ABSTRACT
Vitamin D3 is produced in the skin in response to UVB irradiation, from either sun exposure or UVB sunbeds. The objective of the current study was to characterize serum 25(OH)D response to regular sunbed use from several lamp outputs following their respective time exposure recommendations. There were three groups that tanned over 12 weeks during the winter months in dedicated sunbeds based on lamp outputs (100 W and 160 W low pressure fluorescent and 700 W high pressure filtered metal halide lamps) and a control group provided serum 25(OH)D samples at baseline and end-of-study. Tanning session lengths were calculated based on Health Canada guidelines to stay below the erythema levels. Mean 25(OH)D were increased by an average of 42 nmol/L in the sunbeds that used 100 W and 160 W fluorescents. Change in 25(OH)D was dependent on baseline 25(OH)D levels and sunbed (p = 0.003) and age (p = 0.03), but was not affected by gender, BMI, Fitzpatrick type or cumulative length of tanning sessions. There was no significant increase in 25(OH)D levels in participants using the 700 W filtered metal halide lamp sunbed or in the control participants. Skin pigmentation, , was markedly increased in all tanners and skin lightness, L*, significantly decreased at 12 weeks. Both L* and were significantly correlated with 25(OH)D concentrations for the sunbeds with fluorescent lamps emitting UVB (100 W and 160W). Participants following standardized exposure schedules meeting Health Canada regulations in sunbeds irradiating adequate UVB showed continuous increases of 25(OH)D to physiological levels even after producing a tan in a controlled manner.
ClinicalTrials.gov Registration: NCT02334592
KEYWORDS: Sunbed, tanning, vitamin D, phototherapy, ultraviolet A, ultraviolet B, ultraviolet radiation
Introduction
The production of vitamin D in the skin is a demonstrably positive aspect of tanning.1 UVA radiation from a sunbed also induces immediate pigment darkening which is not involved in vitamin D synthesis and also produces nitric oxide in the skin which leads to relaxation of blood vessels, lower blood pressure and improved cardiovascular health.2 There are risks to any type of UV exposure, whether from the sun or a sunbed. It is widely accepted that excessive and overexposure of UV can increase the risk of skin cancer. Squamous cell carcinoma (SCC) seems to be related to excessive UV exposure where basal cell carcinoma (BCC) and melanoma is related to intermittent exposure such as sun burning.3
Ultraviolet-B (UVB) radiation initiates vitamin D synthesis,4 and wintertime sun exposure at northern latitudes (above 44°N) does not contain sufficient UVB radiation to stimulate vitamin D synthesis.5 Natural vitamin D production is limited by season depending on latitude. In fact, vitamin D production is not possible even on exposed skin for four or more months of the year depending on how far north one resides due to lack of UVB radiation. Sunlamps with a UVB component similar to solar summer output may provide an alternative during the winter.
Health Canada follows the recommendations made by the Institute of Medicine 2010 to achieve 25-hydroxyvitmain D [25(OH)D] concentrations, the accepted measure of vitamin D status, of greater than 50 nmol/L for bone health.6 On the other hand, the Endocrine Society, which also based its recommendations on clinical trials, recommended 25(OH)D concentrations above 75 nmol/L7. The European Vitamin D Association, which based its recommendations on observational studies, recommended 25(OH)D levels of 75–125 nmol/L8, as did another set of vitamin D experts in setting guidelines for those with intellectual and/or developmental disabilities.9 A recent paper suggests that 25(OH)D concentrations of 120 nmol/L are required to promote optimal health10 and to improve overall quality of life11,12 and prevent cancer.13
Sunbed tanning has been demonstrated to increase serum 25(OH)D levels, the accepted measure of vitamin D status.14-16 Over 5 weeks of tanning resulted in mean serum 25(OH)D levels of 80 nmol/L with the greatest increase seen in individuals with the lowest starting 25(OH)D concentrations.17 A recent systematic review examined the relationship between UV exposure and vitamin D production and found that UVB phototherapy, whether narrow or broadband, increased serum 25(OH)D levels.18–20 It has been reported that UV from artificial sources is 8 times more efficient in generating 25(OH)D.21
People who utilize indoor tanning equipment (sunbeds) represent a distinct subset of people who have reported several motivations for tanning including “top-up tanning” (i.e. to extend a tanned appearance), pre-vacation tanning to generate a base tan to prevent burning,22 vitamin D production through winter, and to look or feel good.23–25 In general, an improved feeling of well-being is associated with tanning.26–28 Sunbeds emitting UVB have been demonstrated to stimulate vitamin D production.29–32 Further, sunbeds may confer the benefits associated with obtaining optimal vitamin D status.
Physiological levels of serum 25(OH)D are represented by those levels that are achieved by natural exposure to the sun without sun screen and uninhibited by season. Ancestral people living at the equator, as measured in the Hadzabe and Maasai tribes of Tanzania, had mean serum 25(OH)D concentrations of 109 nmol/L and 119 nmol/L33. Two other lines of evidence suggest that 120 nmol/L is the level of serum 25(OH)D that meets physiological requirements including the amount that is required for vitamin D to be present in breast milk34 as well as the point at which the compensatory mechanism for deficiency is turned off.35 Mean 25(OH)D levels of 115 nmol/L have been achieved in people who use sunbeds regularly.17
The protocols of the tanning industry in Canada are governed by the Radiation Emitting Devices Act (RED Act).36 Health Canada's RED Act provides exposure time regulations for sunbed use to stay below erythema doses. These regulations are intended as recommendations based on a user's Fitzpatrick Skin Type to protect sunbed users from overexposure to UV radiation. Overexposure is defined as “exposure that exceeds the amount needed for vitamin D production and could lead to skin or eye damage. Acute overexposure induces sunburn.36”
The result of these regulations, the recommended exposure times in sunbeds, on vitamin D production has not been investigated. It is unknown if maximum time exposures achieve or maintain optimal 25(OH)D levels. The objective of the current study is to characterize serum 25(OH)D response to regular sunbed use, using several lamp outputs, and following the time exposure schedule recommended by the RED Act and the manufacturer of the equipment. A secondary goal was to quantify changes in skin colour throughout the study period and the effect this had on vitamin D levels.
