ABSTRACT
Bacillus subtilis is known as an endospore- and biofilm-forming bacterium with probiotic properties. We have recently developed a method for displaying heterologous proteins on the surface of B. subtilis biofilms by introducing the coding sequences of the protein of interest into the bacterial genome to generate a fusion protein linked to the C terminus of the biofilm matrix protein TasA. Although B. subtilis is a regular component of the gut microflora, we constructed a series of recombinant B. subtilis strains that were tested for their ability to be used to immunize dogs following oral application of the spores. Specifically, we tested recombinant spores of B. subtilis carrying either the fluorescent protein mCherry or else selected antigenic peptides (tropomyosin and paramyosin) from Echinococcus granulosus, a zoonotic intestinal tapeworm of dogs and other carnivores. The application of the recombinant B. subtilis spores led to the colonization of the gut with recombinant B. subtilis but did not cause any adverse effect on the health of the animals. As measured by enzyme-linked immunosorbent assay and immunoblotting, the dogs were able to develop a humoral immune response against mCherry as well as against E. granulosus antigenic peptides. Interestingly, the sera of dogs obtained after immunization with recombinant spores of E. granulosus peptides were able to recognize E. granulosus protoscoleces, which represent the infective form of the head of the tapeworms. These results represent an essential step toward the establishment of B. subtilis as an enteric vaccine agent.
KEYWORDS: Bacillus subtilis, Echinococcus granulosus, antigen specificity, biofilms, endospores, intestinal colonization, intestinal immunity, mucosal immunity, oral immunization, parasite
INTRODUCTION
The enteric immunization of mammalian species, including canids, is notably challenging. In the particular case of dogs, oral vaccines against parvovirus (1, 2), rabies (3), or canine infectious respiratory disease (4, 5) are based on live attenuated organisms (viruses and bacteria). Nevertheless, an environmentally safe method of immunization for the delivery of antigens that prevents the shedding of the live attenuated pathogen has not yet been established in dogs. Several carriers of enteric antigens based on a viral or bacterial background have recently been developed; unfortunately, they have had only moderate success (6–9). Bacillus subtilis is a well-described and versatile microorganism able to form endospores, to develop into biofilms, and even to be used as a probiotic in humans or livestock (10–12). We recently engineered B. subtilis for the display of heterologous proteins on the surface of the biofilm. To this end, the matrix protein TasA was fused to various peptides via its C terminus, and the fusions were expressed under biofilm-inducing conditions (13). To optimize expression, we used the tasA sinR genetic background. On the one hand, SinR is a repressor of the tapA-sipW-tasA operon and other genes. Therefore, the sinR mutant had enhanced expression levels of the fusion protein. On the other hand, the tasA mutant background prevented competition of the endogenous TasA with the fusion protein (14, 15).
An interesting pathogen is the zoonotic canine intestinal cestode Echinococcus granulosus sensu lato (16), responsible for causing cystic echinococcosis (CE) in mammalian and marsupial intermediate hosts, but also in humans as accidental hosts. The worldwide-distributed CE (17) is considered one of the most important neglected tropical diseases (18). Thus, CE is responsible for high rates of human morbidity and mortality and is also responsible for significant economic losses in livestock (19). The E. granulosus life cycle includes canids (mainly domestic dogs) as definitive hosts, which become infected by ingesting the lungs or livers of intermediate hosts rich in Echinococcus cysts containing protoscoleces. Once the protoscoleces get in close contact with the intestinal mucosa of dogs, they develop into the parasitic form, i.e., 5- to 7-mm-long, egg-producing tapeworms (16). The eggs of E. granulosus are shed in the feces of the definitive host by either the release of the last proglottid in the intestine or the direct excretion of the tapeworms. Intake of these tapeworm eggs by intermediate or susceptible accidental hosts then initiates a new round in the parasite's life cycle. Since dogs are the main host and they spread disease by excreting parasite eggs in their feces, control programs that include regular deworming of the dog population are required (20). A vaccine that immunologically attacks the egg-producing tapeworm might greatly help in reducing the risk of disease for humans. In previous studies, two potential antigens were proposed to be vaccine targets against E. granulosus intestinal infections, namely, the proteins tropomyosin (E. granulosus Trp [EgTrp]) and paramyosin (E. granulosus A31 [EgA31]) (21–24).
In the present study, we investigated the ability of recombinant B. subtilis to induce an antibody response in dogs upon oral application of spores containing TasA linked to mCherry or E. granulosus peptides (EgTrp and EgA31).
RESULTS
The intestinal microflora of dogs contains B. subtilis.
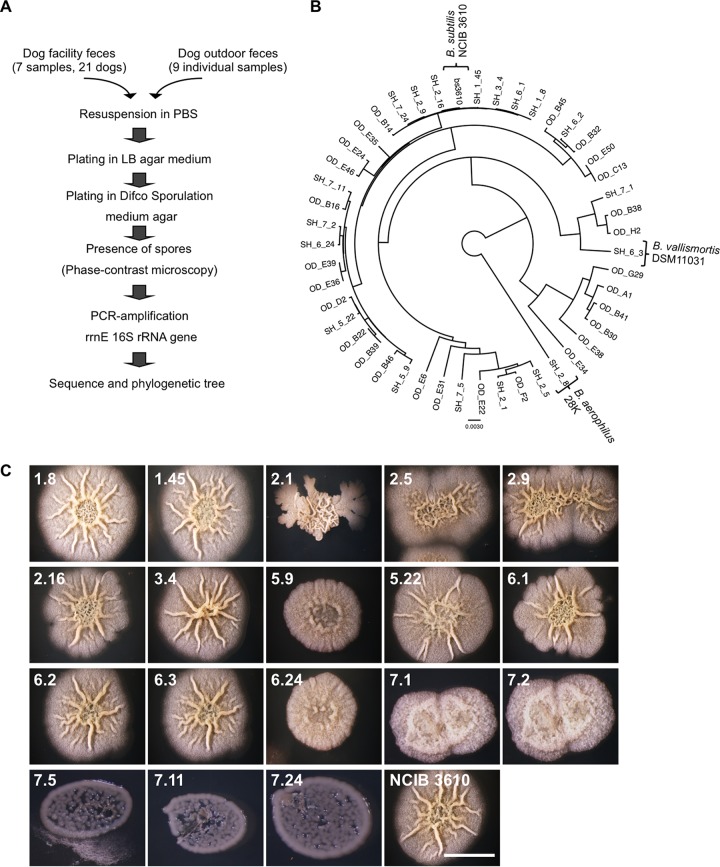
In a first instance, we wondered if the intestinal microflora of dogs could be disturbed by the presence of exogenous B. subtilis. To answer this question, we first investigated whether B. subtilis was part of the intestinal microflora in healthy dogs. For this purpose, feces were collected from either the University of Zurich (UZH) dog facility or from privately owned dogs. Each fecal sample was processed as indicated in the scheme depicted in Fig. 1A. Our data imply that all the isolated bacteria that were able to sporulate and form biofilms in MSgg semisolid medium belonged to Bacillus spp. (Fig. 1B and C). Additionally, the isolated bacteria did not show a significant genetic divergence (Fig. 1B) from the B. subtilis nondomesticated strain NCIB 3610. Interestingly, we could also find close relatives, such as B. vallismortis and B. aerophilus. Taken together, our data indicate that B. subtilis is ubiquitous in the intestinal microflora of healthy dogs and that it is likely that the nondomesticated B. subtilis NCIB 3610 strain will not significantly disturb the balance of the intestinal microflora.
FIG 1.
Bacillus subtilis is present in the dog intestinal microflora. (A) Schematic representation of the procedure used to sequence the 16S rrnE gene from sporulating bacteria isolated from the feces of dogs of the UZH animal facility and privately owned dogs. (B) Bayesian phylogeny of the 16S rrnE gene isolated from sporulating bacteria isolated from fecal samples from privately owned dogs and dogs from the UZH animal facility. The sequences were compared with the reference sequence obtained from the undomesticated B. subtilis NCIB 3610 strain. Alignment gaps and missing data were eliminated in pairwise sequence comparisons. Bar, 0.0030 change per nucleotide position. (C) Top view of colonies of bacterial clones isolated from the feces of dogs from the UZH animal facility. Bacteria were grown on MSgg solid medium for 72 h at 30°C. The clone numbers assigned to the bacteria are denoted at the upper left corner of each panel. Bar, 1 cm.
Luminescent B. subtilis bacteria could be detected in the dog gut.
