Summary
Osteoarthritis is a degenerative disease of joints with destruction of articular cartilage associated with subchondral bone hypertrophy and inflammation. OA is the leading cause of joint pain resulting in significant worsening of the quality-of-life in the elderly. Numerous efforts have been spent to overcome the inherently poor healing ability of articular cartilage. Mesenchymal stem cells (MSCs) have been in the limelight of cell-based therapies to promote cartilage repair. Despite progressive advancements in MSC manipulation and the introduction of various bioactive scaffolds and growth factors in preclinical studies, current clinical trials are still at early stages with preliminary aims to evaluate safety, feasibility and efficacy. This review summarises recently reported MSC-based clinical trials and discusses new research directions with particular focus on the potential application of MSC-derived extracellular vehicles, miRNAs and advanced gene editing techniques which may shed light on the development of novel treatment strategies.
The translational potential of this article: This review summarises recent MSC-related clinical research that focuses on cartilage repair. We also propose a novel possible translational direction for hyaline cartilage formation and a new paradigm making use of extra-cellular signalling and epigenetic regulation in the application of MSCs for cartilage repair.
Keywords: epigenetics, MSCs, osteoarthritis, secretome, translation
Introduction
Osteoarthritis (OA) is a condition of progressive erosion of articular cartilage characterised by worsening pain during movement of joints and decreasing ability of the joint to withstand mechanical stress, eventually limiting joint mobility and function. Any synovial joint can develop OA, but knees, hips and small hand joints are the most commonly affected sites [1]. It often severely impedes elderlies' daily activities. In the United States, data from 2010 to 2012 showed that 21.4%, or around 52.5 million adults reported doctor-diagnosed arthritis during that period; and 9.2% among them had arthritis-attributable activity limitations [2]. Damage to articular cartilage due to traumatic injury or other pathological conditions is traditionally considered to be the main cause of OA. Articular cartilage in diarthroidal joints is composed of hyaline cartilage with a specialised anatomical structure and composition facilitating low friction and painless movement. Articular cartilage is hyaline cartilage composed of chondrocytes residing in a dense extracellular matrix (ECM). The specialised composition of the ECM renders unique viscoelastic properties allowing smooth movement. Collagen is the major constitute making up to 60% of the dry weight. Type II collagen accounts for 90–95% of total collagen and the formed fibres are intertwined with proteoglycan aggregates [3]. Fibrocartilage and elastic cartilage are another two types of cartilage in the human body with different ECM and cell compositions [4], [5]. Disruption of the articular cartilage severely affects the knee joint's load-bearing functions, restricting both the ease and range of movement thus highlighting the importance of chondrocytes for joint health. Chondrocytes originate from mesenchymal progenitor cells and contribute about 2% of the total volume of cartilage; their survival relies on a suitable microenvironment and mechanical stress. However, despite their importance, they have limited potential for replication, leading to a limited capacity of the cartilage to recover in response to injury. The exact pathophysiology of OA is still disputed, but a cardinal feature of OA is the thinning of articular cartilage associated with pain, inflammation, and radiologically observable changes such as sclerosis and osteophytes. Synovial irritation, inflammation and bone remodelling processes result in a more uneven joint surface which could further accelerate joint damage. Emerging evidence indicates that OA is not just a cartilage disease, but rather a dynamic pathological conditions of all of the whole joint tissues including the synovium and subchondral bone [6], [7], [8], [9].
Current treatments for OA and their limitations
According to the OA Research Society International (OARSI) and the American Academy of Orthopaedic Surgeons, the main OA treatments can be categorised into physical measures, pharmacological therapy and surgery [10], [11]. Physical measures help to reduce the mechanical imbalance and thus, the risk of OA. Drugs, including paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs) and opioid analgesics are optional for moderate to severe pain, which are reported to be associated with considerable side effects, including liver toxicity, gastrointestinal complications like bleeding and perforated gastric ulcers, nausea, dizziness, constipation, tiredness, etc. Intra-joint injections of hyaluronic acid was one option for the treatment of cartilage lesions, but recent clinical studies showed that the use of hyaluronic acid did not significantly improve clinical outcomes compared with the placebo group [12]. Arthroscopic lavage and debridement have previously been recommended as treatment modalities up until the early 2000s [13]. Previous uncontrolled studies have signified pain improvement in more than half of the patients [14]. It was thought that the debridement procedure prevented further degradation of the joint during movement, delaying the progression of osteoarthritis and reducing pain. Studies later proved that both procedures performed no better than the placebo in improving knee pain and self-reported function [15], therefore this line of treatment was subsequently abandoned. Minimally invasive microfracture procedures had brought hope to patients, but growing evidence showed that the generated fibrocartilage-like repair tissue was less optimal for long-term benefits [16], [17]. All these treatment solutions may benefit some subjects as reflected by symptom relief, but none of them can prevent the affected articular cartilage from progressive destruction. These patients would eventually need a surgical joint replacement surgery to regain joint function. Apart from the long waiting time in local public hospitals for this surgery, many of these patients who suffer from cartilage lesions are relatively young, and they would outlive the useful life of a total joint replacement [18], implying the need for a second joint replacement surgery. In view of these limitations, extensive efforts have been spent to search for alternative strategies to promote cartilage repair. In this Review, we discuss the rationale and recent clinical trial experiences of the use of mesenchymal stem cells (MSCs) for OA therapy, and summarise some novel research directions to facilitate the application and outcome of MSC-based therapy for the clinical management of OA.
Cell-based therapy for OA
Articular chondral or osteochondral lesions usually cannot regenerate into hyaline cartilage. It is speculated that the lack of vasculature within damaged cartilage might limit the infiltration of progenitor cells which are required for the tissue regeneration process [19]. Tissue engineering has come into the limelight of modern science since its introduction in the 1980s. In recent years, cellular components, engineered scaffolds and bioactive substances have been explored as an alternative to traditional surgical methods to facilitate functional tissue regeneration. Autologous chondrocyte implantation (ACI) is a typical example of tissue engineering widely accepted to treat small to moderate-sized osteochondral defects. The first arthroscopic operation is required to obtain a cartilage biopsy for the expansion of chondrocytes in vitro, which will be implanted into the debrided defect site and covered by a membrane in the second operation. This technique with autologous cells could avoid eliciting immune complications and minimise site morbidity when compared with autologous osteochondral implantation. The drawback of a lack of mechanical and biocompatible scaffolds to support chondrocyte proliferation and function is solved by the introduction of matrix-induced autologous chondrocyte implantation (MACI) technology. Unlike ACI, MACI requires the incubation of expanded autologous chondrocytes on an absorbable porcine-derived mixed collagen membrane before implantation. Several short-term follow-up studies and large animal studies have reported improvement in histological assessments and physical functions by MACI [13], [14], [15], [20]; however, the superiority of MACI over existing microfracture techniques remains debatable [15], [21]. One possible explanation for the compromised outcome of ACI is that chondrocytes will lose their cartilage-like properties and turn into a dedifferentiation status during in vitro expansion [22], leading to the formation of fibrocartilage, with less satisfactory long-term mechanical endurance [23]. Despite these limitations, the most recent approval by the U.S. Food and Drug Administration on the clinical use of autologous cultured chondrocytes on porcine collagen membrane (MACI) for the repair of symptomatic, full-thickness cartilage defects of the knee is indeed a positive movement to encourage further research on cell-based therapy for different cartilage problems.
