Key Points
Question
What types and quantity of ingredients are found in products sold through the internet and advertised to contain selective androgen receptor modulators?
Findings
Chemical analyses of 44 products sold via the internet as selective androgen receptor modulators revealed that only 52% contained selective androgen receptor modulators and another 39% contained another unapproved drug. In addition, 25% of products contained substances not listed on the label, 9% did not contain an active substance, and 59% contained substance amounts that differed from the label.
Meaning
Selective androgen receptor modulators, which have not been approved by the US Food and Drug Administration, were available through the internet and were inaccurately labeled.
Abstract
Importance
Recent reports have described the increasing use of nonsteroidal selective androgen receptor modulators, which have not been approved by the US Food and Drug Administration (FDA), to enhance appearance and performance. The composition and purity of such products is not known.
Objective
To determine the chemical identity and the amounts of ingredients in dietary supplements and products marketed and sold through the internet as selective androgen receptor modulators and compare the analyzed contents with product labels.
Design and Setting
Web-based searches were performed from February 18, 2016, to March 25, 2016, using the Google search engine on the Chrome and Internet Explorer web browsers to identify suppliers selling selective androgen receptor modulators. The products were purchased and the identities of the compounds and their amounts were determined from April to August 2016 using chain-of-custody and World Anti-Doping Association–approved analytical procedures. Analytical findings were compared against the label information.
Exposures
Products marketed and sold as selective androgen receptor modulators.
Main Outcomes and Measures
Chemical identities and the amount of ingredients in each product marketed and sold as selective androgen receptor modulators.
Results
Among 44 products marketed and sold as selective androgen receptor modulators, only 23 (52%) contained 1 or more selective androgen receptor modulators (Ostarine, LGD-4033, or Andarine). An additional 17 products (39%) contained another unapproved drug, including the growth hormone secretagogue ibutamoren, the peroxisome proliferator-activated receptor-δ agonist GW501516, and the Rev-ErbA agonist SR9009. Of the 44 tested products, no active compound was detected in 4 (9%) and substances not listed on the label were contained in 11 (25%). In only 18 of the 44 products (41%), the amount of active compound in the product matched that listed on the label. The amount of the compounds listed on the label differed substantially from that found by analysis in 26 of 44 products (59%).
Conclusions and Relevance
In this limited investigation involving chemical analyses of 44 products marketed as selective androgen receptor modulators and sold via the internet, most products contained unapproved drugs and substances. Only 52% contained selective androgen receptor modulators and many were inaccurately labeled.
This study conducted web-based searches for products marketed as selective androgen receptor modulators, which are performance-enhancing drugs that have not been approved by the US Food and Drug Administration, and compared the chemical identities and the amount of ingredients in each product with those listed on the product label.
Introduction
Anabolic steroids are the most frequently abused appearance- and performance-enhancing drugs.1,2,3 Use of these drugs was largely limited to athletes prior to the 1980s, but since then use of these substances has increased among men with the goal of building muscle and appearing lean and muscular.1,3 Selective androgen receptor modulators (ligands that bind to the androgen receptor and exert tissue-selective effects such as muscle growth4) have emerged as the new appearance- and performance-enhancing substances; however, the extent of product use is unknown. Several oral nonsteroidal selective androgen receptor modulators4,5 are under development for treating functional limitations associated with aging or muscle wasting disorders, but none has been approved by the US Food and Drug Administration (FDA).
In response to reports of increased use of nonsteroidal selective androgen receptor modulators purchased through the internet by athletes, recreational bodybuilders, and members of the US armed forces,6,7 a systematic investigation of the availability of products marketed as selective androgen receptor modulators through the internet was conducted. In addition, the chemical identity of the products sold as selective androgen receptor modulators and the amount of the compound in the product were determined by chemical analysis and compared with the label information.
