Abstract
Beige adipocytes in white adipose tissue (WAT) have received considerable recognition because of their potential protective effect against obesity. Pycnogenol (PYC), extracted from French maritime pine bark, has anti-inflammatory and antioxidant properties and can improve lipid profiles. However, the effect of PYC on obesity has never been explored. In this study, we investigated the effects of PYC on obesity and WAT browning in apolipoprotein E- (ApoE-) deficient mice. The results showed that PYC treatment clearly reversed body weight and the mass of eWAT gain resulting from a high-cholesterol and high-fat diet (HCD), but no difference in food intake. The morphology results showed that the size of the adipocytes in the PYC-treated mice was obviously smaller than that in the HCD-fed mice. Next, we found that PYC upregulated the expression of genes related to lipolysis (ATGL and HSL), while it decreased the mRNA level of PLIN1. PYC significantly increased the expression of UCP1 and other genes related to beige adipogenesis. Additionally, PYC increased the expression of proteins related to the protein kinase A (PKA) signaling pathway. The findings suggested that PYC decreased obesity by promoting lipolysis and WAT browning. Thus, PYC may be a novel therapeutic target for obesity.
1. Introduction
The prevalence of obesity, which has increased regardless of region and country in recent decades, is associated with abnormal adipose tissue distribution [1, 2]. Obesity, especially central obesity, is an independent risk factor for the onset of type 2 diabetes. Excessive body fat accumulation has a profound effect on tissue insulin sensitivity, which in turn affects systemic glucose homeostasis; the underlying molecular mechanism is not entirely clear. In mammals, adipose tissue is composed of white adipose tissue (WAT) and brown adipose tissue (BAT), and their dysfunction can lead to overweight and obesity [2]. WAT stores energy in the form of triglycerides, and excess WAT leads to metabolic abnormalities [3]. However, BAT is specialized to burn fat and generate energy in the form of heat because of its higher mitochondrial content with UCP1 expression, which plays an important role in the regulation of energy balance [3, 4]. Recently, a new type of adipocyte, brown-like adipocytes (also called beige adipocytes), was discovered in WAT. Beige adipocytes are also thermogenic adipocytes because of their large mitochondrial content with UCP1 expression, and they generate heat under cold exposure. Beige fat exhibits similar metabolic properties to BAT, and both control energy homeostasis [4–7]. Thus, brown fat and beige fat are potential therapeutic targets for obesity [4, 7].
UCP1, a key regulator of thermogenesis, is regarded as a BAT-specific transporter protein, and its activation gives BAT and beige adipocytes the capability of thermogenesis [4, 8, 9]. It is recognized that the function of UCP1 is activated by the sympathetic nervous system (SNS). The SNS is known to promote lipolysis via the β3-adrenergic signaling pathway in adipose tissue. β3-adrenergic receptors (β3-ARs) are expressed on the surface of adipocytes and activate protein kinase A (PKA), leading to increased lipolysis by activating hormone-sensitive lipase (HSL). PKA further activates p38 mitogen-activated protein kinase (MAPK) and increases the expression of UCP1, thereby promoting thermogenesis in BAT [10, 11]. However, the increase in UCP1 expression manifests as white adipocytes, which induce the browning of WAT [12, 13]. Many transcriptional regulators, such as PPARγ, PGC1α, PRDM16, and Cidea, participate in the process of WAT browning and enable WAT to achieve similar biological functions as BAT [12, 13].
Pycnogenol (PYC), one of the natural antioxidants extracted from French maritime pine bark, is composed of procyanidins, phenolic acids, and bioflavonoids [14, 15]. Previous studies have shown that PYC has a beneficial protective effect against cardiovascular disease via its anti-inflammatory effects [16, 17]. Clinical studies have also shown that PYC significantly reduced waist circumference and triglyceride and cholesterol levels [18, 19]. In vitro, PYC supplementation decreased the triglyceride level by stimulating lipolysis in adipocytes [11]. However, few studies have focused on the effects of PYC on the browning of white adipocytes. Apolipoprotein E- (ApoE-) knockout mice are the most widely used atherosclerosis mouse model [20]. We also chose this model to explore the effect of PYC on atherosclerosis in the original study, the results of which have been published [16]. At the same time, we found that ApoE-knockout mice fed with a high-cholesterol and high-fat diet (HCD) developed obesity, which was improved in the PYC-treated groups. In this study, we investigated, for the first time, the effects of PYC on the expression of genes related to the browning of WAT in ApoE-deficient mice fed with an HCD, which may provide a novel strategy for preventing and treating obesity.
2. Materials and Methods
2.1. Animals and Treatment
The animal experiment was approved by the Animal Research Committee of China Medical University (Shenyang, China). One hundred male ApoE-deficient mice (7 weeks) were obtained from Vital River (Beijing, China), and they had free access to food and water. After adapting to the feeding regimen, the mice were randomly divided into 3 groups, namely, the blank group (n = 50), the 30 mg·kg−1·day−1 PYC plus HCD group (LoPYC + HCD group, n = 25), and the 100 mg·kg−1·day−1 PYC plus HCD group (HiPYC + HCD group, n = 25), which were treated via oral gavage for 10 weeks with distilled water, 30 mg·kg−1·day−1 PYC, and 100 mg·kg−1·day−1 PYC, respectively. PYC, which was generously provided by Horphag Research (Geneva, Switzerland), was dissolved in 0.1 ml of distilled water and administered orally for 10 weeks. During the first 2 weeks, these mice were fed a regular rodent chow diet. After 2 weeks, the mice in the blank group were divided into 2 subgroups, that is, the normal diet group (ND group, n = 25) and the HCD group (n = 25), which were fed a regular rodent chow diet and an HCD (Research Diet; NJ, USA), respectively, for 8 weeks. The mice in the PYC-treated groups were switched to an HCD for 8 weeks. Body weight and food intake were measured once per week. At the end of the experiment, all mice were sacrificed, and epididymal fat was harvested and stored at −80°C.