Bottleneck of MSC-based therapy for OA
MSCs are multipotent cells capable of differentiating into osteocytes, adipocytes, chondrocytes and other cells under defined conditions and can be isolated from various tissues, including bone marrow, adipose, and even peripheral blood [24], [25]. MSCs exhibit low levels of major histocompatibility complex, therefore they are believed to possess low immunogenicity [26], [27]. There are no definitive markers for the identification of MSCs, but several surface positive phenotypes such as CD90, CD44, CD73, CD105 and CD146, and negative markers including CD11b, CD34, CD45, CD31 and CD117 are suggested representative markers for MSCs characterisation [28], [29]. Despite the lower differentiation potential of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), MSCs derived from adult somatic tissues are of less ethical and safety concerns regarding their clinical applications. At the time of writing, adult tissues are the dominant source of MSCs for clinical trials for OA. Emerging evidence has suggested the therapeutic potential of MSCs derived from human fetal tissues in various aspects; however, it is not covered in this review. Recent studies indicated that the biological activities of MSCs could be achieved by MSC-conditioned medium containing bioactive secretory factors [30], suggesting the potential to develop cell-free therapeutic strategies for tissue repair. Now, MSC-based therapy is categorised into autologous and allogeneic, based on the source of MSCs. Autologous MSCs are a widely selected source to minimise the immune response which is often the major concern of the Institutional Review Board reviewing exercise. Moreover, this patient-specific MSC therapy also means a higher chance of variation in potency. MSC differentiation potential and proliferation capacity are reported to be age-dependent [31], [32], which could result in the decrease of number and activity of MSCs in the final product and thus, the healing outcome. As OA affects mostly aged people, allogeneic MSCs appear to be a more tangible source which could be isolated, expanded, characterised and activity tested in advance to provide more homogenous MSCs in terms of number and activity, and to provide off-the-shelf products to allow for emergency application.
Unstable phenotypes, manifested as reduced chondrogenic matrix formation, more undesired mineralisation and rapid cell death after injection have been reported in MSCs after long-term ex vivo culture [33], [34], [35]. Various attempts have been proposed to retain or promote the chondrogenic potential of MSCs in culture, including identification of new sources of MSCs, addition of growth factors, enrichment of sub-populations and modification of culture conditions [36], [37], [38], [39], [40]. Yet there is no consensus on the source and cell manipulation; a number of clinical trials have been conducted to evaluate the safety, feasibility and potential efficacy of using MSCs for cartilage repair on the basis of successful preclinical studies. In clinicaltrials.gov, a search with the keywords osteoarthritis AND stem cell results in 45 registered trials including those with an ‘Unknown’ and ‘Recruiting’ status. A PubMed search with “osteoarthritis OR chondral OR defect AND stem cell” showed 18 clinical trials (excluding two case reports) published over the past 15 years (Table 1) [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]. From the limited available information, bone marrow and adipose tissue were the major sources for MSC isolation owing to more mature cell isolations, culture protocols, preclinical experiences and evidence. Most of these trials used autologous MSCs to eliminate immune rejection, while there were three out of 18 studies attempting to investigate the potential application of allogeneic MSCs. Of particular interest, de Windt et al [42] reported a one-surgery-two-cells technique which combined allogeneic MSCs with recycled autologous chondrocytes with a native pericellular matrix, and Akgun et al.[57] showed superior healing outcomes of MSCs compared with chondrocytes for the treatment of isolated chondral lesions. Except for one trial with a five-year follow-up of four cases [41], the remaining studies have shorter follow-up periods from six months to two years to provide sufficient data to justify the long-term efficacy of MSC therapy. The pioneer team led by Wakitani reported the absence of local tumours and infections between five and 137 (mean 75) months of follow-up in 41 patients who received 45 transplantations of autologous bone marrow-derived MSCs [59]. This is the longest follow-up on the safety of MSC therapy for cartilage repair. A similar long-term safety follow-up for allogeneic MSCs is warranted.
Table 1.
Chronological list of publications of MSC application for cartilage repair.
| Author/year | Sample size/gender | Age (mean or average) | Type of cartilage lesions | Stages of OA | Stem cell source/amount | Mode of delivery | Co-treatment | Control groups | Longest follow-up time point | Assessments | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | de Windt TS/2017 | 10 (8M, 2F) | 18–45 | Symptomatic isolated cartilage defect (femoral condyle/trochlea) | Modified Outerbridge Grade 3 or 4 | Allogeneic bone marrow-derived MSCs/NS | Single defect site-specific implantation | 10 or 20% autologous chondrons | Nil | 12 months | KOOS, VAS, EQ5D, MRI, second-look arthroscopy, histology (biopsy) | [42] |
| 2 | Pers YM/2016 | 18 (8M, 10F) | 50–75 | Knee OA | KL: 3 or 4 | Adipose-derived MSCs/Low: 2 × 106; Mid: 10 × 106; High: 50 × 106 | Single intra-articular injection | Nil | Nil | 6 months | Primary outcome: Safety of the dose-escalation protocol Secondary outcome: WOMAC, VAS, PGA, SAS, KOOS, histology (biopsy) |
[48] |
| 3 | Koh YG/2016 | 80 (30M, 50F) | 18–50 | Symptomatic cartilage defect (≥3 cm2) on femoral condyle | ICRS: 3 or 4 | Adipose-derived MSCs/5 × 106 | Single defect site-specific implantation | Microfracture; fibrin glue | Microfracture | 24 months | MRI, Lysholm score, KOOS, VAS, second-look arthroscopy and histology (biopsy) | [46] |
| 4 | Fodor PB/2016 | 6 (1M, 5F) | 51–69 | Knee OA | KL: 1 to 3 | Adipose-derived stromal vascular cells/14 × 106 | Single intra-articular injection | Nil | Nil | 12 months | WOMAC, VAS, ROM, TUG, MRI | [43] |
| 5 | Davatchi F/2016 | 4 (2M, 2F) | 54–65 | Knee OA | KL: 2 or 3 | Bone marrow-derived MSCs/8–9 × 106 | Single intra-articular injection | Glucosamine was permitted if the patients was taking it before being enrolled. | Nil | 60 months | Standing knee X-ray, VAS, ROM, walking time for pain to appear. | [41] |
| 6 | Akgun I/2015 | 14 (8M, 6F) | 18–46 | Isolated full thickness knee chondral defect >2 cm2 | KL: 1 or 2 | Autologous synovium-derived MSCs/4 × 106 | Implantation of cells preloaded collagen membrane into the debrided site | Type I/III collagen membrane (2 × 2 cm) | Matrix-induced autologous chondrocyte implantation | 24 months | Primary outcome: pain assessment and KOOS Secondary outcome: clinical knee examination and MRI |
[57] |
