Abstract
Objective:
The use of early colonoscopy in the management of acute lower gastrointestinal bleeding (LGIB) is controversial, with disparate evidence. We aim to formally characterize the utility of early colonoscopy (within 24 h) in managing acute LGIB.
Design:
A systematic literature search to August 2016 identified fully published and abstracts of randomized controlled trials (RCTs) and observational studies assessing early colonoscopy in acute LGIB. Single-arm studies were also included to define incidence. Primary outcomes were overall rebleeding rates and time to rebleeding. Secondary outcomes included mortality, surgery, length of stay (LOS), definite cause of bleeding and adverse events (AEs). Odds ratios (OR) and weighted mean differences (WMD) were calculated.
Results:
Of 897 citations, 10 single-arm, 9 observational studies, and 2 RCTS were included (25,781 patients). Rebleeding was no different between patients undergoing early colonoscopy and controls (seven studies, OR = 0.89, 95% CI 0.49–1.62), or RCT data only (OR = 1.00, 95% CI 0.52–1.62). Early colonoscopy detected more definitive sources of bleeding (OR = 4.12, 95% CI 2.00–8.49), and was associated with shorter LOS colonoscopy (WMD = −1.52, 95% CI −2.54 to −0.50 days). No other differences were noted between early and late colonoscopy. AEs occurred in 4.0%, (95% CI 2.9%; 5.4%) of early colonoscopies. Included studies were of low quality, with significant heterogeneity for some outcomes.
Conclusion:
Early colonoscopy in acute LGIB does not decrease rebleeding, mortality or need for surgery, but is associated with increased detection of definitive sources of bleeding, shorter LOS, with low complication incidence. However, the quality of evidence is low, highlighting the need for additional high-level studies.
Keywords: acute LGIB, early colonoscopy, meta-analyses, rebleeding
What is already known about this subject:
Colonoscopy is used routinely in the acute management of patients with lower GI bleeding.
Society guidelines exist but are based on limited high-quality data.
Recently published meta-analyses in this topic have yielded disparate conclusions, and their adopted analyses have not allowed the confident reconciliation of practice with existing consensus recommendations.
What are the new findings:
Early colonoscopy in acute LGIB does not decrease rebleeding, mortality or need for surgery.
Early colonoscopy in acute LGIB is associated with increased detection of definitive sources of bleeding and a low incidence of complication.
Early colonoscopy results in a shorter length of stay (LOS) for patients hospitalized with acute LGIB.
How might it impact on clinical practice in the foreseeable future?
It is important to understand the evidence with regards to what can be expected from performing early colonoscopy to optimally select patients with acute LGIB for this management approach.
Additional high-quality data are required to better define the role of endoscopic hemostasis in this setting.
Introduction
The management of acute LGIB includes hemodynamic resuscitation, followed by attempts to localize and treat the bleeding source with endoscopic or angiographic interventions, and in refractory cases, surgery. Diagnostic approaches include endoscopy, radionuclide red blood cell scan, CT angiography and mesenteric angiography.1 Colonoscopy, with its high diagnostic yield, is the initial procedure of choice for most. However, it remains controversial whether early colonoscopy – performed within 12–24 h of admission – provides any clinical benefits. For example, a prospective, case historical control study of diverticular bleeding found early colonoscopy decreased rebleeding and need for surgery,2 whereas a randomized controlled trial (RCT) of 100 patients found it only improved diagnostic yield; a second RCT of 72 patients demonstrated no differences in outcomes between early and delayed colonoscopy. Guidelines by the American Society for Gastrointestinal Endoscopy (ASGE),3 and the American College of Gastroenterology (ACG)1 recommend early colonoscopy (within the initial 24 h) in high-risk patients, with a low quality of evidence. Because existing studies are of small sample size and have come to varying conclusions, we performed a systematic review and meta-analysis to determine the impact of early colonoscopy on clinical outcomes in acute LGIB.
Methods
Search strategy
A comprehensive literature search was performed from 1978 to August 2016 using OVID MEDLINE, EMBASE, Cochrane Library, and ISI Web of Knowledge databases with validated search terms specified for acute LGIB and endoscopy (see Online Appendix 1). Abstracts presented at major gastroenterology conferences in the past 5 years were also hand-searched. Additional relevant studies were identified from cross-referencing and hand-searches of references of the retrieved articles. All human adult studies published in English were considered.
Study selection and patient population
We selected all randomized and observational comparative studies that included early colonoscopy in at least one group of patients presenting with symptoms suggestive of acute LGIB. Single-arm studies were identified to define clinical characteristics, incidence and overall outcomes; comparative studies were used to identify relative harm or benefit attributable to early colonoscopy. Early colonoscopy was defined as performed within 24 h of presentation for prospective observational studies and RCTs.4 Because we anticipated definitions would vary among retrospective cohorts, we categorized such studies when at least 75% of patients had undergone a colonoscopy within 24 h of presentation or if the mean (or median) time to colonoscopy was 24 h or less. We excluded studies assessing pediatric patients, no acute LGIB, initial colonoscopy performed only after 24 h, diagnostic testing only (such as radionuclide red blood cell scan or CT angiography) other than colonoscopy unless these represented a control group. Care was taken to avoid double-counting across studies.
Outcome measures
The primary outcomes of the study were overall rebleeding rates and time to rebleeding, defined as time of rebleeding following presentation to hospital or admission. Secondary outcomes included mortality (related to LGIB and overall), surgery, total duration of hospital LOS, identification of a definite cause of LGIB and adverse events (AEs). Additional secondary outcomes included: length of ICU stay, blood transfusions received, definite or probable cause of bleeding (including presence of endoscopic stigmata of recent hemorrhage if the latter was not available) and the performance of endoscopic hemostasis. Finally, etiological endoscopic findings were described for definite or probable causes of acute LGIB. The incidence was reported first for all arms combined.
Validity assessment and data abstraction
Two reviewers evaluated the eligibility of all identified citations independently, with a third resolving disagreements. Study quality was assessed using the Cochrane risk of bias tool for randomized trials,5 and the Ottawa–Newcastle criteria for observational studies.6 The GRADE rating of evidence characterized the body of literature for each outcome.7 Available demographic data were extracted, such as mean age, gender, hemodynamic instability and use of antithrombotic therapies, as were interventions for acute LGIB, and the corresponding aforementioned patient outcomes.
Sources of possible clinical heterogeneity
Possible clinical heterogeneity was evaluated by reviewing patient populations, nature of the interventions and definitions of outcomes across studies. Findings of heterogeneity were used to guide subsequent subgroup analyses.
