Abstract
Background & Aims
There is debate over the best way to stage hepatocellular carcinoma (HCC). We attempted to validate the prognostic and clinical utility of the recently developed Hong Kong Liver Cancer (HKLC) staging system, a hepatitis B-based model, and compared data with that from the Barcelona Clinic Liver Cancer (BCLC) staging system in a North American population who underwent intra-arterial therapy (IAT).
Methods
We performed a retrospective analysis of data from 1009 patients with HCC who underwent intra-arterial therapy from 2000 through 2014. Most patients had hepatitis C or unresectable tumors; all patients underwent IAT, with or without resection, transplantation, and/or systemic chemotherapy. We calculated HCC stage for each patient using 5-stage HKLC (HKLC-5) and 9-stage HKLC (HKLC-9) system classifications, as well as the BCLC system. Survival information was collected up until end of 2014 at which point living or unconfirmed patients were censored. We compared performance of the BCLC, HKLC-5, and HKLC-9 systems in predicting patient outcomes using Kaplan-Meier estimates, calibration plots, c-statistic, Akaike information criterion, and the likelihood ratio test.
Results
Median overall survival time, calculated from first IAT until date of death or censorship, for the entire cohort (all stages) was 9.8 months. The BCLC and HKLC staging systems predicted patient survival times with significance (P<.001). HKLC-5 and HKLC-9 each demonstrated good calibration. The HKLC-5 system outperformed the BCLC system in predicting patient survival times (HKLC c=0.71, Akaike information criterion=6242; BCLC c=0.64, Akaike information criterion=6320), reducing error in predicting survival time (HKLC reduced error by 14%, BCLC reduced error by 12%), and homogeneity (HKLC χ2=201; P<.001; BCLC χ2=119; P<.001) and monotonicity (HKLC linear trend χ2=193; P<.001; BCLC linear trend χ2=111; P<.001). Small proportions of patients with HCC of stages IV or V, according to the HKLC system, survived for 6 months and 4 months, respectively.
Conclusion
In a retrospective analysis of patients who underwent IAT for unresectable HCC, we found the HKLC-5 staging system to have the best combination of performances in survival separation, calibration, and discrimination; it consistently outperformed the BCLC system in predicting survival times of patients. The HKLC system identified patients with HCC of stages IV and V who are unlikely to benefit from IAT.
Keywords: liver cancer, risk factors, prognosis, predicted outcome
BACKGROUND & AIMS
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second most common cause of cancer-related mortality in the world1. It has been increasing worldwide and has nearly tripled in the last decades in the Unites States 2, 3. Etiologic and clinical heterogeneity in HCC populations has hampered efforts in establishing a universally adopted treatment scheme to improve patient care. An ideal staging system would be able to provide accurate prognosis, stratify patients into distinct prognostic groups, and suggest up-to-date therapeutic strategies 4.
The uniquely challenging aspect of staging HCC is the interplay of two diseases, liver cirrhosis and cancer 5, 6. Liver cirrhosis ultimately results in both reduced liver function and can lead to cancer. Cancer in turn worsens liver function by mass effect and parenchymal invasion. Staging is further complicated by patient’s social history and co-morbidities often relevant in this patient population. There has been at least eight proposed HCC staging systems, including the Barcelona Clinic Liver Cancer (BCLC) staging system7.
The BCLC staging system is currently the most widely used system in Europe and the Unites States given its comprehensive algorithm tied to treatment recommendations. It has been externally validated and provides a common framework to enable comparison between patient cohorts and institutions7, 9–11. However, BCLC staging often draws criticism because of conservative treatment recommendations in patients for whom aggressive treatment approaches have become commonplace due to technical safety and effectiveness12–16. Although widely quoted in the scientific literature, efforts have been underway to modify and update the BCLC staging.
The recently developed Hong Kong Liver Cancer (HKLC) staging system has garnered international attention because of its comprehensive nature, based on the largest patient cohort to date for HCC staging, and tied to treatment recommendations that addresses the aforementioned limitations of the BCLC system 17. Compared to BCLC, HKLC staging system provided superior survival discrimination in their internal validation cohort. However, the 3958 patients in the HKLC study were mainly Asians with hepatitis B virus (HBV). Hence, the authors called for external validation outside of Asia, where there are more heterogeneous etiologies of HCC 17. The purpose of our study is to assess the external prognostic validity and clinical utility of the recently developed HKLC staging system in a North American population with unresectable HCC and compare the performance with the BCLC system.
METHODS
Patient population
This Institutional Review Board-approved and Health Insurance Portability and Accountability Act compliant study was performed at a single tertiary referral hospital in North America (Johns Hopkins Hospital, Baltimore, MD, USA). 1009 consecutive HCC patients derived from an ongoing database tracking HCC patients from 2000 – 2014 and who underwent at least one IATs (Lipiodol, drug-eluting beads, or radio-embolization) +/− systemic chemotherapy, liver transplantation, resection, and/or ablation were included 18. 131 patients (representing 13.0% of total) had missing lab or radiologic data that prevented calculation of either HKLC or BCLC stages. A complete-case analysis was performed. A comprehensive review of medical charts, imaging and outcomes were included in the database.
