Abstract
Problematic prescription opioid use is cited as a primary contributor to the current ‘opioid epidemic’ in the United States, which is characterized by recent rapid increases in individuals seeking treatment for opioid dependence and staggering rates of opioid overdose deaths. Individuals with chronic pain are commonly prescribed opioids to treat pain, and by this mere exposure are at increased risk for the development of problematic opioid use. However, the factors contributing to variation in risk across patients have only recently begun to be unraveled. In the present review, we describe the recent and expanding literature on interactions between pain and reward system function in an effort to inform our understanding of risk for problematic opioid use in chronic pain. To that end, we describe the limited experimental evidence regarding opioid abuse liability under conditions of pain, and offer suggestions for how to advance a research agenda that better informs clinicians about the factors contributing to opioid addiction risk in patients with chronic pain. We raise mechanistic hypotheses by highlighting the primary conclusions of several recent reviews on the neurobiology of pain and reward, with an emphasis on describing dopamine deficits in chronic pain, the role of the reward system in mediating the affective and motivational components of pain, and the role of opponent reward/anti-reward processes in the perpetuation of pain states and the development of problematic opioid use behaviors. Finally, we also argue that positive affect—which is directly regulated by the mesolimbic reward system—is a key pain inhibitory factor that, when deficient, may increase risk for problematic opioid use, and present a model that integrates the potential contributions of pain, reward system function, and positive affect to problematic opioid use risk.
The U.S. is presently grappling with a nationwide ‘opioid epidemic’ characterized by widespread problematic use of prescription opioids, defined as misuse, abuse, and/or addiction (O’Connor et al., 2013, Smith et al., 2013). According to recent meta-analyses, approximately 25% of patients with chronic pain misuse prescription opioids (e.g., use of prescription opioids in a manner other than how they were prescribed), and 10% fulfill conventional criteria for addiction [e.g., the repeated, intentional use of an opioid for the purpose of altering mood or cognition, despite known harmful consequences; (Vowles et al., 2015)], compared to just 0.2% of the general population (Degenhardt et al., 2014). Although prescription opioids are generally effective for providing analgesia to acute pain, they lack efficacy for the treatment of chronic pain (Chou et al., 2015, Shaheed et al., 2016, Von Korff et al., 2008). Nonetheless, sales of opioid analgesics increased approximately 400% between 1999 and 2010 in the U.S.(Prevention, 2011), with the majority of opioid prescriptions associated with long-term opioid therapy treatment plans (Von Korff et al., 2011). Over this period, problematic opioid use substantially increased, leading to increased heroin initiation (Pollini et al., 2011) and a dramatically increased incidence of opioid overdose deaths (Bohnert et al., 2011). Opioid-related overdose is currently the leading cause of accidental death in the United States (Rudd et al., 2016). In reaction to the recent trends, federal agencies, such as the Centers for Disease Control (CDC), have recently produced guidelines intended to curb opioid prescribing practices in cases when there is no clear indication, an aspiration that would apply to most long-term chronic pain treatment plans. Physicians and other prescribers have little empirical guidance to inform such an adjustment in prescribing practice. Improved assessment of upstream factors predicting risk for problematic prescription opioid use would aid in that adjustment of clinical care standards.
Presently, we are underinformed about what contributes to problematic opioid use risk in chronic pain. Drug use history is a clear indicator of increased risk for prescription opioid addiction (Morasco et al., 2013), but sensitivity estimates for the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R; CITE), a common prescription opioid risk screener for patients with chronic pain that measures affective styles, substance-related social function, and drug use behaviors, are modest, ranging from 0.49–0.77 (Chou et al., 2009). This suggests that there is substantial variation in opioid risk explained by within-person and between-person factors that have not yet been reliably delineated. Evidence from Koob and colleagues (Koob and Le Moal, 1997, Koob and Le Moal, 2005, Koob and Volkow, 2010) suggest that opioid addiction is behaviorally driven by a disturbance of the affective system that promotes a compensatory impulsive drive to recover affective function through the consumption of opioids, which powerfully enhance hedonic tone and decrease negative affect. Those behavioral changes are mediated by neurocircuitry within the mesolimbic reward system, which includes dopaminergic and opioidergic projections connecting subcortical regions involved in hedonic valuation, including the ventral tegmental area and striatum, to cortical regions involved in decision making and motivated behavior, such as the prefrontal cortex and the anterior cingulate cortex (Fields, 2004, Koob and Volkow, 2010). Importantly, these neural circuits are critically involved in the regulation of positive emotions, the appraisal of pain-related stimuli, and the experience of pain relief. The present review will consider whether and to what extent disruptions to these behavioral and neurobiological systems, which have been observed in patients with chronic pain, may increase risk for problematic opioid use.
To that end, we begin by describing the limited experimental evidence regarding the direct assessment of opioid abuse liability under conditions of pain, and offer suggestions for how to advance a research agenda that better informs clinicians about the factors contributing to opioid addiction risk in patients with chronic pain. Next, we present an overview of the recent and expanding literature on abnormalities within the mesolimbic reward system in chronic pain, which offer putative mechanisms to better understand risk for problematic opioid use in chronic pain. A comprehensive review of the literature on the neurobiological basis for the intersection of pain and reward is outside the scope of this article, and has been amply covered in several recent reviews (Becker et al., 2012, Borsook et al., 2016, Navratilova and Porreca, 2014, Navratilova et al., 2013, Porreca and Navratilova, 2017, Taylor et al., 2016). Therefore, one goal of this article is to offer a high-level summary of the primary conclusions and hypotheses generated from those more expansive review papers. Finally, we describe the evidence in support of the argument that positive affect—which is directly regulated by the mesolimbic reward system—is a key pain inhibitory factor for patients with chronic pain. This line of evidence will be presented to evaluate the overarching hypothesis that chronic pain may engender neurobiological changes that result in positive affective deficits which, in turn, may increase risk for problematic opioid use.
Opioid Abuse Liability in the Context of Pain
Opioid abuse liability under conditions of pain has been directly evaluated through human laboratory experiments using randomized, placebo-controlled designs to assess the subjective and physiological effects of opioids across a range of clinically-relevant doses. Abuse liability testing is a standardized method of empirically evaluating whether the subjective experience of a medication or drug suggests it may be abused (Comer et al., 2012), and is commonly used to characterize new products or determine the relative risk of existing products. Though the abuse liability of various prescribed opioids has been thoroughly demonstrated, the majority of those studies were not conducted under conditions of pain (e.g., in clinical pain populations or with healthy subjects exposed to noxious stimuli). The broad conclusion from those studies is that opioids that have either partial or full μ-receptor agonist properties have a high potential for abuse (Walsh and Babalonis, 2016).
