Abstract
Osteoimmunology encompasses all aspects of the cross-regulation of bone and the immune system, including various cell types, signalling pathways, cytokines and chemokines, under both homeostatic and pathogenic conditions. A number of key areas are of increasing interest and relevance to osteoimmunology researchers. Although rheumatoid arthritis has long been recognized as one of the most common autoimmune diseases to affect bone integrity, researchers have focused increased attention on understanding how molecular triggers and innate signalling pathways (such as Toll-like receptors and purinergic signalling pathways) related to pathogenic and/or commensal microbiota are relevant to bone biology and rheumatic diseases. Additionally, although most discussions relating to osteoimmune regulation of homeostasis and disease have focused on the effects of adaptive immune responses on bone, evidence exists of the regulation of immune cells by bone cells, a concept that is consistent with the established role of the bone marrow in the development and homeostasis of the immune system. The active regulation of immune cells by bone cells is an interesting emerging component of investigations that seek to understand how to control immune-associated diseases of the bone and joints.
The emergence of the field of osteoimmunology has led to a conceptual rethinking of various biological phenomena that connect the functions of bone and the immune system1. The recognition that bone provides a microenvironment that is crucial for the development of haematopoietic stem cells (HSCs)2,3 (from which all cells of the mammalian immune system are derived), coupled with the discovery that bone-resorbing osteoclasts are also derived from HSCs4, has provided substantial impetus for this line of inquiry. At a molecular level, perhaps the most critical work in osteoimmunology of the past two decades has been the discovery and characterization of the receptor activator of nuclear factor-κB (RANK)–RANK ligand (RANKL)–osteoprotegerin (OPG) axis5. This receptor–cytokine–decoy receptor system provides key signals that control intercellular communication between bone cells and immune cells and is an important regulator of bone homeostasis and the development of bone-related autoimmune diseases5,6. RANK–RANKL–OPG interactions were initially characterized during investigations into their crucial role in maintaining bone homeostasis and turnover7–11. Specifically, RANK expressed on the cell surface of pre-osteoclasts and osteoclasts must bind to RANKL on other cells in the bone microenvironment, such as osteoblasts, to trigger differentiation and activation programmes; however, the triggering threshold for cell activation via RANK–RANKL interaction is determined by the relative expression of OPG, which interferes with RANK–RANKL binding by acting as a decoy receptor for RANKL5,12. This axis can also be seen in other cell types that utilize RANK for biological functions, including immune cells. Importantly, this discovery has led to the development of a successful treatment for osteoporosis and metastasis-related bone loss, in which RANKL is targeted with a therapeutic antibody13,14. A similar treatment could potentially be useful for patients with rheumatoid arthritis (RA)15.
Outside of the clinical setting, there have been numerous technical developments that have greatly improved the study of biological phenomena related to bone cell– immune cell interactions, including improved genetic and imaging tools. For these reasons and others, it is important to continue to uncover and contextualize the mechanisms of interactions between bone and the immune system. In this Review, we discuss key emerging areas of interest in osteoimmunology research, including the role of microbiota and related innate signalling pathways in bone homeostasis, and the increasing appreciation of the effects that bone cells have on immune cells. This Review is not a comprehensive overview of osteoimmunology; for a detailed picture of signalling pathways related to osteoimmunology, see other reviews6,16–18. Inevitably, some important studies might be overlooked in this Review but, in itself, this fact is testament to the rapidity of progress in osteoimmunology research.
Bone damage in rheumatoid arthritis
Bone homeostasis has an important role in regulating the quality of an immune response, and dysregulation of the immune system can have deleterious consequences for bone integrity. RA, one of the most common auto-immune diseases in which bone integrity is compromised, is characterized by chronic inflammation and synovial hyperplasia that eventually lead to the destruction of cartilage and bone. RA is the result of a loss of immune tolerance to a specific type of modified self-antigen, which is processed by antigen-presenting cells and presented on MHC molecules to CD4+ effector T cells that then recruit other cells of the adaptive immune system (CD8+ T cells and B cells). T cells have important roles in the onset and pathogenesis of RA, particularly in the initial phase of the autoimmune reaction and in inducing local inflammation in the joints19; however, the bone destruction seen in patients with RA is caused by hyperactivation of osteoclasts, leading to increased bone resorption20,21 (FIG. 1).
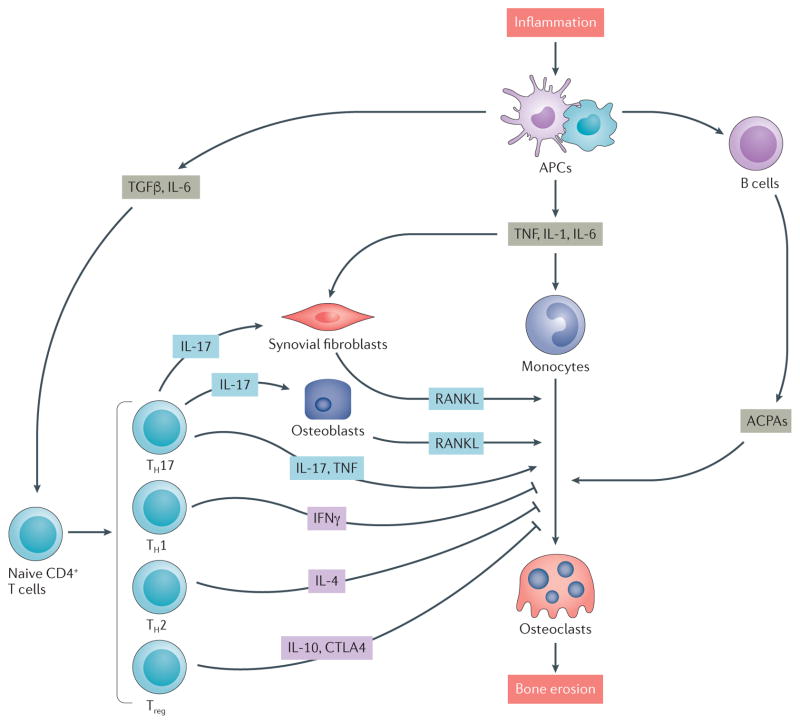
Figure 1. Immune regulation of bone destruction in rheumatoid arthritis (RA).
The interaction of immune cells with bone cells in RA leads to destructive inflammation that involves the activation of cytokine-mediated pathways that result in bone remodelling. Autoimmune responses are induced by inflammation: T cells and B cells are activated by antigen presenting cells (APCs) such as dendritic cells and macrophages in the inflamed synovium. Synovial fibroblasts produce the osteoclastogenic cytokine receptor activator of nuclear factor-κB ligand (RANKL), and CD4+ T cells produce osteoclastogenic cytokines such as IL 17 and TNF (produced by T helper 17 (TH17) cells) but also produce anti osteoclastogenic cytokines such as IFNγ (TH1 cells), IL 4 (TH2 cells) and IL 10 and cytotoxic T lymphocyte protein 4 (CTLA4; produced by regulatory T (Treg) cells). TH17 cells also stimulate osteoblastogenic activity by producing IL 17. Anti citrullinated protein antibodies (ACPAs) are produced by B cells in the RA joint and are also able to promote osteoclastogenesis and osteoclast activity. TGFβ, transforming growth factor β.
Osteoclasts are formed when RANK-expressing monocytes differentiate following the binding of RANK to RANKL, which is expressed as both surface-bound and soluble forms by synovial fibroblasts and T cells in joints affected by RA or adjuvant-induced arthritis22–25. Since RANKL is expressed by various subsets of activated T cells, T cell-derived RANKL was initially proposed to be a main contributor to the enhanced osteoclastogenesis seen in RA26,27. However, co-culture experiments revealed that CD4+ T helper (TH) cells could inhibit osteoclastogenesis by producing anti-osteoclastogenic cytokines such as IFNγ28. TH cells, as well as regulatory T (Treg) cells, can also produce strong inhibitors of osteoclastogenesis, such as IL-10, IL-4, OPG or cytotoxic T lymphocyte protein 4 (CTLA4), which counterbalance the action of RANKL29–31, implying that the contribution made by T cells towards osteoclastogenesis is not straightforward. Anti-citrullinated protein antibody (ACPA)-producing B cells can also promote the differentiation of monocytes into osteoclasts in RA32,33, and immune complexes can directly promote osteoclast differentiation in human and mouse cells in vitro34,35.