Treatment Criteria & Modality
The diagnosis of HCC was made by imaging appearance and/or by biopsy as defined by guidelines 8, 10, 19, 20. Patients with newly diagnosed HCC referred to our center were discussed at weekly multidisciplinary conference for treatment decision. Patients eligible for immediately curative treatments were routed to the corresponding specialties. The remaining patients ineligible for immediate curative therapy were considered for IAT +/− sorafenib, transplantation, and/or delayed resection/ablation as part of individualization of patient care; these were often outside of BCLC treatment guidelines. The cohort consisted of mainly unresectable HCC that included liver transplantation candidates undergoing bridging using IAT, questionably resectable candidates undergoing down-staging with IAT, and select BCLC D patients.
A majority of patients underwent TACE in this cohort. For conventional TACE (cTACE), a mixture of ethiodized oil, doxorubicin (Adriamycin; Pharmacia & Upjohn, Kalamazoo, Mich), mitomycin-C (Bedford Laboratories, Bedford, Ohio), and cisplatin (Bristol-Myers Squibb, Princeton, NJ) was injected in the hepatic arterial vasculature through a microcatheter. This was followed by injection of up to 4 mL of 100–300-μm microsphere particles (Embosphere; Biosphere Medical, Boston, Mass). For TACE with drug-eluting beads (DEB-TACE), patients received 2 mL of 100–300-μm-diameter microsphere particles (LC Beads; BioCompatibles, Surrey, England) loaded with 50 mg of doxorubicin hydrochloride (25 mg/mL) and mixed with nonionic contrast material (300 mg of iodine per milliliter, Oxilan; Guerbet, Bloomington, Ind)21.
Study Design & Data Analysis
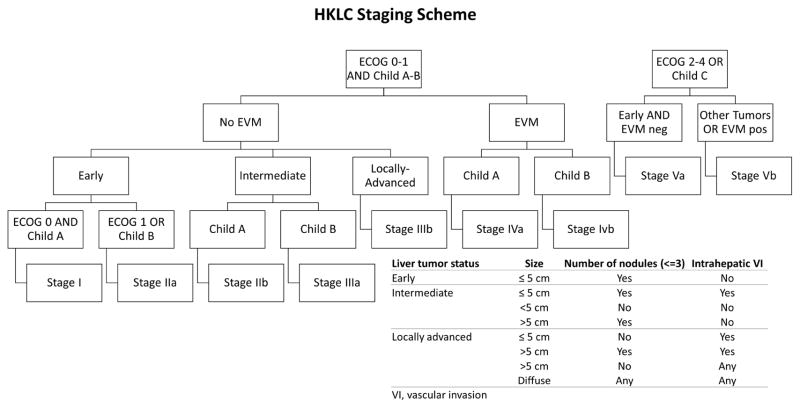
The TRIPOD checklist was adopted to ensure accuracy and comprehensiveness of the external validity assessment 22. The HKLC staging scheme is shown in Figure 1 17. HKLC staging with both five major classifications (stages I, II, III, IV, V; here referred as HKLC-5) and the full nine sub-classifications (stages I, IIa, IIb, IIIa, IIIb, IVa, IVb, Va and Vb; here referred as HKLC-9) were considered. All laboratory values were taken from the time immediately before the first IAT session at our institution. Treatment information was gathered from structured data as well as freestyle clinician notes in the institutional electronic medical record (EMR).
Figure 1. Hong Kong Liver Cancer staging.
The Hong Kong Liver Cancer staging scheme as proposed by Yau et al (2014).
The outcome of interest, overall survival (OS), was computed from the date of first IAT at our institution to death or last known clinic follow-up. OS was ascertained by two methods. First, the institutional EMR was checked to confirm patient’s vital status and death date if available. Second, all patients with unknown vital status were queried once on the United States Office of Vital Records registry in late 2014. All patients who were alive or had unknown vital status were censored on the last known clinical follow-up date. Kaplan-Meier survival curves were plotted and the log-rank test was used to assess the significance of the survival curve separation 23, 24. A difference with a two-tailed P-value of < .05 was considered statistically significant.
Model calibration, discrimination, reduction in error, monotonicity of gradient, and homogeneity were reported as important measures of staging system performance 25–28. Measurement of discrepancy between observed and predicted overall survival time was made through calibration plots of validation cohort versus original HKLC predictions 29 The ability of the staging system to assign distinct stages to patient groups with different survival was measured using Harrell’s c-statistic, a rank-order statistic that relates to the area under the ROC curve 30. Akaike information criterion (AIC) from the Cox proportional hazards model was used to further confirm survival discrimination 31. Each staging system’s ability to reduce error in predicting survival time was calculated as previously reported in literature 32. Cox model with likelihood ratio (LHR) chi-square test was used to measure homogeneity and monotonicity of gradients 33. Monotonicity of gradient was calculated to measure the consistency of worsening patient survival with worsening stages 25. Homogeneity was used to measure similarity in patient survival within a given stage 25. Greater C-statistic, reduction in error, and LHR chi-square values corresponded to better staging system performance while the opposite was the case for AIC.
