Abstract
A chemosensor, 3-aminophenol-based rhodamine conjugate (ARC) has been developed for visualisation of diethylchlorophosphate (DCP), mimic of a chemical warfare agent, in Catfish brain. The simple detection of DCP by “turn-on” fluorescence property of the chemosensor makes it unique for easy and rapid in vivo and in vitro detection of DCP with the detection limit of 5.6 nM.
Introduction
1995, The terrorist attack on Tokyo subway introduces the whole world with a new threat to mankind- Chemical Warfare Agents (CWAs)1–3. The simple organophosphates, present in pesticides eventually become more popular chemical weapon due to its very simple method of manufacturing, availability, and low cost along with its dispensability. Despite of its common use as a pesticide, various analogues of such organophosphates are found to be very potent nerve agent, which irreversibly damage functions of nerve cells. Among all the highly toxic, volatile nerve agents, Sarin (GB), Soman (GD) and Tabun (GA) are of most common. If the nerve agents are being inhaled or absorbed through skin, their reactive phosphate group irreversibly react with the hydroxyl group of cellular acetylcholinesterase, which is responsible for breaking down the acetylcholine neurotransmitter, leads to its inactivation. The fatal consequences are neurological imbalance at cholinergic synapses, failure of several organs, paralysis of central nervous system and rapid death4,5. On 4th April 2017, Khan Shaykhun, a city of Syria faced the destructive activities of suspected chemical warfare with nerve agent Sarin6. For public safety concern and national security, a reliable, facile and reactive detection method of such nerve agents is of great global interest towards the development of practical ‘in field’ devices.
Till today, several detection methods, such as- colorimetry7–10, fluorometry11–17, interferometry18, electrochemistry19–22, surface acoustic wave (SAW) devices23,24, photoacoustic spectroscopy25, moleculer imprinting coupled with lanthanide luminescence and fibre optics26–28, enzymatic assay29,30, and gas chromatography-mass spectrometry31 have been explored to detect substantial number of CWAs. But the problem arises when these existing methods show one or more drawbacks such as low response and sensitivity, limited selectivity, non-specificity and non-portability, difficulties in real time detection, false positive readings and operational complexity. The chemosensors mediated detection is an extremely convenient and simple method based on optical outputs such as color change or change in fluorescence intensity after the exposure to the target analytes32–35. Chemosensors make such detection method attractive and widely acceptable by fulfilling the key features of a practical sensing system i.e. high selectivity, sensitivity, specificity, portability, ability of real time sensing along with cost effectiveness36,37. Implementation of a reliable chemosensor is a highly challenging but it brings a considerable importance for their wide application in medical, environmental and biological field. Generally, diethylchlorophosphate (DCP) is used as mimic of the nerve agent Sarin. DCP is much less toxic than Sarin but its mode of action is similar.
With this stand point in this particular research field, we have described a chemosensor ARC, 3-aminophenol based rhodamine conjugate. This chemosensor is simple and efficient in showing fluorescence signal in presence of DCP in aqueous medium both in in vivo and in vitro systems. There are reports that described recognition of deadly and relevant molecules like DCP and other organophosphates. A comparative assessment of our chemosensor has been provided with that of the published one (Table S1). In our present work with the help of a very simply designed chemosensor we established the detection of DCP in a direct, fast and selective way in living system. Over usage of organophosphate pesticides or use of similar compounds through chemical warfare can essentially disseminate such compound in ecosystem. Hence detection of such compound from contaminated samples is very much awaiting. With a unique approach, we have applied the probe ARC in catfish brain to visualize DCP which was also treated subsequently and the success of this experiment help to advance its practical field-application. As per our knowledge this is the first report where a chemosensor is used for visualizing DCP in vivo.
Results and Discussion
Design and scheme
Probe ARC was synthesized from a simple reaction of rhodamine B and POCl3, followed by reaction with 3-amino phenol with 74% overall yield (detailed in method section). Characterization of the probe ARC has been done by 1H NMR, 13C NMR, 31P NMR and MS analysis (Figs S1–S3). ARC shows fluorescence “turn-on” response upon binding with DCP in aqueous medium at neutral pH (Fig. 1). Several studies such as absorption, fluorescence, NMR titrations and TDDFT calculations have been carried out to establish the interactive properties of ARC towards DCP, our target analyte.
Figure 1.
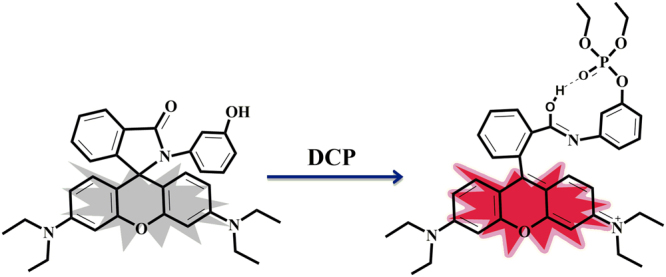
“Turn-on” fluorescence sensing mechanism of ARC upon addition of DCP.