2.2. Histology
The epididymal fat samples were cut into sections (10 μm) using a Leica cryostat and were then stained using hematoxylin and eosin (H&E) to visualize the size of adipocytes in epididymal white adipose tissue (eWAT) using a microscope (OLYMPUS BX51, Japan) (n = 5 mice per group). In each section from each mouse, the number of cells within four randomly chosen areas (100 × 100 μm) was counted, and the mean value was calculated.
2.3. Immunohistochemistry
For UCP1 staining, tissues were embedded in paraffin, and tissue sections were cut to a thickness of 5 μm. Paraffin sections were deparaffinized in xylene, hydrated with ethanol, and subjected to heat-mediated antigen retrieval. Then, the sections were incubated with 3% H2O2 for 15 minutes and blocked with 1/100 diluted goat serum for 15 minutes. The sections were then incubated with a rabbit antibody against UCP1 (1 : 200, Abcam, Cambridge, UK) overnight at 4°C, which was followed by incubation with a biotinylated secondary antibody (Beyotime, China) for 30 minutes at room temperature and treatment with streptavidin-peroxidase complex (Beyotime, China) for 15 minutes. Staining was performed using diaminobenzidine (DAB). Finally, the sections were counterstained with hematoxylin and dehydrated in ethanol.
2.4. Real-Time Quantitative PCR
Total RNA was isolated from eWAT using RNAiso Plus (Takara, Dalian, China) according to the manufacturer's instructions, and cDNA was synthesized from RNA using the PrimeScript™ RT Master Mix (Takara). Primers for the analysis were designed as follows: β-actin, 5′-GTTCCGATGCCCTGAGGCTC and 5′-CAGACAGCACTGTGTTGGCA; UCP1, 5′-ACTGCCACACCTCCAGTCATT and 5′-CTTTGCCTCACTCAGGATTGG; PRDM16, 5′-CAGCACGGTGAAGCCATTC and 5′-GCGTGCATCCGCTTGTG; Cidea, 5′-TGCTCTTCTGTATCGCCCAGT and 5′-GCCGTGTTAAGGAATCTGCTG; PGC1a, 5′-CCCTGCCATTGTTAAGACC and 5′-TGCTGCTGTTCCTGTTTTC; HSL, 5′-TCCTGGAACTAAGTGGACGCAAG and 5′-CAGACACACTCCTGCGCATAGAC; ATGL, 5′-AACACCAGCATCCAGTTCAA and 5′-GGTTCAGTAGGCCATTCCTC; PPARa, 5′-CCCTGAACATCGAGTGTCGAA and 5′-TCGCCGAAAGAAGCCCTTA; PLIN1, 5′-GTCGTCATGGCTCTCATCCT and 5′-GGCCAACACTCTTTCTCGAC; PPAR?, 5′-ATTCTGGCCCACCAACTTCGG and 5′-TGGAAGCCTGATGCTTTATCCCCA; and AP2, 5′-TGGGAACCTGGAAGCTTGTCTC and 5′-GAATTCCACGCCCAGTTTGA. Quantitative real-time PCR was performed to analyze gene expression using the SYBR Premix Ex Tap II (Takara) and the LightCycler 480 System (Roche, Germany) (n = 6–8 mice per group). The mRNA levels were normalized to β-actin.
2.5. Western Blot Analysis
Proteins were extracted from eWAT using a lysis buffer containing protease and phosphatase inhibitors (KeyGen Biotech, Nanjing, China) by homogenization and were then centrifuged at 13000g for 10 minutes at 4°C (n = 3 mice per group). The protein concentration was determined using a BCA kit (Beyotime Biotechnology, China). The protein extracts were boiled for 8 minutes at 100°C and then stored at −20°C. After 12% SDS-acrylamide gel electrophoresis, the proteins were transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, USA) and were then blocked using 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween 20 for 2 h. The membranes were incubated with primary antibody dilutions overnight at 4°C, including a mouse antibody against β-actin (1 : 2000, Zhongshan Golden Bridge, China), rabbit antibody against UCP1 (1 : 1000, Abcam, Cambridge, UK), rabbit antibody against phospho-p38 MAPK (Thr180/Tyr182) (1 : 1000, Cell Signaling Technology, USA), rabbit antibody against PKA (1 : 1000, Cell Signaling Technology), rabbit antibody against phospho-PKA (Thr197) (1 : 1000, Cell Signaling Technology), rabbit antibody against HSL (1 : 1000, Cell Signaling Technology), and rabbit antibody against PGC1a (1 : 1000, Wanleibio, China). The membranes were washed three times using Tris-buffered saline with 0.05% Tween 20 and were incubated for 2 h with horseradish peroxidase- (HRP-) labeled anti-rabbit or anti-mouse IgG (1 : 5000, Zhongshan Golden Bridge, China). Antibody-specific expression was detected using the DNR MicroChemi System (DNR Bio-Imaging Systems Ltd., USA) and was analyzed using ImageJ software.
2.6. Statistical Analysis
All data are presented as the means ± SE. The statistical analyses were performed using one-way analysis of variance (ANOVA) and SPSS 22.0 software (SPSS Inc., Chicago, IL, USA), followed by the least significant difference (LSD) test for multiple comparisons. P < 0.05 was considered statistically significant.
