Abstract
To examine the association between obesity and urothelial cancer, we used a representative data from the National Health Insurance System (NHIS). Participants included 826,170 men aged 20 years and older who experienced a health examination at least one time between 2004 and 2008. The study thus excluded people aged <20 years and women. We used a multivariate adjusted Cox regression analysis to examine the association between urothelial cancer and body mass index (BMI) via a hazard ratio (HR) and 95% confidence interval (CI).
The age- or multivariable-adjusted HR for urothelial cancer was stratified by BMI. Men with a higher BMI were more likely to acquire urothelial cancer independent of variables. In the population with diabetes, there showed a considerable, increasing trend in the risk of urothelial cancer in the overweight and obesity group, compared to the group with the same BMI but without diabetes. This population-based study showed evidence of an association between obesity and the development of urothelial cancer, where the presence of diabetes increased the risk of urothelial cancer. Additionally, the higher the BMI, the higher the risk for urothelial cancer.
Keywords: Carcinoma, Urothelial Cancer; Obesity; Diabetes Mellitus.
Introduction
Being overweight or obese is linked to an increased risk for several chronic diseases, including diabetes, heart disease, and cancer 1, 2. Recently, multiple epidemiologic studies have suggested that obesity may also increase the incidence and/or mortality of liver, gallbladder, pancreatic and stomach cancers 3-5. Postulated mechanisms for the increased risk emphasize the systemic inflammation associated with obesity 6, 7. In addition, Asian populations have a higher body fat percentage for a given body mass index (BMI) compared to Caucasians; accordingly, the consequences of obesity relevant to cancer risk may manifest at lower levels of BMI among Asians 8. Therefore, Asians' risk of cancer, as associated with being overweight or obese, might differ from Caucasians 9.
Urothelial cancer is the sixth-most commonly diagnosed malignancy in the United States, and the eighth-most common cancer in males in South Korea 10. Established risk factors for urothelial cancer include cigarette smoking, occupational exposure to specific carcinogens like aromatic amines, drinking tap water with arsenic, and a familial history of bladder cancer 11. Previous epidemiological studies have reported inconsistent associations between BMI and urothelial cancer 12-14. Some studies suggest the association between obesity and urothelial cancer may depend on the presence of diabetes 15. Other studies have reported a heterogeneity in the effects regarding different patient subgroups 16.
These differences might be because of poor representations about study populations or small sample sizes. Therefore, in South Korea, we managed a nationwide, population-based study to determine the relationship between obesity and the development of urothelial cancer. This study spanned the course of 10 years. We examined the association according to the change of BMI category, using nationally representative data from the National Health Insurance System (NHIS).
Materials and Methods
In South Korea, almost all citizens are registered in the National Health Insurance System (NHIS) as either an employee or a member of a community. As a result, it contains an extensive information about the health problem of most Korean population. It contains eligibility, medical treatment, health examination, and medical care institution databases.
For this study, we developed a customized database. Using NHIS, we generated a sample size of 1,025,340 subjects selected by a systematic sampling method to generate a representative sample from the total Korean population existing in 2004. We retrieved data on sex, birthdate, and diagnostic codes based on the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Urothelial cancer, including renal pelvic cancer, ureter cancer, and bladder cancer was coded C65, C66, and C67. In addition, subjects diagnosed with urothelial cancer underwent at least one related surgery, such as transurethral resection of bladder tumors, nephroureterectomy, and segmental resection of the ureter.
Of the 826,170 participants who underwent a health examination at least one time from 2004-2008, those aged <20 years or women (n = 351,575) were excluded. In additional to age and sex, the health examination revealed information on diabetes and body mass index (BMI).
