Abstract
Background
Physical activity (PA) promotes weight maintenance, potentially due to its beneficial effects on feeding behavior regulation via diminished food cue reactivity within brain reward regions. We examined how levels of PA and sedentary behavior (SB) relate to brain responses to food cues.
Methods
Participants (22 lean, 18 obese) completed 3–5 PA recalls over 2 months. Average minutes/day of moderate to vigorous PA (MVPA) and SB were calculated. Participants completed a functional magnetic resonance imaging session, viewing food and non-food images following glucose ingestion. Region of interest (ROI) analysis examined associations between MVPA and brain percent signal change in response to food vs. non-food images, controlling for obesity and sex. Secondary analysis examined associations between SB and brain responses to food cues.
Results
Greater MVPA was associated with decreased food cue reactivity after glucose across brain ROIs (B=−0.00057, p=0.005), controlling for obesity and sex. Greater SB was associated with increased food cue reactivity after glucose across brain ROIs in unadjusted analyses (B=0.00041, p=0.026).
Conclusions
PA may have beneficial effects on brain regulation of feeding behavior after caloric intake in lean individuals and individuals with obesity.
Keywords: physical activity, imaging, neuroscience, brain, food
Introduction
There is interest in understanding the effects of physical activity (PA) on body weight regulation for obesity prevention (1). PA is essential for weight maintenance (2,3), perhaps through its effects on appetite regulation. Exercise training and an acute exercise bout alter postprandial circulating appetite hormones (e.g. peptide YY (PYY), glucagon-like polypeptide-1 (GLP-1), ghrelin) leading to decreased hunger and food intake (4,5). PA may also have beneficial effects on brain systems involved in processing food reward. In functional magnetic resonance imaging (fMRI) studies, exercise training and acute exercise are associated with reduced responses to high-calorie food cues in brain regions involved in reward (e.g. orbitofrontal cortex, insula), memory (e.g. hippocampus) and visual processing (e.g. visual cortex) (6–9), thereby decreasing neural food cue reactivity and the propensity to overeat. Prior studies have assessed the influence of exercise (10) on brain systems. However, less is known about whether usual PA (not exercise) positively influences neural regulation of feeding behavior. Prior work examined effects of exercise on brain food cue reactivity in a fasted state. Our study is the first to examine how levels of usual PA relate to brain responses to high-calorie food cues following glucose ingestion and the influence of obesity on this relationship.
The health effects of sedentary behaviors (SB) are of growing interest. SB are any waking activities of low energy expenditure performed in a sitting or reclining posture. Some SB, such as screen time (e.g., TV viewing) and occupational sitting, are associated with worse executive cognitive function (11). Screen-based SB may interrupt physiologic food regulation through distraction or disruption of memory formation (12). However, to our knowledge, no prior studies have investigated the SB-neural food cue reactivity relationship.
The primary study aims were to: 1) determine the relationship between time spent in moderate to vigorous PA (MVPA) and brain responses to high-calorie food cues within a priori brain regions of interest (ROIs); and 2) examine the effects of obesity on this relationship. A secondary aim was to examine the relationship between time spent in SB and brain responses to high-calorie food cues within a priori brain ROIs. We hypothesized that individuals with higher MVPA would have diminished brain responses to high-calorie food cues. Previous findings suggest that exercise is associated with reduced neural food cue reactivity among individuals with obesity (6,8) and lean individuals (7,9). Thus we further hypothesized that this relationship would occur in both lean individuals and individuals with obesity. Conversely, we predicted that participants with greater SB would exhibit greater brain food cue reactivity in lean individuals and individuals with obesity.
Methods
Participants
Forty healthy volunteers (see Table 1) gave written informed consent and experimental procedures were approved by the Institutional Review Board of the University of Southern California (USC). Participants were all right-handed and had normal or corrected to normal vision, had no history of eating disorders, diabetes or other medical illnesses, were nonsmokers, and were not on weight-loss diets or taking medications (except oral contraceptives). Participants were asked to maintain their typical diet and PA levels throughout this study, and brain imaging was performed on female participants during the follicular phase of their menstrual cycle.
Table 1.
