Key Points
Question
What is the association between modifiable biological, neighborhood, psychosocial, socioeconomic, and behavioral factors during young adulthood and the higher risk for type 2 diabetes in black adults vs white adults?
Findings
In this retrospective analysis of a cohort study of 4251 participants, there was a statistically significant increased risk for incident type 2 diabetes in black vs white individuals. However, after adjustment for biological, neighborhood, psychosocial, socioeconomic, and behavioral factors during young adulthood, the disparity was no longer statistically significant (hazard ratio for women, 0.79; hazard ratio for men, 0.92).
Meaning
Differences in traditional modifiable diabetes risk factors between black and white individuals may contribute to the racial disparity in diabetes incidence in middle age.
Abstract
Importance
In the United States, black individuals are twice as likely to develop type 2 diabetes compared with white individuals, and these disparities are particularly pronounced in young and middle age. Prior studies have identified differences in traditional risk factors that may be associated with racial disparities in diabetes incidence but have not simultaneously adjusted for risk factors measured across multiple domains (eg, the individual and the environment) and updated over time.
Objective
To determine the relative associations of modifiable biological, neighborhood, psychosocial, socioeconomic, and behavioral factors in young adulthood with the observed racial disparity in diabetes incidence between middle-aged black and white individuals.
Design, Setting, and Participants
Black and white men and women from the observational Coronary Artery Risk Development in Young Adults study, aged 18 to 30 years, without diabetes at baseline (1985-1986; N = 4251) were observed through 2015-2016. Sex-stratified multivariable-adjusted Cox proportional hazards modeling, with adjustment for time-updated covariates, was used to estimate risk for incident diabetes. Percent reduction in the β coefficient (the logarithm used to calculate the hazard ratio [HR]) was calculated to compare black to white participants.
Exposures
Self-identified race and factors including biological (eg, fasting glucose, body mass index), neighborhood (racial segregation and tract-level poverty), psychosocial (depressive symptoms), socioeconomic (eg, personal and parental educational attainment, current employment), and behavioral (eg, regular alcohol consumption, smoking) domains.
Main Outcomes and Measures
Incident type 2 diabetes mellitus.
Results
The mean (SD) age at baseline was 25 (3.6) years, 49% (n = 2066) of the sample was black, and 54% (n = 2304) were women. Over a mean follow-up of 24.5 years, 504 cases of incident diabetes were identified. Using sex-stratified multivariable-adjusted Cox proportional hazards models, black women and men were more likely to develop diabetes than white men and women (black women: HR, 2.86 [95% CI, 2.19-3.72] and risk difference [RD], 89 cases/1000 people [95% CI, 61-117]; black men: HR, 1.67 [95% CI, 1.28-2.17] and RD, 47 cases/1000 people [95% CI, 15-78]) after adjustment for age and center. Biological factors were most strongly associated with the disparity in diabetes risk between black and white individuals for women (percent reduction in β, 112%) and men (percent reduction in β, 86%). There was no longer disparity in diabetes risk between black and white middle-aged adults after adjustment for biological, neighborhood, psychosocial, socioeconomic, and behavioral factors measured over time (HR for women, 0.79 [95% CI, 0.55-1.14]; HR for men, 0.92 [95% CI, 0.62-1.38]).
Conclusions and Relevance
In this cohort study comparing black and white participants, there was a statistically significant increased risk of incident type 2 diabetes among black women and men. However, after adjustment for modifiable risk factors during young adulthood, the disparity was no longer statistically significant.
This study uses data from the observational Coronary Artery Risk Development in Young Adults study to assess disparity in diabetes risk between black and white middle-aged adults.
Introduction
The prevalence of type 2 diabetes among black adults in the United States is nearly twice that of their white counterparts, and the difference in prevalence and incidence between black and white adults has increased between 1980 and 2012. Brancati et al first described the relative association of established diabetes risk factors with the racial differences observed in diabetes incidence in middle-aged and older adults in 2000. However, racial and ethnic minority populations remain at higher risk for incident diabetes and its complications, leading the American Diabetes Association to prioritize the elimination of disparities in diabetes research, treatment, and education. Epidemiological studies have identified factors at the individual and neighborhood levels that are associated with the excess prevalence of diabetes among black vs white individuals, but those studies include middle-aged and older-aged populations. However, since 2002, diabetes incidence has increased most rapidly among black youth and young adults as compared with white individuals in the same age groups.
The objective of this study was to characterize exposure to modifiable biological, neighborhood, psychosocial, socioeconomic, and behavioral factors during young adulthood and quantify the extent to which these factors and their changes over time are associated with the disparity in diabetes incidence between black and white participants over 30 years of follow-up. The study hypothesis was that differences in biological factors during young adulthood would be most strongly associated with the disparity in diabetes incidence in middle age between black and white individuals.
Methods
The Coronary Artery Risk Development in Young Adults (CARDIA) is a multicenter observational cohort study. Briefly, in 1985-1986, 5115 black and white adults (study participants self-reported race as non-Hispanic black or white) aged 18 to 30 years were recruited from 4 US urban communities (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California). Study recruitment was approximately balanced on age (<25 years and ≥25 years) sex, race, and education (≤high school graduation and >high school education). In-person examinations occurred at 2, 5, 7, 10, 15, 20, 25, and 30 (2015-2016) years after baseline with annual telephone interviews to update health status and contact information.
Institutional review boards at each study center approved research protocols, and participants provided written informed consent at each examination.
Ascertainment of Diabetes
Serum glucose was measured using the hexokinase ultraviolet method at baseline and using hexokinase coupled to glucose-6-phosphate dehydrogenase at subsequent examinations; values were standardized across examinations. Hemoglobin A1c was measured using Tosoh G7 (variant mode) high-performance liquid chromatography (National Glycohemoglobin Standardization Program–certified assays). Diabetes status was determined at each examination using a combination of available information on fasting glucose greater than or equal to 126 mg/dL, postchallenge (75 g) glucose greater than or equal to 200 mg/dL, hemoglobin A1c greater than or equal to 6.5%, or use of diabetes medications (eAppendix in the Supplement).
