Key Points
Question
Does antibiotic prophylaxis with a single preoperative dose of intravenous cefazolin (1000 mg) reduce the risk of surgical site infection following removal of orthopedic implants used for treatment of fractures below the knee?
Findings
In this randomized clinical trial that included 470 patients who were undergoing surgery for removal of orthopedic implants used for treatment of below-the-knee fractures, surgical site infection occurred in 12.9% of patients in the cefazolin group and 14.9% of patients in the saline group, a nonsignificant difference.
Meaning
A single preoperative dose of intravenous cefazolin did not reduce the risk of surgical site infection within 30 days following removal of orthopedic implants used for treatment of fractures below the knee.
Abstract
Importance
Following clean (class I, not contaminated) surgical procedures, the rate of surgical site infection (SSI) should be less than approximately 2%. However, an infection rate of 12.2% has been reported following removal of orthopedic implants used for treatment of fractures below the knee.
Objective
To evaluate the effect of a single dose of preoperative antibiotic prophylaxis on the incidence of SSIs following removal of orthopedic implants used for treatment of fractures below the knee.
Design, Setting, and Participants
Multicenter, double-blind, randomized clinical trial including 500 patients aged 18 to 75 years with previous surgical treatment for fractures below the knee who were undergoing removal of orthopedic implants from 19 hospitals (17 teaching and 2 academic) in the Netherlands (November 2014-September 2016), with a follow-up of 6 months (final follow-up, March 28, 2017). Exclusion criteria were an active infection or fistula, antibiotic treatment, reimplantation of osteosynthesis material in the same session, allergy for cephalosporins, known kidney disease, immunosuppressant use, or pregnancy.
Interventions
A single preoperative intravenous dose of 1000 mg of cefazolin (cefazolin group, n = 228) or sodium chloride (0.9%; saline group, n = 242).
Main Outcomes and Measures
Primary outcome was SSI within 30 days as measured by the criteria from the US Centers for Disease Control and Prevention. Secondary outcome measures were functional outcome, health-related quality of life, and patient satisfaction.
Results
Among 477 randomized patients (mean age, 44 years [SD, 15]; women, 274 [57%]; median time from orthopedic implant placement, 11 months [interquartile range, 7-16]), 470 patients completed the study. Sixty-six patients developed an SSI (14.0%): 30 patients (13.2%) in the cefazolin group vs 36 in the saline group (14.9%) (absolute risk difference, −1.7 [95% CI, −8.0 to 4.6], P = .60).
Conclusions and Relevance
Among patients undergoing surgery for removal of orthopedic implants used for treatment of fractures below the knee, a single preoperative dose of intravenous cefazolin compared with saline did not reduce the risk of surgical site infection within 30 days following implant removal.
Trial Registration
clinicaltrials.gov Identifier: NCT02225821
This randomized clinical trial compares the effects of a single preoperative dose of cefazolin vs saline on the incidence of surgical site infection following removal of orthopedic implants used for treatment of below-knee fractures.
Introduction
Metal implants are often used in open reduction and internal fixation of fractures. With the use of antibiotic prophylaxis the surgical site infection (SSI) rate following fracture fixation was reduced from 8.3% to 3.6%, and therefore, antibiotic prophylaxis is routinely administered prior to fracture surgery.1
In most patients, removal of implants is not routinely indicated following fracture healing. Still, removal of orthopedic implants is one of the most frequently performed orthopedic procedures worldwide. For example, 28% to 79% of implants are removed following lower leg, ankle, or foot fracture surgery.2,3,4,5,6,7,8 SSI rates of 0% to 20% following removal of orthopedic implants have been reported, but in only 1 of these studies SSI was the primary outcome measure.2,3,4,5,6,7,9,10 In that study, the overall SSI rate following removal of orthopedic implants was 11.6%, with the highest incidence in the foot, ankle, or lower leg region (12.2%).3
Because removal of orthopedic implants is considered a clean (without skin contamination or local infection) surgical procedure with an expected SSI rate of 2% to 3.3%, preoperative administration of antibiotic prophylaxis is not indicated according to the latest guidelines of the US Centers for Disease Control and Prevention (CDC).11,12,13,14 However, higher SSI rates than anticipated (compared with rates following fracture fixation) are reported following removal of orthopedic implants.2,3,4,5,6,7,9,10,15 Due to these high SSI rates, some advocate that antibiotic prophylaxis should be administered prior to removal of orthopedic implants.15 Despite the large number of implant removal procedures performed worldwide, there is insufficient evidence on this subject and the potential beneficial effect of antibiotic prophylaxis on SSI rates following removal of orthopedic implants.
The aim of this study was to evaluate the effect of a single dose of antibiotic prophylaxis on the incidence of SSIs following removal of orthopedic implants used for treatment of fractures below the knee, the area with highest rate of infection. The study tested the hypothesis that a single dose of prophylactic antibiotics would lower the SSI rate following orthopedic implant removal in the lower leg.