Methods
Product Acquisition
Selective androgen receptor modulator products were found by conducting web-based searches from February 18, 2016, to March 25, 2016, using the Google search engine on the Chrome and Internet Explorer web browsers and the search terms SARMs Supplements, SARMs, selective androgen receptor modulators, and buy. The products purchased were typically supplied as capsules, tablets, or syrups in sealed containers.
Chain-of-Sample Custody
From time of receipt, the product samples were subjected to rigorous chain-of-custody procedures similar to those used during acquisition of biological samples from athletes being tested for banned substances. Records of the chain of custody were maintained and the samples were stored in secured areas.
Product Analyses
The products were tested between April 2016 and mid-August 2016. The extraction, screening, and confirmation (additional details appear in the Supplement) were performed using procedures8 that have been approved by the World Anti-Doping Agency (WADA) for detecting the use of banned substances by athletes. Briefly, the equivalent of 1 serving of the product was extracted when that information was provided by the supplier; otherwise, 1 capsule or 1000 μL of solution was extracted.
The product contents were subjected to both targeted and untargeted analysis. In the targeted analysis, the mass-spectrometric precursor to the fragment transitions specific to the compounds of interest were monitored at the known retention times for the compounds to provide the highest degree of sensitivity in detecting their presence. In the untargeted analysis, full mass-spectrometric scans were acquired with and without molecular fragmentation across the full chromatogram to allow detection of all ionizing species (ie, without predefining which molecular masses to identify). The mass-spectrometric data were acquired with high mass accuracy (mass error of <10 ppm) and at high resolution (35 000 Da) to enable determination of the candidate molecular formulas for the detected masses.
Initially, the compounds were tested using general-purpose screenings meeting all the requirements set forth by WADA for detecting the use of performance-enhancing drugs by athletes.9 If no banned substances were detected in the general-purpose screening, further analyses were performed using untargeted screenings as described above. The use of general-purpose screening procedures enabled detection of additional performance-enhancing drugs other than selective androgen receptor modulators.
When the screening analysis revealed a selective androgen receptor modulator or another performance-enhancing substance, the findings were confirmed by more specific methods and reference materials for comparison of the characteristics from mass-spectral fragmentation. WADA-specified criteria were used for positive identification of compounds.8,9 The amount of compound was estimated based on comparison of the response with those obtained from standards containing known quantities.
