Abstract
Objective
Body mass index (BMI) is a widely used indicator of obesity status in clinical settings and population health research. However, there are concerns about the validity of BMI as a measure of obesity in post-menopausal women. Unlike BMI, which is an indirect measure of obesity and does not distinguish lean from fat mass, dual energy x-ray absorptiometry (DXA) provides a direct measure of body fat and is considered a gold standard of adiposity measurement. The goal of this study is to examine the validity of using BMI to identify obesity in post-menopausal women relative to total body fat percent measured by DXA scan.
Methods
Data from 1329 post-menopausal women participating in the Buffalo OsteoPerio study were used in this analysis. At baseline, women ranged in age from 53 years to 85 years. Obesity was defined as BMI >30kg/m2 and body fat percent (BF%) greater than 35%, 38% or 40%. We calculated sensitivity, specificity, positive predictive value and negative predictive value to evaluate the validity of BMI defined obesity relative BF%. We further explored the validity of BMI relative to percent body fat using graphical tools, such as scatterplots and receiver operating characteristic (ROC) curves. Youden’s J index was used to determine the empirical optimal BMI cut point for each level of BF% defined obesity.
Results
The sensitivity of BMI-defined obesity was 32.4% for 35% body fat, 44.6% for 38% body fat and 55.2% for 40% body fat. Corresponding specificity values were 99.3%, 97.1% and 94.6%, respectively. The empirical optimal BMI cut-point to define obesity is 24.9kg/m2 for 35% BF, 26.49 kg/m2 for 38% BF and 27.05 kg/m2 for 40% BF according to the Youden Index.
Conclusions
Results demonstrate that a BMI cut-point of 30 kg/m2 does not appear to be an appropriate indicator of true obesity status in post-menopausal women. Empirical estimates of the validity of BMI from this study may be used by other investigators to account for BMI-related misclassification in older women.
Keywords: obesity, postmenopausal women, validation study, BMI, body fat
Introduction
Over the past three decades, the prevalence of obesity has increased substantially across all age groups.1,2 Although the effect of obesity on morbidity and mortality in childhood and middle age has been studied extensively, there is still a debate about the health risks of obesity at older ages.3–5 Questions about the effect of obesity in older adults are particularly salient in postmenopausal women because in the years following the menopausal transition women often experience changes in body composition.6–8 Before it is possible to answer questions about the effect of obesity in postmenopausal women, it is crucial to empirically validate tools to measure excess body weight in this population.
Body mass index (BMI) is the most widely used indicator of obesity status in clinical settings and population health research. According to the World Health Organization, BMI greater than 30kg/m2 is considered obese. Despite its widespread use in the general population, several authors have questioned whether BMI is a valid measure of obesity status in older adults.9–11 Prior studies indicate that BMI may be particularly limited as a measure of obesity status in older adults.12,13 BMI is an indirect measure of adiposity, and does not account for the location of adipose tissue (e.g., subcutaneous vs. visceral fat), differentiate between fat mass or lean mass, or account for variation in body composition.11,14,15
Concerns about the validity of BMI to correctly categorize obesity status may be amplified in older women. It is well documented that significant physical changes occur in the post-menopausal period, including body weight changes, redistribution of adipose tissue, decreased skeletal muscle mass, and loss of height.16–18 Evidence suggests that menopause contributes to a change in body composition, such as an accumulation of visceral fat, irrespective of body weight change.17,19 As an example, consider a woman who loses height as she ages. Since BMI is a ratio of weight-for-height, age-related height loss would result in an increased BMI value by virtue of decreasing the value of the denominator, even if body weight remained constant.18 Additionally, post-menopausal women lose bone mineral density over time, which may lead to reduced overall body weight, smaller numerator and overall BMI value, even if the amount of fat mass remains the same.20 Considering the changes in body composition that occur as part of the natural aging process, it is unlikely that BMI corresponds to the same degree of adiposity in young or middle aged women and older postmenopausal women and use of BMI in this population may result in misclassification of obesity status and an inaccurate representation of obesity-related risks.18
Aside from BMI, there are several other indirect measures of adiposity that are often reported in the literature, such as waist circumference and waist to hip ratio, but each has its own set of limitations. Ultimately, no indirect measure of adiposity is able to differentiate between adipose tissue, lean body mass, and bone mass. Direct measures of adiposity, such as dual energy x-ray absorptiometry (DXA) scan, provide a much more accurate measurement of body fat, but require access to expensive specialized equipment and skilled technicians.21,22 Validation studies have indicated that body fat percent (BF%) from DXA scan is an appropriate reference standard, or gold standard, for the measurement of adiposity.12,21–23
The objective of the present study is to explore the relationship between BMI and percent body fat (BF%) in post-menopausal women and examine the validity of using a BMI cut-point of 30kg/m2 to identify obesity in post-menopausal women relative to body fat percentage (BF%) measured by DXA scan.