Methods
Trial Oversight and Design
The Wound Infections Following Implant Removal (WIFI) trial was a multicenter, double-blind, randomized clinical trial in which patients undergoing removal of orthopedic implants to treat fractures below the knee were recruited. The trial protocol and statistical analysis plan are available in the eAppendix in Supplement 1. The study protocol was approved by the medical ethics committee at the Academic Medical Center of the University of Amsterdam and published.16 After publication of the study protocol, type 1 and 2 diabetes was added as a second stratification factor and approved by the medical ethics committee. The study was conducted according to the principles of the Declaration of Helsinki17 and in accordance with the Medical Research Involving Human Subjects Act. Independent monitors assessed the overall performance of study conduct and other issues. The trial was performed in 17 teaching hospitals and 2 academic hospitals in the Netherlands (eAppendix in Supplement 2).
Patient Population
Patients aged 18 to 75 years who were undergoing removal of orthopedic implants following treatment of fractures of the foot, ankle, and lower leg were eligible for inclusion. Exclusion criteria were an active SSI or fistula, antibiotic treatment at the time of implant removal for a concomitant disease or infection, reimplantation of material in the same session, an allergy for cephalosporins, a known kidney disease, immunosuppressant use, or pregnancy. Patients were screened for eligibility at the outpatient clinic. Inclusion ran from November 2014 through September 2016, follow-up ran from November 2014 through March 2017, and the study was ended after inclusion of 500 patients. Date of final follow-up was March 28, 2017.
Intervention, Randomization, and Blinding
After obtaining written informed consent, eligible patients were randomly assigned to receive either 1000 mg of cefazolin in a bolus of sodium chloride (0.9%) intravenously (cefazolin group) or a bolus of sodium chloride (0.9%) intravenously (saline group) preoperatively. This bolus, which was identical in appearance, was prepared and administered 15 to 60 minutes prior to incision in the holding or operation theater by the anesthesiologist or nurse anesthetist in the absence of the surgeon. The dose of 1000 mg of cefazolin was in accordance with guidelines on antibiotic prophylaxis in orthopedic trauma surgery.13,18
Participants were assigned to the cefazolin group or the saline group in a 1:1 ratio. Randomization was performed within each site and randomization sequence was generated by a dedicated computer randomization software program (Alea Software, Netherlands Cancer Institute), version 2.2. Randomization was stratified by center and presence of diabetes mellitus and performed preoperatively by a theater assistant or the anesthesiologist using a dedicated, password-protected, Secure Sockets Layer–encrypted website, ensuring allocation concealment for the patient and the surgeon.
Outcomes
Primary outcome was an SSI within 30 days after removal of orthopedic implants as defined by the criteria applied by the CDC.12,13 Each SSI was characterized as superficial or deep. A superficial SSI involves only skin or subcutaneous tissue of the incision and at least 1 of the following: (1) purulent drainage from the incision with or without laboratory confirmation; (2) organisms isolated from an aseptically obtained culture of fluid or tissue from the superficial incision; (3) at least 1 of the following signs or symptoms of infection: pain or tenderness, localized swelling, redness, or heat; and deliberate opening of superficial incision by surgeon unless incision is culture-negative; (4) diagnosis with subsequent treatment for superficial SSI by a (orthopedic) trauma surgeon.
A deep SSI involves deep tissues and at least 1 of the following: (1) purulent drainage from the incision; (2) the incision spontaneously dehisces or is deliberately opened by a surgeon when the patient has at least 1 of the following signs or symptoms: temperature higher than 38°C or localized pain or tenderness, unless site is culture-negative; (3) an abscess or other evidence of infection involving the incision is found on direct examination, during reoperation, or by histopathologic or radiologic examination; (4) diagnosis with subsequent treatment for deep SSI by a (orthopedic) trauma surgeon.12
Secondary outcomes were health-related quality of life (measured by the EuroQol 5-Dimension 3-Level [EQ-5D-3L] questionnaire [index range, 0-1; 1 indicates maximal health-related quality of life]),19 functional outcome (assessed with the Lower Extremity Functional Scale [LEFS]),20 and patient satisfaction with treatment (measured using a visual analog scale [VAS], ranging from 0 [completely not satisfied] to 10 [completely satisfied]). Questionnaires were sent electronically preoperatively (EQ-5D-3L and LEFS) and 6 months following the procedure (EQ-5D-3L, LEFS, and VAS). If a participant did not have access to the internet, questionnaires were sent by regular mail. All participants received at least 1 reminder in case a questionnaire was not completed.