Sensitivity and subgroup analyses
Sensitivity analyses were planned according to the varying control groups to which early colonoscopy groups were compared, including elective or delayed colonoscopy, no colonoscopy and other comparators. Rebleeding definitions were expected to vary between studies; therefore, additional sensitivity analyses were performed based on the availability of a clearly identified definition for this outcome.
Because of the anticipated varied nature of reported approaches, definitions of outcomes and study designs are reported for all early colonoscopy cohorts <24 h as well as <12 h to characterize overall incidence.
Although RCTs were analyzed with controlled observational studies for the main inferential analyses, as the advantages of including observational studies with randomized trials in a meta-analysis could outweigh the disadvantages,8 RCTs were also analyzed separately.
In addition, we report on one very large study using a nationwide database (22,720 patients)9 separately.
Statistical analysis and possible sources of statistical heterogeneity
Descriptive results were reported as proportions and 95% confidence intervals (CIs), and summary statistics expressed as means and standard deviations (SDs) for continuous variables and proportions for categorical variables. Effect size was calculated with weighted mean differences (WMDs) for continuous variables; medians were used if means were not available, and SDs were calculated or imputed when possible.10 Odds ratios (ORs) were calculated for categorical variables. The Mantel–Haenszel method for fixed effect models was applied to determine corresponding overall effect sizes and their CIs, except when statistical heterogeneity was noted, in which case a random-effects model was used according to the DerSimonian and Laird method.11 WMDs were handled as continuous variables using the inverse variance approach. The presence of heterogeneity across studies was defined using a chi-square test of homogeneity with a 0.10 significance level.10 The Higgins I2 statistic12 was calculated to quantify the proportion of variation in treatment effects attributable to between-study heterogeneity; values of 25%, 50% and 75% represent low, moderate and high heterogeneity, respectively. For all comparisons, publication bias was evaluated using funnel plots as well as the Begg adjusted rank correlation13 and Egger regression asymmetry tests14 if at least three citations were identified. In order to ensure that zero-event trials did not significantly affect the heterogeneity or p values, a continuity correction was added to each trial with zero-events using the reciprocal of the opposite treatment arm size.15 All statistical analyses were done using Meta package in R version 2.13.0 (R Foundation for Statistical Computing, Vienna, Austria, 2008).
Results
Study selection and interventions
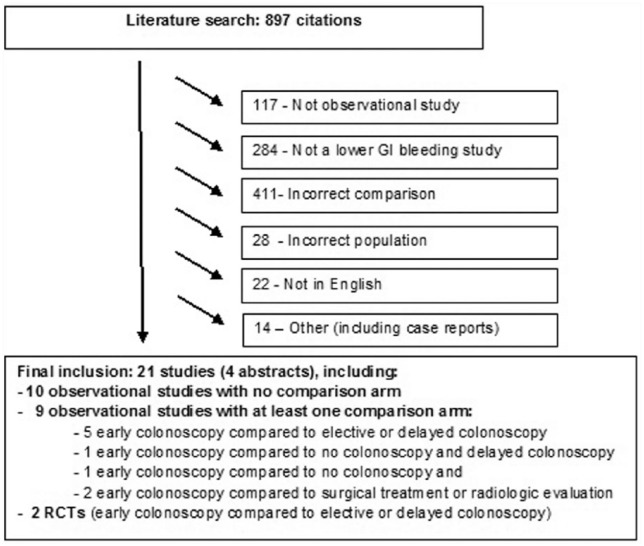
Study selection: we initially identified 897 citations. After review, a total of 876 studies were excluded. The corresponding PRISMA diagram is shown in Figure 1. We finally selected a total of 21 citations, including 2 RCTs and 19 observational studies (4 of which were abstracts, 4 prospectives and 15 retrospectives). One 2005 study from Strate and colleagues published analyses from a subgroup from a previous 2003 publication by Strate and colleagues. Only non-overlapping data from the original population were retrieved.
Figure 1.
PRISMA diagram of trial selection.
Observational studies included 10 early colonoscopy cohorts without a comparison arm (2 prospective16,17 and 8 retrospective18–25); in one we combined two arms of a study assessing bowel preparation (both groups were early colonoscopy).20 Eleven studies included a comparison group, including 2 RCTs that compared colonoscopy within 12 h to standard of care,26 or elective colonoscopy.27 Among observational studies, early colonoscopy was compared to elective colonoscopy (>24 h) in three studies (one prospective28 and two retrospective29,30), or delayed colonoscopy in two retrospective studies.9,31 The control group was no colonoscopy in one study (retrospective),32 surgical treatment in one study2 (prospective) and radiographic evaluation in another study (retrospective).33 Data were extracted as possible in one retrospective study that combined elective (>24 h) and no colonoscopy as the comparator.4 Because of marked heterogeneity in the literature, we accepted a broader definition of acute LGIB than defined a priori, detailed in Supplementary Table 1. This table also lists corresponding patient and design characteristics for each study. Most studies used clinical symptoms as evidence of rebleeding, with endoscopic confirmation.
Study quality assessment and risk of publication bias
The quality scores attributed to each study with a comparison are displayed in Supplementary Table 1, except for the Cochrane risk of bias assessment tool summaries for the 2 RCTs that are included in the Online Appendix. Both RCTs exhibited a high risk of bias due to lack of blinding of study personnel (blinding was not possible). A high risk of bias was also attributed to the RCT by Laine and colleagues since the trial was terminated before reaching the calculated sample size. The Newcastle–Ottawa scale yielded an average score of 8.0 ± 1.2 stars (range 5–9 stars; the highest-quality studies are given nine stars).
Table 1.
Characteristics of the patient population.
| Early colonoscopy, all included studies |
All observational studies that included a comparison |
Only RCTs |
|||
|---|---|---|---|---|---|
| All early colonoscopies <24 h (including single-arm studies) | All urgent colonoscopies <24 h |
Control arm of any type |
Early colonoscopy arm | Control arm | |
| Number of studies* | 21 (4 abstracts) | 11 (1 abstract) | 11 (1 abstract) | 2 | 2 |
| Mean age (years) | Range 51–78** | Range 52–68 | Range 52–71 | 60 (11.3) | 61.5 (13.4) |
| Total N | 11,391 | 9803 | 14,544 | 86 | 86 |
| Female (%) | 4957 (48.5%) | 4641 (48.8%) | 7874 (62.6%) | 33 (38.4%) | 32 (37.2%) |
| Hemodynamic instability (%) | 147 (28.7%) | 91 (33.3%) | 78 (65.5%) | 57 (66.3%) | 65 (75.6%) |
| Use of anticoagulant agents (%) | 19 (6.6%) | 11 (5.9%) | 13 (6.6%) | – | – |
| Use of anti-platelet agents (%) | 57 (23.7%) | 29 (15.5%) | 34 (17.3%) | – | – |
Not all studies included variables below.
