Abstract
Background
Current efforts to develop stem cell therapy as a novel treatment for neurointestinal diseases are limited by the unavailability of a model system to study cell transplantation in the human intestine. We propose that xenograft models support enteric nervous system (ENS) development in the fetal human intestine when transplanted into mice subcutaneously or intra-abdominally.
Methods
Fetal human small and large intestine were grafted onto the small intestinal mesentery and into the subcutaneous tissue of immunodeficient mice for up to 4 months. Intestinal cytoarchitecture and ENS development were studied using immunohistochemistry.
Key Results
In both abdominal and subcutaneous grafts, the intestine developed normally with formation of mature epithelial and mesenchymal layers. The ENS was patterned in two ganglionated plexuses containing enteric neurons and glia, including cholinergic and nitrergic neuronal subtypes. c-Kit-immunoreactive interstitial cells of Cajal were present in the gut wall.
Conclusions
Abdominal xenografts represent a novel model that supports the growth and development of fetal human intestine. This in vivo approach will be a useful method to study maturation of the ENS, the pathophysiology of neurointestinal diseases, and the long-term survival and functional differentiation of neuronal stem cells for the treatment of enteric neuropathies.
Keywords: enteric nervous system, xenotransplant, enteric neuropathies, fetal human intestine
Graphical Abstract
Introduction
Much of our understanding of developmental and pathophysiological processes in the human gut has been based on results of studies of experimental animal models. However, translational research requires models that use human intestine. Most studies of the human gastro-intestinal system at the cellular and molecular level have relied either on biopsy material, organ cultures, or human cell lines. However, the ability to study development, physiology and pathophysiology of the human enteric nervous system (ENS) using these models is limited. In particular, the potential development of enteric neuronal stem cell (ENSC) therapy as a promising approach to replace missing or abnormal neurons in patients with enteric neuropathies would be facilitated by an experimental platform in which 1) the tissue is of human origin and 2) the disease can be induced and therapy implemented and evaluated over time.
One approach to the study of the human gut has been to transplant human fetal gut subcutaneously in immunosuppressed mice (1, 2). This model has been described in detail, and has primarily been used for study of enteropathogen-host interactions (3–10). However, with the exception of one acknowledgement that the myenteric plexus exists in these human xenografts (1), there has been no mention of the ENS in any studies of this model. Here, we describe the characteristics of the ENS in human fetal gut transplanted and maintained in SCID mice over the course of several months. We show that the fundamental neuronal and glial cell types and neuronal organization in the xenografts are as expected from samples of normal human tissue. We also describe a novel experimental model which entails transplanting human fetal gut onto the mesentery in the mouse abdominal cavity, adjacent to the host gut. Here too, the donor gut demonstrates normal cytoarchitecture and cellular differentiation, including formation of a normal enteric nervous system. These novel experimental platforms provide a robust system for long-term studies of human intestine and for furthering the development of cell-based therapies for neurointestinal diseases.
Materials and Methods
SCID mouse human intestinal xeno-transplant models
C.B-17/IcrHsd-Prkdcscid (SCID) mice were purchased from Harlan Biotech Israel (Rehovot, Israel). All mice were housed in a pathogen-free facility in individually ventilated cages (IVC), given autoclaved food and water. All animal use was in accordance with the guidelines and approval of the Animal Care and Use Committee (IACUC) of the Hebrew University of Jerusalem. IRB and IACUC approvals were obtained (Ethics Committee for Animal Experimentation, Hebrew University of Jerusalem (MD-11-12692-4) and the Helsinki Committee of the Hadassah University Hospital (81-23/04/04).