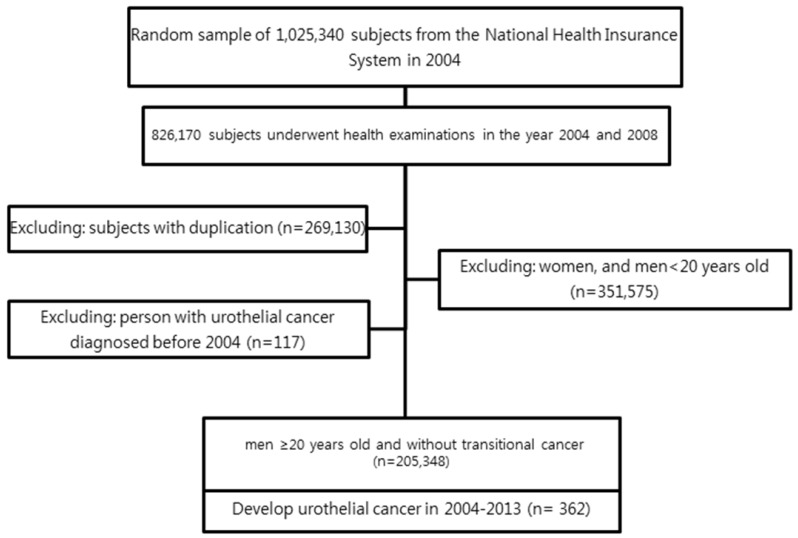
Figure 1 shows a flowchart for selecting cases for the study. The Institutional Review Board of The Catholic University of Korea approved this study.
Figure 1.
Study design and disposition of subjects.
BMI was calculated as weight in kilograms divided by height in square meters. The Korean Society for the Study of Obesity recommends the use of the following BMI ranges: underweight (under 18.5); normal weight (18.5 to 22.9); overweight (23 to 24.9); and obese (over 25) 17. Blood pressure was measured while the subject was in a seated position after five minutes of rest at daytime. Blood samples were collected after an overnight fasting and measured for serum levels of glucose, total cholesterol, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol.
The definition of lifestyle variables was as follows. Smoking status was categorized into three groups: non-smokers, current smokers who had smoked 100 cigarettes or more in their lifetime, and ex-smokers who had smoked in the past but had since quit at least one month. Alcohol consumption status was categorized into three groups: non-drinker, those who drank two-to-three times a month, and those who drank weekly. Exercise status was also divided into three groups: non-exerciser, physical activity performed for at least 30 minutes at less than four times a week, and more than five times a week.
SAS version 9.3 (SAS Institute, Cary, NC, USA) was used for statistical analysis. This study presents data as the mean ± standard error (SE) or proportions for continuous or categorical variables. If necessary, a logarithmic transformation was performed to achieve a normal distribution. Multivariate adjusted Cox regression analysis was conducted to examine the hazard ratio (HR) and 95% confidence interval (CI) for the association between urothelial cancer and BMI, both with and without diabetes. Calculations were made adjusting for age, smoking status, alcohol consumption, and exercise. For each Cox regression analysis, a p-value for linear trend across categories was calculated by introducing the ordinal variable in the model. A p-value < 0.05 was considered statistically significant.
Results
During the follow-up, 362 incident cases of urothelial cancer developed between the beginning of 2004 and end-2013, and the incidence density was 1.76 cases per 1,000 person-years.
Comparison of clinical characteristics according to BMI
Table 1 summarizes the general characteristics of the study population and subgroups. Among a total of 205,348 participants, 73,390 (35.74%) were normal weight and 72,152 (35.14%) were in the obesity group. Mean total cholesterol and the proportion of dyslipidemia were significantly higher in the obesity group. The presence of hypertension or diabetes increased with increasing BMI.
Table 1.