Analysis was performed in the full cohort as well as some in sub-cohorts with HBV carriers and hepatitis C virus (HCV) carriers to assess any clinically relevant trends in different subgroups. Pre-2008 and Post-2008 subgroup analysis was performed to assess for temporal validity. Further subgroup analysis of only confirmed dead patients on pre and post-2008 sub-cohorts was done to explore the effect of censoring. The full validation cohort was primarily analyzed for comparison with the original HKLC cohort while both HBV and HCV sub-cohorts were primarily analyzed for comparison with the full validation cohort. All statistical analysis and plotting were done in R: Statistical Programming Language Version 3.8.11 (Vienna, Austria) and Microsoft Excel 2010.
RESULTS
Validation Cohort & Survival Information
A total of 881 patients were included in the full validation cohort. Detailed baseline clinico-pathologic characteristics are summarized in Table 1. Notable distinguishing characteristics of this validation cohort from the original development cohort was the predominance of Caucasian (n=520, 59.0%) male (n=698, 79.2%) patients with HCV (n=427, 48.5%) and alcohol (n=269, 30.5%) as major HCC etiologies. Other major characteristics of this cohort included Eastern Cooperative Oncology Group (ECOG) performance score 0 (n=426, 48.4%) and Child-Pugh Class A (n=534, 60.6%) (Table 1). All patients received cTACE (n=534, 60.6%), DEB-TACE (n=326, 37.0%), or radioembolization with yttrium-90 (n=21, 2.4%). One-third of the patients received treatment using liver transplantation, resection, or sorafenib at some point in the treatment course.
Table 1.
Characteristics of the Validation Cohort
| Full cohort (N=881) | HBV group (N=132) | HCV group (N=427) | ||||
|---|---|---|---|---|---|---|
| Median age in y (IQR) | 62 | (56–71) | 58 | (51–69) | 59 | (55–65) |
| Sex | ||||||
| Male | 698 | 79.2% | 111 | (84.1%) | 349 | (81.7%) |
| Female | 183 | 20.8% | 21 | (15.9%) | 78 | (18.3%) |
| Ethnicity | ||||||
| White | 520 | 59.0% | 36 | (27.3%) | 232 | (54.3%) |
| Black | 200 | 22.7% | 24 | (18.2%) | 152 | (35.6%) |
| Asians | 69 | 7.8% | 49 | (37.1%) | 8 | (1.9%) |
| Others | 92 | 10.4% | 23 | (17.4%) | 35 | (8.2%) |
|
| ||||||
| Etiologies of HCC** | ||||||
| HCV | 427 | 48.5% | - | - | 427 | (100%) |
| HBV | 132 | 15.0% | 132 | (100%) | - | - |
| Alcoholism | 269 | 30.5% | - | - | - | - |
| NASH | 60 | 6.8% | - | - | - | - |
| Cryptogenic | 48 | 5.4% | - | - | - | - |
|
| ||||||
| Clinico-pathologic Factors | ||||||
| ECOG PS | ||||||
| 0 | 426 | 48.4% | 66 | (50.0%) | 217 | (50.8%) |
| 1 | 407 | 46.2% | 60 | (45.5%) | 188 | (44.0%) |
| 2 | 38 | 4.3% | 6 | (4.5%) | 17 | (4.0%) |
| 3 | 10 | 1.1% | 0 | (0%) | 5 | (1.2%) |
| 4 | 0 | 0.0% | 0 | (0%) | 0 | (0%) |
| Child-Pugh grade | ||||||
| A | 534 | 60.6% | 90 | (68.2%) | 240 | (56.2%) |
| B | 292 | 33.1% | 35 | (26.5%) | 157 | (36.8%) |
| C | 55 | 6.2% | 7 | (5.3%) | 30 | (7.0%) |
| AFP level, ng/mL | ||||||
| Median (IQR) | 64 | (9–1131) | 96 | (18–4604) | 102 | (14–923) |
|
| ||||||
| Liver tumor factors | ||||||
| Size | ||||||
| ≤ 2cm | 62 | 7.0% | 5 | (3.8%) | 39 | (9.1%) |
| > 2cm to ≤ 5cm | 327 | 37.1% | 41 | (31.1%) | 194 | (45.4%) |
| > 5cm | 480 | 54.5% | 83 | (62.9%) | 191 | (44.7%) |
| Diffuse | 12 | 1.4% | 3 | (2.3%) | 3 | (0.7%) |
| No. of nodules | ||||||
| Solitary (1) | 270 | 30.6% | 43 | (32.6%) | 122 | (28.5%) |
| Oligonodular (2–3) | 237 | 26.9% | 31 | (23.5%) | 125 | (29.3%) |
| Multinodular (> 3) | 374 | 42.5% | 58 | (44.0%) | 180 | (42.2%) |
| Intrahepatic VI | 227 | 25.8% | 37 | (28.0%) | 105 | (24.6%) |
| EVM | 195 | 22.1% | 35 | (26.5%) | 84 | (19.7%) |
