Abstract
Rationale
Elevated brain kynurenic acid (KYNA) levels are implicated in the pathology and neurodevelopmental pathogenesis of schizophrenia. In rats, embryonic treatment with kynurenine (EKyn) causes elevated brain KYNA levels in adulthood, and cognitive deficits reminiscent of schizophrenia.
Objectives
Growing evidence suggests that people with schizophrenia have a narrowed attentional focus, and we aimed at establishing whether these abnormalities may be related to KYNA dysregulation.
Methods
To test whether EKyn rats display broad monitoring deficits, kynurenine was added to the chow of pregnant Wistar dams on embryonic days 15–22. As adults, 20 EKyn and 20 control rats were trained to stable performance on the 5-choice serial reaction time task, requiring the localization of 1-s light stimuli presented randomly across 5 apertures horizontally arranged along a curved wall, equating the locomotor demands of reaching each hole.
Results
EKyn rats displayed elevated omission errors and reduced anticipatory responses relative to control rats, indicative of a lower response rate, and showed reduced locomotor activity. The ability to spread attention broadly was measured by parsing performance by stimulus location. Both groups displayed poorer stimulus detection with greater target location eccentricity, but this effect was significantly more pronounced in the EKyn group. Specifically, the groups differed in the spatial distribution of correct but not incorrect responses. This pattern cannot be explained by differences in response rate and is indicative of a narrowed attentional focus.
Conclusions
The findings suggest a potential etiology of broad monitoring deficits in schizophrenia, which may constitute a core cognitive deficit.
Keywords: kynurenic acid, prenatal, attention, broad monitoring, 5-choice serial reaction time
Introduction
Schizophrenia is marked not just by hallucinations, delusions, and negative symptoms, but also by a broad range of distinct neurocognitive deficits that predict long-term functional disease outcome (Green 1996; Green et al. 2004). The pathogenesis of the symptoms of schizophrenia is increasingly thought of as a convergence of neurodevelopmental risk factors, namely the combination of genetic susceptibilities and environmental perturbations during pre- and perinatal periods (Fatemi and Folsom 2009; Lewis and Levitt 2002). In experimental animals, several of these risk factors cause an increase in the peripheral levels of kynurenine (Erhardt et al. 2017), a major tryptophan metabolite, which readily crosses the blood-brain barrier (Fukui et al. 1991). In the brain, kynurenine is promptly converted to kynurenic acid (KYNA), an antagonist of the glycine co-agonist site of the N-methyl-D-aspartic acid glutamate receptor (NMDAR) and of the ±7 nicotinic acetylcholine receptor (nAChR) (Hilmas et al. 2001; Kessler et al. 1989), or metabolized to other neuroactive metabolites including 3-hydroxykynurenine and quinolinic acid (Schwarcz et al. 2012). Interestingly, KYNA levels have been consistently shown to be elevated in cerebrospinal fluid and postmortem brains of people with schizophrenia (PSZ) (Erhardt et al. 2001; Linderholm et al. 2012; Miller et al. 2006; Nilsson et al. 2005; Sathyasaikumar et al. 2011; Schwarcz et al. 2001). This increase may be causally related to cognitive impairments since NMDARs and ±7 nAChRs are critically involved in physiological events underlying learning, memory, and other cognitive processes (Albuquerque et al. 2009; MacDonald et al. 2006), and hypofunction of these receptors has been plausibly linked to cognitive deficits in PSZ (Balu and Coyle 2015; Young and Geyer 2013).
The physiological mechanisms linking neurodevelopmental risk factors to elevations in KYNA are beginning to be understood. Thus, pre- and perinatal infection, inflammation, and stress activate indoleamine 2,3-dioxygenase (IDO), an enzyme that can convert tryptophan to kynurenine (Erhardt et al. 2017; Kiank et al. 2010; Muller and Schwarz 2006; Widner et al. 2000). Furthermore, increased expression of tryptophan 2,3-dioxygenase (TDO2), an enzyme that specifically catalyzes the formation of kynurenine from tryptophan, has been observed in post-mortem brains of PSZ (Miller et al. 2004; Miller et al. 2006). Brain KYNA elevations in PSZ may also be secondary to reductions in expression and activity of kynurenine 3-monooxygenase (KMO), an enzyme that normally channels kynurenine down an alternative branch of the metabolic cascade (Holtze et al. 2012; Sathyasaikumar et al. 2011; Wonodi et al. 2011).
The behavioral consequences of central KYNA elevations have been studied in rats. Systemic application of kynurenine in early life induces a transient increase in brain KYNA and decreases social behavior in adulthood (Iaccarino et al. 2013). Similarly, systemic injection of kynurenine during adolescence impairs social behavior (Trecartin and Bucci 2011), contextual fear and novel object recognition memory (Akagbosu et al. 2012), as well as long-term potentiation (LTP) (DeAngeli et al. 2014) in adulthood. In adult rats, acute kynurenine-induced increases in brain KYNA levels disrupt sensory gating (Erhardt et al. 2004; Nilsson et al. 2006; Shepard et al. 2003) and impair spatial working memory in the radial arm maze (Chess et al. 2007), contextual fear conditioning (Chess et al. 2009), and attentional set shifting (Alexander et al. 2012). Importantly, impairments in adult set shifting (Alexander et al. 2013) and in hippocampus-dependent memory tasks (passive avoidance and Morris water maze) (Pocivavsek et al. 2012) are also observed when rats are exposed to kynurenine pre- and perinatally by feeding the compound to the mother from embryonic day 15 to postnatal day 21, a treatment that leads to long-lasting elevations of brain KYNA in the offspring (Alexander et al. 2013; Pocivavsek et al. 2012). Comparable cognitive abnormalities and increased brain KYNA levels are seen in the adult offspring when kynurenine feeding is restricted to the last week of gestation (embryonic days 15 to 22) (Pershing et al. 2015; Pocivavsek et al. 2014), and a very similar intervention causes decreased hippocampal LTP in adulthood (Forrest et al. 2015).