Clinical Measurements and Questionnaires
The diabetes risk factors that were selected were modifiable, available in the study, known to vary by race in the literature, and categorized into the following groups: biological, neighborhood, psychosocial, socioeconomic, and behavioral.
Biological
At baseline, participants self-reported their parents’ history of diabetes. The following measurements were assessed by research staff during each clinical examination with participants wearing light clothing but no shoes. Waist circumference was measured midway between the xiphoid process and the iliac crest and determined as the mean of the 2 measurements. Body weight was measured with a calibrated scale, and height was measured using a vertical ruler. Body mass index was calculated as weight in kilograms divided by height in meters squared. Medications were assessed using the medication inventory. Fasting lipids were measured from ethylenediaminetetraacetic acid plasma. High-density lipoprotein cholesterol was determined after precipitation with dextran sulfate-magnesium chloride, triglycerides were measured enzymatically, and a ratio of the 2 analytes was used for analysis. Blood pressure was assessed at each examination in triplicate with the average of the last 2 measures used for analysis. For years 0 through 15 blood pressure was measured with a random-zero sphygmomanometer. At years 20, 25, and 30 blood pressure was measured using an automated oscillometric blood pressure monitor and values were standardized across examinations to sphygmomanometric measures.
The following measurements were assessed by research staff at other time points. Maximum forced vital capacity was measured at examination years 0, 2, 5, 10, and 20 by forced spirometry (maximum of 5 trials) using an 8-L water-sealed spirometer and a microprocessor.
Neighborhood
Census tracts from the 1980 US Census data were used as proxies for neighborhoods. Residential racial segregation was quantified using the Getis and Ord local G statistic (Gi*), a spatial autocorrelation measure that estimates the extent to which the racial composition (in this case, percentage of black race) of a participant’s neighborhood and surrounding neighborhoods deviates from the racial composition of the surrounding metropolitan area or county. The G statistic returns a z score (higher positive values indicate living in a neighborhood with a higher than expected percentage of black residents; higher negative values indicate living in a neighborhood with a lower than expected percentage of black residents). Neighborhood poverty was defined as the tract-level percentage of population living in poverty as defined by the US Census. Neighborhood data were measured at examination years 0, 7, 10, 15, 20, and 25.
Psychosocial
Depressive symptoms were measured with the 20-item Center for Epidemiologic Studies–Depression questionnaire at examination years 5, 10, 15, 20, 25, and 30 (score range, 0 [low depressive symptomatology] to 60 [high depressive symptomatology]). The validated cut point score of 16 or greater was used to indicate clinically depressive symptoms at each examination.
Socioeconomic
Level of education of participants and their respective parents, current employment status (full-time, part-time, in school, home or child caretaker, unemployed, and other), and marital status were self-reported at examination years 0, 5, 7, 10, 15, 20, 25, and 30. Participants reported the difficulty for their household in paying for basics (eg, food, medical care, and heating), with the responses of very hard, hard, somewhat hard, not very hard, or don’t know.
Behavioral
Self-administered questionnaires were used to collect information on smoking status and alcohol consumption at each examination. Participants who reported regular consumption of beer, wine, and liquor over the previous week were categorized according to habitual consumption. Dietary intake for the previous 28 days was assessed by a diet history questionnaire at examination years 0, 7, and 20, which included 1609 unique food items and provided participants with food and serving size models for reference. We quantified diet according to the American Heart Association’s Life’s Simple 7 metric for meeting ideal consumption of fruits and vegetables, fiber-rich whole grains, fish, sodium, and sugar-sweetened beverages, previously modeled in this cohort. Physical activity was assessed at each examination as the frequency of participation over the previous 12 months for 13 moderate- and vigorous-intensity activities and reported as a score in exercise units, showing high test-retest reliability (0.77-0.84) over a 2-week period.
Statistical Analysis
Cox proportional hazards models were used to estimate the hazard ratio (HR) for incident diabetes comparing black to white participants (reference standard) with time at risk estimated as time from the baseline examination until date of examination at which diabetes was first diagnosed or administrative censoring at the last attended examination. We decided a priori to stratify models by sex based on literature suggesting that the racial disparity in diabetes risk between black and white individuals is stronger for women than for men. We estimated the extent to which groups of covariates were associated with the disparity in incident diabetes between black and white participants by calculating the percent reduction in β estimates associated with adjustment according to the formula ([β0]−[βn])÷(β0)×100.
Our base model adjusted for age and field center (model 1 [M1]). We used multiple modeling strategies to better understand the cumulative and individual association of each risk factor group. First, we modeled sequential adjustment adding each group to the previous model, beginning with the biological (M2) factors added to the base model. We then added neighborhood (M3), psychosocial (M4), socioeconomic (M5), and behavioral risk factor groups (M6), and estimated the percent reduction in β estimates for each sequentially adjusted model relative to the base model. The final adjusted model (M6) included all risk factor groups. In our second modeling approach, we estimated the percent reduction in β estimates by each group of covariates as compared with the base model without adjustment for the other groups. In our third modeling approach, we estimated the percent reduction in β estimates when excluding each group from the final adjusted model (excluding 1 group) to reflect the remaining difference when one group is not included in the model for adjustment. For example, a model adjusted for biological, neighborhood, psychosocial, and socioeconomic factors would reflect the excess diabetes risk when not adjusting for behavioral factors. Models were run with risk factors assessed and updated at each examination (primary) and when assessed at baseline only (secondary). The last observation was carried forward for risk factors that were not measured at each examination.
The proportional hazards assumption was tested by adding a term reflecting the product of race and natural-log of time at risk, and no violations of this assumption were detected. We also assessed the sensitivity of our estimates to usual medical care services and when using multiple imputation to impute missing risk factor group data (eAppendix in the Supplement). A 2-sided P value of less than .05 was considered statistically significant. SAS software version 9.4 was used for all analyses.