Results
Demographics
The baseline demographics for all participants are reported in Table 1. Significant differences in 25(OH)D values were found at baseline between groups. Group 1 had the highest mean 25(OH)D while the control group had the lowest, with groups 2 and 3 in between. The control group had more male participants. More than half of participants in groups 2, 3 and 4 were vitamin D deficient (<50 nmol/L) at baseline while only a quarter of group 1 were deficient. The owner of the salon where group 1 used the sunbed was a nurse and since most of the participants had been clients of the salon in the past, we suspect that they may have been educated about vitamin D deficiency; hence, the reason for the higher levels at baseline. More than 75% of all groups were considered vitamin D insufficient (<75 nmol/L) at baseline.
Table 1.
Baseline Demographics of Study Participants.
| Group (Sunbed) |
||||
|---|---|---|---|---|
| 1 (LP 100W) | 2 (LP 160W) | 3 (HP) | 4 (Controls) | |
| N | 20 | 20 | 19 | 26 |
| Female:Male | 16:4 | 16:3 | 11:3 | 16:10 |
| Age (yr)* | 37.1 ± 13a | 37.0 ± 10a | 30.5 ± 15b | 39.5 ± 14a |
| BMI* | 24.7 ± 3.7 | 23.9 ± 3.3 | 24.4 ± 3.3 | NA |
| Fitzpatrick Skin type: | ||||
| II | 1 | 2 | 1 | NA |
| III | 12 | 15 | 13 | NA |
| IV | 7 | 3 | 5 | NA |
| Baseline 25(OH)D (nmol/L) | 66 ± 24a | 46 ± 14b,c | 51 ± 24 b,c | 40 ± 17 c |
| Vitamin D deficient at baseline (<50 nmol/) | 26% a | 59% b | 53% b | 67% c |
| Vitamin D insufficiency at baseline (<75 nmol/) | 79% a | 94% a | 82% a | 92% a |
Groups with different superscripts denote significant differences between groups
Mean ± SD
Participants were enrolled between January 1 and February 28th to allow for study completion before summer and limit the effect of any outdoor UVB exposure. The target was to enroll 20 participants in each group which was calculated to allow for the detection of a difference in mean 25(OH)D of 25 nmol/L. Groups 1 (LP 100W) and 2 (LP 160W) enrolled 20 participants and enrollment was closed at the end of February, for group 3 (HP) with 19 participants enrolled. Of those, 20 (100%), 19 (95%) and 14 (74%) completed the study, respectively. Of the 26 participants recruited in the control group, all 26 completed the study. Participants completed blood spot collection kits for 25(OH)D measurements and some collections were rejected by the laboratory for reasons such as too little sample. As a result, the number of samples varies between measurements (Table 2).
Table 2.
Comparison of 25(OH)D Concentrations between groups over time.
| Week 0 |
Week 5 |
Week 9 |
Week 12 |
||||
|---|---|---|---|---|---|---|---|
| Group | 25(OH)D (nmol/L) [n] | N with 25(OH)D >100 nmol/L | 25(OH)D (nmol/L) [n] | 25(OH)D (nmol/L) [n] | 25(OH)D (nmol/L) [n] | N with 25(OH)D >100 nmol/L | Mean Change in 25(OH)D (nmol/L) |
| 1 (LP 100W) | 66 ± 24 [19] | 2/20 | 84 ± 29 [17] | 94 ± 28 [20] | 111 ± 36* [19] | 12/19 | 45 ± 24a [19] |
| 2 (LP 160W) | 46 ± 14 [17] | 0/17 | 61 ± 29 [12] | 79 ± 26 [18] | 84 ± 40 [12] | 4/12 | 38 ± 37a [12] |
| 3 (HP) | 51 ± 24 [17] | 2/17 | 60 ± 37 [15] | 58 ± 28 [12] | 58 ± 29 [13] | 2/13 | 7 ± 39b [13] |
| 4 (Controls) | 40 ± 17 [22] | 1/20 | — | — | 46 ± 17 [24] | 1/20 | 6 ± 20b [20] |
Denotes significant difference from baseline (p<0.05)
Groups with different superscripts denote significant differences between groups
25(OH)D. Concentrations
Our primary objective was to characterize 25(OH)D concentrations resulting from regular tanning sessions for the 3 types of sunbeds in comparison with no tanning (Table 2). Fig. 1 depicts changes in 25(OH)D over the 12 weeks of the study. In comparison with baseline, serum 25(OH)D increased significantly in group 1 at week 9 (p<0.001) and week 12 (p<0.001) and in group 2 at week 12 (p = 0.05) only. Serum 25(OH)D values did not change over time in groups 3 (HP) or 4. Mean 25(OH)D were increased by an average of 42 nmol/L in both LP fluorescent sunbeds, 100 W (group 1) and 160 W (group 2). The 100 W group had a mean increase in 25(OH)D from 66 nmol/L at baseline to 111 nmol/L at study completion (paired t, p<0.001). The 160 W group started with lower 25(OH)D, at 46 nmol/L, and increased to 84 nmol/L (paired t, p = 0.007). These increases included 12 people in group 1, 63% up from 15%, and 4 people in group 2, 33% up from 0, that achieved 25(OH)D concentrations above 100 nmol/L (Table 2). Conversely, the number of vitamin D- deficient individuals, with serum 25(OH)D <50 nmol/L, was reduced significantly in groups 1 (down from 5 to 0) and 2 (down from 10 to 2). In groups 3 and 4 all of the participants that were 25(OH)D deficient (<50 nmol/L) at baseline were still deficient at the end of the study – 53% and 67%, respectively. The length of tanning sessions varied by group as per tanning protocols (detailed in Supplemental Tables 1–3), but each received a maximum exposure in accordance to skin type and the erythema reference action spectrum.