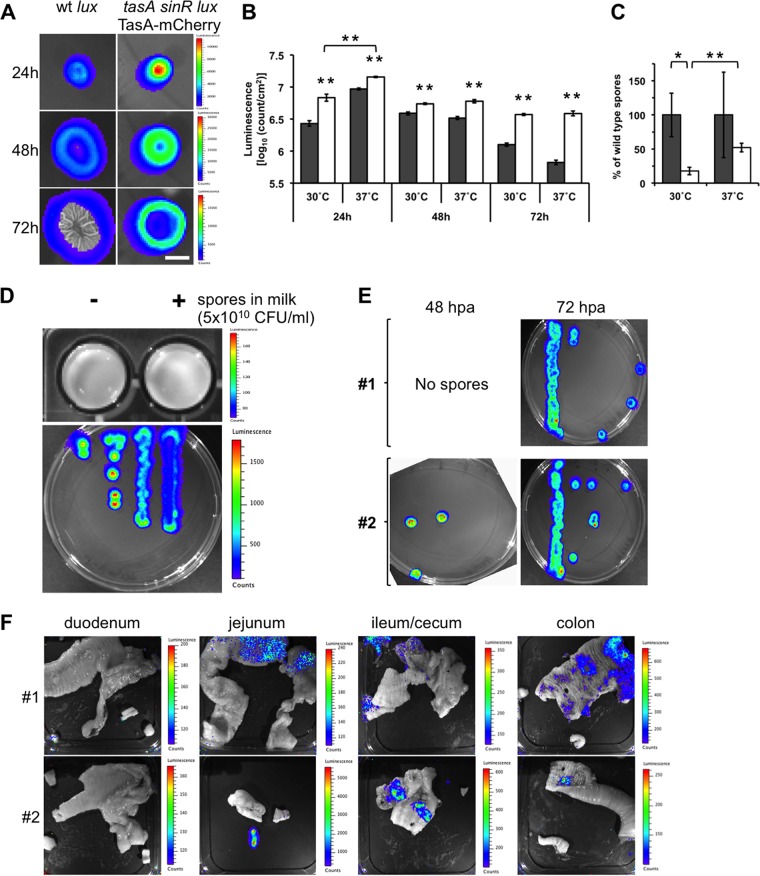
We next wondered whether recombinant spores of B. subtilis could germinate in the gut of dogs and display an antigen of interest. For this purpose, we incorporated the luxCDABE operon from Photorhabdus luminescens (25) driven by the tapA promoter into a B. subtilis tasA sinR strain also harboring the tapA-sipW-tasA-mCherry operon (Table 1), generating the B. subtilis tasA sinR lux TasA-mCherry strain. The tasA sinR genetic background in B. subtilis improves the expression of heterologous genes driven by the tapA promoter (13). We hypothesized that this newly engineered strain would permit the easy tracking of B. subtilis by luminescence when it is in a vegetative state. As expected, enhanced bioluminescence was observed when the expression of the wild-type (wt) B. subtilis luxCDABE (lux) strain was compared with that of B. subtilis tasA sinR lux TasA-mCherry biofilms at 24, 48, and 72 h postinoculation (Fig. 2A). As observed in Fig. 2B, such enhancement of luminescence expression was also improved when the biofilms were incubated at 37°C instead of 30°C. Interestingly, the expression of B. subtilis tasA sinR lux TasA-mCherry was already significantly increased at 24 h postinoculation when it was incubated at 37°C rather than at 30°C. Consistent with the findings of our previous research (13), the sporulation ability of the B. subtilis tasA sinR lux TasA-mCherry strain was decreased compared to that of wt strain B. subtilis lux when it was grown at either 30°C or 37°C (Fig. 2C). As depicted in Fig. 2D (top), no luminescence was detected in B. subtilis tasA sinR lux TasA-mCherry endospores when they were diluted in milk, possibly since the tapA promoter is not active in the endospores. Milk was used as the medium for the oral application of recombinant B. subtilis spores to dogs. Instead, vegetative cells (germinated from the same spore aliquot) were luminescent (Fig. 2D, bottom). The switch from nonluminescence in spores to luminescence in vegetative cells permits the easy tracking of germination of the recombinant B. subtilis tasA sinR lux TasA-mCherry spores in vivo by using in vivo imaging system (IVIS) equipment.
TABLE 1.
B. subtilis strains used in this study
| Strain | Genotypea | Reference or source |
|---|---|---|
| lux (wt) | lacA::PtasA-luxCDABE Ermr | This study |
| tasA sinR lux TasA-mCherry | tasA::Kmr SinR::Spcr lacA::PtasA-luxCDABE MLSr amyE::yqxM-sipW-tasA-mCherry Cmr | This study |
| tasA sinR | tasA-sinR::Kmr | Vogt et al. (13) |
| tasA sinR TasA-(102-207) EgTrp | tasA-sinR::Kmr amyE::yqxM-sipW-tasA-(102-207)EgTrp Spcr | Vogt et al. (13) |
| tasA sinR TasA-(370-583)EgA31 | tasA-sinR::Kmr amyE::yqxM-sipW-tasA-(370-583)EgA31 Spcr | Vogt et al. (13) |
Kmr, kanamycin resistance; Spcr, spectinomycin resistance; Cmr, chloramphenicol resistance; MLSr, macrolide-lincosamide-streptogramin B (erythromycin and lincomycin) resistance.
FIG 2.
Tracking of luminescent Bacillus subtilis in the guts of dogs. (A) Top view of luminescent biofilms from wt strain B. subtilis lux and B. subtilis tasA sinR lux TasA-mCherry incubated in MSgg semisolid medium for 24, 48, and 72 h at 30°C. A color luminescence scale is shown for each row. Bar, 1 cm. (B) Comparison of the luminescence of wt strain B. subtilis lux (gray bars) and B. subtilis tasA sinR lux TasA-mCherry (white bars) biofilms at 30°C and 37°C when incubated for 24, 48, and 72 h. The data represent the mean ± SEM (**, P < 0.01, t test, n = 4). (C) Sporulation ability of wt strain B. subtilis lux and B. subtilis tasA sinR lux TasA-mCherry from 72-h-old biofilms when incubated at 30°C and 37°C. The data represent the mean ± SEM (*, P < 0.05; **, P < 0.01; P values were determined by t test [n = 4]). (D) Comparison of the luminescence of spores resuspended in milk (top) and vegetative cells (bottom) from the B. subtilis tasA sinR lux TasA-mCherry strain. A color luminescence scale is shown for each panel. (E) Germination of spores isolated from anal swab samples from dogs 1 and 2 after the first oral application of B. subtilis tasA sinR lux TasA-mCherry spores. The anal swab samples were collected at 48 and 72 hpa. The spores were incubated in selective LB semisolid medium, and vegetative cells were monitored for luminescence. (F) Detection of luminescence from B. subtilis tasA sinR lux TasA-mCherry vegetative cells in the guts of dogs 1 and 2 after oral application of three doses of recombinant B. subtilis spores (5 × 1010 CFU on days 1, 21, and 42). Each picture corresponds to open portions of representative sections of the duodenum, jejunum, ileum/cecum, and colon of each animal. A color luminescence scale is shown for each picture. All luminescent images were acquired with a Xenogen IVIS camera and analyzed using Living Image (v4.0) software (Caliper Life Sciences, USA).
As a proof of principle, we used oral application to inoculate spores from B. subtilis tasA sinR lux TasA-mCherry into 2-week-old puppies (Table 2, dogs 1 and 2). First, we followed the spore shedding in the puppies' feces for 3 days (at 24, 48, and 72 h postapplication [hpa]). For the detection of the recombinant spores, germinated spores in selective Luria-Bertani (LB) semisolid medium were initially monitored for luminescence expression. As observed in Fig. 2E, the bacterial luminescence was detected at 48 hpa in puppy 2 and 72 hpa in both puppies. As the gastrointestinal transit in puppies is close to 24 h (26), our data suggest that the spores were retained in the gut of the animals and a small fraction was shed 3 days later (72 hpa).
TABLE 2.
Dog identification number, gender, age, and type of B. subtilis spore orally applied
| Animal no. | Gendere | Age (wk)a | B. subtilis spores appliedb |
|---|---|---|---|
| 1, 2 | M, M | 2 | tasA sinR lux TasA-mCherry |
| 3 | F | 9 | Placeboc |
| 4, 5 | M, F | 9 | tasA sinR lux TasA-mCherry |
| 1, 2 | F, M | 9 | Placebo |
| 1, 2 | M, F | 9 | tasA sinR TasA-(102-207)EgTrp |
| 1, 2 | M, F | 9 | tasA sinR TasA-(370-583)EgA31 |
| 1, 2 | M, F | 9 | Mixtured |
The age at the time that the first B. subtilis spores were received.
A total of 5 × 1010 CFU of orally applied recombinant B. subtilis spores per dose.
The placebo consisted of milk.
A total of 2.5 × 1010 CFU B. subtilis tasA sinR TasA-(102-207)EgTrp and 2.5 ×1010 CFU B. subtilis tasA sinR TasA-(370-83)EgA31 spores per dose.
F, female; M, male.