Results
We excluded 1 participant who withdrew consent for study participation, 35 participants with prevalent or unknown diabetes status at baseline, and 158 participants missing follow-up information after baseline necessary to classify diabetes status. We excluded 670 participants (14% of remaining sample) who were missing information for at least 1 of the risk factors of interest, resulting in an analytic sample of 4251. Exclusions are enumerated in eTable 1 in the Supplement, and a comparison of characteristics between participants included and excluded from analysis is shown in eTable 2 in the Supplement. Descriptive baseline characteristics of participants who were not identified as an incident diabetes case over follow-up are presented according to the last attended examination in eTable 3 in the Supplement. Of the 2637 black participants enrolled in this study, 2066 (78%) were included in this analysis, while 2185 (88%) of the 2478 enrolled white participants were included. Participant characteristics at baseline are presented in Table 1. The mean (SD) age at baseline was 25 (3.6) years, 49% of the sample was black, and 54% (2304) were women. On average, black participants had less favorable socioeconomic, behavioral, and neighborhood profiles as compared with whites.
Table 1. Participant Characteristics at Baseline (1985-1986) According to Sex and Race.
| Patient Characteristics and Risk Factors | Women (n = 2304) | Men (n = 1947) | ||
|---|---|---|---|---|
| Black (n = 1159) |
White (n = 1145) |
Black (n = 907) |
White (n = 1040) |
|
| Age, mean (SD), y | 24.5 (3.9) | 25.5 (3.4) | 24.3 (3.7) | 25.5 (3.3) |
| Socioeconomic, No. (%) | ||||
| Education >16 y | 32 (3) | 184 (16) | 41 (5) | 188 (18) |
| Married | 235 (20) | 328 (29) | 174 (19) | 237 (23) |
| Financial statusa | 438 (38) | 366 (32) | 353 (39) | 274 (26) |
| Employed full time | 542 (47) | 722 (63) | 495 (55) | 716 (69) |
| Mother’s education >16 y | 50 (4) | 131 (11) | 43 (5) | 114 (11) |
| Father’s education >16 y | 44 (4) | 246 (21) | 27 (3) | 216 (21) |
| Neighborhood | ||||
| G statistic of black segregation, mean (SD)b | 4.7 (3.0) | 1.5 (3.1) | 4.7 (3.1) | 1.6 (3.3) |
| Percentage of census tract living in poverty, mean (SD) | 23 (13) | 13 (10) | 24 (13) | 13 (10) |
| Psychosocial | ||||
| CES-Depression score, mean (SD)c | 13.3 (9.3) | 10.2 (8.0) | 11.5 (7.3) | 9.5 (6.9) |
| Behavioral | ||||
| Smoking, No. (%) | ||||
| Never | 691 (60) | 607 (53) | 514 (57) | 605 (58) |
| Former | 107 (9) | 236 (21) | 84 (9) | 161 (15) |
| Current | 361 (31) | 302 (26) | 309 (34) | 274 (26) |
| Daily alcohol intake, No. (%) | ||||
| None | 669 (58) | 427 (37) | 309 (34) | 246 (24) |
| Moderate | 414 (36) | 535 (47) | 490 (54) | 637 (61) |
| Heavy | 76 (6) | 183 (16) | 108 (12) | 157 (15) |
| Physical activity, mean (SD), exercise unitsd | 278 (226) | 400 (255) | 514 (327) | 508 (296) |
| Ideal diet score, mean (SD)e | 1.1 (1.0) | 1.7 (1.1) | 0.9 (0.9) | 1.3 (1.1) |
| Biological, mean (SD) | ||||
| Fasting glucose, mg/dL | 79 (8) | 81 (8) | 83 (8) | 84 (8) |
| Prediabetes (fasting glucose ≥100 mg/dL), No. (%) | 18 (2) | 19 (2) | 16 (2) | 32 (3) |
| Body mass indexf | 26.1 (6.5) | 23.1 (4.3) | 24.6 (4.1) | 24.3 (3.6) |
| Waist circumference, cm | 77 (13) | 72 (9) | 81 (10) | 83 (9) |
| Systolic blood pressure, mm Hg | 108 (10) | 105 (9) | 116 (10) | 114 (10) |
| Diastolic blood pressure, mm Hg | 67 (10) | 66 (8) | 71 (10) | 71 (9) |
| Use of blood-pressure lowering medications, No. (%) | 42 (4) | 11 (1) | 19 (2) | 15 (1) |
| High-density lipoprotein cholesterol, mg/dL | 55 (13) | 56 (13) | 53 (13) | 47 (11) |
| Triglycerides, mg/dL | 63 (32) | 70 (39) | 70 (41) | 90 (69) |
| Parental history of diabetes (mother or father), No. (%) | 220 (19) | 132 (12) | 131 (14) | 109 (10) |
| Forced vital capacity, L | 3.4 (0.5) | 3.9 (0.5) | 4.6 (0.7) | 5.5 (0.7) |
Abbreviation: CES, Center for Epidemiologic Studies.
SI conversion factors: To convert glucose to mmol/L, multiply by 0.0556; high-density lipoprotein to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113.
Described by participants as somewhat hard, hard, or very hard to pay for basics.
Interpretation of the G statistic of black segregation is similar to a z score (>1.96 would indicate living in a residential area with a significantly greater than expected percentage of black residents; <−1.96 would indicate a significantly lower than expected percentage). Values between −1.96 and 1.96 would indicate no appreciable difference in actual percentage of black residents compared with the expected.
A validated cut point of ≥16 was used to indicate clinically depressive symptoms at each examination (score range, 0 [low depressive symptomatology] to 60 [high depressive symptomatology]).
Physical activity was assessed at each examination as the frequency of participation over the previous 12 months for 13 moderate- and vigorous-intensity activities and reported as a score in exercise units.
