 0.63 | |
| Peak PRA ≥ 10% | 21.5% | 22.0% | 0.66 | 20.6% | 22.3% | 0.043 | 0.39 | |
| Primary kidney disease | ||||||||
| Hypertensive | 38.7% | 24.6% | <0.01 | 38.9% | 40.6% | 0.034 | 0.32 | |
| Diabetic | 26.9% | 26.3% | 0.61 | 26.6% | 24.7% | 0.044 | 0.27 | |
| Glomerulonephropathy | 17.8% | 24.9% | <0.01 | 18.3% | 19.0% | 0.017 | 0.65 | |
| Polycystic kidney | 5.0% | 10.5% | <0.01 | 5.4% | 4.7% | 0.03 | 0.42 | |
| Others | 9.0% | 10.6% | 0.06 | 9.0% | 9.2% | 0.005 | 0.89 | |
| Unknown | 1.8% | 2.3% | 0.21 | 1.8% | 1.8% | 0.006 | 0.89 | |
| Donor factor | ||||||||
| Age (year)a | 40.0 ± 13.9 | 39.4 ± 14.8 | 0.14 | 40.1 ± 13.9 | 40.7 ± 15.6 | 0.041 | 0.28 | |
| Male | 54.5% | 51.3% | 0.02 | 54.5% | 54.6% | 0.001 | 0.97 | |
| Diabetes mellitus | 4.0% | 3.6% | 0.49 | 3.8% | 5.5% | 0.08 | 0.05 | |
| Hypertension | 23.4% | 16.7% | <0.01 | 23.4% | 22.7% | 0.018 | 0.73 | |
| Deceased donor | 75.2% | 57.2% | <0.01 | 75.4% | 74.6% | 0.02 | 0.52 | |
| Transplant factor | ||||||||
| No. of HLA mismatches | ||||||||
| 0~1 | 9.4% | 14.7% | <0.01 | 9.6% | 10.9% | 0.041 | 0.27 | |
| 2~4 | 41.8% | 47.6% | <0.01 | 42.2% | 42.4% | 0.006 | 0.88 | |
| 5~6 | 48.4% | 37.4% | <0.01 | 48.2% | 46.7% | 0.031 | 0.41 | |
| Cold ischemia time | ||||||||
| ≤24 hours | 69.9% | 69.1% | 0.51 | 70.4% | 69.0% | 0.03 | 0.44 | |
| >24 hours | 16.3% | 11.7% | <0.01 | 16.2% | 15.3% | 0.024 | 0.53 | |
BMI, body mass index; PRA, panel reactive antibody; HLA, human leukocyte antigen;
Variables used in the propensity score included recipient age and gender, BMI, ethnicity, diabetes mellitus, hypertension, length of time on dialysis, primary kidney diagnosis, donor age and gender, donor type, the number of HLA mismatches and cold ischemia time.
In the propensity score matching procedure, 30,721 of 33,357 patients had no missing data for all input variables.
Number of subjects with missing data included 311 for BMI, 437 for diabetes, 1,159 for hypertension, 807 for time on dialysis, 5,513 for peak PRA, and 284 for primary kidney diagnosis among recipient factors; 2,179 for diabetes and, 2,262 for hypertension among donor factors; and 111 for the number of HLA mismatches among transplant factors.
Mean±standard deviation
Median (first quartile – third quatile)
The incidence rate for death or graft failure was calculated for HCV-positive and –negative patients using the Kaplan-Meier method. In calculating the graft failure rate, deaths unrelated to graft failure were censored. In the PS-matched cohort, recipient and graft survival was compared between HCV-positive and –negative patients using the Cox regression analysis. Non-proportionality in some of the variables (eg, recipient age, recipient length of time on dialysis and donor type for recipient survival model and donor gender for graft survival) was corrected by considering time-dependent covariates. Recipient and graft survival was compared using the test proposed by Klein and Moeschberger.7 Finally, we performed sensitivity analyses by repeating the multivariable Cox regression analysis for recipient and graft survival up to 10 years, only including living donor kidney transplant recipients.