The profile of cognitive impairments in schizophrenia includes basic attentional functions (Green 1996; Nuechterlein et al. 2004), typically assessed with simple stimulus detection paradigms. Evidence has been accumulating that PSZ have a narrowed attentional focus, with exaggerated filtering of locations or stimuli not currently focused on. This was first demonstrated using a Posner-type paradigm with 4 possible target locations. With decreasing precision of a central cue and the need to monitor a wider array of locations, performance deficits as reflected by reaction time and omission errors became incrementally more prominent in PSZ (Hahn et al. 2013; Hahn et al. 2016; Hahn et al. 2012b). Subsequent behavioral and event-related potential studies using other paradigms confirmed a narrowed and more intense attentional focus both in the visuospatial domain (Gray et al. 2014; Kreither et al. 2017) and against the backdrop of item features maintained in working memory (Gray et al. 2014; Hahn et al. 2012a; Kreither et al. 2017; Leonard et al. 2013; Luck et al. 2014; Sawaki et al. 2017). Such broad monitoring deficits, or narrowed focusing, may explain a wide range of cognitive deficits in schizophrenia, from attentional abnormalities and working memory impairment to executive dysfunction. Indeed, indices of narrowed focusing correlate with working memory capacity and performance of tasks that predict real-world outcomes (Gray et al. 2014; Kreither et al. 2017).
The present study in rats was designed to test the hypothesis that prenatal treatment with kynurenine on embryonic days 15 to 22 (EKyn), as described above (Pershing et al. 2015; Pocivavsek et al. 2014), and the previously documented resulting increase in brain KYNA, will result in abnormalities in spreading attention across a wide array of locations, resembling the broad monitoring deficits described in PSZ. This would suggest that KYNA dysregulation, for which there is plentiful evidence in schizophrenia, is involved in the etiology of this potential cognitive core deficit.
Methods
Animals
Sixteen pregnant Wistar dams were obtained from Charles River Laboratories (Frederick, MD, USA) on embryonic day 2, weighing 180–200g. To generate EKyn rats, 100 mg of kynurenine was added daily to the chow of 8 of the 16 dams on embryonic days 15 to 22. After birth, all animals had access to normal rodent chow. The pups were weaned on postnatal day 21 and grouped-housed. Starting at 6 weeks of age, the animals were housed individually. Experiments were performed with a total of 20 male EKyn and 20 male control (Econ) rats, using one to four animals from each litter.
Rats were housed in a temperature- and humidity-controlled room, fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and maintained on a 12-h light-dark cycle with lights on at 7 a.m. The animals had free access to water and received a food-restricted diet starting at 8 weeks of age, to maintain them at 85% of their age-appropriate free-feeding weights. The treatment of animals followed the ‘Principles of Laboratory Animal Care’ (NIH publication No. 86–23, 1996) and was approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
After the 5-Choice Serial Reaction Time Task (5-CSRTT) training phase and prior to locomotor activity recording, at 6 months of age, two animals from the EKyn group were euthanized due to onset of health concerns (lethargy, weight loss). Furthermore, one EKyn and one ECon rat displayed health problems (one lethargy, one musculoskeletal) and were euthanized prior to completion of the 4 weeks of recording for location-dependent analyses. Thus, 17 EKyn and 19 ECon animals were available for this final analysis.
Apparatus
Eight operant conditioning chambers measuring 26 cm3 (Med Associates, Inc., Fairfax, VT) were housed in sound-insulated enclosures. The curved rear wall of each chamber contained a horizontal array of five 2.5 cm2 apertures, located 2 cm above the grid floor. At the entrance of each hole a photocell monitored interruptions of a beam of infrared light, and at the rear there was a white light-emitting diode. A food trough was located in the opposite wall, equidistant from each aperture. Illumination was provided by a house light situated in the top portion of the front panel. The apparatus and data collection were controlled by Med-PC software.
Behavioral Procedures
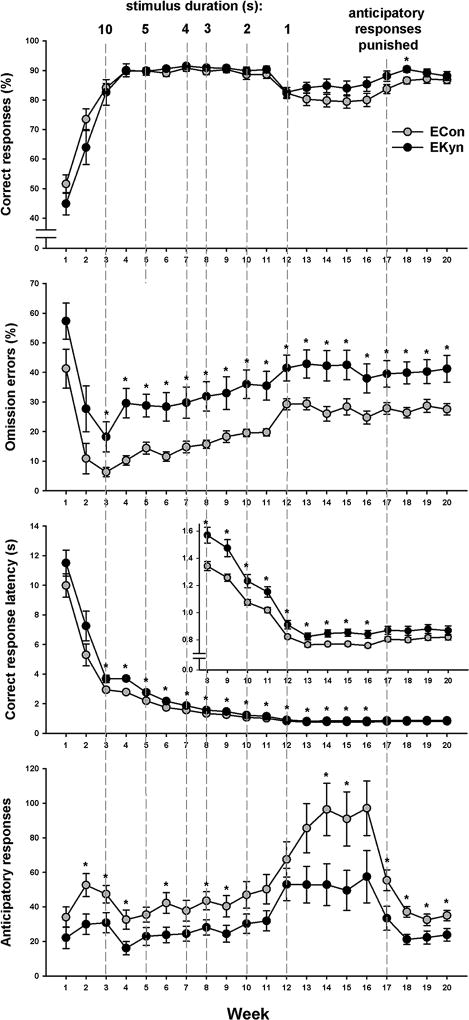
Rats started 5-CSRTT training at 8 weeks of age according to procedures described previously(Mirza and Stolerman 1998). Light stimuli were presented randomly in one of the five holes after an intertrial interval (ITI) of variable duration (1–9 s, average 5 s). A nose-poke into the hole while it was illuminated or within 5 s after the light had terminated (limited hold) was registered as a correct response and resulted in the delivery of a 45-mg food pellet into the trough, followed by a 2-s reward retrieval period. A response into any other hole was recorded as incorrect and resulted in a 5-s time-out, during which the house light was extinguished. A failure to respond before the end of the limited hold was registered as an omission error. A new trial began with the initiation of an ITI after a reward retrieval period, or after time-outs or limited holds in cases of incorrect responses or omission errors. Training commenced with a long stimulus duration of 30 s. As soon as all rats had acquired the basic task contingencies, the stimulus duration was shortened to 10 s for all rats, and thereafter was progressively shortened to 5, 4, 3, 2, and 1 s (final stimulus duration) as shown in Figure 2. The change was always introduced on the same day for all rats.