Three studies have examined the abuse liability of opioids within chronic pain populations. One study reported incidence of medication abuse over 12 months in patients taking tramadol, hydrocodone, or NSAIDs (Adams et al., 2006). Results revealed significantly greater proportion of patients abused a full μ-receptor agonist (hydrocodone, 4.9%), compared to a weaker opioid (tramadol; 2.7%) or non-opioid medications (NSAIDs; 2.5%). Another study compared patients’ craving after exposure to opioids that differed principally in duration of action, and revealed that both short-acting and long-acting opioids produced similar abuse liability risk as compared to placebo (Wilsey et al., 2009). Finally, a small, within-subject study enrolled patients seeking treatment for pain and maintained them on a various doses of buprenorphine (2, 8, 16mg) to examine the abuse liability of several concurrent doses of oxycodone (0, 10, 20, 40, 60mg/70kg) (Jones et al., 2011). The study reported that oxycodone dose-dependently increased withdrawal latencies from a painful cold water hand immersion task but did not reduce ratings of clinical pain. Interestingly, though self-report ratings of abuse liability increased dose-dependently, the amount of oxycodone that patients were willing to self-administer did not vary from placebo at any dose tested.
Additional studies have examined the abuse liability of opioids in healthy individuals using laboratory-evoked pain designs. One within-subject study compared the abuse liability of oxycodone (15, 30 mg/70kg) versus placebo administered during painful cold water immersion in pain-free healthy volunteers and individuals meeting criteria for prescription opioid abuse (Comer et al., 2010). Results showed that the controls dose dependently self-administered opioids under cold water conditions only, whereas the prescription opioid users, who reported greater liking of the drug, self-administered oxycodone to a similar level in the context of either painful or non-painful water immersion. A second study examined the abuse liability of snorted oxycodone (15, 30mg/70kg) under conditions of pain among prescription opioid users, and found that abuse liability ratings did not differ between cold and warm water immersion (Lofwall et al., 2012). A third study compared the relative abuse liability of oxycodone and oxymorphone during cold water immersion and pressure pain, and reported greater abuse liability for oxycodone in the context of both experimental pain stimuli (Babalonis et al., 2016). A final within-subject study found that varying the schedule of dosing of oxycodone enhanced the positive drug effects and analgesic benefits, whereas repeated dosing in a consistent schedule diminished the positive drug effects without altering the analgesic profile (Cooper et al., 2012).
Though it was originally assumed that persons with no history of substance use were unlikely to develop problematic opioid use following opioid narcotic treatment (Porter and Jick, 1980), there is growing empirical evidence that μ-opioid receptor agonists can still produce measureable levels of abuse liability in the context of both clinical and laboratory-induced pain. The level of abuse liability appears to be specific to the dose administered and the potency of the opioid, though the degree to which the absence of pain increases opioid abuse liability has not been well-articulated. While evidence from some of the reviewed studies suggests that measures of analgesia and abuse liability operate independently, no studies have specifically evaluated the relative contributions that positive subjective effects and analgesia make to abuse liability ratings of “opioid liking”. It will also be important to characterize the extent to which variation in opioid analgesic ef ficacy is predictive of actual opioid misuse behavior. It may be expected that patients who fail to experience analgesia at prescribed opioid doses would be more likely to consume more opioids than originally prescribed in order to achieve adequate analgesia. Whether such behavior is associated with opioid abuse liability, as measured in controlled laboratory studies, is an open question that should be taken up in future investigations.
Finally, additional studies are needed to evaluate the potential importance of the chronic pain disease state, its duration, and its effect on putative neurobiological mechanisms, such as reward system function, on opioid abuse liability. It may be hypothesized that patients with a longer history of chronic pain will demonstrate greater opioid abuse liability than those with recent diagnoses due to neuroplastic changes in reward system function that may develop over time. Such mechanistic hypotheses may be enhanced or better targeted by drawing upon the basic science on the interaction between pain and reward processing within areas of the brain responsible for regulating opioid use behavior.
Evidence for Reward Circuitry Involvement in Pain Appraisal and Relief
In recent years, we have seen a sharp rise in the number of preclinical and human investigations into the interactions of pain and reward. Several review papers have described this literature in a level of detail that is beyond the scope of the present article. Here we attempt to summarize the basis for three broad conclusions that have been reached in those reviews, and that bear relevance to our understanding of the development of risk for problematic opioid use in patients with chronic pain. First, we discuss evidence summarized by Becker et al. (Becker, Gandhi, 2012) and Taylor et al. (Taylor, Becker, 2016) suggesting that chronic pain is a hypodopaminergic state. Second, we discuss evidence summarized by Porreca, Navratilova and colleagues (Navratilova and Porreca, 2014, Porreca and Navratilova, 2017), suggesting that the mesolimbic reward system is integral to the affective and motivational aspects of pain and pain relief. Finally, we discuss evidence summarized by Borsook and colleagues suggesting that chronic pain is associated with a neurobiological dysfunction in the opponent processes of reward and ‘anti-reward’, an aversive behavioral state with properties similar to opioid withdrawal (Borsook, Linnman, 2016).
Chronic Pain as a Hypodopaminergic State
Dopamine is a key monoaminergic neurotransmitter in the mesolimbic reward system, classically implicated in the mediation of reward-associated behaviors. Dopamine’s role in reward processing is complex, but there is consensus that critical functions are to motivate behavior to seek rewards and to reinforce learned reward associations. It is also indirectly involved—via interactions with opioid and endocannabinoid neurocircuitry—with the hedonic experience of reward (i.e., liking) (Berridge and Kringelbach, 2015). In addition to its roles in reward processing, the dopaminergic system comprises a heterogeneous population of neurons that respond to aversive stimuli, diversifying its function with respect to motivated behavior (Brischoux et al., 2009, Cohen et al., 2012, Lammel et al., 2011, Matsumoto and Hikosaka, 2009, Mirenowicz and Schultz, 1996). Neuronal subpopulations activated by reward but inhibited by punishment are thought to encode motivational valence, typically associated with reward seeking, valuation, and reinforcement of behavior (Berridge et al., 2009, Schultz, 2007b), whereas dopaminergic neurons activated by both rewarding and aversive stimuli are more likely to encode motivational salience, and are responsible for stimulus detection and prediction of behavioral response to both rewarding and punishing stimuli (Redgrave and Gurney, 2006). In addition to serving distinct functional roles, these dopaminergic subpopulations may also be separated based upon their neuroanatomical projections to the nucleus accumbens (NAc). Dopaminergic neurons originating from the lateral ventral tegmental area and ventromedial substantia nigra project to the NAc shell and encode valence discrimination, while dopaminergic neurons originating in the dorsolateral substantia nigra project to the NAc core and encode salience detection (Brooks and Berns, 2013, Matsumoto and Hikosaka, 2009, Mirenowicz and Schultz, 1996, Nomoto et al., 2010).