3. Results
3.1. PYC Reduced Body Weight Gain in ApoE-Deficient Mice
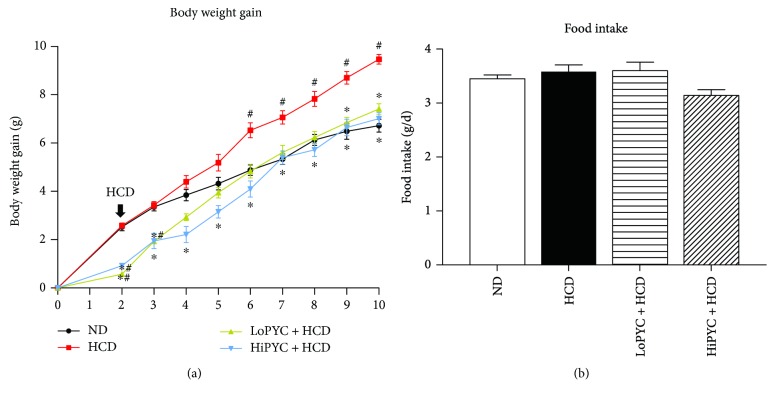
To investigate the preventative effect of PYC on obesity, mice received the PYC treatment for 10 weeks. After 2 weeks, the body weight gain was 0.76, 0.92, and 2.55 g for the LoPYC + HCD, HiPYC + HCD, and blank groups, respectively (Figure 1(a)). The significant difference between the PYC-treated and blank groups suggested that PYC may have a preventative effect on obesity. The blank group was divided into the ND group and the HCD group. The mice in the HCD and PYC-treated groups were given an HCD for 8 weeks, and body weight was measured weekly. Body weight gain occurred faster in the HCD group than in the ND group. This increase differed significantly between the HCD and ND groups starting at 6 weeks. The PYC decreased the HCD-induced body weight gain throughout the experiment, although there were significant differences only between the LoPYC + HCD group and the HCD group starting at 9 weeks (Figure 1(a)). The data confirmed the preventative effect of PYC on obesity. However, there was no difference in food intake between the HCD and PYC-treated groups (Figure 1(b)).
Figure 1.
PYC lowered body weight gain. (a) Change in body weight gain and (b) daily food intake. The data are expressed as the means ± SE for 20 to 25 mice per group. #p < 0.05 versus the ND group; ∗p < 0.05 versus the HCD group.
3.2. PYC Reduced Visceral Fat and the Size of Adipocytes within eWAT
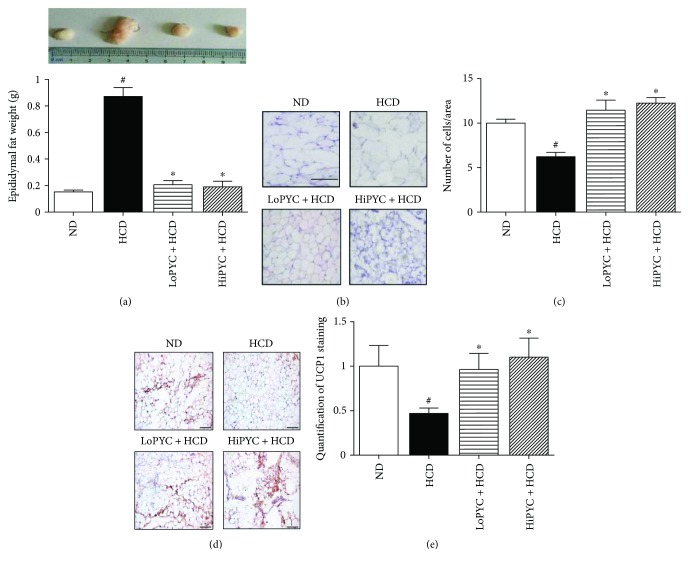
The HCD-induced excessive fat accumulation was assessed by measuring the eWAT mass. The eWAT mass in the HCD mice was greater than that in the ND mice (0.87 ± 0.06 g versus 0.15 ± 0.01 g). The eWAT mass in the low-dose and high-dose PYC-treated mice was significantly lower than that in the HCD mice (0.21 ± 0.03 g, 0.19 ± 0.04 g versus 0.87 ± 0.06 g) (Figure 2(a)). The H&E staining revealed that PYC supplementation significantly decreased the size of adipocytes in eWAT (Figure 2(b)). The number of cells/area in the H&E-stained sections was counted, and the results suggested that the number of cells in the PYC-treated mice was significantly greater than that in the HCD mice (Figure 2(c)).
Figure 2.
PYC decreased the eWAT mass and the size of adipocytes within eWAT. (a) The eWAT mass; (b) H&E staining of eWAT to observe the size of adipocytes, scale bar = 50 μm; and (c) the number of cells/area in H&E-stained sections. (d) UCP1 staining in eWAT. Pictures are shown at 20x magnification; scale bar = 100 μm. (e) Quantification of UCP1 staining in eWAT. The data are expressed as the means ± SE for 5 mice per group. #p < 0.05 versus the ND group; ∗p < 0.05 versus the HCD group.
