Abstract
During bone marrow transplantation (BMT), hematopoietic stem and progenitor cells (HSPCs) respond to signals from the hematopoietic microenvironment (HM) by coordinately activating molecular pathways through Rho GTPases, including Rac. We have previously shown that deletion of Vav1, a hematopoietic-specific activator of Rac, compromises engraftment of transplanted adult HSPCs without affecting steady-state hematopoiesis in adult animals. Here, we show that Vav1−/− fetal HSPCs can appropriately seed hematopoietic tissues during ontogeny but cannot engraft into lethally irradiated recipients. We demonstrate that the engraftment defect of Vav1−/− HSPCs is abrogated in the absence of irradiation and demonstrate that Vav1 is critical for the response of HSPCs to the proinflammatory cytokine interleukin-11 (IL-11) that is upregulated in the marrow of irradiated recipients. Vav1−/− HSPCs display abnormal proliferative responses to IL-11 in vitro and dysregulated activation of pathways critical to engraftment of HSPCs. The engraftment of Vav1−/− HSPCs can be partially rescued in irradiated recipients treated with an anti-IL-11 antibody. These data suggest that HSPCs may respond to different functional demands by selective usage of the IL-11-Vav-Rac pathway, contextualizing further the recent view that HSPCs capable of reconstituting the blood system following transplantation might be distinct from those supporting hematopoiesis during homeostatic conditions.
Graphical abstract
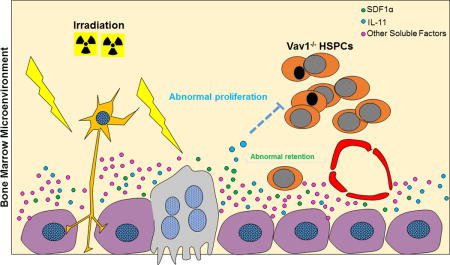
Introduction
Hematopoietic stem cell transplantation (HSCT) is a curative treatment for hematological malignancies and a variety of genetic diseases.[1–3] HSCT success depends on the capacity of transplanted hematopoietic stem cells (HSCs) to home to the bone marrow medullary cavity, migrate and localize to specific anatomically defined niches within the hematopoietic microenvironment (HM) and contribute to the production of donor-derived blood cells. This process is termed hematopoietic engraftment and the molecular mechanisms regulating it are still incompletely understood.[4, 5]
We have previously demonstrated that Rac proteins play a crucial role in regulating homing and engraftment of HSPCs during HSCT.[6–8] Rac belongs to the Rho family of small GTPases, which act as molecular switches and integrate a variety of extracellular stimuli to activate multiple effectors that coordinate a broad range of cellular processes including cytoskeletal reorganization, adhesion, migration and cell division (reviewed in [9, 10]). Rho GTPases cycle between active, GTP-bound and inactive, GDP-bound forms. Three classes of proteins regulate this cycle: guanine-nucleotide exchange factors (GEFs), which accelerate GTP loading and function as activators; GTPase-activating proteins (GAPs) and guanine nucleotide-dissociation inhibitors (GDIs), which accelerate the intrinsic GTPase activity and sequester GTPases in the cytoplasm, respectively, and act to return and maintain the GTPase in a GDP-bound (inactive) form.[11–14]
Studies of the role of GTPases in mammalian cells are complicated by the fact that multiple GEFs and effectors are shared between different GTPases, reflecting cell and agonist-specific regulation of these pathways. Additional complexity results from differences in spatio-temporal responses and extensive cross-talk between various related GTPases, often resulting in opposite cellular outputs.[15–17] We have utilized a variety of genetic models and analysis of primary cells to define both overlapping and unique roles of Rac GTPases in hematopoiesis.[18]
We have previously demonstrated a role for the hematopoietic-specific GEF Vav1 in HPSCs function. Vav1 is a GEF for Rac but can also bind and activate (although with reduced affinity) CDC42 and RhoA, two other Rho GTPase family members.[19–21] Vav1−/− mice have normal steady-state haematopoiesis, but adult Vav1−/−HSPCs fail to engraft in lethally irradiated recipients during HSCT, both in a competitive and in a non-competitive setting.[22] Vav1−/−HSPCs display reduced responses to SDF1α, a chemokine produced by bone marrow stromal cells that regulates migration of HSPCs to the bone marrow during transplantation and ontogenesis.[23–25]
Traditionally, HSPCs have been defined in transplantation studies as cells possessing self-renewal and multilineage differentiation capabilities. However, using a transposon-based cellular tracking system, Sun et al. have recently raised the possibility that transplanted HSCs might represent a distinct class of primitive cells.[26] Their study and others ([27]) suggest that HSCs contributing to hematopoiesis during homeostatic conditions are not found in HSC pools defined by transplantation assays.
Here, we demonstrate that Vav1−/−fetal HSPCs appropriately colonize successive anatomic hematopoietic sites during ontogeny, but, similar to Vav1−/−HSPCs from adult mice, are unable to engraft lethally irradiated recipients. Unexpectedly, we show that Vav1−/−HSPCs are capable of engrafting non-irradiated recipients and display a specific proliferation defect in the presence of irradiated stroma. We identify IL-11, a pro-inflammatory cytokine induced by irradiation, as a potential mediator of the reduced engraftment of Vav1−/−HSPCs, and we conclude that the profound engraftment defect of Vav1−/−HSPCs in irradiated recipients represents a combinatorial defect in their function due to their inability to integrate multiple extracellular signals converging on Rac.