UV-vis and fluorescence spectral studies
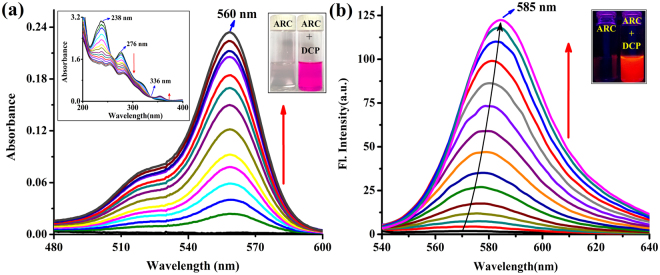
From the UV-vis absorption spectra of the chemosensor ARC it has been revealed that the addition of DCP gradually enhanced the absorption of ARC in aqueous medium at neutral pH. The UV-vis absorption spectra of the probe ARC showed a prominent isosbestic point at 336 nm that proves stronger complexation with three absorption maxima at 238, 276, 355 nm through change in color from colorless to deep pink along with gradual enhancement of ARC absorption during DCP accumulation (Fig. 2a).
Figure 2.
(a) UV-vis absorption spectra of ARC (1 µM) upon gradual addition of DCP upto 1.1 equiv. in H2O-CH3CN (10:1, v/v). [Inset: left- UV-vis spectra of the region 200–400 nm, right- photograph of visual color changes of ARC and ARC + DCP complex in aqueous medium] (b) Fluorescence emission spectra (λex = 520 nm) of ARC (1 µM) toward DCP at varied concentrations (0, 0.01, 0.05, 0.10, 0.15, 0.20, 0.30, 0.40, 0.50, 0.60, 0.70, 0.80, 0.90, 1.0, 1.05, 1.10 µM) in H2O-CH3CN (10:1, v/v). [Inset: Fluorescence color changes of ARC and ARC + DCP complex in aqueous medium].
ARC shows a very weak fluorescence quantum yield (Фfs = 0.006). Within a few seconds in contact with DCP, ARC gives a prompt fluorescence response with a full signal emission maxima at 585 nm (λex = 520 nm) - a 100 fold increased fluorescence signal (Фfs = 0.58) compared to its own (Fig. 2b, Table S2). Non-linear fitting analysis from fluorometric titration curves deduced the binding constant of ARC-DCP complex is 7.89 × 106 M−1 (Fig. S4). This fluorometric titration of ARC with DCP also results the detection limit of ARC which is 5.6 nM of DCP (Fig. S5). All these results explained the deep pink color development of the ARC from colorless and non-fluorescent entity, through the ring opening process of the spirolactam ring of the probe by irreversible coordination with DCP.
Selectivity test has also been performed with different organophosphates (OPs) e.g. diethylcyanophosphonate (DCNP), diethyl(1-phenylethyl)phosphonate (DPEP), diethyl(methylthiomethyl)phosphonate (DMTMP), diethyl-(2-oxopropyl)phosphonate (DOPP), Ethyl methylphosphonate (EMP), Diethyl methylphosphonate (DEMP) by UV-vis and fluorescence method (Figs S6 and S7). Interestingly probe ARC can only recognise DCP distinctly among all of the OPs (Fig. S8). We have also investigated the fluorescence behaviour of ARC on the addition of various interfering substances like dimethylmethylphosphate (DMMP) - a nonreactive analogue of DCP; some common metal ions such as Zn2+, Cu2+, Co2+, Hg2+; reactive oxygen species (ROS), i.e. NaOCl, tBuOOH, H2O2; common acid chlorides, i.e. acetyl chloride, benzoyl chloride; and common pesticides, i.e. benzene hexachloride (BHC), phorate and chlorothalonil (Fig. S9). From the comparative fluorescence experiment we can conclude that there is no interference of the above mentioned substances with ARC. So, it’s clear that ARC can be a simple, selective and efficient chemosensor which can directly measure DCP by a fluorescence “turn-on” mechanism.
A kinetic investigation of the reaction between ARC and DCP showed that the reaction is complete within 20 seconds (Fig. S10). We then examined the kinetic profiles of the reaction under pseudo-first-order conditions with a large excess of DCP (10 equiv.) over the chemosensor ARC (1 µM) in aqueous medium (at neutral pH) at room temperature. The pseudo-first-order rate constant (k′) has been calculated as k′ = 0.172 s−1 (Fig. S11). This rate constant (k′) has been calculated according to the equation38:
where Ft and Fmax are the fluorescence intensities at 585 nm (λex = 520 nm) at time ‘t’ and the maximum value obtained after the reaction is complete, respectively, and k′ is the observed pseudo-first order rate constant.