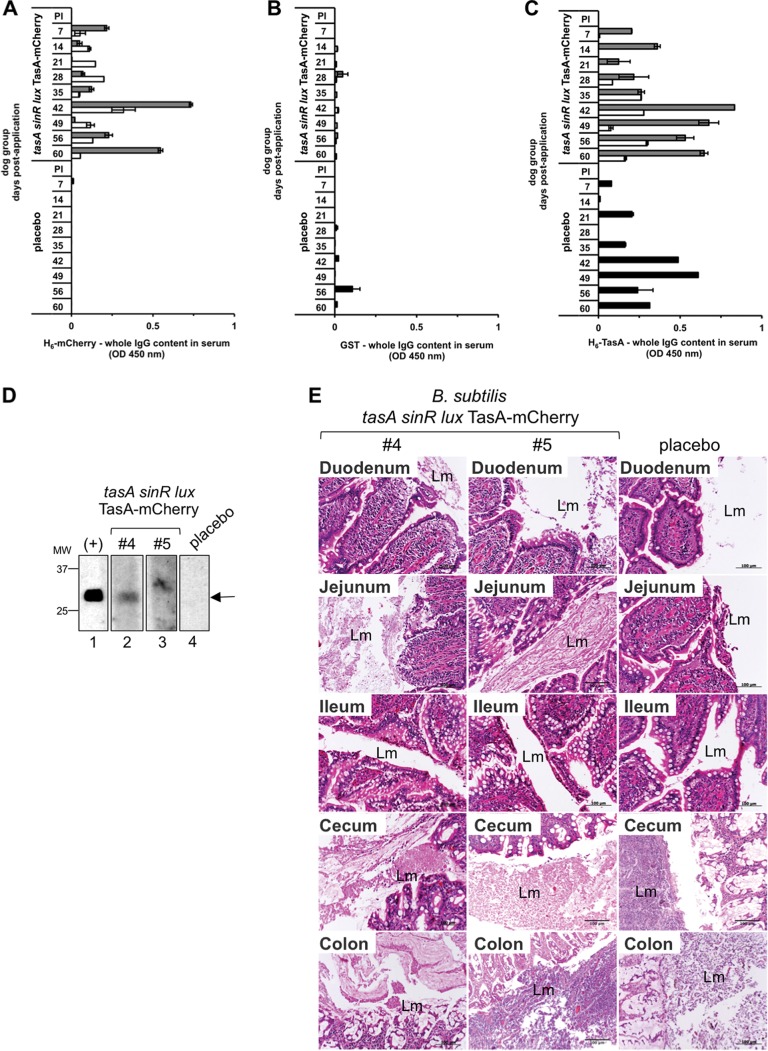
We next explored whether puppies could elicit a humoral immune response against the TasA-mCherry fusion. For this purpose, we provided the dogs with recombinant spores of the B. subtilis tasA sinR lux TasA-mCherry strain on days 21 and 42 after the first application. Interestingly, the dogs elicited a specific humoral immune response of both the IgG and IgA types against the two tested recombinant proteins (H6-mCherry [see Fig. S1, A, and C in the supplemental material] and H6-TasA [Fig. S1, B, and D]). At day 60, the different intestinal sections (duodenum, jejunum, ileum/cecum, and colon) were inspected for luminescence using IVIS equipment (Fig. 2F). The monitoring of the opened intestinal sections evidenced luminescence mainly in the jejunum, ileum/cecum, and colon but not in the duodenum. Consistent with this observation, we could also determine the presence of our recombinant bacteria by immunohistochemistry against TasA (anti-TasA) and mCherry (anti-dsRed2) (Fig. S1E). Our results indicate that recombinant spores of B. subtilis tasA sinR lux TasA-mCherry can germinate in the gut and get established in the intestinal microflora, permitting the heterologous expression of both the Lux and TasA-mCherry proteins.
Recombinant spores of B. subtilis induce a specific humoral response against TasA-mCherry in dogs.
We next administered recombinant spores of B. subtilis to dogs by starting the application in 9-week-old animals, which is in agreement with the Swiss schedule for dog vaccination. To this end, we included three dogs in our experiments, separated into one group of two dogs (Table 2, dogs 4 and 5) to which recombinant B. subtilis tasA sinR lux TasA-mCherry spores were orally applied and a second group composed of a single dog to which milk (placebo) was orally applied (Table 2, dog 3). The animals received three doses of recombinant B. subtilis spores on days 1, 21, and 42. The elicitation of a systemic humoral immune response against the fusion TasA-mCherry was monitored by indirect enzyme-linked immunosorbent assay (ELISA) using serum samples collected weekly. Only dogs to which recombinant B. subtilis spores were orally applied were able to elicit a specific humoral immune response against H6-mCherry (Fig. 3A and S2A). This response was more pronounced for dog 4 (Fig. 3A, gray bars). Remarkably, this humoral immune response consisted of the IgG isotype but not the IgA isotype (Fig. S2A). In contrast, indirect ELISA against the nonrelated antigen glutathione S-transferase (GST) did not show any measurable response from the dogs' serum either for the IgG antibody isotype or for the IgA antibody isotype (Fig. 3B and S2B). Consistent with our data, we could also observe a specific H6-mCherry humoral immune response by immunoblotting. Indeed, we also found an antibody response of the IgG isotype (Fig. 3D) but not one of the IgA isotype (Fig. S2D) when serum was checked at day 60. Interestingly, we could observe that all the tested animals (inoculated with recombinant B. subtilis spores or placebo) were able to elicit both IgA and IgG antibody isotype responses to the H6-TasA protein (Fig. 3C and S2C).
FIG 3.
Immune response after oral administration of recombinant Bacillus subtilis tasA sinR lux TasA-mCherry spores to dogs. On the indicated days postapplication, dog sera were tested by an indirect ELISA using plates coated with E. coli purified H6-mCherry (A), GST (B), and H6-TasA (C), followed by incubation with specific dog anti-IgG conjugated with HRP. Gray, white, and black bars, dogs 4 and 5 and the placebo-treated dog, respectively. Recombinant Bacillus subtilis tasA sinR lux TasA-mCherry spores were orally applied to dogs 4 and 5. The placebo-treated dog received only milk. The data represent the mean ± SEM from three independent experiments. (D) Detection of E. coli purified H6-mCherry by immunoblotting test strips incubated with the indicated dog serum (diluted 1:100) at day 60 postapplication, followed by incubation with specific anti-dog whole IgG-HRP. The positive control (+) (lane 1) was incubated with a specific mouse anti-dsRed2 followed by anti-mouse immunoglobulin-HRP. Arrow, position of mCherry. MW, molecular weights (in thousands). (E) Histological sections from intestinal samples from dogs orally inoculated with recombinant B. subtilis spores (dogs 4 and 5) or the placebo-treated dog stained with hematoxylin and eosin. Lm, intestinal lumen. Bars, 100 μm.
We further investigated if the recombinant spores were shed in the dogs' feces after each application. For this purpose, anal swab samples were collected daily for up to 6 days after the first and second oral applications (on days 1 and 21, respectively) of recombinant B. subtilis spores (Table 3) and cultivated on selective solid medium, and the grown colonies were later examined in an IVIS for the detection of luxCDABE operon luminescence. Only dog 4 shed recombinant spores on the second day after the first and second oral applications. For the third oral application of recombinant B. subtilis spores (Table 4), feces were collected daily in a random manner since the Swiss rules for animal experiments prohibit separate caging of dogs. Under these conditions, three random samples (RS1 to RS3) were collected daily for 3 days postapplication. The presence of recombinant B. subtilis spores in feces was confirmed by germination in selective LB semisolid medium, followed by luxCDABE luminescence detection and further quantification of the positive colonies. Our data showed that random fecal samples contained spores on days 1 to 3 following the third oral application. Interestingly, and consistent with our experimentation schedule, only two fecal samples contained recombinant B. subtilis spores on day 1 (RS1 and RS2) and day 2 (RS1 and RS3), suggesting that these samples were from the two dogs of the group orally inoculated with the recombinant B. subtilis spores. Then, on day 3, however, only one fecal sample (RS2) contained recombinant B. subtilis spores. On day 60 (Fig. 3F), different intestinal sections were inspected, and inflammatory cells were not found either in the placebo-treated dog or in the dogs orally inoculated with the recombinant B. subtilis spores. This result indicates that the recombinant B. subtilis spores or the vegetative cells do not have any detrimental effect on the intestinal structures. This finding is consistent with the probiotic abilities of B. subtilis (27). Collectively, our data show that dogs to which recombinant B. subtilis tasA sinR lux TasA-mCherry spores were orally applied can elicit a specific humoral response against the TasA-mCherry fusion.
TABLE 3.
Summary of presence of recombinant spores of the B. subtilis tasA sinR lux TasA-mCherry strain in the feces of dogs after the first and second oral applications
| DPAa | Presence of recombinant spores in the indicated dog afterb: |
|||||
|---|---|---|---|---|---|---|
| First oral application |
Second oral application |
|||||
| 4 | 5 | Placebo-treated dog | 4 | 5 | Placebo-treated dog | |
| 1 | − | − | − | − | − | − |
| 2 | + | − | − | + | − | − |
| 3 | − | − | − | − | − | − |
| 4 | − | − | − | − | − | − |
| 5 | − | − | − | − | − | − |
| 6 | − | − | − | − | − | − |
DPA, days postapplication.
Dogs 4 and 5 were treated with 5 × 1010 CFU spores/dose of the B. subtilis tasA sinR lux TasA-mCherry strain diluted in milk, and the placebo-treated dog was treated with milk only. Anal swab samples samples were then collected and tested. The samples were considered positive (+) when they were resistant to heat treatment (80°C for 20 min) and displayed luminescence after germination, as inspected with an IVIS camera.
TABLE 4.
Presence and quantification of B. subtilis tasA sinR lux TasA-mCherry spores in feces after a third oral application
| DPAa | RS1b |
RS2 |
RS3 |
|||
|---|---|---|---|---|---|---|
| Luminescencec | No. of sporesd | Luminescence | No. of spores | Luminescence | No. of spores | |
| 1 | + | 2.4 × 105 | + | 3.0 × 106 | − | ND |
| 2 | + | 6.5 × 105 | − | ND | + | 1.3 × 104 |
| 3 | − | ND | + | 1.7 × 105 | − | ND |
DPA, days postapplication.