Materials and Methods
Mice
All procedures involving mice followed Boston Children’s Hospital Institutional Animal Care and Use Committee guidelines. Vav1−/− mice have been previously reported [28] and were backcrossed into a C57BL/10J (CD45.2) background. Age- and sex-matched C57BL/10J mice (Jackson Laboratory, Bar Harbor, ME) were used as WT controls. C57BL/6J (CD45.2) and B6.SJL (CD45.1) mice were obtained from Jackson Laboratories. Lethal irradiation was performed before BM transplantation using a 137Cs source, with a total dose of 11.5 Gy split in two administrations three hours apart. Recipients were irradiated 24 h before transplantation. Rag2−/− γc−/− KitW/Wv mice used as recipients for transplantation experiments without conditioning were a kind gift from Prof. HR Rodewald, Heidelberg, Germany and they have been previously described.[29]
HSPC isolation, transplantation and homing experiments
Lineage depletion was performed using the kit from Miltenyi Biotech, San Diego, CA according to the manufacturer’s instructions. For transplantation experiments, a single cell suspension obtained from BM of adult or E13.5 fetal liver (FL) donor mice was depleted of red blood cells using the PharmLyse® buffer from BD Biosciences, San Jose, CA. Viable cells were then counted and resuspended at the desired concentration in PBS supplemented with 1% BSA, and transplanted into recipient mice by tail vein injections. For transplantation experiments, 2×106 whole BM or FL cells were injected into lethally irradiated or non-conditioned recipients. For competitive transplants, 1×105 BM cells isolated from heterozygous CD45/1.CD45.2 mice were used as supportive cells.
Homing was performed according to our previously published protocol.[30]
For transplantation experiments using an anti-IL-11 antibody, lethally irradiated recipients were treated 6 h and 24 h after the first dose of irradiation with IL-11 blocking antibody or IgG2A isotype control (both from R&D Systems, Minneapolis, MN) at the dose of 1 mg/kg of body weight (by tail vein injection).[31] For these experiments, 3×106 whole BM cells were injected into lethally irradiated recipients by tail vein injection.
Colony-forming unit (CFU) assay and Cobblestone area-forming cell (CAFC) assay
CFU assays were performed as previously described.[22] Briefly, base methylcellulose medium (Methocult M3134, StemCell Technologies, Vancouver, BC) was supplemented with cytokines specific for fetal or adult HSPCs growth. In some experiments, semisolid media was supplemented with the indicated dose of mouse recombinant IL-11. All cytokines were purchased from Peprotech, Rock Hill, NJ. Colonies were scored 7-10 days after seeding using an inverted microscope. The following number of cells was plated per 35 mm dish: 25.000 live cells (from both adult BM and FL samples), 100.000 nucleated cells from embryonic blood and newborn BM samples.
Limiting-dilution CAFC assays were performed as described.[32] For some experiments, primary bone marrow stromal cells were isolated from C57BL/6J mice 24 h after treatment with a lethal dose of irradiation (11.5 Gy). For other experiments, stromal cell lines (MS-5) with or without irradiation (25 Gy) were used as supportive layers. Both MS-5 and primary stromal cells were cultured in Myelocult M5300 (StemCell Technologies) supplemented with 10−5 M hydrocortisone (Spectrum Chemicals MFG, New Brunswick, NJ). For primary bone marrow stromal cell isolation, BM was harvested from irradiated mice. After a single cell suspension was generated, cells were left to adhere onto gelatin-coated flasks. After stromal cells had adhered to the wells for three hours, the suspension fraction (containing hematopoietic cells) was removed, and stromal cell lines were cultured according to the method in Nadri et al.[33] Feeders-containing 96-well plates were then seeded with freshly isolated lineage negative cells at the desired dilution for CAFC assay. Primary stromal cells were also grown under long-term bone marrow culture conditions in 6-well plates and irradiated at the dose of 25 Gy or left untreated for analysis of IL-11 levels in the supernatant by ELISA. For all CAFC experiments, lineage-depleted BM cells from WT and Vav1−/− mice were seeded on stromal cells at 6 serial 2-fold dilutions (from 20.000 to 625 cells/well) in 15 replicate wells. Co-cultures were kept at 33°C and 10% CO2. Half of the culture media in each well was exchanged every week. The frequency of CAFC was calculated by Poisson statistics using L-Calc software (StemCell Technologies).
For quantification of IL-11 mRNA transcript after irradiation, MS-5 cells cultured as above were plated in 6 well plates, irradiated at the dose of 25 Gy or left untreated, and then harvested 24 h after irradiation for qPCR analysis.
Cytokine array and ELISA assays
BM samples were obtained from three age- and sex-matched untreated C57BL/6J mice, irradiated C57BL/6J mice 6 or 24 h after lethal irradiation (11.5 Gy), or untreated Rag2−/− γc−/− KitW/Wv mice. Two femurs of every mouse were flushed using 400 μl of IMDM medium, spun down, and BM supernatants were collected after centrifugation. Cytokine array was performed using the C2000 kit from RayBiotech, Norcross GA following manufacturer’s instructions. BM supernatant from three independent mice per group was pooled for this experiment and arrayed on the chip as an individual sample. Data analysis was performed using the accompanying company analysis tool. Concentration of mouse IL-11 in the BM supernatants was confirmed using enzyme-linked immunosorbent assay (ELISA) kit EK0420 from BosterBio, Pleasanton, CA. For this assay, BM supernatant from each individual mouse was treated as independent replicates (n=5-8 per group). For the determination of IL-11 levels induced by irradiation in primary bone marrow stromal cells cultures, individual mice were treated as independent replicates (n=4-7 per condition).
Statistical methods
Descriptive statistics (means, medians, standard deviation [SD]) were used to summarize continuous measures. The Student’s t-test was used to compare continuous measures between groups. Kaplan-Meier survival curves were generated, and the log-rank test was used to compare survival between groups. Statistical analyses were performed using R version 3.2.1[34] and GraphPad Prism version 7.00, GraphPad Software, La Jolla California USA. Two-sided p-values < 0.05 were considered statistically significant.