Patients were seen routinely by a physician (a nonmember of the surgical team) who was blinded to patient treatment group assignment at the outpatient clinic within 4 weeks after the surgical procedure. Patients also reported on wound problems in their online questionnaires and were contacted by telephone if a problem was reported on the questionnaire by a physician. Signs of SSI (warmth, redness, pain, swelling, wound dehiscence, purulent drainage, or temperature >38°C) were documented on a case report form. Patients were instructed to visit the emergency department or outpatient clinic sooner in case of any signs of SSI. In case of SSI, appropriate treatment was started according to local policy.
Data Collection
Patient and surgical characteristics were collected. Patient characteristics were sex, age at the time of removal of orthopedic implants, location of index fracture, SSI following the index fracture, reason of implant removal, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), diabetes, and use of alcohol or drugs or nicotine. Treatment regimens in case of SSI were documented; subdivided into 4 categories: local wound care without antibiotics, with oral antibiotics, with intravenous antibiotics, or with surgical debridement.
The following surgical characteristics were documented: time from initial fracture treatment with orthopedic implant placement to implant removal, location of removal, complete or partial removal of implants, duration of surgery, tourniquet use, and resident or consultant performing surgery. If a wound or perioperative culture was collected, the cultured microorganisms were documented.
Missing data for the LEFS questionnaire was handled according to the instructions of the developers of the questionnaire; up to 4 items (≤2 within 1 domain) within 1 questionnaire can be imputed by using predefined rules.22 In case of more missing values or nonresponse, multiple imputation (10 data sets) using predictive mean matching was used, data were subsequently pooled using the Rubin rule.23
Sample Size Calculation and Statistical Analysis
An incidence of 3.3% of SSI was expected in clean-contaminated, elective orthopedic procedures for the cefazolin group.12,14 In recent Dutch retrospective studies at the time of study design, the incidence of SSI following removal of orthopedic implants from the foot, ankle, or lower leg was 9.2% to 19%.3,9,10 An SSI rate of 10% at the lower end of this range was chosen for the saline group to have at least 80% power to detect a clinically relevant decrease in SSI rate by two-thirds, from 10% to 3.3%.16
It was calculated that a sample size of 216 patients per study group would provide 80% power to detect a 6.7% difference with a 2-group χ2 test at a 2-sided α level of .05. To allow for an anticipated dropout of 10% to 15%, a total of 250 patients were included per study group.
An interim analysis was performed midway through the study (after inclusion of 216 patients) by an independent statistician to estimate the SSI rate in the saline group to reevaluate the sample size calculation. Because only an estimation of the SSI rate in the saline group was performed and no treatment effect was tested, the overall type I error rate was maintained.
An intention-to-treat analysis was performed according to plan described in the published protocol.16 Continuous data are presented as means and standard deviations or medians and interquartile ranges (IQRs) where appropriate; categorical data are presented as counts and percentages. For continuous data, t tests or Mann-Whitney U tests were used and, for categorical24 data, χ2 tests or Fisher exact tests were used, where appropriate. A 95% CI was calculated for the absolute risk differences by using the Wilson score method, continuity-corrected in case of low cell counts. In a sensitivity analysis, generalized linear mixed modeling was applied to assess the effect of cefazolin on the SSI rate while correcting for the stratification factors of diabetes and treatment center. Diabetes was modeled as a fixed variable and treatment center as a random variable. SSI rate was assumed to have a binomial distribution (logit link). In all analyses, statistical uncertainties were quantified using corresponding 2-sided 95% CIs, and a P value of .05 was used as the threshold for statistical significance. Patients who underwent randomization in error were excluded from analysis (ie, they did not meet the inclusion criteria or did meet an exclusion criterion). Post hoc, exploratory, unadjusted subgroup analyses comparing patients with and without antibiotics by weight (<60 kg and ≥60 kg13) and by type of removal of orthopedic implants (Kirschner wire, screw, syndesmotic screw, plate and screws, and intramedullary nail) were performed with logistic regression by testing for interactions with type of treatment. Furthermore, univariable analyses to identify factors associated with SSIs were performed. Analyses were performed using WINPEPI, version 3.2,25 and the SPSS (IBM), version 24.0.