Methods
Study data
Participants were 1329 post-menopausal women aged 53–85 years in the Buffalo OsteoPerio study, an ancillary study to the Women’s Health Initiative (WHI).24 All OsteoPerio participants were also in the WHI observational study and recruited from the WHI Buffalo Clinical Center.24 Between 1997 and 2000, participants completed baseline OsteoPerio questionnaires and visited the clinical center for anthropometric measures, including weight and height, as well as a dual energy x-ray absorptiometry (DXA) scan with a Hologic QDR-4500A dual x-ray bone densitometer (Hologic Inc, Bedford, MA). Participants returned for 5-year follow-up visits from 2002 to 2005 (n=1,013) and 17-year follow-up is currently underway (n=390 available for the present analysis). Six particpants were missing BMI (1) or BF% (5) Anthropometric measures and DXA scans were measured at baseline and repeated at follow-up visits. This analysis includes data collected at both baseline and follow-up visits, resulting in an analytic cohort of 2726 observations.
Whole body DXA scan was used to measure body composition (fat mass, fat distribution, lean mass) as well as total body and site-specific bone density (hip, spine, wrist). Positioning and analysis of DXA scan results were performed according to standard protocol by trained technicians. Prior to the scan, participants were asked to change into medical gowns and asked to remove all jewelery.22 The validity and precision of the DXA measurements used in the WHI study has been reported elsewhere22. Chen and colleagues demonstrated that DXA-derived measures of fat mass are highly correlated with results from MRI scan (fat mass: Pearson’s correlation coefficient=0.94; lean mass: Pearson’s correlation coefficient=0.99).16,22 From the DXA scan, whole body fat percentage was calculated as total body fat mass over total mass multiplied by 100.25,26
Body Mass Index
Measured height and weight were used to calculate BMI. Having a BMI value greater than 30 kg/m2 indicates obesity, while individuals with BMI value less than 30 kg/m2 are considered non-obese.8 This cut point is widely accepted in the biomedical literature to define obesity and also is used in clinical settings. It has been endorsed by the World Health Organization, Centers for Disease Control, National Institutes of Health, and various other disease-specific organizations.8,27–29
Percent body fat (BF%)
Unlike BMI, there is a lack of consensus regarding a cut point that should be used to define obesity according to BF%. In women, a BF% greater than 35% is frequently used as the cut point to define obesity, but this is largely based on expert opinion and repeated use in the published literature rather than scientific evidence that 35% is a meaningful cutoff value.9,12 The 1995 WHO Technical Report that created the BMI threshold for obesity does not make a recommendation about the BF% that should be used to define obesity.8,30,31 Published cut points to define obesity according to BF% in women range from 25% to 40%.13,32,33 Considering the absence of a well-established BF% cut-point to classify postmenopausal women as obese, we will examine 35%, 38% and 40% BF from DXA scan as the reference standard to define obesity.
Statistical analysis
In the current analysis, we compared obesity defined by BMI (<30 kg/m2 vs. ≥30 kg/m2) with obesity defined by BF% (35%, 38% and 40%). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated to assess the diagnostic accuracy of BMI-defined obesity compared with obesity defined by BF%. Further description of calculations is provided in Supplemental Digital Content file 1. Sensitivity, specificity, PPV and NPV are all proportions that are calculated from counts of true positives (TP), false negatives (FN), true negatives (TN), and true negatives (TN). Sensitivity (Pr(X*=1|X=1) is defined as the proportion of individuals that are truly obese (X*=1) and classified as obese by BMI (X=1). It is calculated as Se=TP/(TP+FN). Specificity (Pr(X*=0|X=0) is defined as the proportion of individuals that are truly non-obese (X*=0) and classified as non-obese by BMI (X=0), calculated as Sp=TN/(TN+FP). Positive predictive value (Pr(X=1|X*=1) is the probability that someone who is classified as obese by BMI is truly obese, calculated as PPV= TP/(TP+FP), and negative predictive value (Pr(X=0|X*=0) is the probability that someone classified as non-obese is truly non-obese, calculated as NPV=TN/(TN+FN). To account for the correlation between observations, pooled logistic regression was used to provide a valid estimate of the 95% confidence interval for sensitivity, specificity, PPV and NPV values. Percent correctly classified is the proportion of women who were classified as true positives and true negatives divided by the total population, calculated as (TP+TN)/(TP+TN+FP+FN). The area under the curve (AUC) was calculated as a metric to evaluate the discriminatory ability of a BMI cut point of 30 kg/m2, a higher AUC value indicates a stronger discrimination ability while an AUC equal to 0.5 means that BMI is no better than chance at identifying individuals according to their true obesity status.34
Scatterplots were used to graphically explore the validity of BMI as a continuous variable against continuous BF%. Comparing Figure 1a and 1b additionally provides a basis for exploring the effect of changing BF% cut points on the prevalence of obesity and the number of women who are correctly, or incorrectly, classified.