For additional details on experimental procedures, please see Supplementary Material and Methods.
Results
Vav1 is dispensable for proper colonization of hematopoietic sites during ontogeny, but required for engraftment of fetal HSPCs following transplantation
Migration of HSPCs during ontogeny is an SDF-1α dependent process. To determine if Vav1 is required for migration of HSPCs during development, we analyzed defined anatomic sites of hematopoiesis in the embryo. The number of immunophenotypically defined HSPCs in Vav1−/− E13.5 FL did not differ significantly from WT numbers (Fig. 1). The percentage of c-Kit/CD34 double positive cells in a single cell suspension from E13.5 FL was 2.1 ± 0.9% in WT vs. 2.2 ±0.6% in Vav1−/− (n=18, mean +/− SD) while the percentage of lineage− Sca-1+ c-Kit+ (LSK) cells in a single cell suspension from E13.5 FL was 3.8 ±1.2% in WT vs. 3.5 ± 1.3% in Vav1−/− (n=8-9) (Fig. 1A and 1B). Similarly, functional analysis of the hematopoietic progenitor compartment of E13.5 FL by colony-forming unit (CFU) assay showed no differences between WT and Vav1−/− embryos with 42.0 ±10.4 CFUs in WT cells vs. 37.6 ± 7.27 in Vav1−/− (n=8-10) (Fig. 1C).
Figure 1. Immunophenotypic and functional characterization of Vav1−/− fetal HSPCs.
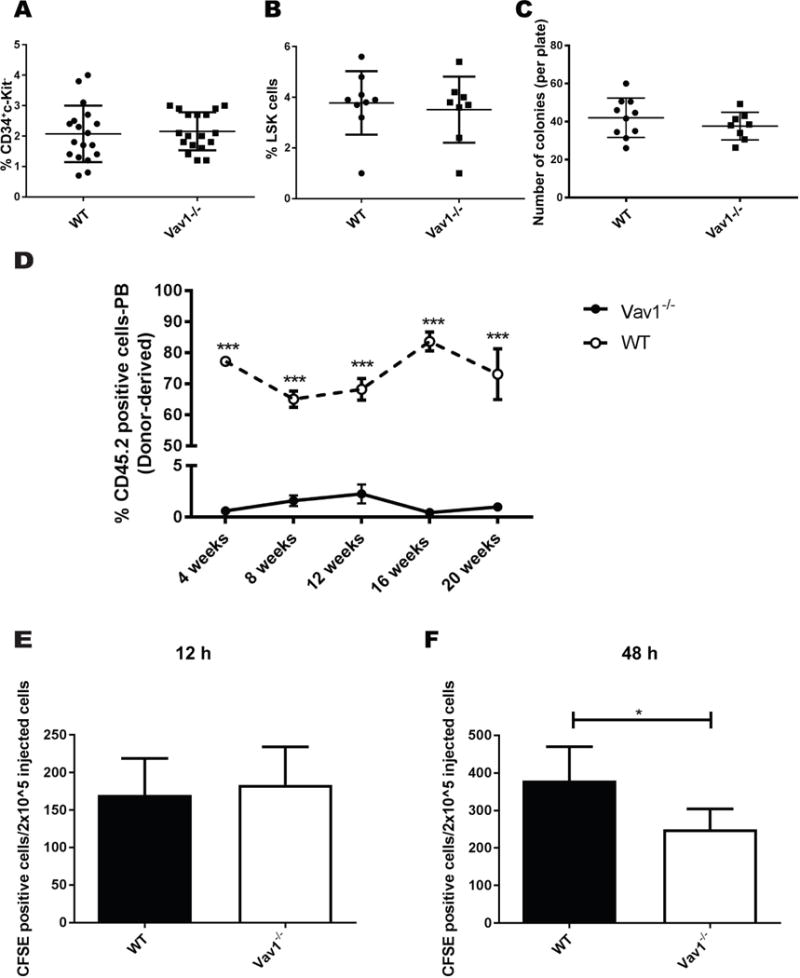
A) Percentage of CD34/c-Kit double positive cells in single cell suspensions prepared from E13.5 FLs. B) Percentage of Lineage− Kit+ Sca1+ (LSK) cells in single cell suspensions prepared from E13.5 FLs. C) Total number of colonies formed in methylcellulose by 25.000 nucleated cells from E13.5 FLs. Each dot in graphs A-C represents an individual embryo. Horizontal lines represent average values. Error bars represent SEM, n=10-18 embryos. D) Engraftment of WT or Vav1−/− E13.5 FL HSPCs into lethally irradiated syngeneic recipients. Graph represents the percentage of CD45.2 donor-derived cells in the peripheral blood (PB) of mice transplanted with both genotypes at the indicated time point. Data represents average of 5 recipients per group per data point. E-F) Homing and retention of E13.5 FL HSPCs into lethally irradiated syngeneic recipients. Cells were labeled with the CFSE dye before infusion, and fluorescence was recorded using an LSRII analyzer 12 h (homing) or 48 h (retention) after injection. Bar graphs represent average values from 5 recipients per condition. Error bars represent standard deviation (SD). *P <05; *** P < .001.