Vapour phase detection studies
Sensing ability of ARC to DCP has also been successfully established in gaseous phase, as our probe can satisfy an important role of a convenient chemosensor kit. We did the experiment using a Whatman-41 filter paper strip immersed in ARC, keeping inside in a glass chamber. A rapid “turn-on” fluorescence response was noticed after adding a few drops of DCP in that glass chamber (Fig. S12). From this observation it has been concluded that ARC strongly binds with DCP even from gaseous phase.
pH titration studies
As rhodamine derivatives are very sensitive in acidic medium, we are always aware to maintain pH while doing experiments with rhodamine derivatives. In acidic condition the spirolactam ring opens up to show its original deep pink color. So it’s very important to fix the pH range where rhodamine derivatives will be non-fluorescent. From the pH titration it has been shown that ARC is stable and non-fluorescent at pH range 5–13. It means the spirolactam ring of ARC opens in highly acidic condition (below pH 5). We therefore maintained the pH of ARC at 7 while doing all experiments. However, while adding DCP at neutral pH the probe shows its deep pink color even at pH 8 (Fig. S13)39,40, which demonstrates the practical applicability of the chemosensor.
NMR titration studies
Interactive properties of the chemosensor ARC towards DCP was further investigated via 1H, 13C, 31P NMR titrations carried out using solvent DMSO-d6. Upon DCP addition, aromatic protons of the xanthene moiety of ARC display downfield shift. This is due to the decrease in electron density of the xanthene moiety upon binding with DCP (Fig. S14). In 13C NMR titration the aromatic region of ARC becomes downfield while comparing with the original one (Fig. S15). The spiro cycle carbon peak at 67 ppm was shifted to 143 ppm. This coordination led to the spiro cycle opening and changes to the absorption and emission spectra. From 31P titration it has also been shown that the only one singlet peak of DCP at −0.39 ppm drastically shifts to lower frequency −5.06 ppm upon binding with DCP which indicates that there is a strong interaction between ARC and phosphate group of DCP (Fig. S16).
Density Functional Theoretical (DFT) studies
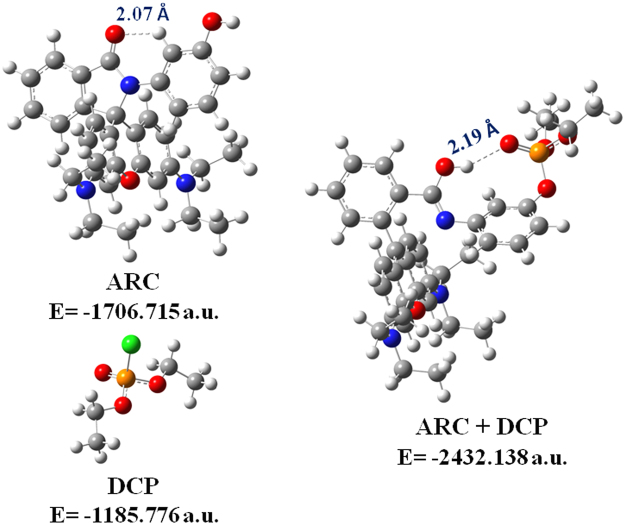
The interaction of the receptor ARC with DCP has further been comprehended by quantum chemical calculations at the TDDFT level with the B3LYP/6-31 + G(d,p) method using the Gaussian 09 program (Tables S3 and S4). The optimized structures and their corresponding energies are shown in Fig. 3. The energy gap between the HOMO and LUMO of the ARC-DCP complex structure is smaller (3.01 eV) than that of the chemosensor ARC (4.61 eV) (Fig. S17). The main contributing transitions for S0 → S1 energy state arises from HOMO-1 → LUMO. This is consistent with the absorbance band at 560 nm obtained experimentally.
Figure 3.
Optimized structures of ARC, DCP and ARC-DCP complex with their corresponding energies. The hydrogen bond interactions are denoted by dotted lines.