Due to variation in age reporting, only lowest and highest mean ages are reported.
Excluding studies of diverticular bleeding alone.
Statistical heterogeneity was observed in overall rebleeding for urgent colonoscopies compared to elective colonoscopies; results are detailed in Table 3. The funnel plots, as well as Begg adjusted rank correlation and Egger regression asymmetry tests, did not suggest publication bias for any of the outcomes assessed (data available upon request).
Table 3.
Primary and secondary outcomes: studies with comparison group.
| N studies | N patients | OR (95% CI) | p value | I2 | |
|---|---|---|---|---|---|
| Primary outcomes | |||||
| Overall rebleeding rate | |||||
| • All: urgent colonoscopy versus any comparisons | 7 | 932 | 0.89 (0.49; 1.62) | 0.13 | 39% |
| • RCT: urgent colonoscopy versus elective colonoscopy | 2 | 172 | 1.00 (0.52; 1.94) | 0.28 | 16% |
| Secondary outcomes | |||||
| Mortality (all causes) | |||||
| • All: urgent colonoscopy versus any comparisons | 6 | 905 | 0.89 (0.35; 2.31) | 0.32 | 15% |
| • RCT: urgent colonoscopy versus elective colonoscopy | 2 | 172 | 1.15 (0.04; 35.61) | 0.07 | 70.5% |
| Mortality (related to LGIB) | |||||
| • All: urgent colonoscopy versus any comparisons | 4 | 522 | 0.61 (0.12; 3.23) | 0.98 | 0% |
| • RCT: urgent colonoscopy versus elective colonoscopy | 2 | 172 | 0.59 (0.08; 4.59) | 0.76 | 0% |
| Surgery | |||||
| • All: urgent colonoscopy versus any comparisons | 5 | 608 | 0.78 (0.39; 1.55) | 0.81 | 0% |
| • RCT: urgent colonoscopy versus elective colonoscopy | 2 | 172 | 0.87 (0.31; 2.46) | 0.27 | 19% |
| LOS | |||||
| • All: urgent colonoscopy versus any comparisons | 4 | 785 | −0.55 (–2.11; 1.01) | <0.01 | 82% |
| • RCT: urgent colonoscopy versus elective colonoscopy | 1 | 72 | 0.40 (–1.62; 2.42) | – | – |
| Definite cause of acute LGIB (including SHR) | |||||
| • All: urgent colonoscopy versus any comparisons | 6 | 1065 | 4.12 (2.00; 8.49)* | 0.02 | 65% |
| • RCT: urgent colonoscopy versus elective colonoscopy | 2 | 172 | 2.75 (1.19; 6.35)* | 0.66 | 0% |
| Adverse events | |||||
| • All: urgent colonoscopy versus any comparisons | 3 | 498 | 0.53 (0.19; 1.47) | 0.51 | 0% |
| • RCT: urgent colonoscopy versus elective colonoscopy | 2 | 172 | 0.11 (0.02; 0.58)* | 0.46 | 0% |
| Other secondary outcomes | |||||
| Length of ICU stay | |||||
| • All: urgent colonoscopy versus any comparisons | 1 | 57 | −3.00 (–6.02, 0.02) | – | – |
| • RCT: urgent colonoscopy versus elective colonoscopy | 0 | – | – | – | – |
| Blood transfusion (initial) | |||||
| • All: urgent colonoscopy versus any comparisons | 3 | 399 | −0.22 (–0.72, 0.27) | 0.08 | 61% |
| • RCT: urgent colonoscopy versus elective colonoscopy | 1 | 100 | 0.00 (–0.10; 0.10) | – | – |
| Blood transfusion (total) | |||||
| • All: urgent colonoscopy versus any comparisons | 4 | 282 | −0.27 (–1.60; 1.06) | <0.01 | 84% |
| • RCT: urgent colonoscopy versus elective colonoscopy | 2 | 172 | −0.06 (–1.62; 1.50) | <0.01 | 92% |
| Definite or probable cause of acute LGIB | |||||
| • All: urgent colonoscopy versus any comparisons | 6 | 796 | 2.94 (0.81; 10.64) | <0.01 | 82% |
| • RCT: urgent colonoscopy versus elective colonoscopy | 2 | 172 | 2.93 (1.30; 6.59)* | 0.11 | 61% |
| Endoscopic therapy | |||||
| • All: urgent colonoscopy versus any comparisons | 3 | 542 | 4.17 (2.32; 7.49)* | 0.75 | 0% |
| • RCT: urgent colonoscopy versus elective colonoscopy | 1 | 72 | 5.3 (0.25; 114.47) | – | – |
LOS, length of stay; OBS, observational study; RCT, randomized controlled trial; * Bold values are significantly different.
The GRADE score of evidence for every outcome was very low; these data are shown in Online Appendix 3.
Patient and study characteristics
A total of 25,781 patients were included, with 22,720 from a single database study.9 There were 24,193 patients from studies with comparative arms (9734 in the intervention and 14,459 in the control groups) and 172 from RCTs (86 patients in each arm). The mean age of patients ranged from 51 to 78 years. A total of 48.5% of patients were female; 28.7% presented with hemodynamic instability; 23.7% were on anti-platelet agents and 6.6% were taking anticoagulants. Detailed patient and study characteristics for all studies as well as comparative observational and RCTs are shown in Table 2. In all studies, it was specified that the colonoscopies were performed by an expert, defined as a physician trained in gastroenterology, internal medicine or general surgery.
Table 2.