Results
Patients
From the OPTN database, 52,294 KTx recipients were identified for 2004–2006, of whom 33,357 primary adult recipients met the eligibility criteria. Among them, 1,470 patients (4.4%) were HCV-positive (Figure 1). The left panel of Table 1 demonstrates that without propensity score matching, HCV-positive recipients were characterized by higher proportions of men and African Americans, higher prevalence of diabetes and hypertension despite lower BMI, and longer time on dialysis, compared to recipients who were HCV-negative. Hypertensive nephropathy, diabetic nephropathy and glomerulonephropathy were the most common causes of primary kidney disease in both groups. Donors of HCV-positive recipients were more likely to be male, hypertensive, and deceased than those for HCV-negative patients. HCV-positive recipients had a higher proportion of 5 or more HLA mismatches.
Figure 1.
In the right panel of Table 1, PS-based matching produced 1,364 pairs of patients (Figure 1). The matching virtually eliminated the differences in recipient, donor, and transplant characteristics between HCV-positive and –negative recipients with standardized differences < 0.1 with nonsignificant p values (Table 1, right panel). The score included a number of recipient, donor and transplant-related variables (Table 1, footnote). In the matched cohort, 383 (14%) of the donors were anti-HCV-positive, of whom 375 were implanted in HCV-positive recipients.
Incidence and Causes of Death and Graft Failure
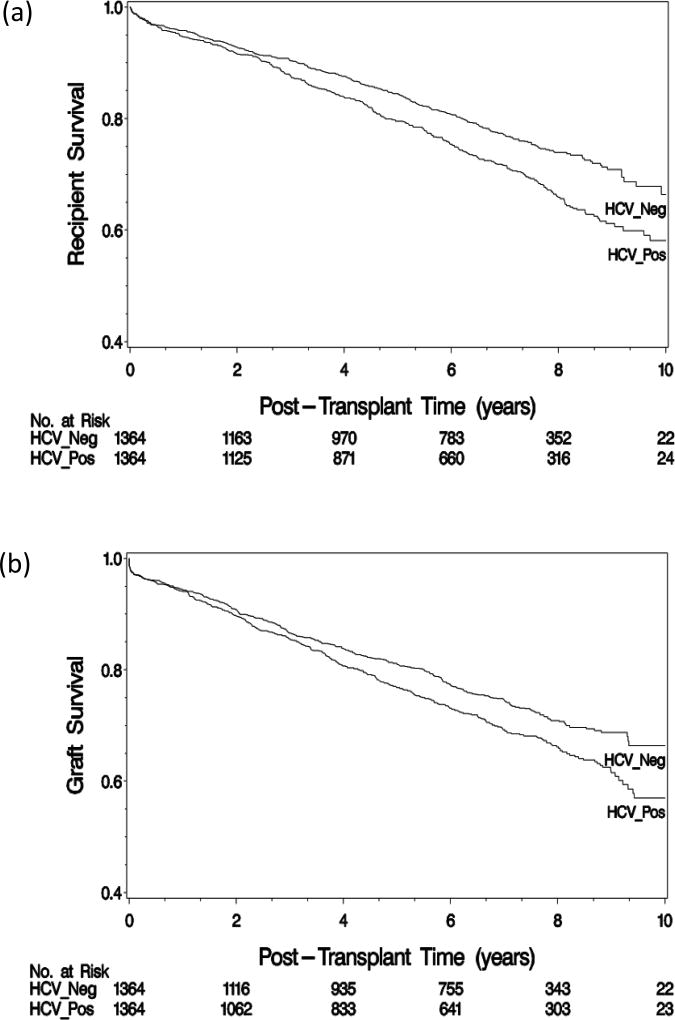
In the PS-matched cohort, the median follow-up was 6.3 (interquartile range, 3.0–8.0) years. There were 666 deaths, 367 among HCV-positive and 299 among HCV–negative recipients. The 1-, 5-, and 10-year recipient survival was 94.7%, 79.5%, and 58.2% in HCV-positive recipients and 95.8%, 84.4%, and 66.3% in HCV-negative recipients, respectively (Figure 2A). Patient survival was significantly lower in HCV-positive recipients than HCV-negative recipients (p<0.01). Cardiovascular disease, infection, and malignancy were the most common causes of death (Table 2, Tables S1 and S2, SDC). Mortality due to infection was significantly higher in HCV-positive than in HCV-negative recipients (0.85% per year versus 0.50% per year; hazard ratio [HR]=1.64; 95% confidence interval [CI], 1.12–2.42). Death due to liver failure occurred at 0.23% per year among HCV-positive recipients, whereas there was no death from liver failure among HCV-negative recipients.