Imaging of Catfish brain and A549 cells
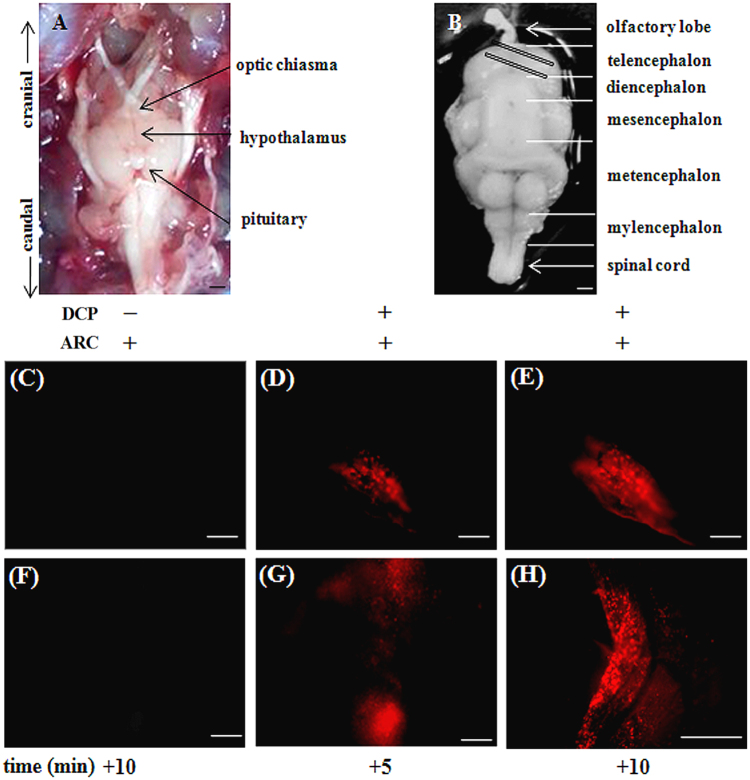
Furthermore, we attempted to visualize DCP from Catfish brain through the fluorescence microscopic imaging upon binding with ARC in Catfish brain. To test the efficiency of binding of DCP to ARC and subsequent fluorescence emission, fish brain, more specifically the slices from optic tectum– the region which have earlier been shown to possess high level of acetylcholinesterase activity41, were used in the present study. As shown in Fig. 4, incubation of the brain slices with the chemosensor ARC alone does not produce detectable fluorescence signal, while incubation of ARC followed by DCP, promoted a robust increase in fluorescence emission indicating specificity of interaction. The experiment has also been done in reverse way, i.e. first incubation of DCP followed by ARC which gives the same result as earlier. These also suggest successful penetration of ARC and DCP into the Catfish brain tissue.
Figure 4.
Representative photomicrographs of Catfish brain (ventral side up) showing cranial and caudal ends, hypothalamus, pituitary and optic chiasma during dissection (A) and after harvesting in chilled PBS (B). Brain slices from telencephalon (marked as diagonal rectangles in (B) were pre-incubated with ARC, washed with PBS, treated with the analyte DCP, washed again to remove excess DCP and observed under fluorescence inverted microscope at 5 min intervals (D,E from upper slice and G,H from lower slice). Parallel slices treated with ARC only served as negative control (C,F). Figures are representative of three independent observations from separate donors, scale bar ~1 mm (A,B); ~200 µm from (C to G) and ~400 µm for (H).
In addition, we have also tested permeability and toxicity of ARC in A549 human cancer cell line (Fig. S18). It is also revealed successful entrance and detection of DCP by the probe ARC. The ARC doesn’t show major cytotoxicity (Fig. S19)42 to the tested cells and could be a choice of probe to detect DCP in simple and complex cellular matrix.
Conclusion
In summary, we have successfully developed a rhodamine based chemosensor ARC for in vivo and in vitro detection of DCP with very low concentration at neutral pH. The simple detection method exhibits high sensitivity of ARC towards DCP both in solution and gas phase and the interaction of ARC with DCP can even noticed with nacked eye. The above method was successfully applied to visualize DCP in catfish brain through “turn-on” fluorescence signal. This proves the potentiality of the probe ARC as a simple, fast, cost-effective and very useful tool to determine DCP in biological samples.
Materials and Methods
Materials
Rhodamine B, phosphorus oxychloride and 3-amino phenol were purchased from Sigma-Aldrich Pvt. Ltd. (India). Unless mentioned otherwise, materials were obtained from commercial suppliers and were used without further purification. Solvents were dried according to the standard procedures. Elix Millipore water was used throughout all experiments. 1H and 13C NMR spectra were recorded on a Bruker 400 MHz instrument. For NMR spectra, DMSO-d6 and for NMR titration DMSO-d6 and D2O were used as solvent using TMS as an internal standard. Chemical shifts are expressed in δ ppm units and 1H–1H and 1H–C coupling constants in Hz. The mass spectrum (HRMS) was carried out using a micromass Q-TOF MicroTM instrument by using methanol as a solvent. Fluorescence spectra were recorded on a Perkin Elmer Model LS 55 spectrophotometer. UV spectra were recorded on a SHIMADZU UV-3101PC spectrophotometer. The following abbreviations are used to describe spin multiplicities in 1H NMR spectra: s = singlet; d = doublet; t = triplet; m = multiplet.