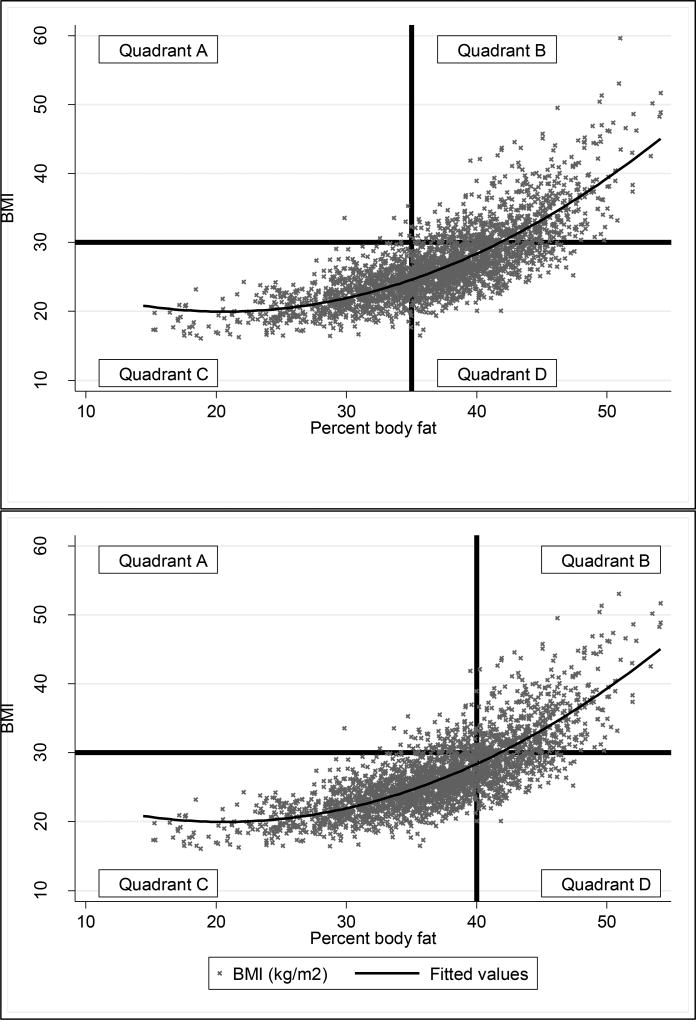
Figure 1.
a–b. Scatterplots comparing BMI (kg/m2) with percent body fat (%). The black horizontal line represents BMI greater than 30 kg/m2. The curved line represents a quadratic prediction line. The vertical line indicates percent body fat greater than 35% (Figure 1a) and 40% (Figure 1b). Participants in Quadrants A and D are misclassified, participants in Quadrants B and C are correctly classified. In Figure 1a, 0.26% of women are in quadrant A, 21% are in quadrant B, 34% are in quadrant C, and 44% are in quadrant D. In figure 1b, 3.7% are in quadrant A, 18% are in quadrant B, 64% are in quadrant C, and 14% in quadrant D.
Finally, although BMI ≥30 kg/m2 is well accepted as the cut point to define obesity according to BMI, we investigated the empirical optimal BMI cut point for each level of BF% defined obesity (35%, 38% and 40%). This analysis demonstrates the BMI cut point that should be used to maximize the sum of sensitivity and specificity at each BF% cut point. The Youden Index (also known as Youden’s J statistic) was used to determine the optimal threshold value for BMI.35,36 For all possible threshold values (t), the Youden Index is defined as:
The critical threshold value, t*, is the point at which the sensitivity and specificity is maximized.36 We subsequently created a Receiver Operating Characteristic (ROC) curve of sensitivity versus 1-specificity for each of the BF% cut points and plotted the optimal BMI cut point on the respective ROC graphs.34,37 All analyses were conducted with Stata 14 Software. Annotated software code is provided (see Supplemental Digital Content 2) to facilitate greater understanding of the results presented here.
Results
Table 1 presents descriptive characteristics of the study cohort. The mean BMI of the participants was 26.7 kg/m2 (SD=5.2, range: 15.4–57.4 kg/m2) and mean BF% was 36.8% (SD=6.0, range: 14.4–54.1%). At baseline, average age was 66.1 years (SD=7.0). The sample was predominantly composed of white women. Based on BMI classification, 35% of the participants were classified as overweight and 21% were obese. There was a positive increase in percent body fat as BMI category increased; for the underweight, normal weight, overweight, and obese categories, the mean percent body fat values were 22.7%, 32.6%, 38.5%, and 43.4%, respectively.
Table 1.