The analytical methods have an estimated intra-assay coefficient of variation of 20% and an interassay coefficient of variation of 20%. Label claims were considered to match the experimentally measured values when they were within the estimated uncertainty windows of the analyses. We compared the compounds found in the product and their amounts with the product label.
Results
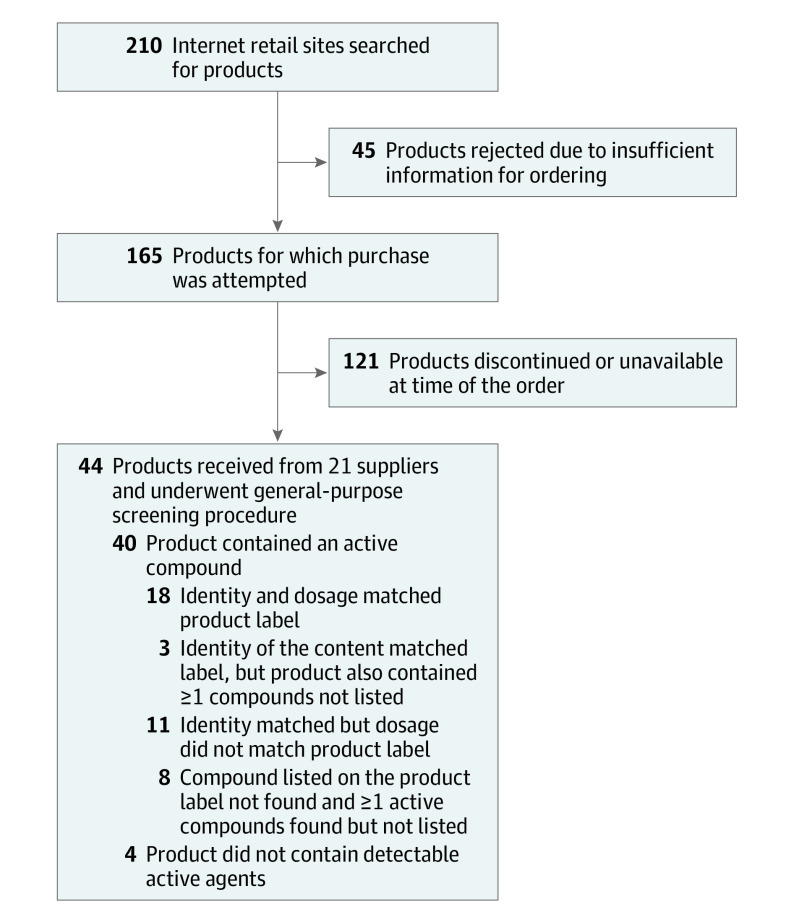
Internet searches yielded 210 products from 51 supplier sites offering selective androgen receptor modulators and other muscle-building compounds (Figure 1). Of these, 45 products could not be purchased due to insufficient information on the websites, and 121 products could not be obtained because they were out of stock, discontinued, or had other restrictions. Forty-four products purchased from 21 suppliers were analyzed (Figure 2 and Table). The number of products purchased from various suppliers ranged from 1 to 5.
Figure 1. Flow of Tested Products Through the Systematic Search and Analytical Process.
Figure 2. Pharmacological Classes of Appearance and Performance Enhancing Substances Found in the Search.
SARM indicates selective androgen receptor modulator; and PPAR-δ, peroxisome proliferator-activated receptor-δ.
aIndicates trade name.
Table. Products, Suppliers, Labeling, and Chemical Testing of 44 Products Sold as Selective Androgen Receptor Modulators via the Internet.
| Product IDa | Supplier IDb |
Label Information for Use | Form and Product Contents |
Retail Cost, $ | Label Claims | Findings |
|---|---|---|---|---|---|---|
| Identity and Dosage Match | ||||||
| 337083 | C | Not for human consumption; Research use only |
60 capsules | 89.99 | 10 mg of GW501516 per capsule | 10-50 mg of GW501516 per capsule |
| 337232 | F | 240 capsules | 64.99 | 5 mg of Andarine per capsule | 1-10 mg of Andarine per capsule | |
| 337235 | H | 90 capsules | 44.99 | 4 mg of LGD-4033 per capsule | 1-5 mg of LGD-4033 per capsule | |
| 337236 | H | 90 capsules | 69.95 | 10 mg of ibutamoren per capsule | 5-20 mg of ibutamoren per capsule | |
| 337238 | I | Research use only | 30-mL solution | 196.59 | 25 mg/mL of ibutamoren | 10-50 mg/mL of ibutamoren |
| 337395 | J | 30 tablets | 74.99 | 15 mg of LGD-4033 per tablet | 5-20 mg of LGD-4033 per tablet | |
| 337396 | F | 90 capsules | 59.99 | 10 mg of Ostarine per capsule | 1-5 mg of Ostarine per capsule | |
| 342066 | K | 90 capsules | 59.95 | 11 mg of GW501516 per capsule | 10-20 mg of GW501516 per capsule | |
| 342067 | K | 90 capsules | 59.95 | 6 mg of LGD-4033 per capsule | 1-10 mg of LGD-4033 per capsule | |
| 342068 | K | 90 capsules | 59.95 | 12 mg of Ostarine per capsule | 1-10 mg of Ostarine per capsule | |
| 342069 | L | 90 capsules | 70.00 | 20 mg of Ostarine per capsule | 10-20 mg of Ostarine per capsule | |