Preparation of ARC
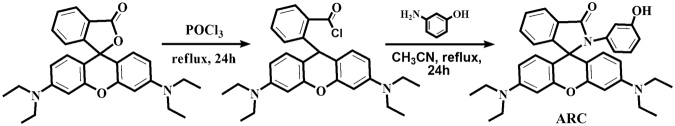
Rhodamine B (4.80 g, 10 mmol) was taken in a 250 mL RB flask containing 100 mL freshly distilled POCl3 and allowed to reflux for 24 h under a dinitrogen blanket. The excess amount of POCl3 was removed in a rotary evaporator. The acid chloride was dried under vacuum and used for the next step without further purification. This was dissolved in dry acetonitrile (100 mL) then 3-amino phenol (1.63 g, 15 mmol) followed by triethylamine (5 mL) were added slowly. Once the addition was complete, the resulting mixture was heated to reflux for 24 h. The reaction mixture was then concentrated in a rotary evaporator under low pressure and then extracted with dichloromethane. After drying it over anhydrous Na2SO4, the organic layer was evaporated completely. The residue was purified by column chromatography with the eluent chloroform: ethyl acetate (6:1, v/v) to get the product ARC with 74% yield (Fig. 5). ARC was then recrystallized from methanol as a pale pink solid. 1H NMR (400 MHz, DMSO-d6): d (ppm) = 9.34 (-OH, s, 1 H), 7.84–7.86 (d, 1 H, J = 8 Hz), 7.51–7.55 (m, 2 H, J = 16 Hz), 7.00–7.02 (d, 1 H, J = 8 Hz), 6.88–6.92 (t, 1 H, J = 16 Hz), 6.47–6.52 (m, 3 H, J = 20 Hz), 6.35–6.40 (m, 3 H, J = 20 Hz), 6.280–6.286 (d, 2 H, J = 2 Hz), 6.19–6.20 (d, 1 H, J = 4 Hz). 13C NMR (400 MHz, DMSO-D6): d (ppm) = 167.18, 157.54, 154.15, 152.65, 148.76, 138.42, 133.74, 129.95, 129.29, 128.82, 124.16, 123.26, 116.78, 114.14, 113.75, 108.55, 106.18, 97.72, 44.07, 12.87. HRMS (TOF MS): (m/z, %): Calcd. for C34H35N3O3: 533.27. Found: m/z = 534.27 (M + H+; 100%). 1H, 13C NMR and HRMS spectra are shown in Figs S1–S3.
Figure 5.
Synthesis of the chemosensor ARC.
UV-vis and fluorescence titration
A stock solution of ARC (1 × 10–6 M) was prepared in water-acetonitrile (10:1, v/v). 1 × 10−5 M DCP solution was prepared in Millipore water. All experiments were carried out in aqueous medium at neutral pH. During titration, each time a 1 × 10−6 M solution of ARC was filled each time in a quartz optical cell with 1 cm optical path length and DCP stock solution was added into the quartz optical cell gradually by using a micropipette. For all fluorescence measurements, excitations were provided at 520 nm, and emissions were collected from 540 to 640 nm.
Kinetic Study
The kinetic study of the reaction between ARC and DCP has been done by measuring the fluorescence spectra after mixing ARC and DCP in a cubic 4-sided quartz cell of 3 ml. The reaction was carried out at room temperature under the excess amount of DCP (initial concentration [ARC] ≪ [DCP]) and the reaction was expected to reach 100% conversion of chemosensor ARC to ARC-DCP complex. ARC and DCP solution of concentration 1 µM and 10 µM, respectively, were prepared in aqueous medium and mixed to investigate the kinetic. The excitation wavelength was 520 nm.
Live Cell Imaging
Cell Culture
A549 cell (Human cell A549, ATCC No CCL-185) lines were prepared from continuous culture in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma Chemical Co., St. Louis, MO) supplemented with 10% fetal bovine serum (Invitrogen), penicillin (100 μg/mL), and streptomycin (100 μg/mL). Cells were initially propagated in 75 cm2 polystyrene, filter-capped tissue culture flask in an atmosphere of 5% CO2 and 95% air at 37 °C in CO2 incubator. When the cells reached the logarithmic phase, the cell density was adjusted to 1.0 × 105 per/well in culture media. The cells were then used to inoculate in a glass bottom dish, with 1.0 mL (1.0 × 104 cells) of cell suspension in each dish. After cell adhesion, culture medium was removed. The cell layer was rinsed twice with phosphate buffered saline (PBS) (pH 7.0), and then treated with ARC or ARC and DCP according to the experimental need.
Cell imaging study
For confocal imaging studies, 1 × 104 A549 cells in 1000 μL of medium, were seeded on sterile 35 mm glass bottom culture dish (ibidi GmbH, Germany), and incubated at 37 °C in a CO2 incubator for 10 hours. Then cells were washed with 500 μL DMEM followed by incubation with ARC (1 µM) dissolved in 1000 μL DMEM at 37 °C for 1 h in a CO2 incubator and cells were washed thrice with phosphate buffered saline (PBS) (pH 7.0) to remove excess ARC observed under an Olympus IX81 microscope equipped with a FV1000 confocal system using 1003 oil immersion Plan Apo (N.A. 1.45) objectives. Images obtained through section scanning were analyzed by fluorochrome filter POPO-3 with excitation at 534 nm monochromatic laser beams, and emission spectra were integrated over the range 580 nm (single channel). The cells were again incubated with DCP (10 µM) for 10 min and excess DCP was washed thrice with PBS (pH 7.0) followed by observations under microscope. For all images, the confocal microscope settings, such as transmission density, and scan speed, were held constant to compare the relative intensity of intracellular fluorescence. All images were captured using 2% lesser light.