Demographic characteristics of study participants at baseline (n=1,329)
| Age, mean ± SD | 66.1 ± 7.0 | |
|
| ||
| Highest level of education | ||
| High school | 280 (21.3%) | |
| College | 585 (44.5%) | |
| Post-graduate | 451 (34.3%) | |
|
| ||
| Race | ||
| White | 1,302 (97.6%) | |
| Other | 35 (2.60%) | |
|
| ||
| Cigarette smoking status | ||
| Never | 705 (52.8%) | |
| Former | 587 (43.9%) | |
| Current | 44 (3.30%) | |
|
| ||
| BMI category | ||
| Underweight (<18.5 kg/m2) | 16 (1.20%) | |
| Normal weight (18.5–24.9 kg/m2) | 567 (42.7%) | |
| Overweight (25–29.9 kg/m2) | 462 (34.76%) | |
| Obese (>30kg/m2) | 284 (21.4%) | |
|
| ||
| Percent body fat (%; mean ± SD) | 36.8% ± 6.02 | |
|
| ||
| Total body fat (kg; mean ± SD) | 26.8 ± 9.20 | |
|
| ||
| Lean body mass (kg; mean ± SD) | 42.0 ± 5.56 | |
|
| ||
| Whole body bone mineral density (g/cm2; mean ± SD) | 1.06 ± 0.11 | |
|
| ||
| Hormone therapy use | ||
| Never | 436 (32.6%) | |
| Former | 269 (20.1%) | |
| Current | 632 (47.3%) | |
|
| ||
| Parity | ||
| 0 | 168 (12.6) | |
| 1–2 | 375 (28.1) | |
| 3–4 | 570 (42.8) | |
| ≥5 | 220 (16.5) | |
|
| ||
| Age at menopause, mean ± SD | 49.3 ± 5.7 | |
|
| ||
| Physical activity (MET-hour/week) | 14.3 ± 14.3 | |
Results presented in Table 2 demonstrate the validity of using BMI-defined obesity (BMI ≥ 30kg/m2) relative to referent standards of 35%, 38% and 40% BF. Using a BMI cut-point of 30kg/m2, sensitivity and specificity ranged from 32.4% and 99.3% for 35% BF to 44.6% and 97.1% for 38% BF and 55.2% and 94.6% for 40% BF. These results demonstrate the low sensitivity of using 30 kg/m2 to correctly identify an individual’s true obesity status according to BMI. Even with 40 BF% as the reference criterion to define obesity according to BF%, only half of women (55%) who are truly obese are being identified as obese according to BMI. Increasing the BF% used as the referent standard from 35% to 40% produces a noticeable increase in sensitivity (+23%) at the expense of a moderate decrease in specificity (−4.6%). The NPV demonstrated the most notable change when comparing across levels of BF%, from 43.6% for 35% BF and 81.8% for 40% BF, indicating a 38% increase in the probability that someone who is truly non-obese is classified as non-obese by BMI.
Table 2.
Classification of obesity using BMI >30kg/m2 and varying percent body fat cut-points to define obesity (N=2726)
| 35% Body Fat | 38% Body Fat | 40% Body Fat | |
|---|---|---|---|
| Prevalence of BMI >30kg/m2 | 66% | 45% | 32% |
| Sensitivity (95%CI) | 32.4% (29.5, 35.3) | 44.6% (40.9, 48.2) | 55.2% (50.9 59.3) |
| Specificity (95%CI) | 99.3% (98.6, 99.8) | 97.1 (96.0, 98.1) | 94.6% (93.2 95.9) |
| Positive predictive value (95%CI) | 98.8% (97.8, 99.8) | 92.5% (89.8, 95.1) | 82.9% (79.0 86.8) |
| Negative predictive value (95%CI) | 43.6 % (40.7, 46.4) | 68.5% (65.9, 71.0) | 81.5% (79.4, 83.6) |
| Percent correctly classified | 55.4% | 73.7% | 81.8% |
| Area under ROC curve | 0.658 | 0.708 | 0.749 |
Note: Details of calculation of sensitivity, specificity, positive predictive value, negative predictive value, and percent correctly classified are provided in the Supplemental Digital Content.
Changing the BF% cut point used to define obesity has a substantial impact on the prevalence of the study population defined as obese; as the cut point increases, the proportion of women categorized as obese decreases. Using a cut point of 35% BF to define obesity, the prevalence of obesity was 66% in the study sample, using a cut point of 38% BF resulted in 45% of women being categorized as obese, and using a cut point of 40% BF resulted in 32% being considered obese.
The scatterplots presented in Figure 1a and 1b are a visual representation of the degree of misclassification present when using BMI ≥30kg/m2 to define obesity against varying BF% cut points. Figures 1a and 1b depicts the relationship between BMI and BF% as continuous variables. Quadrant A represents individuals who were defined as obese by BMI but non-obese by BF% (upper left), quadrant B represents individuals who were classified as obese by BMI and BF% (upper right), quadrant C represents individuals who were non-obese by BMI and BF% (lower left), and quadrant D represents individuals who were classified as non-obese by BMI and obese by BF% (lower right). Participants in quadrants A and D are considered misclassified (i.e., false positive or false negatives), while participants in quadrants B and C are considered correctly classified (i.e., true positive or true negative).. In Figure 1a, a cut point of 35% BF is used to define obesity and in Figure 1b a cut point of 40% BF is used to define obesity, indicated by the black vertical lines at 35 and 40 percent body fat on the respective graphs. In both Figures 1a and b, BMI greater than 30 kg/m2 is used to define obesity, indicated by the black horizontal line on each graph. The fitted line demonstrates a quadratic relationship between BMI and BF%. Upon visual inspection, it is clear that a greater proportion of the sample is considered obese using a cut point of 35% BF rather than 40% BF. A scatterplot demonstrating the cut point of 38% is presented in Supplemental Digital Content 3. Figures 1a and b provide information about the concordance between BMI-defined obesity and % BF-defined obesity. Using a cut point of 35% BF to define obesity, 55% of women were correctly classified by BMI and BF% and using a cut point of 40% BF results in 82% of women being correctly classified. In Figure 1a, the greatest proportion of women (44%) are in Quadrant D, and therefore misclassified, while in Figure 1b the greatest proportion of women (64%) are in Quadrant C, meaning they are correctly classified.