Women undergoing legal termination of pregnancy gave written, informed consent for use of fetal tissue in this study. Human fetal small bowel 12–18 weeks gestational age was implanted subcutaneously on the dorsum of the mouse as described previously (3–5) or intra-abdominally as described below. All surgical procedures were performed in an aseptic environment in a laminar flow HEPA-filtered hood with isoflurane inhalation anesthesia (1.5 to 2% v/v isofluorane in O2). Before surgery, carprofen (5 mg/kg, Rimadyl, Pfizer Animal health) was administered subcutaneously. The surgical area was shaved and depilated and the skin was scrubbed and disinfected with betadine and 70% (v/v) ethanol. For abdominal transplant, a midline vertical incision was made through the skin and abdominal wall exposing the peritoneal cavity and a small segment of mouse jejunum was exteriorized. Next, a 20–25 mm long segment of human fetal gut was placed on the intestinal mesentery adjacent to the jejunum. 3M Vetbond Tissue Adhesive (n-butyl cyanoacrylate) was used to seal both ends of the fetal gut segment and to fix it to the jejunum. Care was taken to avoid injuring the mouse mesenteric vessels. The abdomen was closed using standard surgical techniques. After surgery the mice were provided with enrofloxacin-medicated water (Bayer Animal HealthCare AG) for 7 days and closely monitored daily for behavior, reactivity, appearance, and defecation. Grafts developed in situ for 12–16 weeks prior to manipulation.
Tissue processing
Mice were sacrificed and xenograft tissues trisected for histology, fluorescence staining, and electron microscopy. Samples for histological analysis were fixed in neutral buffered 4% paraformaldehyde (PFA) and paraffin embedded. Alkaline phosphatase staining using Fast red (Roche) was used to detect neutral mucins in goblet cells and on the villi. For immunofluorescence staining fresh xenograft tissue was fixed in 2.5% PFA, incubated with 15% (w/v) sucrose for 12 hours at 4°C and frozen in Tissue-Tek® (EMS, Hatfield, PA) embedding medium. For electron microscopy, samples were fixed in 4 % buffered glutaraldehyde followed by dehydration in graded ethanol, and the samples were embedded in a Polybed/Araldite 6500 mixture (Polysciences, Warrington, Pennsylvania, USA). The ultrathin sections were contrasted with uranyl acetate and lead citrate and studied with a Tecnai 12 (Phillips, Eindhoven, The Netherlands) TEM equipped with MegaView II CCD camera and AnalySIS version 3.0 software.
Immunohistochemistry
Paraffin embedded tissues were sectioned at a thickness of 5 μm, deparaffinized with xylene and hydrated through a graded series of ethanol. After antigen retrieval with 0.1 M sodium citrate, slides were blocked with 10% goat serum for 30 minutes. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide in methanol. The sections were incubated with the primary antibodies PCNA (sc-25280; Santa Cruz Biotechnology) and anti-α-SMA antibody (ab5694; Abcam) and then with a biotinylated horse anti-mouse IgG (Vector Laboratories Inc.) and the avidin-biotinylated horseradish peroxidase complex. Staining was developed using 3,3′-diaminobenzidine (Vector)
For immunofluorescence staining paraffin sections were incubated for 45 minutes with the following primary antibodies: mouse anti-Tuj1 (Covance), human anti-neuronal nuclear antibody-1 (HuC/D; 1:4,000; generous gift from Dr. Vanda Lennon); rabbit anti-nNOS (1:500, Santa Cruz Biotechnology), rabbit anti-human c-kit (DAKO), rabbit anti-PGP9.5 (Cedarlane), rabbit anti-S-100 (NeoMarkers), pan-cytokeratin (Santa Cruz Biotechnology), huCD45 (Abcam), muCD45 (Abcam). Secondary antibodies included Alexa Fluor 594 conjugated anti–mouse IgG, Alexa Fluor 594 and 488 conjugated anti–rabbit, and Alexa Fluor 594 conjugated anti–human IgG (Life Technologies; Carlsbad, CA, USA). Cell nuclei were stained with DAPI (Vector). Images were captured with a Nikon 80i microscope.