Figure 1.
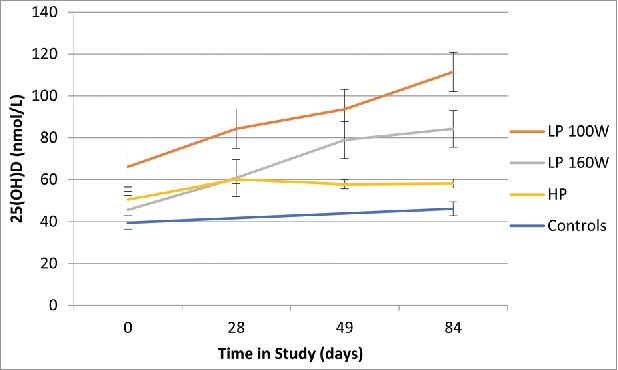
Serum 25-hydroxyvitamin D concentrations over 12 weeks of the study by group. Red line: Tanners in 100 W Low Pressure sunbed; Green line: Tanners in 160 W Low Pressure sunbed; Purple line: Tanners in 700 W filtered High Pressure sunbed; Blue line: Controls (no tanning).
Repeated measures ANOVA revealed a significant effect of time (p = 0.002), treatment group (p = 0.001) and an interaction effect (p = 0.001). Post-hoc testing revealed that the effect of time was driven by groups 1 (100W) and 2 (160W). Linear regression analysis found that change in 25(OH)D was dependent on group (p = 0.003) and age (P = 0.03), but was not influenced by gender, BMI, Fitzpatrick type or the cumulative length of tanning (Table 3). There was no significant change in 25(OH)D levels in the high pressure (HP) 700 W filtered metal halide sunbed group or in control participants.
Table 3.
Effect of study parameters on change in serum 25(OH)D concentration.
| Predictor | B | Standardized Coeffecient (β) | p-value | 95% Confidence Interval |
|---|---|---|---|---|
| Treatment Group | −28.0 | −1.29 | 0.003 | −46.04, −9.89 |
| Gender | 4.71 | 0.067 | 0.53 | −10.39, 19.80 |
| Age | −0.60 | −0.26 | 0.03 | −1.15, −0.05 |
| Fitzgerald skin type | −5.95 | −0.24 | 0.34 | −18.65, 6.45 |
| BMI (kg/m2) | −0.14 | −0.02 | 0.90 | −2.27, 1.99 |
| Cumulative time (min.) | −0.07 | −0.41 | 0.46 | −0.27, 0.13 |
Skin pigmentation
Overall, , a measure of skin lightness, was markedly lower at 12 weeks in all tanning groups at all 5 sites measured (inner upper arm, outer upper arm, cheek, forehead and lower back, RM ANOVA, p < 0.001; Fig. 2). There was an overall increase in at all sites (RM ANOVA, p < 0.001; Fig. 2). There was no change in , meaning yellow and blue pigmentation levels did not change. Skin pigmentation, as measured by , was significantly increased over the course of the 12 weeks of tanning at the lower back and inner arm (RM ANOVA, p < 0.001; Fig. 2; remaining cites are depicted in Supplemental Figure 1).
Figure 2.
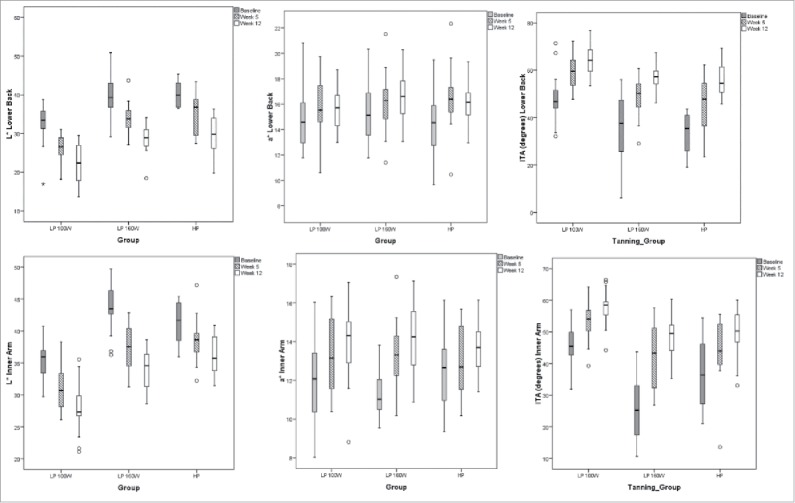
L*, a* and ITA for the lower back and inner arm measurements. Baseline measures are represented by the solid box plots, hatched boxes from mid-study and white boxes after 12 weeks of tanning. Boxplots depict the central 50% with the median as the line and the 25th and 75th percentiles as the whiskers. Outliers are depicted as circles.
L* and were significantly correlated with 25(OH)D concentrations whereas no relationship was observed between a* and 25(OH)D. , , or were not dependent on the type of sunlamp (100 W LP, 160 W LP or 700 W filtered metal halide HP).
The control group was not skin typed or measured for pigmentation changes since the outdoor UV exposure was negligible due to the time of year and clothing worn in winter.