Figure 2.
Table 2.
Cause-specific incidence of death and graft failure and hazards ratios for HCV positivity in the PS-matched cohort
| HCV+ | HCV− | Comparisonb | ||||
|---|---|---|---|---|---|---|
| No. of events |
Incidence ratea |
No. of events |
Incidence ratea |
HR (95% CI) | P | |
| Recipient death | ||||||
| Cardiovascular disease | 66 | 0.9 | 51 | 0.64 | 1.40 (0.97–2.02) | 0.07 |
| Infection | 62 | 0.85 | 40 | 0.5 | 1.64 (1.12–2.42) | 0.01 |
| Malignancy | 53 | 0.72 | 38 | 0.48 | 1.54 (1.01–2.36) | 0.05 |
| Graft failure | 5 | 0.07 | 5 | 0.06 | 1.10 (0.31–3.84) | 0.88 |
| Liver failure | 17 | 0.23 | ||||
| Others | 60 | 0.82 | 66 | 0.83 | 0.99 (0.70–1.39) | 0.95 |
| Unknown | 104 | 1.42 | 99 | 1.24 | 1.17 (0.89–1.53) | 0.25 |
| Graft failure | ||||||
| Chronic rejection | 143 | 2.03 | 127 | 1.65 | 1.25 (0.99–1.59) | 0.06 |
| Acute rejection | 55 | 0.78 | 51 | 0.66 | 1.17 (0.80–1.70) | 0.42 |
| Primary failure | 31 | 0.44 | 37 | 0.48 | 0.88 (0.54–1.41) | 0.59 |
| Recurrent disease | 27 | 0.38 | 15 | 0.19 | 2.00 (1.06–3.78) | 0.03 |
| BK virus | 14 | 0.2 | 14 | 0.18 | 1.08 (0.51–2.25) | 0.85 |
| Infection | 18 | 0.26 | 11 | 0.14 | 1.77 (0.84–3.74) | 0.13 |
| Graft thrombosis | 14 | 0.2 | 21 | 0.27 | 0.68 (0.35–1.31) | 0.24 |
| Non-compliance | 17 | 0.24 | 13 | 0.17 | 1.40 (0.68–2.88) | 0.37 |
| Glomerular pathologyb | 9 | 0.13 | 8 | 0.1 | 1.24 (0.47–3.24) | 0.66 |
| Others | 25 | 0.35 | 20 | 0.26 | 1.33 (0.73–2.42) | 0.35 |
| Unknown | 23 | 0.33 | 16 | 0.21 | 1.60 (0.84–3.04) | 0.15 |
rate per 100 patient per year.
de novo glomerular pathology which is different from the primary kidney disease.
During the follow-up, 376 HCV-positive and 333 HCV-negative recipients experienced graft failure. The 1-, 5-, and 10-year graft survival rate was 94.4%, 76.8%, and 57.0% in HCV-positive recipients and 94.0%, 81.1%, and 66.4% in HCV-negative recipients, respectively (Figure 2B). The difference between the two groups did not reach statistical significance (p=0.15). Chronic rejection, acute rejection, and primary failure were most frequently reported causes of graft failure in both HCV-positive and negative groups (Table 2). The incidence of graft failure due to recurrent disease was significantly higher in HCV-positive than in HCV-negative recipients (0.38% vs. 0.19% per year, respectively; HR=2.00; 95% CI, 1.06–3.78).
Predictors of Death and Graft Failure
In the multivariable Cox proportional hazards regression analysis summarized in Table 3, recipient death was associated with recipient factors including age (HR=1.04; 95% CI, 1.02–1.05) and diabetes (HR=1.34; 95% CI, 1.04–1.73), donor factors including age (HR=1.01; 95% CI, 1.00–1.01) and deceased status (HR=1.64; 95% CI, 1.09–2.44), and a transplant factor of 5 or 6 HLA mismatches (HR= 1.50; 95% CI, 1.11–2.02). After adjusting for these variables, HCV was associated with a significant increase in the risk of death (HR=1.50; 95% CI, 1.28–1.75).
Table 3.