| 342075 | N | 120 capsules | 64.95 | 12.5 mg of Ostarine per capsule | 10-20 mg of Ostarine per capsule | |
| 342076 | O | 90 capsules | 54.95 | 10 mg of Ostarine per capsule | 5-10 mg of Ostarine per capsule | |
| 342077 | O | 60 capsules | 79.95 | 12.5 mg of ibutamoren per capsule | 10-20 mg of ibutamoren per capsule | |
| 342078 | O | 90 capsules | 69.95 | 5 mg of LGD-4033 per capsule | 1-10 mg of LGD-4033 per capsule | |
| 342952 | T | Not for human consumption; Research use only |
30-mL solution | 99.99 | 10 mg/mL of RAD140 | Formula match for RAD140 |
| 342953 | T | Not for human consumption; Research use only |
30-mL solution | 59.99 | 10 mg/mL of GW501516 | 10-50 mg/mL of GW501516 |
| 342954 | T | Not for human consumption; Research use only |
30-mL solution | 99.99 | 25 mg/mL of ibutamoren | 20-80 mg/mL of ibutamoren |
| Identity Matches Label but Additional Compounds Found | ||||||
| 342082 | R | Not for human consumption; Research use only |
60-mL solution | 119.99 | 20 mg/mL of GW501516 | 10-50 mg/mL of GW501516, <0.1 mg/mL of tamoxifen |
| 342086 | R | Not for human consumption; Research use only |
60-mL solution | 119.99 | 50 mg/mL of Andarine | 10-50 mg/mL of Andarine, <0.1 mg/mL of tamoxifen |
| 342089 | S | Research use only | 2 capsules | Free sample | 10 mg of ibutamoren per capsule | 10-20 mg of ibutamoren per capsule, <0.1 mg of GW501516 per capsule |
| Identity Match but Dosage Does not Match | ||||||
| 336800 | A | Not for human consumption; Research use only | 60 capsules | 109.99 | 25 mg of Ostarine per capsule | 1-5 mg of Ostarine per capsule |
| 336801 | B | Not for human consumption; Research use only | 90 capsules | 59.95 | 10 mg of GW501516 per capsule | 0.1-1 mg of GW501516 per capsule |
| 337084 | D | 120 capsules | 49.99 | 25 mg of Ostarine per capsule | 1-5 mg of Ostarine per capsule | |
| 337233 | G | 90 capsules | 69.99 | 12.5 mg of Ostarine per capsule | 1-5 mg of Ostarine per capsule | |
| 342079 | P | Not for human consumption | 90 capsules | 59.95 | 20 mg of Ostarine per capsule | 1-5 mg of Ostarine per capsule |
| 342080 | F | Not for human consumption; Research use only | 180 capsules | 63.95 | 5 mg of GW501516 per capsule | 0.1-1 mg of GW501516 per capsule |
| 342083 | R | Not for human consumption; Research use only | 60-mL solution | 159.99 | 50 mg/mL of ibutamoren | 10-20 mg/mL of ibutamoren |
| 342085 | R | Not for human consumption; Research use only | 60-mL solution | 129.99 | 30 mg/mL of LGD-4033 | 1-10 mg/mL of LGD-4033 |
| 342088 | S | 90 capsules | 54.99 | 5 mg of GW501516 per capsule | 10-20 mg of GW501516 per capsule | |
| 342950 | T | Not for human consumption; Research use only | 30-mL solution | 54.99 | 10 mg/mL of LGD-4033 | 0.1-1 mg/mL of LGD-4033 |
| 342951 | T | Not for human consumption; Research use only | 30-mL solution | 44.99 | 50 mg/mL of Andarine | 10-20 mg/mL of Andarine |