CD4+ TH cells, which can be divided into TH1, TH2, and TH17 cell subsets, are indispensable for the initiation of RA. Collagen-induced arthritis (CIA) and the K/B×N mouse model36, together the most widely used mouse models of RA, both require CD4+ T cells for the full induction of disease36,37, and antibody-mediated CD4+ T cell depletion can substantially diminish disease severity in these models. CD4+ and CD8+ T cells that infiltrate synovial tissue in patients with RA express high levels of CD40 ligand (CD40L)38,39, and the expression of CD40L on these cells positively correlates with disease activity, suggesting a possible role for the CD40–CD40L pathway in the pathogenesis of RA39. Cytokines typically produced by TH1 cells (such as IFNγ) and TH2 cells (such as IL-4) exert inhibitory effects on osteoclastogenesis, and these TH cell subsets are not thought to be involved in bone damage in RA19,31.
TH17 cells, which promote the development of autoimmune diseases by producing IL-17, are recognized to be a pathogenic subset of CD4+ T cells in RA. The development of CIA is markedly diminished in IL-17A-deficient mice40 or by antibody-mediated IL-17 blockade41, and IL-17 promotes osteoclastogenesis by upregulating RANKL expression on osteoblasts and synovial fibroblasts42,43. When naive CD4+ T cells from SKG mice, which have a mutation in Zap70 that results in the spontaneous development of RA-like disease, are adoptively transferred into Rag2−/− mice, the recipient mice developed arthritis and had increased numbers of joint-infiltrating TH17 cells44. However, disease was not observed when CD4+ T cells from Il17−/− SKG mice were transferred to Rag2−/− mice, demonstrating the need for TH17 cells in disease development44. TH17 cells can also induce osteoclastogenesis by producing IL-17A, which stimulates RANKL expression on osteoclastogenesis- supporting cells, including osteoblasts and synovial fibroblasts42,45. Furthermore, IL-17 produced by TH17 cells induces innate immune cells, including macrophages and dendritic cells, as well as synovial fibroblasts and endothelial cells, to express pro-inflammatory cytokines (such as IL-6 and TNF) and matrix-degrading enzymes, which leads to further upregulation of RANKL in synovial fibroblasts and an amplification of chronic inflammation45,46. TH17 cells express RANKL, but TH17 cell-derived RANKL seems not to be directly involved in osteoclastogenesis45. Interestingly, a subset of FOXP3+ Treg cells in the joints of mice with CIA can be converted into a pathogenic subset of TH17 cells that have a distinct pattern of gene expression and potent osteoclastogenic capacity47. Taken together, these results indicate that TH17 cells are a pathogenic subset of CD4+ T cells in RA that should continue to be researched to further our understanding of the pathogenesis of RA.
Microbiota, innate immunity and bone
In the past few years, increased attention has been paid to understanding how molecular triggers and innate signalling pathways related to pathogenic and/or commensal microbiota are related to the activation and regulation of immune cells48,49. Similarly, how these immune stimuli regulate bone-related homeostasis and pathology, including rheumatic diseases, is also of interest50,51. The following section highlights studies that explain the ways in which microbiota, innate immune signalling pathways and bone biology interact. For comprehensive reviews of osteoimmune-related signalling pathways that involve adaptive pathways and cytokine production, see previously published works6,52,53.
Microbiota and bone homeostasis
The vast majority of cells in the body are not self- derived, but instead are commensal, symbiotic or pathogenic microbiota. The more than 1,000 different strains of bacteria (mostly from the phyla Firmicutes and Bacteroidetes) reside primarily in the gut, but are also found on other mucosal tissues, such as those lining the lung, oral cavity and genitourinary tract and vary between individuals54. Microbiota do not only affect the physiology of the gut, as dysbiosis of the commensal microbiota lining the gut and other mucosal tissues can also affect non-mucosal organ systems55. Microbiota affect host biology by putting direct pressure on nutrient homeostasis (large populations of resident bacterial cells have high nutrient needs that can adapt or change depending on diet of the host); by the systemic dissemination of pathogen-associated molecular patterns (PAMPs) or microbiota-associated molecular patterns (MAMPs) that act on, or trigger, pattern-recognition receptors (such as Toll-like receptors (TLRs)) on organ-specific tissue cells; and by activating mucosal immune responses48,56,57 that can exert effects on distal tissues including bone58,59. A defining feature of the immune system is the ability to differentiate self from non-self, and mechanisms have evolved to determine whether, and under what conditions, immune cells tolerate microbiota48. Therefore, the presence of both commensal and pathogenic microbiota, and the indirect effects their presence has on the host, is regulated by the immune system48,60. In the past few years, however, increased attention has been paid to how microbiota affect bone, during both development and homeostasis50, and to how these interactions might be mediated by the immune system (FIG. 2).
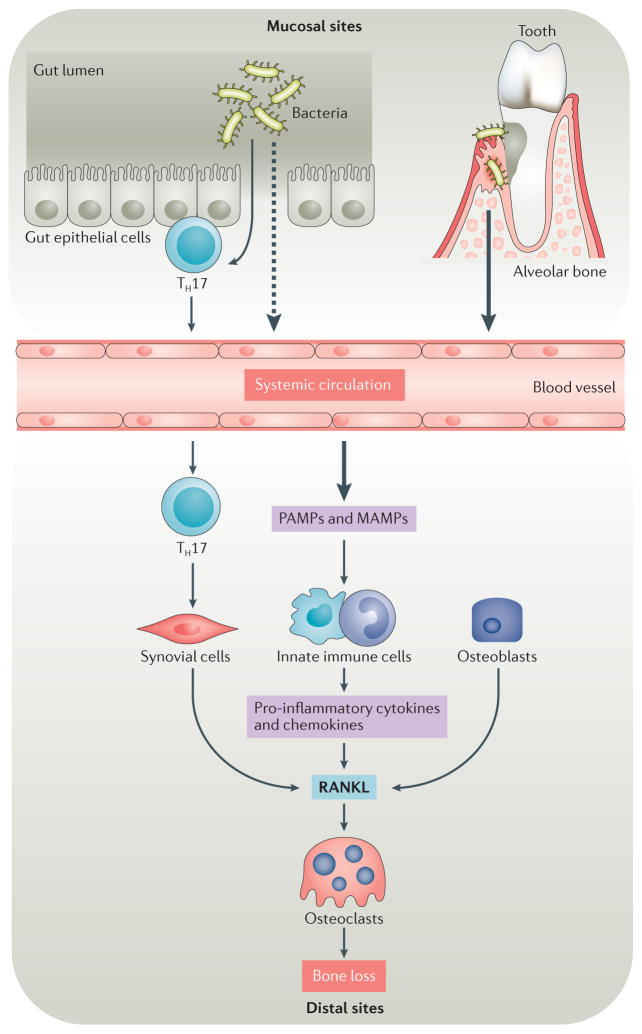
Figure 2. Microbiota and osteoimmunology.
Under homeostatic or inflammatory conditions, microbiota can affect bone resorption at sites proximal to and distal to the site of colonization. Inflammatory conditions are often triggered by dysbiosis of microbiota, which can be associated with the outgrowth of particular phyla and species. Molecular triggers from gut microbiota cross the epithelial boundary more readily under dysbiotic and/or inflammatory conditions than during homeostasis and might eventually influence bone at distal sites, either by entering systemic circulation or by activating local adaptive immune elements, such as T helper 17 (TH17) cells within the mucosal tissue that can transit to distal sites and therein exert their effector function. By contrast, stimuli from microbiota in the subgingival crest between the tooth and the gum act proximally on the alveolar bone that anchors teeth. Oral microbiota can also contribute to inflammatory bone loss at distal sites, such as the joints of patients with rheumatoid arthritis. Pathogen-associated molecular patterns (PAMPs) and microbiota-associated molecular patterns (MAMPs) trigger signalling pathways in innate immune cells and synovial fibroblasts that induce the production of pro-inflammatory cytokines and chemokines. These factors contribute to increased expression of receptor activator of nuclear factor-κB ligand (RANKL) and increased osteoclast differentiation and activation, which increase inflammatory bone loss at both distal sites and proximal sites.