| Extrahepatic VI | 124 | 14.1% | 27 | (20.5%) | 53 | (12.4%) |
| Extrahepatic metastasis | 92 | 10.4% | 11 | (8.3%) | 41 | (9.6%) |
|
| ||||||
| Treatment Received | ||||||
| Intra-arterial Therapy | ||||||
| cTACE | 534 | 60.6% | 85 | (64.4%) | 245 | (57.4%) |
| DEB-TACE | 326 | 37.0% | 39 | (29.5%) | 176 | (41.2%) |
| Y-90 | 21 | 2.4% | 8 | (6.1%) | 6 | (1.4%) |
| Other Treatments*** | ||||||
| Sorafenib | 176 | 20.0% | 32 | (24.2%) | 83 | (19.4%) |
| Resection | 19 | 2.2% | 5 | (3.8%) | 2 | (0.5%) |
| Liver Transplantation | 94 | 10.7% | 13 | (9.8%) | 66 | (15.5%) |
| Follow-up summary | ||||||
| Enrollment Pre-2008 | 327 | 37.1% | * | * | * | * |
| Vital Status | ||||||
| Dead, confirmed | 560 | 63.6% | 83 | (62.9%) | 242 | (56.7%) |
| Alive or censored | 321 | 36.4% | 49 | (37.1%) | 185 | (43.3%) |
| Overall Survival, mo | ||||||
| Median (IQR) | 9.8 | (3.4–22.2) | 11.3 | (3.5–29.6) | 9.2 | (3.4–21.3) |
Abbreviations: ECOG PS, Eastern Coooperative Oncology Group performance status; HBV, Hepatitis B Virus;
Numbers do not add up to N due to one person potentially carrying multiple etiologies
These represent treatments in addition to IAT
The full validation cohort on average had slightly more advanced disease compared to the original HKLC cohort 17, with smaller proportions of Child-Pugh A (60.6% vs. 72.6%, validation cohort vs. the original HKLC cohort, respectively), ECOG performance score 0 (48.4% vs. 56.9%, respectively), patients with tumor size < 2cm (7.0% vs. 9.3%, respectively), and patients with a solitary nodule (30.6% vs. 47.3% respectively) (Table 1). There was larger presence in our study of BCLC C patients (60.8% vs. 39.1% for the original HKLC cohort) (Table 2).
Table 2.
Median Overall Survival by Stage
| Full Cohort | HBV Cohort | HCV Cohort | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Stage | mOS [95% CI]* | N | mOS [95% CI]* | N | mOS [95% CI]* | N | |
| BCLC | |||||||
| A | 52 [45,71] | 137 | 63 [45,NA] | 22 | 52 [39,92] | 79 | |
| B | 24 [21,31] | 146 | 27 [18, NA] | 21 | 27 [20, NA] | 78 | |
| C | 12 [11,14] | 536 | 13 [9,25] | 83 | 11 [9,15] | 235 | |
| D | 4 [3,7] | 62 | 1 [1,NA] | 6 | 6 [3,9] | 35 | |
|
| |||||||
| HKLC-5 | |||||||
| I | 63 [48, 92] | 100 | 63 [52, NA] | 14 | 55 [47, NA] | 67 | |
| II | 29 [23, 34] | 261 | 32 [18, 45] | 42 | 29 [23, 45] | 114 | |
| III | 12 [10, 16] | 262 | 25 [11, 27] | 34 | 12 [9,20] | 129 | |
| IV | 6 [5, 11] | 164 | 6 [3, 18] | 31 | 6 [4, 9] | 69 | |
| V | 3 [2, 6] | 94 | 2 [1, NA] | 11 | 6 [3, 7] | 48 | |
|
| |||||||
| HKLC-9 | |||||||
| I | 63 [48, 92] | 100 | 63 [52, NA] | 14 | 55 [47, NA] | 67 | |
| IIa | 36 [26, 48] | 105 | 32 [16, NA] | 14 | 30 [23, NA] | 63 | |
| IIb | 24 [21, 31] | 156 | 31 [18, NA] | 28 | 28 [21, NA] | 51 | |
| IIIa | 11 [9, 25] | 63 | 28 [25, NA] | 5 | 21 [9, NA] | 36 | |
| IIIb | 12 [10, 15] | 199 | 15 [10, 27] | 29 | 12 [9, 16] | 93 | |
| IVa | 11 [8, 14] | 85 | 13 [6, NA] | 22 | 8 [5, 15] | 30 | |
| IVb | 4 [3, 8] | 79 | 3 [2, NA] | 9 | 4 [3, 12] | 39 | |
| Va | 11 [7, N/A] | 22 | 26 [2, N/A] | 3 | 15 [7, NA] | 13 | |
| Vb | 2 [1, 5] | 72 | 1 [1, NA] | 8 | 5 [3, 7] | 35 | |
mOS is in months; Abbreviations: mOS, median overall survival
Median OS, as calculated from first IAT date at our institution to date of death or censorship, for the entire cohort across all stages was 9.8 months. Detailed median OS information and the number of patients corresponding to each stage for BCLC, HKLC-5, and HKLC-9 are reported in Table 2. Three most represented groups in cross-tabulations were HKLC III / BCLC C patients (n=193, 21.9%), HKLC IV / BCLC C (n=162, 18.3%), and HKLC II / BCLC C (n=145, 16.5%) (Supplemental Table 1).