Participant Characteristics by Obesity Status
| Combined (n=40) |
Lean Individuals (n=22) |
Individuals with Obesity (n=18) |
||
|---|---|---|---|---|
| N (%) | N (%) | N (%) | p-value | |
|
| ||||
| Male | 19 (47.5) | 10 (45.5) | 9 (50.0) | 0.7746 |
|
| ||||
| M (SD) | M (SD) | M (SD) | p-value | |
|
| ||||
| BMI | 28.2 (7.0) | 22.6 (1.9) | 35.2 (4.0) | <0.0001 |
| Age | 21.7 (2.0) | 21.2 (2.1) | 22.2 (1.8) | 0.1198 |
| Daily SB minutes | 624.8 (111.0) | 646.8 (93.2) | 597.9 (127.1) | 0.1685 |
| Daily MVPA minutes | 129.3 (97.1) | 125.4 (83.5) | 134.0 (113.9) | 0.7915 |
p-values for continuous variables from t-test and for categorical variables from chi-square
Overview of Study Design
MRI sessions were performed in the morning between 9:00–11:00 a.m. after a 12-hour overnight fast at the USC Dana & David Dornsife Cognitive Neuroscience Imaging Center. The food cue task (details below) was performed between 20–30 minutes after ingestion of 75 g of glucose (in 300mL water). A water (300 mL) drink session was conducted as control in a subset of participants (19 lean; 15 obese) on a separate day, and drink order was randomized. Non-sweetened cherry flavoring was added to drinks to improve palatability. The primary imaging outcome of this study was brain food cue reactivity after glucose ingestion. The water session served as a control session and was not performed in our first 6 participants, thus the sample size was smaller. PA and SB were measured using 24-hour Physical Activity Recalls (PAR) collected over the span of two months.
Anthropometric and dietary data measurements
Height was measured to the nearest 0.1 cm using a portable stadiometer and weight to the nearest 0.1 kg using a portable scale. Body mass index (BMI; kg/m2) was calculated, and participants were categorized into their corresponding BMI category using CDC guidelines.
Activity assessments
PA and SB were assessed by trained study staff using the PAR, which demonstrates reasonably accurate recall of activity behaviors (28). Participants were assessed at up to 5 visits (range: 3 to 5 visits) over a 2-month time period, and PAR was collected at each visit. All available data were included in the analysis, with the exception of time spent sleeping between 7:00am - midnight, which was excluded from analyses. Participants reported on their primary activity and self-rated intensity levels in 30-minute increments from 7:00am - midnight on separate days, including the day preceding the MRI imaging session. At least one weekend day was assessed in order to better capture PA variability. Participants selected from 73 possible activity codes and four possible intensity ratings, which ranged from light to very hard. Each activity code and self-rated intensity combination was assigned a metabolic equivalent (MET) from the Compendium of Energy Expenditure (13). Activity categories ranged from sedentary, representing non-sleeping activities with MET ≤1.5, to vigorous, representing activities with MET ≥6. We defined usual PA as each individual’s average MVPA minutes/day (i.e., MET ≥3) across all assessments; usual SB was defined as the average sedentary minutes/day (i.e., METS ≤1.5) across all assessments. MVPA was selected as our primary indicator of PA, as MVPA is associated with numerous health benefits and decreased risk of many chronic diseases, such as diabetes (14) and certain cancers (15,16). Sedentary minutes was a secondary outcome of interest, as time spent in SB is associated with poor health outcomes (17).
Food Cue Task
Participants completed the food cue task in the MRI scanner. Using a randomized block design, participants were presented with a total of 12 food cue and non-food cue visual activation blocks using Matlab (The MathWorks, Inc., Natick, Massachusetts) and Psychtoolbox on a Mac laptop. There were four photographs presented in a random order in each block, and each photograph was presented once for four seconds with one second wait time between photographs. Participants were instructed to view these pictures attentively and asked to rate their hunger and desire to eat after each block. Food cues consisted of high-calorie food items, such as cookies and pizza. The control stimuli consisted of non-food pictures, such as books and baskets. Pictures were previously matched for visual appeal (18–18). The duration for this food cue task was ~9 minutes.
MRI Imaging Parameters
Food cue and structural MRI data were collected using 3T Siemens MAGNETOM Tim/Trio scanner (N=24) and MAGNETOM Prismafit MRI scanner (N=16) due to a scanner upgrade in the middle of our study. Participants laid supine on a scanner bed, viewing stimuli through a mirror mounted over the head coil. Functional blood oxygen level dependent (BOLD) signals were acquired with a single-shot gradient echo planar imaging (EPI) sequence. 4 mm thick slices that cover the whole brain were acquired using the following parameters: Repetition time (TR)=2,000 msec, echo time (TE)=25 msec, bandwidth=2520 Hz/pixel, flip angle=85°, field of view (FOV)=220×220 mm, matrix=64900D7;64. A high resolution 3D Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence (TR=2530 ms; TE=2.62 ms; bandwidth=240 Hz/pixel; flip angle=9°; slice thickness=1mm; FOV=256×256 mm; matrix=256×256) was used to acquire structural images for multi-subject registration.