Based on the American Heart Association’s Life’s Simple 7 metric for meeting ideal consumption of fruits and vegetables, fiber-rich whole grains, fish, sodium, and sugar-sweetened beverages (score range, 0-5 with a higher score indicating better adherence to the Simple 7 metric).
Body mass index was calculated as weight in kilograms divided by height in meters squared.
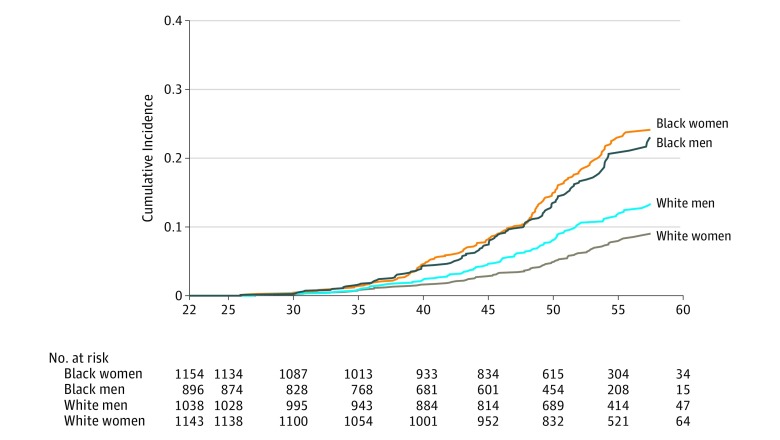
Over a mean follow-up of 24.5 years, 315 cases of incident diabetes were identified among black participants and 189 incident cases were identified among white participants. Ascertainment of diabetes cases is detailed in the eAppendix in the Supplement. The Figure presents cumulative incidence curves according to race and sex. The unadjusted incidence for diabetes was 86 cases/1000 people for white individuals and 152 cases/1000 people for black individuals, resulting in an excess of 66 cases of diabetes/1000 people for black compared with white individuals. When adjusted for age, sex, and field center, this risk difference estimate was 67 cases/1000 people for black compared with white individuals (95% CI, 48/1000-89/1000). The HR for diabetes and percent reduction in β estimates according to each model are presented in Table 2. Models stratified by sex showed evidence for effect by sex in our base model (χ2 = 7.48; 1 degree of freedom; P = .006) based on prior literature suggesting the racial disparity in diabetes risk between black and white individuals is stronger for women than for men. In the base model, black participants had greater risk for incident diabetes compared with white participants (HR, 2.22 [95% CI, 1.84-2.67]); for men, the HR was 1.67 (95% CI, 1.28-2.17); whereas for women, the HR was 2.86 (95% CI, 2.19-3.72). In the primary analysis, which included updated data for each risk factor, the disparity in diabetes HR estimates for women (HR, 0.88 [95% CI, 0.64-1.21]) and men (HR, 1.08 [95% CI, 0.77-1.51]) were no longer observed after adjustment for biological factors, indicating no significant difference in diabetes risk between black and white individuals. Further adjustment for the updated neighborhood, psychosocial, socioeconomic, and behavioral risk factors did not change this finding. Table 3 presents HRs per standard deviation unit increment for continuous characteristics or according to categorical characteristics for the final model when including updated risk factor data.
Figure. Cumulative Incidence of Diabetes for Black and White Women and Men From 1985 to 2016.
The median follow-up time was 29.9 years (interquartile range [IQR], 25.2-30.2) for white women, 29.6 years (IQR, 19.9-30.1) for black women, 29.7 years (IQR, 20.8-30.1) for white men, and 29.3 years (IQR, 15.2-30.1) for black men.
Table 2. Hazard Ratios for Incident Diabetes in Black vs White Participants and Percent Reduction in Parameter Estimatesa.
| Women | Men | |||
|---|---|---|---|---|
| Black | White | Black | White | |
| No. | 1159 | 1145 | 907 | 1040 |
| Cases/person-years | 188/27 694 | 81/29 957 | 127/20 644 | 108/26 051 |
| Risk difference/1000 people (95% CI)b | 89 (61-117) | 47 (15-78) | ||
| HRs by Model | ||||
| Race HR (95% CI) | Reduction in β, %c | Race HR (95% CI) | Reduction in β, %c | |
| Updated Risk Factor Information Adjustment | ||||
| Model 1: age and field center | 2.86 (2.19-3.72) | [Reference] | 1.67 (1.28-2.17) | [Reference] |
| Model 2: model 1 + biologicald | 0.88 (0.64-1.21) | 112 | 1.08 (0.77-1.51) | 86 |
| Model 3: model 2 + neighborhoode | 0.87 (0.61-1.23) | 113 | 0.97 (0.66-1.42) | 106 |
| Model 4: model 3 + psychosocialf | 0.86 (0.60-1.22) | 115 | 0.96 (0.66-1.41) | 107 |
| Model 5: model 4 + socioeconomicf | 0.80 (0.56-1.16) | 121 | 0.90 (0.60-1.34) | 121 |
| Model 6: model 5 + behavioralg | 0.79 (0.55-1.14) | 122 | 0.92 (0.62-1.38) | 116 |
| Baseline Risk Factor Adjustment | ||||
| Model 2: model 1 + biologicald | 1.56 (1.14-2.14) | 58 | 1.48 (1.07-2.05) | 23 |
| Model 3: model 2 + neighborhoode | 1.36 (0.95-1.95) | 71 | 1.66 (1.15-2.40) | 1 |
| Model 4: model 3 + psychosocialf | 1.36 (0.95-1.95) | 71 | 1.66 (1.14-2.39) | 1 |
| Model 5: model 4 + socioeconomicg | 1.20 (0.83-1.74) | 82 | 1.59 (1.08-2.34) | 9 |
| Model 6: model 5 + behavioralh | 1.22 (0.84-1.78) | 81 | 1.46 (0.99-2.16) | 25 |
Abbreviation: HR, hazard ratio.