3.3. PYC Altered the Expression of Genes Related to Lipid Metabolism
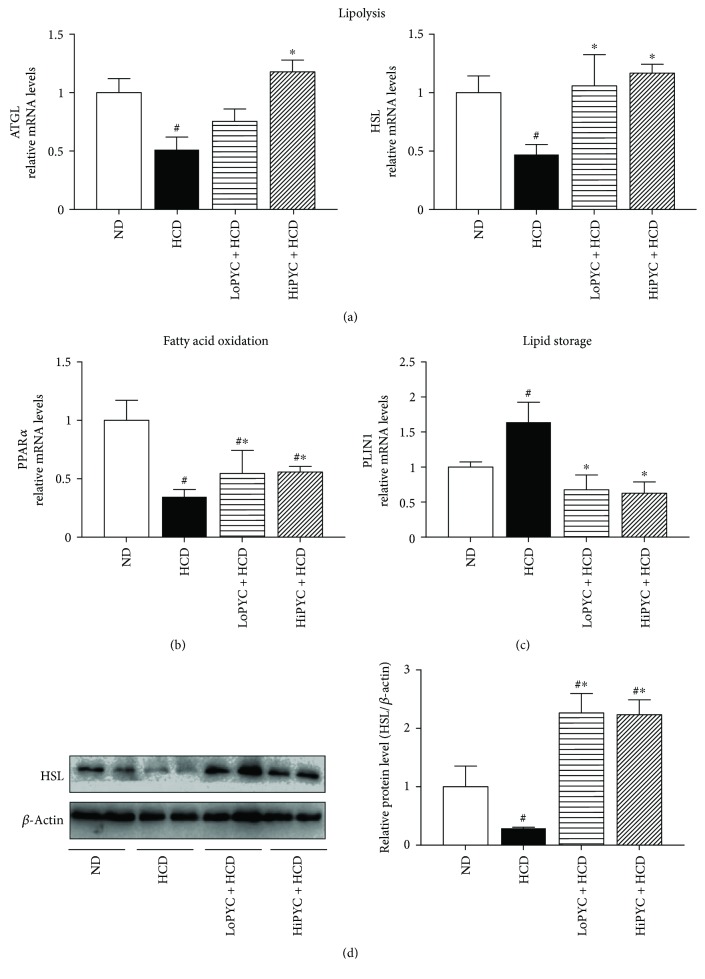
To determine the likely mechanism by which PYC decreased the mass of eWAT, we assessed the expression of genes related to lipid metabolism. The expression of ATGL and HSL, genes that are related to lipolysis, was significantly increased in the PYC-treated groups compared with that in the HCD group. The expression of HSL mRNA was more than 2-fold greater in the PYC-treated groups than in the HCD group. Consistent with these results, the expression of ATGL mRNA in the HiPYC + HCD group was 2-fold higher than that in the HCD group, although this increase was not significantly different between the LoPYC + HCD group and the HCD group (Figure 3(a)). In addition, the results of Western blot analysis showed that the protein level of HSL in the PYC-treated groups was higher than that in the HCD group (Figure 3(d)). Moreover, PPARα has been shown to play a role in regulating fatty acid oxidation [21]. The mRNA level of PPARα in the low-dose and high-dose PYC-treated mice was 2-fold higher than that in the HCD mice (Figure 3(b)). PLIN1, a member of the PAT family, is a key gene that regulates lipid storage in WAT [22–24]. The mRNA level of PLIN1 in the HCD mice was 1.6-fold greater than that in the ND mice, while the levels in the low-dose and high-dose PYC-treated mice were approximately 58% and 62% lower than those in the HCD mice, respectively (Figure 3(c)).
Figure 3.
PYC altered the expression of genes related to lipid metabolism. (a) The mRNA levels of genes (ATGL and HSL) related to lipolysis; (b) the mRNA level of PPARα (related to fatty acid oxidation); (c) the mRNA level of PLIN1; and (d) the protein level of HSL. The data are expressed as the means ± SE for 6 to 8 mice per group. #p < 0.05 versus the ND group; ∗p < 0.05 versus the HCD group.
3.4. PYC Enhanced the Expression of Genes Related to WAT Browning.
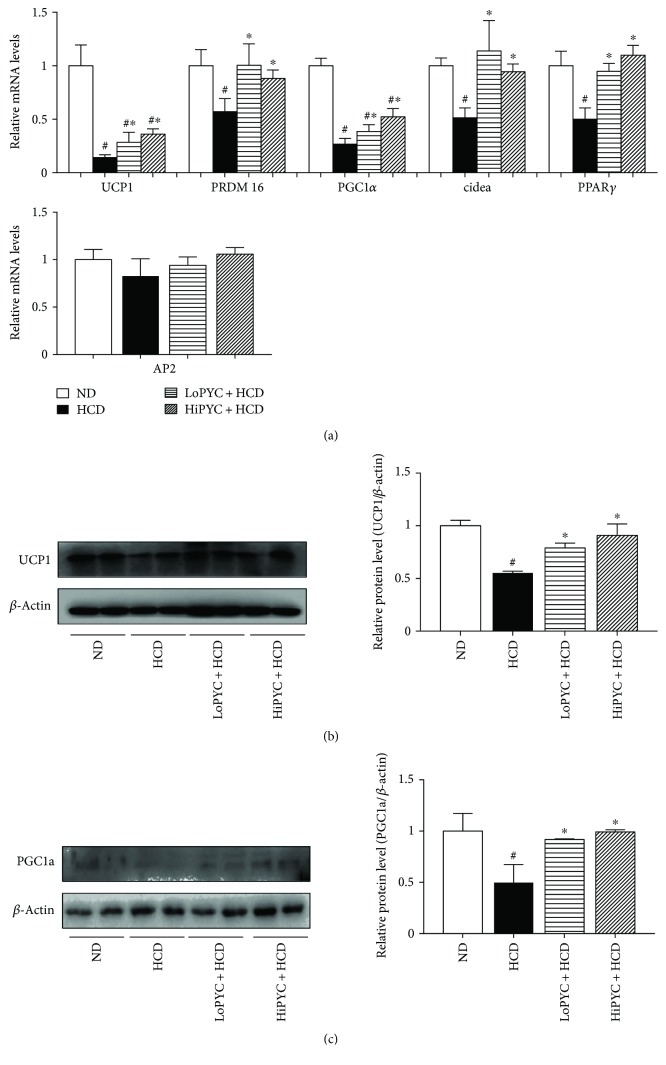
The increase in brown fat-specific proteins, such as UCP1, PRDM16, Cidea, and PGC1α, in white adipocytes induces its acquisition of brown adipocyte features [12, 13]. To investigate the effect of PYC on WAT browning, we analyzed brown fat-specific gene expression in eWAT. The mRNA level of UCP1 in the HCD-fed mice was 80% less than that in the ND-fed mice (Figure 4(a)). The level of UCP1 in the LoPYC + HCD group was 2-fold higher than that in the HCD group, while that in the HiPYC + HCD group was 2.5-fold greater (Figure 4(a)). Consistent with these results, the mRNA levels of genes related to WAT browning, such as PRDM16, PGC1α, Cidea, and PPARγ, showed a similar trend (Figure 4(a)). The mRNA levels of PRDM16, PGC1α, Cidea, and