| Compound on Label not Found but Unlisted Active Compounds Found | ||||||
| 337231 | E | 90 capsules | 59.95 | 8 mg of Ostarine, 6 mg of GW501516, 35 mg of 5-aminoimidazole-4-carboxamide ribonucleotide per capsule | 0.1-1 mg of Ostarine per capsule, 1-5 mg of androstatrienedione per capsule | |
| 337237 | I | Research use only | 30-mL solution | 179.49 | 50 mg/mL of Ostarine | 0.1-1 mg/mL of Ostarine, 10-50 mg/mL of Andarine, <0.1 mg/mL of GW501516 |
| 337394 | C | Not for human consumption; Research use only | 90 capsules | 119.99 | 3 mg of RAD140 per capsule | 2.1 mg of Ostarine, <0.1 mg of Andarine per capsule |
| 342072 | M | Not for human consumption; Research use only | 40 capsules | 62.99 | 20 mg of SR9009 per capsule | 1-5 mg of androstatrienedione per capsule |
| 342073 | M | Not for human consumption; Research use only | 40 capsules | 61.99 | 30 mg of ibutamoren per capsule | 1-5 mg of LGD-4033 per capsule |
| 342081 | Q | 60-mL solution | 159.99 | 20 mg/mL of LGD-4033, 25 mg/mL of Ostarine, 20 mg/mL of ibutamoren | 1-10 mg/mL of LGD-4033, 1-10 mg/mL of GW501516, 1-5 mg/mL of ibutamoren, <0.1 mg/mL of tamoxifen | |
| 342084 | R | Not for human consumption; Research use only | 60-mL solution | 129.99 | 50 mg/mL of Ostarine | 10-50 mg/mL of GW501516, <0.1 mg/mL of tamoxifen, <0.1 mg/mL of Ostarine |
| 342955 | U | 60 capsules | 60.00 | 20-mg blend of Ostarine and GW501516 per capsule | 5-10 mg of Ostarine, 5-10 mg of LGD-4033 per capsule | |
| No Active Agents Detected in Product | ||||||
| 337234 | F | 90 capsules | 39.95 | 5 mg of LGD-4033 per capsule | No active agents detected | |
| 342070 | M | Not for human consumption; Research use only | 40 capsules | 54.99 | 25 mg of Ostarine per capsule | No active agents detected |
| 342071 | M | Not for human consumption; Research use only | 40 capsules | 62.99 | 70 mg of 5α-hydroxylaxogenin per capsule | No active agents detected |
| 342074 | M | Not for human consumption; Research use only | 40 capsules | 59.99 | 15 mg of RAD140 per capsule | No active agents detected |
Internal laboratory identifier.
Coded indicator of the company the product was branded under.
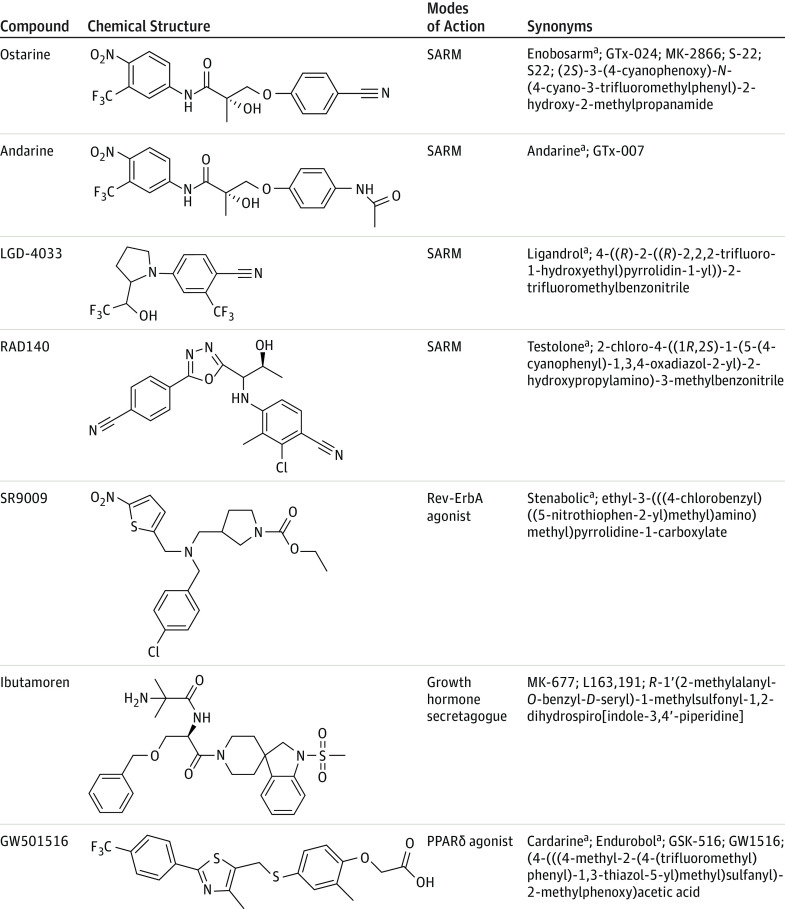
Some companies used the term SARM in their product label or in advertising, even though the product was not a selective androgen receptor modulator (eg, growth hormone secretagogue ibutamoren) (Figure 2). Of the 44 purchased products, the following compounds were listed on the labels: Ostarine (also known as enobosarm, GTx-024, MK-2866, S-22, and S22), Andarine (also known as GTx-007), LGD-4033 (also known as Ligandrol), RAD140 (also known as Testolone), ibutamoren (also known as MK-677, L163,191), GW501516 (also known as Cardarine, Endurobol, GSK-516, and GW1516), and SR9009 (also known as Stenabolic).