Subsequent seeding of the BM from the FL was also not affected by deletion of Vav1. The number of LSK cells and CFU-derived colonies from single cell suspensions prepared from the BM of newborn mice was 2.6 ± 1.4% in WT vs. 4.0 ±2.3% in Vav1−/− (n=5) and 52.9 ± 34.4 in WT cells vs 38.2 ± 40.9 in Vav1−/−, respectively (n=14-15) (Fig. S1A and S1B). Similarly, CFU assay performed on the peripheral blood (PB) of E18.5 embryos, a developmental age when the colonization of BM from the FL reaches its peak, showed no differences with 49.1 ± 22.2 CFUs per 5×10^5 nucleated cells in WT vs 55.1 ± 38.7 in Vav1−/− (n=5) (Fig. S1C). These data demonstrate that in spite of the defect in SDF-1 signaling Vav1 is not required for the establishment of proper migratory routes and colonization of successive anatomic sites during embryonic development of the hematopoietic system.
To verify that the engraftment defect previously demonstrated using HSPCs from the BM of adult mice is also a phenotype of FL cells from Vav1−/− mice, we transplanted freshly isolated E13.5 FL cells with un-manipulated WT supportive BM cells into irradiated recipient mice. As previously seen in transplants utilizing HSPCs from the BM of adult mice, Vav1−/− FL HSPCs displayed a significant reduction in engraftment compared with WT FL cells at both early and late time points. Transplanted Vav1−/− FL cells lead to 0.6 ±0.4% donor-derived cells compared with 77.2 ±1.2% WT cells in the PB at 4 weeks and 1±0.4% engraftment compared with 73.1±8.1% in WT at 20 weeks (n=5) (Fig. 1D). Mechanistically, this profound engraftment defect was not apparently due to an alteration in the initial homing of Vav1−/− FL HSPCs to the BM, as assed by homing of CFSE-labeled cells. Homing assessed 12 h after transplantation showed 170.1 ± 48.7 CFSE positive cells per 2×10^5 injected cells in WT vs. 183.6 ± 50.43 in Vav1−/− (n=5) (Fig. 1E). In contrast, the number of Vav1−/− CFSE positive cells recovered from the BM of recipient mice 48 h after transplantation was significantly lower than WT with 379.9 ± 89.7 CFSE positive cells per 2×10^5 injected cells in WT vs. 250.0 ± 54.3 in Vav1−/−(n=5) (Fig. 1F). Similar to previous observations using HSPCs derived from adult animals, these data suggest reduced retention, survival or proliferation of Vav1−/− HSPCs in the medullary HM. In summary, while different anatomic sites are repopulated during ontogeny in normal numbers in Vav1−/− mice, corresponding to normal numbers of HSPCs in adult Vav1−/− mice, FL HSPC cells from these mice, similar to HSPCs derived from adult animals, have a significant engraftment defect in conditioned recipients.
Vav1−/− adult and fetal HSPCs can engraft and repopulate non-conditioned recipients
Based on these data, we hypothesized that the Vav1−/− HSPC engraftment defect seen in conditioned animals may be related to changes in the HM induced by irradiation. While the signals regulating HSPC self-renewal and differentiation after transplantation into conditioned recipients are incompletely understood, conditioning using irradiation leads to marked changes in the HM, including induction of proinflammatory cytokines. We next tested if a non-irradiated BM microenvironment could be permissive for the engraftment of Vav1−/− HSPCs. We transplanted freshly isolated E13.5 FL or adult BM cells into Rag2−/− γc−/− KitW/Wv, a mouse strain that can be successfully engrafted with hematopoietic stem cells in the absence of conditioning (Fig. 2A). These mice lack T, B and NK cells and display severe macrocytic anemia and mast cell deficiency due to defective c-kit signaling in the stem cell compartment (Fig. 2A). Donor-derived engraftment was evaluated at 12 weeks after transplantation based on the correction of macrocytic anemia. Mean red cell volume (MCV) was 74.4± 4.8 fL for un-manipulated Rag2−/− γc−/− KitW/Wv mice and corrected to 46.7± 2.0 fL for mice transplanted with WT FL cells and to 48.4±8.6 fL for mice transplanted with Vav1−/− FL cells (n=6-10) (Fig. 2B). Correction of macrocytosis was accompanied by a correction of B lymphocyte numbers in the PB of transplanted animals. B cell numbers in the PB as measured by the percentage of B220+ cells were 0.3 ± 0.2% for un-manipulated Rag2−/− γc−/− KitW/Wv mice and corrected to 58.2 ±11.8% for mice transplanted with WT FL cells and to 62.2 ± 9.6% for mice transplanted with Vav1−/− FL cells (n=6-10) (Fig. 2C). Similar correction of the hematopoietic defects in Rag2−/− γc−/− KitW/Wv mice was demonstrated with HSPCs derived from adult Vav1−/− mice (Fig. 2D, E). Vav1−/− and WT HSPCs were able to correct total white blood cells (WBC), hemoglobin (Hb) and red blood cell count (RBC) values (Fig. S2A and S2C), but not T cell numbers as assayed by the percentage of CD3+ cells in the PB (Fig. S2B), a finding consistent with the known cell-autonomous block in T cell maturation and T lymphopenia present in Vav1-deficient animals.[28]
Figure 2. Vav1−/− fetal and adult HSPCs engraft in non-irradiated recipients.