PPARγ in the HCD-fed mice were approximately 45%, 73%, 50%, and 50% lower than those in the ND-fed mice, respectively. The low and high PYC doses reversed the phenomenon that resulted from the HCD. The level of PRDM16 was increased approximately 1.75- and 1.5-fold in the LoPYC + HCD and HiPYC + HCD mice, respectively, compared with that in the HCD-fed mice. The level of PGC1α was increased approximately 1.7-fold in the LoPYC + HCD mice compared with that in the HCD-fed mice, while there was nearly a 2-fold increase in the HiPYC + HCD group compared with the HCD group. Cidea and PPARγ mRNA levels exhibited an approximately 2-fold increase in the LoPYC + HCD and HiPYC + HCD mice compared with those in the HCD-fed mice. However, PYC did not affect the expression of AP2 (Figure 4(a)). PYC increased the number of UCP1-positive cells (Figures 2(d) and 2(e)). In addition, the protein levels of UCP1 and PGC1 in eWAT from the PYC-treated groups were higher than those in the HCD group (Figures 4(b) and 4(c)).
Figure 4.
PYC enhanced the expression of genes and proteins related to WAT browning. (a) The expression of genes related to WAT browning and the protein levels of (b) UCP1 and (c) PGC1α. The data are expressed as the means ± SE for 6 to 8 mice per group. #p < 0.05 versus the ND group; ∗p < 0.05 versus the HCD group.
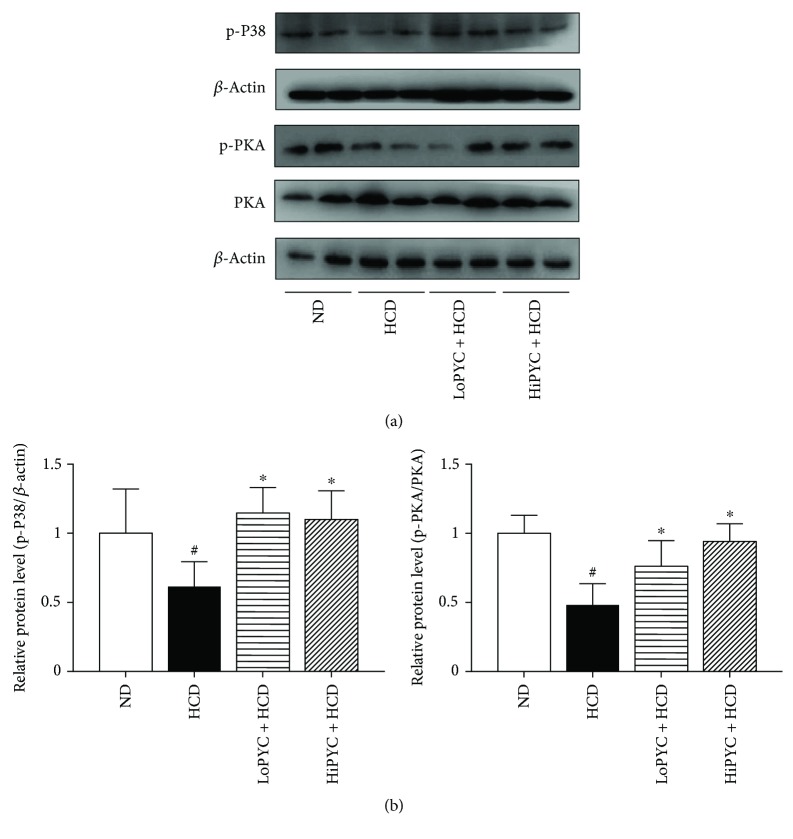
3.5. PYC Increased the Expression of Proteins Related to the PKA Signaling Pathway
PKA signaling activates UCP1 expression, which has a protective effect on metabolic health [25]. To assess whether PYC could enhance the expression of UCP1 through the PKA-dependent pathway, we detected the expression of proteins related to the PKA signaling pathway. As expected, PYC significantly increased the phosphorylation of PKA and p38 proteins in eWAT (Figure 5).
Figure 5.
PYC increased the expression of proteins related to the PKA signaling pathway. (a) Results of the Western blot analysis of PKA signaling in eWAT, including p-p38 MAPK, p-PKA, and PKA, and (b) quantification of the bands. The data are expressed as the means ± SE for 3 mice per group. #p < 0.05 versus the ND group; ∗p < 0.05 versus the HCD group.
4. Discussion
Obesity is a common chronic disease that results from an imbalance of food intake and energy expenditure. Generally, classic brown adipocytes and beige adipocytes increase energy expenditure by generating heat, which protects against the development of overweight and obesity. In our study, we investigated for the first time the effect of PYC on the browning of WAT by altering gene expression related to beige adipogenesis in ApoE-deficient mice, which provides new insight into the effect of PYC on obesity.