Cytotoxicity assay
In vitro studies established the ability of the chemosensor ARC to detect DCP in biological system. Human cell A549 (ATCC No CCL-185) were used as models. However, to materialize this objective, it is a prerequisite to assess the cytotoxic effect of ARC and ARC-DCP complex on live cells. The well-established MTT assay was adopted to study cytotoxicity of above mentioned complexes at varying concentrations. A cytotoxicity measurement for each experiment shows that the chemosensor ARC does not have any toxicity on the tested cells and ARC-DCP complex does not exert any significant adverse effect on cell viability at tested concentrations.
Imaging of Catfish brain
Methodology
Live specimens of adult male catfish, Clarias batrachus (Actinopterygii, Siluriformes, Clariidae), average body weight 65.48 ± 3.91 g, were maintained in laboratory condition as described earlier43. All the experimental protocols were approved by the Institutional Animal Ethical Committee (Reg. no.: 1819/GO/Ere/S/15/CPCSEA) of Visva-Bharati University, Santiniketan, India. We also confirm that all the experiments were performed in accordance with the relevant guidelines and regulations. During autopsy, fishes were killed by decapitation; brain was dissected out and placed immediately in phosphate buffer saline, PBS (0.1 M phosphate buffer; pH 7.4 containing 0.6% NaCl and 0.01% Triton X-100). Catfish brain, specifically the optic tectum (telencephalon) region was sliced using sharp blades and incubated in 6 well culture plate (SPL, India), either in PBS alone or supplemented with the ARC (1 µM) for 15 min in a humidified chamber at 23 ± 20 C. Brain slices were washed thrice with PBS, probed with the guest (DCP: 10 µM), for 5 min with gentle shaking (40 rpm), washed again to remove unbound guest and observed under an inverted fluorescent microscope (DMi8, Leica Microsystems, Germany) using HI PLAN I 10 × /0.22 PH1 objective and ROHD filter (excitation and emission ranges at 520 nm and 580 nm respectively). Images were acquired through Leica EC4 Digital Camera and DFC 3000 G and processed using LAS X software.
Electronic supplementary material
Acknowledgements
P.S. acknowledges SERB-DST, Govt. of India for awarding her the young scientist grant [Project file no. SB/FT/CS-021/2014]. H.S.S. thanks UGC, India for research fellowship.
Author Contributions
H.S.S. performed the experiments and wrote the manuscript. A.G. and S.D. prepared some figures and supported technically. S. Maitra performed the Catfish experiment. P.K.M. and S. Mandal performed the cell imaging and cytotoxicity assay experiments. P.S. proposed the original idea and supervised the project. All the authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21780-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Croddy E. Urban terrorism: chemical warfare in Japan. Jane’s Intelligence Rev. 1995;7:520–527. [Google Scholar]
- 2.Masuda N, et al. Sarin poisoning in Tokyo subway. The Lancet. 1995;345:1446–1447. doi: 10.1016/S0140-6736(95)92637-2. [DOI] [PubMed] [Google Scholar]
- 3.Volans AP. Sarin: guidelines on the management of victims of a nerve gas attack. J. Accid. Emerg. Med. 1996;13:202–206. doi: 10.1136/emj.13.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eubanks LM, Dickerson TJ, Janda KD. Technological advancements for the detection of and protection against biological and chemical warfare agents. Chem. Soc. Rev. 2007;36:458–470. doi: 10.1039/b615227a. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Qare AW, Abou-Donia MB. Sarin: health effects, metabolism, and methods of analysis. Food and Chemical Toxicology. 2002;40:1327–1333. doi: 10.1016/S0278-6915(02)00079-0. [DOI] [PubMed] [Google Scholar]
- 6.“Chemical attack of 4 April 2017 (Khan Sheikhoun): Clandestine Syrian chemical weapons programme”. Retrieved 2017-04-26.