Fig. 2.
Average (± SEM) performance of rats embryonically exposed to kynurenine (EKyn; N=20) and control rats (ECon; N=20) in each of the 20 weeks of training. The vertical dashed lines demarcate weeks in which a shorter stimulus duration was introduced; the new stimulus duration is indicated on top. Insert: larger scale for the weeks indicated, to allow improved visualization of group differences in response latency. * P<0.05 in independent-samples t-test.
The animals were trained Monday through Friday for 20 weeks. In the first 16 weeks of training, responses during ITIs (anticipatory responses) had no programmed consequences. However, because of the large group difference in anticipatory responses when the shortest stimulus duration was introduced, and the confounding effects of this difference on stimulus detection (see Results, Figure 2), anticipatory responses were punished by a 5-s time-out starting in week 17. All sessions lasted 30 minutes.
The time of day at which 5-CSRTT sessions were performed was held constant for individual rats and was counterbalanced between ECon and EKyn rats. Eight rats were trained or tested at the same time, and care was taken to include at least three rats from each group into each of the five runs. The number of animals from each group that was run in each individual testing chamber was also counterbalanced. The chambers were wiped with 70% alcohol after each animal.
For group comparisons of training performance, the following behavioral measures are reported:
Percentage of correct responses (accuracy): 100 × [correct responses / (correct + incorrect responses)]. Response accuracy is a measure of response choice. It is based only on stimulus-contingent responses that have been emitted and does not take into account omission error trials.
Percentage of omission errors: 100 × (omission errors / stimuli presented). Errors of omission are influenced by stimulus detection, but also by the general rate of responding.
Latency of correct responses: the mean time between stimulus onset and a nose-poke in the correct hole.
Anticipatory responses: total number of responses in ITIs per session. In the first 16 weeks of training, anticipatory responses were unpunished, and therefore had no direct influence on reward payoff. Starting in week 17, anticipatory responses were punished by time-outs.
Locomotor activity
Locomotor activity was measured to further the interpretation of the observed group differences in response rate-dependent 5-CSRTT measures. 5-CSRTT training was continued during this time period, although not on days on which an animal's locomotor activity was recorded. For each rat, locomotor activity was recorded twice for 1 hour, 24 hours apart, in 42 × 42 × 42 cm chambers constructed from acrylic with plastic floors. Walls were externally covered with black construction paper, and floor trays were painted black. Ambulation was recorded with a video camera and analyzed off-line by Any-Maze tracking software. Two dependent variables were analyzed: total distance traveled in meters, and average speed in meters/second.
Over the following 4 consecutive weeks, 5-CSRTT performance was averaged and parsed as a function of stimulus location, to measure the animals' ability to spread attention broadly. To reduce variability, Mondays were excluded from analyses because performance was consistently worse than on all other days of the week.
For location-dependent analyses, the following behavioral indices were calculated:
Percentage of correct responses (accuracy): 100 × [correct responses at this location / (correct responses at this location + incorrect responses, i.e., responses at any other location when the target had been presented at this location)].
Percentage of all correct responses at this location: 100 × [correct responses at this location / all correct responses emitted]. This measure describes the spatial distribution of correct responses, independent of response rate.
Percentage of all incorrect responses at this location: 100 × [incorrect responses at this location / all incorrect responses emitted]. This measure describes the spatial distribution of incorrect responses, independent of response rate.
Percentage of omission errors: 100 × (omission errors at this location / stimuli presented at this location).
Latency of correct responses: the mean time between stimulus onset and nose-poke at this location.
Note: anticipatory responses were not recorded by location.
Data analysis
Training 5-CSRTT performance was averaged over sessions within each week of training and analyzed by 2-factor ANOVA with Group (ECon, EKyn) as between-subjects factor and Week (1–20) as within-subject factor.
Locomotor activity was analyzed by 2-factor ANOVA with Group as between-subjects factor and Day of recording (day 1 vs. 2) as within-subject factor.
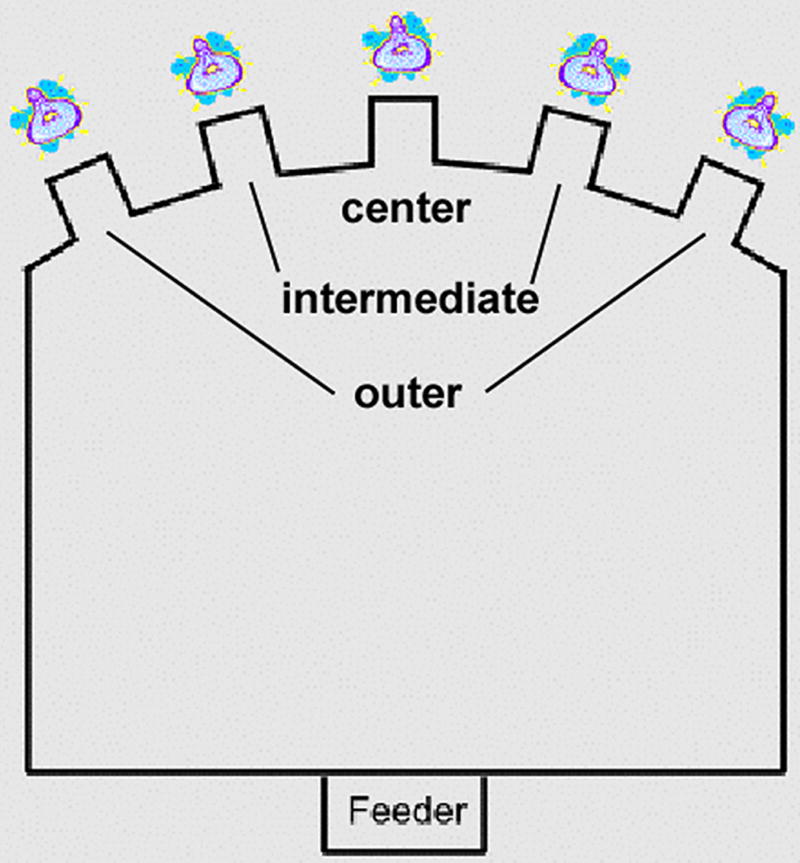
For analyses of 5-CSRTT performance by location, data were averaged over the two outermost locations, and over the two intermediate locations, resulting in three levels of eccentricity (Figure 1). Each performance index was analyzed by 2-factor ANOVA with Group as between-subjects factor and Location (outer, intermediate, center) as within-subject factor. Note that the curvature of the rear wall serves to equate the locomotor demands of reaching each location.