The role of the mesolimbic reward system in pain is, in part, dictated by variation in dopamine signaling associated with antinociception versus motivational valence/salience. Manipulation of dopamine and dopamine 2 (D2) receptor availability modulates the affective component of pain and motivated behavioral responses to pain relief (Navratilova et al., 2012a, Scott et al., 2006, Tiemann et al., 2014, Treister et al., 2013a, Wood et al., 2007), while little to no change in the somatosensory processing of pain is observed by altering D2 availability (Becker et al., 2013, Tiemann, Heitmann, 2014, Treister, Pud, 2013a, Treister et al., 2013b). Becker et al. found that modulation of synaptic dopamine altered endogenous pain inhibition induced by reward, and conversely enhanced pain facilitation by punishment (Becker, Gandhi, 2013). Taken together, they postulated that dopamine is context-dependent and is important in modulating the salience of pain stimuli and mediating the motivation to avoid or endure pain.
Human neuroimaging studies in chronic pain patients have demonstrated a reduced presence of dopamine and D2 receptor availability (Hagelberg et al., 2003a, Hagelberg et al., 2003b, Martikainen et al., 2015, Wood, Schweinhardt, 2007). Additionally, the potential involvement of dopamine abnormalities in fibromyalgia and chronic low back pain patients may be indirectly inferred from functional magnetic resonance imaging studies showing alterations in blood oxygen level-dependent response of the mesolimbic reward valuation circuitry to salient stimuli (Loggia et al., 2014, Martikainen, Nuechterlein, 2015). Similar results have been shown in animal models of chronic pain as well (Chang et al., 2014, Narita et al., 2003, Ren et al., 2016, Taylor et al., 2014, Wu et al., 2014). Thus, Taylor et al. suggest that chronic pain promotes a “hypodopaminergic” tone that can impair motivated behavior (Taylor, Becker, 2016). While this hypothesis is intriguing, it requires further testing and clarification, as different subtypes of dopaminergic neurons have been shown to phasically respond to either reward or punishment, or both (Cohen, Haesler, 2012, Matsumoto and Hikosaka, 2009), suggesting that there is heterogeneity among dopaminergic neurons and some subtypes of dopamine neurons may be downregulated in chronic pain while others may be upregulated (Sagheddu et al., 2015, Zhang et al., 2017a).
Mesolimbic Reward System Mediates the Affective Component of Pain and Pain Relief
Pain is composed of sensory-discriminative, affective, and cognitive components, and is dependent upon environmental context (Melzack R, 1968). Although the somatosensory component of pain is commonly associated with aversive behavioral responses (Fields, 1999, Gold and Gebhart, 2010), the affective component of pain is the primary driver of the behavioral responses to withdraw from harm or the threat of harm in an effort to seek pain relief (Navratilova and Porreca, 2014, Navratilova, Xie, 2013, Porreca and Navratilova, 2017). Affective-motivational states associated with pain elicit behavioral responses that promote both avoidance (i.e., escape from pain), and approach (i.e., seeking pain relief) behaviors. Pain relief, then, can be conceptualized as rewarding in that it engenders motivated ‘seeking’ behavior through reinforcement learning (Navratilova and Porreca, 2014, Porreca and Navratilova, 2017). Conserved neural circuitry including the thalamus, somatosensory cortices, insula, and anterior cingulate cortex (ACC) are critically involved in coding the affective aspects of pain (Apkarian et al., 2005).
The ACC, in particular, is commonly associated with the descending, affective modulation of pain (Fields, 2004, Porreca et al., 2002, Rainville et al., 1997, Vogt, 2005). But it also performs a key role in both reward and aversion learning, and neuroimaging studies have shown that opioid receptors densely populate the ACC of both humans and rats (Vogt et al., 2001). Indeed, increases in pain, reductions in pain unpleasantness, and placebo analgesia all result in endogenous opioid release in the ACC (Scott et al., 2008, Wager et al., 2007). Loss of ACC function in the context of chronic pain inhibits the drive to seek pain relief without altering reflexive withdrawal responses to evoked painful stimuli (Johansen and Fields, 2004, Johansen et al., 2001, Qu et al., 2011), suggesting its function with respect to pain is predominantly motivational.
Interestingly, the ACC is functionally connected to the mesolimbic reward circuitry, subserving its role in reward valuation and decision-making (Apkarian et al., 2011, Navratilova et al., 2015). Administration of morphine into the ACC of rats not only produces conditioned place preference (CPP) in injured animals, but also elicits dopamine release in the NAc shell in response to relief of ongoing pain (Navratilova, Xie, 2015). Inhibition of dopaminergic signaling from the ventral tegmental area (VTA) to the NAc, or direct blockade of dopamine release in the NAc, blocks pain-relief induced CPP, providing evidence of a direct link between pain-motivated behaviors and the mesolimbic reward valuation system (Navratilova et al., 2012b). Thus, pain relief appears to be less rewarding when dopamine levels are attenuated.
There is limited evidence to suggest that chronic pain alters the function of the reward system in the context of pain-related motivated behavior. Baliki et al. observed NAc deactivation when a noxious thermal stimulus was removed from the back of patients with chronic low back pain. In contrast, healthy subjects evidenced an increased NAc activation pattern during the same ‘pain offset’ period. The authors attributed these findings to the competition between experimental and clinical pain for patients’ attention, suggesting that the acute painful stimuli were able to provide temporary relief of the ongoing pain and represented in NAc deactivation, which was phasically restored when the exogenous stimulus was removed (Baliki et al., 2010). This interpretation is advocated by Porreca and colleagues, who have found greater phasic dopamine response to peripheral analgesics (e.g., lidocaine) in rats with, versus without, chronic pain (Navratilova, Xie, 2012a, Navratilova, Xie, 2013, Remeniuk et al., 2015), and argue that chronic pain is associated with a shift in motivational behavior toward pain relief via alterations in molecular (dopamine and opioid neurotransmission), structural (gray matter volume, white matter connectivity), and functional (BOLD activation) brain changes (Apkarian et al., 2009, Geha et al., 2008, May, 2008, Tracey and Bushnell, 2009).
An alternative possibility, though, is that patients’ ability to extract reward from pain relief may be impaired in patients with chronic pain, and represented in reduced NAc activity in the context of pain relief, as was observed in the Baliki et al. study (Baliki, Geha, 2010). This alternative interpretation would follow from the hypothesis from Taylor, Becker, and colleagues that chronic pain is a hypodopaminergic state, and the findings from Navratilova and Porreca’s group (Navratilova, Xie, 2012b) showing that disrupted dopamine neurotransmission prevented CPP to pain relief. This interpretation is also supported by a recent finding that deficits in behavioral reward processing and striatal function during a reward task in early adolescence predict future development of chronic pain in late adolescence, partly as a function of polymorphisms in the μ-opioid receptor gene (Nees et al., 2017).
Taken together, the data as reviewed by Porreca, Navratilova and colleagues indicate that pain and reward share overlapping neurocircuitry between the ACC and NAc that under normal conditions help to drive pain relief and its associated behavior. However, chronic pain may produce maladaptive alterations, increasing attention to pain and creating deficits in motivational salience and decision-making.