Analyses were according to sequential adjustment for each risk factor domain when including updated risk factor information for model adjustment and when adjusting for baseline measurement of risk factors.
Risk difference indicates age and field center adjusted presenting excess cases in black compared with white participants.
Percent reduction in β estimate ([β0–βn]÷[β0]×100). β0 indicates an age and field center–adjusted reference model. β0 as an exponent with the base e equals the HR estimate for an age- and field center–adjusted model. The percent reduction in β estimate from β0 to βn is the percent reduction in risk for diabetes on the log-scale comparing black individuals to white individuals that is associated with adjustment for the factors in the βn model.
Biological factors: fasting glucose, body mass index, waist circumference, parental history of diabetes, triglycerides to high-density lipoprotein cholesterol ratio, forced vital capacity, systolic blood pressure, and blood pressure–lowering medication use.
Neighborhood factors: G statistic for racial segregation and tract-level percentage of population living in poverty.
Psychosocial factor: Center for Epidemiologic Studies–Depression score.
Socioeconomic factors: education, current employment status, paying for basics, marital status, and mother’s and father’s educational attainment
Behavioral factors: regular alcohol consumption, smoking status, diet score from the American Heart Association’s Life’s Simple 7, and regular physical activity.
Table 3. Hazard Ratios for Incident Diabetes per Standard Deviation Unit Increment in Continuous Factors or According to Category When Including Updated Risk Factor Informationa.
| Risk Factor | Hazard Ratio (95% CI) | |
|---|---|---|
| Women | Men | |
| Black race | 0.79 (0.55-1.14) | 0.92 (0.62-1.38) |
| Age, per 3.6 y | 1.08 (0.94-1.23) | 1.33 (1.14-1.56) |
| Site, Birmingham, Alabama | [Reference] | [Reference] |
| Chicago, Illinois | 0.86 (0.59-1.25) | 0.80 (0.53-1.20) |
| Minneapolis, Minnesota | 0.80 (0.54-1.19) | 0.75 (0.50-1.12) |
| Oakland, California | 0.78 (0.54-1.13) | 0.67 (0.44-1.01) |
| Socioeconomic | ||
| Mother’s education, ≤12 y | [Reference] | [Reference] |
| Unknown | 1.26 (0.78-2.04) | 1.04 (0.62-1.74) |
| 13-15 y | 1.05 (0.75-1.47) | 1.24 (0.84-1.81) |
| ≥16 y | 0.95 (0.50-1.8) | 1.02 (0.56-1.84) |
| Father’s education, ≤12 y | [Reference] | [Reference] |
| Unknown | 1.17 (0.83-1.66) | 1.33 (0.87-2.03) |
| 13-15 y | 0.89 (0.60-1.32) | 1.04 (0.68-1.59) |
| ≥16 y | 0.90 (0.48-1.70) | 1.06 (0.60-1.85) |
| Participant’s education, ≥16 y | [Reference] | [Reference] |
| 13-15 y | 1.40 (0.93-2.12) | 1.35 (0.90-2.03) |
| ≤12 y | 1.13 (0.69-1.86) | 1.27 (0.78-2.08) |
| Marriage status, single | [Reference] | [Reference] |
| Married | 0.80 (0.57-1.13) | 0.91 (0.62-1.34) |
| Separated or widowed | 0.72 (0.50-1.04) | 1.18 (0.78-1.79) |
| Financial statusb | 1.35 (1.01-1.80) | 0.91 (0.65-1.28) |
| Employment status, full-time | [Reference] | [Reference] |
| Part-time | 0.83 (0.55-1.26) | 0.82 (0.48-1.39) |
| Home or child care | 1.12 (0.80-1.57) | 1.59 (1.01-2.50) |
| In school | 2.46 (0.88-6.87) | 1.94 (0.27-14.1) |
| Unemployed | 0.59 (0.33-1.07) | 0.79 (0.47-1.33) |
| Neighborhood | ||
| G statistic of black segregation, per 3.52 units | 1.01 (0.81-1.26) | 1.21 (0.96-1.54) |
| Percent of census tract living in poverty, per 12% | 0.99 (0.83-1.17) | 0.88 (0.72-1.08) |
| Psychosocial, CES-Depression score ≥16 | 1.1 (0.81-1.47) | 1.25 (0.88-1.79) |
| Behavioral | ||
| Smoking, never | [Reference] | [Reference] |
| Former | 0.89 (0.64-1.24) | 1.50 (1.05-2.14) |
| Current | 1.05 (0.73-1.50) | 1.32 (0.89-1.95) |
| Daily alcohol consumption, none | [Reference] | [Reference] |
| Moderate | 0.92 (0.68-1.24) | 1.07 (0.78-1.46) |
| Heavy | 0.51 (0.29-0.93) | 0.96 (0.59-1.56) |
| Physical activity, per 291 exercise units | 1.25 (1.04-1.49) | 0.96 (0.82-1.14) |
| Diet score, per 1 component | 1.10 (0.96-1.25) | 1.02 (0.88-1.19) |
| Biological | ||
| Fasting glucose, per 8.2 mg/dL | 1.21 (1.19-1.24) | 1.20 (1.18-1.23) |
| Body mass index, per 4.9 kg/m2 | 0.93 (0.81-1.08) | 1.24 (0.97-1.58) |
| Waist circumference, per 11.2 cm | 1.73 (1.47-2.03) | 1.32 (1.02-1.70) |
| Systolic blood pressure, per 10.9 mm Hg | 1.07 (0.98-1.17) | 1.02 (0.92-1.13) |
| Use of blood pressure–lowering medications | 1.98 (1.49-2.62) | 1.81 (1.30-2.52) |
| Triglycerides to HDL-cholesterol ratio, per 1.5 | 1.00 (0.94-1.07) | 1.06 (1.03-1.09) |
| Parental history of diabetes | 1.80 (1.37-2.37) | 1.45 (1.05-1.99) |
| Forced vital capacity, per 1.0 L | 0.78 (0.62-0.99) | 0.84 (0.69-1.03) |
Abbreviations: CES, Center for Epidemiologic Studies; HDL, high-density lipoprotein.