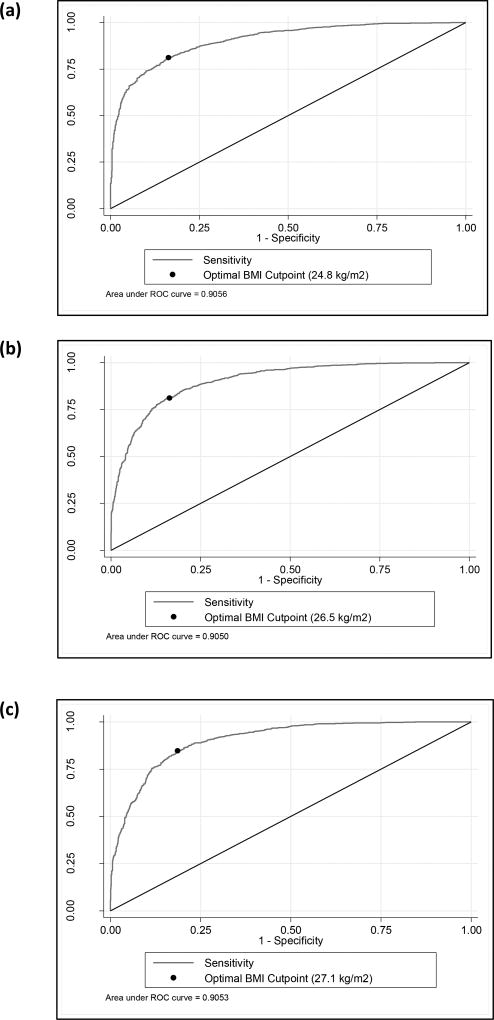
Further analyses (Table 3) demonstrated that the optimal cut point to define obesity using BMI is 24.84 kg/m2 if 35% BF is considered the true referent standard to define obesity, a cut point of 26.49 kg/m2 if 38% BF is considered the true referent standard, and a cut point of 27.04 kg/m2 if 40% BF is the true referent standard. Using the empirical optimal BMI cut point for each of the BF% levels results in over 80% of women being correctly classified. The ROC curves of sensitivity versus 1-specificity comparing the BF% cut points (e.g., 35%, 38% and 40%) and BMI can be found in Figure 2a–c. The optimal BMI cut point for each BF% is indicated on the respective ROC curves.
Table 3.
The optimal BMI cut point values and empirical estimates of validity at varying levels of BF%-defined obesity (N=2726)
| Cut Point | Empirical optimal BMI cutpoint |
Sensitivity at optimal BMI cutpoint(%) |
Specificity at optimal BMI cutpoint (%) |
Youden’s Index (SE) |
Correctly Classified (%) |
|---|---|---|---|---|---|
| TBF > 35% | 24.85 | 0.81 | 0.84 | 0.65 (0.01) | 81.9 |
| TBF > 38% | 26.49 | 0.81 | 0.85 | 0.66 (0.01) | 82.9 |
| TBF > 40% | 27.05 | 0.85 | 0.81 | 0.66 (0.01) | 82.5 |
Figure 2.
a–c. Receiver operating characteristic (ROC) curves comparing BMI with a) 35%, b) 38%, and c) 40% BF cut points to define obesity. The black point on the curve of each graph demonstrates the optimal BMI threshold value for that BF% cutpoint.
Discussion
The results of the present study indicate that a substantial proportion of older women who are truly obese are misclassified as non-obese by BMI, regardless of the BF% used as the reference standard. It is important for researchers and clinicians to recognize the substantial misclassification that may result from using BMI ≥30kg/m2 as a criterion to define obesity in postmenopausal women. BMI additionally underestimates the prevalence of obesity compared with obesity defined directly from BF%. Using 40% BF as the reference standard maximizes the indices of validity relative to a BMI cut point of 30kg/m2. Furthermore, we demonstrated that whether 35%, 38% or 40% BF is used as the reference standard, a BMI cut point of 30kg/m2 is too high to accurately classify women as obese or non-obese, and should potentially be replaced with BMI cut points of 24.9 kg/m2, 26.5 kg/m2 or 27.1 kg/m2, respectively. The optimal BMI cut point to define obesity increased as the BF% to define obesity increased. This demonstrates that while BMI may not be a perfect indicator of BF%, there is still concordance between an increasing amount of body fat and an increasing BMI value. Further research is required to understand the impact of changing the cut-point for BMI-defined obesity and how it relates to health outcomes or future disease risk.