Incidence of primary and secondary outcomes for all studies.
| All groups |
Early colonoscopy all included studies |
All early colonoscopies <12 h |
All comparison groups |
|
|---|---|---|---|---|
| Combination of early colonoscopies <24 h and comparative group (including single-arm studies) | All early colonoscopies <24 h (including single-arm studies) | All early colonoscopies <12 h (including single-arm studies) | Delayed colonoscopy, no colonoscopy or other | |
| Primary outcome | ||||
| Overall rebleed rate | 13.5%; 11.8–15.5% (13 studies, n = 1374) |
12.9%; 10.8–15.3% (13 studies, n = 823) |
21.7%; 16.9–27.3% (5 studies, n = 240) |
14.5%; 11.8–17.7% (5 studies, n = 551) |
| Secondary outcomes | ||||
| Mortality (all causes) | 0.4%; 0.3–0.5% (13 studies, n = 24,520) 1.3%; 0.9–2.0% (12 studies without Navaneethan*, n = 1800) |
0.4%; 0.3–0.6% (13 studies, n = 10,422) 1.3%; 0.8–2.0% (12 studies without Navaneethan*, n = 1266) |
1.9%; 1.0–3.4% (4 studies, n = 532)) |
0.4%; 0.3–0.6% (7 studies, n = 14,098) 1.5%; 0.8–2.9% (6 studies without Navaneethan*, n = 534) |
| Mortality (related) | 1.1%; 0.6–1.8% (6 studies, n = 1303) |
1.1%; 0.6–2.0% (6 studies, n = 965) |
1.7%; 0.9–3.2% (3 studies, n = 522) |
0.9%; 0.3–2.6% (4 studies, n = 338) |
| Surgery | 6.8%; 5.2–8.8% (9 studies, n = 752) |
7.1%; 5.1–9.9% (9 studies, n = 450) |
8.1%; 4.0–15.9% (2 studies, n = 86) |
6.3%; 4.1–9.6% (5 studies, n = 302) |
| LOS (days) | 5.7 ± 5.2 (8 studies) | 4.5 ± 2.7 (8 studies) | 4.3 ± 2.0 (3 studies) | 5.9 ± 5.4 (7 studies) |
| Definite cause of rebleeding (including SHR) | 42.0%; 40.0–44.0% (15 studies, n = 2301) |
52.0%; 49.6–54.3% (15 studies, n = 1722) |
30.8%; 27.2–34.6% (5 studies, n = 601) |
12.3%; 9.8–15.2% (6 studies, n = 579) |
| Adverse events | 4.0%; 3.0–5.3% (13 studies, n = 1204) |
4.0%; 2.9–5.4% (10 studies, n = 282) |
0.9%; 0.2–4.8% (3 studies, n = 115) |
4.0%; 2.2–7.2% (3 studies, n = 429) |
| Other outcomes | ||||
| Length of ICU stay | 1.9 ± 0.4 (3 studies) | 1.8 ± 0.3 (3 studies) | 1.8 (no SD available) (1 study) | 5.0 ± 5.7 (1 study) |
| Blood transfusion (initial) | 1.3 ± 0.3 (2 studies) | 1.3 ± 0.4 (2 studies) | 1.5 ± 0.3 (1 study) | 1.3 ± 1.3 (2 studies) |
| Blood transfusion (total) | 3.4 ± 2.2 (7 studies) | 3.7 ± 1.9 (7 studies) | 2.9 ± 1.9 (2 studies) | 2.7 ± 2.8 (4 studies) |
| Definite or probable cause of LGIB | 69.6%; 67.3–71.8% (14 studies, n = 1596) |
81.6%; 79.3–83.7% (14 studies, n = 1147) |
87.9%; 82.0–92.0% (4 studies, n = 165) |
39.0%; 34.6–43.6% (6 studies, n = 449) |
| Endoscopic therapy | 33.4%; 32.8–34.0% (13 studies, n = 23,999) 18.5%; 16.4–20.7% (12 studies without Navaneethan*, n = 1279) |
34.3%; 33.4–35.3% (13 studies, n = 10,161) 21.5%; 19.2–24.1% (12 studies without Navaneethan*, n = 1074) |
24.2%; 18.3–31.3% (4 studies, n = 165) |
32.8%; 32.0–33.5% (4 studies, n = 13,838) 5.8%; 3.6–9.3% (3 studies without Navaneethan*, n = 274) |
Navaneethan and colleagues.9
Primary outcomes
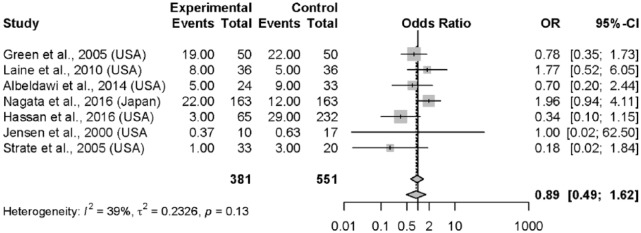
The incidence of rebleeding was 13.5% (95% CI, 11.8–15.5%) across all arms for all studies (Table 2). Rebleeding rates were reported in seven studies with control groups,2,26–30,33 totaling 381 patients undergoing early colonoscopy compared to 551 controls. There was no significant difference in rebleeding rates between early colonoscopy versus all other comparators (OR = 0.89; 95% CI, 0.49–1.62) (Figure 2). Time to rebleeding was specified in only two studies, preventing a pooled estimate for rebleeding stratified by time (Table 3). Among the two RCTs, rebleeding rates did not demonstrate any significant between-group differences (OR = 1.00; 95% CI, 0.52–1.62). The time to rebleeding was only specified in the trial by Green and colleagues26 (early rebleeding defined as bleeding prior to hospital discharge), in which 22.0% of subjects rebled after a mean LOS of 5.8 days in the early colonoscopy group versus 30.0% after a mean LOS of 6.6 days in the standard care control group (p = 0.50).
Figure 2.
Forest plot: rebleeding.
Secondary outcomes
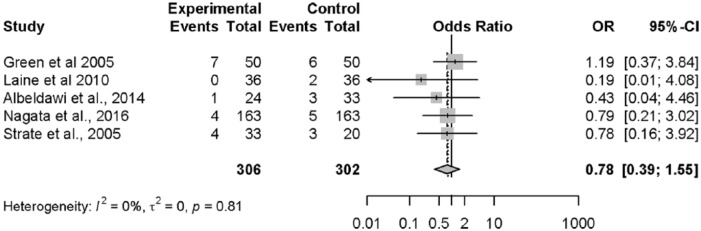
The incidence of surgery during the hospital stay was 6.8% (95% CI, 5.2–8.8%) (Figure 3). In five studies26–29,33 (n = 608) that assessed early colonoscopy versus any other comparison, rates of surgery were not significantly different (OR = 0.78; 95% CI, 0.39–1.55). The conclusion was similar when solely assessing the two RCTs (OR = 0.87; 95% CI, 0.31–2.46). Additional data relating to incidences and subgroup analyses are shown in Tables 2 and 3.
Figure 3.
Forest plot: surgery.