fMRI Analysis
We used fMRI Expert Analysis Tool version 6.00 (http://www.fmrib.ox.ac.uk/fsl) to process fMRI data. Four functional volumes acquired at the beginning of each MRI session were discarded in order to account for magnetic saturation effects. One participant’s glucose session (lean, male) was excluded from data analysis due to an acquisition error. FMRI preprocessing steps included motion correction, high-pass filtering (100s) and spatial smoothing with a Gaussian kernel of full-width at half-maximum=5mm. The functional data were first mapped to each participant’s anatomical image, and then registered into standard space [Montreal Neurological Institute (MNI)] using affine transformation with FLIRT (21) to the avg152 T1 MNI template. Food and non-food cue events were added to the General Linear Model after convolution with a canonical hemodynamic response function. Temporal derivatives and filtering were added to increase statistical sensitivity. Group level mixed-effects analyses were performed to compute food vs. non-food contrast across individuals after each drink. An extra explanatory variable was included to account for variance related to imaging data collected using two scanners. Locations for ten ROIs were selected based on a meta-analysis of brain regions that have been consistently shown to be responsive to visual food cues and included: left orbital frontal cortex (OFC), left ventral striatum (VS), left amygdala, left hippocampus, left and right anterior insula, bilateral middle insula, left and right precuneus, and left postcentral gyrus (22). ROIs were defined by drawing a 4 mm radius sphere around the peak voxel reported in the meta-analysis, except for the VS, in which a 2 mm radius sphere was drawn because of its smaller size. Individual percent signal change was calculated for food vs. non-food contrast in each ROI (averaged over all voxels within each ROI) for each subject.
Statistical Analysis
Differences in demographic and activity characteristics between weight groups were examined using t-tests (continuous) and chi-square tests (categorical). Linear regression was used to examine the association of 1) average MVPA (minutes/day) and 2) average SB (minutes/day) on brain response to food vs. non-food cues in the mean signal change of ten ROIs after each drink. Due to the study design, the MRI scans could have occurred before or after the PAR assessments. However, analysis of variance (ANOVA) by both visit number and by drink condition revealed no significant differences in MVPA or SB (p's > 0.05). Our primary outcome was the mean signal change to food vs. non-food cues after glucose across the ROIs, and subsequent post-hoc analyses isolated each individual brain region and its association with MVPA and SB. Models were run unadjusted (Model 1), adjusted for obesity (Model 2), and adjusted for both obesity and sex (Model 3). Sex was included as a covariate in Model 3, as previous studies reported sex differences in brain reactivity to food cues (23). False discovery rate (FDR) was used within each model to examine significance of each brain region controlling for multiple comparisons. Analyses were also performed after stratifying into weight groups. Exploratory analyses on PA-food cue relationships were performed on the whole group stratified by sex and between water and glucose conditions. Average MVPA and SB (minutes/day) were negatively correlated (r= −0.7383, p<0.0001); due to multicollinearity, MVPA and SB were not mutually adjusted for in models.
Results
Participant characteristics
The sample consisted of 22 lean (BMI mean (SD): 22.58 (1.89) kg/m2) and 18 participants with obesity (BMI: 35.15 (3.99) kg/m2). The mean age was 21.7 (2.0) years, about half of the sample self-identified as male (47.5%) and as Hispanic (45%). There were no significant differences across weight groups in sex, race or age (p’s all >0.05; Table 1).
Activity Behaviors
Thirty participants completed all five PAR assessments, nine participants completed four days, and one participant completed three days. ANOVA revealed no differences in mean MVPA minutes for participants with 5, 4, or 3 days of PAR assessments (p=0.31). Participants spent 74.1% (mean (SD)=624.8 (111.0) minutes/day) and 15.1% (129.3 (97.1) minutes/day) of non-sleeping time in SB and MVPA, respectively. MVPA and SB did not differ by weight status (Table 1). We did not observe sex differences in either MVPA (average minutes/day) (Males: 149.1 (127.6); Females: 111.4 (54.9), p=0.225), or SB (average minutes/day) (Males: 610.9 (138.3); Females: 637.4 (80.4), p=0.457).