Fig. 1.
Bird's-eye view of an operant conditioning chamber used for the 5-choice serial reaction time task. For analysis of location-dependent data, the five apertures in which target stimuli were presented were categorized as the two outer locations, the two intermediate locations, and the center location.
Results
5-CSRTT training performance
Figure 2 illustrates average performances in each week of training. Over the course of the training, the stimulus duration was progressively shortened from 30 to 1 s.
Over the first 3 weeks, response accuracy and anticipatory responding increased, and omission errors and response latency decreased sharply as the animals acquired the basic task contingencies. Two-factor ANOVA including only these 3 weeks confirmed significant main effects of Week on each measure [all Fs(2,76)>6.1, all Ps<0.004]. The effects of Week did not interact with Group on any measure [all Fs(2,76)<1.38, all Ps>0.25], suggesting that there was no group difference in the initial rate of task acquisition.
In a 2-factor ANOVA (Group × Week) including all 20 weeks, significant main effects of Group supported higher omission errors [F(1,38)=13.3, P<0.001], longer response latency [F(1,38)=8.20, P=0.007], and fewer anticipatory responses [F(1,38)=5.84, P=0.021] in EKyn as compared with ECon rats. On omission errors, the group difference stayed robust over the course of the training [Group × Week interaction: P>0.9], but the difference in response latency appeared to diminish over time, as supported by a significant Group × Week interaction [F(19,722)=1.90, P=0.011]. The accuracy of responding did not differ between groups overall [main effect of Group: P>0.6]. Accuracy was almost identical between groups until week 12, when the stimulus duration was shortened to 1 s and both groups displayed a sharp reduction in accuracy. Hereafter, however, the accuracy of the EKyn rats slowly recovered while that of the ECon rats kept declining, resulting in greater accuracy in the EKyn group. This pattern was supported by a Group × Week interaction [F(19,722) = 2.63, P<0.001]. Importantly, it was the mirror image of the pattern on anticipatory responding [Group × Week: F(19,722)=1.75, P=0.025], which displayed a sharp increase in both groups in week 12, and kept increasing in the ECon but not EKyn rats thereafter.
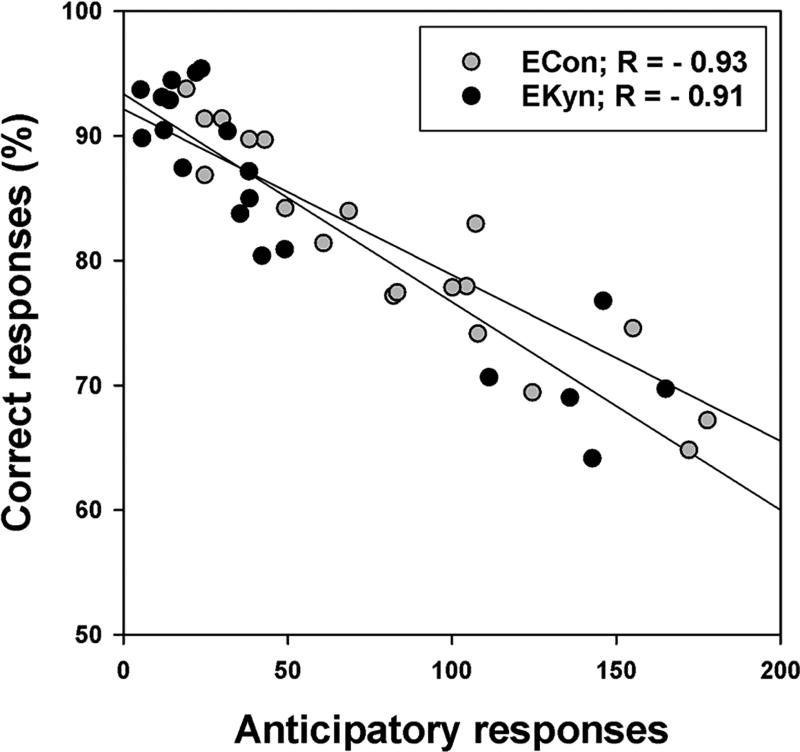
Anticipatory responses and response accuracy in the 5-CSRTT are known to be inversely related (e.g.(Hahn et al. 2002); thus, the lower response rate may have elevated the accuracy of responding in the EKyn group relative to the ECon group, especially after week 12 when the group difference in anticipatory responding was the largest. Indeed, when we correlated response accuracy and anticipatory responses, averaged over weeks 13 to 16, the two measures were highly negatively correlated (Figure 3), both in the EKyn [R= −0.91, P<0.001] and the ECon group [R= −0.93, P<0.001]. To reduce the confounding effects of response rate differences on accuracy, starting in week 17, we punished anticipatory responses with 5-s time-outs, identical to the time-outs following incorrect responses. As illustrated in Figure 2, this resulted in a sharp decrease in anticipatory responding and a recovery of response accuracy in both groups, but particularly in the ECon group, so that accuracy barely differed between groups by the end of the training period. Thus, the temporarily lower response accuracy in the ECon group most likely was a confound of the higher rate of anticipatory responding.