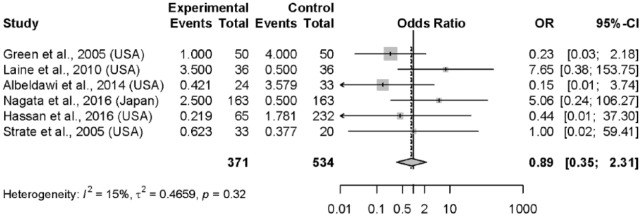
The incidence of all-cause mortality was 0.4% (95% CI, 0.3–0.5%) overall (Figure 4) and 1.3% (95% CI, 0.9–2.0%) when excluding the study by Navaneethan and colleagues. The incidence of mortality related to acute LGIB was 1.1% (95% CI, 0.6–1.8%). No significant difference was observed for mortality among the six observational studies (n = 905 without Navaneethan and colleagues; OR = 0.89; 95% CI, 0.35–2.31), nor for the two RCTs (OR = 1.15; 95% CI, 0.04–35.61) when comparing early colonoscopy to controls (Table 3). No difference was noted in the study by Navaneethan and colleagues (n = 22,720) for in-hospital death (0.3% early colonoscopy (⩽ 24 h) versus 0.4% for delayed (>24 h) colonoscopy (p = 0.24). There were no significant differences between early colonoscopy and controls when assessing mortality specifically related to LGIB in four observational studies26,27,30,33 (n = 522; OR = 0.61; 95% CI, 0.12–3.23) nor in the two RCTs (OR = 0.59; 95% CI, 0.08–4.59).
Figure 4.
Forest plot: mortality (all-cause).
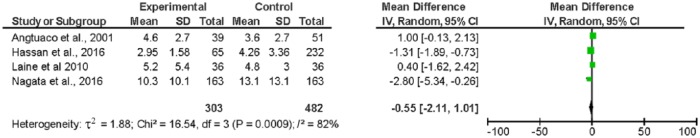
Mean length of hospital stay was 5.7 ± 5.2 days for all patients. No significant between-group difference was noted in four studies27,29,30,32 (n = 785) for which the data were available (WMD = −0.55; 95% CI, −2.11 to 1.01) (Figure 5). In the RCT by Laine and colleagues, urgent colonoscopy did not result in a significantly different hospitalization LOS (5.2 ± 0.9 days for early compared to 4.8 ± 0.5 days for elective colonoscopy). Similar results were noted in the RCT by Green and colleagues (5.8 versus 6.6 days, no SD provided), although we could not use these data as presented in the summary findings.
Figure 5.
Forest plot: length of stay.
For all included studies, a definite source of acute LGIB was found in 42.0% (95% CI, 40.0–44.0%) of patients. Among the six studies (n = 1065) that included a comparison, a greater number of lesions felt to be the ‘definite cause of bleeding’ were found after early colonoscopy compared to all other comparator groups (OR = 4.12; 95% CI, 2.00–8.49). The conclusion was similar when considering solely the two RCTs (OR = 2.75; 95% CI, 1.19–6.35).
AEs were found in 4.0% (95% CI; 3.0–5.3%) of all patients. Complications included perforation, cardiovascular complications, as well as minor events such as fever and hypotension. Hemo-dynamic instability and death were not considered to be AEs related to the colonoscopy since, in all studies, the former was recorded prior to endoscopy only, and death was categorized as all-cause or disease-related rather than procedure-related.
Analyzable data were provided in three studies26,27,29 (n = 498) with a comparison group. The study by Navaneethan and colleagues provided information for each separate AE, but could not be combined since each AE type could be counted more than once for the same patient. There was no significant between-group differences (OR = 0.53; 95% CI, 0.19–1.47). When assessing the two RCTs, AEs were significantly lower in the early colonoscopy group (OR = 0.11; 95% CI, 0.02–0.58).
Among the additional secondary outcomes, when grouping definite or probable cause of bleeding, no significant differences were noted (OR = 2.94; 95% CI, 0.81–10.64) for all studies with a comparison arm, but early colonoscopy yielded significantly more such lesions when solely assessing both RCTs (OR = 2.93; 95% CI, 1.30–6.59). Endoscopic therapy was described in four studies.8,17,20,34 When only studies with delayed or elective colonoscopy as a comparator were included, early colonoscopy increased the likelihood of a therapeutic intervention (OR = 4.17; 95% CI, 2.32–7.49). No difference in endoscopic intervention was noted in the large study by Navaneethan and colleagues (35.7% for early colonoscopy versus 33.3% for delayed/later colonoscopy) (p = 0.19). There was no difference in the use of endoscopic therapy in the early and elective colonoscopy groups in the RCTs (OR = 5.29; 95% CI, 0.25–114.47).
Length of ICU stay, initial blood transfusion and total blood transfusion requirements were not significantly different between groups for either observational studies, or in the two RCTs. Additional recorded incidences comprising pre-planned secondary outcomes and subgroup analyses are shown in Tables 2 and 3.
Three studies2,19,23 included only patients with diverticular bleeding as a definite or probable cause of bleeding. Excluding diverticular bleeding, the most common cause of probable or definite bleeding was colitis among patients undergoing colonoscopy within 24 h. Diverticular bleeding was the most frequent finding in colonoscopies performed within 12 h (Table 4). Endoscopic therapy was performed at the time of early colonoscopy in 34.2% (95% CI, 33.3–35.1%) of patients in all studies and 21.5% (95% CI, 19.2–24.1%) when excluding the study by Navaneethan and colleagues). Data available on the nature of endoscopic therapy are detailed in Table 4.
Table 4.
Endoscopic findings and nature of endoscopic therapy.