MVPA and Food Cue Reactivity after Glucose Ingestion
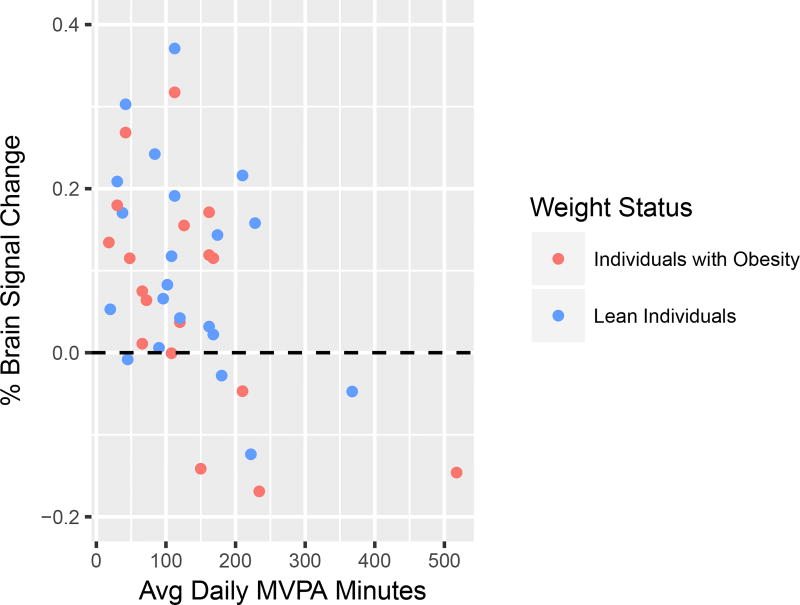
There was a significant negative association between MVPA and brain response to food cues in unadjusted (B=−0.00065, p=0.0015) and adjusted analyses, controlling for obesity status (B=−0.00064, p=0.0017) and for obesity status and sex (B=−0.00057, p=0.0048) (Table 2). For each additional 30 minutes/day spent in MVPA, the mean % signal change decreased by 0.017% (Figure 1). One participant had an extreme MVPA value, and after screening the data it was determined that the participant spent several hours a day working at a moderate intensity job. When this participant was excluded, results remained largely the same (Model 3 B=−0.00067, p=0.0110). Further examination of individual brain regions highlighted specific brain regions that were associated with MVPA: the middle insula (B=−0.00076, p=0.0031) and left postcentral gyrus (B=−0.00147, p=0.0049) (FDR correction for multiple ROIs at q=.05), controlling for sex and obesity (Supplemental Figure S1, S2).
Table 2.
Association of Daily MVPA Minutes with Brain Response to Food (vs. Non-Food) Cues
| Average Daily MVPA Minutes | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Model 1∞ | Model 2 | Model 3 | ||||
|
| ||||||
| B (SE) | P-value | B (SE) | P-value | B (SE) | P-value | |
| Composite ROI | −0.00065 (0.0002) | 0.0015 | −0.00064 (0.0002) | 0.0017 | −0.00057 (0.0002) | 0.0048 |
| R Precuneus | −0.00086 (0.0004) | 0.0243 | −0.00085 (0.0004) | 0.0267 | −0.00080 (0.0004) | 0.0439 |
| L Precuneus | −0.00080 (0.0005) | 0.1325 | −0.00081 (0.0005) | 0.1273 | −0.00054 (0.0005) | 0.2907 |
| Middle Insula | −0.00067 (0.0002) | 0.0081* | −0.00066 (0.0002) | 0.0088* | −0.00076 (0.0002) | 0.0031* |
| R Anterior Insula | −0.00036 (0.0003) | 0.2719 | −0.00035 (0.0003) | 0.2826 | −0.00030 (0.0003) | 0.3747 |
| L Anterior Insula | −0.00002 (0.0003) | 0.9278 | −0.00002 (0.0002) | 0.9371 | −0.00007 (0.0003) | 0.8211 |
| L Ventral Striatum | −0.00009 (0.0003) | 0.7879 | −0.00009 (0.0004) | 0.7956 | 0.00006 (0.0003) | 0.8533 |
| L Postcentral Gyrus | −0.00158 (0.0005) | 0.0020* | −0.00157 (0.0004) | 0.0022* | −0.00147 (0.0005) | 0.0049* |
| L Orbital Frontal Cortex | −0.00059 (0.0005) | 0.2106 | −0.00058 (0.0005) | 0.2199 | −0.00027 (0.0004) | 0.5203 |
| L Hippocampus | −0.00047 (0.0002) | 0.0530 | −0.00046 (0.0002) | 0.0563 | −0.00045 (0.0002) | 0.0776 |
| L Amygdala | −0.00100 (0.0004) | 0.0230 | −0.00099 (0.0004) | 0.0246 | −0.00091 (0.0004) | 0.0438 |
Model 1 is unadjusted; Model 2 controls for obesity status; Model 3 controls for obesity status and sex
Bold font indicates result is statistically significant at p < 0.05
Indicates result remained significant after adjusting for multiple comparisons within each model
Figure 1.
Correlation of average daily minutes spent in moderate-to-vigorous physical activity (MVPA) with brain response to food vs. non-food cues in the mean signal change across all ten regions of interest (ROI). Blue indicates lean individuals and red indicates individuals with obesity.