Results
Abdominal xenografts
Intestinal tissue, including either small or large intestine, was harvested from human fetuses at 12–18 weeks gestation and transplanted into immunocompromised SCID mice. Recipient mice were 6–8 weeks of age. The graft was positioned along the mesenteric side of the jejunum of recipient mice (Figure 1A). Prior to transplantation, the intestine was confirmed to have normal cytoarchitecture, including a normal-appearing ENS (Figure 1B). A total of 18 mice were transplanted with fetal gut segments from 9 different donors. Recipients were maintained for 6–8 weeks and graft survival was 100%. At the time of harvest the graft shows obvious growth (Figure 1C) and preserved histology, as shown in Figure 1D, which demonstrates the grafted human intestine adjacent to the host mouse intestine. Despite the large size of the transplanted graft relative to the host intestine, no evidence of obstruction of the mouse intestine was observed.
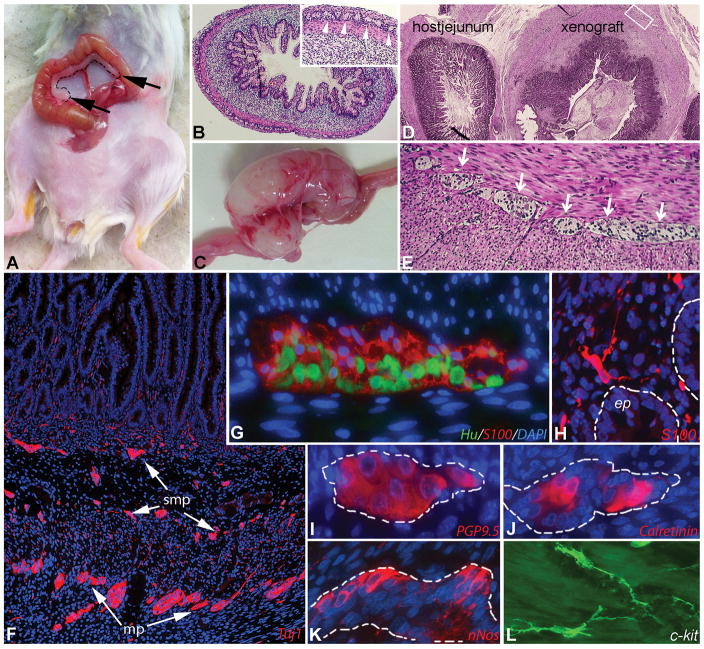
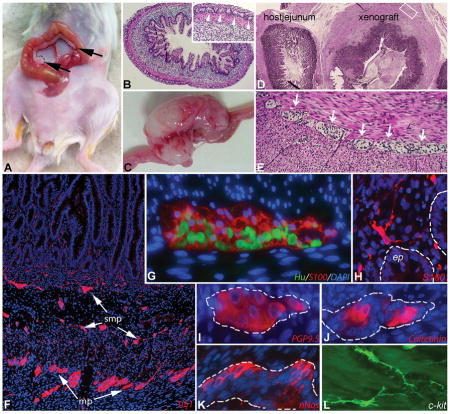
Figure 1. Abdominal human gut xenograft model in SCID mice.
Human 15-week-old fetal gut (dashed line) was transplanted onto the mesentery adjacent to the mouse jejunum (A, graft marked with black arrows). Cross-section of hematoxylin-eosin stained fetal large intestine before abdominal transplantation showing the normal cytoarchitecture of the grafted intestine (B; inset shows magnified view of myenteric plexus; arrowheads). The transplant grows and develops over 2–3 months into grossly normal-appearing intestinal tissue (C). Histology of the mature xenograft is seen adjacent to the normal host jejunum (D). A well-developed myenteric plexus (arrows) is seen between the smooth muscle layers (E). Immunofluorescence of the xenograft ENS shows Tuj1+ neurons and processes in both plexuses (F). Hu+ myenteric neurons and S100+ myenteric glia (G), S100+ mucosal glia (H) and multiple neuronal subtypes (I–K) are present in the xenograft. Interstitial cells of Cajal are seen with c-kit staining (L).