| 7 | Vega A/2015 | 30 (13M, 17F) | 36–73 | Knee OA | KL: 2 to 4 | Allogeneic bone marrow-derived MSCs/40 × 106 | Single intra-articular injection | Nil | Single dose of HA | 12 months | VAS, WOMAC, Lequesne, SF-12, MRI | [51] |
| 8 | Jo CH/2014 | Phase I: 9 patients (n = 3 each group) | 18–75 | Knee OA | KL: 2 to 4 | Adipose-derived stromal cells/Low: 1 × 107; Mid : 5.0 × 107; High : 1 × 108 cells | Single intra-articular injection | Nil | Nil | 6 months | Primary outcomes: Safety and WOMAC Secondary outcomes: Clinical (VAS & KSS); Radiological (X-ray and MRI); Arthroscopic (Size and ICRS), and Histology (biopsy) |
[44] |
| Phase II: 12 patients (including 3 (high dose) from phase I | Adipose-derived stromal cells/1 × 108 cells | |||||||||||
| 9 | Vangsness Jr CT/2014 | 55 (35M, 20F) | 18–60 | Patient underwent partial medial meniscectomy | Not specified | Allogeneic bone marrow-derived MSCs/Group A: 50 × 106; Group B: 150 × 106 | Superolateral knee injection | Injection given 7–10 days after partial medial meniscectomy | Vehicle control (sodium hyaluronate, human serum albumin and PlasmaLyte A) | 24 months | Safety assessment, serological measurement of immune cell markers, MRI, VAS, Lysholm Knee Scale Score | [50] |
| 10 | Wong KL/2013 | 56 (29M, 27F) | 18–55 | Uni-compartmental OA knee (medial) & genu varum | ICRS: 2 to 4 | Bone marrow-derived MSCs/1.46 ± 0.29 × 107 cells | Intra-articular injection | MSCs injection in conjunction with microfracture & High tibial osteotomy; MSCs in HA (2 mL); followed by 2 extra doses of 2 mL HA injection repeated at weekly intervals for all patients | HA | 24 months | Primary Outcome: IKDC Secondary Outcome: Tegner and Lysholm clinical scores; MOCART |
[52] |
| 11 | Orozco L/2013 | 12 (6M, 6F) | 49 ± 5 | Knee OA | KL: 2 to 4 | Bone marrow-derived MSCs/40 × 106 cells | Intra-articular injection | Ringer lactate, 0.5% human albumin and 5 mM glucose | Nil | 12 months | VAS, WOMAC, Lequesne, MRI, | [47] |
| 12 | Saw KY/2013 | 49 (17M, 32F) | 18–50 | Focal Chondral lesion | ICRS: 3 or 4 | Autologous peripheral blood progenitor cells/NS | Intra-articular injection | PBSC + HA; 5 weekly injections 1 week after surgery followed by 3 additional injections weekly intervals at 6 months | HA | 24 months | IKDC; MRI; second-look arthroscopy; histology (biopsy) | [49] |
| 13 | Koh YG/2013 | 18 (6M, 12F) | 54.6 | Knee OA | ICRS: Grade 3 or 4KL: Grade 3 or 4 | Infrapatellar fat pad MSCs/1.18 × 106 | IA injection - lateral approach | Arthroscopy debridement; MSCs in PRP (Avg 1,280,000/μL platelets); PRP injection every 7 days for 2 weeks; CaCl2 added to PRP for activation | Nil | 26 months | Lysholm score; Tegner activity scale; VAS; MRI | [53] |
| 14 | Koh YG/2012 | 25 (8M, 17 F) | 54.1 | Knee OA | ICRS: Grade 3 or 4KL: Grade 3 or 4 | Infrapatellar fat pad MSCs/1.89 × 106 | IA injection - lateral approach | Arthroscopy debridement; MSCs in PRP (Avg 1,280,000/μL platelets); PRP injection every 7 days for 2 weeks; CaCl2 added to PRP for activation | PRP | 18 months | Lysholm score; Tegner activity scale; VAS | [45] |
| 15 | Saw KY/2011 | 5 (1M, 4F) | 39.4 | Focal Chondral lesion | ICRS 3 or 4 | Autologous peripheral blood progenitor cells/NS | Intra-articular injection | Arthroscopic subchondral drilling; 5 weekly injections 1 week after surgery; 3 additional injections of either HA or PBSC + HA weekly intervals 6 months after surgery | Nil | 26 months | Second look arthroscopy; Histology (H&E, Safranin O, IHC) | [54] |
| 16 | Nejadnik H/2010 | 72 (38M, 34F) | 43.25 | Symptomatic chondral lesion | Lesion grade 3 or 4 | Autologous bone marrow derived MSCs/NS | Implantation upon autologous periosteal patch | Fibrin glue | First-generation autologous chondrocyte implantation | 24 months | ICRS cartilage injury evaluation package, IKDC, Lysholm score; Tegner activity scale | [58] |
| 17 | Davatchi F/2011 | 4 (2M, 2 F) | 57.75 | Bilateral OA | Nil | Autologous bone marrow derived MSCs from iliac crest/8 or 9 × 106 | Intra-articular injection | Saline with 2% human serum albumin | Nil | 6 months | X-rays; Clinical; VAS | [55] |
| 18 | Wakitani/2002 | 24 (9M, 15F) | 63 | Medial unicompartmental OA | Ahlback changes: Stage 1–2; Outerbridge stage 4 lesion on the tibial plateau and femoral condyle | Autologous bone marrow derived MSCs from iliac crest/NS | Implantation of collagen cell sheets | High tibial osteotomy; Gel-cell composite; Covered with autologous periosteum | Cell free | 95 weeks | Arthroscopic photography; Histological samples (cell morphology); Clinical evaluation (Pain Function Range of motion Muscle strength Flexion deformity Instability Subtraction etc) | [56] |
EQ5D = EuroQoL 5-Dimension Health Questionnaire; F = female; HA = hyaluronic acid; H&E = haematoxylin and eosin staining; ICRS = International Cartilage Repair Society score; IHC = immunohistochemistry; IKDC = International Knee Documentation Committee; KL = Kellgren–Lawrence Scale; KOOS = Knee injury and Osteoarthritis Outcome Scoring; KSS = Knee Society Clinical Rating System score; Lequesne = Lequesne algo-functional indices; M = male; MOCART = Magnetic Resonance Observation of Cartilage Repair Tissue score; MRI = magnetic resonance imaging; MSC = mesenchymal stem cell; NS = not specified; OA = osteoarthritis; PBSC = peripheral blood stem cell; PGA = Patient Global Assessment; PRP = platelet-rich plasma; ROM = range of motion; SAS = Short Arthritis Assessment Scale; SF-12 = short form-12 life quality questionnaire; TUG = timed up-and-go; VAS = visual analogue scale for pain; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Based on our experience and other reported trials, one common problem affecting the healing outcome is the tendency of MSCs to differentiate into fibro-like tissue instead of hyaline cartilage [60]. How to eliminate or reduce hypertrophic chondrogenesis of the injected/implanted MSCs is one of the major hurdles to successful cartilage repair. Genetic modification is a promising tool to induce the persistent chondrogenic phenotype of MSCs in cellular and animal studies [61], [62]; however, the required well-trained personnel and specific facilities are only available in a few clinical centres. It is of clinical interest to develop more feasible and effective methods to obtain more chondrogenic capable MSCs in order to promote its clinical application. One research direction is to identify new sources of MSCs for cartilage repair. It is believed that epigenetic memory may render significant impact on the lineage-differentiation specificity of MSCs. Recently, synovium stem cells (SSCs) [63] have been used for OA treatment. Since it is isolated from the synovium membrane, which is a tissue attached at the surface of articular cartilage, a better outcome in chondrogenic differentiation is expected. Fetal tissue-derived stem cells, particularly fetal cartilage- derived stem cells have shown higher chondrogenic activity [64]. Due to the higher plastic and proliferation ability of fetal stem cells as compared to their adult counterpart, fetal stem cells may be a better choice for tissue engineering.