RS, random fecal sample.
The samples were considered positive (+) when they were resistant to heat treatment (80°C for 20 min) and displayed luminescence after germination, as inspected with an IVIS camera.
The number of recombinant B. subtilis spores is represented as the average of two independent measurements. The data correspond to the number of CFU per gram of feces. ND, not detected.
Induction of intestinal immunity against E. granulosus antigens in dogs.
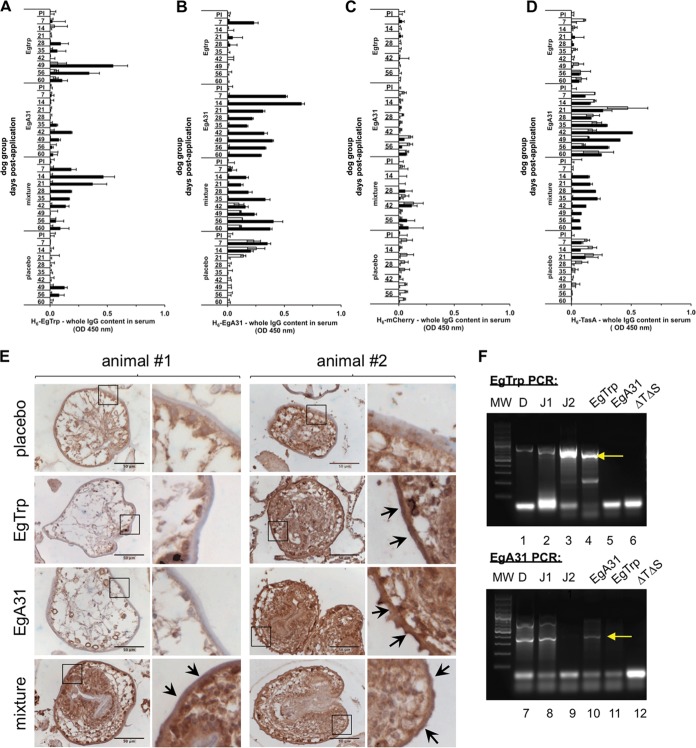
Next, we wondered whether a specific humoral immune response against the integumental antigens from the parasite E. granulosus (EgTrp and EgA31) could be elicited by using the immunization methodology described above. For this purpose, we used two different recombinant B. subtilis spores engineered to express the fusion proteins TasA plus EgTrp from residues 102 to 207 [TasA-(102-207)EgTrp] or TasA plus EgA31 from residues 370 to 583 [TasA-(370-583)EgA31] (Table 1) (13). For simplification, we refer to these as B. subtilis TasA-EgTrp and B. subtilis TasA-EgA31, respectively. As described by Vogt et al. (13), these strains can display the E. granulosus antigenic peptides (EgTrp and EgA31) fused in frame to the C terminus of TasA on the surface of in vitro biofilms. For this experiment, four groups of two dogs each (Table 2) were employed. Recombinant B. subtilis spores harboring the TasA-EgTrp fusion (group EgTrp), the TasA-EgA31 fusion (group EgA31), and a mixture of both recombinant spores (TasA-Egtrp and TasA-EgA31) (the mixture group) were orally applied to these three groups on days 1, 21, and 42. The fourth group was the placebo control group, which received only milk. The animals were 9 weeks old when they received the first oral application. In agreement with our previous results, no variations in the body weights among the animals in all the experimental groups were observed during the whole procedure (Fig. S3A). The elicitation of the humoral response to TasA fused to E. granulosus antigens in sera collected from the treated dogs was monitored weekly. For this purpose, an indirect ELISA was developed for the detection of specific antibodies against the purified proteins H6-EgTrp, H6-EgA31, and H6-TasA and the nonrelated antigen H6-mCherry. Of note, maternal antibodies can be detected as an early signal (7 to 15 days postimmunization) in young dogs by ELISA (28), and therefore, this signal was considered not to be significant. We noticed that at least one of the dogs in each of the groups receiving a single kind of recombinant B. subtilis spores was positive for the respective antigen (dog 2 in the group immunized with Tas-EgTrp [Fig. 4A, black bars] and dog 2 in the group immunized withTasA-EgA31 [Fig. 4B, black bars]), when they were tested for specific IgG antibodies against H6-EgTrp (Fig. 4A) and H6-EgA31 (Fig. 4B). However, the dogs that received the mixture of recombinant spores mainly responded to H6-EgA31, especially dog 2 (mixture group; Fig. 4B, black bars). Placebo-treated dogs did not react to either of these two antigens (Fig. 4A and B). None of the animals responded to the nonrelated H6-mCherry antigen (Fig. 4C), showing the specific nature of the immune response to the recombinant spores that was mounted. Consistent with our previous result (Fig. 3), all the animals presented a specific immune response against H6-TasA (Fig. 4D). However, when the sera were tested for specific IgA antibodies (Fig. S3B to E) targeting H6-Egtrp, H6-EgA31, H6-mCherry, and H6-TasA, no immune response to any of these antigens was detected.
FIG 4.
A specific humoral response is elicited in dogs after oral application of recombinant B. subtilis spores harboring Echinococcus granulosus antigens. Dog serum was collected on the indicated days postapplication and tested by indirect ELISA using plates coated with E. coli purified H6-EgTrp (A), H6-EgA31 (B), H6-mCherry (C), and H6-TasA (D). The samples were then incubated with specific dog anti-IgG conjugated with HRP. The dog groups are indicated on the vertical axis. White and black bars, dogs 1 and 2 in each group, respectively. The data represent the mean ± SEM from three independent experiments. (E) Immunohistochemistry of sheep E. granulosus protoscoleces incubated with the indicated dogs sera (1:100) followed by secondary anti-IgG dog-HRP and stained with diaminobenzidine (brown). Nuclei were counterstained with hematoxylin (blue). Each row corresponds to the indicated dog group (groups treated with placebo, EgTrp, EgA31, and the mixture of EgTrp and EgA31). The number of the dog to which the treatment was orally applied is reported at the top of each panel. The right columns of the two panels correspond to enlargements of the insets marked with a black frame in the pictures on the left. Black arrows, positive sera recognizing the subtegument/tegument membrane of the protoscoleces. Bars, 50 μm. (F) PCR characterization of intestinal recombinant B. subtilis bacteria isolated from the small intestine of dog 2 from the mixture group. Colonies isolated from one duodenal section (lanes D) and two jejunal sections (lanes J1 and J2) were analyzed by PCR for the detection of the B. subtilis tasA sinR TasA-EgTrp (top) and B. subtilis tasA sinR TasA-EgA31 (bottom) strains. Each panel also includes the results for a set of controls, namely, EgTrp, EgA31, and ΔTΔS, which correspond to laboratory strains B. subtilis tasA sinR TasA-EgTrp, B. subtilis tasA sinR TasA-EgA31, and B. subtilis tasA sinR, respectively. Lanes MW, molecular weight markers; yellow arrows, the position of each E. granulosus-specific band.
We next questioned if the sera of the dogs collected on day 60 could also recognize the antigens in the parasite context. The two antigens used in this study, EgA31 and EgTrp, have previously been described to be localized in the tegument and subtegument outer membranes of the E. granulosus protoscolex (23, 29, 30). For this purpose, immunohistochemistry of E. granulosus protoscoleces was performed by incubation with serum samples from the dogs. For analysis of the data, we particularly considered the staining of the subtegument/tegument membranes, shown in the enlargements of the insets shown in Fig. 4E. The brownish color observed in the internal regions of the protoscoleces by immunohistochemistry was considered background because it is not possible to distinguish between serum incubated with placebo and spore-treated serum. Our results reveal that the subtegument/tegument membranes presented a brown color (Fig 4E, inset, arrowheads) when they were incubated with serum from dog 2 in the EgTrp group, dog 2 in the EgA31 group, and both dogs in the mixture group. In contrast, serum from the placebo-treated dogs, dog 1 in the EgTrp group, and dog 1 in the EgA31 group revealed a negative color (blue) for the subtegument/tegument membranes of the protoscoleces. These data are consistent with the immune response observed by ELISA (Fig. 4A to D) and strongly suggest that the dogs treated with recombinant B. subtilis spores harboring E. granulosus antigens could recognize the parasite.
In an attempt to reisolate the applied recombinant B. subtilis from the guts of the treated animals, we recovered the intestinal microflora of the duodenum, jejunum, and ileum of two dogs which received the recombinant B. subtilis spore mixture. Recombinant B. subtilis was isolated in selective medium (Table 1). Interestingly, recombinant B. subtilis bacteria could be separated from both the duodenum and the jejunum of dog 2, but no recombinant bacteria were isolated from the intestine of dog 1. The presence of recombinant bacteria was confirmed by PCR of colonies isolated from the duodenum and jejunum of dog 2 from the mixture group (Fig. 4F).
Importantly, neither inflammatory nor degenerative lesions could be detected by histology in the intestinal sections. Likewise, no undesirable clinical signs were observed in the dogs after treatment with spores (Fig. S3F). These data confirm that the use of recombinant B. subtilis spores is safe for dogs. Our results indicate that orally applied recombinant B. subtilis spores harboring TasA fusions to E. granulosus antigens (EgTrp and EgA31) get germinated in the gut and elicit a specific humoral response in dogs.