| All urgent colonoscopy cohorts |
All observational studies that include a comparison |
Only RCTs |
|||
|---|---|---|---|---|---|
| All urgent colonoscopies <24 h |
All urgent colonoscopies <24 h |
Control arm of any type |
Urgent endoscopy arm | Control arm | |
| Endoscopic findings of probable or definite cause of bleeding* | |||||
| N = 888 | N = 182 | N = 86 | N = 86 | N = 86 | |
| Diverticula | 15.2%; 13.0–17.7% | 39.6%; 32.7–46.8% | 40.7%; 30.9–51.3% | 58.1%; 47.6–68.0% | 40.7%; 30.9–51.3% |
| Ulcer | 10.2%; 8.4–12.4% | 6.6%; 3.8–11.2% | 3.5%; 1.2–9.8% | 2.3%; 0.6–8.1% | 3.5%; 1.2–9.8% |
| Angiodysplasia | 2.3%; 1.5–3.5% | 5.5%; 3.0–9.8% | 2.3%; 0.6–8.1% | 8.1%; 4.0–15.9% | 2.3%; 0.6–8.1% |
| Cancer | 3.9%; 2.9–5.4% | 2.2%; 0.9–5.5% | 5.8%; 2.5–12.9% | 1.2%; 0.2–6.7% | 5.8%; 2.5–12.9% |
| Colitis | 27.4%; 24.5–30.4% | 4.4%; 2.2–8.4% | 8.1%; 4.0–15.9% | 8.1%; 4.0–15.9% | 8.1%; 4.0–15.9% |
| Small bowel bleeding | 7.0%; 5.5–8.9% | 0.0%; 0.0–2.1% | 0.0%; 0.0–4.3% | 0.0%; 0.0–4.3% | 0.0%; 0.0–4.3% |
| Post-polypectomy | 10.5%; 8.6–12.7% | 6.0%; 3.4–10.5% | 0.0%; 0.0–4.3% | 0.0%; 0.0–4.3% | 0.0%; 0.0–4.3% |
| Other | 13.7%; 11.6–16.2% | 17.6%; 12.7–23.8% | 10.5%; 5.6–18.7% | 10.5%; 5.6–18.7% | 10.5%; 5.6–18.7% |
| Non-diagnostic | 9.8%; 8.0–11.9% | 18.1%; 15.2–32.2% | 29.1%; 20.5–39.4% | 11.6%; 6.4–20.1% | 29.1%; 20.5–39.4% |
| Nature of endoscopic therapy in patients that received endoscopic hemostasis | |||||
| N = 28 | N = 8 | N = 2 | N = 2 | N = 2 | |
| Injection | 57.1%; 39.1–73.5% | 50.0%; 21.5–78.5% | 0.0%; 0.0–65.7% | 0.0%; 0.0–65.7% | 0.0%; 0.0–65.7% |
| Ligation | 10.7%; 3.7–27.2% | 37.5%; 13.7–69.4% | 0.0%; 0.0–65.7% | 0.0%; 0.0–65.7% | 0.0%; 0.0–65.7% |
| Thermal | 7.1%; 2.0–22.7% | 12.5%; 2.2–47.1% | 0.0%; 0.0–65.7% | 0.0%; 0.0–65.7% | 0.0%; 0.0–65.7% |
| Injection + thermal | 17.9%; 7.9–35.6% | 0.0%; 0.0–32.4% | 0.0%; 0.0–65.7% | 100.0%; 34.2–100.0% | 0.0%; 0.0–65.7% |
Excluding studies of diverticular bleeding alone.
Subgroups and sensitivity analyses
Subgroup analyses for the primary and secondary outcomes including studies of patients undergoing colonoscopy within 12 h, or studies using alternative control groups (elective colonoscopy, no colonoscopy or non-colonoscopic interventions (such as surgery or angiography)) resulted in similar conclusions to the main analyses. However, LOS was significantly shorter in the early colonoscopy group compared to elective colonoscopy (WMD = −1.52; 95% CI, −2.54 to −0.50)
Sensitivity analyses including studies with varying definitions of acute LGIB also yielded similar conclusions (see Supplementary Table 2).
Discussion
We performed a formal summary assessment of the existing literature on the management of patients with acute LGIB – in particular the role of early colonoscopy (as defined a priori as within 24 h following admission).1 Our review of 21 studies, including 25,935 patients, demonstrated no differences in rebleeding rates among patients undergoing early colonoscopies compared to controls. These findings remained even when assessing colonoscopy within 12 h in the two RCTs. Early colonoscopy detected more definitive sources of bleeding (OR = 4.12; 95% CI, 2.00–8.49), and was associated with shorter LOS versus later colonoscopy (WMD = −1.52; 95% CI, −2.54 to −0.50 days). As benefits in therapeutic impact are marginal, clinicians need to ensure hemodynamic stability before initiating the bowel preparation that is critical to performing a high-quality early colonoscopy. No other differences were noted between early and late colonoscopy. AEs occurred in 4.0% (95% CI, 3.0–5.3%) of early colonoscopies. However, the included studies were of low quality, with only 2 small RCTs among the 21 included studies, and there existed significant heterogeneity for some outcomes. Given the conflicting evidence in the literature, mostly from small or observational studies, our results help to shed light on the optimal management of patients presenting with acute LGIB. It was noted that patients receiving a colonoscopy within 12 h had higher rates of mortality and surgery compared with patients in all comparison groups. Patient instability may explain the increase in the former as showed in upper GI bleeding; however, these observations are based principally on observational, uncontrolled data.35,36
Importantly, recent society recommendations favor early colonoscopy for patients with high-risk features, rating quality of evidence as low and the strength of recommendations as conditional,1 indicating the need for additional research. These conclusions are supported by our findings that included additional data published since then. The recommendation supporting early colonoscopy is based on the belief that an early colonoscopy approach identified more patients with a definitive cause of bleeding1 – a finding borne out in the current meta-analysis, bearing in mind the difficulty in adjudicating such a subjective outcome. This result was consistent whether grouping all studies, only prospective studies, and only the two RCTs. The suggested inference is that the detection of additional lesions allows more endoscopic therapy, which may result in decreased rebleeding. However, this benefit was not demonstrated in our analysis.
The reason for improved detection of bleeding sources not resulting in an observed decrease in rebleeding rates is unclear, but may be related to many factors, including clinical heterogeneity in study design, inclusion of older studies with limited options for hemostasis or adopted management schemes, variability in endoscopic expertise impacting both diagnosis and therapy, as well as methodological considerations such as small sample sizes in high-quality studies. The shortened hospital stay noted may result from earlier decision-making following a prompt endoscopic diagnosis (finding a bleeding lesion or excluding one). The data comparing 12–24 h are harder to interpret because of widely varying study methodologies.
Not surprisingly, considering their low incidences and the paucity of patients included in comparative trials, no significant differences were noted in mortality or need for surgery (1.1%, and overall 6.8%, respectively), nor in disease-related mortality (due to LGIB), with existing limitations in such adjudication. Studies were underpowered to show any difference in these outcomes. Additionally, we found significant shortening in LOS attributable to early colonoscopy (WMD = −1.52; 95% CI, −2.54 to −0.50 days), albeit only among the subgroup analysis comparing early to later colonoscopy – a benefit further justifying recent society recommendations.1
Large cohort studies are probably more useful than smaller comparative trials for characterizing both feasibility and safety of an early colonoscopy. We noted a low incidence of AEs among patients undergoing an intervention for acute LGIB. Early colonoscopy, in particular, was not associated with increased AEs. However, the source data were disparate in their categorization of AEs and difficult to amalgamate.