Our results are consistent with previous research demonstrating poor diagnostic accuracy of BMI in older women.12,32 In line with the conclusions of Evans et al. (2006) and Batsis et al. (2016), our findings suggest that using a cut point of 30 kg/m2 to define obesity in postmenopausal women is too high. Using this BMI cut point results in a low true positive rate (i.e., sensitivity) and a high true negative rate (i.e., specificity). There is a well-known trade-off between sensitivity and specificity in diagnostic research; lowering the BMI cut-off value will increase sensitivity (identifying more true positives) at the cost of specificity, producing more false positives.38 Thus, the cost of lowering the BMI cut point to define obesity in postmenopausal women will come in the form of more women being erroneously diagnosed as obese according to BMI, when, in fact, they are not truly obese. In making this decision, it is important to weigh the implications of keeping the cut point at 30kg/m2, and potentially missing the diagnosis of true cases of obesity versus lowering the cut point and potentially misclassifying non-obese women as obese.
Our study highlights two important challenges faced by researchers and clinicians focused on obesity in postmenopausal women. Firstly, although it is evident that BMI≥30kg/m2 is suboptimal for classifying truly obese postmenopausal women as obese, it may be challenging to come up with a superior method to assess obesity that could realistically be implemented in busy clinical settings with limited resources. Aside from its use in research, BMI is frequently used to inform clinical decisions, such as identifying whether an individual patient is an appropriate candidate for surgery or to predict future risk of morbidity (i.e., risk of fracture, coronary disease, cancer)39–42. It is unrealistic to assume that direct methods of assessing adiposity are available to or affordable for clinicians responsible for treating postmenopausal women. One potential solution is using inverse BMI (iBMI=1000/BMI, cm/kg2) to classify post-menopausal women as obese, as it has been demonstrated to be a better proxy for percent body fat and true obesity status.43 The relationship between BMI and BF% is non-linear, as previously reported by other authors and indicated by the quadratic lines of best fit in Figures 1a and 1b.11,43,44 However, prior research has demonstrated that the relationship between iBMI and BF% is linear, indicating that iBMI may be a better predictor of true obesity status. There is currently no recognized cut point to identify obesity according to iBMI, so this represents an interesting opportunity for future research.
The second challenge raised by the present results is that if BMI is being used as a proxy to indicate true adiposity status, it is evident that 30kg/m2 may not be the appropriate cut point to use to define obesity in postmenopausal women. Although BMI≥30 kg/m2 is widely recognized as the cut point to define obesity, and its use is ubiquitous, the scientific rationale for using a threshold of 30 kg/m2, as opposed to an alternative BMI value, such as 28 kg/m2 or 29 kg/m2, is relatively weak. The 1995 WHO Technical Report, responsible for initially creating the BMI categories, states “the method used to establish BMI cut-off points has been largely arbitrary. In essence it has been based on visual inspection of the relationship between BMI and mortality: the cut-off of 30 is based on the point of flexion in the curve” (p.313).8 The present results provide empirical evidence that it may be appropriate to re-evaluate whether the current threshold of 30kg/m2 should continue to be used to define obesity in women’s health research, considering its weak validity and poor diagnostic accuracy relative to true obesity status.
Studies have documented that the effect of obesity on mortality may be attenuated in older women resulting in an apparently reduced risk of obesity-related morbidity.4,20 Bea and colleagues reported a consistent decrease in obesity-related mortality risk by age among women in the Women’s Health Initiative, ranging from 1.46 (95% CI: 0.90,2.36) to 1.17 (95% CI: 0.90, 1.52) to 1.02 (95% CI: 0.80, 1.30) for women with BMI 30–35 kg/m2 aged ≤60, 60–69, and ≥70.16 The present results raise an interesting question about whether the attenuated effect of obesity in older women might be attributed to incorrect classification of exposure. Many women who are truly obese are being misclassified as non-obese by BMI, potentially leading to a systematic underestimation of obesity-related mortality risks in older women.
In any research study, accurate exposure measurement is crucial to producing valid and reliable effect estimates. The sensitivity, specificity, PPV and NPV values presented here can be used to adjust for BMI-related misclassification in future studies on postmenopausal women.45 This represents an important strength and contribution of the present work. With a large sample of post-menopausal women and an analysis that considered the effect of varying values for the BF% referent standard, these results provide researchers and clinicians with important information that can be used to improve the validity of research focused on the effect of obesity in older women. Another potential avenue for future research would be to explore the validity of other BMI categories (e.g., overweight; BMI ≥25 kg/m2) in older women. The present study also has several limitations. Due to the small number of non-white participants, we were unable to explore whether misclassification of obesity status varied by race. The results also are only generalizable to post-menopausal women, as our sample was exclusively composed of older females.
Conclusion
This study adds to the growing body of knowledge highlighting the limitations of using BMI to define obesity.13 These findings represent an important addition to the literature because the body composition of postmenopausal women is distinct from pre-menopausal women or men at any stage of the life course. As life expectancies continues to rise, women are expected to spend more than a third of their lifetime beyond the menopausal transition6. Understanding the effect of obesity in postmenopausal older women is critical to preventing morbidity and mortality and ensuring high quality of life as women age. Though BMI is frequently used as a measure of obesity, our findings indicate that using a BMI cut point of 30 kg/m2 may lead to bias in measuring the effects of obesity on health outcomes in postmenopausal women.11 Our results add an important perspective focused on aging women to a growing body of literature emphasizing the shortcomings of using BMI to define obesity.