SI conversion factor: to convert glucose to mmol/L, multiply values by 0.0556.
Model adjustments include baseline age, field center, mother’s and father’s educational attainment, and updated data for the participants’ education, current employment status, paying for basic essentials, marital status, G statistic for racial segregation, tract-level percentage of population living in poverty, CES-Depression score, regular alcohol consumption, smoking status, diet score from the American Heart Association’a Life’s Simple 7, regular physical activity, fasting glucose, body mass index, waist circumference, parental history of diabetes, triglycerides to high-density lipoprotein cholesterol ratio, forced vital capacity, systolic blood pressure, and blood pressure–lowering medication use.
Described by participants as somewhat hard, hard, or very hard to pay for basics.
In our secondary modeling analysis, we observed substantial attenuation in the HR with adjustment for baseline measurement of biological factors for women (58% reduction in β estimates) and men (23% reduction in β estimates). Adjustment for baseline neighborhood factors was associated with further attenuation in diabetes HR comparing black to white women (HR, 1.36 [95% CI, 0.95-1.95]) and strengthening of the HR for men (HR, 1.66 [95% CI, 1.15-2.40]). For both sexes, sequential adjustment for depressive symptoms had minimal effect on diabetes HRs. After adjustment for all risk factor groups, the 95% CIs for diabetes HRs for both sexes included 1. For men, adjustment for all risk factor groups resulted in less attenuation of HRs comparing black to white participants than for women. Results for these same modeling schemes of women and men combined are presented in eTable 4 in the Supplement. Including adjustment for usual medical care did not alter the HR estimates (eAppendix in the Supplement). HR estimates from the multiply imputed data were attenuated for women and stronger for men as compared with the nonimputed data (eAppendix in the Supplement).
In the primary analysis, which included updated data for each risk factor, adjustment for biological factors only was associated with the greatest percent reduction in β estimates (Table 4) for women (112%) and men (86%), and exclusion of biological factors was associated with the least attenuation in β estimates (35% for women; 47% for men). The risk factor group associated with the second strongest attenuation in β estimates was socioeconomic factors for women and also for men when the modeling scheme included updated data for each risk factor. When compared with the base model and without adjustment for other risk factor groups, adjustment for baseline measurement of biological factors only was associated with the greatest percent reduction in β estimates when comparing black with white women (58%), whereas among men, adjustment for baseline socioeconomic factors only was associated with the greatest percent reduction in β estimates (39%). For women, when compared with the base model, omitting adjustment for baseline measurement of biological factors from a model simultaneously adjusted for all risk factor groups was associated with the least attenuation of diabetes β estimates. For men, omitting adjustment for baseline behavioral factors from a simultaneously adjusted model was associated with the least attenuation of diabetes β estimates when compared with the base model.
Table 4. Hazard Ratios for Incident Diabetes in Black Compared With White Participants and Percent Reduction in Parameter Estimates According to Individual Risk Factor Groupsa.
| Individual Risk Factor Groups | Women | Men | ||
|---|---|---|---|---|
| Race HR (95% CI) | Percent Reduction in β, %b | Race HR (95% CI) | Percent Reduction in β, %b | |
| Base model: Age and field center | 2.86 (2.19-3.72) | [Reference] | 1.67 (1.28-2.17) | [Reference] |
| Updated Risk Factor Information Adjustment | ||||
| Base + biologicalc | 0.88 (0.64-1.21) | 112 | 1.08 (0.77-1.51) | 86 |
| Base + neighborhoodd | 2.70 (2.00-3.65) | 5 | 1.54 (1.12-2.12) | 15 |
| Base + psychosociale | 2.79 (2.14-3.64) | 2 | 1.62 (1.24-2.12) | 5 |
| Base + socioeconomicf | 2.20 (1.63-2.97) | 25 | 1.34 (0.99-1.82) | 42 |
| Base + behavioralg | 2.29 (1.73-3.02) | 21 | 1.56 (1.18-2.05) | 14 |
| Risk Factors Adjusted for All Groups but Excluding 1 Group | ||||
| Excluding biologicalc | 1.98 (1.42-2.77) | 35 | 1.31 (0.93-1.84) | 47 |
| Excluding neighborhoodd | 0.79 (0.56-1.12) | 122 | 0.97 (0.67-1.41) | 106 |
| Excluding psychosociale | 0.80 (0.55-1.15) | 122 | 0.93 (0.62-1.39) | 115 |
| Excluding socioeconomicf | 0.86 (0.60-1.22) | 115 | 0.98 (0.67-1.45) | 103 |
| Excluding behavioralg | 0.80 (0.56-1.16) | 121 | 0.90 (0.60-1.34) | 121 |
| Baseline Risk Factor Adjustment | ||||
| Base + biologicalc | 1.56 (1.14-2.14) | 58 | 1.48 (1.07-2.05) | 23 |
| Base + neighborhoodd | 2.24 (1.63-3.08) | 23 | 1.68 (1.22-2.31) | −1 |
| Base + psychosociale | 2.77 (2.12-3.61) | 3 | 1.64 (1.25-2.14) | 3 |
| Base + socioeconomicf | 2.09 (1.56-2.80) | 30 | 1.36 (1.01-1.84) | 39 |
| Base + behavioralg | 2.41 (1.81-3.20) | 16 | 1.56 (1.19-2.05) | 13 |
| Risk Factors Adjusted for All Groups but Excluding 1 Group | ||||
| Excluding biologicalc | 1.67 (1.18-2.36) | 51 | 1.38 (0.97-1.95) | 37 |
| Excluding neighborhoodd | 1.34 (0.95-1.90) | 72 | 1.30 (0.91-1.86) | 49 |
| Excluding psychosociale | 1.22 (0.84-1.78) | 81 | 1.48 (1.00-2.18) | 24 |
| Excluding socioeconomicf | 1.34 (0.93-1.92) | 72 | 1.50 (1.03-2.18) | 21 |
| Excluding behavioralg | 1.20 (0.83-1.74) | 82 | 1.59 (1.08-2.34) | 9 |
Abbreviation: HR, hazard ratio.