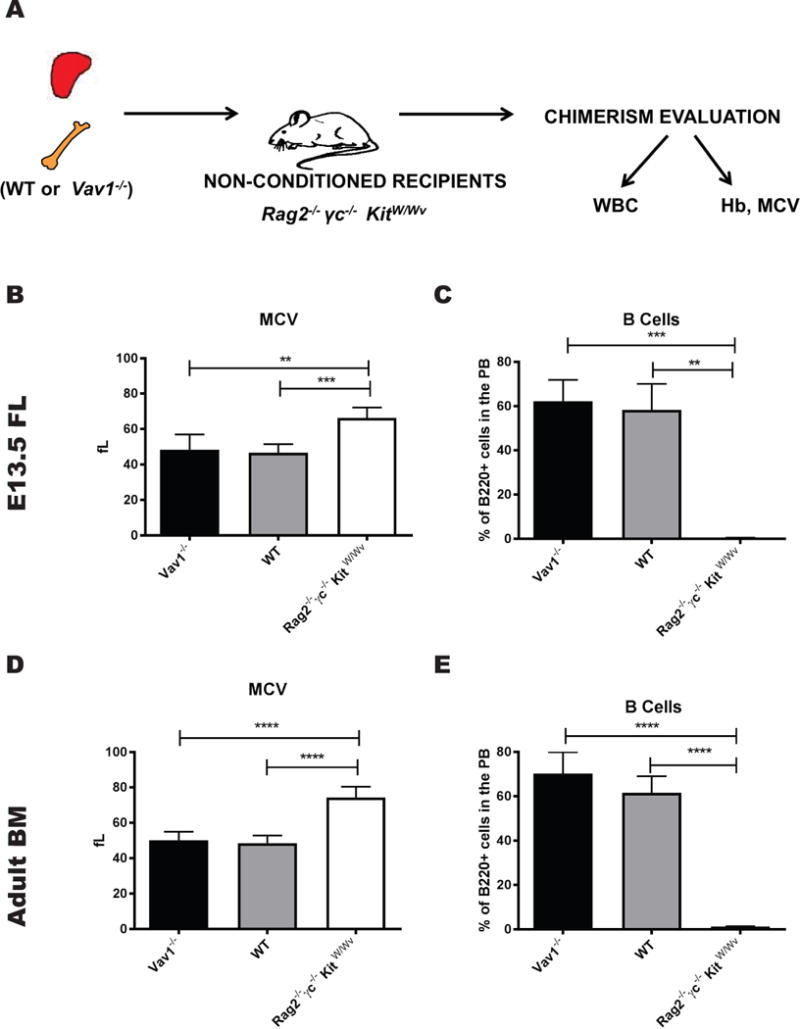
A) Schema of the experimental design used for the transplantation studies with non-conditioned recipients. Vav1−/− and WT HSPCs isolated from E13.5 FLs or adult BM were transplanted into Rag2 −/− γc−/− KitW/Wv recipient mice without any conditioning. Rag2 −/− γc−/− KitW/Wv display macrocytic anemia and B, T and NK cell deficiency; donor-derived engraftment can be evaluated in this model based on the correction of these phenotypes. (B, D) MCV values from mice transplanted with E13.5 or adult WT and Vav1−/− HSPCs 12 weeks after transplantation. (C, E) Peripheral B cell counts (% of B220+ cells in the PB 12 weeks after transplantation. Bar graphs represent average values from 8-10 recipients per condition. Error bars represent standard deviation (SD). **P <.01; *** P < .001; **** P < .0001.
Irradiation impairs the interaction of Vav1−/− HSPCs with the BM hematopoietic microenvironment
To directly test if irradiation leads to a detrimental effect on Vav1−/− HSPCs via the HM, we modeled the interaction between irradiated BM microenvironment and HSPCs in vitro. We conditioned mice with the same dosage of irradiation utilized in HSPC transplantation experiments and isolated primary stromal cells from the irradiated mice. Vav1−/− or WT HSPCs were then seeded onto the primary stromal cells and the number of cobblestone area-forming cells (CAFCs) was analyzed every week for 5 weeks (Fig. 3A). We observed a substantial reduction in the frequency of CAFCs derived from Vav1−/− HSPCs compared to WT at all time points analyzed on irradiated stroma. The mean number of CAFCs at 2, 3, 4 and 5 weeks was, respectively, 37.2 ±15.5, 17.9 ±1.8, 10.6 ± 2.5, 4.6 ± 0.9 for WT cells and 16.3 ± 2.7, 8.91± 0.7, 5.4 ± 0.9, 2.4 ± 0.4 for Vav1−/− cells (Fig. 3B). The difference between WT and Vav1−/− was slightly more pronounced at early time points, suggesting that the detrimental effect of the irradiated microenvironment may be more pronounced on Vav1−/− progenitors than stem cells. A similar trend was observed in CAFC assays performed using an independent stromal cell line system (MS-5) (Fig. 3C–D, and Suppl. Table 1). Although there was variability in absolute numbers among independent experiments, in each experiment we noted a difference in CAFC frequency between Vav1−/− and WT when hematopoietic cells were plated on irradiated MS-5, while no difference was observed when using non-irradiated MS-5 (Fig. 3D and Suppl. Table 1). From these data, we conclude that Vav1−/− HSPCs display a defect in proliferation that is specific to the irradiated HM.
Figure 3. Lethal irradiation of stromal cells impairs proliferation of Vav1−/− HSPCs.