Supplementary Material
Acknowledgments
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (University of Nevada, Reno, NV) Robert Brunner; (University of Minnesota, Minneapolis, MN) Karen L. Margolis
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Mark Espeland
Sources of financial support: The Women’s Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C; JW-W: R01DE013505 from the National Institute of Dental and Craniofacial Research, National Institutes of Health (NIH), U.S. Army, Medical Research and Materiel Command, grant OS950077 and NIH/National Heart Lung and Blood Institute contract N01WH32122; AS: National Center for Health Statistics R03SH000037; HB: Canadian Institute of Health Research (CIHR) Banting Postdoctoral Fellowships Program
Footnotes
Conflicts of Interest: A Stokes has received research funding from Johnson & Johnson
Supplemental Digital Content 1. Hand calculations of sensitivity, specificity, PPV and NPV .doc
Supplemental Digital Content 2. Annotated Stata code for analyses presented in manuscript .doc
Supplemental Digital Content 3. Scatterplot demonstrating 38% BF cutpoint .doc
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Gotay CC, Katzmarzyk PT, Janssen I, Dawson MY, Aminoltejari K, Bartley NL. Updating the Canadian obesity maps: An epidemic in progress. Can J Public Health. 2013;104(1):64–68. doi: 10.1007/BF03405657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy R, Kuh D. BMI and mortality in the elderly—a life course perspective. International Journal of Epidemiology. 2006;35(1):179–180. doi: 10.1093/ije/dyi302. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simmonds M, Burch J, Llewellyn A, Griffiths C, Yang H, Owen C, Duffy S, Woolacott N. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: a systematic review and meta-analysis. Health Technol Assess. 2015;19(43) doi: 10.3310/hta19430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Safi ZA, Polotsky AJ. Obesity and Menopause. Best Practice & Research Clinical Obstetrics & Gynaecology. 2015;29(4):548–553. doi: 10.1016/j.bpobgyn.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, Villaseca P. Understanding weight gain at menopause. Climacteric. 2012;15(5):419–429. doi: 10.3109/13697137.2012.707385. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization Expert Committee. WHO Technical Report Series. Geneva: 1995. Physical status: The use and interpretation of anthropometry. [PubMed] [Google Scholar]
- 9.Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, Harris TB, Everhart JE, Schenker N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89(2):500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durazo-Arvizu RA, Cooper RS. Issues related to modeling the body mass index-mortality association: the shape of the association and the effects of smoking status. Int J Obes. 2008;32(S3):S52–S55. doi: 10.1038/ijo.2008.86. [DOI] [PubMed] [Google Scholar]
- 11.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes. 2008;32(s3):s56–s59. doi: 10.1038/ijo.2008.87. [DOI] [PubMed] [Google Scholar]
- 12.Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes. 2016;40(5):761–767. doi: 10.1038/ijo.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes. 2010;34(5):791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 14.Banack HR, Kaufman JS. The obesity paradox: Understanding the effect of obesity on mortality among individuals with cardiovascular disease. Prev Med. 2014;62:96–102. doi: 10.1016/j.ypmed.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Kopelman P. Obesity as a medical problem. Nature. 2000;404(6778):635. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 16.Bea JW, Thomson CA, Wertheim BC, Nicholas JS, Ernst KC, Hu C, Jackson RD, Cauley JA, Lewis CE, Caan B, Roe DJ, Chen Z. Risk of Mortality According to Body Mass Index and Body Composition Among Postmenopausal Women. American Journal of Epidemiology. 2015;182(7):585–596. doi: 10.1093/aje/kwv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Bassford T, Green SB, Cauley JA, Jackson RD, LaCroix AZ, Leboff M, Stefanick ML, Margolis KL. Postmenopausal hormone therapy and body composition—a substudy of the estrogen plus progestin trial of the Women's Health Initiative. The American Journal of Clinical Nutrition. 2005;82(3):651–656. doi: 10.1093/ajcn.82.3.651. [DOI] [PubMed] [Google Scholar]
- 18.Sorkin JD, Muller DC, Andres R. Longitudinal Change in Height of Men and Women: Implications for Interpretation of the Body Mass Index: The Baltimore Longitudinal Study of Aging. American Journal of Epidemiology. 1999;150(9):969–977. doi: 10.1093/oxfordjournals.aje.a010106. [DOI] [PubMed] [Google Scholar]
- 19.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes. 2008;32(6):949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. The American Journal of Clinical Nutrition. 2005;82(5):923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 21.Sun Q, van Dam RM, Spiegelman D, Heymsfield SB, Willett WC, Hu FB. Comparison of Dual-Energy X-Ray Absorptiometric and Anthropometric Measures of Adiposity in Relation to Adiposity-Related Biologic Factors. American Journal of Epidemiology. 2010;172(12):1442–1454. doi: 10.1093/aje/kwq306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Wang Z, Lohman T, Heymsfield SB, Outwater E, Nicholas JS, Bassford T, LaCroix A, Sherrill D, Punyanitya M, Wu G, Going S. Dual-Energy X-Ray Absorptiometry Is a Valid Tool for Assessing Skeletal Muscle Mass in Older Women. The Journal of Nutrition. 2007;137(12):2775–2780. doi: 10.1093/jn/137.12.2775. [DOI] [PubMed] [Google Scholar]