Perspectives of MSC-based therapy for OA
Paracrine action of MSCs on tissue regeneration
How MSCs facilitate tissue regeneration is seemingly not a simple process. Increasing evidence indicates that the life span of injected/implanted MSCs at the damaged sites was much shorter than expected [65], [66]. It appears that the bioactive paracrine factors secreted by MSCs play some, if not all, beneficial effects in modulating the microenvironment of the damaged tissue, leading to more favourable conditions for tissue regeneration [67]. MSCs secrete a spectrum of paracrine factors, collectively termed as secretome, consisting of various proteins for diverse biological functions, including immune regulation, angiogenesis, antiapoptotic, antioxidative, cell homing and promotion of cell differentiation [68]. MSCs release cytokines to initiate cartilage repair, which is followed by chondrogenic proliferation together with secretion of ECM proteases and growth factors like transforming growth factor-β (TGF-β), insulin-like growth factor-1 (IGF-1), and fibroblast growth factor (FGF) [28], [29], [30], [31], [32], [33]. These factors collectively comprise an important part of the MSCs secretome and stimulate cartilage repair. A recent study revealed that MSCs secreted various chemokine (C-X-C motif) ligands and chemokine (C-C motif) ligands, vascular endothelial growth factor A, and interleukin 6 under the exposure of synovial fluid from early- and late-stage OA patients [69]. The secreted paracrine factors could be enclosed in extracellular vehicles (EVs), which are comprised of diverse molecules including lipids, proteins, RNA (mRNA and non-coding RNAs), and DNA subtypes [70]. The diameter of EVs range from 40 nm to 150 nm. The biological nature of EVs facilitate crossing the blood brain barrier, cell targeting, and protection of the components from degradation in circulation [71]. Increasing evidence has shown the beneficial effects of EV-treatment on various tissues, including cardiovascular tissues, kidney, liver and skin [72]. Its lower tumourigenicity [73] suggests that EVs could be a standalone therapeutic product or a co-administered agent to enhance the effectiveness of MSC therapy.
Delivery efficiency is decided by two key features: the durability of EVs presence in blood and tissue distribution of EVs. EVs with a diameter between 80 nm and 150 nm exhibited the longest circulation time owing to selective uptake by the liver (<80 nm) and spleen (>250 nm) [74], [75]. Besides the size, the loading efficiency also governs the effectiveness of EVs. EVs are a bilayer membrane structure, which is of limited loading efficiency. There are two major strategies for loading; first, gene overexpression vectors are transferred into cells before EV isolation. The bioactive proteins will be enveloped in EVs via endogenous RNA secretory mechanisms. The second strategy is loading after EV isolation, in which proteins of interest are loaded into pre-isolated EVs by physical or chemical treatment, including electroporation and incubation at room temperature, repeat freeze-thaw cycle, ultrasonication, detergent treatment and extrusion [71].
Clinical application potential of EVs
Though there are still ambiguous areas of mechanisms, EVs have been employed in many clinical trials. Until now, only one group reported that EVs derived from gene-modified bone marrow-derived dendritic cells could delay collagenase-induced arthritis [76]. Exosomes have been isolated from various sources of stem cells, including adipose, bone marrow, fetal tissue and ESC/iPSC [77], [78], [79], [80]. Studies report encouraging results of exosome therapy. Exosomes derived from MSCs have been used as a tool to treat graft-versus-host-diseases, since MSCs are considered immune modulating cells [81]. Another group report that MSC exosomes could prevent adverse remodelling after myocardial ischemia/reperfusion injuries through regulation of the energy metabolism [82]. Additionally, MSC exosomes could induce fibroblast proliferation and angiogenesis which contribute to wound healing [83]. A recent study showed the positive effect of exosomes isolated from ESC-derived MSCs, which promoted osteochondral regeneration in rats [84]. This study demonstrated the potential application of exosomes in OA treatment. Until now, there are few studies on EVs in the field of OA. Intra-articular injection of EVs derived from human embryonic MSCs resulted in remarkable restoration of hyaline cartilage in adult rats with a groove model while the phosphate-buffered saline control group resulted in fibrocartilage generation. Another study proved that miR-140 overexpression synovium-derived stem cell-derived exosomes promoted cartilage regeneration in rats [85]. On the other hand, EVs derived from IL-1β-pretreated MSCs induced OA changes in chondrocyte culture [86]. Further study indicated enriched content of OA genes target miR-23b and miR-92a in conditioned medium of MSC culture [87]. These clues suggest that the biological function of EVs is dependent from where the cells originated. EVs per se could be an indicator of the cellular response to the surrounding microenvironment, and contain information for cell-to-cell communication.
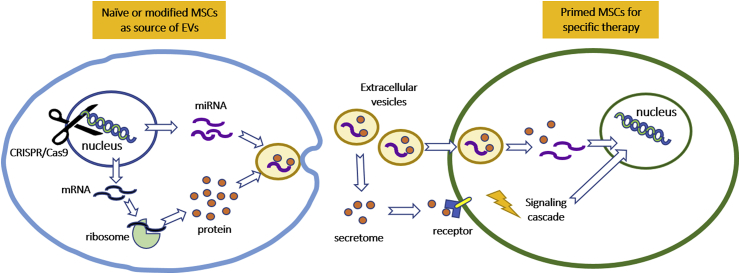
With this concept, EVs derived from specifically cultured or engineered MSCs could be used for the treatment of particular pathological conditions, while EVs derived from juvenile MSCs could ameliorate some age-associated impairments in aging MSCs (Figure 1). It remains elusive how these secreted proteins and EVs as a cocktail promote cartilage regeneration and what factors determine the secretary profile. A standardised and validated protocol for preparation and quality control would be indispensable to avoid batch-to-batch variation.
Figure 1.
Schematic illustration of the proposed strategy of hyaline cartilage regeneration with MSCs and secreted EVs. Naïve or genetically modified MSC-secreted EVs consisting of bioactive proteins and miRNAs could be used directly to modulate the microenvironment of the damaged cartilage tissue to promote cartilage repair, or be used to enhance the chondrogenic ability of the primed MSCs before injection or implantation for cartilage repair.
Epigenetics: a new player in MSC therapy for OA?
Until now, the exact mechanism underlying the biological activity of EVs is unclear. The above evidence suggests that microRNA (miRNA) could be a determining factor for the biological activity of EVs. As a key regulator of epigenetic regulation, miRNA has been under investigation for decades [88], [89]. Epigenetic regulation can generally be defined as post-genetic or non-genetic regulation. This post-transcript modulation will evenly affect the gene expression without modifying the gene sequence. It is safer to control the fate of the stem cell’s self-renewal or differentiation through post-transcript modulation. Interestingly, although the genome sequence did not change, the epigenetic pattern was still inheritable [90], this provides a clue that extracellular signalling can possibly be passed to descendants. Epigenetic regulation of MSCs can be achieved through DNA methylation [91], [92], histone modification [93] and chromatin remodelling [94]. For instance, histone methylation on H3K9 and H3K14 could control the replicative senescence of MSCs [93], as well as the histone acetylation [95]. The differentiation ability of MSCs is also under the regulation of the epigenetic gene network. Epigenetics has emerged to provide additional information on the pathomechanism of many complex diseases [96]. Several studies have revealed the epigenetic changes during chondrogenesis. In general, the expression of critical chondrogenic genes such as Sox9, Runx2 and Fgfr3 are influenced by DNA methylation [97]. Two histone acetylates, HDAC1 and HDAC4, could inhibit the gene expression of Comp and Runx2, respectively [98], [99]. HDAC also activates type II collagen expression through Wnt signalling [100]. High throughput screening has identified a few histone modification enzymes, including H3K4me3, H3K9ac and H3K36me3, which were enriched near the promoter regions of genes related to chondrogenesis and ECM synthesis [101]. Recent studies reported that Col10A1 and Mmp13 promoters could be demethylated under OA conditions [102], [103], indicating the link between the hypertrophic phenotype of chondrocytes and DNA methylation. For histone modification, HDAC overexpression was reported to repress Acan, Col2A1 and Mmp13 [104], [105].