Opponent Processes of Reward and Anti-Reward in Chronic Pain
Borsook and colleagues approach the pain/reward interaction from the standpoint of opponent process theory (Solomon and Corbit, 1973, 1974), which argues that reward is homeostatically balanced against an opposing process termed ‘anti-reward’ that may be experienced as stress, negative affect, pain, or a combination of those aversive states (Borsook, Linnman, 2016). Koob et al. hypothesize that repeated opioid use shifts the reward/anti-reward balance, whereby the ability to experience positive emotions from natural rewards (i.e., non-drug rewards) degrades over time, and is accompanied by an increase in anti-reward state, which fuels further opioid use to compensate for the reward deficit (Koob et al., 2014, Koob and Le Moal, 2005). Borsook argues that chronic pain may also upset the reward/antireward balance through repeated sensitization of nociceptive circuits within the mesolimbic reward system (primarily the NAc), along with accompanying inhibitory signaling from the habenula, which is activated by aversive stimuli and negative reward prediction errors (Matsumoto, 2009, Matsumoto and Hikosaka, 2009).
The NAc and lateral habenula (LHb) have distinct dopaminergic neural circuitry, in which projections from the VTA to the NAc promote reward signaling, and projections from the LHb inhibit dopaminergic signaling in the VTA via GABAergic neurons, known as the aversion circuit (Balcita-Pedicino et al., 2011, Barrot et al., 2012, Lammel et al., 2012, Omelchenko et al., 2009). Borsook et al. argue that a reward deficit state can be promoted either by chronic pain or repeated opioid use, resulting in a blunted positive affective system, which may result in the clinical condition of anhedonia (Borsook, Linnman, 2016). Anti-reward, in contrast, is characterized by increases in sensitivity of the aversion system, which responds to stress by releasing the hormones corticotropin-releasing factor, norepinephrine, glutamate and dynorphin (Nestler and Carlezon, 2006). These factors contribute to the perception of stress and negative affective states and may alter phasic dopaminergic burst firing to become temporarily more responsive to opioids, ultimately contributing to the reward deficit.
Synergy in the Pain/Reward Literature and Relevance to Opioid Addiction Risk
Pain is a complex sensory, emotional, and cognitive experience. The affective and sensory components of pain are modulated by both the opioidergic and dopaminergic systems in the NAc and ACC which promote motivated behavior to seek pain relief and restore the relative balance of reward and anti-reward processes. However, chronic pain and repeated opioid use both may promote a hypodopaminergic state, or a reward deficit, which alters dopamine signaling and can increase the sensitivity of aversion circuitry (anti-reward), leading to elevations in stress and negative affect, which is a primary risk factor for problematic opioid use, and would be expected to further facilitate increased pain sensitivity (Borsook, Linnman, 2016). Interestingly, stimulation of the habenula under conditions of acute pain can produce analgesia (Shelton et al., 2012), but this region becomes tonically activated in chronic pain patients, leading to chronically aversive states, including further pain chroni fication (Erpelding et al., 2014). In the addiction literature, cross sensitization refers to conditions in which exposure to a stimulus increases future responses to it. This may also hold true in chronic pain, in which hypersensitivity of the anti-reward system creates greater awareness of pain-associated cues (Zhang et al., 2017b), and increased compensatory opioid use which, over time, may give way to problematic prescription opioid use in patients maintained on long-term opioid therapy.
Positive Affect Deficits in Chronic Pain
In patients with chronic pain, problematic opioid use risk increases in the context of maladaptive cognitive and emotional coping processes. Much of the research attention in this domain has been focused on the correlation of negative patterns of thought and emotion with indices of opioid misuse. For example, patients with chronic pain and a history of substance use disorder are at greater risk for prescription opioid misuse (based on self-report) if they believe their opioid medications are unlikely to have an analgesic benefit (Schieffer et al., 2005). Similarly, pain catastrophizing, a negative cognitive style associated with helplessness, rumination, and magnification of pain’s potential for harm, is correlated with risk for opioid misuse in patients with comorbid chronic pain and substance use disorder (Morasco, Turk, 2013) and non-substance abusing patients with chronic pain (Jamison et al., 2009, Martel et al., 2013). Numerous studies have also shown that high levels of negative affect are associated with increased risk for opioid misuse in patients with chronic pain (Martel, Wasan, 2013, Martel et al., 2014, Wasan et al., 2007).
Relatively less attention has focused on the potential role of positive affect as a mechanism of problematic opioid use risk. Positive affect is a pleasant feeling state that is characterized by either a high (e.g., jubilation) or low (e.g., serenity) level of physiological activation. Positive affect may occur in the context of anticipation of an impending reward or in the course of reward consumption (Gard et al., 2006). Both anticipatory and consummatory positive affect drive behavior to approach future potential rewards. Decades of preclinical research demonstrates that anticipatory positive affect is associated with phasic dopamine signals that burst fire in response to a positive reward prediction error (i.e., receiving a reward of greater value than expected) and promote learning that rapidly alters behavior (Ashby and Isen, 1999, Schultz, 2007a, 2013). Disruptions to dopaminergic neurotransmission inhibit the experience of positive emotions, such as excitement or eagerness, thereby attenuating reward learning and diminishing motivated behavior to approach future rewards.
Positive affect has been identified as a critical “resilience resource” for individuals exposed to a range of physiological and psychological insults, including pain (Ong et al., 2010, Sturgeon and Zautra, 2010, Zautra et al., 2005c) and stress (Ong et al., 2006, Tugade and Fredrickson, 2004). Numerous human laboratory studies have shown that evoked positive affect, relative to evoked neutral and negative affects, inhibits pain [for a review see: (Finan and Garland, 2015b)]. The mechanisms by which positive affect confers benefits to patients with chronic pain, however, are multifactorial. Frederickson and colleagues (Fredrickson, 1998, Fredrickson and Branigan, 2005, Fredrickson et al., 2008, Fredrickson and Joiner, 2002) have demonstrated that positive affect broadens thought-action repertoires, increases openness to experience, and fosters creativity. These cognitive skills enable individuals to engage social supports, develop meaningful interpersonal relationships, and adapt to stressful environmental demands (Kok et al., 2013, Kok and Fredrickson, 2010). People with chronic pain face substantial challenges to their ability to regulate affect, engage in valued activities, and maintain social connections. Such difficulties are at least partly, if not largely, owing to the fact that pain is both a physiological and psychological stressor that promotes a narrow cognitive orientation toward the perception of negative affective states (Davis et al., 2004). For example, women with rheumatoid arthritis report substantially higher negative affect and lower positive affect on days characterized by high levels of stress (Zautra et al., 2005a). However, when positive affect is maintained through elevations of stress and/or pain, it buffers the negative health consequences of negative affect, and serves to decouple the push-pull association of pain and negative affect (Finan and Garland, 2015a). In support of this notion, daily diary studies have shown that patients with chronic pain experience less negative affective reactivity to spikes in pain on days when positive affect is elevated (Zautra et al., 2005b, Zautra, Johnson, 2005c).