Comparison of clinical characteristics according to the body mass index
| BMI (kg/m2) | < 18.5 | 18.6-22.9 | 23 - 24.9 | ≥25 |
|---|---|---|---|---|
| No of individuals | 5448 | 73390 | 54358 | 72152 |
| Age (Mean±S.E) | 47.0±18.0 | 44.2±15.0 | 45.3±13.5 | 44.5±12.8 |
| < 65 | 4221(773.5) | 64509(87.9) | 49019(90.2) | 66616(92.3) |
| 65 ≤ | 1227(22.5) | 8881(12.1) | 5339(9.8) | 5536(7.7) |
| Smoking (%) | ||||
| Non | 1796(36.8) | 26668(40.66) | 21598(45.09) | 28020(44.19) |
| Ex. | 260(5.33) | 4690(7.15) | 4294(8.96) | 5772(9.1) |
| Current | 2824(57.87) | 34226(52.19) | 22008(45.95) | 29618(46.71) |
| Exercise | ||||
| Non | 3446(64.67) | 37404(51.88) | 24573(46.04) | 31625(44.6) |
| 1~4/week | 1576(29.57) | 29139(40.42) | 24015(44.99) | 33133(46.73) |
| ≥5/week | 307(5.76) | 5550(7.7) | 4789(8.97) | 6151(8.67) |
| Alcohol consumption | ||||
| Non | 2249(42.23) | 24919(34.68) | 17421(32.73) | 22545(31.91) |
| 2~3/month | 1080(20.28) | 15962(22.22) | 11657(21.9) | 14744(20.87) |
| ≥1/week | 1996(37.48) | 30963(43.1) | 24145(45.37) | 33353(47.21) |
| Current Hypertension (%) | ||||
| YES | 868 (17.44) | 14060(20.58) | 14591(27.92) | 26355(38.00) |
| NO | 4109(82.56) | 54268(79.42) | 37664(72.08) | 42996(62.00) |
| Current Diabetes (%) | ||||
| YES | 360(6.61) | 5111(6.96) | 4779(8.79) | 8171(11.32) |
| NO | 5088(93.39) | 68279(93.04) | 49579(91.21) | 63981(88.68) |
| Current Dyslipidemia (%) | ||||
| YES | 216(3.96) | 5858(8) | 7206(13.26) | 13594(18.84) |
| NO | 5232(96.04) | 67532(92) | 47152(86.74) | 58558(81.16) |
| BMI, kg/m2 | 17.6±0.8 | 21.3±1.2 | 24±0.6 | 27.2±2 |
| TC, mg/dl | 173.2±32 | 184.3±34.6 | 193.7±35.2 | 200.3±36.7 |
| Glucose, mg/dl | 94.3±31.7 | 95.1±25.8 | 97.6±26.3 | 100.2±28.1 |
Data are presented as the means ± SE, or % (SE). BMI: body mass index, TC: total cholesterol, SE: standard error
Hazard ratios for urothelial cancer according to BMI
Table 2 shows a regression analyses for urothelial cancer development, adjusted for variables, including age, smoking status, alcohol consumption, and exercise. Being elderly (over 65 years old), currently smoking, and presently diabetic were remarkable risk factors in both of the age-adjusted and multivariable-adjusted models. Data on alcohol consumption, the presence of hypertension, hyperlipidemia, and exercise did not create a statistically significant, increasing trend in risk of urothelial cancer.
Table 2.