Fig. 3.
Correlations between the accuracy of responding (% correct responses) and anticipatory responses, averaged over weeks 13 to 16, in rats embryonically exposed to kynurenine (EKyn; N=20) and control rats (ECon; N=20).
Locomotor activity
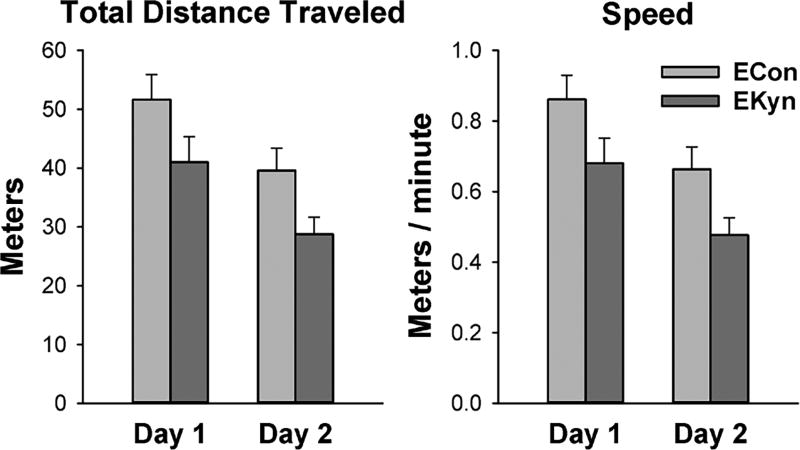
To test whether the reduced response rate in the EKyn animals, as indicated by higher omission errors and lower anticipatory responses, was specific to food-reinforced operant task performance or reflected a more general reduction in non-contingent activity levels, we recorded unconstrained locomotor activity twice, 24 hours apart, for 1 hour each time. Both total distance traveled and speed of ambulation were lower in EKyn than in ECon animals (Figure 4), as supported by a significant main effect of Group on both measures [F(1,36)>5.6, P<0.025]. Locomotor activity was lower on the second than on the first day, supported by main effects of Day on both measures [F(1,36)>14, P<0.001]. This habituation effect did not differ between groups, as indicated by a lack of Group × Day interactions [F(1,36)<0.01, P>0.9].
Fig. 4.
Indices of locomotor activity, averaged (± SEM) over 1-hour recording session on 2 consecutive days, in rats embryonically exposed to kynurenine (EKyn; N=18) and control rats (ECon; N=20).
5-CSRTT performance by location
Unlike overall responding, the relative distribution of responses across the array of apertures, given the curvature of the rear wall, is devoid of confounds due to general motor activity and response rate.
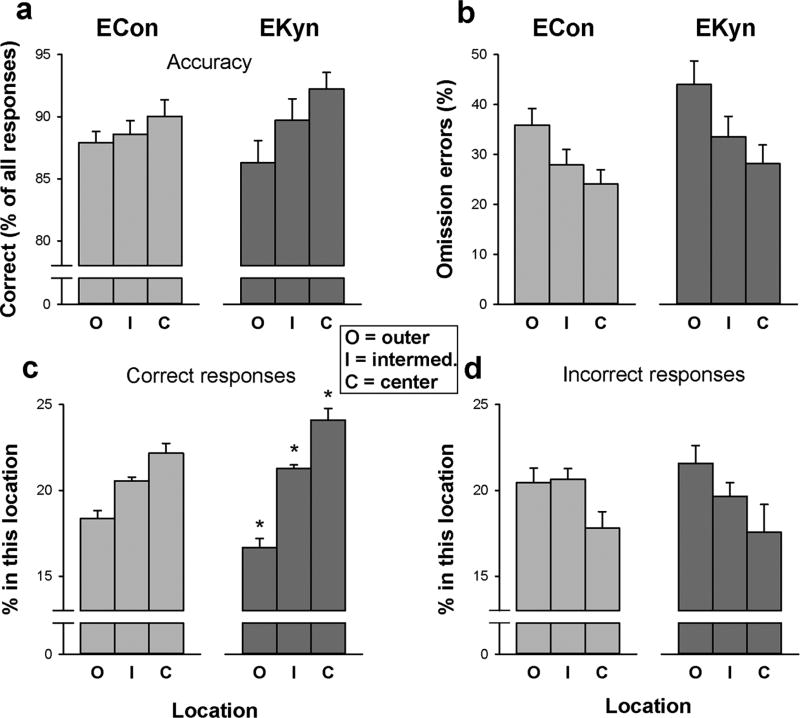
Response accuracy was overall highest at the center and lowest at the outer locations (Figure 5a), supported by a main effect of Location [F(2,68)=17.4, P<0.001]. The effect of location was more pronounced in EKyn rats, with numerically higher accuracy at the center and lower accuracy at the outer locations as compared with ECon rats, resulting in a significant Group × Location interaction [F(2,68)=4.19, P=0.019].
Fig. 5.
Average (± SEM) performance of rats embryonically exposed to kynurenine (EKyn; N=17) and control rats (ECon; N=19) for the outer (O), intermediate (I), and center (C) locations as illustrated in Figure 1. * P<0.05, independent samples t-tests comparing groups at each target eccentricity, with significance controlled for three comparisons by the Benjamini Hochberg procedure.