In our study, PYC supplementation reduced body weight gain compared with the HCD group, which is consistent with previous in vivo studies [11, 26, 27]. The morphological results showed that the PYC-treated mice appeared to have smaller-sized adipocytes within eWAT than the obese mice, suggesting that PYC may improve the lipid accumulation within eWAT caused by HCD. It is recognized that obesity is characterized by abnormal fat deposition, suggesting that it is necessary to investigate changes in genes or proteins in adipose tissue. To explore the likely mechanism of the obesity-preventative effect of PYC, we analyzed genes and proteins related to the browning of WAT and lipid metabolism.
The browning of WAT provides a new perspective in the identification of therapeutic strategies for weight loss. Several studies have focused on the relationship between the antiobesity effects of plant extracts and WAT browning [28–31]. In our study, we found that PYC significantly increased the expression of UCP1, including the mRNA and protein levels. UCP1, a thermogenic protein, is abundantly expressed in BAT. The increase in UCP1 expression in WAT induces the formation of beige adipocytes [12, 13]. The UCP1-positive beige adipose tissue also has a thermogenic capability that is similar to that of BAT [9, 32, 33]. PYC increased the UCP1 expression in eWAT, indicating that PYC possesses the ability to promote WAT browning, thereby increasing energy expenditure and thermogenesis. A recent study showed that UCP1 activity is stimulated by the cAMP-dependent PKA pathway. The activation of PKA triggers p38 MAPK phosphorylation, which phosphorylates PGC1α [10, 25, 34]. PGC1α is a transcriptional coactivator that modulates the expression of UCP1 and then stimulates thermogenesis and the browning of WAT [35–37]. The present study showed that PYC significantly increased PKA phosphorylation and that the protein expression of p-p38 MAPK and PGC1α was higher in the PYC-treated groups than in the HCD group. The findings suggested that the increased expression of UCP1 was regulated by the PKA-p38 MAPK-PGC1α signaling pathway by PYC in ApoE-deficient mice fed with an HCD. Furthermore, the expression levels of other brown fat-specific markers, such as PRDM16, Cidea, and PPARγ, were increased in the PYC-treated mice, which explains the effect of PYC on WAT browning.
Lipid metabolism is a complex process that involves multiple genes. Among them, ATGL and HSL both hydrolyze triglycerides (TGs) within lipid droplets, leading to the release of diacylglycerol (DG) and fatty acids [38, 39]. HSL is capable of simultaneously hydrolyzing DG [40]. This process is called lipolysis, and a portion of the fatty acids produced in the process is oxidized to provide energy. PYC supplementation increased the levels of ATGL and HSL in adipose tissue; however, PKA inhibitor (H89) treatment clearly blocked the level of HSL, suggesting that PYC promotes lipolysis via a PKA-dependent pathway [11, 41]. The activation of PKA further induces downstream HSL phosphorylation. As a result, a large number of fatty acids are released, and free fatty acids also stimulate UCP1 activity [42–45]. Consistent with these findings, we also found that ATGL and HSL levels were upregulated in the PYC-treated mice, which may explain why there was less lipid droplet accumulation in the PYC-treated groups than in the obese mice. Additionally, HCD significantly increased the expression of PLIN1, while PYC treatment clearly decreased PLIN1 expression. PLIN1, a member of the PAT family, is located on the surface of lipid droplets and plays a role in preventing lipolysis under basal conditions [44, 46]. PYC also promoted lipolysis by reducing the expression of PLIN1. These results also revealed that PYC may promote lipolysis through the PKA signaling pathway and that lipolysis induced by PYC promoted the expression of UCP1. Furthermore, we found that PYC could reverse the reduction in PPARα expression resulting from an HCD. PPARα is a nuclear receptor that plays an important role in the transcriptional control of genes encoding fatty acid β-oxidation enzymes [21, 47]. It has been reported that a PPARα agonist induced beige cell formation [48]. Together with the results of our study, these results suggest that PYC may promote WAT browning by upregulating PPARα expression.
5. Conclusion
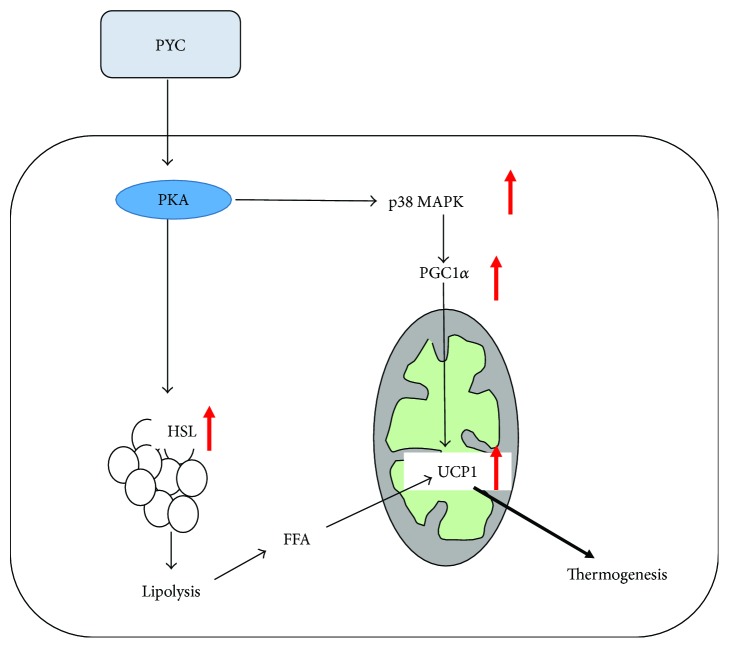
In conclusion, our data demonstrate that PYC can effectively reduce body weight and body fat deposition. Regarding the underlying mechanism, PYC promotes lipolysis and WAT browning via the PKA signaling pathway in eWAT (Figure 6). This finding suggests that PYC administration may be a novel strategy for preventing and treating obesity and associated metabolic diseases.