DISCUSSION
Current evidence indicates that B. subtilis endospores can germinate in the guts of mice, rabbits, and humans (31, 32). Consequently, engineered B. subtilis spores may be used as carriers for the administration of antigens for immunization against enteric pathogens, such as viruses, bacteria, and parasites, or even for the induction of tolerance in the treatment of chronic inflammatory diseases (33). In this study, we provide evidence that the oral application of recombinant B. subtilis spores to dogs resulted in spore germination in the gut. The presence of vegetative recombinant bacteria could favor the formation of an intestinal biofilm, allowing the stimulation of gut-associated lymphoid tissue (GALT) (31), which elicited a humoral response against TasA fused to an epitope. In fact, our results show that a humoral immune response against the fusion protein TasA-mCherry and the TasA-E. granulosus tapeworm antigen fusions TasA-EgTrp and TasA-EgA31 was mounted in dogs, suggesting spore germination and GALT stimulation by the recombinant B. subtilis spores.
In contrast to other methodologies based on the surface expression or surface adsorption of antigens on spores of B. subtilis (34), our approach consisted of the expression of heterologous peptides after spore germination. This method is particularly advantageous for oral application since the spores can bypass the stomach barrier without damaging the structure of the heterologous peptide. Another advantage of using B. subtilis as an antigen carrier is its probiotic nature (27, 35), which permits its safe application. We demonstrated that there was no significant difference in the body weights of the dogs in the different groups during the whole procedure and that there were no undesirable clinical signs in the animals receiving recombinant B. subtilis spores. Hematoxylin-eosin (H&E)-stained histological sections from different intestinal sections showed neither inflammatory nor degenerative lesions after the oral application of the recombinant B. subtilis spores. The fact that B. subtilis, as shown here and by others (36, 37), is part of the dog's natural intestinal microflora reinforces the safety of the use of this organism for the delivery of antigens in the intestine. Altogether, our data show that B. subtilis can safely deliver heterologous peptides to the intestine of dogs so that they may subsequently mount an immune response. Interestingly, and in line with our results, the biofilm formation ability of B. subtilis strains isolated from the intestinal microflora has also been described in species as diverse as the grass carp (38) and humans (39, 40). It has been suggested that the biofilm-forming properties of B. subtilis strains are relevant for the growth and formation of biofilms in the intestine (32, 40). In this study, we used the nondomesticated B. subtilis NCIB 3610 strain to engineer recombinant spores. This strain has been extensively studied because of its remarkable biofilm-forming abilities (15). Thus, the recombinant spores used in this study (Fig. 2) (13) have the potential to germinate and then form a biofilm in the mucosa of the intestine. We present strong evidence indicating that recombinant B. subtilis bacteria colonize and express the heterologous proteins in the dog intestine, as denoted by (i) the visualization of vegetative recombinant B. subtilis by luminescence (Fig. 2F), immunohistochemistry against the specific antigens (see Fig. S1E in the supplemental material), and specific PCR of perfused intestinal content (Fig. 4F) and (ii) quantification of recombinant B. subtilis spores shed in feces, in which spores were present in amounts less than the initial load of the spores, suggesting intestinal retention and germination of the spores (Tables 3 and 4).
By using indirect ELISA and immunoblotting with dog serum, we demonstrated that dogs generate a humoral response against the presented antigens, mCherry or E. granulosus peptides. Interestingly, the tested dogs also developed a humoral response against TasA, and placebo-treated dogs were also positive for TasA in all experimental settings. This result suggests that native TasA derived from the dogs' intestinal microflora could stimulate local immunity, which relates to the maturation of the intestinal microflora of the dogs. It has been proposed by others (41) that the generation of antibodies against the intestinal microflora promotes host-microbiota mutualism through a reduction of the inflammatory response toward the microbiota, allowing the exclusion of an immune response to the bacteria in direct contact with the intestinal mucosa.
Even if the humoral response recognizes recombinant E. granulosus antigens EgA31 and EgTrp, further research will unveil whether this technology can neutralize parasitic infections. A good indication in this direction is the recognition of the tegument and subtegument from the isolated E. granulosus protoscoleces by the serum of the recombinant B. subtilis spore-treated dogs.
Collectively, our results demonstrate that the oral application of recombinant B. subtilis spores elicited a specific humoral response in dogs. This technology could be the foundation for the development of vaccines for dogs that carry B. subtilis in their intestinal microbiota.
MATERIALS AND METHODS
Ethics statement.
All the dog experiments were performed according to the guidelines of the animal experimentation law (SR 455.163; TVV) of the Swiss federal government. The Cantonal Veterinary Office of Zurich, Switzerland, approved the protocols under animal experimentation number 100/2010.
B. subtilis strains, media, and culture conditions.
The B. subtilis strains used in this study are described in Table 1. For routine growth and spore quantification, cells were propagated on Luria-Bertani (LB) medium. For biofilm assays, cells were scraped from overnight growth on LB agar plates and resuspended in LB liquid medium to an optical density at 600 nm (OD600) of 1, and then 2 μl of this suspension was spotted onto MSgg solid medium (42). The biofilms were incubated at 30°C or 37°C. The final concentrations of antibiotics used for the B. subtilis strains were as follows: 100 μg/ml for spectinomycin (Spc), 10 μg/ml for kanamycin (Km), 1 μg/ml for erythromycin, 25 μg/ml for lincomycin (the macrolide-lincosamide-streptogramin B [MLS] group), and 5 μg/ml for chloramphenicol (Cm).
Plasmid constructions.
pQE80L-EgA31PstI/DraI was obtained from A.-F. Pétavy (Université Claude Bernard Lyon 1, Lyon, France) (43). pQE32-(102-278)EgTrp was achieved by PCR amplification of (102-278)EgTrp fragments from the construct pQIA-EgTrp, kindly provided by Adriana Esteves (Universidad de la República, Montevideo, Uruguay) (21), using specific primers containing flanking BamHI and PstI restriction sites, followed by ligation between BamHI and PstI in pQE32 (Qiagen). pQE32-mCherry was obtained by PCR amplification of mCherry from pRSET-mCherry (44) using specific primers containing BamHI and HindIII restriction sites, followed by ligation between BamHI and HindIII in pQE32 (Qiagen). pET22b-TasA (45) was provided by Diego Romero (University of Malaga, Malaga, Spain). pDR-PtapA-luxCDABE was obtained by PCR amplification of luxABE genes from pSB403 (25) using specific primers containing AgeI and SacI restriction sites, followed by ligation between AgeI and SacI in pDR183-PtapA-luxA. The plasmid pDR183-PtapA-luxA was obtained by PCR amplification of the luxCDA genes from pSB403 using specific primers to insert an EcoRI restriction site followed by a ribosome binding site (RBS) at the 5′ end and AgeI and SacI restriction sites at the 3′ end. The PCR fragment was ligated between EcoRI and SacI in pDR183-PtapA. The plasmid pDR183-PtapA was obtained by PCR amplification of PtapA from pBS-TapAop-mCherry (13) using specific primers containing SacII and EcoRI restriction sites, followed by ligation between SacII and EcoRI in pDR183 (lacA::erm) (46) (kindly provided by David Rudner, Harvard University, Cambridge, MA, USA). All oligonucleotides were obtained from Microsynth AG, Switzerland, and are described in Table S1.
Antibodies and reagents.
Rabbit polyclonal anti-TasA was a gift from R. Losick (Harvard University, Cambridge, MA, USA). Mouse polyclonal anti-EgTrp and mouse polyclonal anti-EgA31 were gifts from A.-F. Pétavy (Université Claude Bernard Lyon 1, Lyon, France). Goat anti-GST was purchased from GE Healthcare Life Sciences. Mouse anti-dsRed2 was purchased from Santa Cruz Biotechnology, USA. Rabbit F(ab′)2 anti-dog IgG (H&L)-peroxidase was acquired from Abcam. Goat anti-dog IgA antibody-peroxidase was obtained from Bethyl Laboratories, Inc. (Montgomery, TX, USA). Rabbit anti-goat IgG (whole molecule)-peroxidase and goat anti-mouse IgG Fab′-peroxidase were obtained from Sigma-Aldrich. Isopropyl-β-d-thiogalactopyranoside (IPTG) was acquired from Biosolve Chimie, France.
Transformation of B. subtilis.
Bacillus subtilis strain 168 was transformed as described previously by Cutting and Vander Horn (47). The transformants were selected with the appropriate antibiotics for a double-crossover recombination at the amyE locus or lacA locus (48). The different operons were then transferred to the undomesticated B. subtilis NCIB 3610 strain by SPP1-mediated generalized transduction (49). The positive clones were identified by direct PCR of the selected colonies using specific primers.
Characterization and identification of B. subtilis in dog intestinal microflora.