Age- and multivariable-adjusted hazard ratios for urothelial cancer
| event | Total F/U | Incidence rate per 1,000 person-years | HR (95% CI) | ||
|---|---|---|---|---|---|
| Age adjusted | Multivariables adjusted1 | ||||
| AGE | |||||
| <64 | 213 | 1419176.52 | 1.50 | 1 | 1 |
| 65 ≤ | 149 | 155465.4 | 9.58 | 1.09(1.08,1.09)* | 1.09(1.08,1.10)* |
| Smoking | |||||
| NO | 148 | 598226.44 | 2.47 | 1 | 1 |
| Ex | 32 | 114108.38 | 2.80 | 1.47(1.00,2.15)* | 1.47(0.99,2.15) |
| Current | 135 | 680143.27 | 1.98 | 1.42(1.12,1.80)* | 1.49(1.17,1.90)* |
| Alcohol consumption | |||||
| 0 | 147 | 512797.92 | 2.87 | 1 | |
| 1 | 60 | 338730.39 | 1.77 | 1.316(0.97,1.79) | |
| 2 | 146 | 692702.87 | 2.11 | 1.158(0.92,1.46) | |
| Exercise | |||||
| 1 | 116 | 678904.72 | 1.71 | 0.98(0.77,1.24) | |
| 2 | 46 | 128868.75 | 3.57 | 0.967(0.70,1.33) | |
| Current Diabetes | |||||
| Yes | 72 | 138487.64 | 5. 20 | 1.35(1.04,1.75)* | 1.35(1.03,1.79)* |
| Current Hypertension | |||||
| Yes | 178 | 500534.79 | 3.56 | 0.99(0.8,1.23) | |
| Current Dyslipidemia | |||||
| Yes | 61 | 204071.98 | 2.99 | 1.08(0.82,1.42) | |
Data are presented as HR (95% confidence interval).
1Adjusted for age, smoking status, alcohol consumption, and exercise
*p-value < 0.05
HR: Hazard ratio, CI: confidence interval, BMI: body mass index
The HR for urothelial cancer was stratified by BMI level in the age- and multivariable- adjusted models (Table 3). The HR for urothelial cancer was lowest in those with a BMI < 18.5 and highest for those with a BMI ≥ 25. Furthermore, there was a statistically significant, increasing trend in risk of urothelial cancer in the overweight and obese groups (p < 0.0001).
Table 3.
Age- and multivariable-adjusted hazard ratios for urothelial cancer according to body mass index
| BMI | Event | Total F/U | Incidence rate per 1,000 person-years, | Age adjusted | Multivariables adjusted2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% C.I | P-value | H.R | 95% C.I | P-value | ||||
| < 18.5 | 14 | 41467.73 | 3.38 | 1.11 | 0.63-1.94 | <0.01 | 1.20 | 0.66-2.14 | <0.01 |
| 18.5 - 22.9 | 108 | 562792.15 | 1.92 | Ref. | Ref. | ||||
| 23 - 24.9 | 108 | 419114.78 | 2.58 | 1.47 | 1.12-1.92 | <0.01 | 1.58 | 1.18-2.10 | <0.01 |
| 25≤ | 132 | 551267.26 | 2.39 | 1.55 | 1.99-2.00 | <0.01 | 1.62 | 1.22-2.14 | <0.01 |
Data are presented as HR (95% confidence interval).
1All rates are expressed as number per 1000 person-years.
2Adjusted for age, hypertension, dyslipidemia, smoking status, alcohol consumption, and exercise.
BMI: body mass index, HR: Hazard ratio, CI: confidence interval
The presence of diabetes influenced the association between BMI and urothelial cancer
Table 4 shows an association between urothelial cancer and increasing BMI according to the presence of diabetes. The data shows that as the BMI increased, the HR of bladder cancer became more vulnerable. In the same BMI range, categories of diabetes impacted the HR (p<0.05). There was a marked, increasing risk for urothelial cancer in the overweight and obesity group compared to the group with the same BMI without diabetes.
Table 4.
Association between urothelial cancer and increasing BMI according to the presence of diabetes.
| HR(95%CI) | ||
|---|---|---|
| non | DM | |
| Normal | 1 | 1.98* |
| Overweight | 1.81 | 2.41* |
| Obesity | 1.66 | 2.88* |
Data are presented as HR (95% confidence interval).
*p-value < 0.05
HR: Hazard ratio, CI: confidence interval, BMI: body mass index
Discussion
The main findings of this population-based study are as follows: (1) men with a higher BMI were more likely to develop urothelial cancer independent of confounding variables; (2) this positive association between urothelial cancer and BMI is differentiated by the existence of diabetes; and (3) in the population with diabetes, there showed a marked, increasing risk for urothelial cancer in the overweight and obesity groups, compared to the group with the same BMI without diabetes.