Prognostic Validation
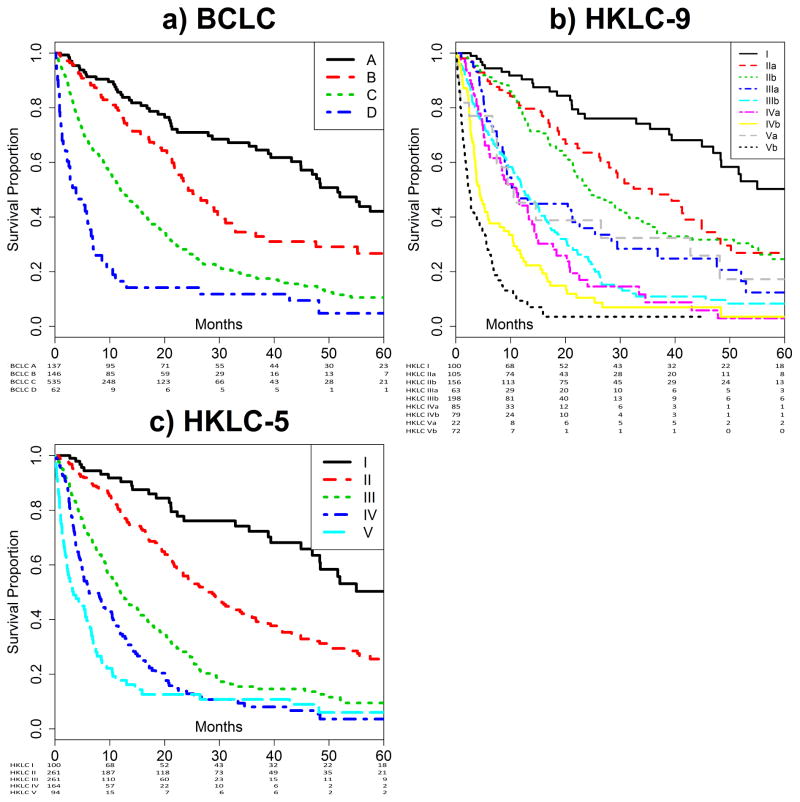
HKLC and BCLC staging systems predicted survival with significance (P<0.001). Specifically, BCLC and HKLC-5 staging achieved separation of the survival curves (Figure 2A and 2B), whereas the survival curves for HKLC-9 had overlaps in survival for stages IIIa, IIIb, IVa, and Va, all of which had median OS of approximately 11 months (Figure 2C).
Figure 2. Kaplan-Meier Curves.
Stratification by BCLC staging (A), HKLC-5 staging (B) and HKLC-9 staging (C). All three staging systems demonstrate separation of survival curves. The HKLC-9 staging has several overlaps, notably in stages IIIa, IIIb, IVa, and Va. Number at risk tables are attached below each subfigure.
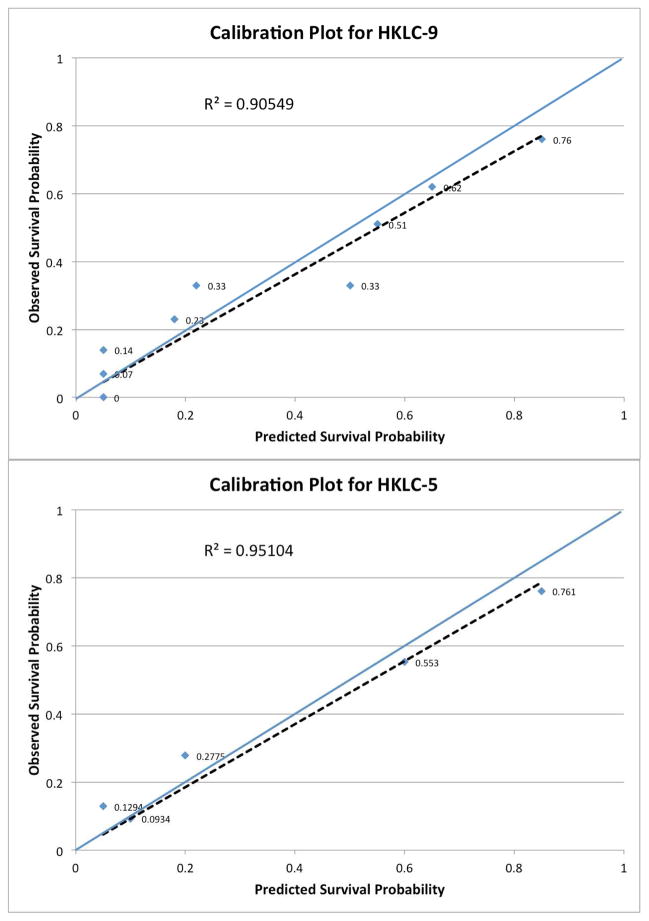
Both calibration curves for HKLC-5 and HKLC-9 demonstrated matching of OS between the validation cohort and the original cohort (Figure 3). Both plots result in R2 values > 0.9 signifying goodness of fit, with HKLC-5 (R2 = 0.95) being slightly better than HKLC-9 (R2 = 0.90).