MVPA and Food Cue Reactivity Stratifying into Obese and Lean Groups
In participants with obesity, MVPA was negatively associated with brain response to food vs. non-food cues (B=−0.00069, p=0.0105), and although in the same direction, this relationship was not statistically significant among lean participants (B=−0.00056, p=0.0905) (Figure 1).
Sedentary Behavior and Food Cue Reactivity after Glucose Ingestion
We observed a significant positive association between SB and brain response to food cues in unadjusted (B=0.00041, p=0.0258) and in adjusted analysis controlling for obesity status (B=0.00039, p=0.0383); when controlling for both obesity status and sex, results remained in the same direction but were attenuated (B=0.00035, p=0.0538) (Table 3). For each additional 30 minutes spent in SB, the mean % signal change of food cue reactivity increased by 0.012%. When excluding data from one participant with very low SB, results remained in the same direction, but were attenuated (Model 3 B=0.00035, p=0.1641). Examination of individual brain regions showed a positive association between SB and food cue reactivity in the middle insula (B=0.00066, p=0.0032, FDR corrected) controlling for obesity and sex (Supplemental Figure S3).
Table 3.
Association of Daily SB Minutes with Brain Response to Food (vs. Non-Food) Cues
| Average Daily SB Minutes | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Model 1∞ | Model 2 | Model 3 | ||||
|
| ||||||
| B (SE) | P-value | B (SE) | P-value | B (SE) | P-value | |
| Composite ROI | 0.00041 (0.0002) | 0.0258 | 0.00039 (0.0002) | 0.0383 | 0.00035 (0.0002) | 0.0538 |
| R Precuneus | 0.00037 (0.0003) | 0.2775 | 0.00036 (0.0003) | 0.3091 | 0.00032 (0.0003) | 0.3677 |
| L Precuneus | 0.00032 (0.0005) | 0.4970 | 0.00043 (0.0005) | 0.3683 | 0.00030 (0.0004) | 0.5048 |
| Middle Insula | 0.00064 (0.0002) | 0.0031* | 0.00062 (0.0002) | 0.0052 | 0.00066 (0.0002) | 0.0032* |
| R Anterior Insula | 0.00033 (0.0003) | 0.2528 | 0.00027 (0.0003) | 0.3629 | 0.00024 (0.0003) | 0.4193 |
| L Anterior Insula | 0.00012 (0.0002) | 0.6149 | 0.00010 (0.0002) | 0.6791 | 0.00012 (0.0002) | 0.6266 |
| L Ventral Striatum | −0.00020 (0.0003) | 0.5063 | −0.00023 (0.0003) | 0.4639 | −0.00030 (0.0003) | 0.3150 |
| L Postcentral Gyrus | 0.00094 (0.0004) | 0.0408 | 0.00091 (0.0005) | 0.0557 | 0.00084 (0.0005) | 0.0750 |
| L Orbital Frontal Cortex | 0.00021 (0.0004) | 0.6095 | 0.00017 (0.0004) | 0.6788 | 0.00003 (0.0004) | 0.9334 |
| L Hippocampus | 0.00023 (0.0002) | 0.2836 | 0.00019 (0.0002) | 0.3822 | 0.00018 (0.0002) | 0.4314 |
| L Amygdala | 0.00090 (0.0004) | 0.0187 | 0.00085 (0.0004) | 0.0298 | 0.00081 (0.0004) | 0.0404 |
Model 1 is unadjusted; Model 2 controls for obesity status; Model 3 controls for obesity status and sex
Bold font indicates result is statistically significant at p < 0.05
Indicates result remained significant after adjusting for multiple comparisons within each model
Sedentary Behavior and Food Cue Reactivity Stratified into Obese and Lean Groups
In stratified analyses, participants with obesity (B=0.00040, p=0.1188) and lean participants (B=0.00037, p=0.2076) had a positive but non-significant association between SB and brain responses to food cues.
Food Cue Reactivity and Relationship to Physical Activity Stratified by Sex
Post-hoc analyses revealed that males (mean: 0.04) and females (mean: 0.13) displayed different mean % brain signal changes to food vs. non-food cues following glucose (p=0.0257). The association between MVPA and mean % signal change to food cues in males was B= −0.0007 (p=0.0014), while in females it was B= 0.0002 (p=0.5875). The association between SB and mean % signal change in males was B= 0.0004 (p=0.075), while in females it was B= 0.0002 (p=0.4803).
MVPA and Food Cue Reactivity after Water vs. Glucose Ingestion
Among the sample of the 34 participants who completed both sessions, there were no significant differences between water vs. glucose condition in neural food cue reactivity (water: 0.1015; glucose: 0.0892 mean % signal change; p=0.7398). In the water condition, we observed a negative, non-significant association between MVPA and mean % signal change of B= −0.0004 (p=0.1348).