Cox propotional hazards regression analyses for recipient death and graft failure in the PS-matched cohort
| Recipient death | Graft failure | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Recipient factor | ||||
| Anti-HCV | 1.50 (1.28–1.75) | <0.01 | 1.26 (1.08–1.47) | <0.01 |
| Age (year) | 1.04 (1.02–1.05) | <0.01 | 0.98 (0.98–0.99) | <0.01 |
| Male | 1.10 (0.91–1.32) | 0.33 | 1.05 (0.88–1.25) | 0.62 |
| BMI (kg/m2) | 1.00 (0.98–1.02) | 0.96 | 1.00 (0.99–1.02) | 0.68 |
| African American | 0.93 (0.78–1.11) | 0.43 | 1.56 (1.31–1.85) | <0.01 |
| Diabetes mellitus | 1.34 (1.04–1.73) | 0.02 | 0.97 (0.73–1.29) | 0.83 |
| Hypertension | 1.13 (0.87–1.47) | 0.35 | 1.00 (0.79–1.27) | 0.99 |
| Length of time on dialysis (year) | 1.08 (1.05–1.12) | <0.01 | 0.99 (0.97–1.02) | 0.62 |
| Primary kidney disease | ||||
| Hypertensive nephropathy | 1.11 (0.54–2.28) | 0.77 | 0.86 (0.49–1.53) | 0.62 |
| Diabetic nephropathy | 1.35 (0.64–2.82) | 0.43 | 0.88 (0.48–1.63) | 0.69 |
| Glomerulonephritis/nephropathy | 0.88 (0.42–1.85) | 0.74 | 0.91 (0.52–1.62) | 0.76 |
| Polycystic kidney disease | 0.58 (0.25–1.34) | 0.20 | 0.60 (0.30–1.21) | 0.15 |
| Others | 1.16 (0.54–2.50) | 0.70 | 0.97 (0.52–1.79) | 0.91 |
| Donor factor | ||||
| Age (year) | 1.01 (1.00–1.01) | <0.01 | 1.02 (1.01–1.02) | <0.01 |
| Male | 1.01 (0.86–1.19) | 0.90 | 0.78 (0.60–1.01) | 0.06 |
| Deceased donor | 1.64 (1.09–2.44) | 0.02 | 1.78 (1.43–2.23) | <0.01 |
| Transplant factor | ||||
| No. of HLA mismatches | ||||
| 0–1 | ref | ref | ||
| 2~4 | 1.22 (0.90–1.65) | 0.21 | 1.87 (1.31–2.65) | <0.01 |
| 5~6 | 1.50 (1.11–2.02) | <0.01 | 1.98 (1.40–2.82) | <0.01 |
| Cold ischemic time | ||||
| ≤24 hours | ref | ref | ||
| >24 hours | 0.99 (0.80–1.22) | 0.90 | 0.88 (0.71–1.08) | 0.22 |
In the multivariable Cox analysis predicting graft failure, HCV was found to be significantly associated with graft failure (HR=1.26; 95% CI=1.08–1.47). Other statistically significant factors in the model included African American race (HR=1.56; 95% CI, 1.31–1.85), donor age (HR=1.02; 95% CI, 1.01–1.02), deceased donor (HR=1.78; 95% CI, 1.43–2.23), 2 to 4 HLA mismatches (HR=1.87; 95% CI, 1.31–2.65), and 5 or 6 HLA mismatches (HR=1.98; 95% CI, 1.40–2.82). In contrast, recipient age was inversely associated with risk of graft failure (HR=0.98; 95% CI, 0.98–0.99).
Sensitivity Analysis
When the analysis was repeated restricting the study to living donor KTx, among 14,025 primary living donor adult KTx recipients, 364 were HCV-positive (Figure S1, SDC). PS-matching resulted in 335 pairs of HCV-positive and -negative recipients (Table S3, SDC). Results of the recipient and graft survival analyses mirrored those of the overall results. For example, in the multivariable proportional hazards regression analyses, HCV-positivity was independently and significantly associated with recipient death (HR=1.90; 95% CI, 1.33–2.74) and graft failure (HR=1.54; 95% CI, 1.06–2.24) (Table S4, SDC).
We examined whether the inferior outcome of HCV-positive recipients was attributable to the use of HCV-positive donor kidneys. In the PS-matched pairs, 375 (27.5%) HCV-positive recipients and 8 (0.6%) HCV-negative recipients were given a HCV-positive organ. In unadjusted analysis, patient and graft survival was indeed inferior in recipients with HCV+ donors (Figure S2A and S2B, SDC). Then we repeated the multivariable analysis excluding pairs at least one of whom received a HCV-positive organ from a HCV+ donor. The hazard ratios remained virtually unchanged (Table S5, SDC), suggesting that recipient HCV serostatus remains an independent predictor of their outcome.