Figure 3. Calibration plots for HKLC-5 and HKLC-9.
Calibration plots for HKLC-9 (A) and for HKLC-5 (B). They plot the validation cohort patients’ actual survival time (y-axis) versus the HKLC staging system’s predicted survival (x-axis). The closer points are to the reference line (blue), the better the calibration. The dotted line represents the least-squares trendline. Both HKLC-9 and HKLC-5 demonstrate moderate to good calibration with R2 = 0.9055 and R2 = 0.9510, respectively.
HKLC-5 and HKLC-9 demonstrated stronger survival discrimination than BCLC as illustrated by both Harrell’s c-statistic and AIC (Table 3). Greater homogeneity was observed with HKLC compared to BCLC (Table 3), suggesting similar overall survival within each given stage. The sum of errors in survival prediction for the entire cohort when using median OS as the sole survival predictor was 782 days. Compared to using the median OS alone, when using the BCLC staging as the survival predictor, 12% overall error reduction was observed whereas greater error reductions of 14% and 16% were measured using HKLC-5 and HKLC-9, respectively, (Table 3).
Table 3.
Summary of Staging System Performance Measures
| BCLC | HKLC-5 | HKLC-9 | ||
|---|---|---|---|---|
| Full | ||||
| Harrell’s c-statistic | 0.643 ± 0.012 | 0.707 ± 0.013 | 0.717 ± 0.014 | |
| AIC* | 6321 | 6241 | 6200 | |
| Likelihood Ratio χ2 | 119 | 201 | 250 | |
| Error Reduction (%) | 12% | 14% | 16% | |
| HBV | ||||
| Harrell’s c-statistic | 0.677 ± 0.032 | 0.747 ± 0.035 | 0.759 ± 0.036 | |
| AIC* | 642 | 629 | 612 | |
| Likelihood Ratio χ2 | 29 | 45 | 69 | |
| Error Reduction (%) | 13% | 16% | 18% | |
| HCV | ||||
| Harrell’s c-statistic | 0.668 ± 0.019 | 0.715 ± 0.020 | 0.732 ± 0.021 | |
| AIC* | 2426 | 2399 | 2387 | |
| Likelihood Ratio χ2 | 63 | 92 | 113 | |
| Error Reduction (%) | 13% | 14% | 16% |
smaller the better
Abbreviation: AIC, Akaike’s Information Criterion; LHR, lik elihood ratio;
Sub-cohort Analysis: HBV vs HCV and pre-2008 vs post-2008
The predominant population in the HBV sub-cohort was an Asian (n = 49, 37.1%), male (n=111, 85.1%) patient with ECOG performance score 0 (n=66, 50.0%), and Child-Pugh Class A (n=90, 68.2%) (Table 1). Predominant patient characteristics in the HCV sub-cohort was Caucasian (n=232, 54.3%), male (n=349, 81.7%), ECOG performance score 0 (n=217, 50.8%), and Child-Pugh Class A (n=240, 56.2%) (Table 1). Overall, the survival curves demonstrate similar qualitative degree of separation among HBV and HCV cohorts, though with some more overlaps, possibly due to smaller sample size in each stage in this subcohort compared to the full validation cohort (Table 2, Supplemental figure 1).
Pre-2008 (n = 554) and Post-2008 (n=327) subgroup Kaplan-Meier curves (supplemental figure 2) demonstrated excellent survival curve separation in both subgroups. The most represented patients in pre-2008 were White (n = 313, 56%), male (n = 427, 77%) patients with ECOG performance score 0 (n = 240, 43%), and Child-Pugh Class A (n = 342, 62%). Similarly, the most represented patients in post-2008 were White (n = 207, 63%), male (n = 270, 83%) patients with ECOG performance score 1 (n = 167, 51%), and Child-Pugh Class A (n = 327, 59%). Most notable difference in pre and post-2008 groups was improvements in survival of HKLC stage I and BCLC stage A patients. Of note, censorship proportions for pre-2008 and post-2008 groups were 19% (n = 63) and 47% (n = 258), respectively. Sub-analysis of only confirmed dead patients for pre and post 2008 sub-cohorts (supplemental figure 3) demonstrated similar trends of overall survival changes with worsening stages, except in HKLC stage I in post-2008 sub-cohort where most patients were censored. Overall, HKLC demonstrated excellent survival discrimination and error reduction in various sub-cohorts. The sub-cohorts demonstrated consistent worsening of survival with worsening stages, similar to the full validation cohort, as expected of a staging system (Table 3).
DISCUSSION
The present study showed prognostic external validity of the HKLC staging system in a North American HCC cohort whose main treatment modality was TACE and primary HCC etiology was HCV and/or EtOH. Specifically, the HKLC-5 staging demonstrated significantly improved performance over BCLC based on calibration, discrimination, monotonicity/homogeneity, and survival curve separation. Furthermore, HKLC-5 identified stages IV and V patients who had very poor survival despite IAT. In light of the superior predictive power and more practically relevant treatment decision support, the HKLC staging system may have a significant impact on HCC patient stratification for research design, clinical decision recommendation, and patient care.