Our systematic review includes an overall quantification of outcome rates considering observational and interventional trials with and without comparators, as well as a more traditional meta-analysis of comparative trials comparing early colonoscopy to controls, including targeted subgroup analyses. As a comparison, a recently published meta-analysis addressing the role of early colonoscopy noted increased endoscopic interventions in the absence of increased diagnostic yield and decreased duration of LOS and costs attributable to early colonoscopy;37 the rest of the findings were qualitatively similar to our own. The difference in colonoscopic therapies is hard to interpret in the face of control groups that did not include colonoscopy, and in fact disappeared when limiting the analysis to the control group also receiving a colonoscopy, but after 24 h. Although adopting an overall sound methodological approach, this systematic review performed subgroup analyses combining the two RCTs with the controlled study by Jensen and colleagues that was mistakenly considered a prospective comparative cohort study (whereas it in fact included a historical control group), and the matched propensity analysis by Nagata and colleagues, a statistical approach that fails to obviate residual confounding in contrast to randomized patient allocation. Both of these studies are thus at greater methodological risk of bias than RCTs, and their inclusion can be questioned.
Another meta-analysis yielded similar results, but identified only six studies.38 Furthermore, the authors chose to exclude patients in whom cecal intubation could not be achieved – a decision that limits the interpretation and generalizability of any endoscopic approach when managing patients with acute LGIB. Indeed, adequacy of the preparation and feasibility of a complete colonoscopy are inherently critical components when assessing impact on patient outcomes.
The main shortcoming of the current systematic review relates somewhat to statistical and more importantly to the clinical heterogeneity of existing literature with disparate studies exhibiting and methodological limitations. Other than two small RCTs, all other included studies were heterogeneous observational studies with possibility of bias and confounding, lack of adequate/consistent control groups and varying timings of colonoscopy, endoscopic treatments, patient inclusion criteria and definitions of outcomes. Moreover, there was a paucity of adequately controlled studies. Additionally, there exist possible issues of generalizability as most of the cohort studies, and both RCTs (published at least seven years ago) enrolled patients who were predominantly male and over 50, with a high proportion with hemodynamic instability – ranging from 29% to 76%. The increased representation of such sicker patients may not be representative in acute LGIB and may imply some selection, with the need in such patients to exclude an upper GI source of bleeding that can be found in up to 15%.1,27 Recent guidelines define LGIB as the onset of hematochezia originating from either the colon or the rectum, but in our meta-analysis only one study excluded small bowel bleeding,39 with only one other specifying that small bowel was diagnosed by capsule endoscopy or double-balloon endoscopy.29 In the RCT by Laine and colleagues, selected patients went on to small bowel contrast radiography after colonoscopy.27 As for the choice of optimal endoscopic therapy, the heterogeneity in approaches for given lesions or locations and the lack of patient-level information prevents any meaningful conclusions. The definition and timing of rebleeding varied widely, further limiting the validity of available summary data, even across both RCTs.26,27 Finally, the approach to patients in the control groups also varied widely, with included studies spanning 16 years, and a noticeable paucity of data assessing the role of CT angiography and modern embolization techniques.1
Such realizations do not detract from the importance of this analysis and its dissemination in light of published recommendations so that clinicians can understand the level of evidence and rationale for contemporary guidelines. Just as importantly, however, we feel the aforementioned limitations in level of evidence should deter endoscopists from proceeding with a colonoscopy within the first 24 h following admission if there remain issues of adequate stabilization, patient safety or feasibility concerns related to patient status and comorbidity, availability of resources and endoscopic expertise.
Conclusion
Early colonoscopy is feasible and safe in acute LGIB. Although it does not result in decreased rebleeding, mortality or need for surgery, an increase in the identification of bleeding sources and a decrease in length of hospitalization were noted with the performance of early colonoscopy. The quality of the evidence is limited by clinical and statistical heterogeneity, and is further hampered by a paucity of controlled studies. Additional high-quality data are needed to better determine whether colonoscopy performed within 24 h of admission can improve clinical outcomes such as rebleeding.
Supplementary Material
Footnotes
Authors’ contribution: Study concept and design: I. Roshan Afshar, M. Seyed Sadr, L.L. Strate, M. Martel, C. Menard, A.N. Barkun.
Acquisition of data: I. Roshan Afshar, M. Seyed Sadr, M. Martel, C. Menard, A.N. Barkun.
Analysis and interpretation of data: I. Roshan Afshar, M. Seyed Sadr, M. Martel, A.N. Barkun.
Drafting of the manuscript: I. Roshan Afshar, M. Seyed Sadr, L.L. Strate, M. Martel, C. Menard, A.N. Barkun.
Critical revision of the manuscript for important intellectual content: I. Roshan Afshar, M. Seyed Sadr, L.L. Strate, M. Martel, C. Menard, A.N. Barkun.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Ira Roshan Afshar, Division of Gastroenterology, McGill University Health Centre, McGill University, Montréal, Québec, Canada.
Mo Seyed Sadr, University of British Columbia, Division of Neurosurgery, BC, Canada.
Lisa L. Strate, Division of Gastroenterology, University of Washington School of Medicine, Seattle, Washington, USA
Myriam Martel, Division of Gastroenterology, McGill University Health Centre, McGill University, Montréal, Québec, Canada.
Charles Menard, Medicine, University of Sherbrooke, Sherbrooke, Canada.
Alan N. Barkun, McGill University and the McGill University Health Centre, 1650 Cedar Avenue, D7.346, Montréal, Québec, H3G1A4, Canada.
References
- 1. Strate LL, Gralnek IM. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol 2016; 111: 755. [DOI] [PubMed] [Google Scholar]
- 2. Jensen DM, Machicado GA, Jutabha R, et al. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med 2000; 342: 78–82. [DOI] [PubMed] [Google Scholar]
- 3. Davila RE, Rajan E, Adler D, et al. ASGE guideline: the role of endoscopy in the diagnosis, staging, and management of colorectal cancer. Gastrointest Endosc 2005; 61: 1–7. [DOI] [PubMed] [Google Scholar]
- 4. Strate LL, Syngal S. Timing of colonoscopy: impact on length of hospital stay in patients with acute lower intestinal bleeding. Am J Gastroenterol 2003; 98: 317–322. [DOI] [PubMed] [Google Scholar]
- 5. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, www.ohri.ca/programs/clinical_epidemiology/oxford.htm (2009, 1 February 2009).