Finally, we analyzed whether the difference in liver failure mortality between HCV-positive and –negative patients may have been exaggerated because hepatitis B patients were excluded from the analysis. In the overall cohort, 560 patients (1.7%) were HBsAg-positive, including 50 who were HCV-positive and 510 HCV-negative. When HBsAg-positive patients were added back into the sample, there was only one additional death attributed to liver failure which occurred in a HCV-patients. The incidence of liver failure deaths and hazard ratios associated with HCV did not change (data not shown).
Discussion
In this study, HCV infection was common among KTx recipients: 4.4% of KTx recipients were HCV-positive, highlighting the burden of HCV in KTx patients. More importantly, when HCV-positive and –negative KTx recipients were carefully matched, HCV was associated with significantly lower long-term recipient survival, which was attributable to infection and liver failure. Similarly, long-term death-censored graft survival was also lower in HCV-positive KTx recipients, in part as a result of higher incidence of recurrent disease. These results were similar between deceased donor and live donor recipients.
While it makes intuitive sense that HCV infection would worsen the outcome of KTx, published data have been inconsistent. Earlier studies were inconclusive because of their small sample size and short-term follow-up.5,6,8,9 More recent papers began to incorporate better statistical power.10–22 Most, but not all, showed that HCV infection posed a negative impact on recipient survival,5,11,13–15,17,19–22 in part because very few directly addressed the substantial differences in demographic and clinical characteristics between HCV-positive and –negative patients. Given the known deleterious effects of immunosuppression on the progression of HCV liver disease and lack of safe and effective antiviral therapy until very lately, those recipients, if followed long enough, are expected to experience higher incidence of liver-related morbidity and mortality.
Data are less certain when the question is about the impact of HCV on survival of the renal graft, apart from the decrement in recipient’s outcome related to liver disease. Majority of studies assessing graft survival considered the recipient’s death as graft loss as well, making the interpretation of the data tricky.10–12,14,15,17–22 There have been three studies which considered death-censored graft survival and none of them showed significant impact of HCV on graft survival.16,18,19 None of these studies incorporated statistical matching of HCV-positive and -negative patients to account for the substantial differences between those patients. Finally, Kucirka investigated the impact of donor HCV-positivity.23 They found that the utilization of HCV-positive kidneys was associated with an increase in adverse liver outcomes, while there was a significant reduction in the waiting time. As discussed below, given the recent availability of effective antiviral therapy, these data will need to be updated in the near future.
Based on propensity score-matched comparisons, we demonstrated that survival of HCV-positive recipients is decreased significantly (multivariable hazards ratio=1.5), which is likely driven by the progression of liver disease. Deaths from liver failure were only seen in HCV-positive recipients – which is also consistent with the fact that the survival curves in Figure 2 begin to separate after several years after KTx. Further, we suspect that the significantly increased mortality from infection and malignancy may also in part be explained by advanced liver disease, where sepsis and multi-organ failure and/or hepatocellular carcinoma are important causes of death. It is also noteworthy that cardiovascular mortality was increased, albeit marginally significantly, in HCV-positive KTx recipients. HCV infection may cause systemic inflammation which has been associated with accelerated atherosclerosis and cardiovascular morbidity and mortality.24
With regard to graft survival, while the difference between HCV-positive and -negative patients did not reach statistical significance in the unadjusted analysis, the multivariable Cox model incorporating other relevant predictors showed that HCV-positivity was associated with significantly increased risk of graft failure (multivariable HR=1.26). Table 2 strongly indicates that an important driver of this difference is recurrence of the original renal disease. There are several renal diseases associated with HCV infection, including mixed cryoglobulinemia, membranoproliferative glomerulonephritis (MPGN), membranous nephropathy (MN) and polyarteritis nodosa. Some of these lesions such as MPGN and MN are known to recur in the allograft.25,26 In addition, certain de novo lesions such as renal thrombotic microangiopathy and transplant glomerulopathy are more frequently reported in HCV-positive recipients.27,28
Safe and highly effective anti-HCV agents are now available for patients with chronic kidney disease, including grazoprevir/elbasvir and ombitasvir/paritaprevir/ritonavir/dasabuvir. With these agents, there has been a significant change in the treatment of HCV-positive patients with chronic kidney disease. While antiviral regimens are limited in patients with severely decreased glomerular filtration rate, essentially all of them including sofosbuvir may be used in KTx recipients with satisfactory renal function with very high rates of cure.29–32 It is likely that data will accumulate soon which will demonstrate HCV-positive KTx recipients’ survival has improved. It is less clear, however, to what extent antiviral therapy will also improve graft survival. While clearance of HCV may halt the generation of immune complexes, the natural course of post-KTx glomerulopathies remains uncertain. These data are urgently needed because it is a common policy to withhold anti-HCV therapy in patients awaiting KTx in the hopes of shortening the waiting time with utilization of HCV-positive organs.