Results
Patients
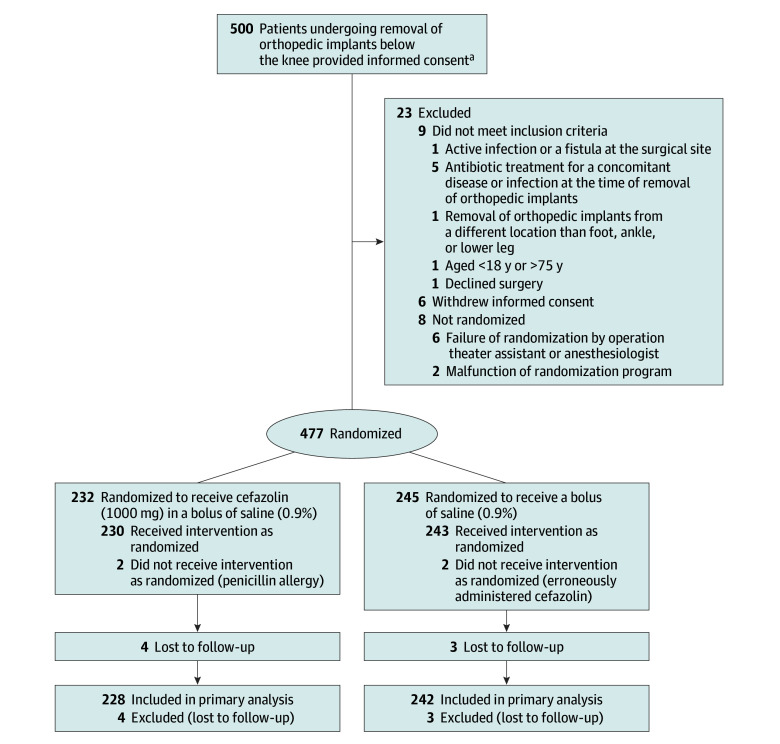
In total, 500 patients were included in 19 Dutch hospitals, of which 2 were university hospitals (eAppendix in Supplement 2). Two hundred thirty-two patients received antibiotic prophylaxis intravenously (cefazolin group) and 245 patients received sodium chloride only (saline group) (Figure).26 Eighty-eight patients were treated in an academic center. After randomization 7 patients were lost to follow-up. A total of 470 patients were available for analysis (228 in the cefazolin group and 242 in the saline group). Baseline characteristics are displayed in Table 1.
Figure. Flow of Patients Through the WIFI Trial.
WIFI indicates Wound Infections Following Implant Removal.
aThe number of patients screened for eligibility was not available.
Table 1. Baseline Characteristics of the Patients in the WIFI Trial.
| Characteristics | No. of Patients/Total (%) | |
|---|---|---|
| Cefazolin Group (n = 228) |
Saline Group (n = 242) |
|
| Men | 93/228 (40.8) | 109/242 (45.0) |
| Age, mean (SD), y | 43.4 (14.8) | 45.0 (15.4) |
| BMI, mean (SD)a | 26.5 (5.4) | 26.8 (5.5) |
| Diabetes mellitus | 5/228 (2.2) | 7/242 (1.7) |
| Any nicotine use | 56/218 (24.6) | 62/231 (26.8) |
| Any alcohol use | 57/211 (27.0) | 65/224 (29.0) |
| Illegal drug use | 6/228 (2.6) | 10/242 (4.1) |
| SSI following ORIF | 15/222 (6.8) | 12/238 (5.0) |
| Reason for removal of orthopedic implantb | ||
| Pain | 163/228 (71.5) | 182/242 (75.2) |
| Implant failure | 7/228 (3.1) | 8/242 (3.3) |
| Functional problem | 15/228 (6.6) | 12/242 (5.0) |
| Patients request | 111/228 (48.9) | 113/242 (46.7) |
| Planned procedure | 30/228 (13.2) | 30/242 (12.4) |
| Time to removal of orthopedic implant, median (IQR), mo |
11 (7-16) | 11 (7-17) |
| Location removal of orthopedic implantc | ||
| Forefoot or midfoot | 15/228 (6.6) | 12/242 (5.0) |
| Tarsus | 30/228 (13.2) | 29/242 (12.0) |
| Ankle | 124/228 (54.4) | 149/242 (61.6) |
| Lower leg (tibia or fibula) | 59/228 (25.9) | 52/242 (21.5) |
| Type of removal of orthopedic implantb | ||
| Intramedullary nail | 28/228 (12.3) | 11/242 (4.4) |
| Syndesmotic screw | 24/228 (10.5) | 29/242 (12.0) |
| Screw only | 52/228 (22.8) | 49/242 (20.2) |
| Plate and screws | 132/228 (57.9) | 163/242 (67.4) |
| Kirschner wire | 11/228 (4.8) | 13/242 (5.4) |
| Duration of surgery, mean (SD), mind | 35.2 (18.5) | 34.8 (20.2) |
| Resident performing surgery | 167/227 (74.6) | 173/242 (71.5) |
| Tourniquet use | 28/225 (12.4) | 31/237 (13.1) |
| Incomplete removal of orthopedic implant | 170/228 (74.6) | 172/242 (71.1) |
| Preoperative quality-of-life scores, mean (95% CI) | ||
| Health-related quality of lifee | 0.71 (0.68-0.74) | 0.71 (0.68-0.75) |
| Self-reported quality of lifef | 77.92 (74.67-81.17) | 75.56 (72.67-78.45) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; ORIF, open reduction and internal fixation; SSI, surgical site infection; VAS, visual analog scale.
A BMI of 30 or higher was defined as obese. Data are missing for 20 patients in the cefazolin group and 25 patients in the saline group.
More than 1 option was possible.