Adverse events
There were no adverse events or sunburns reported throughout the duration of the trial.
Discussion
The current study provides the first evidence that following recommended sunbed tanning protocols in a typical tanning salon can achieve physiological levels of vitamin D when the lamp emits UVB in the range equivalent to outdoor summer sunshine. The majority of participants tanning in the LP 100 W sunbed attained mean 25(OH)D concentrations above 100 nmol/L. While the LP 160 W group reached a mean 25(OH)D concentration of 84 nmol/L, the relative increase in 25(OH)D levels was the similar in both LP groups. With higher baseline 25(OH)D concentrations those in the LP 160 W may have reached 25(OH)D levels above 100 nmol/L as well, since neither group appeared to plateau in 25(OH)D concentrations by the end of 12 weeks. In addition, the lack of a plateau in 25(OH)D concentrations (Fig. 1) suggests that higher 25(OH)D levels may be attained with further tanning sessions. The LP beds produced a 75–100% increase in 25(OH)D concentrations in 12 weeks and 28 tanning sessions. The current study supports earlier research that showed that skin pigmentation/tan did not decrease the vitamin D production after 12 weeks. The 25(OH)D concentration levels continued to increase, especially in the lower baseline 25(OH)D concentrations.37,38 Further research extending the period to 120 days of exposure may be required to see an effect on leveling of 25(OH)D concentrations.39 The current study supports the suggestion that artificially produced UVB radiation sources that mimic sunlight, in this case fluorescent sunbed lamps with 2.2% and 4.2% UVB, could be a surrogate for sunlight when the UV index is low in northern countries for vitamin D production.
Not surprisingly, the 700 W filtered HP sunbed, which lacked appreciable UVB (0.8%), and contained UVB wavelengths outside that required to stimulate vitamin D production, did not produce an increase in 25(OH)D levels. As such, the use of 700 W filtered HP sunbeds to increase vitamin D status is not supported or recommended.
The control group was meant to verify that 25(OH)D concentrations would not increase by normal means – through diet, consuming supplements up to the amount of the recommended dietary allowance (600 IU/d), or by sun (UVB) exposure which was not expected during the winter.40 Baseline 25(OH)D concentrations were found to be significantly lower in the control group. This may be due to a difference in behavior particularly given that we recruited family and friends of sunbed users as control participants who chose not to participate as tanners and were therefore less likely to sunbathe or practice behaviors that exposed them to the sun at any time of year.
The proportion of participants who were vitamin D deficient at baseline was alarming and supports the recent claim that a calculation error was made for the RDA. Further recommended intakes of vitamin D are much too low to achieve optimal levels41 and suggests either the need for further time in a UV environment or increased levels of supplementation.42 More than half of participants at baseline had 25(OH)D concentrations below 50 nmol/L and more than 80% were below the suggested level for optimal bone health at 75 nmol/L43. Surprisingly this was true even for those who were interested in participating in the tanning groups of this study. Clearly, public health messages for vitamin D need to be stronger.
There was an expected increase in skin pigmentation as measured by L*, a* and ITA° that was independent of the type of the sunbed utilized, age, sex, BMI or cumulative time tanning. Skin lightness, L*, negatively correlated with 25(OH)D, whereas skin color, ITA°, positively correlated with 25(OH)D concentrations. The same ultraviolet wavelengths (290 to 320 nm) that initiate vitamin D synthesis also trigger melanin production and thus skin pigmentation. The current study demonstrates that the dose of UV from a sunbed that is required to raise 25(OH)D levels to 100 nmol/L will also produce a tan. However, the amount of skin pigmentation produced was not dependent on vitamin D production as the 700 W filtered HP bed produced similar changes in L*, a* and ITA° without an increase in 25(OH)D.
There are a number of adaptive responses that are triggered in the skin in response to increased sunlight exposure including development of pigmentation (tan), an increase in the thickness of the epidermal layer, which reduces UV penetration, and an up-regulation of DNA repair processes.44 The current study protocol involved exposure times that correspond to a starting UV dose of 100 J/m2/session and increased to 625 J/m2/session, a 6-fold increase in UV exposure over 12 weeks. No sunburns or adverse reactions were reported throughout the study period, demonstrating the safety and validity of exposure regulations and manufacturer recommendations outlined in the RED Act. Also, the amount of skin pigmentation achieved throughout the study afforded a “sun protection value” such that the skin acclimation to UV exposure skin was six times greater at the end of 12 weeks.
There are several limitations to the present study. For ethical reasons recruitment was limited to persons actively seeking sunbed use, introducing a selection bias. Because a dedicated sunbed was used at each salon which were in different cities, this may have resulted in a unique sample of participants at each site. While the study design was not randomized, the outcome of the study was an objective biomarker that was measured at consecutive time points. Lastly, the method of sample collection by individuals, blood spot analysis, resulted in several samples that did not meet the specifications for testing, a random error, and thus were not available for analysis.
Overall, the present study confirms that serum 25(OH)D levels improved on average by 42 nmol/L through regular use of either 100 W (4.2% UVB) or 160 W (2.2% UVB) low pressure fluorescent lamp sunbeds following the regulations set forth by Health Canada in the RED Act. Creating a tan after 4 weeks showed no effect on increasing 25(OH)D levels, but 12 weeks was effective at increasing 25(OH)D levels. Responsible exposure to summer sunshine and the majority of sunbeds sold in Canada (containing appreciable levels of UVB) are effective means by which to increase our serum 25(OH)D levels. In a tanning bed the exposure to UV light can be controlled more precisely than casual sun exposure.
Materials and methods
Study population
Study participants included both men and women, 18 years of age and older. Exclusion criteria included a history of skin cancer, granulomatous disease, liver disease, or kidney disease, pregnancy, use of photosensitive medications, vitamin D supplementation intake >1,000 IU/d within the last 60 days, planned travel to a sunny destination during the study period (January to April 2014), and sunbed use within the last 60 days.