Feces from seven samples (21 dogs) from dogs from the UZH animal facility and nine individual fecal samples collected from privately owned dogs were analyzed. All the samples were from healthy animals that had not been treated with antibiotics or probiotics for at least 1 year. The feces were resuspended at 10 times weight volume in phosphate-buffered saline (PBS), serially diluted, plated in LB agar medium, and incubated for 2 days at 37°C. The isolated colonies were streaked onto Difco sporulation agar medium for 24 h at 37°C and selected for rod shape by inspection under a microscope using a ×63 lens. Then, the selected isolates were reinoculated in 3 ml of Difco sporulation medium, incubated on an orbital shaker for 4 days at 37°C, and inspected under the microscope for the presence of endospores. The selected sporulating rod bacteria were stored at −80°C in 15% glycerol for further analysis. The sequence of the rrnE gene of 16S rRNA was analyzed as described by Hoa et al. (50). Briefly, PCR amplification was performed using primers specific for the B. subtilis rrnE gene of 16S rRNA (primers P1 [5′-GCGGCGTGCCTAATACATGC-3′] and P2 [5′-CACCTTCCGATACGGCTACC-3′]) and then sequenced by the Sanger method at Microsynth AG, Switzerland. Sequences were aligned by use of the Clustal X program (51). The optimal model of DNA evolution was evaluated for the best fit of the data set using the MrAIC.al program (which is distributed by the author, J. A. A. Nylander, Evolutionary Biology Centre, Uppsala University, 2004). The Bayesian phylogeny was inferred using the BEAST program (version 1.5.3). A Markov chain Monte Carlo simulation with a GTR (general time reversible) substitution matrix and a strict clock was run over 10,000,000 generations. The tree files were combined into one consensus tree by using the LOGCOMBINER program. The consensus tree representing the similarities between the 16S rRNA genes was displayed with the FIGTREE program (52). The rrnE gene sequence from the undomesticated B. subtilis NCIB 3610 strain was used as a reference. A list of the assigned bacterial strains from the isolated clones is provided in Table S2.
Quantification of spores in a biofilm.
The ability of recombinant B. subtilis to sporulate in a biofilm was determined as described by Vlamakis et al. (15). Briefly, B. subtilis cultured in LB medium was diluted to an OD600 of 1, and 10 μl of the suspension was inoculated in duplicate over 2.5 ml of MSgg medium in 12-well culture plates. The culture plates were incubated at room temperature with no agitation. Samples of cells were taken after 48 h and subjected to mild sonication conditions (10 s at 14 kHz) to obtain intact single cells. After sonication, each preparation was normalized to an OD600 of 1, incubated for 20 min at 80°C to kill vegetative cells, and serially diluted to determine viable spore counts in selective LB agar plates.
Production of recombinant B. subtilis spores.
The recombinant spores were produced and purified as described by Vogt et al. (13). A list of the B. subtilis strains used in this study is provided in Table 1.
Expression and purification of H6-tagged proteins.
pQE31-mCherry, pQE32-(102-278)EgTrp, and pQE80L-EgA31PstI/DraI were expressed in Escherichia coli M15(pREP4) (Qiagen). pET22b-TasA was expressed in E. coli BL21 (New England BioLabs, Inc.). The culture was induced with isopropyl-β-d-thiogalactoside (1 mM), grown for 4 h, and centrifuged at 3,500 rpm for 15 min. The pellet was resuspended in 6 ml of PBS and incubated for 15 min on ice with 0.1 mg/ml lysozyme, 5 mM dithiothreitol, 1.5% lauryl sarcosine, and cOmplete protease inhibitor cocktail (Roche, Switzerland). The lysate was sonicated (six times for 10 s each time) and centrifuged at 12,000 × g for 15 min. The supernatant was supplemented with 1% Triton X-100 and loaded onto an Ni Sepharose 6 Fast Flow column (GE Healthcare) that had previously been equilibrated with 5 volumes of 20 mM imidazole in 50 mM NaH2PO4 for 2 h at 4°C in a rotation wheel. Subsequently, the resin was washed with 10 volumes of 60 mM imidazole, 200 mM NaCl, 0.1% Triton X-100, and 50 mM NaH2PO4, then with 10 volumes of 60 mM imidazole, 300 mM NaCl, 0.1% Triton X-100, and 50 mM NaH2PO4, and finally, with 10 volumes of 60 mM imidazole, 400 mM NaCl, 0.1% Triton X-100, and 50 mM NaH2PO4. The protein was eluted four times for 10 min each time by incubation with 250 μl of 250 mM imidazole, 400 mM NaCl, 0.1% Triton X-100, and 50 mM NaH2PO4.
The GST protein was expressed from pGEX-6P-1 (GE Healthcare) in E. coli BL21 and purified following the instructions provided by the manufacturer.
The eluted proteins were pooled in a single fraction and stored at 4°C in 30% glycerol. The protein concentration was obtained by comparison to a bovine serum albumin calibration curve in an SDS-polyacrylamide gel followed by Coomassie blue staining. The estimation of the protein concentration was performed by densitometry using the gel algorithm from ImageJ (v1.48) software (Wayne Rasband, NIH, USA).
Biofilm imaging.
Whole colonies were photographed, and the images were processed as described by Vogt et al. (13).
Experimental administration of recombinant B. subtilis spores to dogs.
In the Vetsuisse animal facility of the University of Zurich, 2 to 4 beagle dogs were housed in pens with a surface area of 1.45 m by 4.5 m and with access to an outside area of 1.45 m by 5.5 m. The pens were also enriched with installations permitting the dogs to play in a three-dimensional space, to rest, and to retreat. Beagle dogs were raised for laboratory use in the Vetsuisse animal facility of the University of Zurich. All the dogs were vaccinated against leptospirosis, canine distemper, canine hepatitis, parvovirus, and parainfluenza viruses (Canigen L, Virbac, Canigen, and SHA2PPi). A total of 5 × 1010 CFU per dose of recombinant B. subtilis spores was orally applied to the dogs three times, on days 1, 21, and 42. Each dose of recombinant B. subtilis spores was diluted in 1 ml of cat milk (Whiskas Mars, VA, USA) to increase palatability. The placebo group received 1 ml of cat milk. Each oral gavage was performed in the morning hours after the dogs were fed. All the animals were clinically monitored in a weekly routine and every day after each oral administration. Data on the animal number assigned, sex, age, and kind of recombinant B. subtilis spore applied to each dog are provided in Table 2. Blood samples (6 to 8 ml) were collected from the cephalic vein weekly, starting 1 day before the first oral gavage. The serum was used to test the humoral response against purified E. coli recombinant proteins.
Animal necropsy.
The dogs were sacrificed with an intravenous administration of acepromazine at 0.1 ml/10 kg of body weight (Prequillan; Arovet AG, Switzerland), followed by three doses of 80 mg/kg of sodium pentobarbitone (Esconarkon; Streuli Pharma AG, Switzerland). During the postmortem examination blood and fecal samples and intestinal tissue sections were collected.
Immunoblotting.
Identical amounts of purified recombinant protein (3 μg) were mixed with sample buffer (8% SDS, 40% glycerol, 200 mM Tris, pH 6.8, 4% 2-mercaptoethanol, 0.4% bromophenol blue), and the mixture was heated for 5 min at 95°C and consecutively loaded onto a 12% SDS-polyacrylamide gel. After migration, the proteins were transferred to an Amersham Protan 0.45-μm-pore-size nitrocellulose blotting membrane (GE Healthcare), and the lanes were cut into strips. The strips were incubated in diluted dog serum (1:100) in 5% milk-PBS. The samples were further processed as described by Gluck et al. (53).
Indirect ELISA.
Recombinant purified protein (500 ng/well) in 0.2 M bicarbonate buffer (pH 9.4) was coated for 16 h at 4°C in 96-well multiwell plates (Nunc-Immuno MaxiSorp; Thermo Scientific). Sample wells were incubated for 2 h at room temperature with blocking buffer (5% milk, 0.1% Tween 20 in PBS). The dog serum was diluted to 1:500 in blocking buffer, 50 μl was added to each well, and the plate was incubated overnight at 4°C. Samples were incubated with the corresponding secondary anti-dog immunoglobulin antibody conjugated to horseradish peroxidase (HRP) diluted in blocking buffer for 1 h at 37°C in a moist chamber. Between each incubation, the plates were washed three times with PBS containing 0.05% Tween 20. The ELISA was developed by the addition of 100 μl of tetramethylbenzidine substrate (Thermo Fisher Scientific) per well in the dark for 30 min at room temperature, and the reaction was stopped by adding 100 μl 1 M H2SO4. The plate's absorbance was read at OD450 using an SLT 340 ATTC Tecan microplate reader (Tecan US Inc.). The data were analyzed and processed using Microsoft Excel for Mac 2011 software. The cutoff was determined to be the average for three negative controls. The negative-control value was obtained by incubation of the antigen only with the secondary antibody conjugated to HRP. The cutoff was subtracted from all the sample values. Each value was subtracted from its corresponding preimmune value (PI).
Histology and immunohistochemistry.
The dogs' intestines were sectioned into the duodenum, jejunum, ileum, cecum, and colon. Each intestinal section was knotted at both ends before sectioning to avoid the loss of the intestinal contents. Samples of approximately 2 cm in length were fixed in 4% formaldehyde. After fixation, each sample was dehydrated in alcohol solutions of increasing concentration and embedded in paraffin. The embedded samples were cut into sections 2 to 3 μm thick, placed onto slides, and stained with hematoxylin and eosin (H&E).