Figure 6.
The likely mechanism underlying the effect of PYC on the browning of WAT. PYC may stimulate UCP1 expression through the PKA-p38 MAPK-PGC1α pathway and the PKA-HSL pathway.
Acknowledgments
This work was supported by the National Natural Science Foundation of China Youth Fund Project (Grant no. 81100216) and the National Natural Science Foundation of China (Grant no. 81370905). The authors acknowledge the technical support from the Department of Endocrinology and Metabolism of the First Affiliated Hospital of China Medical University.
Contributor Information
Bin Fan, Email: fanbin@hotmail.co.jp.
Jianqiu Gu, Email: gujianqiu1112@163.com.
Conflicts of Interest
The authors have no conflicts of interest, financial or otherwise, to declare.
References
- 1.Ng M., Fleming T., Robinson M., et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. The Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gesta S., Tseng Y. H., Kahn C. R. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131(2):242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Giralt M., Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology. 2013;154(9):2992–3000. doi: 10.1210/en.2013-1403. [DOI] [PubMed] [Google Scholar]
- 4.Diaz M. B., Herzig S., Vegiopoulos A. Thermogenic adipocytes: from cells to physiology and medicine. Metabolism. 2014;63(10):1238–1249. doi: 10.1016/j.metabol.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Sidossis L., Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. The Journal of Clinical Investigation. 2015;125(2):478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J., Bostrom P., Sparks L. M., et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J., Cohen P., Spiegelman B. M. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes & Development. 2013;27(3):234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirschberg V., Fromme T., Klingenspor M. Test systems to study the structure and function of uncoupling protein 1: a critical overview. Front Endocrinol. 2011;2:p. 63. doi: 10.3389/fendo.2011.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamatsu-Ogura Y., Fukano K., Tsubota A., et al. Thermogenic ability of uncoupling protein 1 in beige adipocytes in mice. PLoS One. 2013;8(12, article e84229) doi: 10.1371/journal.pone.0084229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao W., Medvedev A. V., Daniel K. W., Collins S. β-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. Journal of Biological Chemistry. 2001;276(29):27077–27082. doi: 10.1074/jbc.M101049200. [DOI] [PubMed] [Google Scholar]
- 11.Ho J. N., Kim O. K., Nam D. E., Jun W., Lee J. Pycnogenol supplementation promotes lipolysis via activation of cAMP-dependent PKA in ob/ob mice and primary-cultured adipocytes. Journal of Nutritional Science and Vitaminology. 2014;60(6):429–435. doi: 10.3177/jnsv.60.429. [DOI] [PubMed] [Google Scholar]
- 12.Tiraby C., Tavernier G., Lefort C., et al. Acquirement of brown fat cell features by human white adipocytes. The Journal of Biological Chemistry. 2003;278(35):33370–33376. doi: 10.1074/jbc.M305235200. [DOI] [PubMed] [Google Scholar]
- 13.Inagaki T., Sakai J., Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nature Reviews Molecular Cell Biology. 2016;17(8):480–495. doi: 10.1038/nrm.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maimoona A., Naeem I., Saddiqe Z., Jameel K. A review on biological, nutraceutical and clinical aspects of French maritime pine bark extract. Journal of Ethnopharmacology. 2011;133(2):261–277. doi: 10.1016/j.jep.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 15.Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. International Journal of Clinical Pharmacology and Therapeutics. 2002;40(04):158–168. doi: 10.5414/CPP40158. [DOI] [PubMed] [Google Scholar]
- 16.Liu R., Fan B., Cong H., Ikuyama S., Guan H., Gu J. Pycnogenol reduces toll-like receptor 4 signaling pathway-mediated atherosclerosis formation in apolipoprotein E-deficient mice. Journal of Cardiovascular Pharmacology. 2016;68(4):292–303. doi: 10.1097/FJC.0000000000000415. [DOI] [PubMed] [Google Scholar]
- 17.Luo H., Wang J., Qiao C., Ma N., Liu D., Zhang W. Pycnogenol attenuates atherosclerosis by regulating lipid metabolism through the TLR4–NF-κB pathway. Experimental & Molecular Medicine. 2015;47(10, article e191) doi: 10.1038/emm.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuard S., Belcaro G., Cesarone M. R., et al. Kidney function in metabolic syndrome may be improved with Pycnogenol®. Panminerva Medica. 2010;52(2, Supplement 1):27–32. [PubMed] [Google Scholar]
- 19.Belcaro G., Cornelli U., Luzzi R., et al. Pycnogenol® supplementation improves health risk factors in subjects with metabolic syndrome. Phytotherapy Research. 2013;27(10):1572–1578. doi: 10.1002/ptr.4883. [DOI] [PubMed] [Google Scholar]
- 20.Berbee J. F., Boon M. R., Khedoe P. P., et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nature Communications. 2015;6:p. 6356. doi: 10.1038/ncomms7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefebvre P., Chinetti G., Fruchart J. C., Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. The Journal of Clinical Investigation. 2006;116(3):571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bickel P. E., Tansey J. T., Welte M. A. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2009;1791(6):419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brasaemle D. L. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. Journal of Lipid Research. 2007;48(12):2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Souza S. C., Muliro K. V., Liscum L., et al. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. The Journal of Biological Chemistry. 2002;277(10):8267–8272. doi: 10.1074/jbc.M108329200. [DOI] [PubMed] [Google Scholar]