Recruitment of participants occurred within the selected salons and on social media. Participants recruited as “tanners” were most likely to have been former clients. Controls were friends and family of the tanners. As the study was open label, participants used the salon they were recruited from (different cities) where a dedicated sunbed was provided for study use. The target enrollment for each group was 20 participants to allow for a difference in vitamin D status to be detected between groups. The flow of trial progress is depicted in.Fig. 3
Figure 3.
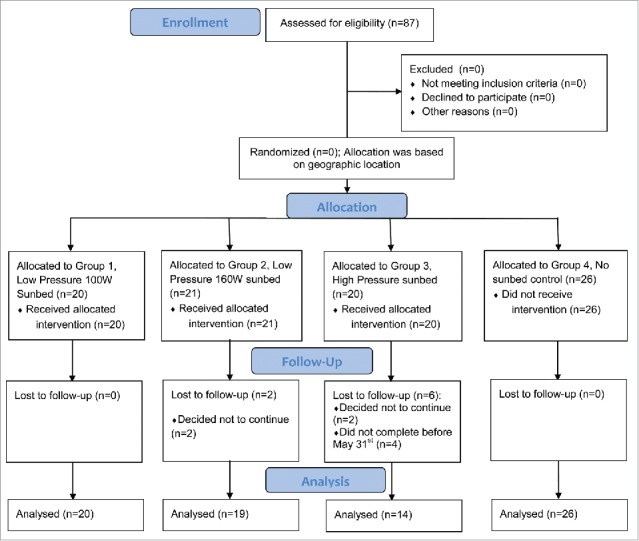
Flow of trial progress and analysis.
Tanning protocol
The study was conducted at three different salons located within Southern Ontario, Canada with a dedicated sunbed at each site. Enrollment of participants occurred from January 1st to February 28th, 2014 with study completion on May 31st, 2014. Sunbed lamps were changed prior to the beginning of the study and run for 100 hours before enrollment of participants to level out the exposure of the lamps. Spectral outputs from baseline are found in Fig. 4. The tanning protocol was 12 weeks in length. The following lamps were used at the location specified:
-
1)
100 Watt (W) low pressure (LP) fluorescent sunbed with 4.2% UVB mix in Hamilton, Ontario;
-
2)
160 W LP fluorescent sunbed with 2.2% UVB mix in Tillsonburg, Ontario;
-
3)
High Pressure (HP) 700 W filtered metal halide sunbed with 0.8% UVB mix in Stittsville, Ontario;
-
4)
Control group (No tanning) from Southern Ontario.
Figure 4.
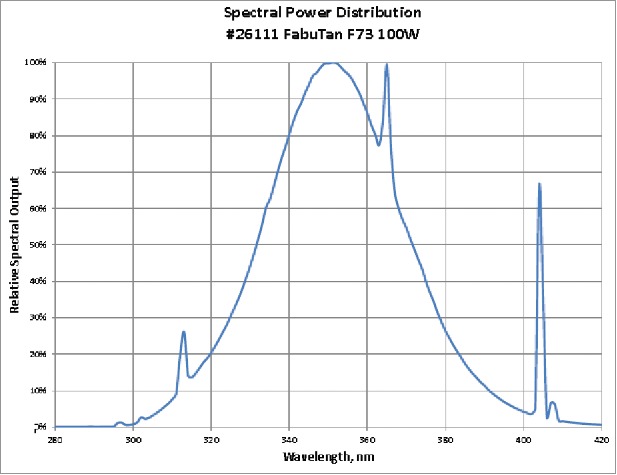
Lamp Spectral Outputs and CIE Vitamin D Action Spectrum a) 100 W LP fluorescent sunbed lamps, b) 160 W LP fluorescent sunbed lamps, c) High pressure (HP) 700 W filtered metal halide sunbed lamps, d) CIE Vitamin D Action Spectrum (Red Line – 100 w LP; Green Line – 160 w LP; Purple Line – 700 w HP), e) Normalized Spectral Irradiance Distribution (Blue Line – CIE 2006 Vitamin D Action Spectrum; Red Line – 100 w LP; Green Line – 160 w LP; Purple Line – 700 w HP).
Tanning sessions occurred three times per week for the first four weeks (sessions 1–12) to build photoprotection (tan), which means starting at approximately two to three minute exposures and gradually increasing the time to the maximum exposure time set by Health Canada. For the remaining eight weeks of the study (sessions 13–28) tanners used sunbeds twice per week based on manufacturers recommendations to maintain a tan. All exposure times are based on sub-erythema doses set out by recommendations from the equipment manufacturers except for the first and last session set by Health Canada. Participants were asked to expose ∼90% of their body surface area, i.e. they could wear undergarments and/or cover their face.
Exposure times
Time exposure schedules were determined according to the Health Canada RED Act for the first and maximum session time and manufacturers standards for remaining sessions. First exposure is ≤100J/m2 and gradually increases over a four-week period to 625J/m2; weeks following the first exposure increase according to the manufacturers recommendation to the maximum exposure. Supplemental Tables 1 to 3 show exposure times and dose amount for each session for each specific piece of equipment. The 100 W fluorescent lamp had a UVB mix of 4.2%, the 160 W fluorescent lamp had a 2.2% UVB content and the 700 W filtered metal halide had 0.8% UVB content. All exposure time calculations are in accordance with the erythema reference action spectrum set out by Health Canada below.