- 7. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shrier I, Boivin JF, Steele RJ, et al. Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. Am J Epidemiol 2007; 166: 1203–1209. [DOI] [PubMed] [Google Scholar]
- 9. Navaneethan U, Njei B, Venkatesh PG, et al. Timing of colonoscopy and outcomes in patients with lower GI bleeding: a nationwide population-based study. Gastrointest Endosc 2014; 79: 297–306. e12. [DOI] [PubMed] [Google Scholar]
- 10. Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med 1998; 17: 841–856. [DOI] [PubMed] [Google Scholar]
- 11. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 13. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 14. Egger M, Davey Smith G, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 2004; 23: 1351–1375. [DOI] [PubMed] [Google Scholar]
- 16. Repaka A, Atkinson MR, Faulx AL, et al. Immediate unprepared hydroflush colonoscopy for severe lower GI bleeding: a feasibility study. Gastrointest Endosc 2012; 76: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berrozpe A, Rodriguez-Moranta F, Botargues JM, et al. Colonoscopy predicts the risk of recurrence in lower gastrointestinal bleeding. Gastrointest Endosc 2010; 71: AB194-AB. [Google Scholar]
- 18. Ohyama T, Sakurai Y, Ito M, et al. Analysis of urgent colonoscopy for lower gastrointestinal tract bleeding. Digestion 2000; 61: 189–192. [DOI] [PubMed] [Google Scholar]
- 19. Bloomfeld RS, Rockey DC, Shetzline MA. Endoscopic therapy of acute diverticular hemorrhage. Am J Gastroenterol 2001; 96: 2367–2372. [DOI] [PubMed] [Google Scholar]
- 20. Lim DS, Kim HG, Jeon SR, et al. Comparison of clinical effectiveness of the emergent colonoscopy in patients with hematochezia according to the type of bowel preparation. J Gastroenterol Hepatol 2013; 28: 1733–1737. [DOI] [PubMed] [Google Scholar]
- 21. Smoot RL, Gostout CJ, Rajan E, et al. Is early colonoscopy after admission for acute diverticular bleeding needed? Am J Gastroenterol 2003; 98: 1996–1999. [DOI] [PubMed] [Google Scholar]
- 22. Lin CK, Liang CC, Chang HT, et al. Acute hemorrhagic rectal ulcer: an important cause of lower gastrointestinal bleeding in the critically ill patients. Dig Dis Sci 2011; 56: 3631–3637. [DOI] [PubMed] [Google Scholar]
- 23. Ishii N, Setoyama T, Deshpande GA, et al. Endoscopic band ligation for colonic diverticular hemorrhage. Gastrointest Endosc 2012; 75: 382–387. [DOI] [PubMed] [Google Scholar]
- 24. Jensen DM, Ohning GV, Kovacs TO, et al. Sa1736 differences in diagnoses, prevalence, and outcomes of definitive, presumptive, and incidental diverticular hemorrhage. Gastrointest Endosc 2016; 83: AB280. [Google Scholar]
- 25. Vitale G, Tremolaterra F, Iosca N, et al. Does urgent colonoscopy for lower gastrointestinal bleeding need oral bowel preparation? Dig Liver Dis 2016; 48: E209-E. [Google Scholar]
- 26. Green BT, Rockey DC, Portwood G, et al. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: a randomized controlled trial. Am J Gastroenterol 2005; 100: 2395–2402. [DOI] [PubMed] [Google Scholar]
- 27. Laine L, Shah A. Randomized trial of urgent vs. elective colonoscopy in patients hospitalized with lower GI bleeding. Am J Gastroenterol 2010; 105: 2636–2641; quiz 42. [DOI] [PubMed] [Google Scholar]
- 28. Albeldawi M, Ha D, Mehta P, et al. Utility of urgent colonoscopy in acute lower gastro-intestinal bleeding: a single-center experience. Gastroenterol Rep (Oxf) 2014; 2: 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nagata N, Niikura R, Sakurai T, et al. Safety and effectiveness of early colonoscopy in management of acute lower gastrointestinal bleeding on the basis of propensity score matching analysis. Clin Gastroenterol Hepatol 2016; 14: 558–564. [DOI] [PubMed] [Google Scholar]
- 30. Hassan M, Meighani A, Rao B, et al. Sa1735 early versus late colonoscopy in patients presenting with diverticular bleeds: a single center experience. Gastrointest Endosc 2016; 83: AB280. [Google Scholar]
- 31. Niikura R, Nagata N, Aoki T, et al. Predictors for identification of stigmata of recent hemorrhage on colonic diverticula in lower gastrointestinal bleeding. J Clin Gastroenterol 2015; 49: e24–e30. [DOI] [PubMed] [Google Scholar]
- 32. Angtuaco TL, Reddy SK, Drapkin S, et al. The utility of urgent colonoscopy in the evaluation of acute lower gastrointestinal tract bleeding: a 2-year experience from a single center. Am J Gastroenterol 2001; 96: 1782–1785. [DOI] [PubMed] [Google Scholar]
- 33. Strate LL, Syngal S. Predictors of utilization of early colonoscopy vs. radiography for severe lower intestinal bleeding. Gastrointest Endosc 2005; 61: 46–52. [DOI] [PubMed] [Google Scholar]
- 34. Vernava AM, III, Longo WE. Complications of endoscopic polypectomy. Surg Oncol Clin N Am 1996; 5: 663–673. [PubMed] [Google Scholar]
- 35. Kumar NL, Cohen AJ, Nayor J, et al. Timing of upper endoscopy influences outcomes in patients with acute nonvariceal upper GI bleeding. Gastrointest Endosc 2017; 85: 945–952.e1. [DOI] [PubMed] [Google Scholar]
- 36. Rotondano G. Epidemiology and diagnosis of acute nonvariceal upper gastrointestinal bleeding. Gastroenterol Clin North Am 2014; 43: 643–663. [DOI] [PubMed] [Google Scholar]
- 37. Kouanda AM, Somsouk M, Sewell JL, et al. Urgent colonoscopy in patients with lower gastrointestinal bleeding: a systematic review and meta-analysis. Gastrointest Endosc 2017; 86: 107–117.e1. [DOI] [PubMed] [Google Scholar]
- 38. Sengupta N, Tapper EB, Feuerstein JD. Early versus delayed colonoscopy in hospitalized patients with lower gastrointestinal bleeding: a meta-analysis. J Clin Gastroenterol 2017; 51: 352–359. [DOI] [PubMed] [Google Scholar]
- 39. Strate LL, Syngal S. Timing of colonoscopy: impact on length of hospital stay in patients with acute lower intestinal bleeding. Am J Gastroenterol 2003; 98: 317–322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.