Experimental models for microbiota research
Currently, there is limited evidence for the effects of microbiota on bone in a clinical context, but important advances in this field have been made in experimental animal studies50. The most direct, although somewhat resource-intensive, approach for determining the effects of microbiota on bone and bone cell–immune cell dynamics is to use gnotobiotic animals, such as germ-free mice, that are bred and housed in aseptic conditions so that their exposure to microbiota is extremely limited61,62. However, it is important to understand that mice kept in these conditions exhibit developmental and homeostatic irregularities that affect the immune system61,63,64, in particular in the Treg cell–TH17 cell balance that might be critical for controlling autoimmune conditions like RA65,66. Studies using germ-free mice have shown that the absence of microbiota affects bone growth; for example, mature female germ-free C57BL/6J mice have increased bone mass, decreased numbers of osteoclasts on the bone surface, decreased numbers of T cells in the bone marrow and decreased levels of TNF in the bone compared with mice kept under specific pathogen-free (SPF) conditions, results which the authors suggest provide a mechanistic explanation for microbiota-associated effects on the immune system affecting bone homeostasis67. Importantly, the observed phenotypes were reversed by treating germ-free mice with faecal microbiota from syngeneic SPF mice, suggesting that the presence of gut microbiota promotes bone resorption67.
To complement studies using germ-free mice, the treatment of SPF mice with antibiotics can be a useful means of demonstrating the effects of microbiota on bone. SPF mice treated with low-dose antibiotics experienced a significant, if temporary, increase in bone mineral density, suggesting that the presence of microbiota had a catabolic effect on bone68. By contrast, the results of another study suggested that microbiota actually promote bone growth, as newborn SPF mice were found to have a higher bone mass, cortical thickness and femur length than germ-free neonates69. In fact, microbiota can promote both bone growth and bone resorption; the key to determining the net effects of these processes might be related to the duration of exposure to microbiota70. In a study exploring this concept70, the production of insulin growth factor 1 (IGF1) by liver and adipose tissue was induced by microbiota, and was a key factor in promoting bone growth. Modulation of serum IGF1 levels could be observed in germ-free mice colonized with SPF microbiota whereas SPF mice had reduced IGF1 expression and associated bone formation. Interestingly, provision of antibiotic-treated mice with short-chain fatty acids (a microbial metabolite) restored bone phenotypes (as well as serum IGF1 levels) to those of mice not treated with antibiotics70. The results of this study70 that demonstrate of the effects of treatment with antibiotics or common metabolites might have important implications for the treatment of bone-related diseases.
Sex hormones, microbiota and bone
Sex hormone- related bone loss is a widespread problem associated with osteoporosis that is triggered during menopause; however, the mechanism(s) by which hormonal changes lead to increased bone formation, increased numbers of T cells or increased levels of macrophage activation are not well understood71. A 2016 study demonstrated a connection between sex hormone-related bone loss and microbiota-related inflammation by examining the effects of chemical castration on germ-free mice72. Although induced hypogonadism caused an inflammatory immune response characterized by increased levels of TNF, IL-1β, RANKL and the chemokine CCL2 that led to heightened osteoclast activity and bone loss in SPF mice, germ-free mice that received the same induced hypogonadism did not display these phenotypes72. Furthermore, oestrogen promoted intestinal integrity, which prevented inflammatory microbiota-associated factors from passing through gap junctions72. Notably, it seems that probiotics might act in a similar manner to oestrogen in promoting osteo-immune regulation by increasing bone formation through increased Treg cell activity, increased expression of transforming growth factor β and inhibition of inflammatory cytokine production72.
Oral microbiome
Studies that utilize gnotobiotic animals are useful for identifying how systematic alterations to the microbiota can indirectly affect bone that is not in direct contact with mucosal tissue. By contrast, the oral microbiome provides a direct example of the interactions between microbiota, mucosal surfaces and bone. Inflammatory diseases of the subgingival tissue include periodontitis and gingivitis, and the subgingival community of microbiota consists of typically more than 500 species of bacteria under normal conditions73. Periodontitis results in the destruction of the alveolar bone and periodontal ligaments that make up the structure of the teeth and jaw; osteoclast-mediated bone loss leads to erosion of these structures and to eventual tooth loss74,75. The key factors that control the progression and severity of periodontal disease are the production of chemokines and cytokines and the profile of immune cells that are recruited to the site of inflammation, which ultimately lead to a localized ratio of RANKL to OPG that is capable of increasing osteoclast differentiation and activity to pathogenic levels76.
Periodontal disease is thought to be triggered by species residing within the bacterial biofilm on the tooth surface, a community that is largely made up of Gram-negative anaerobic bacteria such as Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis75. Research suggests that periodontitis is the result of polymicrobial synergy and dysbiosis, in which disease is caused by and/or exacerbated by the synergistic activity of multiple bacterial species rather than the simple outgrowth of one or a few select species77,78. This model of pathogenesis is characterized by the ability of a few ‘keystone’ pathogens to not only modulate and impair the immune response, but to also enhance the virulence of pathogenic bacteria77. In particular, a group of bacteria known as the ‘red complex’, which contains P. gingivalis, Treponema denticola and Tannerella forsythia, is strongly associated with synergistic outgrowth at sites of inflammatory disease79. Population studies have identified varying frequencies of red complex species between different patient cohorts, making it somewhat unclear as to what the specific contributions or requirements for pathogenicity are for each species78. Studies have also been performed using gnotobiotic mice to determine the effects of systemic alterations to the microbiota on periodontal disease. A comparison of SPF mice and germ-free mice matched for sex and age showed that the germ-free mice had fewer RANKL+ and IL-17+ cells in the periodontium and less associated alveolar bone erosion than the SPF mice, which suggests that in the absence of inflammation or dysbiosis, the oral microbiota might still erode bone locally over time80. As understanding of the osteoimmune mechanisms of pathogenesis in periodontal disease grows, the research community is beginning to appreciate that periodontal disease might have systemic effects in other diseases with strong associations with microbiota and bone loss, such as RA81.
Innate immunity and bone loss
Innate immune signalling pathways
For cellular activators and promoters of osteoimmunologic bone loss, such as TH17 cells, to develop, typically there must first be an inflammatory trigger(s)82,83. Microbiota produce many molecular triggers of inflammation, such as PAMPs and MAMPs84, which initiate signalling pathways that can act either directly or indirectly (by inducing the production of inflammatory cytokines by myeloid cells) on bone. The ways in which microbiota-associated stimuli might initiate pathways that eventually lead to cell-mediated and cytokine-mediated pathology are currently of great interest. Innate immune signalling pathways have been intensely studied for the past few decades, and various types of pattern-recognition receptors have been identified that are specific for various PAMPs or MAMPs84. The best-characterized group of pattern-recognition receptors are the TLRs, which are expressed on innate immune cells and some tissue-resident cells, as well as on bone cells such as osteoclasts and pre-osteoclasts82. TLR ligands include various microbiota-derived components, including lipoprotein (TLR1, TLR2 and TLR6), lipopolysaccharide (TLR4), flagella (TLR5), and bacterial cytosine–phosphate–guanine DNA (TLR9), and can exert a diverse array of effects on bone and immune cell–bone interactions, primarily by increasing the expression of RANKL on various cells and inducing the production of pro-osteoclastogenic cytokines such as TNF and IL-1β84. In addition to de novo transcription of IL1B, IL-1β maturation is promoted by inflammasomes (intracellular protein complexes that activate the caspase-1 cascade), which are mainly found in myeloid cells, including osteoclasts, but which have also been detected in osteoblasts85–88. A variety of upstream activators of inflammasomes exist, including receptors that respond to external stimuli. One such class of stimulus is extracellular nucleotides, such as ATP, which might be present in the extracellular space as a result of tissue damage or as a by-product of microbiota growth and turnover89,90. ATP is also released by cultured osteoblastic cells via homeostatic mechanisms and as a result of mechanical loading91, and has been implicated in alveolar bone loss caused by oral microbiota-triggered inflammatory processes90,92. Dephosphorylation of extracellular ATP through bone cell-associated mechanisms produces ADP and AMP, which themselves regulate inflammatory processes in bone93.
Purinergic signalling pathways
Purine signalling is one of the most primitive intercellular signalling systems and was originally identified as a key factor in neuron biology, but is now recognized as an important pathway in immunity and inflammation94–96. Purines such as ATP, ADP and AMP bind to and signal through purinergic receptors that belong to two families: the P2X receptors (seven known family members), which are ligand-gated ion channels, and the P2Y receptors (eight known family members), which are G-protein coupled receptors97. In bone cells, as in other cells, purinergic receptor signalling is regulated, in part, through the heteromeric coupling of different family members, and through the regulation of expression of different family members, which exhibit distinct sensitivities for ligand binding92,97. Additionally, the conversion of ATP to ADP and AMP alters ligand binding and receptor avidity, thereby changing ion fluxes and potentially altering signalling outcomes that control biological functions.