Deficits in positive affect and positive affect regulation have been observed in several chronic pain patient populations, including fibromyalgia (Zautra, Fasman, 2005b), comorbid osteoarthritis and fibromyalgia (Finan et al., 2009), rheumatoid arthritis (Connelly et al., 2007), temporomandibular joint disorder (Marbach and Lund, 1981), and sickle cell disease (Gil et al., 2004). Epidemiological studies reveal that chronic pain and major depressive disorder are highly comorbid (Bair et al., 2003, Ohayon and Schatzberg, 2003). Although the association between chronic pain and depression is bidirectional, longitudinal studies suggest that chronic pain more commonly precedes depression than vice versa (Fishbain et al., 1997), suggesting that pain-related processes stably degrade affective function in a substantial subset of patients. To the extent that chronic pain precipitates alterations to the brain reward system, it follows that such neuroplastic changes may drive the positive affective deficits commonly observed in patients with chronic pain.
This problem may be compounded by long-term opioid exposure, as positive affective deficits at baseline could theoretically expedite the rate of development of problematic opioid use following initial exposure. Stable positive affective deficits may develop in opioid-treated chronic pain patients as a result both of interactions between nociceptive signaling and reward system function, and prolonged exposure to opioids. Such effects would likely depend on multiple interacting neurotransmitter circuits. For example, microinjection of morphine into the ventral tegmental of rats with chronic pain failed to produce conditioned place preference, but pretreatment with the microglia inhibitor minocycline recovered this effect (Taylor et al., 2015), suggesting that the effect of opioid exposure influences the degree of interaction of pain and reward through dopamine, opioid, and glial circuits.
Chronically deficient positive affect may drive problematic opioid use as patients seek to recover both the hedonic experience of pleasure and the analgesic benefit it confers. Over time, these recursive interactions between pain, opioid use, and positive affect would be expected to increase patients’ risk for the development of opioid addiction. Indeed, a recent clinical trial of a mindfulness-based intervention in patients with chronic pain and current opioid misuse [Mindfulness-Oriented Recovery Enhancement; (Garland et al., 2014b)] revealed that reductions in opioid misuse over the course of treatment were mediated by improvements in daily positive affect regulation (Garland, 2017). Interestingly, this hybrid mindfulness intervention, which emphasizes cognitive techniques such as savoring pleasant experiences and positively reappraising negative thoughts, has previously been shown to reduce pain severity, enhance both physiological and psychological aspects of natural reward responsiveness in laboratory tasks (Garland et al., 2014a, 2015), and increase ventral striatal responses to natural rewards in smokers without pain (Froeliger et al., 2017). These findings suggest that mindfulness meditation may modulate of the reward system independent of pain relief in a manner that drives upward shifts in natural hedonic tone that could translate into both improved pain and opioid use outcomes.
Summary and Conclusions
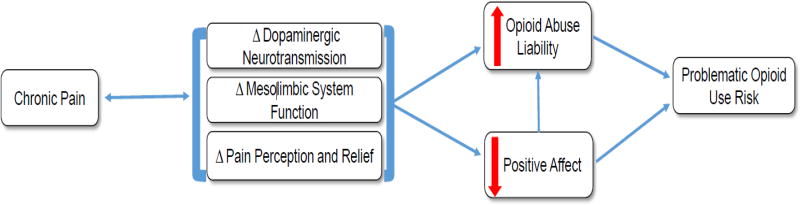
Although patients with chronic pain are at elevated risk to develop problematic opioid use, the mechanisms and processes contributing to that risk profile have not been elucidated. We offer a putative model for problematic opioid use risk that integrates the contributions of neurobiological changes within the mesolimbic reward system, as well as accompanying, and possibly related, changes in positive affective functioning (see Figure 1). The animal and human literature on the interaction of pain and reward offers several putative mechanistic pathways of vulnerability to problematic opioid use for patients in pain, including: 1) alterations in dopamine function, leading to a hypodopaminergic state and reward processing deficits; 2) neuroplastic changes influencing the affective and motivational components of pain, leading to impaired neural and behavioral function in the context of pain relief; and 3) dysregulation of the opponent processes of reward (NAc function) and anti-reward (lHb function), leading to increased pain sensitivity, and an affective imbalance favoring aversive to pleasurable states. Together, this group of neurobiological mechanisms likely contributes to the experience and regulat ion of positive affect, which has been found to be deficient in subsets of patients with chronic pain. The absence of positive affect challenges patients’ endogenous pain inhibitory system and could increase opioid seeking to both achieve analgesia in the context of a heightened analgesic threshold and recover a diminished capacity to feel pleasure. While opioid abuse liability is observed in chronic pain patients, pain (either clinical or experimentally-induced) does not appear to increase the likelihood of self-administration of prescription opioids in controlled experimental designs. However, the human experimental literature base is limited, and future studies will need to test the possibility that impairments in reward processing and positive affect regulation influence the extent to which individuals are liable to misuse and abuse opioids.
Figure 1. Putative pathways from chronic pain to problematic opioid use risk.
Chronic pain may engender (and be perpetuated by) changes within the mesolimbic dopaminergic reward system, which overtime may alter the perception of pain and its relief. These neurobiological alterations may increase opioid abuse liability either directly or indirectly via changes in positive affective system functioning. Together, these biological mechanisms and behavioral processes may influence the likelihood to misuse, abuse, and or develop addiction to prescription opioids.
Highlights.