Figure 2 shows the most common compounds detected in the 44 products. Selective androgen receptor modulators, peroxisome proliferator-activated receptor-δ (PPAR-δ) agonists, and growth hormone secretagogues were the 3 most frequently found classes of compounds among those tested. Selective androgen receptor modulators accounted for 23 of the 44 tested compounds (52%). Ostarine and LGD-4033 were found in more than 80% of selective androgen receptor modulator products. The PPAR-δ agonist GW501516 was the second most frequently found compound, and the growth hormone secretagogue ibutamoren was the third most common.
In only 18 of the 44 products (41%), the amount of active compound in the product matched that listed on the label. In 11 products (25%), the listed compound was detected, but in an amount that differed from the label (Table and Figure 1). In 8 products (18%), the compound listed on the label was not found; however, different compounds not listed on the label were found. Three products (7%) contained the amount listed on the label but also contained an additional compound not listed. Four products contained minimal amounts of the selective estrogen receptor modulator tamoxifen. In another 4 products (9%), no active compounds were detected.
The amount of the compounds listed on the label differed substantially from that found by analysis in 26 of 44 products (59%). Seventeen products (39%) contained another unapproved drug, including the growth hormone secretagogue ibutamoren, the peroxisome proliferator-activated receptor-δ agonist GW501516, and the Rev-ErbA agonist SR9009. None of the 5 products purchased from 1 supplier contained the compounds listed on the label (Table). In 2 of 5 products obtained from this supplier, the analyses detected banned substances not listed on the label.
Of the 44 products, 20 (45%) were sold as dietary supplements and contained Supplement Fact panels on their labels. No product label included a Drug Fact panel. There were 24 products (55%) labeled as being for research use only, not for human consumption, or both (Table). The retail cost, product form (capsules or solution), and contents (quantity or solution volume) appear in the Table.
Discussion
This limited investigation found that products containing investigational selective androgen receptor modulators and other performance-enhancing drugs not approved by the FDA can be purchased from internet sites without a prescription. Although the tested products were advertised as selective androgen receptor modulators, some products contained growth hormone secretagogues,10 PPAR-δ agonists,11 and Rev-ErbA agonists,12 all of which are recognized by WADA as banned substances.13 Neither the efficacy nor the safety of these investigational drugs has been demonstrated. For many products, the ingredients and their amounts found in these analyses did not match the label information. These findings suggest the need for greater regulatory oversight of products sold on the internet.