External prognostic validation is an important step in staging system establishment because prediction models almost always perform better on the development cohort 34. Furthermore, validation process allows assessment of model robustness in a new patient cohort where underlying assumptions, such as patient demographics, treatment strategy, and disease etiology are violated by measurable amounts.
Asian and North American HCC cohorts have key differences in treatment strategies, etiologic factors, and socioeconomic background. Measurable differences included disease etiology and baseline patient characteristics. Unmeasured differences include patient social history and medical comorbidity that could have influence on prognosis but difficult to quantify in a research study. Hence, the value of staging system validation in an independent cohort cannot be overstated.
The treatment course for HCC patients is often driven by availability of local expertise, which is mainly TACE at our institution (>70% of HCC patients). Compared to the original HKLC cohort, our validation cohort had fewer small-sized (< 2cm) single HCC patients, especially those classified as Child-Pugh Class A. This is because a large number of very early stage patients proceeded to ablation or resection without requiring IAT. Furthermore, it explains why our validation cohort had percent-wise higher proportion of BCLC C patients and slightly lower median OS compared to the development cohort. Similar to the development cohort however, the validation cohort consisted of a wide variety of patients ranging from BCLC A to D and HKLC I to V. A large number of patients in our validation cohort as well as those who received IATs in the original HKLC cohort were BCLC C patients for whom systemic chemotherapy was recommended per BCLC scheme.
An important secondary finding from this study is the HKLC system’s identification of patient groups who did not appear to benefit from current treatment strategy. HKLC stages IV and V had uniformly poor survival at 6 months and 4 months, respectively. A large percentage of patients in this cohort were treated with TACE, hence very poor survival in these two stages implicate TACE should not be used in this particular group. Consequently, an important benefit of the HKLC system may be the identification of exclusion criteria for IAT. The 9.8 months mOS for the full validation cohort appeared particularly poor compared to other studies, but explained by difference in mOS calculation methodology (i.e. start from first IAT date instead of first imaging/pathology diagnosis).
Furthermore, temporal validity of BCLC and HKLC as shown in supplemental figure 1 raises two important questions. First, despite active ongoing research and clinical trials, HCC patients in “intermediate or advanced” stages continue to survive poorly. The sorafenib era from 2008 – 2014 did not appear to change the poor survival outcome seen in this cohort. Second, BCLC A and HKLC I patients have shown substantial improvement in mOS over time. Patients in these stages were often receiving IAT to bridge for transplantation or to downstage prior to resection.
There were several limitations to our study. First, all patients in this validation cohort underwent IAT at some point in the treatment course. This underrepresented resectable HCC cases as well as primary advanced/metastatic cases that were determined ineligible for IAT and treated with systemic chemotherapy only. However, >70% of HCC patients received IAT at some point in their treatment course at our institution, in conjunction with resection, transplantation, and systemic chemotherapy. Furthermore, accurate staging would arguably be of greatest relevance in unresectable HCC cases. Second, this is a single-institution study at a tertiary center subject to referral bias. Further study in community setting would be beneficial. Third, the study had high proportion of censored patients (36.4%), including those with incomplete survival data, which resulted in increased variance and potential bias.
Despite the stated limitations, HKLC staging system’s demonstrated external prognostic validity has important implications. As in this North American study, in three patient cohorts of more than 5000 patients from Europe and Asia, HKLC outperformed BCLC as a survival classification system in two of the three cohorts and similarly performed to BCLC in one cohort 17, 35, 36. The HKLC might become the first HCC staging system accepted across the West and the East, for the purposes of patient stratification for research studies, accurate prediction of patient prognosis, and clinical decision support.
CONCLUSION
HKLC-5 demonstrated the best combination of performances in survival separation, calibration, and discrimination, while consistently outperforming BCLC staging as a prognostic classification system in this cohort. Furthermore, HKLC identified stages IV and V patients who are unlikely to benefit from IAT.
Supplementary Material
Acknowledgments
Grant Support
This study was funded by NIH/NCI R01 CA160771, P30 CA006973, NCRR UL1 RR 025005, Philips Research North America, Cambridge, MA.
We thank Dr. Ruben Hernaez, MD, PhD for suggesting the TRIPOD checklist.
Abbreviations
- AFP
alpha-fetoprotein
- BCLC
Barcelona Clinic Liver Cancer
- ECOG
Eastern Cooperative Oncology Group
- EVM
extravascular metastasis
- IAT
intra-arterial therapy
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HKLC
Hong Kong Liver Cancer
- NASH
non-alcoholic steatohepatitis
- TACE
transarterial chemoembolization
- TRIPOD
Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis
Footnotes
Writing Assistance:
None
Disclosures:
JHS, RD, YZ, FF, JC, SPS, RES, TQ, HL, LZ, JH, CF, RS - None;
ML – Philips Employee, NIH RO1 grant;
JG – Consultant: Biocompatibles/BTG, Bayer HealthCare, Guerbet, Nordion/BTG, Philips Healthcare and Jennerex; Grant Support: Biocompatibles/BTG, Bayer HealthCare, Philips Medical, Nordion/BTG, Threshold, Guerbet, DOD, NCI-ECOG and NIH-R01; Founder and CEO PreScience Labs, LLC.