The ENS of the xenograft contains normal-appearing enteric ganglia (Figure 1E), shown by immunofluorescent labeling of both submucosal and myenteric plexuses with the enteric neuronal antibody, Tuj1 (Figure 1F). S100+ glial cells are demonstrated both in the ganglia (Figure 1G) and in the lamina propria (Figure 1H). The ganglia also contain Hu+ neurons (Figure 1G), as well as enteric neurons immunoreactive to PGP9.5 (Figure 1I), calretinin (Figure 1J), and nNOS (Figure 1K). c-Kit-expressing interstitial cells of Cajal (ICC) are also present (Figure 1L).
Subcutaneous xenografts
Human fetal intestine was transplanted subcutaneously into SCID mice (Figure 2A), where they were maintained for 12–16 weeks. Following harvesting of the xenograft, the intestine appeared viable and grossly normal. Epithelial cell proliferation within the crypts was confirmed by PCNA staining (Figure 2B). Normal development of the smooth muscle layers (Figure 2C) and ENS (Figure 2D) were also confirmed by immunostaining. As shown in Figure 2D, enteric ganglia containing Hu+ neurons and S100+ glia are present in the submucosal and myenteric plexuses. Villi have a normal structure, with epithelial cells staining for cytokeratin (Figure 2E). Interestingly, CD45+ immune cells from both the mouse host and the human graft are present within the transplanted tissue (Figure 2F). Alkaline phosphatase staining demonstrates the presence of a normal epithelial layer with an intact brush border, suggesting the capacity for functional absorption (Figure 2G), and electron microscopy confirms normal microvillus architecture (Figure 2H).
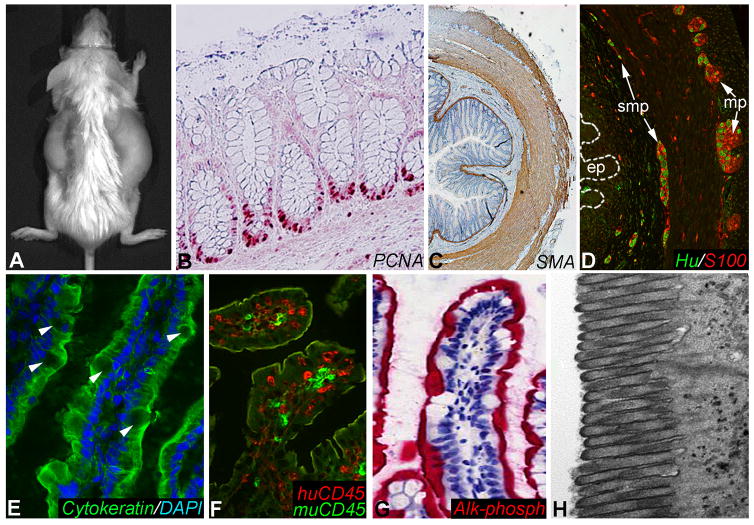
Figure 2. Subcutaneous human gut xenografts.
Human fetal gut was transplanted subcutaneously into a SCID mouse (A). Histology of the mature gut xenograft shows proliferative PCNA+ cells at the base of the epithelial crypts (B), actin+ smooth muscle cells (C), and Hu+ enteric neurons with S100+ glial cells within the submucosal (smp) and myenteric (mp) plexuses (D). Villous epithelial cells in the xenografts stain with anti-cytokeratin antibodies (E; arrowheads shows goblet cells). Species-specific CD45 antibodies demonstrate the presence of both human and mouse immune cells in the xenografts (F). Xenografts show strong brush border alkaline phosphatase activity with Fast Red staining (G) and well-developed microvilli by electron microscopy (H).
Discussion
Subcutaneous transplantation of fetal rodent intestine into rodent hosts was described over 45 years ago (11) and has been extensively studied since then with respect to its anatomical and functional development (12). Transplantation of fetal human intestine into a nude mouse was first described in 1991 (2). Human fetal small and large intestine was able to be maintained for up to six months in 62% of the transplants and demonstrated neovascularization with age-appropriate maturation and normal gross histology of all intestinal elements. Studies using this platform have focused on investigation of human-specific enteropathogens and the inflammatory and immunological responses they evoke (3–10, 13). There has been no study of the human ENS in this model. However, Tanaka et al (14) performed a careful characterization of the ENS in fetal rat gut transplanted into rats. These workers reported hyperganglionosis and hyperinnervation in the transplanted rat intestine. In the present study of human grafts, we observed normal development and differentiation of enteric neurons and glial cells. Further analyses are needed to more precisely determine whether neuronal and glial cell numbers, morphology, or subtypes differ from normal human intestine. Moreover, we observed spontaneous peristaltic activity when the grafts were harvested, suggesting that the gut remains functionally active throughout the transplantation period, although quantitative analyses of motility are needed.