Inflammation and insulin resistance can partly explain the underlying biological mechanism contributing to the epidemiologically positive association between obesity and urothelial cancer. That is, excessive adipose tissue can increase adipocytokines, which can play a major role for insulin resistance and systemic inflammation 18. Hyperinsulinemia increases the promotion and activity of Insulin-like Growth Factor 1 (IGF-1), which in turn drives the imbalance of cell proliferation, apoptosis, and angiogenesis, therefore playing a role in the development of urothelial cancer 19.
Several epidemiological studies have reported inconsistent associations between obesity and urothelial cancer. A recent meta-analysis from 16 cohort studies suggested a linear relationship between BMI and risk of urothelial cancer 20. However, the relation between BMI and the percentage of body fat depends on age and sex, and differs across ethnic groups 8.
Asian populations generally have a lower BMI for the same age and percentage of body fat than do Caucasian populations 9. In other words, compared to Caucasian populations, Asian populations in same age will have more accumulated body fat and a higher level of adipocytokine at equivalent BMI levels. Accordingly, the consequences of obesity relevant to cancer risk may be manifest at lower levels of BMI among Asians. As a result, an increase in BMI can have a greater effect in the development of urothelial cancer in Asian populations. This theory may explain why the prevalence of obesity and urothelial cancer recently increased 21, 22. Therefore, this study was prompted by the necessity to address obesity and its association with urothelial cancer specifically in Asian populations, as they accumulate more fat with body weight gain.
Diabetes may also be a risk factor for urothelial cancer, but findings from epidemiological studies are inconsistent. The underlying biological mechanisms contributing to the epidemiologically positive association between diabetes and urothelial cancer can be explained by over-expressions of IGF-1 receptors in malignant urothelial cells and IGF-1 stimulations by insulin 23. Through these mechanisms, hyperinsulinemia in individuals with Type-2 diabetes may more strongly promote urothelial cancer than in non-diabetic individuals 24. A previous meta-analysis showed that diabetes was associated with an increased risk of urothelial cancer in case-control studies and cohort studies, excluding cohort studies of patients with diabetes 20. However, a publication bias against studies with small sample sizes and against reporting a low relative risk is possible, and may have resulted in an overestimation of the relationship between diabetes and urothelial cancer 25. Our study also supported that the presence of diabetes increased the risk of urothelial cancer regardless of BMI level.
The other distinctive feature of this study is its use of Korea's NHIS database, which provides a highly representative cohort of the Korean population. The NHIS contains a large sample size and detailed information, such as physical, laboratory, and habitual covariates, like smoking status. A properly-designed NHIS service, like a biennial urine analysis, can serve as a major advantage for urothelial cancer research in Korea.
A possible limitation with this study is for covariates to be misclassified or confounding. For instance, smoking status has been known to be risk factor for urothelial cancer. However, smoking status is self-reported, which means it could have been misclassified to some extent and is probably confounding by the amount smoked. Other habitual covariates that were self-reported included alcohol status and exercise, which are likely to be similarly confounding.
Conclusion
This population-based study showed evidence of an association between BMI and the development of urothelial cancer, and presence of diabetes increased the influence of BMI on the development of urothelial cancer.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government. (No. NRF-2015R1C1A1A01051802).
Abbreviations
- NHIS
National Health Insurance System
- BMI
Body mass index
- SE
standard error
- HR
hazard ratio
- CI
confidence interval
- IGF
insulin/insulin-like growth factor
- TC
total cholesterol.