Discussion
Greater MVPA was associated with decreased brain response to high-calorie food cues after glucose ingestion in regions implicated in processing food rewards among healthy young adults, and particularly among individuals with obesity. In secondary analysis, greater SB was correlated with greater brain food cue reactivity following glucose ingestion. Thus, increasing PA or decreasing SB may diminish brain responses to high-calorie food cues following caloric intake. Increasing time spent in PA may mitigate the negative effects of the frequent exposures in our obesogenic environment to palatable food cues on obesity risk.
Our MVPA findings are consistent with previous studies where brain response to food cues decreased after participation in exercise and after an acute in-lab exercise bout (6–9). We also found that the MVPA-brain response association was significant in fully adjusted models, and the results were stronger among obese participants in stratified analyses.
We assessed PA across the time span of two months, including at least one weekend to capture variability in PA throughout the week. Although the usual levels of MVPA were above the Physical Activity Recommendation Guidelines (24), participants rarely reported engaging in structured exercise but the majority of exercise was aerobic (e.g., basketball). Regular and consistent engagement in aerobic exercise is associated with better regulation of food intake (25,26). Thus, it is possible that while exercise levels were low, the consistent engagement in these behaviors reduced food cue reactivity and may modulate food intake.
We observed a negative relationship between MVPA and mean signal change in the ROIs identified by a meta-analysis as regions that are consistently activated by visual food cues (22). Post-hoc analysis revealed a significant negative correlation between MVPA and food cue reactivity within the left postcentral gyrus and middle insula. The postcentral gyrus is part of the visual processing areas and plays a role in identifying salient cues (22). Although visual areas may not directly modulate appetitive responses, studies have shown they are sensitive to top down influences from the amygdala and OFC, regions involved in emotion and reward processing (27,28). The attenuated response to food cues in these regions suggests a decreased saliency of visual food cues. The insula is involved in visceral interoceptive processing (29) and is part of brain salience network (30). Specific to eating behavior, studies reported that the insula was responsive to food cravings (31), and brain activity in the insula was modulated by hunger state (32,33). Prior studies also observed that both chronic and acute exercise were associated with reduced insula responses to visual food cues in a fasted state (6,7). The attenuated middle insula response to food cues following glucose ingestion suggests that engaging in regular MVPA may also reduce the saliency of food rewards and food cravings after caloric intake.
We also found that SB was significantly associated with increased brain response to food cues across ROIs after glucose consumption. Results remained significant after controlling for obesity but were attenuated after further adjusting for sex. Post-hoc analyses revealed that SB was positively correlated with food cue reactivity within the middle insula. Our findings and prior reports showing that exercise diminishes insula reactivity to high-calorie food cues (6–8) highlight the insula as a brain area that may be particularly sensitive to PA and SB.
Exploratory analyses observed an effect of sex on the relationship between MVPA on brain food cue reactivity following glucose, suggesting that the association may be stronger in males than females. Given the small sample size we were unable to stratify by both obesity status and sex. Larger studies are required to investigate the relationship between sex, PA, and brain reactivity to food cues in the context of obesity.
Although prior studies observed reduced brain food cue reactivity in a fasted state following an exercise intervention or in-lab exercise bout, we did not observe a significant relationship between levels of usual PA and neural food cue reactivity after water ingestion. Compared with exercise, usual PA is less intense, and thus may have smaller effects on neural food cue reactivity under fasting conditions. Moreover, due to the smaller sample size in the water condition, we had limited power to detect an effect, although the MVPA-food cue relationship trended in the same direction under both the water and glucose conditions.
PA was assessed within 7:00am – midnight. Although this time frame was selected to capture the time that young adults typically spend in waking activity, we may have missed activities occurring outside of this time window. In addition, any self-report measure is prone to measurement error and recall bias, which may lead to overestimation of time spent in MVPA. It is important to note that persons with obesity may be more likely to overestimate time spent in self-reported MVPA, potentially due to the misclassification of intensity due to lower levels of cardiorespiratory fitness. Nonetheless, there were no significant differences in mean MVPA between lean and obese subjects in our sample, and therefore errors in self-reported MVPA by weight status were unlikely to have altered relationships between MVPA and food cue reactivity across groups. Also, our study design is correlational and thus cannot determine the directionality of the relationship between PA and brain food cue reactivity. However, our findings are consistent with prior studies, which experimentally manipulated exercise levels and showed that exercise reduced brain food cue reactivity (6,7,9). Our results suggest that PA might attenuate brain appetitive responses to food cues, resulting in reduced food consumption. Another interpretation is that individuals who exhibited less food cue reactivity may be motivated to engage in PA.