Author Contributions:
Study concept and design: JHS, RD, JG
Acquisition of Data: JHS, YZ, FF, HL, LZ
Analysis & Interpretation: JHS, RD, YZ, FF, JC, SS, RES, TQ, JH, CF, ML, RS, JG
Drafting of the manuscript: JHS, RD
Critical revision of the manuscript for intellectual content: RD, JC, RS, ML, JG, JH
Statistical Analysis: JHS, TQ, CF
Obtained Funding / Study Supervision: ML, JG
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60:1767–75. doi: 10.1002/hep.27222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene FL, Sobin LH. The staging of cancer: a retrospective and prospective appraisal. CA Cancer J Clin. 2008;58:180–90. doi: 10.3322/CA.2008.0001. [DOI] [PubMed] [Google Scholar]
- 5.Yang JD, Kim WR, Park KW, et al. Model to estimate survival in ambulatory patients with hepatocellular carcinoma. Hepatology. 2012;56:614–21. doi: 10.1002/hep.25680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 9.Marrero JA. Staging systems for hepatocellular carcinoma: should we all use the BCLC system? J Hepatol. 2006;44:630–2. doi: 10.1016/j.jhep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 10.European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Bruix J, Sherman M American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guglielmi A, Ruzzenente A, Conci S, et al. Hepatocellular carcinoma: surgical perspectives beyond the barcelona clinic liver cancer recommendations. World J Gastroenterol. 2014;20:7525–33. doi: 10.3748/wjg.v20.i24.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329–40. doi: 10.1097/SLA.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 14.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 15.Luo J, Guo RP, Lai EC, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413–20. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 16.Georgiades CS, Hong K, D’Angelo M, et al. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:1653–9. doi: 10.1097/01.RVI.0000182185.47500.7A. [DOI] [PubMed] [Google Scholar]
- 17.Yau T, Tang VY, Yao TJ, et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691–700. e3. doi: 10.1053/j.gastro.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 18.Lee H, Chapiro J, Schernthaner R, et al. How I do it: a practical database management system to assist clinical research teams with data collection, organization, and reporting. Acad Radiol. 2015;22:527–33. doi: 10.1016/j.acra.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell DG, Bruix J, Sherman M, et al. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61:1056–65. doi: 10.1002/hep.27304. [DOI] [PubMed] [Google Scholar]
- 21.Tacher V, Lin M, Duran R, et al. Comparison of Existing Response Criteria in Patients with Hepatocellular Carcinoma Treated with Transarterial Chemoembolization Using a 3D Quantitative Approach. Radiology. 2016;278:275–84. doi: 10.1148/radiol.2015142951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins GS, Reitsma JB, Altman DG, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) Ann Intern Med. 2015;162:735–6. doi: 10.7326/L15-5093-2. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan ELMP. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53(282):457–481. [Google Scholar]
- 24.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B (Methodological) 1972;34:187–220. [Google Scholar]
- 25.Ueno S, Tanabe G, Sako K, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program Hepatology. 2001;34:529–34. doi: 10.1053/jhep.2001.27219. [DOI] [PubMed] [Google Scholar]
- 26.Memon K, Kulik LM, Lewandowski RJ, et al. Comparative study of staging systems for hepatocellular carcinoma in 428 patients treated with radioembolization. J Vasc Interv Radiol. 2014;25:1056–66. doi: 10.1016/j.jvir.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–16. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 28.Cho YK, Chung JW, Kim JK, et al. Comparison of 7 staging systems for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancer. 2008;112:352–61. doi: 10.1002/cncr.23185. [DOI] [PubMed] [Google Scholar]
- 29.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–38. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in Medicine. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19(6):716–723. [Google Scholar]
- 32.Georgiades CS, Liapi E, Frangakis C, et al. Prognostic accuracy of 12 liver staging systems in patients with unresectable hepatocellular carcinoma treated with transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:1619–24. doi: 10.1097/01.RVI.0000236608.91960.34. [DOI] [PubMed] [Google Scholar]
- 33.Cox D. Regression models and life tables. J R Stat Soc. 1972;B34:187–220. [Google Scholar]
- 34.Lemeshow S, Le Gall JR. Modeling the severity of illness of ICU patients. A systems update. JAMA. 1994;272:1049–55. [PubMed] [Google Scholar]
- 35.Adhoute X, Penaranda G, Bronowicki JP, et al. Usefulness of HKLC versus BCLC staging system in a European HCC cohort. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 36.Yan X, Fu X, Cai C, et al. Validation of models in patients with hepatocellular carcinoma: comparison of Hong Kong Liver Cancer with Barcelona Clinic Liver Cancer staging system in a Chinese cohort. Eur J Gastroenterol Hepatol. 2015 doi: 10.1097/MEG.0000000000000418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.