- 25.Dickson L. M., Gandhi S., Layden B. T., Cohen R. N., Wicksteed B. Protein kinase A induces UCP1 expression in specific adipose depots to increase energy expenditure and improve metabolic health. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2016;311(1):R79–R88. doi: 10.1152/ajpregu.00114.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jankyova S., Rubintova D., Janosikova L., Panek P., Foltanova T., Kralova E. The effects of Pycnogenol® as add-on drug to metformin therapy in diabetic rats. Phytotherapy Research. 2016;30(8):1354–1361. doi: 10.1002/ptr.5639. [DOI] [PubMed] [Google Scholar]
- 27.Mei L., Mochizuki M., Hasegawa N. Hepatoprotective effects of pycnogenol in a rat model of non-alcoholic steatohepatitis. Phytotherapy Research. 2012;26(10):1572–1574. doi: 10.1002/ptr.4602. [DOI] [PubMed] [Google Scholar]
- 28.Wang S., Liang X., Yang Q., et al. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. International Journal of Obesity. 2015;39(6):967–976. doi: 10.1038/ijo.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sae-Tan S., Rogers C. J., Lambert J. D. Decaffeinated green tea and voluntary exercise induce gene changes related to beige adipocyte formation in high fat-fed obese mice. Journal of Functional Foods. 2015;14:210–214. doi: 10.1016/j.jff.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X., Tian Y., Zhang H., et al. Curcumin analogues as selective fluorescence imaging probes for brown adipose tissue and monitoring browning. Scientific Reports. 2015;5(1, article 13116) doi: 10.1038/srep13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X., Zhang Q. X., Wang X., et al. Dietary luteolin activates browning and thermogenesis in mice through an AMPK/PGC1α pathway-mediated mechanism. International Journal of Obesity. 2016;40(12):1841–1849. doi: 10.1038/ijo.2016.108. [DOI] [PubMed] [Google Scholar]
- 32.Bartesaghi S., Hallen S., Huang L., et al. Thermogenic activity of UCP1 in human white fat-derived beige adipocytes. Molecular Endocrinology. 2015;29(1):130–139. doi: 10.1210/me.2014-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keipert S., Jastroch M. Brite/beige fat and UCP1—is it thermogenesis? Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2014;1837(7):1075–1082. doi: 10.1016/j.bbabio.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Robidoux J., Cao W., Quan H., et al. Selective activation of mitogen-activated protein (MAP) kinase kinase 3 and p38α MAP kinase is essential for cyclic AMP-dependent UCP1 expression in adipocytes. Molecular and Cellular Biology. 2005;25(13):5466–5479. doi: 10.1128/MCB.25.13.5466-5479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bostrom P., Wu J., Jedrychowski M. P., et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puigserver P., Spiegelman B. M. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocrine Reviews. 2003;24(1):78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 37.Ringholm S., Grunnet Knudsen J., Leick L., Lundgaard A., Munk Nielsen M., Pilegaard H. PGC-1α is required for exercise- and exercise training-induced UCP1 up-regulation in mouse white adipose tissue. PLoS One. 2013;8(5, article e64123) doi: 10.1371/journal.pone.0064123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmermann R., Strauss J. G., Haemmerle G., et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306(5700):1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 39.Zechner R., Strauss J. G., Haemmerle G., Lass A., Zimmermann R. Lipolysis: pathway under construction. Current Opinion in Lipidology. 2005;16(3):333–340. doi: 10.1097/01.mol.0000169354.20395.1c. [DOI] [PubMed] [Google Scholar]
- 40.Haemmerle G., Zimmermann R., Hayn M., et al. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. The Journal of Biological Chemistry. 2002;277(7):4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- 41.Mochizuki M., Hasegawa N. Pycnogenol stimulates lipolysis in 3t3-L1 cells via stimulation of beta-receptor mediated activity. Phytotherapy Research. 2004;18(12):1029–1030. doi: 10.1002/ptr.1612. [DOI] [PubMed] [Google Scholar]
- 42.Richard D., Picard F. Brown fat biology and thermogenesis. Frontiers in Bioscience. 2011;16:1233–1260. doi: 10.2741/3786. [DOI] [PubMed] [Google Scholar]
- 43.Sell H., Deshaies Y., Richard D. The brown adipocyte: update on its metabolic role. The International Journal of Biochemistry & Cell Biology. 2004;36(11):2098–2104. doi: 10.1016/j.biocel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Carmen G. Y., Victor S. M. Signalling mechanisms regulating lipolysis. Cellular Signalling. 2006;18(4):401–408. doi: 10.1016/j.cellsig.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Divakaruni A. S., Humphrey D. M., Brand M. D. Fatty acids change the conformation of uncoupling protein 1 (UCP1) Journal of Biological Chemistry. 2012;287(44):36845–36853. doi: 10.1074/jbc.M112.381780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mishra A. K., Dubey V., Ghosh A. R. Obesity: an overview of possible role(s) of gut hormones, lipid sensing and gut microbiota. Metabolism. 2016;65(1):48–65. doi: 10.1016/j.metabol.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Ferreira A. V., Menezes-Garcia Z., Mario E. G., Delpuerto H. L., Martins A. S., Botion L. M. Increased expression of oxidative enzymes in adipose tissue following PPARα-activation. Metabolism. 2014;63(4):456–460. doi: 10.1016/j.metabol.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Rachid T. L., Penna-de-Carvalho A., Bringhenti I., Aguila M. B., Mandarim-de-Lacerda C. A., Souza-Mello V. Fenofibrate (PPARalpha agonist) induces beige cell formation in subcutaneous white adipose tissue from diet-induced male obese mice. Molecular and Cellular Endocrinology. 2015;402:86–94. doi: 10.1016/j.mce.2014.12.027. [DOI] [PubMed] [Google Scholar]