For immunohistochemistry of the dogs' intestinal sections, the samples were deparaffinized, rehydrated, and incubated for 30 min at room temperature with the primary antibody (rabbit anti-TasA serum or mouse anti-dsRed2 serum). The secondary antibody and aminoethyl carbazole (AEC) from a chromogen A detection kit were subsequently applied according to the manufacturer's protocols (peroxidase/AEC rabbit/mouse kit; Dako).
E. granulosus protoscoleces were isolated from a sheep liver cyst. The samples were treated for immunohistochemistry by fixation, embedment in paraffin, and deparaffinization as described above. Subsequently, the samples in EDTA buffer, pH 9.0 (1.25 mM EDTA, 10 mM Tris, pH 9.0), were heated for 20 min at 98°C using a steamer (Pascal; Dako Cytomation). The slides were blocked for 10 min at room temperature with Dako Real peroxidase blocking solution (catalog number S2023; Dako). The samples were then incubated with the indicated dog serum (diluted 1:50) followed by incubation with rabbit F(ab′)2 anti-dog IgG (H&L) conjugated to HRP (1:100; catalog number ab136759; Abcam), stained with a liquid DAB+ substrate chromogen system (brown; Dako), and counterstained with hematoxylin (blue).
Images were acquired using an Olympus CX41 light microscope equipped with a 40× objective lens and an Olympus Vanox-S AxioCam interface. The acquired images were processed using Image J software (Wayne Rasband, NIH, USA; http://imagej.nih.gov/ij).
Detection of recombinant spores in intestinal contents.
The small intestine was dissected into the duodenum, jejunum, and ileum. Perfusion was used to collect the intestinal contents of each section with vigorous flushes of PBS, and the intestinal contents were centrifuged at 14,000 × g and 20°C for 15 min. The pellet was resuspended in 20 ml PBS and centrifuged at 1,500 × g for 10 min at 20°C. The supernatant was collected and centrifuged at 14,000 × g for 15 min and 20°C, and the final pellet was resuspended in 3 ml of PBS. Serial dilutions of from 1 × 10−1 to 1 × 10−6 were prepared, 10 μl of each dilution was plated on semisolid LB-kanamycin plates (10 μg/ml), and the plates were incubated for 36 h at 37°C. The colonies were counted, and the number of CFU per milliliter was estimated (15).
Colony PCR.
A colony was resuspended in 15 μl lysis buffer (50 mM KCl, 0.1% Tween 20, 10 mM Tris-HCl, pH 8.3), and the mixture was heated to 99°C for 10 min. The PCR amplicons were obtained by thermocycling 5 μl of bacterial lysate with 15 μl of PCR master mix (0.2 mM deoxynucleoside triphosphates, 150 ng of each forward and reverse primers, 0.3 μl Taq DNA polymerase [5 U/μl; New England BioLabs, Inc.], 1 μl dimethyl sulfoxide). The following internal primers were used for detection of B. subtilis recombinant strains: for EgTrp, 5′-ATGCGCGGCCGCCATTATGATGGCAATGAAATTG-3′ and 5′-GATCCCCGGGGGGATCCTTACTCTTGCTCGGAGACTTCGAG-3′, and for EgA31, 5′-ATGCGCGGCCGCCGCAGCTGAAAAACAAGCCATG-3′ and 5′-GATCCCCGGGGGATCCTCACCTTGTTTCAAGCATTTCAAT-3′. The PCR amplicons were monitored by agarose gel electrophoresis. Images were acquired using a Molecular Imager Gel Doc XR system with the incorporated Image Lab software (Bio-Rad) and processed using Image J (v1.48) software (Wayne Rasband, NIH, USA) and Microsoft PowerPoint for Mac 2011 software.
IVIS.
The luminescence of biofilm and intestinal samples was acquired using a Xenogen IVIS100 imaging system (Imaging Technologies) equipped with a charge-coupled-device array scientific camera (Spectral Instruments, Inc.) mounted over a light-tight specimen chamber, a 600 series camera controller, and a camera cooling system. The images were analyzed and processed using Living Image (v4.0) software (Caliper Life Sciences, Inc.).
Supplementary Material
ACKNOWLEDGMENTS
We thank Walter Basso, Manuela Schnyder, and Ruth Fietcher for their veterinary help and assistance. We thank Armin Rudemann and Esther Merz for taking care of the animals during the study. We thank Sabine Wunderlin and Marta R. Figueiredo for their excellent technical assistance.
We all declare that we have no conflict of interest.
This research was funded by a PARAVAC European Commission grant (reference number 265862/FP-7-KBBE).
The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
C.A., M.A., P.D., and C.E. conceived and designed the experiments. C.M.V., M.T.A.-F., M.H., C.A., and C.E. performed the experiments. C.M.V., M.T.A.-F., K.T., M.H., C.A., M.A., P.D., and C.E. analyzed the data. C.M.V., M.T.A.-F., K.T., M.H., C.A., P.D., M.A., and C.E. contributed reagents, materials, and analysis tools. K.T., C.A., M.A., P.D., and C.E. wrote the paper.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00495-17.
REFERENCES
- 1.Decaro N, Desario C, Elia G, Campolo M, Lorusso A, Mari V, Martella V, Buonavoglia C. 2007. Occurrence of severe gastroenteritis in pups after canine parvovirus vaccine administration: a clinical and laboratory diagnostic dilemma. Vaccine 25:1161–1166. doi: 10.1016/j.vaccine.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Decaro N, Crescenzo G, Desario C, Cavalli A, Losurdo M, Colaianni ML, Ventrella G, Rizzi S, Aulicino S, Lucente MS, Buonavoglia C. 2014. Long-term viremia and fecal shedding in pups after modified-live canine parvovirus vaccination. Vaccine 32:3850–3853. doi: 10.1016/j.vaccine.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahl P, Cliquet F, Guiot AL, Niin E, Fournials E, Saint-Jean N, Aubert M, Rupprecht CE, Gueguen S. 2014. Twenty year experience of the oral rabies vaccine SAG2 in wildlife: a global review. Vet Res 45:77. doi: 10.1186/s13567-014-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesnut M. 2012. Bronchi-Shield Oral-First oral Bordetella bronchiseptica vaccine approved for use in dogs. Boehringer Ingelheim, Ingelheim am Rhein, Germany. [Google Scholar]
- 5.Ellis JA. 2015. How well do vaccines for Bordetella bronchiseptica work in dogs? A critical review of the literature 1977-2014. Vet J 204:5–16. doi: 10.1016/j.tvjl.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Cliquet F, Barrat J, Guiot AL, Cael N, Boutrand S, Maki J, Schumacher CL. 2008. Efficacy and bait acceptance of vaccinia vectored rabies glycoprotein vaccine in captive foxes (Vulpes vulpes), raccoon dogs (Nyctereutes procyonoides) and dogs (Canis familiaris). Vaccine 26:4627–4638. doi: 10.1016/j.vaccine.2008.06.089. [DOI] [PubMed] [Google Scholar]
- 7.Dahiya SS, Saini M, Kumar P, Gupta PK. 2011. An oral Sindbis virus replicon-based DNA vaccine containing VP2 gene of canine parvovirus delivered by Escherichia coli elicits immune responses in dogs. Acta Virol 55:289–294. doi: 10.4149/av_2011_04_289. [DOI] [PubMed] [Google Scholar]
- 8.Petavy AF, Hormaeche C, Lahmar S, Ouhelli H, Chabalgoity A, Marchal T, Azzouz S, Schreiber F, Alvite G, Sarciron ME, Maskell D, Esteves A, Bosquet G. 2008. An oral recombinant vaccine in dogs against Echinococcus granulosus, the causative agent of human hydatid disease: a pilot study. PLoS Negl Trop Dis 2:e125. doi: 10.1371/journal.pntd.0000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Liu Y, Fooks AR, Zhang F, Hu R. 2008. Oral vaccination of dogs (Canis familiaris) with baits containing the recombinant rabies-canine adenovirus type-2 vaccine confers long-lasting immunity against rabies. Vaccine 26:345–350. doi: 10.1016/j.vaccine.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 10.Larsen N, Thorsen L, Kpikpi EN, Stuer-Lauridsen B, Cantor MD, Nielsen B, Brockmann E, Derkx PM, Jespersen L. 2014. Characterization of Bacillus spp. strains for use as probiotic additives in pig feed. Appl Microbiol Biotechnol 98:1105–1118. doi: 10.1007/s00253-013-5343-6. [DOI] [PubMed] [Google Scholar]
- 11.Tompkins TA, Xu X, Ahmarani J. 2010. A comprehensive review of post-market clinical studies performed in adults with an Asian probiotic formulation. Benef Microbes 1:93–106. doi: 10.3920/BM2008.1005. [DOI] [PubMed] [Google Scholar]
- 12.Zokaeifar H, Babaei N, Saad CR, Kamarudin MS, Sijam K, Balcazar JL. 2014. Administration of Bacillus subtilis strains in the rearing water enhances the water quality, growth performance, immune response, and resistance against Vibrio harveyi infection in juvenile white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 36:68–74. doi: 10.1016/j.fsi.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Vogt CM, Schraner EM, Aguilar C, Eichwald C. 2016. Heterologous expression of antigenic peptides in Bacillus subtilis biofilms. Microb Cell Fact 15:137. doi: 10.1186/s12934-016-0532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veening JW, Smits WK, Hamoen LW, Kuipers OP. 2006. Single cell analysis of gene expression patterns of competence development and initiation of sporulation in Bacillus subtilis grown on chemically defined media. J Appl Microbiol 101:531–541. doi: 10.1111/j.1365-2672.2006.02911.x. [DOI] [PubMed] [Google Scholar]