The location of the orthopedic implant removal was the same as the location of the index fracture.
The duration of the surgery was from the time of incision to the time of wound closure. Data are missing for 7 patients in the cefazolin group and 44 patients in the saline group.
Measured by the EuroQol 5-Dimension 3-Level questionnaire (index range, 0-1; 1 indicates maximal health-related quality of life).
Measured using a visual analog scale score ranging from 0 (lowest self-reported state of health) to 100 (maximum self-reported state of health).
Primary Outcome
Sixty-six patients (14.0%) developed an SSI (SSI classification: superficial, 58; deep, 8). In the cefazolin group, 30 patients (13.2%) developed an SSI vs 36 patients (14.9%) in the saline group (P = .60; absolute risk difference, −1.7 [95% CI, −8.0 to 4.6]) (Table 2). Adjustment for center and diabetes did not affect the odds ratios (ORs) for the association between cefazolin and risk of SSI (unadjusted OR: 0.867 [95% CI, 0.514 to 1.462] for center to 0.854 [95% CI, 0.505 to 1.445] for diabetes mellitus).
Table 2. Unadjusted Results for Surgical Site Infection (SSI) Within 30 Days After Removal of Orthopedic Implants.
| No. of Patients With an SSI (%) | Absolute Risk Difference, % (95% CI) |
||
|---|---|---|---|
| Cefazolin Group (n = 228) |
Saline Group (n = 242) |
||
| SSIa | 30 (13.2) | 36 (14.9) | −1.7 (−8.0 to 4.6) |
| Superficial | 29 (12.7) | 29 (12.0) | 0.7 (−5.3 to 6.8) |
| Deep | 1 (0.4) | 7 (2.9) | −2.5 (−5.7 to 0.4) |
Primary outcome measure.
In 6 of 8 patients (75%) with a deep SSI, surgical debridement was performed; all 8 patients were admitted and treated with intravenous antibiotics. Seven of 8 patients with a deep SSI were in the saline group (Table 2). In 49 of 58 patients (84.5%) with a superficial SSI, oral antibiotics were prescribed at the outpatient clinic or emergency department and 9 patients (15.5%) were treated conservatively without antibiotics. Two patients (3.4%) had the incision opened locally.
Secondary Outcomes
Patients reported a mean health-related quality of life score measured with the EQ-5D-3L of 0.72 preoperatively and 0.78 postoperatively. The mean health-related quality-of-life score did not statistically significantly differ between the 2 study groups postoperatively (0.78 in the cefazolin group vs 0.79 in the saline group; absolute difference, −0.02 [95% CI, −0.07 to 0.04]). The mean self-reported quality-of-life score on the VAS of the EQ-5D-3L was 76.2 preoperatively and 77.9 postoperatively. The mean self-reported quality-of-life score on the VAS did not statistically significantly differ between the 2 study groups postoperatively (78.5 in the cefazolin group vs 77.4 in the saline group; absolute difference, 1.16 [95% CI, −3.14 to 5.45]). Patients had a mean LEFS score of 54.2 preoperatively and 62.7 postoperatively. The mean LEFS did not statistically significantly differ between the 2 study groups postoperatively (62.3 in the cefazolin group vs 62.2 in the saline group; absolute difference, 0.1 [95% CI, −3.6 to 3.8]). The mean patient satisfaction score with treatment on the VAS was 7.7. This did not statistically significantly differ between the 2 study groups postoperatively (7.5 in the cefazolin group vs 7.8 in the saline group; absolute difference, −0.22 [95% CI, −0.77 to 0.33]).
Post Hoc Analyses
A variety of microorganisms were cultured in patients with an SSI. Thirty of 232 patients (12.9%) in the cefazolin group were diagnosed with an SSI (eTable 1 in Supplement 2). In this group, 19 causative organisms were found in 19 cultures. Fourteen microorganisms (74%) were sensitive for cefazolin and 5 (26%) were not sensitive for cefazolin. Among 2.7% of patients from whom a culture swab was obtained, no growth was detected, and, in 45.5% of patients diagnosed with an SSI, no culture swab was collected. In 87.2% of the cases, the identified microorganisms (both groups) were sensitive to cefazolin.
The tests for interaction for exploratory subgroup analyses revealed that neither weight (ie, <60 kg or ≥60 kg) (P = .14) nor the type of removal of orthopedic implants (P = .63) moderated the effect of treatment with cefazolin or saline on the incidence of SSIs (eTable 2 in Supplement 2).
The absolute risk difference in SSI rate between alcohol users and non–alcohol users equaled 7.5% (95% CI, 0.2% to 16.1%) in favor of the latter (eTable 3 in Supplement 2).
Discussion
In this multicenter, double-blind, randomized clinical trial involving patients who were undergoing surgery for removal of orthopedic implants used for treatment of fractures below the knee, a single preoperative dose of intravenous cefazolin compared with no administration of antibiotic prophylaxis did not reduce the risk of a SSI within 30 days after the surgical procedure.