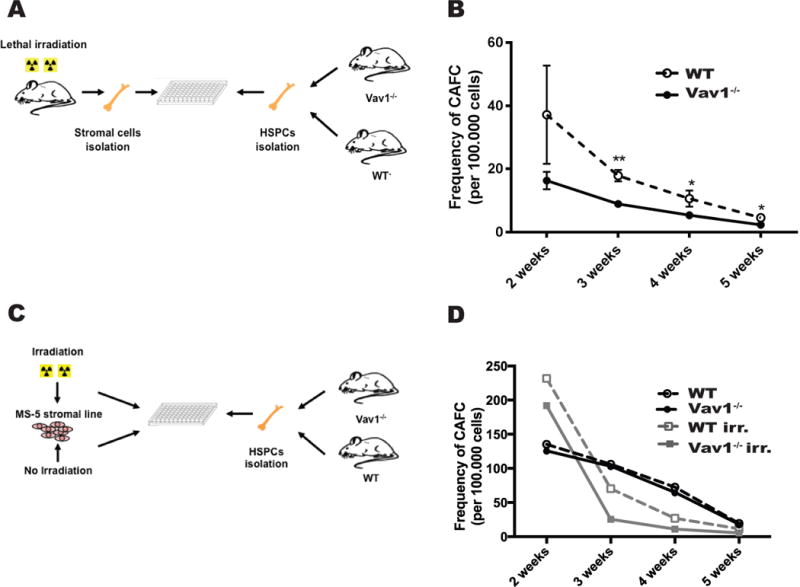
A) Schema of the experimental design for CAFC assay performed using BM cells from WT or Vav1−/− mice cultured on primary stromal cells isolated from irradiated mice (24 h after irradiation). B) Frequency of CAFC in both genotypes using conditions represented in A). The x-axis represents time in culture (weeks); the y-axis represents the frequency of CAFC per 100.000 BM cells at each time point, calculated by limited dilution analysis. n=4. Error bars represent standard deviation (SD). *P <05; **P <.01. C) Schema of the experimental design for CAFC assay performed using BM cells from WT or Vav1−/− mice cultured on the MS-5 stromal cell line, treated with irradiation or left untreated. D) Frequency of CAFC formed by both genotypes on non-irradiated and irradiated stromal cells. The x-axis represents time in culture (weeks); the y-axis represents the frequency of CAFC per 100.000 BM cells at each time point, calculated by limited dilution analysis. Graph represents one of three independent experiments with similar results. Raw values for all three experiments are reported in Suppl. Table 1.
IL-11 is upregulated in the BM of irradiated recipients and after irradiation of stromal cell cultures
To dissect which factors might mediate the sensitivity to irradiation that we observed in Vav1−/− HSPCs, we profiled irradiated and non-irradiated BM supernatants by cytokine array (Fig. 4A and Suppl. Table 2). We noted cytokines that showed significant upregulation at 24 h post irradiation compared to two control groups, including Rag2−/− γc−/− KitW/Wv or unirradiated syngeneic mice (Fig 4B). We focused on the 24 h as this time point is biologically relevant to our experimental setting, since we routinely perform irradiation of recipient mice 24 h before transplantation. Among the top 20 cytokines that showed irradiation-associated induction, we selected interleukin-11 (IL-11) for further studies. IL-11, which was induced >50 fold in the bone marrow 24 h after irradiation, was originally cloned from a bone marrow derived stromal cell line [35] and has previously been characterized by us and others to have effects on HSC function.[36–39] First, we confirmed by ELISA using an independent cohort of sex and age-matched mice that IL-11 was increased in irradiated recipients compared to unirradiated syngeneic controls and Rag2−/− γc−/− KitW/Wv mice (179.1 ± 62.9 pg/mL vs 62.9 ± 13.3 pg/mL vs 83.1 ± 47.8 pg/mL, respectively, n=4-11) (Fig. 4C). By 48 h, the levels of IL-11 were back to baseline (data not shown). Similarly, we detected a 2.5X-fold increase of IL-11 by ELISA in supernatants harvested from primary bone marrow stromal cell cultures 24 h after irradiation, compared with untreated cells (Fig. 4D) (n=4-7 independent cultures). Finally, irradiation of MS-5 stromal cell line induced a 2.6-fold upregulation of the IL-11 transcript measured by qPCR 24 h after treatment (fold change to untreated cells, n=4) (Fig. 4E).
Figure 4. IL-11 is upregulated in the bone marrow of irradiated recipients, but not in Rag2 −/− γc−/− KitW/Wv mice.
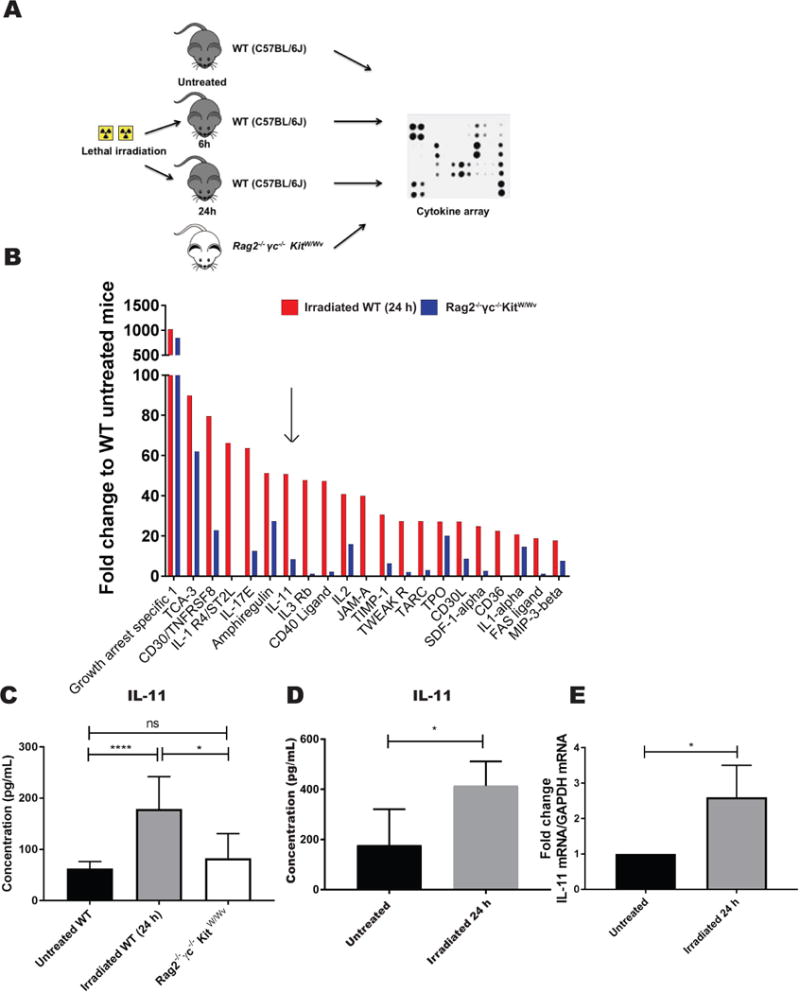