- 7.Bui M-PN, Abbas A. Simple and rapid colorimetric detection of p-nitrophenyl substituent organophosphorous nerve agents. Sensors and Actuators B. 2015;207:370–374. doi: 10.1016/j.snb.2014.10.010. [DOI] [Google Scholar]
- 8.Sayed SE, et al. A Chromogenic Probe for the Selective Recognition of Sarin and Soman Mimic DFP. Chemistry Open. 2014;3:142–145. doi: 10.1002/open.201402014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace KJ, Morey J, Lynch VM, Anslyn EV. Colorimetric detection of chemical warfare stimulants. New J. Chem. 2005;29:1469–1474. doi: 10.1039/b506100h. [DOI] [Google Scholar]
- 10.Gotor R, et al. Nerve agent simulant detection by using chromogenic triaryl methane cation probes. Tetrahedron. 2012;68:8612–8616. doi: 10.1016/j.tet.2012.07.091. [DOI] [Google Scholar]
- 11.Wu X, Wu Z, Han S. Chromogenic and fluorogenic detection of a nerve agent simulant with a rhodamine-deoxylactam based sensor. Chem. Commun. 2011;47:11468–11470. doi: 10.1039/c1cc15250e. [DOI] [PubMed] [Google Scholar]
- 12.Zhang SW, Swager TM. Fluorescent detection of chemical warfare agents: functional group specific ratiometric chemosensors. J. Am. Chem. Soc. 2003;125:3420–3421. doi: 10.1021/ja029265z. [DOI] [PubMed] [Google Scholar]
- 13.Knapton D, Burnworth M, Rowan SJ, Weder C. Fluorescent organometallic sensors for the detection of chemical-warfare-agent mimics. Angew. Chem. Int. Ed. 2006;45:5825–5829. doi: 10.1002/anie.200601634. [DOI] [PubMed] [Google Scholar]
- 14.Goswami S, Manna A, Paul S. Rapid ‘naked eye’ response of DCP, a nerve agent simulant: from molecules to low-cost devices for both liquid and vapour phase detection. RSC Adv. 2014;4:21984–21988. doi: 10.1039/C4RA01060D. [DOI] [Google Scholar]
- 15.So H-S, Angupillai S, Son YA. Prompt liquid-phase visual detection and low-cost vapor-phase detection of DCP, a chemical warfare agent mimic. Sensors and Actuators B. 2016;235:447–456. doi: 10.1016/j.snb.2016.05.106. [DOI] [Google Scholar]
- 16.Mahapatra AK, et al. A cyclization-induced emission enhancement (CIEE)-based ratiometric fluorogenic and chromogenic probe for the facile detection of a nerve agent simulant DCP. Chem. Commun. 2015;51:9729–9732. doi: 10.1039/C5CC02991K. [DOI] [PubMed] [Google Scholar]
- 17.Wallace KJ, et al. Detection of chemical warfare simulants by phosphorylation of a coumarin oximate. Chem. Commun. 2006;37:3886–3888. doi: 10.1039/b609861d. [DOI] [PubMed] [Google Scholar]
- 18.Sohn H, Letant S, Sailor MJ, Trogler WC. Detection of Fluorophosphonate Chemical Warfare Agents by Catalytic Hydrolysis with a Porous Silicon Interferometer. J. Am. Chem. Soc. 2000;122:5399–5400. doi: 10.1021/ja0006200. [DOI] [Google Scholar]
- 19.Lin Y, Lu F, Wang J. Disposable Carbon Nanotube Modified Screen-Printed Biosensor for Amperometric Detection of Organophosphorus Pesticides and Nerve Agents. Electroanalysis. 2004;16:145–149. doi: 10.1002/elan.200302933. [DOI] [Google Scholar]
- 20.Zhou Y, Yu B, Shiu E, Levon K. Potentiometric sensing of chemical warfare agents: surface imprinted polymer integrated with an indium tin oxide electrode. Anal. Chem. 2004;76:2689–2693. doi: 10.1021/ac035072y. [DOI] [PubMed] [Google Scholar]
- 21.Yu D, et al. Aqueous sol-gel encapsulation of genetically engineered Moraxella spp. cells for the detection of organophosphates. Biosens. Bioelectron. 2005;20:1433–1437. doi: 10.1016/j.bios.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Hammond MH, et al. A novel chemical detector using cermet sensors and pattern recognition methods for toxic industrial chemicals. Sensors and Actuators B. 2006;116:135–144. doi: 10.1016/j.snb.2005.12.065. [DOI] [Google Scholar]
- 23.Yang Y, Ji H-F, Thundat T. Nerve agents’ detection using a Cu2+/L-cysteine bilayer-coated microcantilever. J. Am. Chem. Soc. 2003;125:1124–1125. doi: 10.1021/ja028181n. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann-Thompson C, et al. Hydrogen-Bond Acidic Hyperbranched Polymers for Surface Acoustic Wave (SAW) Sensors. Chem. Mater. 2004;16:5357–5364. doi: 10.1021/cm040346z. [DOI] [Google Scholar]
- 25.Pushkarsky MB, Webber ME, Macdonald T, Patel CKN. High-sensitivity, high-selectivity detection of chemical warfare agents. Applied Physics Letter. 2006;88:044103-1–044103-3. doi: 10.1063/1.2166692. [DOI] [Google Scholar]