The exposure time in seconds was calculated using the formula set forth in Health Canada's Red Act (Part XI) for the first and maximum exposure time, as follows:
where:
- X
is a dose not greater than 100 J/m2 for the first session for untanned skin that could progressively increase to a maximum dose of 625 J/m2 for the following sessions,
- λ
is the wavelength in nanometers,
- V
is the weighting factor determined in accordance with the erythema reference action spectrum; and
- R
is the irradiance of the tanning equipment, measured at the minimum exposure distance.
Vitamin D assessment
The primary outcome was the difference between baseline and end-of-study vitamin D status. Serum 25-hydroxyvitamin D [25(OH)D] measurements were assessed four times throughout the tanning procedure for participants in groups 1–3: baseline or week 0, the beginning of weeks 5 and 9 and the end of week 12) and twice in controls, at baseline and at the end of 12 weeks. Serum 25(OH)D blood spots were collected before sunbed use on the first session of the week 5 and 9 and at the last session for week 12. Serum 25(OH)D was tested every four weeks to determine if increased pigmentation/photoprotection interfered with the production of vitamin D and the average person creates a base tan in four weeks. The control group was tested at baseline and 12 weeks only because there was no sunbed exposure nor significant natural UVB exposure for the 12-week period. All participants provided finger-prick samples (via a drop of blood on filter paper). De-identified samples were measured by LC-MS/MS at Purity Labs (Lake Oswego, OR) with an intra-assay CV of 4.78% and inter-assay CV of 6.85%.
Skin pigmentation measurements
A Fitzpatrick skin type was determined for each participant (tanning groups 1–3) at baseline by completing a skin typing form (http://skintype.ca/).
Skin pigmentation, a secondary outcome, was assessed in duplicate at 5 sites: inner upper arm, outer upper arm, forehead, cheek and the small of the back. Measurements were taken by a DSM II colorimeter (Cortex Technology, Hadsund, Denmark) at baseline, mid-way (week 5) and at the end of the study (end of week 12). Calibration against a standard white tile was performed at the start of each day.
Color system coordinates for L* (dark vs. light), a* (red vs. green) and b* (yellow vs. blue) based on the commision internationale de l'eclairage system.45 Skin color measurements in terms of CIELAB color space values. Averages of the two values were used at each site. Changes in L* () and a* () are reported.
Individual typology angle (ITA°) was calculated by the following equation:
Skin color change () was calculated as an objective measure of skin pigmentation development, were the differences in ITA° readings between baseline and end of study.
Statistical analysis
We used SPSS version 22.0 (SPSS Inc, Chicago, IL) statistical software for statistical analysis. Results are reported as mean and standard deviation (± SD). Repeated Measures Analysis of variance (RM ANOVA) was performed to detect differences between groups for 25(OH)D and skin pigment measures. Paired t-tests were used to determine differences within groups between baseline and end-of-study. One-way ANOVA was used to assess changes over time within groups. Linear regression analyses were utilized to test the relationship of categorical variables on changes in 25(OH)D and skin pigmentation measures. Spearman correlation was used to investigate changes in skin pigmentation relative to 25(OH)D. Study data is available by contacting the authors.
Ethics
Approval for this study was granted by the Institutional Review Board (IRB VDS-1234). All study participants provided written informed consent.
Funding Statement
Funding for this study was provided by the Vitamin D Society.
Disclosure of interest
The authors report no conflict of interest. No financial interest or benefit have arisen from the direct application of our research.
Acknowledgments
We would like to thank the owners of the tanning studios that provided a dedicated sunbed for the duration of the study: Shari Hodges at Fabutan, Hamilton, Ontario; Scott and Debbie Gilvesy at Tanners Studio, Tillsonburg, Ontario; Andy Boznar at UTAN, Stittsville, Ontario.
References
- 1.Health Canada. Tanning and its Effects on Your Health. Health. Ottawa (ON); 2012. Sept [accessed 2016 Dec 12] http://www.canada.ca/en/health-canada/services/healthy-living/your-health/lifestyles/your-health-tanning-effects-your-health-health-canada-2012.html. [Google Scholar]
- 2.Weller RB. The health benefits of UV radiation exposure through vitamin D production or non-vitamin D pathways. Blood pressure and cardiovascular disease. Photochemical & Photobiological Sciences. 2017; 16:374–80. doi: 10.1039/C6PP00336B [DOI] [PubMed] [Google Scholar]
- 3.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon (France): World Health Organization; 2012 [Google Scholar]
- 4.MF H. McCollum Award Lecture 1994: Vitamin D – new horizons for the 21st Century. Am J Clin Nutr. 1994; 60:12. [DOI] [PubMed] [Google Scholar]
- 5.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988; 67:373–8. doi: 10.1210/jcem-67-2-373. PMID:2839537 [DOI] [PubMed] [Google Scholar]