As one of the epigenetic mediators, miRNA has attracted researchers' attention in recent years. The biology of miRNA has been reviewed extensively but is not in the scope of the present review [106], [107]. miRNA is a small non-coding RNA (18–24 nucleotides in length) and constitutes part of a large family named non-coding RNA. They exert their function by binding to the target's mRNA 3′ untranslated region (3-UTR) and further repress the translation or increased degradation of mRNA [108]. Differentially expressed miRNAs are found in the whole joint in OA patients. Increasing studies have depicted a complex picture on how miRNA regulates or influences OA. In OA pathogenesis, the role of miRNA is mainly focused on inflammation pathways. They also mediated the anabolic and catabolic balance. In cell therapy, miRNA also regulates the differentiation of MSCs into adipocytes, osteoblasts and chondrocytes. miR-140 is one the most studied miRNAs in OA research [107]. It is highly expressed in chondrocytes and regulates cartilage homeostasis. miR-140 also controls the cartilage via TGF-β and BMP signalling [109]. Recently, a specific group of miRNAs showed their therapeutic potential in OA treatment [69]. MSC-derived EVs contain 150 miRNAs, which target the wide spectrum of signalling pathways, including SMAD, MMP13 and PKA which are all crucial pathways affecting OA pathogenesis. Among them, miR-125 targets ADAMTS4, miR-320 targets MMP-13 and miR-145 targets Sox9. Increasing evidence indicates that MSC-secreted EVs might assist OA treatment through complicated miRNA network regulations [110]. It appears that different cell sources or treatment conditions will produce EVs with various miRNA profiles. In short, along with the increasing studies in the OA field, it becomes more clear that nearly all the representative OA related genes, including Nfat1, Sox9, IL-1beta, TNF-alpha, ADAMTS4/5, Mmp9/13, Col2A1, Col9A1 and Acan are regulated by DNA methylation, histone modification and miRNA, respectively [111]. Epigenetic regulation is a vital player during OA development and progression. Targeting these epigenetic changes directly or indirectly could produce additive or synergistic effects to enhance the therapeutic outcomes of MSC-based therapy.
Advancing MSC abilities with gene editing technology
The success of many of the aforementioned novel strategies relies on sophisticated gene editing technology. Gene editing MSCs have always been criticised due to the potential risk of tumourigenecity. An advanced CRISPR/Cas9 technology has emerged to overcome this obstacle. CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) and CRISPR-associated (Cas) genes play an essential adaptive immunity in some bacteria, allowing the elimination of invading genetic materials. Cas9 is one of the Cas proteins which is unique to other CRISPR systems as only one Cas9 protein is required for gene silencing. This system consists of two elements: a nuclease, which behaves like a pair of scissor to cut genomic DNA, and a guide RNA (gRNA) which responds to recruit the nuclease to the target site. By altering the sequence of the gRNA, Cas9 nucleases can be directed toward the DNA target via Watson-Crick base pairing where it carries out strand-specific cleavage leaving the site for further genetic manipulation [112]. With subsequent improvement since its introduction, the CRISPR/Cas9 system is now able to achieve gene knockout, genome correction, cassette knockin and gene deletion. More importantly, the CRISPR/Cas9 system can be delivered in different forms, including virus, plasmid, RNA, and protein [113]. For example, CRISPR/Cas9 has been employed to regulate TGF-β signalling through precise SMAD protein knockdown [114]. To avoid permanent genome editing, CRISPR/Cas9 could be activated in a reversible manner [115] and block the gene expression in other cell types but not MSCs [116]. One of the advantages of the CRISPR/Cas9 system is the very low effect in gene editing [117]. Despite this, numerous efforts have been spent to optimise the CRISPR/Cas9 system for diverse applications, readers are advised to pay attention to the ethical concerns and safety issues that have been raised regarding this powerful tool, which is not the scope of the present review.
The first clinical trial using CRISPR/Cas9 technology in humans was conducted in China in 2016. CRISPR/Cas9 has been used to treat patients with metastatic non-small cell lung cancer [118]. This breakthrough provides conceptual and practical guidelines on the clinical application of CRISPR/Cas9. The capability of CRISPR/Cas9 has been used in many pre-clinical studies. In the treatment of OA, it is not necessary to modify the patient's genome. However, specifically targeting the key regulators represents a potential strategy to enhance the therapeutic ability of MSCs. In 2016, three research groups have attempted to modify MSCs and chondrocytes with lentivirus-carried CRISPR/Cas9 [119], [120], [121].
Conclusion
Although MSC-based therapies have shown successful cartilage renewal and pain relief, recent trials on MSC cartilage regeneration do not provide a high level of evidence on the restoration of the original hyaline cartilage for long-term OA improvement. Processing techniques of MSCs lacked expansion and differentiation control for optimal chondrogenic potential, and sample sizes and follow-up periods for randomised control trials were not large or long enough to conclude effective regeneration. Furthermore, some studies utilised autologous MSCs in elderly patients, resulting in outcome inconsistencies between trials due to an age-related decrease in MSC proliferation. Unlike pharmaceutical drugs with defined chemical structures and functions, the identification and functional characterisation of MSCs are not standardised yet, thus making it difficult to produce MSCs with consistent biological activities in a large scale for clinical trials. Yet the dosage and mode of delivery of MSCs for cartilage repair in humans remains debatable, recent advancement in the aetiology and pathogenesis of OA, the biology of MSCs, and state-of-the-art gene editing technology have provided insight into the alternative manipulation and application of MSCs. With the combination of EV application and CRISPR/Cas9 technology, there are enormous opportunities to achieve better clinical outcomes from the use of gene modulation.
Conflict of interest
The author(s) have no conflicts of interest relevant to this article.
Funding/support
The authors have no funding to declare for this article.
References
- 1.Gupta S., Hawker G.A., Laporte A., Croxford R., Coyte P.C. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford) 2005;44(12):1531–1537. doi: 10.1093/rheumatology/kei049. [DOI] [PubMed] [Google Scholar]
- 2.Barbour K.E., Helmick C.G., Boring M. Prevalence of doctor-diagnosed arthritis at state and county levels – United States, 2014. Mmwr-Morbid Mortal W. 2016;65:489. doi: 10.15585/mmwr.mm6519a2. 2016;65(20):524. and arthritis-attributable activity limitation—United States, 2010–2012. In: (CDC). CfDCaP, editor. United States, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox A.J., Bedi A., Rodeo S.A. The basic science of human knee menisci: structure, composition, and function. Sports Health. 2012;4(4):340–351. doi: 10.1177/1941738111429419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mow V.C., Ratcliffe A., Poole A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13(2):67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 6.Brandt K.D., Dieppe P., Radin E. Etiopathogenesis of osteoarthritis. Med Clin North Am. 2009;93(1):1–24. doi: 10.1016/j.mcna.2008.08.009. xv. [DOI] [PubMed] [Google Scholar]
- 7.Dieppe P.A., Lohmander L.S. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365(9463):965–973. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 8.Mobasheri A., Kalamegam G., Musumeci G., Batt M.E. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014;78(3):188–198. doi: 10.1016/j.maturitas.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Hügle T., Geurts J. What drives osteoarthritis?—synovial versus subchondral bone pathology. Rheumatology. 2016 doi: 10.1093/rheumatology/kew389. kew389. [DOI] [PubMed] [Google Scholar]