The potential mechanism(s) through which PA may affect brain response to food cues are unclear. We speculate that PA could suppress brain food cue reactivity, especially within brain regions involved in visual attention, saliency, and craving, through improved insulin sensitivity (35–37), reductions in inflammatory cytokines (38), and/or increases in postprandial circulating anorexigenic hormones including PYY and GLP-1 (5,39) that centrally regulate food intake (40). Our study was not designed to address these possibilities, but future studies could provide insights into the underlying mediators of the effects of PA on reductions in brain food cue responsivity.
Supplementary Material
What is already known about this subject?
Prior studies have shown that exercise is associated with diminished food cue reactivity within brain areas involved in attention and reward processing. However, less is known about the effects of habitual physical activity or sedentary behavior on neural regulation of feeding behavior, and whether these relationships are affected by weight status.
What does your study add?
Across all participants, greater physical activity was associated with reduced food cue reactivity in brain regions involved in attention and reward processing, and results remained significant after adjusting for obesity and sex. Greater SB was associated with increased neural food cue reactivity, but this relationship was attenuated after adjusting for both obesity and sex.
Acknowledgments
Funding: This work was supported by the National Institutes of Health, NIDDK R01DK102794 (Page, KA), the Doris Duke Charitable Foundation Clinical Scientist Development Award 2012068 (Page, KA), the National Institutes of Health Cancer Control and Epidemiology Research Training Grant (T32 CA 009492) (O’Connor, SG), and the University of Southern California Graduate School Provost Fellowship (O’Connor, SG).
We would like to thank our study participants, Ana Romero for assistance with study coordination, and the Dornsife Cognitive Neuroimaging Center at USC. A Research Electronic Data Capture, REDCap, was used for this study, which is supported by the Southern California Clinical and Translational Science Institute (SC CTSI) through NIH UL1TR001855.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Jakicic JM. Obesity. Suppl 3(n3s) Vol. 17. Nature Publishing Group; 2009. The effect of physical activity on body weight; pp. S34–8. [DOI] [PubMed] [Google Scholar]
- 2.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;(21):323–41. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 3.Jakicic JM. The Role of Physical Activity in Prevention and Treatment of Body Weight Gain in Adults. Life Sci. 2002;(10):3826–9. doi: 10.1093/jn/132.12.3826S. [DOI] [PubMed] [Google Scholar]
- 4.Hagobian TA, Braun B, Sharoff CG, Braun B, Stephens BR, Wade GN, et al. Effects of exercise on gut peptides, energy intake and appetite. Am J Physiol Regul Integr Comp Physiol. 2007;296(2):R233–42. doi: 10.1152/ajpregu.90671.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broom DR, Batterham RL, King Ja, Stensel DJ. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am J Physiol Regul Integr Comp Physiol. 2009;296:R29–35. doi: 10.1152/ajpregu.90706.2008. (November 2008) [DOI] [PubMed] [Google Scholar]
- 6.Cornier M-A, Melanson EL, Salzberg AK, Bechtell JL, Tregellas JR. Physiol Behav. 4. Vol. 105. Elsevier Inc; 2012. The effects of exercise on the neuronal response to food cues; pp. 1028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evero N, Hackett LC, Clark RD, Phelan S, Hagobian Ta. Aerobic exercise reduces neuronal responses in food reward brain regions. J Appl Physiol. 2012;112:1612–9. doi: 10.1152/japplphysiol.01365.2011. (March 2012) [DOI] [PubMed] [Google Scholar]
- 8.Killgore WDS, Kipman M, Schwab ZJ, Tkachenko O, Preer L, Gogel H, et al. Physical exercise and brain responses to images of high-calorie food. Neuroreport. 2013;24:962–7. doi: 10.1097/WNR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 9.Crabtree DR, Chambers ES, Hardwick RM, Blannin AK. The effects of high-intensity exercise on neural responses to images. Am J Clin Nutr. 2014;99(2):258–67. doi: 10.3945/ajcn.113.071381. [DOI] [PubMed] [Google Scholar]
- 10.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–31. [PMC free article] [PubMed] [Google Scholar]
- 11.Loprinzi PD, Kane CJ. Exercise and Cognitive Function. Mayo Clin Proc. Elsevier. 2017 Aug 10;90(4):450–60. doi: 10.1016/j.mayocp.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Marsh S, Ni Mhurchu C, Maddison R. Appetite. Vol. 71. Elsevier Ltd; 2013. The non-advertising effects of screen-based sedentary activities on acute eating behaviours in children, adolescents, and young adults. A systematic review; pp. 259–73. [DOI] [PubMed] [Google Scholar]