A high rate of SSI was observed; higher than in most available retrospective series.2,3,4,5,6,7,9,10,15 SSI rates are often higher in prospective studies, because adequate reporting of SSIs after hospital discharge has proven to be difficult and underreporting is common.27 However, the incidence of 14.0% was still higher than expected. In contrast to the hypothesis, antibiotic prophylaxis did not lower the SSI rate and it exceeds rates reported following open reduction and internal fixation.1
The high rate of SSIs following removal of orthopedic implants could be a result of a low threshold for start of antibiotic treatment upon the slightest suspicion of a SSI (eg, wound dehiscence without infection), which might have caused overtreatment. We used the CDC criteria as the primary end point criterion; these state that a wound is classified as infected whenever antibiotic treatment has been started. As a result, patients with a wound dehiscence or wound edge necrosis who started antibiotic treatment were registered as patients with an SSI. The relatively high number of patients with an SSI without collection of a wound culture swab supports this hypothesis.
Cefazolin was used in this study—a first-generation cephalosporin, which is a broad spectrum antibiotic with good and rapid bone, soft tissue, and muscle concentrations.28 Cephalosporins have proven to be effective as antibiotic prophylaxis in orthopedic trauma surgery.1 It is widely used, as confirmed by a recent survey among US orthopedic surgeons in which 96% of the respondents reported to use cefazolin as the standard antibiotic prophylaxis.29 Several microorganisms, which were cultured in patients with SSI, were not sensitive for cefazolin (12.8%). However, the majority was sensitive (87.2%); therefore, cefazolin appears to be an adequate option for antibiotic prophylaxis.
Timing of administration of antibiotic prophylaxis has shown to be of importance. Antibiotics should be administered within 1 hour prior to incision.30,31 The allocated intervention was administered within this timespan as randomization was performed in the operating room holding or in the operation theater. Additionally, some advocate repeated doses of antibiotic prophylaxis.29 A Cochrane review32 has shown that a single dose of antibiotic prophylaxis is sufficient and multiple doses do not lower the SSI rate in surgical fixation of long bone fractures. The guideline of the American Academy of Orthopaedic Surgeons therefore only advises redosing if procedure time exceeds 1 to 2 times the half-life of the antibiotic (1.5 to 2.0 hours for cefazolin).28 Removal of orthopedic implants lasted a mean 36 minutes (maximum, 109 minutes). As a result, not repeating prophylaxis would not have influenced the results. Even though the correct type of antibiotic was administered in the correct dose at the right time without need for redosing, a high SSI rate was still observed following removal of orthopedic implants.
There were numerically more deep SSIs in the saline group than in the cefazolin group (1 patient [0.4%] in the cefazolin group vs 7 patients [2.9%] in the saline group), although this difference was not statistically different. These results, however, need to be interpreted with care. First, it was a data-driven analysis on a secondary outcome measure. Second, the study was not powered for detecting a difference in deep SSIs mandating careful interpretation.
Several causes for the failure of the primary outcome in the design of a randomized clinical trial are identified: under powering, inadequate primary outcome, inappropriate population, inappropriate treatment regimen, and deficiencies in trial conduct.33 An adequate sample size calculation was performed based on extensive evaluation of available literature with conservative estimation of treatment effect, with an appropriate and unambiguous primary outcome measure and including the appropriate population and trial regimen.
Postoperative health-related quality of life measured through the EQ-5D-3L questionnaire did not statistically significantly differ between the 2 study groups. It was, however, lower compared with reference data.34 This was also the case for the self-rated health status incorporated in the EQ-5D-3L questionnaire.34 Functional outcome did not differ statistically significantly between the 2 study groups. Functional outcome was considerably lower compared with normative data provided for the LEFS questionnaire. However, when comparing functional outcome in this cohort with patients with a history of a lower extremity fracture in this study, functionality was comparable.35 Overall, patients were satisfied with treatment, as reflected by the mean score of 7.7 on the treatment satisfaction (VAS) scale. This did not statistically significantly differ between the 2 study groups.
In addition to the negative effects of an SSI for the patient, SSIs incur a financial penalty to the health care system. In the current era of increasing burden of health care–related costs on governmental spending, prevention of SSI is of importance as a superficial SSI may cost up to $1700 per case and a deep SSI may cost up to $21 200 per case.36
Awareness that removal of orthopedic implants is not a straightforward procedure is needed. The procedure is accompanied by a high rate of SSIs. Patients should be adequately counseled on the risks of SSI following removal of orthopedic implants.