- 10.Treatment of osteoarthritis of the knee: American Academy of Orthopaedic Surgeons. 2013. [Google Scholar]
- 11.Zhang W., Moskowitz R.W., Nuki G., Abramson S., Altman R.D., Arden N. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr Cartil. 2008;16(2):137–162. doi: 10.1016/j.joca.2007.12.013. Epub 2008/02/19. [DOI] [PubMed] [Google Scholar]
- 12.Colen S., van den Bekerom M.P., Mulier M., Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs. 2012;26(4):257–268. doi: 10.2165/11632580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Basad E., Wissing F.R., Fehrenbach P., Rickert M., Steinmeyer J., Ishaque B. Matrix-induced autologous chondrocyte implantation (MACI) in the knee: clinical outcomes and challenges. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3729–3735. doi: 10.1007/s00167-014-3295-8. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett W., Skinner J.A., Gooding C.R., Carrington R.W., Flanagan A.M., Briggs T.W. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Jt Surg Br. 2005;87(5):640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 15.Basad E., Ishaque B., Bachmann G., Sturz H., Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):519–527. doi: 10.1007/s00167-009-1028-1. [DOI] [PubMed] [Google Scholar]
- 16.Frisbie D.D., Oxford J.T., Southwood L., Trotter G.W., Rodkey W.G., Steadman J.R. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;407:215–227. doi: 10.1097/00003086-200302000-00031. Epub 2003/02/05. [DOI] [PubMed] [Google Scholar]
- 17.Kaul G., Cucchiarini M., Remberger K., Kohn D., Madry H. Failed cartilage repair for early osteoarthritis defects: a biochemical, histological and immunohistochemical analysis of the repair tissue after treatment with marrow-stimulation techniques. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2315–2324. doi: 10.1007/s00167-011-1853-x. Epub 2012/01/10. [DOI] [PubMed] [Google Scholar]
- 18.Somoza R.A., Welter J.F., Correa D., Caplan A.I. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev. 2014;20(6):596–608. doi: 10.1089/ten.teb.2013.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunziker E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil. 2002;10(6):432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 20.Nixon A.J., Rickey E., Butler T.J., Scimeca M.S., Moran N., Matthews G.L. A chondrocyte infiltrated collagen type I/III membrane (MACI(R) implant) improves cartilage healing in the equine patellofemoral joint model. Osteoarthr Cartil. 2015;23(4):648–660. doi: 10.1016/j.joca.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 21.DiBartola A.C., Everhart J.S., Magnussen R.A., Carey J.L., Brophy R.H., Schmitt L.C. Correlation between histological outcome and surgical cartilage repair technique in the knee: a meta-analysis. Knee. 2016;23(3):344–349. doi: 10.1016/j.knee.2016.01.017. Epub 2016/02/24. [DOI] [PubMed] [Google Scholar]
- 22.Kang S.W., Yoo S.P., Kim B.S. Effect of chondrocyte passage number on histological aspects of tissue-engineered cartilage. Biomed Mater Eng. 2007;17(5):269–276. [PubMed] [Google Scholar]
- 23.Peterson L., Vasiliadis H.S., Brittberg M., Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117–1124. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- 24.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 25.Xu L., Li G. Circulating mesenchymal stem cells and their clinical implications. J Orthop Transl. 2014;2(1):1–7. [Google Scholar]
- 26.Huang X.-P., Ludke A., Dhingra S., Guo J., Sun Z., Zhang L. Class II transactivator knockdown limits major histocompatibility complex II expression, diminishes immune rejection, and improves survival of allogeneic bone marrow stem cells in the infarcted heart. FASEB J. 2016;30(9):3069–3082. doi: 10.1096/fj.201600331R. [DOI] [PubMed] [Google Scholar]
- 27.Molina E.R., Smith B.T., Shah S.R., Shin H., Mikos A.G. Immunomodulatory properties of stem cells and bioactive molecules for tissue engineering. J Control Release. 2015;219:107–118. doi: 10.1016/j.jconrel.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 28.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. Epub 2006/08/23. [DOI] [PubMed] [Google Scholar]
- 29.Steinert A.F., Rackwitz L., Gilbert F., Noth U., Tuan R.S. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;1(3):237–247. doi: 10.5966/sctm.2011-0036. Epub 2012/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J.W., Fang X., Krasnodembskaya A., Howard J.P., Matthay M.A. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29(6):913–919. doi: 10.1002/stem.643. Epub 2011/04/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roobrouck V.D., Ulloa-Montoya F., Verfaillie C.M. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314(9):1937–1944. doi: 10.1016/j.yexcr.2008.03.006. Epub 2008/04/29. [DOI] [PubMed] [Google Scholar]
- 32.Steinert A.F., Ghivizzani S.C., Rethwilm A., Tuan R.S., Evans C.H., Noth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9(3):213. doi: 10.1186/ar2195. Epub 2007/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelttari K., Winter A., Steck E., Goetzke K., Hennig T., Ochs B.G. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54(10):3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 34.Farrell M.J., Fisher M.B., Huang A.H., Shin J.I., Farrell K.M., Mauck R.L. Functional properties of bone marrow-derived MSC-based engineered cartilage are unstable with very long-term in vitro culture. J Biomech. 2014;47(9):2173–2182. doi: 10.1016/j.jbiomech.2013.10.030. Epub 2013/11/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Bogt K.E., Schrepfer S., Yu J., Sheikh A.Y., Hoyt G., Govaert J.A. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87(5):642–652. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez-Silos V., Camacho-Morales A., Fuentes-Mera L. Mesenchymal stem cells subpopulations: application for orthopedic regenerative medicine. Stem Cells Int. 2016;2016:3187491. doi: 10.1155/2016/3187491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly D. Mechanical modulation of stem cell chondrogenesis. Eur Cells Mater. 2016;31(1):94. [Google Scholar]
- 38.Marsano A., Medeiros da Cunha C.M., Ghanaati S., Gueven S., Centola M., Tsaryk R. Spontaneous in vivo chondrogenesis of bone marrow-derived mesenchymal progenitor cells by blocking vascular endothelial growth factor signaling. Stem Cells Transl Med. 2016;5(12):1730–1738. doi: 10.5966/sctm.2015-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bian L., Guvendiren M., Mauck R.L., Burdick J.A. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci. 2013;110(25):10117–10122. doi: 10.1073/pnas.1214100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gugjoo M.B., Amarpal, Sharma G.T., Aithal H.P., Kinjavdekar P. Cartilage tissue engineering: role of mesenchymal stem cells along with growth factors & scaffolds. Indian J Med Res. 2016;144(3):339–347. doi: 10.4103/0971-5916.198724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davatchi F., Sadeghi Abdollahi B., Mohyeddin M., Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis. 2016;19(3):219–225. doi: 10.1111/1756-185X.12670. Epub 2015/05/21. [DOI] [PubMed] [Google Scholar]
- 42.de Windt T.S., Vonk L.A., Slaper-Cortenbach I.C., van den Broek M.P., Nizak R., van Rijen M.H. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells. 2017;35(1):256–264. doi: 10.1002/stem.2475. [DOI] [PubMed] [Google Scholar]
- 43.Fodor P.B., Paulseth S.G. Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthet Surg J. 2016;36(2):229–236. doi: 10.1093/asj/sjv135. Epub 2015/08/05. [DOI] [PubMed] [Google Scholar]
- 44.Jo C.H., Lee Y.G., Shin W.H., Kim H., Chai J.W., Jeong E.C. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 45.Koh Y.G., Choi Y.J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902–907. doi: 10.1016/j.knee.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Koh Y.G., Kwon O.R., Kim Y.S., Choi Y.J., Tak D.H. Adipose-Derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy. 2016;32(1):97–109. doi: 10.1016/j.arthro.2015.09.010. Epub 2015/11/21. [DOI] [PubMed] [Google Scholar]