References
- 1.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–25. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolk A, Gridley G, Svensson M. et al. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12:13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 4.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–8. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–65. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 6.Algül H, Treiber M, Lesina M, Schmid RM. Mechanisms of disease: chronic inflammation and cancer in the pancreas-a potential role for pancreatic stellate cells? Nat Clin Pract Gastroenterol Hepatol. 2007;4:454–62. doi: 10.1038/ncpgasthep0881. [DOI] [PubMed] [Google Scholar]
- 7.Gunter MJ, Leitzmann MF. Obesity and colorectal cancer: epidemiology, mechanisms and candidate genes. J Nutr Biochem. 2006;17:145–56. doi: 10.1016/j.jnutbio.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164–71. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 9.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 10.Ok BG, Ji YS, Ko YH, Song PH. Usefulness of urine cytology as a routine work-up in the detection of recurrence in patients with prior non-muscle-invasive bladder cancer: practicality and cost-effectiveness. Korean J Urol. 2014;55:650–5. doi: 10.4111/kju.2014.55.10.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelucchi C, Bosetti C, Negri E, Malvezzi M, La Vecchia C. Mechanisms of disease: The epidemiology of bladder cancer. Nat Clin Pract Urol. 2006;3:327–40. doi: 10.1038/ncpuro0510. [DOI] [PubMed] [Google Scholar]
- 12.Häggström C, Stocks T, Rapp K. et al. Metabolic syndrome and risk of bladder cancer: prospective cohort study in the metabolic syndrome and cancer project (Me-Can) Int J Cancer. 2011;128:1890–8. doi: 10.1002/ijc.25521. [DOI] [PubMed] [Google Scholar]
- 13.Holick CN, Giovannucci EL, Stampfer MJ, Michaud DS. Prospective study of body mass index, height, physical activity and incidence of bladder cancer in US men and women. Int J Cancer. 2007;120:140–6. doi: 10.1002/ijc.22142. [DOI] [PubMed] [Google Scholar]
- 14.Koebnick C, Michaud D, Moore SC. et al. Body mass index, physical activity, and bladder cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2008;17:1214–21. doi: 10.1158/1055-9965.EPI-08-0026. [DOI] [PubMed] [Google Scholar]
- 15.Chen HF, Chen SW, Chang YH, Li CY. Risk of Malignant Neoplasms of Kidney and Bladder in a Cohort Study of the Diabetic Population in Taiwan With Age, Sex, and Geographic Area Stratifications. Medicine (Baltimore) 2015;94:e1494. doi: 10.1097/MD.0000000000001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384:755–65. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization Western Pacific Region. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Communications Australia; 2000. [Google Scholar]
- 18.Gallagher EJ, LeRoith D. Insulin, insulin resistance, obesity, and cancer. Curr Diab Rep. 2010;10:93–100. doi: 10.1007/s11892-010-0101-y. [DOI] [PubMed] [Google Scholar]
- 19.Zhao H, Grossman HB, Spitz MR, Lerner SP, Zhang K, Wu X. Plasma levels of insulin-like growth factor-1 and binding protein-3, and their association with bladder cancer risk. J Urol. 2003;169:714–7. doi: 10.1097/01.ju.0000036380.10325.2a. [DOI] [PubMed] [Google Scholar]
- 20.Sun JW, Zhao LG, Yang Y, Ma X, Wang YY, Xiang YB. Obesity and risk of bladder cancer: a dose-response meta-analysis of 15 cohort studies. PLoS One. 2015;10:e0119313. doi: 10.1371/journal.pone.0119313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim S, Shin H, Song JH. et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care. 2011;34:1323–8. doi: 10.2337/dc10-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh CM, Won YJ, Jung KW. et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2013. Cancer Res Treat. 2016;48:436–50. doi: 10.4143/crt.2016.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rochester MA, Patel N, Turney BW. et al. The type 1 insulin-like growth factor receptor is over-expressed in bladder cancer. BJU Int. 2007;100:1396–401. doi: 10.1111/j.1464-410X.2007.06931.x. [DOI] [PubMed] [Google Scholar]
- 24.Giovannucci E, Harlan DM, Archer MC. et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–85. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang H, Yao B, Yan Y. et al. Diabetes mellitus increases the risk of bladder cancer: an updated meta-analysis of observational studies. Diabetes Technol Ther. 2013;15:914–22. doi: 10.1089/dia.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]