Limitations
This study has several limitations. First, a dose of 1000 mg of cefazolin was administered, which was standard dosage at the time of development of the trial (and is still the standard dosage for surgery lasting <60 minutes).18 In recently published studies, some advocate administration of 2000 mg cefazolin if a patient weighs more than 60 kg13 or 85 kg.28 However, this practice is solely based on expert opinion and pharmacokinetic data and there is no high-quality evidence for this practice.13 A post hoc, exploratory, subgroup analysis including patients weighing less than 60 kg and 60 kg or more was performed. In these subgroup analyses, no statistical differences between the 2 subgroups in the rate of SSI were found, although a potential benefit associated with antibiotic prophylaxis was observed in patients with a body weight less than 60 kg. This analysis was, however, a data-driven, post hoc, underpowered analysis. Research on whether the biological availability of antibiotics (in this concentration) in the lower extremity is adequate enough to prevent development of an SSI is therefore warranted. In addition, antibiotic susceptibility may be reduced thousand-fold when a biofilm is present.37 This is especially important in case of incomplete removal of orthopedic implants. Second, a potential beneficial effect on preventing deep SSIs was observed. However, the study was not powered for analysis on this secondary outcome measure, hampering the interpretation of this finding. Third, the number of eligible patients in the participating centers is not available; as a result, selection bias might be present. Nevertheless a randomized clinical trial using blinded outcome assessment analyzed with appropriate statistical methods was performed ensuring the quality of the presented data.
Conclusions
Among patients undergoing surgery for removal of orthopedic implants used for treatment of fractures below the knee, a single preoperative dose of intravenous cefazolin compared with saline did not reduce the risk of surgical site infection within 30 days following implant removal.
Trial Protocol and Statistical Analysis Plan
eAppendix. Participating Study Centers in the Netherlands With Number of Included Patients
eTable 1. Growth in Culture Swabs of Patients With SSI
eTable 2. Post hoc Exploratory Unadjusted Subgroup Analyses by Weight and by Type of Removal of Orthopedic Implants Concerning the SSI Rate by Study Arm
eTable 3. Patient and Surgical Characteristics and Association With SSI Within 30 Days
References
- 1.Boxma H, Broekhuizen T, Patka P, Oosting H. Randomised controlled trial of single-dose antibiotic prophylaxis in surgical treatment of closed fractures: the Dutch Trauma Trial. Lancet. 1996;347(9009):1133-1137. [DOI] [PubMed] [Google Scholar]
- 2.Vos D, Hanson B, Verhofstad M. Implant removal of osteosynthesis: the Dutch practice: results of a survey. J Trauma Manag Outcomes. 2012;6(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backes M, Schep NW, Luitse JS, Goslings JC, Schepers T. High rates of postoperative wound infection following elective implant removal. Open Orthop J. 2015;9(1):418-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raahave D. Postoperative wound infection after implant and removal of osteosynthetic material. Acta Orthop Scand. 1976;47(1):28-35. [DOI] [PubMed] [Google Scholar]
- 5.Richards RH, Palmer JD, Clarke NM. Observations on removal of metal implants. Injury. 1992;23(1):25-28. [DOI] [PubMed] [Google Scholar]
- 6.Sanderson PL, Ryan W, Turner PG. Complications of metalwork removal. Injury. 1992;23(1):29-30. [DOI] [PubMed] [Google Scholar]
- 7.Minkowitz RB, Bhadsavle S, Walsh M, Egol KA. Removal of painful orthopaedic implants after fracture union. J Bone Joint Surg Am. 2007;89(9):1906-1912. [DOI] [PubMed] [Google Scholar]
- 8.Pot JH, Van Wensen RJA, Olsman JG. Hardware related pain and hardware removal after open reduction and internal fixation of ankle fractures. Foot Ankle Online J. 2011;4(5):1-6. [Google Scholar]
- 9.Schepers T, Van Lieshout EM, de Vries MR, Van der Elst M. Complications of syndesmotic screw removal. Foot Ankle Int. 2011;32(11):1040-1044. [DOI] [PubMed] [Google Scholar]
- 10.Backes M, Schep NW, Luitse JS, Goslings JC, Schepers T. Indications for implant removal following intra-articular calcaneal fractures and subsequent complications. Foot Ankle Int. 2013;34(11):1521-1525. [DOI] [PubMed] [Google Scholar]
- 11.Leaper DJ. Risk factors for surgical infection. J Hosp Infect. 1995;30(suppl):127-139. [DOI] [PubMed] [Google Scholar]
- 12.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR; Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee . Guideline for prevention of surgical site infection, 1999. Am J Infect Control. 1999;27(2):97-132. [PubMed] [Google Scholar]
- 13.Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. ; Healthcare Infection Control Practices Advisory Committee . Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784-791. [DOI] [PubMed] [Google Scholar]
- 14.Wukich DK, Lowery NJ, McMillen RL, Frykberg RG. Postoperative infection rates in foot and ankle surgery: a comparison of patients with and without diabetes mellitus. J Bone Joint Surg Am. 2010;92(2):287-295. [DOI] [PubMed] [Google Scholar]
- 15.Andersen MR, Frihagen F, Madsen JE, Figved W. High complication rate after syndesmotic screw removal. Injury. 2015;46(11):2283-2287. [DOI] [PubMed] [Google Scholar]
- 16.Backes M, Dingemans SA, Schep NW, et al. Wound infections following implant removal below the knee. BMC Surg. 2015;15(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 18.SWAB . SWAB guideline: perioperatieve profylaxe. http://www.swab.nl/swab/cms3.nsf/uploads/6E0F2BC314131641C12580BB0049E90B/$FILE/SWAB%20richtlijn%20perioperatieve%20profylaxe%20algemeen%20def%20290117.pdf. Accessed October 1, 2017.