- 47.Orozco L., Munar A., Soler R., Alberca M., Soler F., Huguet M. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. 2013;95(12):1535–1541. doi: 10.1097/TP.0b013e318291a2da. Epub 2013/05/18. [DOI] [PubMed] [Google Scholar]
- 48.Pers Y.M., Rackwitz L., Ferreira R., Pullig O., Delfour C., Barry F. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med. 2016;5(7):847–856. doi: 10.5966/sctm.2015-0245. Epub 2016/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saw K.Y., Anz A., Siew-Yoke Jee C., Merican S., Ching-Soong Ng R., Roohi S.A. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29(4):684–694. doi: 10.1016/j.arthro.2012.12.008. Epub 2013/02/06. [DOI] [PubMed] [Google Scholar]
- 50.Vangsness C.T., Jr., Farr J., 2nd, Boyd J., Dellaero D.T., Mills C.R., LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Jt Surg Am. 2014;96(2):90–98. doi: 10.2106/JBJS.M.00058. [DOI] [PubMed] [Google Scholar]
- 51.Vega A., Martin-Ferrero M.A., Del Canto F., Alberca M., Garcia V., Munar A. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–1690. doi: 10.1097/TP.0000000000000678. Epub 2015/03/31. [DOI] [PubMed] [Google Scholar]
- 52.Wong K.L., Lee K.B., Tai B.C., Law P., Lee E.H., Hui J.H. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years' follow-up. Arthroscopy. 2013;29(12):2020–2028. doi: 10.1016/j.arthro.2013.09.074. Epub 2013/11/30. [DOI] [PubMed] [Google Scholar]
- 53.Koh Y.G., Jo S.B., Kwon O.R., Suh D.S., Lee S.W., Park S.H. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29(4):748–755. doi: 10.1016/j.arthro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 54.Saw K.Y., Anz A., Merican S., Tay Y.G., Ragavanaidu K., Jee C.S. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 2011;27(4):493–506. doi: 10.1016/j.arthro.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 55.Davatchi F., Abdollahi B.S., Mohyeddin M., Shahram F., Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211–215. doi: 10.1111/j.1756-185X.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 56.Wakitani S., Imoto K., Yamamoto T., Saito M., Murata N., Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr Cartil. 2002;10(3):199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 57.Akgun I., Unlu M.C., Erdal O.A., Ogut T., Erturk M., Ovali E. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: a 2-year randomized study. Arch Orthop Trauma Surg. 2015;135(2):251–263. doi: 10.1007/s00402-014-2136-z. [DOI] [PubMed] [Google Scholar]
- 58.Nejadnik H., Hui J.H., Feng Choong E.P., Tai B.C., Lee E.H. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38(6):1110–1116. doi: 10.1177/0363546509359067. Epub 2010/04/16. [DOI] [PubMed] [Google Scholar]
- 59.Wakitani S., Okabe T., Horibe S., Mitsuoka T., Saito M., Koyama T. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5(2):146–150. doi: 10.1002/term.299. [DOI] [PubMed] [Google Scholar]
- 60.Vinardell T., Sheehy E.J., Buckley C.T., Kelly D.J. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng Part A. 2012;18(11–12):1161–1170. doi: 10.1089/ten.tea.2011.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li W.J., Tuli R., Okafor C., Derfoul A., Danielson K.G., Hall D.J. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26(6):599–609. doi: 10.1016/j.biomaterials.2004.03.005. Epub 2004/07/30. [DOI] [PubMed] [Google Scholar]
- 62.Pagnotto M.R., Wang Z., Karpie J.C., Ferretti M., Xiao X., Chu C.R. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007;14(10):804–813. doi: 10.1038/sj.gt.3302938. Epub 2007/03/09. [DOI] [PubMed] [Google Scholar]
- 63.De Bari C., Dell'Accio F., Tylzanowski P., Luyten F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 64.Choi W.H., Kim H.R., Lee S.J., Jeong N., Park S.R., Choi B.H. Fetal cartilage-derived cells have stem cell properties and are a highly potent cell source for cartilage regeneration. Cell Transpl. 2016;25(3):449–461. doi: 10.3727/096368915X688641. [DOI] [PubMed] [Google Scholar]
- 65.Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang B., Song Y.P., Liao L.M., Han Q., Zhao R.C. Treatment of severe therapy-resistant acute graft-versus-host disease with human adipose tissue-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;38(5):389–390. doi: 10.1038/sj.bmt.1705457. [DOI] [PubMed] [Google Scholar]
- 67.Park C.W., Kim K.S., Bae S., Son H.K., Myung P.K., Hong H.J. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J Stem Cells. 2009;2(1):59–68. doi: 10.15283/ijsc.2009.2.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liang X., Ding Y., Zhang Y., Tse H.F., Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transpl. 2014;23(9):1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 69.Gomez-Aristizabal A., Sharma A., Bakooshli M.A., Kapoor M., Gilbert P.M., Viswanathan S. Stage-specific differences in secretory profile of mesenchymal stromal cells (MSCs) subjected to early- vs late-stage OA synovial fluid. Osteoarthr Cartil. 2016 doi: 10.1016/j.joca.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 70.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vader P., Mol E.A., Pasterkamp G., Schiffelers R.M. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106(Pt A):148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Rani S., Ryan A.E., Griffin M.D., Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Molecular therapy. J Am Soc Gene Ther. 2015;23(5):812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu S., Ju G.-Q., Du T., Zhu Y.-J., Liu G.-H. Microvesicles derived from human umbilical cord Wharton's jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PLoS One. 2013;8(4):e61366. doi: 10.1371/journal.pone.0061366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hasselmann D.O., Rappl G., Tilgen W., Reinhold U. Extracellular tyrosinase mRNA within apoptotic bodies is protected from degradation in human serum. Clin Chem. 2001;47(8):1488–1489. [PubMed] [Google Scholar]
- 75.van der Meel R., Fens M.H., Vader P., van Solinge W.W., Eniola-Adefeso O., Schiffelers R.M. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J Control Release. 2014;195:72–85. doi: 10.1016/j.jconrel.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 76.Gyorgy B., Hung M.E., Breakefield X.O., Leonard J.N. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–464. doi: 10.1146/annurev-pharmtox-010814-124630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lai R.C., Arslan F., Lee M.M., Sze N.S., Choo A., Chen T.S. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 78.Nakano M., Nagaishi K., Konari N., Saito Y., Chikenji T., Mizue Y. Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci Rep. 2016;6:24805. doi: 10.1038/srep24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu G.W., Li Q., Niu X., Hu B., Liu J., Zhou S.M. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;6:10. doi: 10.1186/scrt546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lai R.C., Arslan F., Tan S.S., Tan B., Choo A., Lee M.M. Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles. J Mol Cell Cardiol. 2010;48(6):1215–1224. doi: 10.1016/j.yjmcc.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 81.Kordelas L., Rebmann V., Ludwig A.K., Radtke S., Ruesing J., Doeppner T.R. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 82.Arslan F., Lai R.C., Smeets M.B., Akeroyd L., Choo A., Aguor E.N. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 83.Shabbir A., Cox A., Rodriguez-Menocal L., Salgado M., Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24(14):1635–1647. doi: 10.1089/scd.2014.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang S., Chu W.C., Lai R.C., Lim S.K., Hui J.H., Toh W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr Cartil. 2016;24(12):2135–2140. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 85.Tao S.C., Yuan T., Zhang Y.L., Yin W.J., Guo S.C., Zhang C.Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. doi: 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kato T., Miyaki S., Ishitobi H., Nakamura Y., Nakasa T., Lotz M.K. Exosomes from IL-1beta stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16(4):R163. doi: 10.1186/ar4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen T.S., Lai R.C., Lee M.M., Choo A.B., Lee C.N., Lim S.K. Mesenchymal stem cell secretes microparticles enriched in pre-miRNAs. Nucleic Acids Res. 2010;38(1):215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maumus M., Jorgensen C., Noel D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie. 2013;95(12):2229–2234. doi: 10.1016/j.biochi.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 89.Withrow J., Murphy C., Liu Y., Hunter M., Fulzele S., Hamrick M.W. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2016;18(1):286. doi: 10.1186/s13075-016-1178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trerotola M., Relli V., Simeone P., Alberti S. Epigenetic inheritance and the missing heritability. Hum Genomics. 2015;9:17. doi: 10.1186/s40246-015-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fernandez-Tajes J., Soto-Hermida A., Vazquez-Mosquera M.E., Cortes-Pereira E., Mosquera A., Fernandez-Moreno M. Genome-wide DNA methylation analysis of articular chondrocytes reveals a cluster of osteoarthritic patients. Ann Rheum Dis. 2014;73(4):668–677. doi: 10.1136/annrheumdis-2012-202783. [DOI] [PubMed] [Google Scholar]
- 92.Beerman I., Rossi D.J. Epigenetic control of stem cell potential during homeostasis, aging, and disease. Cell Stem Cell. 2015;16(6):613–625. doi: 10.1016/j.stem.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Z., Liu C., Xie Z., Song P., Zhao R.C., Guo L. Epigenetic dysregulation in mesenchymal stem cell aging and spontaneous differentiation. PLoS One. 2011;6(6):e20526. doi: 10.1371/journal.pone.0020526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Croce L., Helin K. Transcriptional regulation by Polycomb group proteins. Nat Struct Mol Biol. 2013;20(10):1147–1155. doi: 10.1038/nsmb.2669. [DOI] [PubMed] [Google Scholar]
- 95.Yu K.R., Kang K.S. Aging-related genes in mesenchymal stem cells: a mini-review. Gerontology. 2013;59(6):557–563. doi: 10.1159/000353857. [DOI] [PubMed] [Google Scholar]
- 96.Kelly T.K., De Carvalho D.D., Jones P.A. Epigenetic modifications as therapeutic targets. Nat Biotechnol. 2010;28(10):1069–1078. doi: 10.1038/nbt.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ezura Y., Sekiya I., Koga H., Muneta T., Noda M. Methylation status of CpG islands in the promoter regions of signature genes during chondrogenesis of human synovium-derived mesenchymal stem cells. Arthritis Rheum. 2009;60(5):1416–1426. doi: 10.1002/art.24472. [DOI] [PubMed] [Google Scholar]
- 98.Liu C.J., Prazak L., Fajardo M., Yu S., Tyagi N., Di Cesare P.E. Leukemia/lymphoma-related factor, a POZ domain-containing transcriptional repressor, interacts with histone deacetylase-1 and inhibits cartilage oligomeric matrix protein gene expression and chondrogenesis. J Biol Chem. 2004;279(45):47081–47091. doi: 10.1074/jbc.M405288200. [DOI] [PubMed] [Google Scholar]
- 99.Vega R.B., Matsuda K., Oh J., Barbosa A.C., Yang X., Meadows E. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119(4):555–566. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 100.Huh Y.H., Ryu J.H., Chun J.S. Regulation of type II collagen expression by histone deacetylase in articular chondrocytes. J Biol Chem. 2007;282(23):17123–17131. doi: 10.1074/jbc.M700599200. [DOI] [PubMed] [Google Scholar]
- 101.Herlofsen S.R., Bryne J.C., Hoiby T., Wang L., Issner R., Zhang X. Genome-wide map of quantified epigenetic changes during in vitro chondrogenic differentiation of primary human mesenchymal stem cells. BMC Genomics. 2013;14:105. doi: 10.1186/1471-2164-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zimmermann P., Boeuf S., Dickhut A., Boehmer S., Olek S., Richter W. Correlation of COL10A1 induction during chondrogenesis of mesenchymal stem cells with demethylation of two CpG sites in the COL10A1 promoter. Arthritis Rheum. 2008;58(9):2743–2753. doi: 10.1002/art.23736. [DOI] [PubMed] [Google Scholar]
- 103.Roach H.I., Yamada N., Cheung K.S., Tilley S., Clarke N.M., Oreffo R.O. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52(10):3110–3124. doi: 10.1002/art.21300. [DOI] [PubMed] [Google Scholar]
- 104.Hong S., Derfoul A., Pereira-Mouries L., Hall D.J. A novel domain in histone deacetylase 1 and 2 mediates repression of cartilage-specific genes in human chondrocytes. FASEB J. 2009;23(10):3539–3552. doi: 10.1096/fj.09-133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Higashiyama R., Miyaki S., Yamashita S., Yoshitaka T., Lindman G., Ito Y. Correlation between MMP-13 and HDAC7 expression in human knee osteoarthritis. Mod Rheumatol. 2010;20(1):11–17. doi: 10.1007/s10165-009-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nugent M. MicroRNAs: exploring new horizons in osteoarthritis. Osteoarthr Cartil. 2016;24(4):573–580. doi: 10.1016/j.joca.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 107.Vicente R., Noel D., Pers Y.M., Apparailly F., Jorgensen C. Deregulation and therapeutic potential of miRNAs in arthritic diseases. Nat Rev Rheumatol. 2016;12(4):211–220. doi: 10.1038/nrrheum.2015.162. [DOI] [PubMed] [Google Scholar]
- 108.Ghildiyal M., Zamore P.D. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10(2):94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang R., Ma J., Yao J. Molecular mechanisms of the cartilage-specific miRNA-140 in osteoarthritis. Inflamm Res. 2013;62(10):871–877. doi: 10.1007/s00011-013-0654-8. [DOI] [PubMed] [Google Scholar]
- 110.Toh W.S., Lai R.C., Hui J.H., Lim S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2016 doi: 10.1016/j.semcdb.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 111.Zhang M., Wang J. Epigenetics and osteoarthritis. Genes Dis. 2015;2(1):69–75. doi: 10.1016/j.gendis.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cho S.W., Kim S., Kim J.M., Kim J.S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31(3):230–232. doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- 113.Mout R., Ray M., Lee Y.W., Scaletti F., Rotello V.M. In vivo delivery of CRISPR/Cas9 for therapeutic gene editing: progress and challenges. Bioconjug Chem. 2017 doi: 10.1021/acs.bioconjchem.7b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee J.S., Grav L.M., Lewis N.E., Faustrup Kildegaard H. CRISPR/Cas9-mediated genome engineering of CHO cell factories: application and perspectives. Biotechnol J. 2015;10(7):979–994. doi: 10.1002/biot.201500082. [DOI] [PubMed] [Google Scholar]
- 115.Cheng A.W., Wang H., Yang H., Shi L., Katz Y., Theunissen T.W. Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res. 2013;23(10):1163–1171. doi: 10.1038/cr.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qi L.S., Larson M.H., Gilbert L.A., Doudna J.A., Weissman J.S., Arkin A.P. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152(5):1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kleinstiver B.P., Pattanayak V., Prew M.S., Tsai S.Q., Nguyen N.T., Zheng Z. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016 doi: 10.1038/nature16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cyranoski D. Chinese scientists to pioneer first human CRISPR trial. Nat News. 2016;535(7613):476. doi: 10.1038/nature.2016.20302. [DOI] [PubMed] [Google Scholar]
- 119.Gibson G., Yang M. Gene editing in chondrocytes using CRISPR/CAS9. Osteoarthr Cartil. 2016;24:S2–S3. [Google Scholar]
- 120.van den Akker G., van Beuningen H., Davidson E.B., van der Kraan P. CRISPR/CAS9 mediated genome engineering of human mesenchymal stem cells. Osteoarthr Cartil. 2016;24:S231. [Google Scholar]
- 121.Yang M., Zhang L., Stevens J., Gibson G. CRISPR/Cas9 mediated generation of stable chondrocyte cell lines with targeted gene knockouts; analysis of an aggrecan knockout cell line. Bone. 2014;69:118–125. doi: 10.1016/j.bone.2014.09.005. [DOI] [PubMed] [Google Scholar]