We recognize some of the study’s limitations. First, HCV-positivity was defined based on anti-HCV results, not true viremia (ie, HCV RNA-positivity). With modern anti-HCV assays, approximately 30% of individuals with positive anti-HCV antibodies do not have detectable HCV RNA in the serum and are considered to have spontaneously cleared the virus. In patients on hemodialysis, however, up to 20% with virema may lack anti-HCV antibodies.33 Thus, in this study, it is likely that some proportion of patients were misclassified with regard to their HCV infection status. It is important to point out that misclassification of these patients would have tended to negate the differences between HCV-positive and -negative patients, making our observation more conservative. Second, as with any retrospective studies, this analysis was constrained by availability, completeness and quality of data. For example, the cause of death reported to OPTN was missing in approximately 30% of the decedents. Moreover, cause of death may be difficult to determine and inaccurately reported. We note, however, that these difficulties should affect both HCV-positive and –negative patient equally. Other variables such as antiviral therapy, detailed assessment of liver pathology (eg, liver biochemical data, cirrhosis status, and hepatocellular carcinoma) and socioeconomic status were unavailable. Given the various differences between HCV-positive and –negative patients, there may have been undetected confounders. While we attempted to minimize this effect by instituting propensity score matching, some of the potentially important data (eg, socioeconomic status) were not available in the database.
In conclusion, after careful matching of patients, HCV infection was associated with decreased long-term recipient and graft survival, largely as a result of HCV’s impact on the liver and the kidney graft, respectively. When highly effective and safe therapy against HCV is widely available, prospective, large scale studies are needed define its impact on recipient and graft survival. Until such data become available, our data suggest that KTx recipients with HCV infection must be urgently treated, which may prevent progression of liver disease and potentially avert re-establishment of HCV-related glomerular disease.
Supplementary Material
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health (DK-34238 and DK-92336) and from Inje University (No. 20140103).
Abbreviations
- BMI
Body Mass Index
- CI
Confidence Interval
- CKD
chronic kidney disease
- HBsAg
Hepatitis B Surface Antigen
- HCV
Hepatitis C Virus
- HD
Hemodialysis
- HIV
Human Immunodeficiency Virus
- HLA
Human Leukocyte Antigen
- HR
Hazard Ratio
- KTx
Kidney Transplant
- MN
Membranous Nephropathy
- MPGN
Membranoproliferative Glomerulonephritis
- OPTN
Organ Procurement and Transplantation Network
- PRA
Panel Reactive Antibody
- PS
Propensity Score
- STAR
Standard Transplant Analysis And Research
Footnotes
Authorship
Nae-Yun Heo: acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis
Ajitha Mannalithara: acquisition of data; analysis and interpretation of data
Donghee Kim: critical revision of the manuscript for important intellectual content
Prowpanga Udompap: critical revision and reformatting the manuscript
Jane C. Tan: critical revision of the manuscript for important intellectual content
W. Ray Kim: study concept and design; obtained funding; administrative, technical, or material support; study supervision
Disclosure
Dr. Kim has received honoraria for advisory board participation from Gilead Sciences, Abbvie and Merck, all manufacturers of HCV therapeutic agents.