- 23.Kelly TL, Wilson KE, Heymsfield SB. Dual Energy X-Ray Absorptiometry Body Composition Reference Values from NHANES. PLOS ONE. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The women's health initiative observational study: baseline characteristics of participants and reliability of baseline measures. Annals of Epidemiology. 2003;13(9, Supplement):S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 25.Shah NR, Braverman ER. Measuring Adiposity in Patients: The Utility of Body Mass Index (BMI), Percent Body Fat, Leptin. PLoS ONE. 2012;7(4):e33308. doi: 10.1371/journal.pone.0033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, Harris TB, Everhart JE, Schenker N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2008 doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: Evidence in support of current national institutes of health guidelines. Archives of Internal Medicine. 2002;162(18):2074–2079. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 28.Janssen I, Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, Berenson GS. Utility of Childhood BMI in the Prediction of Adulthood Disease: Comparison of National and International References. Obesity Research. 2005;13(6):1106–1115. doi: 10.1038/oby.2005.129. [DOI] [PubMed] [Google Scholar]
- 29.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. 2013 doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Ho-Pham L, Campbell L, Nguyen T. More on body fat cutoff points. Mayo Clinic Proceedings. 2011;86:584+. doi: 10.4065/mcp.2011.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snitker S. Use of Body Fatness Cutoff Points. Mayo Clinic Proceedings. 2010;85(11):1057–1057. doi: 10.4065/mcp.2010.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans EM, Rowe DA, Racette SB, Ross KM, McAuley E. Is the current BMI obesity classification appropriate for black and white postmenopausal women? Int J Obes. 2006;30(5):837–843. doi: 10.1038/sj.ijo.0803208. [DOI] [PubMed] [Google Scholar]
- 33.Blew RM, Sardinha LB, Milliken LA, Teixeira PJ, Going SB, Ferreira DL, Harris MM, Houtkooper LB, Lohman TG. Assessing the Validity of Body Mass Index Standards in Early Postmenopausal Women. Obesity Research. 2002;10(8):799–808. doi: 10.1038/oby.2002.108. [DOI] [PubMed] [Google Scholar]
- 34.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 35.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its Associated Cutoff Point. Biometrical Journal. 2005;47(4):458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 36.Schisterman EF, Faraggi D, Reiser B, Hu J. Youden Index and the optimal threshold for markers with mass at zero. Statistics in medicine. 2008;27(2):297–315. doi: 10.1002/sim.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook NR. Use and Misuse of the Receiver Operating Characteristic Curve in Risk Prediction. Circulation. 2007;115(7):928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 38.Bharti B, Bharti S. Receiver-operating characteristic curve analysis in diagnostic, prognostic and predictive biomarker research: trade-off between sensitivity and specificity with change of test cut-offs. Journal of Clinical Pathology. 2009;62(11):1051–1051. doi: 10.1136/jcp.2009.069591. [DOI] [PubMed] [Google Scholar]
- 39.De Laet C, Kanis JA, Odén A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ, Meunier PJ, Pols HAP, Reeve J, Silman A, Tenenhouse A. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporosis International. 2005;16(11):1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 40.Moore LL, Bradlee ML, Singer MR, Splansky GL, Proctor MH, Ellison RC, Kreger BE. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. Int J Obes Relat Metab Disord. 2004;28(4):559–567. doi: 10.1038/sj.ijo.0802606. [DOI] [PubMed] [Google Scholar]
- 41.Li TY, Rana JS, Manson JE, Willett WC, Stampfer MJ, Colditz GA, Rexrode KM, Hu FB. Obesity as Compared With Physical Activity in Predicting Risk of Coronary Heart Disease in Women. Circulation. 2006;113(4):499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeMaria EJ, Portenier D, Wolfe L. Obesity surgery mortality risk score: proposal for a clinically useful score to predict mortality risk in patients undergoing gastric bypass. Surgery for Obesity and Related Diseases. 2007;3(2):134–140. doi: 10.1016/j.soard.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Nevill AM, Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, Holder RL, Kitas GD, Mohammed MA. Inverted BMI rather than BMI is a better proxy for percentage of body fat. Annals of Human Biology. 2011;38(6):681–684. doi: 10.3109/03014460.2011.606832. [DOI] [PubMed] [Google Scholar]
- 44.Durazo-Arvizu R, McGee D, Li Z, Cooper R. Establishing the Nadir of the Body Mass Index-Mortality Relationship: A Case Study. Journal of the American Statistical Association. 1997;92(440):1312–1319. [PubMed] [Google Scholar]
- 45.Lash T, Fox M, Fink A. Applying Quantitative Bias Analysis to Epidemiologic Data. New York: Springer; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.