- 6.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, et al.. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011; 96:53–8. doi: 10.1210/jc.2010-2704. PMID:21118827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96:1911–30. doi: 10.1210/jc.2011-0385. PMID:21646368 [DOI] [PubMed] [Google Scholar]
- 8.Pludowski P, Karczmarewicz E, Bayer M, Carter G, Chlebna-Sokol D, Czech-Kowalska J, Debski R, Decsi T, Dobrzanska A, Franek E, et al.. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe – recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynologia Polska. 2013; 64:319–27. doi: 10.5603/EP.2013.0012. PMID:24002961 [DOI] [PubMed] [Google Scholar]
- 9.Grant WB, Wimalawansa SJ, Holick MF, Cannell JJ, Pludowski P, Lappe JM, Pittaway M, May P. Emphasizing the health benefits of vitamin D for those with neurodevelopmental disorders and intellectual disabilities. Nutrients. 2015; 7:1538–64. doi: 10.3390/nu7031538. PMID:25734565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baggerly CA, Cuomo RE, French CB, Garland CF, Gorham ED, Grant WB, Heaney RP, Holick MF, Hollis BW, McDonnell SL, et al.. Sunlight and Vitamin D: Necessary for Public Health. J Am Coll Nutr. 2015; 34:359–65. doi: 10.1080/07315724.2015.1039866. PMID:26098394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao YS, Ekwaru JP, Ohinmaa A, Griener G, Veugelers PJ. Vitamin D and health-related quality of life in a community sample of older Canadians. Qual Life Res. 2014; 23:2569–75. doi: 10.1007/s11136-014-0696-6. PMID:24760533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekwaru JP, Ohinmaa A, Veugelers PJ. The effectiveness of a preventive health program and vitamin D status in improving health-related quality of life of older Canadians. Qual Life Res. 2015; 25:661–8. doi: 10.1007/s11136-015-1103-7. PMID:26282006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonnell SL, Baggerly C, French CB, Baggerly LL, Garland CF, Gorham ED, Lappe JM, Heaney RP. Serum 25-Hydroxyvitamin D Concentrations >/ = 40 ng/ml Are Associated with >65% Lower Cancer Risk: Pooled Analysis of Randomized Trial and Prospective Cohort Study. PLoS One. 2016; 11:e0152441. doi: 10.1371/journal.pone.0152441. PMID:27049526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwalfenberg GK, Genuis SJ, Hiltz MN. Addressing vitamin D deficiency in Canada: a public health innovation whose time has come. Public Health. 2010; 124:350–9. doi: 10.1016/j.puhe.2010.03.003. PMID:20413135 [DOI] [PubMed] [Google Scholar]
- 15.Chel VG, Ooms ME, Popp-Snijders C, Pavel S, Schothorst AA, Meulemans CC, Lips P. Ultraviolet irradiation corrects vitamin D deficiency and suppresses secondary hyperparathyroidism in the elderly. J Bone Miner Res. 1998; 13:1238–42. doi: 10.1359/jbmr.1998.13.8.1238. PMID:9718191 [DOI] [PubMed] [Google Scholar]
- 16.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007; 22 Suppl 2:V28−33. doi: 10.1359/jbmr.07s211. PMID:18290718 [DOI] [PubMed] [Google Scholar]
- 17.Tangpricha V, Turner A, Spina C, Decastro S, Chen TC, Holick MF. Tanning is associated with optimal vitamin D status (serum 25-hydroxyvitamin D concentration) and higher bone mineral density. Am J Clin Nutr. 2004; 80:1645–9. PMID:15585781 [DOI] [PubMed] [Google Scholar]
- 18.Kahn S NR, Whiteman D, Kimlin M. Vitamin D production and UV exposure – a systematic review. Auckland (NZ): NIWA A UV Workshop; 2014 [Google Scholar]
- 19.Vahavihu K, Ala-Houhala M, Peric M, Karisola P, Kautiainen H, Hasan T, Snellman E, Alenius H, Schauber J, Reunala T. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010; 163:321–8. doi: 10.1111/j.1365-2133.2010.09767.x. PMID:20331450 [DOI] [PubMed] [Google Scholar]
- 20.Osmancevic A, Landin-Wilhelmsen K, Larko O, Mellstrom D, Wennberg AM, Hulthen L, Krogstad AL. UVB therapy increases 25(OH) vitamin D syntheses in postmenopausal women with psoriasis. Photodermatol Photoimmunol Photomed. 2007; 23:172–8. doi: 10.1111/j.1600-0781.2007.00301.x [DOI] [PubMed] [Google Scholar]
- 21.Datta P, Bogh MK, Olsen P, Eriksen P, Schmedes AV, Grage MM, Philipsen PA, Wulf HC. Increase in serum 25-hydroxyvitamin-D3 in humans after solar exposure under natural conditions compared to artificial UVB exposure of hands and face. Photochem Photobiol Sci. 2012; 11:1817–24. doi: 10.1039/c2pp25093d. PMID:22851263 [DOI] [PubMed] [Google Scholar]
- 22.de Winter S Vink AA, Roza L, Pavel S. Solar-simulated skin adaptation and its effect on subsequent UV-induced epidermal DNA damage. The Journal of investigative dermatology. 2001; 117:678–82. doi: 10.1046/j.0022-202x.2001.01478.x. PMID:11564176 [DOI] [PubMed] [Google Scholar]
- 23.Geller AC, Brooks DR, Colditz GA, Koh HK, Frazier AL. Sun protection practices among offspring of women with personal or family history of skin cancer. Pediatrics. 2006; 117:e688−94. doi: 10.1542/peds.2005-1734. PMID:16585282 [DOI] [PubMed] [Google Scholar]
- 24.Oliver H, Ferguson J, Moseley H. Quantitative risk assessment of sunbeds: impact of new high power lamps. Br J Dermatol. 2007; 157:350–6. doi: 10.1111/j.1365-2133.2007.07985.x. PMID:17650177 [DOI] [PubMed] [Google Scholar]