References
- 1.Appel GB, Radhakrishnan J, D'Agati V. Secondary glomerular disease. In: Skorecki K, Chertow GM, Marsden PA, Taal MW, Yu ASL, Wasser WG, editors. Brenner and Rector's The Kidney. 10. Philadelphia, PA: Elsevier, Inc.; 2016. pp. 1092–1160. [Google Scholar]
- 2.Burra P, Rodriguez-Castro KI, Marchini F, et al. Hepatitis C virus infection in end-stage renal disease and kidney transplantation. Transpl Int. 2014;27(9):877–891. doi: 10.1111/tri.12360. [DOI] [PubMed] [Google Scholar]
- 3.Park S-H, Rehermann B. Immune Responses to HCV and Other Hepatitis Viruses. Immunity. 2014;40(1):13–24. doi: 10.1016/j.immuni.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roche B, Samuel D. Risk factors for hepatitis C recurrence after liver transplantation. J Viral Hepat. 2007;14(Suppl 1):89–96. doi: 10.1111/j.1365-2893.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- 5.Batty DS, Jr, Swanson SJ, Kirk AD, Ko CW, Agodoa LY, Abbott KC. Hepatitis C virus seropositivity at the time of renal transplantation in the United States: associated factors and patient survival. Am J Transplant. 2001;1(2):179–184. [PubMed] [Google Scholar]
- 6.Meier-Kriesche HU, Ojo AO, Hanson JA, Kaplan B. Hepatitis C antibody status and outcomes in renal transplant recipients. Transplantation. 2001;72(2):241–244. doi: 10.1097/00007890-200107270-00013. [DOI] [PubMed] [Google Scholar]
- 7.Klein JP, Moeschberger ML. Survival analysis. Techniques for Censored and Truncated Data. New York: Springer; 1997. [Google Scholar]
- 8.Pol S, Legendre C, Saltiel C, et al. Hepatitis C virus in kidney recipients. Epidemiology and impact on renal transplantation. J Hepatol. 1992;15(1–2):202–206. doi: 10.1016/0168-8278(92)90036-o. [DOI] [PubMed] [Google Scholar]
- 9.Stempel CA, Lake J, Kuo G, Vincenti F. Hepatitis C--its prevalence in end-stage renal failure patients and clinical course after kidney transplantation. Transplantation. 1993;55(2):273–276. doi: 10.1097/00007890-199302000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int. 1994;45(1):238–244. doi: 10.1038/ki.1994.29. [DOI] [PubMed] [Google Scholar]
- 11.Periera BJ, Wright TL, Schmid CH, Levey AS. The impact of pretransplantation hepatitis C infection on the outcome of renal transplantation. Transplantation. 1995;60(8):799–805. [PubMed] [Google Scholar]
- 12.Orloff SL, Stempel CA, Wright TL, et al. Long-term outcome in kidney transplant patients with hepatitis C (HCV) infection. Clin Transplant. 1995;9(2):119–124. [PubMed] [Google Scholar]
- 13.Legendre C, Garrigue V, Le Bihan C, et al. Harmful long-term impact of hepatitis C virus infection in kidney transplant recipients. Transplantation. 1998;65(5):667–670. doi: 10.1097/00007890-199803150-00011. [DOI] [PubMed] [Google Scholar]
- 14.Gentil MA, Rocha JL, Rodriguez-Algarra G, et al. Impaired kidney transplant survival in patients with antibodies to hepatitis C virus. Nephrol Dial Transplant. 1999;14(10):2455–2460. doi: 10.1093/ndt/14.10.2455. [DOI] [PubMed] [Google Scholar]
- 15.Mathurin P, Mouquet C, Poynard T, et al. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999;29(1):257–263. doi: 10.1002/hep.510290123. [DOI] [PubMed] [Google Scholar]
- 16.Breitenfeldt MK, Rasenack J, Berthold H, et al. Impact of hepatitis B and C on graft loss and mortality of patients after kidney transplantation. Clin Transplant. 2002;16(2):130–136. doi: 10.1034/j.1399-0012.2002.1o034.x. [DOI] [PubMed] [Google Scholar]
- 17.Bruchfeld A, Wilczek H, Elinder CG. Hepatitis C infection, time in renal-replacement therapy, and outcome after kidney transplantation. Transplantation. 2004;78(5):745–750. doi: 10.1097/01.tp.0000131948.29742.24. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoud IM, Elhabashi AF, Elsawy E, El-Husseini AA, Sheha GE, Sobh MA. The impact of hepatitis C virus viremia on renal graft and patient survival: a 9-year prospective study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2004;43(1):131–139. doi: 10.1053/j.ajkd.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Aroldi A, Lampertico P, Montagnino G, et al. Natural history of hepatitis B and C in renal allograft recipients. Transplantation. 2005;79(9):1132–1136. doi: 10.1097/01.tp.0000161250.83392.73. [DOI] [PubMed] [Google Scholar]