Review of experimental literature on opioid abuse liability in the context of pain
Review of the interaction of pain and reward within the mesolimbic dopaminergic reward system
Discussion of positive affect as a putative mechanism linking chronic pain and problematic opioid use
Presentation of a synergistic model offering hypothesized links between chronic pain, mesolimbic reward system function, and positive affective function with opioid abuse liability and risk for problematic opioid use
Acknowledgments
This work is funded by a grant from the National Institutes of Health K23DA035915 (PHF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams EH, Breiner S, Cicero TJ, Geller A, Inciardi JA, Schnoll SH, et al. A comparison of the abuse liability of tramadol, NSAIDs, and hydrocodone in patients with chronic pain. J Pain Symptom Manage. 2006;31:465–76. doi: 10.1016/j.jpainsymman.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:S49–64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG, Isen AM. A neuropsychological theory of positive affect and its influence on cognition. Psychol Rev. 1999;106:529. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Babalonis S, Lofwall MR, Nuzzo PA, Walsh SL. Pharmacodynamic effects of oral oxymorphone: abuse liability, analgesic profile and direct physiologic effects in humans. Addict Biol. 2016;21:146–58. doi: 10.1111/adb.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The Inhibitory Influence of the Lateral Habenula on Midbrain Dopamine Cells: Ultrastructural Evidence for Indirect Mediation via the Rostromedial Mesopontine Tegmental Nucleus. J Comp Neurol. 2011;519:1143–64. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–60. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC. Braking Dopamine Systems: A New GABA Master Structure for Mesolimbic and Nigrostriatal Functions. J Neurosci. 2012;32:14094–101. doi: 10.1523/JNEUROSCI.3370-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Gandhi W, Elfassy NM, Schweinhardt P. The role of dopamine in the perceptual modulation of nociceptive stimuli by monetary wins or losses. Eur J Neurosci. 2013;38:3080–8. doi: 10.1111/ejn.12303. [DOI] [PubMed] [Google Scholar]
- Becker S, Gandhi W, Schweinhardt P. Cerebral interactions of pain and reward and their relevance for chronic pain. Neurosci Lett. 2012;520:182–7. doi: 10.1016/j.neulet.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86:646–64. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: 'liking', 'wanting', and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–21. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev. 2016;68:282–97. doi: 10.1016/j.neubiorev.2016.05.033. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–9. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AM, Berns GS. Aversive stimuli and loss in the mesocorticolimbic dopamine system. Trends Cogn Sci. 2013;17:281–6. doi: 10.1016/j.tics.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Chang PC, Pollema-Mays SL, Centeno MV, Procissi D, Contini M, Baria AT, et al. Role of nucleus accumbens in neuropathic pain: linked multi-scale evidence in the rat transitioning to neuropathic pain. Pain. 2014;155:1128–39. doi: 10.1016/j.pain.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. The Journal of Pain. 2009;10:131–46. e5. doi: 10.1016/j.jpain.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276–86. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–8. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer S, Sullivan M, Vosburg S, Kowalczyk W, Houser J. Abuse liability of oxycodone as a function of pain and drug use history. Drug Alcohol Depend. 2010;109:130–8. doi: 10.1016/j.drugalcdep.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Zacny JP, Dworkin RH, Turk DC, Bigelow GE, Foltin RW, et al. Core outcome measures for opioid abuse liability laboratory assessment studies in humans: IMMPACT recommendations. PAIN®. 2012;153:2315–24. doi: 10.1016/j.pain.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly M, Keefe FJ, Affleck G, Lumley MA, Anderson T, Waters S. Effects of day-to-day affect regulation on the pain experience of patients with rheumatoid arthritis. Pain. 2007;131:162–70. doi: 10.1016/j.pain.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Sullivan MA, Vosburg SK, Manubay JM, Haney M, Foltin RW, et al. Effects of repeated oxycodone administration on its analgesic and subjective effects in normal, healthy volunteers. Behav Pharmacol. 2012;23:271. doi: 10.1097/FBP.0b013e3283536d6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MC, Zautra AJ, Smith BW. Chronic pain, stress, and the dynamics of affective differentiation. J Pers. 2004;72:1133–60. doi: 10.1111/j.1467-6494.2004.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Charlson F, Mathers B, Hall WD, Flaxman AD, Johns N, et al. The global epidemiology and burden of opioid dependence: results from the global burden of disease 2010 study. Addiction. 2014;109:1320–33. doi: 10.1111/add.12551. [DOI] [PubMed] [Google Scholar]
- Erpelding N, Sava S, Simons LE, Lebel A, Serrano P, Becerra L, et al. Habenula functional resting-state connectivity in pediatric CRPS. J Neurophysiol. 2014;111:239–47. doi: 10.1152/jn.00405.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–75. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Fields HL. Pain: an unpleasant topic. Pain. 1999;(Suppl 6):S61–9. doi: 10.1016/S0304-3959(99)00139-6. [DOI] [PubMed] [Google Scholar]
- Finan PH, Garland EL. The role of positive affect in pain and its treatment. Clin J Pain. 2015a;31:177–87. doi: 10.1097/AJP.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Garland EL. The role of positive affect in pain and its treatment. The Clinical journal of pain. 2015b;31:177. doi: 10.1097/AJP.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan PH, Zautra AJ, Davis MC. Daily affect relations in fibromyalgia patients reveal positive affective disturbance. Psychosom Med. 2009;71:474–82. doi: 10.1097/PSY.0b013e31819e0a8b. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. The Clinical journal of pain. 1997;13:116–37. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL. What good are positive emotions? Rev Gen Psychol. 1998;2:300. doi: 10.1037/1089-2680.2.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Branigan C. Positive emotions broaden the scope of attention and thought-action repertoires. Cogn Emot. 2005;19:313–32. doi: 10.1080/02699930441000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Cohn MA, Coffey KA, Pek J, Finkel SM. Open hearts build lives: positive emotions, induced through loving-kindness meditation, build consequential personal resources. J Pers Soc Psychol. 2008;95:1045. doi: 10.1037/a0013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Joiner T. Positive emotions trigger upward spirals toward emotional well-being. Psychol Sci. 2002;13:172–5. doi: 10.1111/1467-9280.00431. [DOI] [PubMed] [Google Scholar]
- Froeliger B, Mathew A, McConnell P, Eichberg C, Saladin M, Carpenter M, et al. Restructuring Reward Mechanisms in Nicotine Addiction: A Pilot fMRI Study of Mindfulness-Oriented Recovery Enhancement for Cigarette Smokers. Evid Based Complement Alternat Med. 2017;2017 doi: 10.1155/2017/7018014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. Journal of research in personality. 2006;40:1086–102. [Google Scholar]
- Garland EL, Froeliger B, Howard MO. Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology (Berl) 2014a;231:3229–38. doi: 10.1007/s00213-014-3504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Howard MO. Neurophysiological evidence for remediation of reward processing deficits in chronic pain and opioid misuse following treatment with Mindfulness-Oriented Recovery Enhancement: exploratory ERP findings from a pilot RCT. J Behav Med. 2015;38:327–36. doi: 10.1007/s10865-014-9607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol. 2014b;82:448. doi: 10.1037/a0035798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland ELBCJ, Finan PH, Thomas EA, Priddy SE, Riquino MR, Howard MO. Pain, hedonic regulation, and opioid misuse: Modulation of momentary experience by Mindfulness-Oriented Recovery Enhancement in opioid-treated chronic pain patients. Drug Alcohol Depend. 2017 doi: 10.1016/j.drugalcdep.2016.07.033. In Press. [DOI] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The Brain in Chronic CRPS Pain: Abnormal Gray-White Matter Interactions in Emotional and Autonomic Regions. Neuron. 2008;60:570–81. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil KM, Carson JW, Porter LS, Scipio C, Bediako SM, Orringer E. Daily mood and stress predict pain, health care use, and work activity in African American adults with sickle-cell disease. Health Psychol. 2004;23:267. doi: 10.1037/0278-6133.23.3.267. [DOI] [PubMed] [Google Scholar]
- Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16:1248–57. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagelberg N, Forssell H, Aalto S, Rinne JO, Scheinin H, Taiminen T, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003a;106:43–8. doi: 10.1016/s0304-3959(03)00275-6. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Forssell H, Rinne JO, Scheinin H, Taiminen T, Aalto S, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003b;101:149–54. doi: 10.1016/s0304-3959(02)00323-8. [DOI] [PubMed] [Google Scholar]
- Jamison RN, Link CL, Marceau LD. Do pain patients at high risk for substance misuse experience more pain?: A longitudinal outcomes study. Pain Med. 2009;10:1084–94. doi: 10.1111/j.1526-4637.2009.00679.x. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2001;98:8077–82. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Sullivan MA, Manubay J, Vosburg SK, Comer SD. The subjective, reinforcing, and analgesic effects of oxycodone in patients with chronic, non-malignant pain who are maintained on sublingual buprenorphine/naloxone. Neuropsychopharmacology. 2011;36:411–22. doi: 10.1038/npp.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok BE, Coffey KA, Cohn MA, Catalino LI, Vacharkulksemsuk T, Algoe SB, et al. How positive emotions build physical health: Perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychol Sci. 2013 doi: 10.1177/0956797612470827. [DOI] [PubMed] [Google Scholar]
- Kok BE, Fredrickson BL. Upward spirals of the heart: Autonomic flexibility, as indexed by vagal tone, reciprocally and prospectively predicts positive emotions and social connectedness. Biol Psychol. 2010;85:432–6. doi: 10.1016/j.biopsycho.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76:370–82. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nat Neurosci. 2005;8:1442–4. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-Specific Modulation of Dopamine Neuron Synapses by Aversive and Rewarding Stimuli. Neuron. 2011;70:855–62. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212-+. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall MR, Nuzzo PA, Walsh SL. Effects of cold pressor pain on the abuse liability of intranasal oxycodone in male and female prescription opioid abusers. Drug Alcohol Depend. 2012;123:229–38. doi: 10.1016/j.drugalcdep.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Berna C, Kim J, Cahalan CM, Gollub RL, Wasan AD, et al. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 2014;66:203–12. doi: 10.1002/art.38191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbach J, Lund P. Depression, anhedonia and anxiety in temporomandibular joint and other facial pain syndromes. Pain. 1981;11:73–84. doi: 10.1016/0304-3959(81)90140-8. [DOI] [PubMed] [Google Scholar]
- Martel M, Wasan A, Jamison R, Edwards RR. Catastrophic thinking and increased risk for prescription opioid misuse in patients with chronic pain. Drug Alcohol Depend. 2013;132:335–41. doi: 10.1016/j.drugalcdep.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MO, Dolman AJ, Edwards RR, Jamison RN, Wasan AD. The association between negative affect and prescription opioid misuse in patients with chronic pain: The mediating role of opioid craving. The Journal of Pain. 2014;15:90–100. doi: 10.1016/j.jpain.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martikainen IK, Nuechterlein EB, Pecina M, Love TM, Cummiford CM, Green CR, et al. Chronic Back Pain Is Associated with Alterations in Dopamine Neurotransmission in the Ventral Striatum. J Neurosci. 2015;35:9957–65. doi: 10.1523/JNEUROSCI.4605-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M. Role of the lateral habenula and dopamine neurons in reward processing. Brain Nerve. 2009;61:389–96. [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–U4. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Melzack RCK. Sensory, motivational, and central control determinants of pain: new conceptual model. In: K DR, editor. The skin senses. Springfield: Thomas; 1968. pp. 423–43. [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–51. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Morasco BJ, Turk DC, Donovan DM, Dobscha SK. Risk for prescription opioid misuse among patients with a history of substance use disorder. Drug Alcohol Depend. 2013;127:193–9. doi: 10.1016/j.drugalcdep.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Ozaki S, Narita M, Ise Y, Yajima Y, Suzuki T. Change in the expression of c-fos in the rat brain following sciatic nerve ligation. Neurosci Lett. 2003;352:231–3. doi: 10.1016/j.neulet.2003.08.052. [DOI] [PubMed] [Google Scholar]
- Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci. 2014;17:1304–12. doi: 10.1038/nn.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie J, Okun A, Qu C, Eyde N, Ci S, et al. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A. 2012a;109:20709–13. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, King T, Porreca F. Evaluation of reward from pain relief. Ann Ny Acad Sci. 2013;1282:1–11. doi: 10.1111/nyas.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Meske D, Qu CL, Morimura K, Okun A, et al. Endogenous Opioid Activity in the Anterior Cingulate Cortex Is Required for Relief of Pain. J Neurosci. 2015;35:7264–71. doi: 10.1523/JNEUROSCI.3862-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, et al. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A. 2012b;109:20709–13. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nees F, Becker S, Millenet S, Banaschewski T, Poustka L, Bokde A, et al. Brain substrates of reward processing and the mu-opioid receptor: a pathway into pain? Pain. 2017;158:212–9. doi: 10.1097/j.pain.0000000000000720. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–9. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nomoto K, Schultz W, Watanabe T, Sakagami M. Temporally Extended Dopamine Responses to Perceptually Demanding Reward-Predictive Stimuli. J Neurosci. 2010;30:10692–702. doi: 10.1523/JNEUROSCI.4828-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AB, Turk DC, Dworkin RH, Katz NP, Colucci R, Haythornthwaite JA, et al. Abuse liability measures for use in analgesic clinical trials in patients with pain: IMMPACT recommendations. PAIN®. 2013;154:2324–34. doi: 10.1016/j.pain.2013.06.035. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry. 2003;60:39–47. doi: 10.1001/archpsyc.60.1.39. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–50. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong AD, Bergeman C, Bisconti TL, Wallace KA. Psychological resilience, positive emotions, and successful adaptation to stress in later life. J Pers Soc Psychol. 2006;91:730. doi: 10.1037/0022-3514.91.4.730. [DOI] [PubMed] [Google Scholar]
- Ong AD, Zautra AJ, Reid MC. Psychological resilience predicts decreases in pain catastrophizing through positive emotions. Psychol Aging. 2010;25:516. doi: 10.1037/a0019384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollini RA, Banta-Green CJ, Cuevas-Mota J, Metzner M, Teshale E, Garfein RS. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Substance abuse and rehabilitation. 2011;2:173. doi: 10.2147/SAR.S24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Navratilova E. Reward, motivation, and emotion of pain and its relief. Pain. 2017;158(Suppl 1):S43–S9. doi: 10.1097/j.pain.0000000000000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–25. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Porter J, Jick H. Addiction rare in patients treated with narcotics. The New England journal of medicine. 1980;302:123. doi: 10.1056/nejm198001103020221. [DOI] [PubMed] [Google Scholar]