To analyze whether the interaction on accuracy was due to group differences in the spatial pattern of correct responses or incorrect responses, we analyzed the percentage of all correct responses emitted per location, and the equivalent for incorrect responses. As shown in Figure 5c, the largest percentage of all correct responses was emitted at the center, and the smallest percentage at the outer locations, resulting in a significant main effect of Location [F(2,68)=49.7, P<0.001]. The effect of location was more pronounced in the EKyn rats, as indicated by a Group × Location interaction [F(2,68)=5.24, P=0.008], mirroring the pattern on response accuracy. In contrast, the percentage of all incorrect responses emitted (Figure 5d) was smallest at the center and largest at the outer locations [main effect of Location: F(2,68)=4.05, P=0.022]. This effect did not differ between groups [Group × Location interaction: P>0.6], which further emphasizes that the observed group differences in the spatial distribution of correct responses did not reflect differences in responding generally, but in stimulus detection specifically.
Omission errors (Figure 5b) were overall lowest at the center and highest at the outer locations [main effect of Location: F(2,68)=83.0, P<0.001]. There was a trend for this effect to be more pronounced in the EKyn animals; however, the Group × Location interaction was not significant [F(2,68)=1.72, P=0.19].
The latency of correct responses (data not shown) was shortest at the center and longest at the outer locations [F(2,69)=76.6, P<0.001]. There was no Group × Location interaction [P>0.7].
Discussion
In the present study, we characterized the performance of rats prenatally exposed to kynurenine in the 5-CSRTT, a stimulus detection paradigm requiring the monitoring of a broad array of locations. ECon and EKyn rats did not differ in their initial acquisition of basic task contingencies. However, robust differences in 5-CSRTT performance quickly emerged, consisting of a persistent increase in omission errors and reduction in anticipatory responses in the EKyn group, both explainable by a reduced response rate, as well as a slower response latency. In separate tests, we also identified a significant reduction in spontaneous ambulation, consistent with findings in rats systemically administered kynurenine in early life (Iaccarino et al. 2013). In combination, the above findings lead us to conclude that the EKyn treatment resulted in overall lower activity levels.
The accuracy of responding, the performance index most closely reflective of attention, is heavily confounded by overall activity, and in particular appears to be reduced by high levels of anticipatory responding. Anticipatory responses are likely to reflect exploratory activity, i.e., rats actively check the apertures rather than scanning them visually, and this active engagement likely comes at the cost of correct stimulus localization. The lower activity levels of the EKyn group, perhaps reflective of reduced exploratory behavior, is thus likely to have artificially augmented accuracy and obscured any potential attentional deficits. Thus, our results do not allow any conclusions as to whether EKyn animals display any basic stimulus detection deficits overall. However, the present results point to a specific attentional abnormality reminiscent of a phenomenon described in PSZ:
When performance was analyzed as a function of target location, both groups displayed better stimulus detection toward the center of the target location array. Notably, this was not a reflection of a higher general response rate at the center because the number of incorrect responses was lowest at the center and highest at the outer locations. Instead, this pattern likely reflects the challenge of distributing attention broadly across the entire array, with an attentional bias towards the center. Broad monitoring was thus taxed in both groups, but the location dependence of stimulus detection was significantly more pronounced in EKyn as compared with ECon rats. Specifically, the larger location effect on response accuracy in the EKyn group was due to a significantly greater concentration of correct responses towards the center. There was no group difference in the distribution of incorrect responses, again emphasizing that the EKyn group did not simply have a greater tendency to respond more centrally; instead, the group difference was specific to correct responses. We thus interpret this finding as a narrowed attentional focus, or broad monitoring deficit, in the EKyn animals, mirroring the findings of broad monitoring deficits in PSZ (Gray et al. 2014; Hahn et al. 2012b; Kreither et al. 2017). A trend effect on omission errors pointed in the same direction.
Previous studies of attention in rats prenatally exposed to elevated KYNA levels showed deficits selectively in reversal and extradimensional set shifting performance (Alexander et al. 2013; Alexander et al. 2012; Pershing et al. 2015), suggesting a specific deficit in cognitive flexibility. In contrast, bottom-up attentional orienting, tested in rats exposed to elevated KYNA in early life (Iaccarino et al. 2013), was not impaired in the presence of other behavioral abnormalities. The attentional deficit described here is of a different kind, but represents a similarly circumscribed functional impairment. Thus, is appears that not all aspects of attention are impacted by KYNA elevation, consistent with findings in schizophrenia (Gold et al. 2009), but those that do show deficits are reminiscent of areas of impaired function in schizophrenia.
Details of the cellular and molecular mechanisms linking prenatal kynurenine exposure and brain KYNA elevations with the attentional abnormalities described here remain to be determined. Several other cognitive deficits induced by dysregulation of the kynurenine pathway can be remediated by galantamine, which is thought to act via ±7 nAChR and NMDAR mechanisms (Alexander et al. 2013; Alexander et al. 2012; Forrest et al. 2015), i.e. the same sites that are inhibited by KYNA (see Introduction). However, just as different cognitive processes are subserved by divergent neuronal structures and mechanisms, the events underlying the specific deficits observed here may involve different or additional processes. Moreover, the present location-dependent analysis was performed on data acquired when the animals were 8 months of age, i.e. they were substantially older than the EKyn rats in studies demonstrating elevated brain KYNA levels in adulthood (Pershing et al. 2015; Pershing et al. 2016; Pocivavsek et al. 2014). It is therefore possible that the broad monitoring deficits described here were not caused by persistent hyperphysiological KYNA concentrations, but were related to downstream consequences. Notably, the neurodevelopmental link between prenatal KYNA elevation and behavioral impairments later in life may also involve reductions in the expression of various neurotransmitter receptors, in dendritic spine density (Pershing et al. 2015; Pershing et al. 2016), abnormal cell differentiation, and inflammatory processes (Bagasrawala et al. 2016).
Future studies will investigate whether the broad monitoring deficits described here were the result of persisting KYNA elevations, or secondary effects of excessive exposure to KYNA early in life. In this context, it will also be of interest to determine whether broad monitoring deficits are specific to the EKyn model or can be observed in other models of pathophysiological aspects of schizophrenia. Effects of gestational methylazoxymethanol (MAM) exposure and acute or subchronic treatment with NMDA receptor antagonists have been studied in the 5-CSRTT (Amitai and Markou 2009; Barnes et al. 2014; Featherstone et al. 2007; Paine and Carlezon 2009), with results largely inconsistent between the MAM, NMDA antagonist, and EKyn models. However, the spatial breadth of attention has not previously been assessed in a rodent model.