In bone, the receptor P2X7 has been the most closely studied, although its role in bone remains incompletely understood. Reports suggest that P2X7 might be involved in regulating osteoclast cell fusion92; in one study, the use of various P2X7 antagonists showed a dose-dependent inhibition of the generation of multi-nucleated osteoclasts in vitro98. The results of another study suggested that P2X7 acted on osteoclast development by regulating ATP release99. In addition, P2X7 deficiency altered the response of bone to mechanical loading and fluid shear stress; the release of prostaglandin E2 in response to these forces was significantly reduced in P2rx7−/− mice100. These findings are important when viewed in the context of data showing that mechanical stress also regulates ATP release91. However, although differentiating osteoclasts lacking P2X7 showed defective fusion in vitro, P2rx7−/− mice exhibited normal osteoclast multi-nucleation in vivo101,102. Notably, researchers reported a decrease in total and cortical bone content in at least one strain of P2rx7−/− mice102. These findings suggested that the role for P2X7 in osteoclasts might be context-dependent, or that external factors or conditions might affect the role of P2X7 in bone biology. Differences between the bone cell phenotypes observed in vitro and in vivo in the context of P2X7 deficiency might also suggest that P2X7 has roles in multiple cell types, the physiologic interactions of which cannot be faithfully replicated in a culture dish.
In a 2017 study that utilized P2rx5−/− mice, the purinergic receptor P2X5 was shown to be required for the formation of large, multi-nucleated osteoclasts in vitro, and for lipopolysaccharide-induced inflammatory bone loss in vivo103. Interestingly, the expression of P2X5 was highly induced specifically during the maturation phase of osteoclast development103, suggesting that P2X5 might be a suitable target for preventing bone loss without affecting bone formation. Although P2X5 might not be a key purinergic receptor in inflammatory bone loss per se, this study103 further highlights the importance of purinergic pathway regulation in general for controlling inflammatory conditions (FIG. 3).
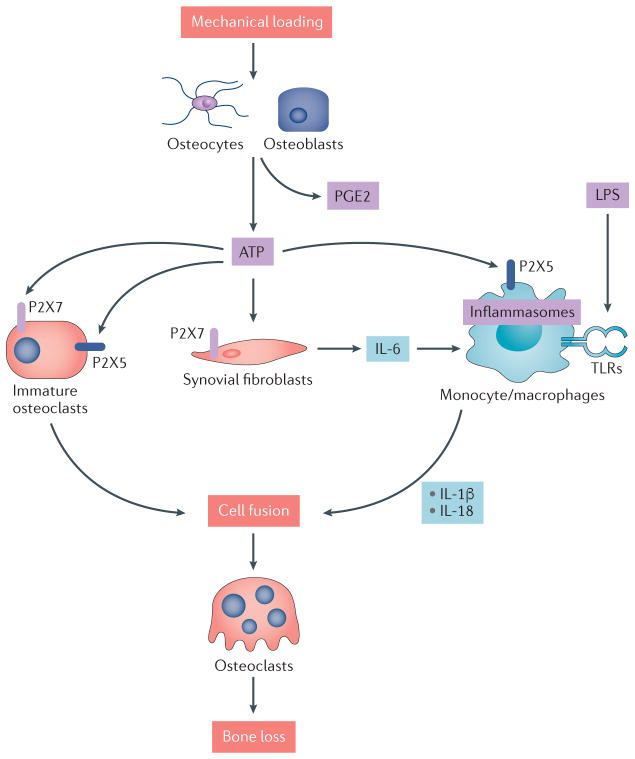
Figure 3. Purinergic signalling regulates bone cells and inflammation.
ATP signals through members of the P2X receptor family of ligand-gated ion channels. Mechanical loading regulates the release of ATP and prostaglandin E2 (PGE2) by osteoblasts and osteocytes. Extracellular ATP signals through P2X7 or P2X5 on immature osteoclasts, triggering cell fusion and resulting in multi-nucleated osteoclasts. ATP also triggers the production of pro inflammatory cytokines such as IL 6 by synovial fibroblasts, and can synergize with lipopolysaccharide (LPS) signalling through Toll like receptors (TLRs) to activate inflammasomes and produce mature forms of IL 1β and IL 18 that also promote osteoclast multi nucleation and maturation. In an experimental setting, LPS triggered inflammatory bone loss requires purinergic signalling through the P2X5 receptor.
Purinergic signalling pathways could possibly be a suitable treatment target for those patients with RA who do not respond well to currently available therapies. ATP signalling pathways are particularly important for the formation of the mature forms of IL-1β and IL-18, important factors in RA pathogenesis104,105. Ex vivo treatment of human blood with either ATP or the potassium ionophore nigericin resulted in increased levels of secreted IL-1β and IL-18, but not of TNF106. P2X7 is also expressed on synoviocytes from the joints of patients with RA, and P2X7 signalling regulates IL-6 release from these cells107. Seemingly, P2X7 signalling might also promote joint-related pathogenesis by controlling the release of cathepsins from macrophages, as shown in human and murine cells in vitro108. Regarding attempts thus far to specifically target P2X7 in the context of RA, the P2X7 antagonist AZD9056 ameliorated joint inflammation and bone destruction in streptococcal cell wall-induced arthritis in rats; however, clinical trials of P2X7 inhibitors in patients with RA have failed to show evidence of altered disease progression109–111. Therefore, although purinergic signalling might have an important role in the intersection between bone and inflammation, and might be relevant to specific conditions such as RA, it seems to be important to consider molecules that target P2X receptor pathways in general, and/or that can be combined with established treatments such as TNF inhibitors. Overall, the examples highlighted indicate that purinergic signalling pathways require additional study before their role in the regulation of inflammation and bone is fully understood.
Immune regulation by bone cells
Although interactions between bone and the immune system have been studied in depth in the context of maintenance of haematopoietic and/or lymphopoietic cells, as well as in inflammatory diseases and postmenopausal osteoporosis, the majority of discussions have focused on how adaptive immune responses affect bone. However, mounting evidence has revealed that the reverse also occurs: bone cells regulate immune cells, a concept that is consistent with the established role of the bone marrow in the development and homeostasis of the immune system. In this section, we focus on active regulation of immune cells by bone cells as an emerging feature of current investigations into osteoimmunology.
Maintenance of haematopoietic cells
Osteolineage cells arise from multipotent mesenchymal progenitor cells and eventually give rise to osteoblasts and osteocytes. Mature osteoblasts line the inner surface of bone and represent a mature direct derivative of mesenchymal reticular stromal cells. Although the primary function of the osteoblast is the secretion of a matrix that can be mineralized extracellularly, osteoblasts can also act as regulatory elements within the bone marrow in the maintenance of the haematopoietic system. Osteoblasts produce a number of cytokines that are implicated in haematopoietic stem cell (HSC) regulation, including granulocyte colony-stimulating factor (G-CSF), IL-1, IL-6, IL-7, thrombopoietin, angiopoietin 1 and the chemokine CXCL12 (REF. 112). Osteoblasts also express many types of adhesion molecules, such as vascular cell adhesion molecule 1 (VCAM1), intercellular adhesion molecule 1, annexin II, N-cadherin, CD44 and CD164, that might facilitate interactions within the HSC niche112. Although it has previously been suggested that osteoblasts fulfil their key role in the regulation of HSCs by providing a supportive niche for HSCs to develop, the current suggestion is that osteoblasts contribute in a limited way to HSC maintenance, as osteoblast numbers do not consistently correlate with numbers of HSCs113. In addition, conditional deletion of CXCL12 or Kit ligand (also known as stem cell factor) from mature osteoblasts had no effect on HSCs114–116. Furthermore, primitive osteolineage cells express higher levels of Kit ligand and CXCL12 and provide better support to HSCs than differentiated osteolineage cells117, suggesting that immature osteolineage cells are required for HSC maintenance.