Analyses were implemented when including updated risk factor information for model adjustment and when adjusting for baseline measurement of risk factors.
Percent reduction in β estimate ([β0–βn])÷[β0]×100). β0 indicates an age and field center–adjusted reference model. β0, as an exponent with the base e, equals the HR estimate for an age- and field center–adjusted model. The percent reduction in β estimate from β0 to βn is the percent reduction in risk for diabetes on the log-scale comparing black individuals to white individuals that is associated with adjustment for the factors in the βn model.
Biological factors: fasting glucose, body mass index, waist circumference, parental history of diabetes, triglycerides to high-density lipoprotein cholesterol ratio, forced vital capacity, systolic blood pressure, and blood pressure–lowering medication use.
Neighborhood factors: G statistic for racial segregation and tract-level percentage of population living in poverty.
Psychosocial factor: Center for Epidemiologic Studies–Depression score.
Socioeconomic factors: education, current employment status, paying for basic essentials, marital status, and mother’s and father’s educational attainment.
Behavioral factors: regular alcohol consumption, smoking status, diet score from the American Heart Association’s Life’s Simple 7, and regular physical activity.
Discussion
In this cohort of adults who were young at study inception and followed up for 30 years, the disparity in diabetes incidence between black and white women and men was primarily associated with differences in biological risk factors; however, differences in neighborhood, psychosocial, socioeconomic, and behavioral factors were additionally associated with disparities in diabetes risk. Among women, nearly all of the excess diabetes risk in black participants was associated with the combined differences in these factors at baseline. By contrast, one-fourth of the excess diabetes risk in black participants was associated with the combined differences in these factors at baseline among men. For women and men, adjustment for changes in these factors was associated with the disparity in diabetes incidence between black and white participants. These findings build on previous epidemiologic research observing disparities in the development of diabetes by race. However, this work extends prior findings by describing these disparities at a younger age and with longer follow-up than previous studies and by including a broader set of explanatory factors and changes in these factors over time.
Recent findings from the cross-sectional Boston Area Community Health III Survey data found individual-level socioeconomic factors at a single point in time explained the greatest percentage of excess prevalence of diabetes in black individuals relative to white individuals. The Northern Manhattan study observed greater risk for developing diabetes in black individuals compared with white individuals over 11 years, finding differential influences of body mass index, smoking, and C-reactive protein on diabetes risk across racial-ethnic groups in older adults. However, data from the Health and Retirement study did not show a higher risk for incident diabetes among black individuals relative to white individuals after accounting for self-rated health status in old age. Findings from the present study are similar to those of the Atherosclerosis Risk in Communities (ARIC) study of adults aged 45 to 64 years, whereby differences in biological factors at baseline were associated with the greatest disparity in diabetes incidence between black and white individuals, and these findings were more prominent among women than among men.
In ARIC, nonobese black women, but not men, had higher levels of fasting insulin than their nonobese white counterparts before developing diabetes, suggesting that sex-specific mechanisms may contribute to excess risk of diabetes in black individuals. This current work is consistent with this observation as greater attenuation in diabetes HRs, according to race, was observed for women compared with men when including biological factors measured at baseline to a model adjusted for age and field center. Using multiple modeling strategies, this study consistently demonstrated that differences in modifiable biological factors, namely body mass index and central adiposity between black and white women, were most associated with racial disparities in diabetes incidence. Among men, differences in modifiable behavioral and individual socioeconomic factors between black and white individuals at baseline were most strongly associated with racial disparities in diabetes incidence. However, when incorporating changes in these risk factors over time, differences in biological factors were most strongly associated with racial disparities in diabetes incidence for women and men. Literature highlighting the social determinants of health suggests that racial disparities in diabetes may be influenced by the psychosocial and environmental determinants of behaviors and health care access that directly influence biological factors. Findings from the present study support this hypothesis as individual-level and neighborhood-level social determinants did contribute significantly to disparities in diabetes. These results suggest prevention efforts that address racial inequalities in socioeconomic factors (eg, educational attainment and income) may be one strategy to reduce racial disparities in diabetes risk.
Limitations
This study has several limitations. First, diagnostic criteria for diabetes changed during follow-up, and ascertainment of diabetes cases identified solely from the medication inventory would be less sensitive prior to 1997. Diabetes was defined at each examination using current American Diabetes Association criteria for laboratory measures, and appreciable differences by race in case-ascertainment by diabetes medication use were not observed. Second, for reasons of missing data, 14% of participants were excluded from analysis. Those who were excluded were more likely to be black and had higher fasting glucose, and estimates from multiple imputation suggest this may underestimate diabetes risk in black men in this study. Third, this is an observational study, and residual confounding may be present in these estimates.
Conclusions
In this cohort study comparing black participants with white participants, there was a statistically significant increased risk of incident type 2 diabetes among black women and men. However, after adjustment for modifiable risk factors during young adulthood, the disparity was no longer statistically significant.