- 26.Jenkins AL, Uy OM, Murray GM. Polymer Based Lanthanide Luminescent Sensors for the Detection of Nerve Agents. Anal. Commun. 1997;34:221–224. doi: 10.1039/a704220e. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins AL, Uy OM, Murray GM. Polymer-based lanthanide luminescent sensor for detection of the hydrolysis product of the nerve agent Soman in water. Anal. Chem. 1999;71:373–378. doi: 10.1021/ac980985r. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins AL, Yin R, Jensen JL. Molecularly imprinted polymer sensors for pesticide and insecticide detection in water. Analyst. 2001;126:798–802. doi: 10.1039/b008853f. [DOI] [PubMed] [Google Scholar]
- 29.Russell AJ, Berberich JA, Drevon GF, Koepsel RR. Biomaterials for mediation of chemical and biological warfare agents. Annu. Rev. Biomed. Eng. 2003;5:1–27. doi: 10.1146/annurev.bioeng.5.121202.125602. [DOI] [PubMed] [Google Scholar]
- 30.Wheelis M. Biotechnology and chemical weapons control. Pure Appl. Chem. 2002;74:2247–2251. doi: 10.1351/pac200274122247. [DOI] [Google Scholar]
- 31.Steiner WE, Klopsch SJ, English WA, Clowers BH, Hill HH. Detection of a chemical warfare agent simulant in various aerosol matrixes by ion mobility time-of-flight mass spectrometry. Anal. Chem. 2005;77:4792–4799. doi: 10.1021/ac050278f. [DOI] [PubMed] [Google Scholar]
- 32.Sahoo P, et al. Pyrene appended thymine derivative for selective turn-on fluorescence sensing of uric acid in live cells. RSC Adv. 2016;6:66774–66778. doi: 10.1039/C6RA15980J. [DOI] [Google Scholar]
- 33.Sarkar HS, Das S, Uddin MR, Mandal S, Sahoo P. Selective Recognition and Quantification of 2,3-Bisphosphoglycerate in Human Blood Samples by a Rhodamine Derivative. Asian J. Org. Chem. 2017;6:71–75. doi: 10.1002/ajoc.201600516. [DOI] [Google Scholar]
- 34.Sarkar HS, Das S, Rissanen K, Sahoo P. First chemosensor for rapid detection and quantification of L-4-Hydroxyproline in collagen and other bio samples. Anal. Chem. 2017;89:13054–13057. doi: 10.1021/acs.analchem.7b04430. [DOI] [PubMed] [Google Scholar]
- 35.Sarkar HS, et al. “Turn-on” fluorescence sensing of cytosine: Development of a chemosensor for quantification of cytosine in human cancer cells. RSC Adv. 2017;7:54008–54012. doi: 10.1039/C7RA11096K. [DOI] [Google Scholar]
- 36.Royo S, et al. Chromogenic and fluorogenic reagents for chemical warfare nerve agents’ detection. Chem. Commun. 2007;46:4839–4847. doi: 10.1039/b707063b. [DOI] [PubMed] [Google Scholar]
- 37.Quang DT, Kim JS. Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem. Rev. 2010;110:6280–6303. doi: 10.1021/cr100154p. [DOI] [PubMed] [Google Scholar]
- 38.Jo J, Lee D. Turn-On Fluorescence Detection of Cyanide in Water: Activation of Latent Fluorophores through Remote Hydrogen Bonds That Mimic Peptide β-Turn Motif. J. Am. Chem. Soc. 2009;131:16283–16291. doi: 10.1021/ja907056m. [DOI] [PubMed] [Google Scholar]
- 39.Reddy GU, Ali F, Taye N, Chattopadhyay S, Das A. A new turn on Pd(2 + )-specific fluorescence probe and its use as an imaging reagent for cellular uptake in Hct116 cells. Chem. Commun. 2015;51:3649–3652. doi: 10.1039/C4CC10171E. [DOI] [PubMed] [Google Scholar]
- 40.Majumdar A, et al. α-Amino Acid Derived Benzimidazole-Linked Rhodamines: A Case of Substitution Effect at the Amino Acid Site toward Spiro Ring Opening for Selective Sensing of Al3+ Ions. Inorganic Chemistry. 2017;56:8889–8899. doi: 10.1021/acs.inorgchem.7b00835. [DOI] [PubMed] [Google Scholar]
- 41.Tripathi A, Rahman M, Chakraborty B. Distribution pattern of acetylcholinesterase in the optic tectum of two Indian air breathing teleosts. Annals of Neurosciences. 2013;20:7–9. doi: 10.5214/ans.0972.7531.200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratha J, et al. A sphingolipid rich lipid fraction isolated from attenuated Leishmania donovani promastigote induces apoptosis in mouse and human melanoma cells in vitro. Molecular and Cellular Biochemistry. 2006;290:113–123. doi: 10.1007/s11010-006-9174-y. [DOI] [PubMed] [Google Scholar]
- 43.Nath P, Maitra S. Role of two plasma vitellogenins from Indian major carp (Cirrhinus mrigala) in catfish (Clarias batrachus) vitellogenesis. General and Comparative Endocrinology. 2001;124:30–44. doi: 10.1006/gcen.2001.7686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.