Cumulative evidence indicates that signals derived from the mature osteoblastic niche are required for the maintenance of lymphoid progenitor cells and for balanced B cell and T cell generation. The dependence of B cell development on osteoblasts was established in vivo by the conditional ablation of osteoblast lineage cells in Col2.3Δtk transgenic mice118,119, which resulted in a loss of HSCs118 and a rapid reduction in the number of B cells119, demonstrating that osteoblasts support B cell commitment and differentiation from HSCs. Notably, this developmental process requires direct physical interaction between HSCs and osteoblasts via VCAM1 and local secretion of IL-7 and CXCL12. Parathyroid hormone (PTH) stimulates osteoblasts through its receptor PTH-related peptide receptor (PPR). Specific genetic deletion of the G-protein subunit GSα (a major downstream mediator of PPR signalling) in osteoblasts resulted in a decrease in the number of B cell precursors in the bone marrow, in addition to a decrease in trabecular bone120. The reduction of B cell precursors was attributed to a decreased production of IL-7 by osteoblasts120. These findings confirm that osteoblasts are part of a specific B cell niche within the bone marrow. The contribution of osteoblasts to T cell development has also been studied. Specific deletion of osteoblast lineage cells in osteocalcin-Cre or osterix-Cre transgenic mice crossed to iDTR mice (in which cell populations can be ablated by systemic diphtheria toxin administration) produced a marked reduction in progenitor cells capable of becoming T cells, and also of thymus-homing receptor expression among bone marrow haematopoietic cells121. The decreased number of T cell precursors, and subsequent decreased generation of mature T cells, was attributed to disrupted Notch receptor signalling through Delta-like protein 4, which is produced by osteoblast lineage cells121. Together, these reports demonstrate the importance of osteoblast lineage cells for lymphopoiesis in the bone marrow (FIG. 4).
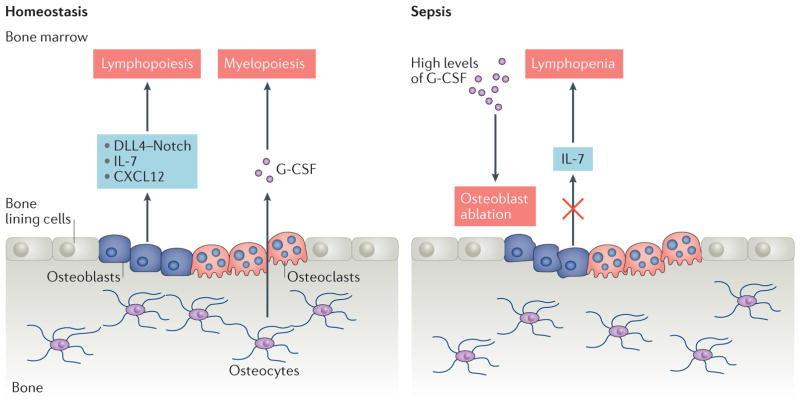
Figure 4. Interaction between immune cells and bone cells in homeostasis and sepsis.
Under homeostatic conditions, osteolineage cells (osteoblasts and osteocytes) provide niches for haematopoiesis, lymphopoiesis and myelopoiesis to occur by producing cytokines and chemokines. Osteoclasts contribute to the provision of a favourable niche by maintaining the bone marrow microenvironment. By contrast, sepsis induces aberrant production of granulocyte colony stimulating factor (G CSF), which suppresses mature osteoblasts, resulting in the depletion of IL 7, an important lymphopoietic factor, and leading to lymphopenia. CXCL12, CXC motif chemokine 12; DLL4, Delta like protein 4.
Osteocytes, another type of osteolineage cell, have also been implicated in the regulation of haematopoiesis. G-CSF is a haematopoietic cytokine with a well-characterized capacity to mobilize HSCs from the bone marrow to the blood. Mice that lack osteocytes or that have a disrupted osteocyte network have comparable numbers of HSCs in the bone marrow but fail to mobilize HSCs in response to G-CSF122, suggesting a role for osteocytes in creating a specific niche for HSCs. Osteocytes might also be involved in myelopoiesis. Specific genetic deletion of the G-protein subunit GSα in osteocytes caused increased G-CSF production by osteocytes, which resulted in dramatic expansion of cells of the myeloid lineage123, suggesting that osteocytes might contribute to the maintenance of bone homeostasis by providing a niche for myelopoiesis (FIG. 4).
Unlike osteoblasts and osteocytes, osteoclasts are derived from HSCs. Several reports have shown that osteoclasts are involved in the retention and mobilization of haematopoietic cells by maintaining bone microenvironments124,125, but are dispensable for HSC regulation126. However, it remains unclear whether osteoclasts are actively required for immune cell regulation.
Acute immune responses
Pathogenic viral and bacterial infections can disrupt bone homeostasis by perturbing osteoblast functions and triggering bone loss127,128. Although haematopoiesis and bone marrow dynamics are highly reactive to challenge with infectious agents, relatively few experimental studies have addressed how pathogenic infections and the ensuing inflammatory responses directly affect the composition, structure and function of immune cells in the bone marrow stroma.
Pathogenic insults can disrupt immune homeostasis by selectively affecting osteoblast survival. In a murine model of sepsis (a host inflammatory response to polymicrobial systemic infection that is associated with high mortality) cecal ligation and puncture induced the acute ablation of bone-lining osteoblasts and led to rapid bone loss129. Numbers of B cells, T cells and common lymphoid progenitor cells were decreased in these mice, whereas the numbers of long-term and short-term HSCs and common myeloid progenitor cells were increased129. This lymphopenia was caused by diminished IL-7 production by osteoblasts129, confirming that osteoblast-derived IL-7 has a crucial role in the regulation of lymphocyte development. Sepsis-induced acute ablation of osteoblasts is TLR-independent, but is mediated by G-CSF129, which is often induced during infection130. Interestingly, sepsis-induced lymphopenia can be ameliorated by the stimulation of osteoblasts with PTH, which induces IL-7 expression by osteoblasts129. These observations tentatively recommend osteoblasts as a potential therapeutic target in sepsis-induced lymphopenia (FIG. 4).
Tissue-specific RANKL
RANKL is produced by a variety of cell types in response to many different stimuli. Tissue-specific deletion of RANKL in mouse models reveals that RANKL exerts unique context-dependent effects depending on which cells it is expressed by. For example, lymphocytes, a source of RANKL, have important effects on bone, as demonstrated by sex hormone deficiency-induced bone loss (such as the bone loss that occurs after menopause or after gonadectomy). Ovariectomy, a mouse model of oestrogen deficiency, alters in vivo levels of RANKL by inducing increased numbers of RANKL+ lymphocytes131. Although both T cells and B cells are reported to be involved in such bone loss, the functional relevance of lymphocyte-derived RANKL in this bone loss is controversial. In mouse models of bone loss, T cells augment osteoclastogenesis as a peripheral effect of increased T cell proliferation and production of TNF and, possibly, RANKL132,133; however, these results have yet to be fully corroborated134. Increased production of RANKL by T cells also occurs in postmenopausal women135,136. In addition, ablation of RANKL specifically in B cells or T cells using conditional knockout mice has revealed that RANKL produced by B cells, but not T cells, contributes to bone loss by inducing osteoclastogene-sis137 (FIG. 5). Reportedly, a lack of mature B cells does not prevent bone loss138, which suggests that RANKL derived from immature B cells, as opposed to mature B cells, might be the key contributor to excess generation of osteoclasts in the context of oestrogen deficiency. Additional studies are required to clarify the specific requirements for lymphocyte-derived RANKL in oestrogen deficiency-induced bone loss. Notably, deletion of RANKL from T cells does not alter the abundance of T cells in the bone marrow, whereas the deletion of RANKL from B cells does alter the abundance of B cells in the bone marrow137, suggesting that RANKL functions in an autocrine manner to promote B cell maturation. However, B cells develop normally in mice that have B cell-specific RANK deficiency139. Further studies are required to clarify the role of RANK signalling in the maintenance of the B cell compartment.
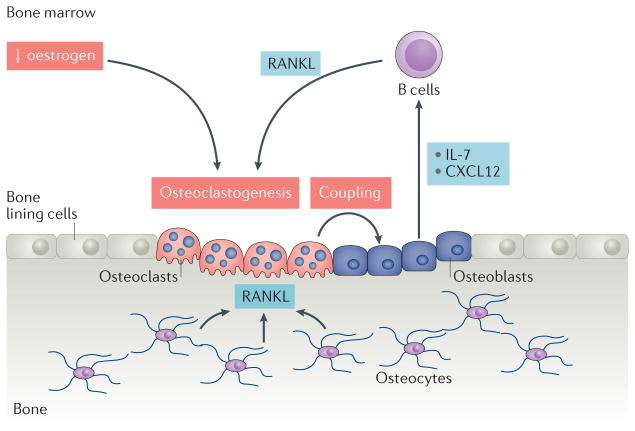
Figure 5. The effects of tissue-specific RANKL on bone and the immune system.