- 20.Mitwalli AH, Alam A, Al-Wakeel J, et al. Effect of chronic viral hepatitis on graft survival in Saudi renal transplant patients. Nephron Clin Pract. 2006;102(2):c72–80. doi: 10.1159/000089090. [DOI] [PubMed] [Google Scholar]
- 21.Einollahi B, Pourfarziani V, Ahmadzad-Asl M, et al. Iranian model of renal allograft transplantation in 3028 recipients: survival and risk factors. Transplant Proc. 2007;39(4):907–910. doi: 10.1016/j.transproceed.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Scott DR, Wong JK, Spicer TS, et al. Adverse impact of hepatitis C virus infection on renal replacement therapy and renal transplant patients in Australia and New Zealand. Transplantation. 2010;90(11):1165–1171. doi: 10.1097/TP.0b013e3181f92548. [DOI] [PubMed] [Google Scholar]
- 23.Kucirka LM, Peters TG, Segev DL. Impact of donor hepatitis C virus infection status on death and need for liver transplant in hepatitis C virus-positive kidney transplant recipients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;60(1):112–120. doi: 10.1053/j.ajkd.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Negro F, Forton D, Craxi A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149(6):1345–1360. doi: 10.1053/j.gastro.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 25.Cruzado JM, Gil-Vernet S, Ercilla G, et al. Hepatitis C virus-associated membranoproliferative glomerulonephritis in renal allografts. J Am Soc Nephrol. 1996;7(11):2469–2475. doi: 10.1681/ASN.V7112469. [DOI] [PubMed] [Google Scholar]
- 26.Morales JM, Pascual-Capdevila J, Campistol JM, et al. Membranous glomerulonephritis associated with hepatitis C virus infection in renal transplant patients. Transplantation. 1997;63(11):1634–1639. doi: 10.1097/00007890-199706150-00017. [DOI] [PubMed] [Google Scholar]
- 27.Cosio FG, Roche Z, Agarwal A, Falkenhain ME, Sedmak DD, Ferguson RM. Prevalence of hepatitis C in patients with idiopathic glomerulopathies in native and transplant kidneys. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1996;28(5):752–758. doi: 10.1016/s0272-6386(96)90260-7. [DOI] [PubMed] [Google Scholar]
- 28.Baid S, Pascual M, Williams WW, Jr, et al. Renal thrombotic microangiopathy associated with anticardiolipin antibodies in hepatitis C-positive renal allograft recipients. J Am Soc Nephrol. 1999;10(1):146–153. doi: 10.1681/ASN.V101146. [DOI] [PubMed] [Google Scholar]
- 29.Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386(10003):1537–1545. doi: 10.1016/S0140-6736(15)00349-9. [DOI] [PubMed] [Google Scholar]
- 30.Pockros PJ, Reddy KR, Mantry PS, et al. Efficacy of Direct-Acting Antiviral Combination for Patients With Hepatitis C Virus Genotype 1 Infection and Severe Renal Impairment or End-Stage Renal Disease. Gastroenterology. 2016;150(7):1590–1598. doi: 10.1053/j.gastro.2016.02.078. [DOI] [PubMed] [Google Scholar]
- 31.Kamar N, Marion O, Rostaing L, et al. Efficacy and Safety of Sofosbuvir-Based Antiviral Therapy to Treat Hepatitis C Virus Infection After Kidney Transplantation. Am J Transplant. 2016;16(5):1474–1479. doi: 10.1111/ajt.13518. [DOI] [PubMed] [Google Scholar]
- 32.Sawinski D, Kaur N, Ajeti A, et al. Successful Treatment of Hepatitis C in Renal Transplant Recipients With Direct-Acting Antiviral Agents. Am J Transplant. 2016;16(5):1588–1595. doi: 10.1111/ajt.13620. [DOI] [PubMed] [Google Scholar]
- 33.Hinrichsen H, Leimenstoll G, Stegen G, et al. Prevalence and risk factors of hepatitis C virus infection in haemodialysis patients: a multicentre study in 2796 patients. Gut. 2002;51(3):429–433. doi: 10.1136/gut.51.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.