A) Schema of the experimental design for the cytokine array analysis. Bone marrow supernatant was isolated from C57Bl/6J mice either untreated or treated with lethal doses of irradiation (6 and 24 h after irradiation) and compared to the bone marrow supernatant isolated from Rag2 −/− γc−/−KitW/Wv mice. B) Bar graph represents the fold change to WT untreated mice of cytokines upregulated in the BM supernatant of irradiated mice (only the top 20 hits ranked by the highest expression at 24 h after irradiation are shown), and the relative levels in Rag2 −/− γc−/−KitW/Wv mice. Arrow points to IL-11. C) IL-11 levels were quantified by ELISA on BM supernatants isolated from irradiated and non-irradiated WT mice at the indicated time point, and Rag2 −/− γc−/−KitW/Wv. Bar graphs represent average values from 5-8 mice per condition. Error bars represent standard deviation (SD). D) IL-11 was measured by ELISA in supernatants harvested from primary stromal cell cultures untreated or 24 h after irradiation. Bar graphs represent mean IL-11 concentration values from 4-7 independent cultures. Error bars represent standard deviation (SD). E) IL-11 mRNA levels were quantified by qPCR in MS-5 stromal cell lines at baseline (untreated) and 24 h after irradiation. Bar graphs represent mean values from 4 biological replicates. Values are presented as fold change to untreated. Error bars represent standard deviation (SD). *P <05; ****P < .0001
Molecular and functional abnormalities in Vav1−/− cells treated with IL-11 in vitro
We next determined if IL-11 treatment of WT cells leads to activation of Vav1 by immunoblot. To better mimic the complexity of the HM, where multiple cytokines act together to support expansion and proliferation of hematopoietic cells, we stimulated WT HSPCs with IL-11 alone and in combination with stem cell factor (SCF), a cytokine known to act synergistically with IL-11 to promote hematopoiesis.[39, 40] Treatment with IL-11 alone or with SCF increased phosphorylation of Vav1 compared with baseline, confirming that IL-11 activates Vav1 in these cells (Fig 5A). To directly assess the biological consequences of IL-11 on Vav1-deficient HSPCs, we supplemented WT and Vav1−/− cells with increasing concentrations of IL-11 in CFU assays. In standard semisolid media, there was no difference in the number of CFU formed by both genotypes (Fig 5B). However, when supplemented with 200 ng/mL of IL-11, WT HSPCs displayed a statistically significant higher number of CFUs compared with Vav1−/− HSPCs (88.6 ±9.5 vs. 66 ±9.1 CFUs per 25,000 plated cells) (Fig. 5C). Complementary data was obtained when BM HSPCs were incubated in liquid cultures supplemented with SCF and IL-11. The fraction of BrdU+ cells after 4 days in culture was significantly lower in Vav1−/− HSPCs than in WT (% BrdU+: 34.0 ±6.1% in WT cells, 16.7 ±12.1% in Vav1−/− cells, n=3) (Fig. 5D). Moreover, addition of IL-11 to an SCF-based liquid culture system also modestly increased the percentage of early and late apoptotic cells in Vav1−/− but not in WT cells when analyzed after 7 days in culture (Fig. 5E). The expression of gp130 (IL-11 receptor common subunit) was not different between Vav1−/− and WT HSPCs (Fig. S3). Taken together, these data indicate a detrimental effect of IL-11 on Vav1−/− HSPC proliferation and survival in vitro.
Figure 5. Vav1−/− HSPCs display reduced proliferation, increased apoptosis and abnormal molecular responses to IL-11.