Most tested products were investigational drugs that have not received FDA approval. The FDA requires all drugs to have an approved application for continued marketing.14,15 Such approval from the FDA requires demonstration of safety and efficacy.15 Some compounds such as Ostarine,16 Andarine, LGD4033,6 and ibutamoren10 have undergone some human studies, but have not received FDA approval. The development of GW50151617 was halted because of safety concerns. Preclinical studies have occurred for SR9009, but no human trials. Internet sales of drugs that are not approved by the FDA and whose benefits and safety have not been demonstrated raise public safety concerns. Individuals who use these products generally do not disclose the use of these products to their physicians; furthermore, clinicians do not have ready access to information about the contents and safety of such unapproved products, further increasing the health risk to the user.
The Fair Packaging and Labeling Act14 requires consumer products to be labeled to disclose the contents and the product identity.8 For drugs, a listing of drug facts with potential adverse effects and guidelines for safe use are required.18 Of the products tested, some did not contain the compound listed on the label and others contained amounts that differed substantially from the label. Nearly one-quarter of tested products contained compounds not included on the label, and some included a mixture of 2 or more compounds.
Nearly half the products were marketed as dietary supplements. The FDA defines dietary supplements as products taken by mouth that contain a “dietary ingredient” such as vitamins, minerals, amino acids, and herbs or botanicals that can be used to supplement the diet.19 None of the tested compounds meets the definition of a dietary supplement.
Antidoping agencies have identified cases of doping with selective androgen receptor modulators by athletes.20 Contamination of dietary supplements by anabolic steroids also has been reported.21 This investigation demonstrates the ease with which unapproved appearance- and performance-enhancing drugs can be purchased over the internet, which could increase abuse by adolescents, military personnel, and recreational weightlifters.
In addition, the ability of small laboratories located inside and outside the United States to synthesize and package pharmaceutical agents has contributed to the ease of global purchasing of these compounds. Vendors easily disseminate information about the products to potential users via the internet. The ease of website creation makes it possible for suppliers to create and remove websites as often as needed to stay ahead of law enforcement, regulators, and posted reviews from users who experienced adverse effects.
This investigation has several strengths. Rigorous testing procedures, which are similar to those used to detect banned substances in the WADA-certified testing program, were used for the analyses of these compounds. The chain of custody was recorded and products were stored in secure locations. The sensitivity of these procedures enabled detection of even small quantities of compounds.
Limitations
This study has several limitations. Because all possible internet sites were not searched, this search should not be viewed as exhaustive. It is possible that additional appearance- and performance-enhancing substances are being sold on the internet as selective androgen receptor modulators. The results apply only to the products purchased. Therefore, these findings should not be viewed as representative of all products sold through internet sites because the sample is limited to a single point in purchase time. Because of the rapidly changing nature of such internet sites, the results of similar searches will vary.
Conclusions
In this limited investigation involving chemical analyses of 44 products marketed as selective androgen receptor modulators and sold via the internet, most products contained unapproved drugs and substances. Only 52% contained selective androgen receptor modulators and many were inaccurately labeled.