One goal of this study was to develop a novel platform that would allow long-term survival, growth, and maturation of fetal human intestine in a vascularized environment and thereby provide a new way to study human fetal ENS maturation and the development of regenerative stem cell-based therapies for neurointestinal diseases. In comparing the merits of intra-abdominal and subcutaneous grafts, we did not detect any histologic differences in their phenotypes. While technically more difficult to perform due to the need for laparotomy, intra-abdominal grafts may have several advantages over subcutaneous grafts. First, they are readily vascularized by the intestinal mesentery on which they develop. Second, their growth may be less constrained than in the subcutaneous space. Third, there may be advantages to being in the microenvironment of the host intestine rather than in a location where the gut does not normally reside.
Neurointestinal diseases, including esophageal achalasia, gastroparesis, chronic intestinal pseudo-obstruction, and Hirschsprung disease, represent major causes of morbidity (15). Despite this, current treatments aim only to alleviate the symptoms rather than correct the underlying neurointestinal pathology. ENSC transplantation has the potential to replace the abnormal or missing enteric neurons and thereby restore gastrointestinal function in these patients, for example in those with Hirschsprung disease. However, existing model systems to develop and test this novel approach are limited due to the host animal’s clinical deterioration and premature mortality as a result of the congenital aganglionosis. Furthermore, for future clinical application, ENSC transplantation studies need to be performed in human intestine, but current organ culture techniques allow only short-term tissue survival.
Transplantation of human fetal gut into SCID mice, especially the novel intra-abdominal xenograft model, will allow prolonged studies of the effects of ESNC transplantation in human intestine. It can also serve as a platform for syngeneic transplants of mouse gut from transgenic lines or experimental manipulations that normally do not survive long enough for these types of experiments. Moreover, while our focus is on the use of this model system for ENSC transplantation studies, the intra-abdominal gut xenografts are also a valuable platform for the study of normal development and mechanisms of disease, especially in those cases where the use of human tissue is of particular value.
Key Messages.
Reliable and experimentally tractable models are needed to study the pathophysiology of enteric neuropathies and to develop novel cell-based treatments for these diseases.
The enteric nervous system of fetal human intestine develops normally in murine subcutaneous and abdominal xenografts.
Abdominal xenografts represent a novel and useful method for studying the enteric nervous system, neurointestinal diseases, and enteric neuronal stem cell transplantation.
Acknowledgments
We thank Dr. Vanda Lennon, MD, PhD (Mayo Clinic, Rochester, MN, USA) for the kind gift of Hu antibody.
Funding
Nandor Nagy is supported by a Bolyai Fellowship of the Hungarian Academy of Sciences. Allan M. Goldstein is supported by the National Institutes of Health (R01DK103785). Nahum Y. Shpigel, Allan M. Goldstein and Mike J. Gutnick were supported by BSF grant 2015157. The work leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2012-2017) under grant agreement no. 305564 as partners of the SysmedIBD research consortium (to Werner Muller, University of Manchester, United Kingdom). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Disclosure
The authors have no conflicts of interest.
Contributions
N.Y.S, N.N, A.M.G and M.J.G conceived the experiments and wrote the paper with contributions from all authors; N.M performed the transplants and maintained the colonies; N.N, R.S.B, and E.A performed the immunohistochemistry; S.Y contributed reagents and materials. N.Y.S, A.M.G and M.J.G secured funding; N.N, N.M, R.S.B, and N.Y.S analyzed data; and all authors reviewed and approved the final manuscript.