Sex hormone deficiency induces aberrant osteoclastogenesis owing to a lack | of inhibition provided by oestrogen. Increased levels of osteoclastogenesis lead to enhanced osteoblastic activity via coupling, a homeostatic process in which bone resorption and new bone formation are temporally and spatially coordinated, which results in the production of chemokines and cytokines that increase the generation of B cells. B cell derived receptor activator of nuclear factor κB ligand (RANKL) can further enhance osteoclastogenesis. CXCL12, CXC motif chemokine 12.
Osteocytes are an important source of RANKL for osteoclastogenesis required for bone remodelling under physiological conditions140,141. Osteocyte-derived RANKL participates in oestrogen deficiency-induced bone loss by indirectly regulating B cell development142, and is required for osteoclastogenesis not only in intact mice, but also in mice that have undergone ovariectomy. Increased numbers of osteoclasts enhance osteoblast activities through a phenomenon known as coupling, and the activated osteoblasts provide factors such as IL-7 and CXCL12 that support B cell development (FIG. 5). Mouse models of oestrogen deficiency are revealing a complex set of interactions between the skeletal and haematopoietic systems that require further investigation.
In RA, RANKL+ T cells extensively infiltrate the inflamed synovium, thereby contributing to the pathogenesis of RA by enhancing osteoclastogenic activity23,26,143. However, several reports suggest that T cell-derived RANKL might not be directly involved in osteoclastogenesis28,46,144. Synovial fibroblasts are another source of RANKL in the joints of patients with RA22,24. A 2016 study showed that synovial fibroblast-derived RANKL is predominantly responsible for the formation of osteoclasts145. Specific genetic deletion of RANKL in T cells or synovial fibroblasts using Lck-Cre mice or Col6a1-Cre mice, respectively, revealed that T cell-derived RANKL is of relatively minor importance compared with synovial fibroblast-derived RANKL in bone erosion associated with collagen antibody-induced arthritis145. These results identify synovial fibroblast-derived RANKL as a primary driver of osteoclastogenesis in RA (FIG. 1); however, whether bone cells actively affect immune cells during the progression of RA, and whether these relationships can be targeted therapeutically, requires further investigation.
Conclusions
In this Review, we have focused on selected areas in which we believe that important advances have been made in our collective understanding of the relationship between bone and the immune system in the context of homeostasis and inflammation. Although the RANK–RANKL–OPG signalling axis remains the foundation for understanding bone cell–immune cell crosstalk, much important new work has involved the characterization of additional stimuli and signalling pathways that act either independently of or in concert with RANK. We have chosen to focus on purinergic and innate signalling pathways, but advances have been made in other signalling pathways that should be considered as well, particularly in the area of immune receptor-mediated regulation of bone146.
Going forward, it will be interesting to further investigate the relationship between immune receptor tyrosine-based activation motif (ITAM)-related signalling146 and innate signalling pathways (such as TLRs and P2X receptors) to determine how additional potential modes of cross-modulation might affect bone homeostasis or disease. It would also be interesting to investigate how the effects of these pathways integrate with the effects of microbiota on bone.
With respect to microbiota and osteoimmunology, in the future it will be important to connect what has been learned using animal models to conditions experienced by patients with bone-related diseases. In particular, it will be of interest to understand how dysbiosis of the microbiota in a distal area, such as the gut or the mouth, can affect bone health in the joints. This area of research is becoming more relevant as we begin to understand the various biological effects that microbiota-associated molecular factors such as TLR ligands and extracellular ATP can have on inflammation, immune cell development and bone homeostasis, especially considering that bone cells, such as osteoblasts, exert control over immune cell development and homeostasis in response to infection and sepsis. The picture of the osteoimmune system and the biological relationships it encompasses is becoming more complex, but with this complexity comes more opportunities to uncover previously unconsidered relationships between localized disruptions in bone and joint health and processes in distant parts of the body.
Key points.
Multiple elements of the adaptive immune system, including B cells and T cells, contribute to autoimmunity-associated bone pathology
Commensal microbiota can affect bone through involvement in immune responses at sites proximal and distal to bone
Various innate immune triggers such as nucleotides can lead to pathologic bone loss
Bone cells actively regulate immune cell maintenance and acute immune responses
Tissue-specific receptor activator of nuclear factor-κB ligand (RANKL) exerts unique effects on bone and the immune system
Acknowledgments
The work of the authors was supported in part by NIH grants AR069546, AR067726, AI64909, and AI125284 awarded to Y.C.
Footnotes
Author contributions
All authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and reviewed and/or edited the manuscript before submission.
Competing interests statement
The authors declare no competing interests.
References
- 1.Arron JR, Choi Y. Bone versus immune system. Nature. 2000;408:535–536. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- 2.Walsh MC, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 3.Yu VW, Scadden DT. Hematopoietic stem cell and its bone marrow niche. Curr Top Dev Biol. 2016;118:21–44. doi: 10.1016/bs.ctdb.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ash P, Loutit JF, Townsend KM. Osteoclasts derived from haematopoietic stem cells. Nature. 1980;283:669–670. doi: 10.1038/283669a0. [DOI] [PubMed] [Google Scholar]
- 5.Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014;5:511. doi: 10.3389/fimmu.2014.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takayanagi H. New developments in osteoimmunology. Nat Rev Rheumatol. 2012;8:684–689. doi: 10.1038/nrrheum.2012.167. [DOI] [PubMed] [Google Scholar]
- 7.Fuller K, Wong B, Fox S, Choi Y, Chambers TJ. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med. 1998;188:997–1001. doi: 10.1084/jem.188.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong YY, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 9.Lacey DL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 10.Simonet WS, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda H, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostenuik PJ. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol. 2005;5:618–625. doi: 10.1016/j.coph.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Gul G, Sendur MA, Aksoy S, Sever AR, Altundag K. A comprehensive review of denosumab for bone metastasis in patients with solid tumors. Curr Med Res Opin. 2016;32:133–145. doi: 10.1185/03007995.2015.1105795. [DOI] [PubMed] [Google Scholar]
- 14.Zaheer S, LeBoff M, Lewiecki EM. Denosumab for the treatment of osteoporosis. Expert Opin Drug Metab Toxicol. 2015;11:461–470. doi: 10.1517/17425255.2015.1000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boleto G, Drame M, Lambrecht I, Eschard JP, Salmon JH. Disease-modifying anti-rheumatic drug effect of denosumab on radiographic progression in rheumatoid arthritis: a systematic review of the literature. Clin Rheumatol. 2017;36:1699–1706. doi: 10.1007/s10067-017-3722-6. [DOI] [PubMed] [Google Scholar]
- 16.Crotti TN, Dharmapatni AA, Alias E, Haynes DR. Osteoimmunology: major and costimulatory pathway expression associated with chronic inflammatory induced bone loss. J Immunol Res. 2015;2015:281287. doi: 10.1155/2015/281287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginaldi L, De Martinis M. Osteoimmunology and beyond. Curr Med Chem. 2016;23:3754–3774. doi: 10.2174/0929867323666160907162546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones D, Glimcher LH, Aliprantis AO. Osteoimmunology at the nexus of arthritis, osteoporosis, cancer, and infection. J Clin Invest. 2011;121:2534–2542. doi: 10.1172/JCI46262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komatsu N, Takayanagi H. Inflammation and bone destruction in arthritis: synergistic activity of immune and mesenchymal cells in joints. Front Immunol. 2012;3:77. doi: 10.3389/fimmu.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catrina AI, Svensson CI, Malmstrom V, Schett G, Klareskog L. Mechanisms leading from systemic autoimmunity to joint-specific disease in rheumatoid arthritis. Nat Rev Rheumatol. 2017;13:79–86. doi: 10.1038/nrrheum.2016.200. [DOI] [PubMed] [Google Scholar]
- 21.Komatsu N, Takayanagi H. Arthritogenic T cells in autoimmune arthritis. Int J Biochem Cell Biol. 2015;58:92–96. doi: 10.1016/j.biocel.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Gravallese EM, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–258. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Kong YY, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 24.Takayanagi H, et al. Involvement of receptor activator of nuclear factor κB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43:259–269. doi: 10.1002/1529-0131(200002)43:2<259::AID-ANR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 25.Tunyogi-Csapo M, et al. Cytokine-controlled RANKL and osteoprotegerin expression by human and mouse synovial fibroblasts: fibroblast-mediated pathologic bone resorption. Arthritis Rheum. 2008;58:2397–2408. doi: 10.1002/art.23653. [DOI] [PubMed] [Google Scholar]