- 19.Lamers LM, Stalmeier PFM, McDonnell J, Krabbe PFM, van Busschbach JJ. Measuring the quality of life in economic evaluations: the Dutch EQ-5D tariff [in Dutch]. Ned Tijdschr Geneeskd. 2005;149(28):1574-1578. [PubMed] [Google Scholar]
- 20.Binkley JM, Stratford PW, Lott SA, Riddle DL; North American Orthopaedic Rehabilitation Research Network . The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther. 1999;79(4):371-383. [PubMed] [Google Scholar]
- 21.Olsen LL, Møller AM, Brorson S, Hasselager RB, Sort R. The impact of lifestyle risk factors on the rate of infection after surgery for a fracture of the ankle. Bone Joint J. 2017;99-B(2):225-230. [DOI] [PubMed] [Google Scholar]
- 22.Stratford P, Hart D, Binkley J, Kennedy D, Alcock G, Hanna S. Interpreting lower extremity functional status scores. Physiother Can. 2005;57:154-162. doi: 10.2310/6640.2005.00023 [DOI] [Google Scholar]
- 23.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 24.Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med. 2007;26(19):3661-3675. [DOI] [PubMed] [Google Scholar]
- 25.Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Visser A, Ubbink DT, Gouma DJ, Goslings JC. Surgeons are overlooking post-discharge complications: a prospective cohort study. World J Surg. 2014;38(5):1019-1025. [DOI] [PubMed] [Google Scholar]
- 28.Prokuski L. Prophylactic antibiotics in orthopaedic surgery. J Am Acad Orthop Surg. 2008;16(5):283-293. [DOI] [PubMed] [Google Scholar]
- 29.Gans I, Jain A, Sirisreetreerux N, Haut ER, Hasenboehler EA. Current practice of antibiotic prophylaxis for surgical fixation of closed long bone fractures: a survey of 297 members of the Orthopaedic Trauma Association. Patient Saf Surg. 2017;11(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35(4):322-332. [DOI] [PubMed] [Google Scholar]
- 31.de Jonge SW, Gans SL, Atema JJ, Solomkin JS, Dellinger PE, Boermeester MA. Timing of preoperative antibiotic prophylaxis in 54 552 patients and the risk of surgical site infection: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(29):e6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bryson DJ, Morris DLJ, Shivji FS, Rollins KR, Snape S, Ollivere BJ. Antibiotic prophylaxis in orthopaedic surgery: difficult decisions in an era of evolving antibiotic resistance. Bone Joint J. 2016;98-B(8):1014-1019. [DOI] [PubMed] [Google Scholar]
- 33.Gillespie WJ, Walenkamp G. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database Syst Rev. 2001;(1):CD000244. [DOI] [PubMed] [Google Scholar]
- 34.Pocock SJ, Stone GW. The primary outcome fails—what next? N Engl J Med. 2016;375(9):861-870. [DOI] [PubMed] [Google Scholar]
- 35.Szende A, Janssen B, Cabases J. Self-Reported Population Health: An International Perspective based on EQ-5D. Dordrecht, the Netherlands: Springer; 2014. [PubMed] [Google Scholar]
- 36.Dingemans SA, Kleipool SC, Mulders MAM, et al. Normative data for the lower extremity functional scale (LEFS). Acta Orthop. 2017;88(4):422-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institute for Public Health and the Environment . Incidence of in-hospital infections 2007-2014 [in Dutch]. http://www.rivm.nl/Onderwerpen/P/PREZIES/Prevalentieonderzoek_Ziekenhuizen/Referentiecijfers_Prevalentieonderzoek_ziekenhuizen/Referentiecijfers_Prevalentie_t_m_oktober_2014.org. Accessed October 1, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eAppendix. Participating Study Centers in the Netherlands With Number of Included Patients
eTable 1. Growth in Culture Swabs of Patients With SSI
eTable 2. Post hoc Exploratory Unadjusted Subgroup Analyses by Weight and by Type of Removal of Orthopedic Implants Concerning the SSI Rate by Study Arm
eTable 3. Patient and Surgical Characteristics and Association With SSI Within 30 Days