In conclusion, the present study identified deficits in distributing attention broadly in rats prenatally exposed to elevated levels of kynurenine, bearing striking similarity to broad monitoring deficits described in PSZ. Thus, the present results suggest that dysregulation of the kynurenine pathway may underlie this phenomenon in PSZ, which may explain other cognitive deficits, as well. The findings thus add to a growing body of evidence that pre- and perinatal disturbances in kynurenine pathway regulation are causally related to a cognitive phenotype associated with schizophrenia. The narrowed attentional focus described here is a highly specific phenomenon and cannot be the result of non-specific changes in response rate or overall activity. Pinpointing the cellular, molecular, and pharmacological underpinnings of broad monitoring deficits in the 5-CSRTT EKyn model, and possible strategies to reverse them, would therefore have high translational relevance to a presumed core cognitive deficit in schizophrenia.
Acknowledgments
This work was supported by a Silvio O. Conte Center grant (P50 MH103222), and R01 DA035813 (B. Hahn). A. Pocivavsek is a trainee on K12 HD43489-14. We thank Ashleigh Wells and Taylor Radden for contributing to data collection.
Funding:
This work was supported by a Silvio O. Conte Center grant (P50 MH103222), and R01 DA035813 (B. Hahn). A. Pocivavsek is a trainee on K12 HD43489-14.
Footnotes
Conflict of Interest:
Britta Hahn, Carolyn Reneski, Ana Pocivavsek, and Robert Schwarcz declare that they have no conflict of interest.
References
- Akagbosu CO, Evans GC, Gulick D, Suckow RF, Bucci DJ. Exposure to kynurenic acid during adolescence produces memory deficits in adulthood. Schizophr Bull. 2012;38:769–78. doi: 10.1093/schbul/sbq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KS, Pocivavsek A, Wu HQ, Pershing ML, Schwarcz R, Bruno JP. Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: reversal with galantamine. Neuroscience. 2013;238:19–28. doi: 10.1016/j.neuroscience.2013.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KS, Wu HQ, Schwarcz R, Bruno JP. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berl) 2012;220:627–37. doi: 10.1007/s00213-011-2539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Markou A. Increased impulsivity and disrupted attention induced by repeated phencyclidine are not attenuated by chronic quetiapine treatment. Pharmacol Biochem Behav. 2009;93:248–57. doi: 10.1016/j.pbb.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasrawala I, Zecevic N, Radonjic NV. N-Methyl D-Aspartate Receptor Antagonist Kynurenic Acid Affects Human Cortical Development. Front Neurosci. 2016;10:435. doi: 10.3389/fnins.2016.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Coyle JT. The NMDA receptor 'glycine modulatory site' in schizophrenia: D-serine, glycine, and beyond. Curr Opin Pharmacol. 2015;20:109–15. doi: 10.1016/j.coph.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Sawiak SJ, Caprioli D, Jupp B, Buonincontri G, Mar AC, Harte MK, Fletcher PC, Robbins TW, Neill JC, Dalley JW. Impaired limbic cortico-striatal structure and sustained visual attention in a rodent model of schizophrenia. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess AC, Landers AM, Bucci DJ. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav Brain Res. 2009;201:325–31. doi: 10.1016/j.bbr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33:797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngeli NE, Todd TP, Chang SE, Yeh HH, Yeh PW, Bucci DJ. Exposure to Kynurenic Acid during Adolescence Increases Sign-Tracking and Impairs Long-Term Potentiation in Adulthood. Front Behav Neurosci. 2014;8:451. doi: 10.3389/fnbeh.2014.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–8. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry. 2004;56:255–60. doi: 10.1016/j.biopsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Imbeault S, Engberg G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology. 2017;112:297–306. doi: 10.1016/j.neuropharm.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–48. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, Rizos Z, Nobrega JN, Kapur S, Fletcher PJ. Gestational methylazoxymethanol acetate treatment impairs select cognitive functions: parallels to schizophrenia. Neuropsychopharmacology. 2007;32:483–92. doi: 10.1038/sj.npp.1301223. [DOI] [PubMed] [Google Scholar]
- Forrest CM, McNair K, Pisar M, Khalil OS, Darlington LG, Stone TW. Altered hippocampal plasticity by prenatal kynurenine administration, kynurenine-3-monoxygenase (KMO) deletion or galantamine. Neuroscience. 2015;310:91–105. doi: 10.1016/j.neuroscience.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–17. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Gold JM, Hahn B, Strauss GP, Waltz JA. Turning it upside down: areas of preserved cognitive function in schizophrenia. Neuropsychol Rev. 2009;19:294–311. doi: 10.1007/s11065-009-9098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray BE, Hahn B, Robinson B, Harvey A, Leonard CJ, Luck SJ, Gold JM. Relationships between divided attention and working memory impairment in people with schizophrenia. Schizophr Bull. 2014;40:1462–71. doi: 10.1093/schbul/sbu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–30. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Hahn B, Harvey AN, Concheiro-Guisan M, Huestis MA, Holcomb HH, Gold JM. A test of the cognitive self-medication hypothesis of tobacco smoking in schizophrenia. Biol Psychiatry. 2013;74:436–43. doi: 10.1016/j.biopsych.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Harvey AN, Gold JM, Fischer BA, Keller WR, Ross TJ, Stein EA. Hyperdeactivation of the Default Mode Network in People With Schizophrenia When Focusing Attention in Space. Schizophr Bull. 2016;42:1158–66. doi: 10.1093/schbul/sbw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Hollingworth A, Robinson BM, Kaiser ST, Leonard CJ, Beck VM, Kappenman ES, Luck SJ, Gold JM. Control of working memory content in schizophrenia. Schizophr Res. 2012a;134:70–5. doi: 10.1016/j.schres.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Harvey AN, Kaiser ST, Leonard CJ, Luck SJ, Gold JM. Visuospatial attention in schizophrenia: deficits in broad monitoring. J Abnorm Psychol. 