References
- 1.Savidge TC, Morey AL, Ferguson DJ, Fleming KA, Shmakov AN, Phillips AD. Human intestinal development in a severe-combined immunodeficient xenograft model. Differentiation. 1995;58:361–371. doi: 10.1046/j.1432-0436.1995.5850361.x. [DOI] [PubMed] [Google Scholar]
- 2.Winter HS, Hendren RB, Fox CH, et al. Human intestine matures as nude mouse xenograft. GASTROENTEROLOGY. 1991;100:89–98. doi: 10.1016/0016-5085(91)90587-b. [DOI] [PubMed] [Google Scholar]
- 3.Canavan JB, Scotta C, Vossenkamper A, et al. Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut. 2016;65:584–594. doi: 10.1136/gutjnl-2014-306919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golan L, Gonen E, Yagel S, Rosenshine I, Shpigel NY. Enterohemorrhagic Escherichia coli induce attaching and effacing lesions and hemorrhagic colitis in human and bovine intestinal xenograft models. Disease models & mechanisms. 2011;4:86–94. doi: 10.1242/dmm.005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golan L, Livneh-Kol A, Gonen E, Yagel S, Rosenshine I, Shpigel NY. Mycobacterium avium paratuberculosis invades human small-intestinal goblet cells and elicits inflammation. The Journal of infectious diseases. 2009;199:350–354. doi: 10.1086/596033. [DOI] [PubMed] [Google Scholar]
- 6.Bertelsen LS, Paesold G, Eckmann L, Barrett KE. Salmonella infection induces a hypersecretory phenotype in human intestinal xenografts by inducing cyclooxygenase 2. Infect Immun. 2003;71:2102–2109. doi: 10.1128/IAI.71.4.2102-2109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Jin L, Champion G, Seydel KB, Stanley SL., Jr Shigella infection in a SCID mouse-human intestinal xenograft model: role for neutrophils in containing bacterial dissemination in human intestine. Infect Immun. 2001;69:3240–3247. doi: 10.1128/IAI.69.5.3240-3247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seydel KB, Zhang T, Champion GA, et al. Cryptosporidium parvum Infection of Human Intestinal Xenografts in SCID Mice Induces Production of Human Tumor Necrosis Factor Alpha and Interleukin-8. Infect Immun. 1998;66:2379–2382. doi: 10.1128/iai.66.5.2379-2382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seydel KB, Li E, Swanson PE, Stanley SL., Jr Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect Immun. 1997;65:1631–1639. doi: 10.1128/iai.65.5.1631-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurent F, Eckmann L, Savidge TC, et al. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect Immun. 1997;65:5067–5073. doi: 10.1128/iai.65.12.5067-5073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zinzar SN, Leitina BI, Tumyan BG, Svet-Moldavsky GJ. Very large organ-like structures formed by syngeneic foetal alimentary tract transplanted as a whole or in parts. Rev Eur Etud Clin Biol. 1971;16:455–458. [PubMed] [Google Scholar]
- 12.Bass BL, Schweitzer EJ, Harmon JW, Tai YH, Sjogren RW, Kraimer J. Anatomic and physiologic characteristics of transplanted fetal rat intestine. Ann Surg. 1984;200:734–741. doi: 10.1097/00000658-198412000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savidge TC, Pan WH, Newman P, O’Brien M, Anton PM, Pothoulakis C. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology. 2003;125:413–420. doi: 10.1016/s0016-5085(03)00902-8. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Ohshiro K, Puri P. Morphological changes in the enteric nervous system of the transplanted fetal rat intestine. J Pediatr Surg. 1997;32:897–901. doi: 10.1016/s0022-3468(97)90646-1. [DOI] [PubMed] [Google Scholar]
- 15.Westfal ML, Goldstein AM. Pediatric enteric neuropathies: diagnosis and current management. Curr Opin Pediatr. 2017;29:347–353. doi: 10.1097/MOP.0000000000000486. [DOI] [PMC free article] [PubMed] [Google Scholar]