- 26.Horwood NJ, et al. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun. 1999;265:144–150. doi: 10.1006/bbrc.1999.1623. [DOI] [PubMed] [Google Scholar]
- 27.Rudd CE, Raab M. Independent CD28 signaling via VAV and SLP-76: a model for in trans costimulation. Immunol Rev. 2003;192:32–41. doi: 10.1034/j.1600-065x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- 28.Takayanagi H, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 29.Kim YG, et al. Human CD4+CD25+ regulatory T cells inhibit the differentiation of osteoclasts from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2007;357:1046–1052. doi: 10.1016/j.bbrc.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 30.Zaiss MM, et al. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. 2007;56:4104–4112. doi: 10.1002/art.23138. [DOI] [PubMed] [Google Scholar]
- 31.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009;5:667–676. doi: 10.1038/nrrheum.2009.217. [DOI] [PubMed] [Google Scholar]
- 32.Amara K, et al. Monoclonal IgG antibodies generated from joint-derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition. J Exp Med. 2013;210:445–455. doi: 10.1084/jem.20121486. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Krishnamurthy A, et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann Rheum Dis. 2016;75:721–729. doi: 10.1136/annrheumdis-2015-208093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harre U, et al. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat Commun. 2015;6:6651. doi: 10.1038/ncomms7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Negishi-Koga T, et al. Immune complexes regulate bone metabolism through FcRγ signalling. Nat Commun. 2015;6:6637. doi: 10.1038/ncomms7637. [DOI] [PubMed] [Google Scholar]
- 36.Kouskoff V, et al. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 37.Ranges GE, Sriram S, Cooper SM. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985;162:1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang YM, et al. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med. 2002;195:1325–1336. doi: 10.1084/jem.20011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacDonald KP, Nishioka Y, Lipsky PE, Thomas R. Functional CD40 ligand is expressed by T cells in rheumatoid arthritis. J Clin Invest. 1997;100:2404–2414. doi: 10.1172/JCI119781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 41.Lubberts E, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 42.Kotake S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lubberts E, et al. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-κB ligand/osteoprotegerin balance. J Immunol. 2003;170:2655–2662. doi: 10.4049/jimmunol.170.5.2655. [DOI] [PubMed] [Google Scholar]
- 44.Hirota K, et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ TH cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato K, et al. TH17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 47.Komatsu N, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 48.Belkaid Y, Harrison OJ. Homeostatic immunity and the microbiota. Immunity. 2017;46:562–576. doi: 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kramer CD, Genco CA. Microbiota, immune subversion, and chronic inflammation. Front Immunol. 2017;8:255. doi: 10.3389/fimmu.2017.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernandez CJ, Guss JD, Luna M, Goldring SR. Links between the microbiome and bone. J Bone Miner Res. 2016;31:1638–1646. doi: 10.1002/jbmr.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van de Wiele T, Van Praet JT, Marzorati M, Drennan MB, Elewaut D. How the microbiota shapes rheumatic diseases. Nat Rev Rheumatol. 2016;12:398–411. doi: 10.1038/nrrheum.2016.85. [DOI] [PubMed] [Google Scholar]
- 52.Kasagi S, Chen W. TGF-β1 on osteoimmunology and the bone component cells. Cell Biosci. 2013;3:4. doi: 10.1186/2045-3701-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nemeth K, et al. The role of osteoclast-associated receptor in osteoimmunology. J Immunol. 2011;186:13–18. doi: 10.4049/jimmunol.1002483. [DOI] [PubMed] [Google Scholar]
- 54.Proctor LM. The National Institutes of Health Human Microbiome Project. Semin Fetal Neonatal Med. 2016;21:368–372. doi: 10.1016/j.siny.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Charbonneau MR, et al. A microbial perspective of human developmental biology. Nature. 2016;535:48–55. doi: 10.1038/nature18845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fraiture M, Brunner F. Killing two birds with one stone: trans-kingdom suppression of PAMP/MAMP-induced immunity by T3E from enteropathogenic bacteria. Front Microbiol. 2014;5:320. doi: 10.3389/fmicb.2014.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:569–578. doi: 10.1038/nrrheum.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu HJ, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Asmakh M, Zadjali F. Use of germ-free animal models in microbiota-related research. J Microbiol Biotechnol. 2015;25:1583–1588. doi: 10.4014/jmb.1501.01039. [DOI] [PubMed] [Google Scholar]
- 62.Faith JJ, et al. Creating and characterizing communities of human gut microbes in gnotobiotic mice. ISME J. 2010;4:1094–1098. doi: 10.1038/ismej.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Tanoue T, Honda K. Induction of Treg cells in the mouse colonic mucosa: a central mechanism to maintain host-microbiota homeostasis. Semin Immunol. 2012;24:50–57. doi: 10.1016/j.smim.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Alunno A, et al. Altered immunoregulation in rheumatoid arthritis: the role of regulatory T cells and proinflammatory TH17 cells and therapeutic implications. Mediators Inflamm. 2015;2015:751793. doi: 10.1155/2015/751793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Min YW, Rhee PL. The role of microbiota on the gut immunology. Clin Ther. 2015;37:968–975. doi: 10.1016/j.clinthera.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 67.Sjögren K, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27:1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cho I, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwarzer M, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351:854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 70.Yan J, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA. 2016;113:E7554–E7563. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iqbal J, Zaidi M. Understanding estrogen action during menopause. Endocrinology. 2009;150:3443–3445. doi: 10.1210/en.2009-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li JY, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126:2049–2063. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paster BJ, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costalonga M, Herzberg MC. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett. 2014;162:22–38. doi: 10.1016/j.imlet.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tompkins KA. The osteoimmunology of alveolar bone loss. Connect Tissue Res. 2016;57:69–90. doi: 10.3109/03008207.2016.1140152. [DOI] [PubMed] [Google Scholar]
- 76.Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 2005;76:2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 77.Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27:409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silva N, et al. Host response mechanisms in periodontal diseases. J Appl Oral Sci. 2015;23:329–355. doi: 10.1590/1678-775720140259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 80.Irie K, Novince CM, Darveau RP. Impact of the oral commensal flora on alveolar bone homeostasis. J Dental Res. 2014;93:801–806. doi: 10.1177/0022034514540173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13:606–620. doi: 10.1038/nrrheum.2017.132. [DOI] [PubMed] [Google Scholar]
- 82.Charles JF, Nakamura MC. Bone and the innate immune system. Curr Osteoporos Rep. 2014;12:1–8. doi: 10.1007/s11914-014-0195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elshabrawy HA, Essani AE, Szekanecz Z, Fox DA, Shahrara S. TLRs, future potential therapeutic targets for RA. Autoimmun Rev. 2017;16:103–113. doi: 10.1016/j.autrev.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bar-Shavit Z. Taking a toll on the bones: regulation of bone metabolism by innate immune regulators. Autoimmunity. 2008;41:195–203. doi: 10.1080/08916930701694469. [DOI] [PubMed] [Google Scholar]
- 85.Bonar SL, et al. Constitutively activated NLRP3 inflammasome causes inflammation and abnormal skeletal development in mice. PLoS ONE. 2012;7:e35979. doi: 10.1371/journal.pone.0035979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCall SH, et al. Osteoblasts express NLRP3, a nucleotide-binding domain and leucine-rich repeat region containing receptor implicated in bacterially induced cell death. J Bone Miner Res. 2008;23:30–40. doi: 10.1359/JBMR.071002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qu C, et al. NLRP3 mediates osteolysis through inflammation-dependent and -independent mechanisms. FASEB J. 2015;29:1269–1279. doi: 10.1096/fj.14-264804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Atarashi K, et al. ATP drives lamina propria TH17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 90.Binderman I, Gadban N, Yaffe A. Extracellular ATP is a key modulator of alveolar bone loss in periodontitis. Arch Oral Biol. 2017;81:131–135. doi: 10.1016/j.archoralbio.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 91.Turner CH. Bone strength: current concepts. Ann NY Acad Sci. 2006;1068:429–446. doi: 10.1196/annals.1346.039. [DOI] [PubMed] [Google Scholar]