- 15.Vlamakis H, Aguilar C, Losick R, Kolter R. 2008. Control of cell fate by the formation of an architecturally complex bacterial community. Genes Dev 22:945–953. doi: 10.1101/gad.1645008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson RC. 2017. Biology and systematics of Echinococcus. Adv Parasitol 95:65–109. doi: 10.1016/bs.apar.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Deplazes P, Rinaldi L, Alvarez Rojas CA, Torgerson PR, Harandi MF, Romig T, Antolova D, Schurer JM, Lahmar S, Cringoli G, Magambo J, Thompson RC, Jenkins EJ. 2017. Global distribution of alveolar and cystic echinococcosis. Adv Parasitol 95:315–493. doi: 10.1016/bs.apar.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. 2012. Accelerating work to overcome the global impact of neglected tropical diseases. A roadmap for implementation. WHO Press, Geneva, Switzerland. [Google Scholar]
- 19.Budke CM, Deplazes P, Torgerson PR. 2006. Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis 12:296–303. doi: 10.3201/eid1202.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig PS, Hegglin D, Lightowlers MW, Torgerson PR, Wang Q. 2017. Echinococcosis: control and prevention. Adv Parasitol 96:55–158. doi: 10.1016/bs.apar.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Alvite G, Esteves A. 2009. Echinococcus granulosus tropomyosin isoforms: from gene structure to expression analysis. Gene 433:40–49. doi: 10.1016/j.gene.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Fraize M, Sarciron ME, Azzouz S, Issaadi N, Bosquet G, Petavy AF. 2005. Immunogenicity of two Echinococcus granulosus antigens EgA31 and EgTrp in mice. Parasitol Res 96:113–120. doi: 10.1007/s00436-005-1322-x. [DOI] [PubMed] [Google Scholar]
- 23.Fu Y, Martinez C, Chalar C, Craig PS, Ehrlich R, Petavy AF, Bosquet G. 1999. A new potent antigen from Echinococcus granulosus associated with muscles and tegument. Mol Biochem Parasitol 102:43–52. doi: 10.1016/S0166-6851(99)00084-5. [DOI] [PubMed] [Google Scholar]
- 24.Muhlschlegel F, Sygulla L, Frosch P, Massetti P, Frosch M. 1993. Paramyosin of Echinococcus granulosus: cDNA sequence and characterization of a tegumental antigen. Parasitol Res 79:660–666. doi: 10.1007/BF00932508. [DOI] [PubMed] [Google Scholar]
- 25.Winson MK, Swift S, Fish L, Throup JP, Jorgensen F, Chhabra SR, Bycroft BW, Williams P, Stewart GS. 1998. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol Lett 163:185–192. doi: 10.1111/j.1574-6968.1998.tb13044.x. [DOI] [PubMed] [Google Scholar]
- 26.Weber MP, Stambouli F, Martin LJ, Dumon HJ, Biourge VC, Nguyen PG. 2002. Influence of age and body size on gastrointestinal transit time of radiopaque markers in healthy dogs. Am J Vet Res 63:677–682. doi: 10.2460/ajvr.2002.63.677. [DOI] [PubMed] [Google Scholar]
- 27.Cutting SM. 2011. Bacillus probiotics. Food Microbiol 28:214–220. doi: 10.1016/j.fm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Niewiesk S. 2014. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol 5:446. doi: 10.3389/fimmu.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esteves A, Senorale M, Ehrlich R. 2003. A tropomyosin gene is differentially expressed in the larval stage of Echinococcus granulosus. Parasitol Res 89:501–502. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins RE, Taylor MJ, Gilvary NJ, Bianco AE. 1998. Tropomyosin implicated in host protective responses to microfilariae in onchocerciasis. Proc Natl Acad Sci U S A 95:7550–7555. doi: 10.1073/pnas.95.13.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhee KJ, Sethupathi P, Driks A, Lanning DK, Knight KL. 2004. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol 172:1118–1124. doi: 10.4049/jimmunol.172.2.1118. [DOI] [PubMed] [Google Scholar]
- 32.Tam NK, Uyen NQ, Hong HA, Duc LH, Hoa TT, Serra CR, Henriques AO, Cutting SM. 2006. The intestinal life cycle of Bacillus subtilis and close relatives. J Bacteriol 188:2692–2700. doi: 10.1128/JB.188.7.2692-2700.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amuguni H, Tzipori S. 2012. Bacillus subtilis: a temperature resistant and needle free delivery system of immunogens. Hum Vaccin Immunother 8:979–986. doi: 10.4161/hv.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang JM, Hong HA, Van Tong H, Hoang TH, Brisson A, Cutting SM. 2010. Mucosal delivery of antigens using adsorption to bacterial spores. Vaccine 28:1021–1030. doi: 10.1016/j.vaccine.2009.10.127. [DOI] [PubMed] [Google Scholar]
- 35.Permpoonpattana P, Hong HA, Khaneja R, Cutting SM. 2012. Evaluation of Bacillus subtilis strains as probiotics and their potential as a food ingredient. Benef Microbes 3:127–135. doi: 10.3920/BM2012.0002. [DOI] [PubMed] [Google Scholar]
- 36.Hooda S, Minamoto Y, Suchodolski JS, Swanson KS. 2012. Current state of knowledge: the canine gastrointestinal microbiome. Anim Health Res Rev 13:78–88. doi: 10.1017/S1466252312000059. [DOI] [PubMed] [Google Scholar]
- 37.Swanson KS, Dowd SE, Suchodolski JS, Middelbos IS, Vester BM, Barry KA, Nelson KE, Torralba M, Henrissat B, Coutinho PM, Cann IK, White BA, Fahey GC Jr. 2011. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J 5:639–649. doi: 10.1038/ismej.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo X, Chen DD, Peng KS, Cui ZW, Zhang XJ, Li S, Zhang YA. 2016. Identification and characterization of Bacillus subtilis from grass carp (Ctenopharynodon idellus) for use as probiotic additives in aquatic feed. Fish Shellfish Immunol 52:74–84. doi: 10.1016/j.fsi.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Hong HA, Khaneja R, Tam NM, Cazzato A, Tan S, Urdaci M, Brisson A, Gasbarrini A, Barnes I, Cutting SM. 2009. Bacillus subtilis isolated from the human gastrointestinal tract. Res Microbiol 160:134–143. doi: 10.1016/j.resmic.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Sirec T, Cangiano G, Baccigalupi L, Ricca E, Isticato R. 2014. The spore surface of intestinal isolates of Bacillus subtilis. FEMS Microbiol Lett 358:194–201. doi: 10.1111/1574-6968.12538. [DOI] [PubMed] [Google Scholar]
- 41.Palm NW, de Zoete MR, Flavell RA. 2015. Immune-microbiota interactions in health and disease. Clin Immunol 159:122–127. doi: 10.1016/j.clim.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Branda SS, Gonzalez-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. 2001. Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci U S A 98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saboulard D, Lahmar S, Petavy AF, Bosquet G. 2003. The Echinococcus granulosus antigen EgA31: localization during development and immunogenic properties. Parasite Immunol 25:489–501. doi: 10.1111/j.1365-3024.2003.00663.x. [DOI] [PubMed] [Google Scholar]
- 44.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 45.Romero D, Vlamakis H, Losick R, Kolter R. 2011. An accessory protein required for anchoring and assembly of amyloid fibres in B. subtilis biofilms. Mol Microbiol 80:1155–1168. doi: 10.1111/j.1365-2958.2011.07653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doan T, Marquis KA, Rudner DZ. 2005. Subcellular localization of a sporulation membrane protein is achieved through a network of interactions along and across the septum. Mol Microbiol 55:1767–1781. doi: 10.1111/j.1365-2958.2005.04501.x. [DOI] [PubMed] [Google Scholar]
- 47.Cutting SM, Vander Horn PB. 1990. Genetic analysis, p 27–74. In Harwood CR, Cutting SM (ed), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 48.Guerout-Fleury AM, Frandsen N, Stragier P. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 180:57–61. doi: 10.1016/S0378-1119(96)00404-0. [DOI] [PubMed] [Google Scholar]
- 49.Yasbin RE, Young FE. 1974. Transduction in Bacillus subtilis by bacteriophage SPP1. J Virol 14:1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoa NT, Baccigalupi L, Huxham A, Smertenko A, Van PH, Ammendola S, Ricca E, Cutting AS. 2000. Characterization of Bacillus species used for oral bacteriotherapy and bacterioprophylaxis of gastrointestinal disorders. Appl Environ Microbiol 66:5241–5247. doi: 10.1128/AEM.66.12.5241-5247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 52.Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gluck S, Buttafuoco A, Meier AF, Arnoldi F, Vogt B, Schraner EM, Ackermann M, Eichwald C. 2017. Rotavirus replication is correlated with S/G2 interphase arrest of the host cell cycle. PLoS One 12:e0179607. doi: 10.1371/journal.pone.0179607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.