2012b;121:119–28. doi: 10.1037/a0023938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl) 2002;162:129–37. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–73. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtze M, Saetre P, Engberg G, Schwieler L, Werge T, Andreassen OA, Hall H, Terenius L, Agartz I, Jonsson EG, Schalling M, Erhardt S. Kynurenine 3-monooxygenase polymorphisms: relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls. J Psychiatry Neurosci. 2012;37:53–7. doi: 10.1503/jpn.100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino HF, Suckow RF, Xie S, Bucci DJ. The effect of transient increases in kynurenic acid and quinolinic acid levels early in life on behavior in adulthood: Implications for schizophrenia. Schizophr Res. 2013;150:392–7. doi: 10.1016/j.schres.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52:1319–28. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Kiank C, Zeden JP, Drude S, Domanska G, Fusch G, Otten W, Schuett C. Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans. PLoS One. 2010;5:e11825. doi: 10.1371/journal.pone.0011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreither J, Lopez-Calderon J, Leonard CJ, Robinson BM, Ruffle A, Hahn B, Gold JM, Luck SJ. Electrophysiological Evidence for Hyperfocusing of Spatial Attention in Schizophrenia. J Neurosci. 2017;37:3813–3823. doi: 10.1523/JNEUROSCI.3221-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CJ, Kaiser ST, Robinson BM, Kappenman ES, Hahn B, Gold JM, Luck SJ. Toward the neural mechanisms of reduced working memory capacity in schizophrenia. Cereb Cortex. 2013;23:1582–92. doi: 10.1093/cercor/bhs148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–32. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, Samuelsson M, Erhardt S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–32. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, McClenon C, Beck VM, Hollingworth A, Leonard CJ, Hahn B, Robinson BM, Gold JM. Hyperfocusing in schizophrenia: Evidence from interactions between working memory and eye movements. J Abnorm Psychol. 2014;123:783–95. doi: 10.1037/abn0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JF, Jackson MF, Beazely MA. Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit Rev Neurobiol. 2006;18:71–84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. [DOI] [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Dulay JR, Barillo MM, Yolken RH, Weis S. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol Dis. 2004;15:618–29. doi: 10.1016/j.nbd.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073–1074:25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Stolerman IP. Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology (Berl) 1998;138:266–74. doi: 10.1007/s002130050671. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox Res. 2006;10:131–48. doi: 10.1007/BF03033242. [DOI] [PubMed] [Google Scholar]
- Nilsson LK, Linderholm KR, Engberg G, Paulson L, Blennow K, Lindstrom LH, Nordin C, Karanti A, Persson P, Erhardt S. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 2005;80:315–22. doi: 10.1016/j.schres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Nilsson LK, Linderholm KR, Erhardt S. Subchronic treatment with kynurenine and probenecid: effects on prepulse inhibition and firing of midbrain dopamine neurons. J Neural Transm (Vienna) 2006;113:557–71. doi: 10.1007/s00702-005-0343-z. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Paine TA, Carlezon WA., Jr Effects of antipsychotic drugs on MK-801-induced attentional and motivational deficits in rats. Neuropharmacology. 2009;56:788–97. doi: 10.1016/j.neuropharm.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershing ML, Bortz DM, Pocivavsek A, Fredericks PJ, Jorgensen CV, Vunck SA, Leuner B, Schwarcz R, Bruno JP. Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia. Neuropharmacology. 2015;90:33–41. doi: 10.1016/j.neuropharm.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershing ML, Phenis D, Valentini V, Pocivavsek A, Lindquist DH, Schwarcz R, Bruno JP. Prenatal kynurenine exposure in rats: age-dependent changes in NMDA receptor expression and conditioned fear responding. Psychopharmacology (Berl) 2016;233:3725–3735. doi: 10.1007/s00213-016-4404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Thomas MA, Elmer GI, Bruno JP, Schwarcz R. Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology (Berl) 2014;231:2799–809. doi: 10.1007/s00213-014-3452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Wu HQ, Elmer GI, Bruno JP, Schwarcz R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Eur J Neurosci. 2012;35:1605–12. doi: 10.1111/j.1460-9568.2012.08064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, Schwarcz R. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 2011;37:1147–56. doi: 10.1093/schbul/sbq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki R, Kreither J, Leonard CJ, Kaiser ST, Hahn B, Gold JM, Luck SJ. Hyperfocusing of attention on goal-related information in schizophrenia: Evidence from electrophysiology. J Abnorm Psychol. 2017;126:106–116. doi: 10.1037/abn0000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–77. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–30. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- Shepard PD, Joy B, Clerkin L, Schwarcz R. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology. 2003;28:1454–62. doi: 10.1038/sj.npp.1300188. [DOI] [PubMed] [Google Scholar]
- Trecartin KV, Bucci DJ. Administration of kynurenine during adolescence, but not during adulthood, impairs social behavior in rats. Schizophr Res. 2011;133:156–8. doi: 10.1016/j.schres.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widner B, Ledochowski M, Fuchs D. Interferon-gamma-induced tryptophan degradation: neuropsychiatric and immunological consequences. Curr Drug Metab. 2000;1:193–204. doi: 10.2174/1389200003339063. [DOI] [PubMed] [Google Scholar]
- Wonodi I, Stine OC, Sathyasaikumar KV, Roberts RC, Mitchell BD, Hong LE, Kajii Y, Thaker GK, Schwarcz R. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch Gen Psychiatry. 2011;68:665–74. doi: 10.1001/archgenpsychiatry.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem Pharmacol. 2013;86:1122–32. doi: 10.1016/j.bcp.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]