- 92.Rumney RM, Wang N, Agrawal A, Gartland A. Purinergic signalling in bone. Front Endocrinol. 2012;3:116. doi: 10.3389/fendo.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strazzulla LC, Cronstein BN. Regulation of bone and cartilage by adenosine signaling. Purinergic Signal. 2016;12:583–593. doi: 10.1007/s11302-016-9527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burnstock G, Boeynaems JM. Purinergic signalling and immune cells. Purinergic Signal. 2014;10:529–564. doi: 10.1007/s11302-014-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16:177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 96.Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal. 2009;2:pe6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 97.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Agrawal A, et al. The effects of P2X7 receptor antagonists on the formation and function of human osteoclasts in vitro. Purinergic Signal. 2010;6:307–315. doi: 10.1007/s11302-010-9181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pellegatti P, Falzoni S, Donvito G, Lemaire I, Di Virgilio F. P2X7 receptor drives osteoclast fusion by increasing the extracellular adenosine concentration. FASEB J. 2011;25:1264–1274. doi: 10.1096/fj.10-169854. [DOI] [PubMed] [Google Scholar]
- 100.Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem. 2005;280:42952–42959. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- 101.Gartland A, et al. Multinucleated osteoclast formation in vivo and in vitro by P2X7 receptor-deficient mice. Crit Rev Eukaryot Gene Expr. 2003;13:243–253. doi: 10.1615/critreveukaryotgeneexpr.v13.i24.150. [DOI] [PubMed] [Google Scholar]
- 102.Ke HZ, et al. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 103.Kim H, et al. The purinergic receptor P2X5 regulates inflammasome activity and hyper-multinucleation of murine osteoclasts. Sci Rep. 2017;7:196. doi: 10.1038/s41598-017-00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giacomelli R, et al. IL-1β at the crossroad between rheumatoid arthritis and type 2 diabetes: may we kill two birds with one stone? Expert Rev Clin Immunol. 2016;12:849–855. doi: 10.1586/1744666X.2016.1168293. [DOI] [PubMed] [Google Scholar]
- 105.Gracie JA, et al. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104:1393–1401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perregaux DG, McNiff P, Laliberte R, Conklyn M, Gabel CA. ATP acts as an agonist to promote stimulus-induced secretion of IL-1β and IL-18 in human blood. J Immunol. 2000;165:4615–4623. doi: 10.4049/jimmunol.165.8.4615. [DOI] [PubMed] [Google Scholar]
- 107.Caporali F, et al. Human rheumatoid synoviocytes express functional P2X7 receptors. J Mol Med (Berl) 2008;86:937–949. doi: 10.1007/s00109-008-0365-8. [DOI] [PubMed] [Google Scholar]
- 108.Lopez-Castejon G, et al. P2X7 receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases. J Immunol. 2010;185:2611–2619. doi: 10.4049/jimmunol.1000436. [DOI] [PubMed] [Google Scholar]
- 109.Chen J, Zhao Y, Liu Y. The role of nucleotides and purinergic signaling in apoptotic cell clearance — implications for chronic inflammatory diseases. Front Immunol. 2014;5:656. doi: 10.3389/fimmu.2014.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Keystone EC, et al. Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann Rheum Dis. 2012;71:1630–1635. doi: 10.1136/annrheumdis-2011-143578. [DOI] [PubMed] [Google Scholar]
- 111.Stock TC, et al. Efficacy and safety of CE-224,535, an antagonist of P2X7 receptor, in treatment of patients with rheumatoid arthritis inadequately controlled by methotrexate. J Rheumatol. 2012;39:720–727. doi: 10.3899/jrheum.110874. [DOI] [PubMed] [Google Scholar]
- 112.Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2011;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lymperi S, et al. Strontium can increase some osteoblasts without increasing hematopoietic stem cells. Blood. 2008;111:1173–1181. doi: 10.1182/blood-2007-03-082800. [DOI] [PubMed] [Google Scholar]
- 114.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Greenbaum A, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakamura Y, et al. Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood. 2010;116:1422–1432. doi: 10.1182/blood-2009-08-239194. [DOI] [PubMed] [Google Scholar]
- 118.Visnjic D, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 119.Zhu J, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 120.Wu JY, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gsα-dependent signaling pathways. Proc Natl Acad Sci USA. 2008;105:16976–16981. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu VW, et al. Specific bone cells produce DLL4 to generate thymus-seeding progenitors from bone marrow. J Exp Med. 2015;212:759–774. doi: 10.1084/jem.20141843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Asada N, et al. Matrix-embedded osteocytes regulate mobilization of hematopoietic stem/progenitor cells. Cell Stem Cell. 2013;12:737–747. doi: 10.1016/j.stem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 123.Fulzele K, et al. Myelopoiesis is regulated by osteocytes through Gsα-dependent signaling. Blood. 2013;121:930–939. doi: 10.1182/blood-2012-06-437160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mansour A, et al. Osteoclast activity modulates B-cell development in the bone marrow. Cell Res. 2011;21:1102–1115. doi: 10.1038/cr.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kollet O, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 126.Miyamoto K, et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med. 2011;208:2175–2181. doi: 10.1084/jem.20101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen W, et al. Arthritogenic alphaviral infection perturbs osteoblast function and triggers pathologic bone loss. Proc Natl Acad Sci USA. 2014;111:6040–6045. doi: 10.1073/pnas.1318859111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tang TT, Zhang L, Bansal A, Grynpas M, Moriatry TJ. The Lyme disease pathogen Borrelia burgdorferi infects murine bone and induces trabecular bone loss. Infect Immun. 2017;85:e00781–16. doi: 10.1128/IAI.00781-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Terashima A, et al. Sepsis-induced osteoblast ablation causes immunodeficiency. Immunity. 2016;44:1434–1443. doi: 10.1016/j.immuni.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 130.Day RB, Bhattacharya D, Nagasawa T, Link DC. Granulocyte colony-stimulating factor reprograms bone marrow stromal cells to actively suppress B lymphopoiesis in mice. Blood. 2015;125:3114–3117. doi: 10.1182/blood-2015-02-629444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kanematsu M, et al. Prostaglandin E2 induces expression of receptor activator of nuclear factor-κB ligand/osteoprotegrin ligand on pre-B cells: implications for accelerated osteoclastogenesis in estrogen deficiency. J Bone Miner Res. 2000;15:1321–1329. doi: 10.1359/jbmr.2000.15.7.1321. [DOI] [PubMed] [Google Scholar]
- 132.Cenci S, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-α. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roggia C, et al. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee SK, et al. T lymphocyte-deficient mice lose trabecular bone mass with ovariectomy. J Bone Miner Res. 2006;21:1704–1712. doi: 10.1359/jbmr.060726. [DOI] [PubMed] [Google Scholar]
- 135.D’Amelio P, et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43:92–100. doi: 10.1016/j.bone.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 136.Eghbali-Fatourechi G, et al. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–1230. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Onal M, et al. Receptor activator of nuclear factor κB ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J Biol Chem. 2012;287:29851–29860. doi: 10.1074/jbc.M112.377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li Y, Li A, Yang X, Weitzmann MN. Ovariectomy-induced bone loss occurs independently of B cells. J Cell Biochem. 2007;100:1370–1375. doi: 10.1002/jcb.21121. [DOI] [PubMed] [Google Scholar]
- 139.Perlot T, Penninger JM. Development and function of murine B cells lacking RANK. J Immunol. 2012;188:1201–1205. doi: 10.4049/jimmunol.1102063. [DOI] [PubMed] [Google Scholar]
- 140.Xiong J, et al. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakashima T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 142.Fujiwara Y, et al. RANKL (receptor activator of NF-κB ligand) produced by osteocytes is required for the increase in B cells and bone loss caused by estrogen deficiency in mice. J Biol Chem. 2016;291:24838–24850. doi: 10.1074/jbc.M116.742452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. Requirement of pp60c-Src expression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Invest. 1992;90:1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ishimi Y, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–3303. [PubMed] [Google Scholar]
- 145.Danks L, et al. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann Rheumat Diseases. 2016;75:1187–1195. doi: 10.1136/annrheumdis-2014-207137. [DOI] [PubMed] [Google Scholar]
- 146.Humphrey MB, Nakamura MC. A comprehensive review of immunoreceptor regulation of osteoclasts. Clin Rev Allergy Immunol. 2016;51:48–58. doi: 10.1007/s12016-015-8521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]