- 13.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, et al. 2011 compendium of physical activities: A second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–81. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 14.Jeon CY, Lokken RP, Hu FB, Van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: A systematic review. Diabetes Care. 2007;30(3):744–52. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 15.Lynch BM. Sedentary behavior and cancer: A systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2691–709. doi: 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 16.Howard RA, Freedman DM, Park Y, Hollenbeck A, Schatzkin A, Leitzmann MF. Physical activity, sedentary behavior, and the risk of colon and rectal cancer in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2008;19(9):939–53. doi: 10.1007/s10552-008-9159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knaeps S, Bourgois JG, Charlier R, Mertens E, Lefevre J, Wijndaele K. Ten-year change in sedentary behaviour, moderate-to-vigorous physical activity, cardiorespiratory fitness and cardiometabolic risk: independent associations and mediation analysis. Br J Sports Med. 2016;(1) doi: 10.1136/bjsports-2016-096083. bjsports-2016-096083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, et al. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest. 2011;121(10):4161–9. doi: 10.1172/JCI57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo S, Romero A, Adam TC, Hu HH, Monterosso J, Page KA. Abdominal fat is associated with a greater brain reward response to high-calorie food cues in hispanic women. Obesity. 2013;21(10):2029–36. doi: 10.1002/oby.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo S, Monterosso JR, Sarpelleh K, Page KA. Differential effects of fructose versus glucose on brain and appetitive responses to food cues and decisions for food rewards. Proc Natl Acad Sci. 2015;112(20):6509–14. doi: 10.1073/pnas.1503358112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 22.Tang DW, Fellows LK, Small DM, Dagher A. Physiol Behav. 3. Vol. 106. Elsevier Inc; 2012. Food and drug cues activate similar brain regions: A meta-analysis of functional MRI studies; pp. 317–24. [DOI] [PubMed] [Google Scholar]
- 23.Cornier M, Salzberg A, Endly D. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99(4):538–43. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. 2. Vol. 67. Washingt DC US: 2008. p. 683. [Google Scholar]
- 25.Long SJ, Hart K, Morgan LM. The ability of habitual exercise to influence appetite and food intake in response to high- and low-energy preloads in man. Br J Nutr. 2002;87(5):517–23. doi: 10.1079/BJNBJN2002560. [DOI] [PubMed] [Google Scholar]
- 26.Blundell JE, Stubbs RJ, Hughes Da, Whybrow S, King Na. Cross talk between physical activity and appetite control: does physical activity stimulate appetite? Proc Nutr Soc. 2003;62(3):651–61. doi: 10.1079/PNS2003286. [DOI] [PubMed] [Google Scholar]
- 27.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin Modulates Brain Activity in Areas that Control Appetitive Behavior. Cell Metab. 2008;7(5):400–9. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 28.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 29.Craig AD. Interoception: The sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 30.Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(s5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: Food-craving activation during fMRI. Neuroimage. 2004;23(4):1486–93. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Gottfried JA, O’Doherty J, Dolan RJ. Encoding Predictive Reward Value in Human Amygdala and Orbitofrontal Cortex. Science (80- ) 2003;301(5636):1104–8. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 33.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate. Brain. 2001;124(9):1720–33. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 34.Welk GJ, Kim Y, Stanfill B, Osthus DA, Calabro MA, Nusser SM, et al. Validity of 24-h physical activity recall: Physical activity measurement survey. 2014:2014–24. doi: 10.1249/MSS.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balkau B, Mhamdi L, Oppert J, Nolan J. Physical activity and insulin sensitivity the RISC study. Diabetes. 2008;57:2613–8. doi: 10.2337/db07-1605. (October) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer-Davis EJ, D’Agostino R, Karter aJ, Haffner SM, Rewers MJ, Saad M, et al. Intensity and amount of physical activity in relation to insulin sensitivity: the Insulin Resistance Atherosclerosis Study. JAMA. 1998;279(9):669–74. doi: 10.1001/jama.279.9.669. [DOI] [PubMed] [Google Scholar]
- 37.Gustat J, Srinivasan SR, Elkasabany A, Berenson GS. Relation of self-rated measures of physical activity to multiple risk factors of insulin resistance syndrome in young adults: The Bogalusa Heart Study. J Clin Epidemiol. 2002;55(10):997–1006. doi: 10.1016/s0895-4356(02)00427-4. [DOI] [PubMed] [Google Scholar]
- 38.Kasapis C, Thompson PD. J Am Coll Cardiol. 10. Vol. 45. Elsevier Masson SAS; 2005. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review; pp. 1563–9. [DOI] [PubMed] [Google Scholar]
- 39.Martins C, Morgan LM, Bloom SR, Robertson MD. Effects of exercise on gut peptides, energy intake and appetite. Journal of Endocrinology. 2007:251–8. doi: 10.1677/JOE-06-0030. [DOI] [PubMed] [Google Scholar]
- 40.Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1187–209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.