Analytical Methods
eTable 1. Chromatographic methods for confirmatory analyses
eTable 2. Mass spectrometric parameters for confirmatory analyses
References
- 1.Pope HG Jr, Wood RI, Rogol A, Nyberg F, Bowers L, Bhasin S. Adverse health consequences of performance-enhancing drugs: an Endocrine Society scientific statement. Endocr Rev. 2014;35(3):341-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westerman ME, Charchenko CM, Ziegelmann MJ, Bailey GC, Nippoldt TB, Trost L. Heavy testosterone use among bodybuilders: an uncommon cohort of illicit substance users. Mayo Clin Proc. 2016;91(2):175-182. [DOI] [PubMed] [Google Scholar]
- 3.Pope HG Jr, Khalsa JH, Bhasin S. Body image disorders and abuse of anabolic-androgenic steroids among men. JAMA. 2017;317(1):23-24. [DOI] [PubMed] [Google Scholar]
- 4.Gao W, Dalton JT. Expanding the therapeutic use of androgens via selective androgen receptor modulators (SARMs). Drug Discov Today. 2007;12(5-6):241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basaria S, Collins L, Dillon EL, et al. The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men. J Gerontol A Biol Sci Med Sci. 2013;68(1):87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan BP, Kanayama G, Pope HG Jr. Performance-enhancing drugs on the web: a growing public-health issue. Am J Addict. 2013;22(2):158-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kao TC, Deuster PA, Burnett D, Stephens M. Health behaviors associated with use of body building, weight loss, and performance enhancing supplements. Ann Epidemiol. 2012;22(5):331-339. [DOI] [PubMed] [Google Scholar]
- 8.World Anti-Doping Agency . WADA World Anti-Doping Code: international standards for laboratories. https://www.wada-ama.org/sites/default/files/resources/files/isl_june_2016.pdf. Accessed May 11, 2017.
- 9.World Anti-Doping Agency Laboratory Expert Group . WADA technical document: TD2015IDCR. https://www.wada-ama.org/sites/default/files/resources/files/td2015idcr_-_eng.pdf. Accessed May 11, 2017.
- 10.Adunsky A, Chandler J, Heyden N, et al. MK-0677 (ibutamoren mesylate) for the treatment of patients recovering from hip fracture: a multicenter, randomized, placebo-controlled phase IIb study. Arch Gerontol Geriatr. 2011;53(2):183-189. [DOI] [PubMed] [Google Scholar]
- 11.Manickam R, Wahli W. Roles of peroxisome proliferator-activated receptor β/δ in skeletal muscle physiology. Biochimie. 2017;136:42-48. [DOI] [PubMed] [Google Scholar]
- 12.Solt LA, Wang Y, Banerjee S, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485(7396):62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Anti-Doping Agency . The 2016 prohibited list. https://www.wada-ama.org/sites/default/files/resources/files/wada-2016-prohibited-list-en.pdf. Accessed May 11, 2017.
- 14.US Food and Drug Administration . What are unapproved drugs and why are they on the market? https://www.fda.gov/aboutfda/transparency/basics/ucm213030.htm. Accessed May 18, 2017.
- 15.Williams CT. Food and Drug Administration drug approval process: a history and overview. Nurs Clin North Am. 2016;51(1):1-11. [DOI] [PubMed] [Google Scholar]
- 16.Dobs AS, Boccia RV, Croot CC, et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013;14(4):335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Health Canada . Health Canada endorsed important safety information on an unauthorized product called GW501516. http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2013/33605a-eng.php. Accessed October 25, 2017.
- 18.US Food and Drug Administration . Guidance for industry: labeling for human prescription drug and biological products—content and format requirements. https://www.fda.gov/downloads/drugs/guidances/ucm075082.pdf. Accessed October 17, 2017.
- 19.US Food and Drug Adminstration . Guidance for industry: a dietary supplement labeling guide. https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/DietarySupplements/ucm2006823.htm. Accessed October 17, 2017.
- 20.Thevis M, Lagojda A, Kuehne D, et al. Characterization of a non-approved selective androgen receptor modulator drug candidate sold via the Internet and identification of in vitro generated phase-I metabolites for human sports drug testing. Rapid Commun Mass Spectrom. 2015;29(11):991-999. [DOI] [PubMed] [Google Scholar]
- 21.Abbate V, Kicman AT, Evans-Brown M, et al. Anabolic steroids detected in bodybuilding dietary supplements—a significant risk to public health. Drug Test Anal. 2015;7(7):609-618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analytical Methods
eTable 1. Chromatographic methods for confirmatory analyses
eTable 2. Mass spectrometric parameters for confirmatory analyses