- Prevention CfDCa. Vital signs: overdoses of prescription opioid pain relievers---United States, 1999--2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487–92. [PubMed] [Google Scholar]
- Qu C, King T, Okun A, Lai J, Fields HL, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011;152:1641–8. doi: 10.1016/j.pain.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–71. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nat Rev Neurosci. 2006;7:967–75. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Remeniuk B, Sukhtankar D, Okun A, Navratilova E, Xie JY, King T, et al. Behavioral and neurochemical analysis of ongoing bone cancer pain in rats. Pain. 2015;156:1864–73. doi: 10.1097/j.pain.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, et al. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat Neurosci. 2016;19:220. doi: 10.1038/nn.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, Matthew Gladden R. Increases in drug and opioid overdose deaths—United States, 2000–2014. Am J Transplant. 2016;16:1323–7. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- Sagheddu C, Aroni S, De Felice M, Lecca S, Luchicchi A, Melis M, et al. Enhanced serotonin and mesolimbic dopamine transmissions in a rat model of neuropathic pain. Neuropharmacology. 2015;97:383–93. doi: 10.1016/j.neuropharm.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Schieffer BM, Pham Q, Labus J, Baria A, Van Vort W, Davis P, et al. Pain medication beliefs and medication misuse in chronic pain. The Journal of Pain. 2005;6:620–9. doi: 10.1016/j.jpain.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends in neurosciences. 2007a;30:203–10. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007b;30:203–10. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013;23:229–38. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26:10789–95. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–31. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Shaheed CA, Maher CG, Williams KA, Day R, McLachlan AJ. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: A systematic review and meta-analysis. JAMA internal medicine. 2016;176:958–68. doi: 10.1001/jamainternmed.2016.1251. [DOI] [PubMed] [Google Scholar]
- Shelton L, Pendse G, Maleki N, Moulton EA, Lebel A, Becerra L, et al. Mapping pain activation and connectivity of the human habenula. J Neurophysiol. 2012;107:2633–48. doi: 10.1152/jn.00012.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Dart RC, Katz NP, Paillard F, Adams EH, Comer SD, et al. Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. PAIN®. 2013;154:2287–96. doi: 10.1016/j.pain.2013.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. II. Cigarette addiction. J Abnorm Psychol. 1973;81:158–71. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–45. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Sturgeon JA, Zautra AJ. Resilience: a new paradigm for adaptation to chronic pain. Current pain and headache reports. 2010;14:105–12. doi: 10.1007/s11916-010-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Becker S, Schweinhardt P, Cahill C. Mesolimbic dopamine signaling in acute and chronic pain: implications for motivation, analgesia, and addiction. Pain. 2016;157:1194–8. doi: 10.1097/j.pain.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Castonguay A, Taylor AJ, Murphy NP, Ghogha A, Cook C, et al. Microglia disrupt mesolimbic reward circuitry in chronic pain. J Neurosci. 2015;35:8442–50. doi: 10.1523/JNEUROSCI.4036-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Murphy NP, Evans CJ, Cahill CM. Correlation between ventral striatal catecholamine content and nociceptive thresholds in neuropathic mice. J Pain. 2014;15:878–85. doi: 10.1016/j.jpain.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemann L, Heitmann H, Schulz E, Baumkotter J, Ploner M. Dopamine precursor depletion influences pain affect rather than pain sensation. PLoS One. 2014;9:e96167. doi: 10.1371/journal.pone.0096167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Bushnell MC. How Neuroimaging Studies Have Challenged Us to Rethink: Is Chronic Pain a Disease? J Pain. 2009;10:1113–20. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Treister R, Pud D, Ebstein RP, Eisenberg E. Dopamine transporter genotype dependent effects of apomorphine on cold pain tolerance in healthy volunteers. PLoS One. 2013a;8:e63808. doi: 10.1371/journal.pone.0063808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treister R, Pud D, Eisenberg E. The dopamine agonist apomorphine enhances conditioned pain modulation in healthy humans. Neurosci Lett. 2013b;548:115–9. doi: 10.1016/j.neulet.2013.05.041. [DOI] [PubMed] [Google Scholar]
- Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. J Pers Soc Psychol. 2004;86:320. doi: 10.1037/0022-3514.86.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt LJ, Sim-Selley LJ, Childers SR, Wiley RG, Vogt BA. Colocalization of mu-opioid receptors and activated G-proteins in rat cingulate cortex. J Pharmacol Exp Ther. 2001;299:840–8. [PubMed] [Google Scholar]
- Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med. 2011;155:325–8. doi: 10.1059/0003-4819-155-5-201109060-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M, Saunders K, Ray GT, Boudreau D, Campbell C, Merrill J, et al. Defacto long-term opioid therapy for non-cancer pain. The Clinical journal of pain. 2008;24:521. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569–76. doi: 10.1097/01.j.pain.0000460357.01998.f1. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A. 2007;104:11056–61. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Babalonis S. The Abuse Potential of Prescription Opioids in Humans—Closing in on the First Century of Research. 2016 doi: 10.1007/7854_2016_448. [DOI] [PubMed] [Google Scholar]
- Wasan AD, Butler SF, Budman SH, Benoit C, Fernandez K, Jamison RN. Psychiatric history and psychologic adjustment as risk factors for aberrant drug-related behavior among patients with chronic pain. The Clinical journal of pain. 2007;23:307–15. doi: 10.1097/AJP.0b013e3180330dc5. [DOI] [PubMed] [Google Scholar]
- Wilsey BL, Fishman S, Li C-S, Storment J, Albanese A. Markers of abuse liability of short-vs long-acting opioids in chronic pain patients: a randomized cross-over trial. Pharmacology Biochemistry and Behavior. 2009;94:98–107. doi: 10.1016/j.pbb.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576–82. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Na X, Zang Y, Cui Y, Xin W, Pang R, et al. Upregulation of tumor necrosis factor-alpha in nucleus accumbens attenuates morphine-induced rewarding in a neuropathic pain model. Biochem Biophys Res Commun. 2014;449:502–7. doi: 10.1016/j.bbrc.2014.05.025. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Affleck GG, Tennen H, Reich JW, Davis MC. Dynamic approaches to emotions and stress in everyday life: Bolger and Zuckerman reloaded with positive as well as negative affects. J Pers. 2005a;73:1511–38. doi: 10.1111/j.0022-3506.2005.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra AJ, Fasman R, Reich JW, Harakas P, Johnson LM, Olmsted ME, et al. Fibromyalgia: evidence for deficits in positive affect regulation. Psychosom Med. 2005b;67:147–55. doi: 10.1097/01.psy.0000146328.52009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. J Consult Clin Psychol. 2005c;73:212. doi: 10.1037/0022-006X.73.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Qian Y-L, Li C, Liu D, Wang L, Wang X-Y, et al. Brain-Derived Neurotrophic Factor in the Mesolimbic Reward Circuitry Mediates Nociception in Chronic Neuropathic Pain. Biol Psychiatry. 2017a doi: 10.1016/j.biopsych.2017.02.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Manders T, Tong AP, Yang R, Garg A, Martinez E, et al. Chronic pain induces generalized enhancement of aversion. eLife. 2017b;6:e25302. doi: 10.7554/eLife.25302. [DOI] [PMC free article] [PubMed] [Google Scholar]