eAppendix. Supplementary Methods, Results, and References
eTable 1. Participant Exclusions
eTable 2. Comparison of Characteristics Between Participants Included in the Analytic Sample and Those Excluded From Analysis
eTable 3. Comparison of Characteristics for Participants Who Were not Identified as an Incident Diabetes Case According to Last Examination Visit
eTable 4. Hazard Ratios (95% CIs) for Incident Diabetes in Blacks Compared With Whites and Percent Reduction in Parameter Estimates for Women and Men Combined According to Sequential Adjustment for Each Risk Factor Domain When Including Updated Risk Factor Information for Model Adjustment and When Adjusting for Baseline Measurement of Risk Factors
References
- 1.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029. [DOI] [PubMed] [Google Scholar]
- 2.McBean AM, Li S, Gilbertson DT, Collins AJ. Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ethnic groups. Diabetes Care. 2004;27(10):2317-2324. [DOI] [PubMed] [Google Scholar]
- 3.Geiss LS, Wang J, Cheng YJ, et al. . Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA. 2014;312(12):1218-1226. [DOI] [PubMed] [Google Scholar]
- 4.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities study. JAMA. 2000;283(17):2253-2259. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association 1. Promoting health and reducing disparities in populations. Diabetes Care. 2017;40(suppl 1):S6-S10. [DOI] [PubMed] [Google Scholar]
- 6.Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13(6):814-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golden SH, Brown A, Cauley JA, et al. . Health disparities in endocrine disorders: biological, clinical, and nonclinical factors. J Clin Endocrinol Metab. 2012;97(9):E1579-E1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143-152. [PubMed] [Google Scholar]
- 9.Carnethon MR, Palaniappan LP, Burchfiel CM, Brancati FL, Fortmann SP. Serum insulin, obesity, and the incidence of type 2 diabetes in black and white adults: the Atherosclerosis Risk in Communities study: 1987-1998. Diabetes Care. 2002;25(8):1358-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee R, Brancati FL, Shafi T, et al. . Non-traditional risk factors are important contributors to the racial disparity in diabetes risk: the Atherosclerosis Risk in Communities study. J Gen Intern Med. 2014;29(2):290-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulick ER, Moon YP, Cheung K, Willey JZ, Sacco RL, Elkind MS. Racial-ethnic disparities in the association between risk factors and diabetes: the Northern Manhattan study. Prev Med. 2016;83:31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccolo RS, Subramanian SV, Pearce N, Florez JC, McKinlay JB. Relative contributions of socioeconomic, local environmental, psychosocial, lifestyle/behavioral, biophysiological, and ancestral factors to racial/ethnic disparities in type 2 diabetes. Diabetes Care. 2016;39(7):1208-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. ; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med. 2017;376(15):1419-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman GD, Cutter GR, Donahue RP, et al. . CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105-1116. [DOI] [PubMed] [Google Scholar]
- 15.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28(6):1379-1388. [PubMed] [Google Scholar]
- 16.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101-123. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society ATS statement—snowbird workshop on standardization of spirometry. Am Rev Respir Dis. 1979;119(5):831-838. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152(3):1107-1136. [DOI] [PubMed] [Google Scholar]
- 19.Getis A, Ord JK. The analysis of spatial association by use of distance statistics. Geogr Anal. 1992;24(3):189-206. doi: 10.1111/j.1538-4632.1992.tb00261.x. [DOI] [Google Scholar]
- 20.Kershaw KN, Osypuk TL, Do DP, De Chavez PJ, Diez Roux AV. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease. Circulation. 2015;131(2):141-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D scale. Appl Psychol Meas. 1977;1(3):385-401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 22.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations. Am J Epidemiol. 1977;106(3):203-214. [DOI] [PubMed] [Google Scholar]
- 23.Matthews KA, Kiefe CI, Lewis CE, Liu K, Sidney S, Yunis C, et al. . Socioeconomic trajectories and incident hypertension in a biracial cohort of young adults. Hypertension. 2002;39(3):772-776. [DOI] [PubMed] [Google Scholar]
- 24.US Department of Health Human Services Dietary Guidelines for Americans 2015-2020. New York NY: Skyhorse Publishing Inc; 2017. [Google Scholar]
- 25.McDonald A, Van Horn L, Slattery M, et al. . The CARDIA dietary history. J Am Diet Assoc. 1991;91(9):1104-1112. [PubMed] [Google Scholar]
- 26.Liu K, Slattery M, Jacobs D Jr, et al. . A study of the reliability and comparative validity of the CARDIA dietary history. Ethn Dis. 1994;4(1):15-27. [PubMed] [Google Scholar]
- 27.Bancks MP, Allen NB, Dubey P, et al. . Cardiovascular health in young adulthood and structural brain MRI in midlife: the CARDIA study. Neurology. 2017;89(7):680-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American College of Sports Medicine The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness in healthy adults. Schweiz Z Sportmed. 1993;41(3):127-137. [PubMed] [Google Scholar]
- 29.Jacobs DJ, Hahn L, Haskell W, Pirie P, Sidney S. Validity and reliability of a short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil. 1989;9(11):448-459. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipton RB, Liao Y, Cao G, Cooper RS, McGee D. Determinants of incident non-insulin-dependent diabetes mellitus among blacks and whites in a national sample: the NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1993;138(10):826-839. [DOI] [PubMed] [Google Scholar]
- 31.Resnick HE, Valsania P, Halter JB, Lin X. Differential effects of BMI on diabetes risk among black and white Americans. Diabetes Care. 1998;21(11):1828-1835. [DOI] [PubMed] [Google Scholar]
- 32.Quiñones AR, Liang J, Ye W. Differences in diabetes mellitus onset for older black, white, and Mexican Americans. Ethn Dis. 2013;23(3):310-315. [PMC free article] [PubMed] [Google Scholar]
- 33.Walker RJ, Smalls BL, Campbell JA, Strom Williams JL, Egede LE. Impact of social determinants of health on outcomes for type 2 diabetes. Endocrine. 2014;47(1):29-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplementary Methods, Results, and References
eTable 1. Participant Exclusions
eTable 2. Comparison of Characteristics Between Participants Included in the Analytic Sample and Those Excluded From Analysis
eTable 3. Comparison of Characteristics for Participants Who Were not Identified as an Incident Diabetes Case According to Last Examination Visit
eTable 4. Hazard Ratios (95% CIs) for Incident Diabetes in Blacks Compared With Whites and Percent Reduction in Parameter Estimates for Women and Men Combined According to Sequential Adjustment for Each Risk Factor Domain When Including Updated Risk Factor Information for Model Adjustment and When Adjusting for Baseline Measurement of Risk Factors