- 25.Sami S. Qutob MOB, Feder Katya, McNamee James, Guay Mireille, Than John. Tanning equipment use: 2014 Canadian Community Health Survey. Health Reports 2017; 28:12–16. [PubMed] [Google Scholar]
- 26.Gambichler T, Bader A, Vojvodic M, Bechara FG, Sauermann K, Altmeyer P, Hoffmann K. Impact of UVA exposure on psychological parameters and circulating serotonin and melatonin. BMC Dermatol. 2002; 2:6. doi: 10.1186/1471-5945-2-6. PMID:11952999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldman SR, Liguori A, Kucenic M, Rapp SR, Fleischer AB Jr., Lang W, Kaur M. Ultraviolet exposure is a reinforcing stimulus in frequent indoor tanners. J Am Acad Dermatol. 2004; 51:45–51. doi: 10.1016/j.jaad.2004.01.053. PMID:15243523 [DOI] [PubMed] [Google Scholar]
- 28.Heckman CJ, Egleston BL, Wilson DB, Ingersoll KS. A preliminary investigation of the predictors of tanning dependence. Am J Health Behav. 2008; 32:451–64. doi: 10.5993/AJHB.32.5.1. PMID:18241130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armas LA, Dowell S, Akhter M, Duthuluru S, Huerter C, Hollis BW, Lund R, Heaney RP. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007; 57:588–93. doi: 10.1016/j.jaad.2007.03.004. PMID:17637484 [DOI] [PubMed] [Google Scholar]
- 30.Moan J, Lagunova Z, Cicarma E, Aksnes L, Dahlback A, Grant WB, Porojnicu AC. Sunbeds as vitamin D sources. Photochem Photobiol. 2009; 85:1474–9. doi: 10.1111/j.1751-1097.2009.00607.x. PMID:19788534 [DOI] [PubMed] [Google Scholar]
- 31.Farrar MD, Webb AR, Kift R, Durkin MT, Allan D, Herbert A, Berry JL, Rhodes LE. Efficacy of a dose range of simulated sunlight exposures in raising vitamin D status in South Asian adults: implications for targeted guidance on sun exposure. Am J Clin Nutr. 2013; 97:1210–6. doi: 10.3945/ajcn.112.052639. PMID:23615828 [DOI] [PubMed] [Google Scholar]
- 32.Thieden E, Jorgensen HL, Jorgensen NR, Philipsen PA, Wulf HC. Sunbed radiation provokes cutaneous vitamin D synthesis in humans–a randomized controlled trial. Photochem Photobiol. 2008; 84:1487–92. doi: 10.1111/j.1751-1097.2008.00372.x. PMID:18513233 [DOI] [PubMed] [Google Scholar]
- 33.Luxwolda MF, Kuipers RS, Kema IP, van der Veer E, Dijck-Brouwer DA, Muskiet FA. Vitamin D status indicators in indigenous populations in East Africa. Eur J Nutr. 2013; 52:1115–25. doi: 10.1007/s00394-012-0421-6. PMID:22878781 [DOI] [PubMed] [Google Scholar]
- 34.Hollis BW, Wagner CL, Howard CR, Ebeling M, Shary JR, Smith PG, Taylor SN, Morella K, Lawrence RA, Hulsey TC. Maternal Versus Infant Vitamin D Supplementation During Lactation: A Randomized Controlled Trial. Pediatrics. 2015; 136:625–34. doi: 10.1542/peds.2015-1669. PMID:26416936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heaney RP. Toward a physiological referent for the vitamin D requirement. J Endocrinol Invest. 2014; 37:1127–30. doi: 10.1007/s40618-014-0190-6. PMID:25308199 [DOI] [PubMed] [Google Scholar]
- 36.Health Canada. Frequently Asked Questions on the Amendments to the Radiation Emitting Devices Regulations (Tanning Equipment): Ottawa (ON), 2014. Jun 02 [accessed 2016 Dec 12]; https://www.canada.ca/en/health-canada/services/environment-workplace-health/radiation/ultraviolet-radiation/frequently-asked-questions-amendments-radiation-emmtting-devices-regulations-tanning-equipment.html [Google Scholar]
- 37.Bogh MK, Schmedes AV, Philipsen PA, Thieden E, Wulf HC. Vitamin D production depends on ultraviolet-B dose but not on dose rate: a randomized controlled trial. Exp Dermatol. 2011; 20:14–8. doi: 10.1111/j.1600-0625.2010.01201.x. PMID:21158934 [DOI] [PubMed] [Google Scholar]
- 38.Bogh MK, Schmedes AV, Philipsen PA, Thieden E, Wulf HC. Vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation. J Invest Dermatol. 2010; 130:546–53. doi: 10.1038/jid.2009.323. PMID:19812604 [DOI] [PubMed] [Google Scholar]
- 39.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003; 77:204–10. PMID:12499343 [DOI] [PubMed] [Google Scholar]
- 40.Webb AR, Holick MF. The role of sunlight in the cutaneous production of vitamin D3. Annu Rev Nutr. 1988; 8:375–99. doi: 10.1146/annurev.nu.08.070188.002111. PMID:2849469 [DOI] [PubMed] [Google Scholar]
- 41.Veugelers PJ, Ekwaru JP. A statistical error in the estimation of the recommended dietary allowance for vitamin D. Nutrients. 2014; 6:4472–5. doi: 10.3390/nu6104472. PMID:25333201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veugelers PJ, Pham TM, Ekwaru JP. Optimal Vitamin D Supplementation Doses that Minimize the Risk for Both Low and High Serum 25-Hydroxyvitamin D Concentrations in the General Population. Nutrients. 2015; 7:10189–208. doi: 10.3390/nu7125527. PMID:26690210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005; 16:713–6. doi: 10.1007/s00198-005-1867-7. PMID:15776217 [DOI] [PubMed] [Google Scholar]
- 44.Gordon-Thomson C, Tongkao-on W, Song EJ, Carter SE, Dixon KM, Mason RS. Protection from ultraviolet damage and photocarcinogenesis by vitamin D compounds. Adv Exp Med Biol. 2014; 810:303–28. PMID:25207373 [DOI] [PubMed] [Google Scholar]
- 45.Weatherall IL, Coombs BD. Skin color measurements in terms of CIELAB color space values. J Invest Dermatol. 1992; 99:468–73. doi: 10.1111/1523-1747.ep12616156. PMID:1402005 [DOI] [PubMed] [Google Scholar]