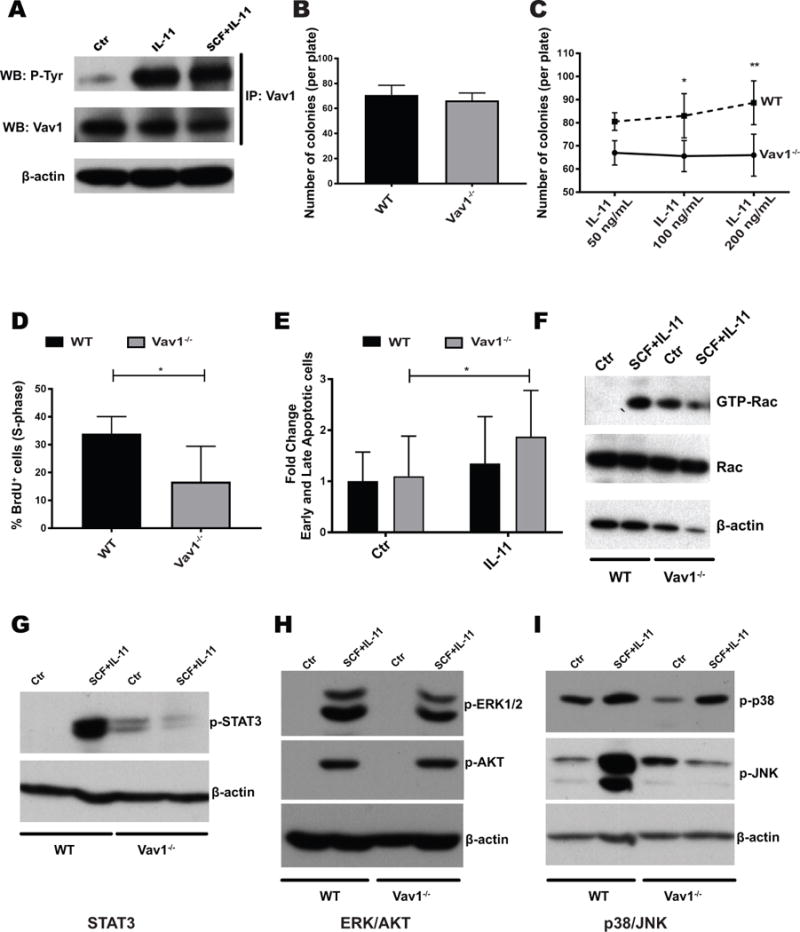
A) Vav1 is phosphorylated upon treatment of HSPCs with IL-11, as demonstrated by immunoprecipitation (IP) with a Vav1-specific antibody, followed by detection with phosphotyrosine antibody (p-Tyr). WT lineage-depleted cells were either left untreated or stimulated with IL-11 alone or SCF and IL-11 (both at 100 ng/mL) for 10 minutes. WB: western blot. B) Number of colonies formed by WT or Vav1−/− BM cells in standard semisolid media (n=12). C) Number of colonies formed by WT or Vav1−/− BM cells in standard semisolid media supplemented with increasing doses of IL-11 (n=4). Error bars represent standard deviation (SD). *P <05; **P <.01. D) Percentage of proliferating cells in liquid cultures established from Vav1−/− and WT HSPCs. Cells were cultured in SCF and IL-11. Bar graphs represent average percentage of cells in S-phase after a 12 h pulse with BrdU (n=3). Error bars represent standard deviation (SD). *P <05. E) Percentage of apoptotic (both early and late) cells per genotype with SCF alone (control) and SCF/IL-11 (IL-11). Bar graphs represent fold change to WT control (n=3). Error bars represent standard deviation (SD). *P <05. F) Levels of active (GTP-bound) and total Rac in WT or Vav1−/− HSPCs. Lineage-depleted cells were starved and left untreated or stimulated with SCF/IL-11. GTP-bound Rac was precipitated with agarose-conjugated PAK1-p21-binding domain (PBD) and detected by western blot. G-I) Activation status of signaling pathways in WT and Vav1−/− lineage-depleted cells by WB with phospho-specific antibodies. Cells were stimulated as in E), and protein lysates were probed with the indicated antibodies. Images display one representative WB of 3 independent experiments.
Since we previously implicated Vav1 as a GEF for Rac in HSPCs [22] and Rac is critical for HSPC engraftment [6, 7, 18], we next investigated the effect of IL-11 on Rac activation in both WT and Vav1-deficient HSPCs. Using a Pak binding domain (PBD) pull-down assay, we observed that treatment of WT HSPCs with IL-11/SCF causes a robust increase in GTP-bound (activated) Rac, whereas Vav1-deficient cells display both an abnormal baseline activation of Rac and decreased Rac activation in response to stimulation with SCF/IL-11 (Fig. 5F). Next, we investigated the activation status of signaling pathways previously implicated downstream of the Vav1/Rac pathway. After stimulation of WT HSPCs with SCF/IL-11, there was clear phosphorylation of ERK, AKT, p38, JNK and STAT3 (Fig. 5G–I). In Vav1−/− HSPCs, we observed an increased baseline phosphorylation of both STAT3 and JNK with reduced activation in response to cytokine stimulation (Fig. 5F and 5H). JNK is a known regulator of stress hematopoiesis [41], and reduced activation of STAT3 has previously been associated with reduced engraftment of HSPCs.[42] These data demonstrate that IL-11 activates Vav and Rac in HSPCs and that lack of Vav1 signaling attenuates IL-11 induced pathways implicated in successful engraftment of hematopoietic cells during transplantation.
IL-11 depletion partially rescues the engraftment defect of Vav1−/− HSPCs in lethally irradiated recipients
To test if attenuation of IL-11 signalling in vivo after irradiation conditioning could reduce the engraftment defect of Vav1−/− HSPCs, we treated irradiated mice with either an IL-11 blocking antibody or an isotype control (IgG) and subsequently transplanted antibody-treated mice with Vav1−/− or WT HSPCs (Fig 6A). We then analyzed recipient mice for engraftment and survival. Treatment of recipient mice transplanted with Vav1−/− HSPCs with an IL-11 blocking antibody significantly extended their survival compared to mice transplanted with Vav1−/− HSPCs and treated with an isotype antibody (log-rank p value=0.02) (Fig. 6B). All mice transplanted with WT cells survived. Six weeks after transplantation, only 50% (95% confidence interval [CI]: 29.6-84.4%) of mice transplanted with Vav1−/− HSPCs and treated with IgG survived, vs. 73.3% (95%CI: 54.0-99.5%) of mice transplanted with Vav1−/− HSPCs and treated with an anti-IL-11 Ab. The improved survival of mice transplanted with Vav1−/− and treated with an IL-11 blocking antibody correlated with an increase in the PB chimerism of donor-derived cells in this group: median percentage of donor-derived cells in the PB at 6 weeks after transplantation was 92.0% and 92.7% for mice transplanted with WT cells, and 44.9% and 82.2% for mice transplanted with Vav1−/− HSPCs and treated with IgG and IL-11, respectively (Fig. 6C).
Figure 6. Depletion of IL-11 in lethally irradiated recipients ameliorates the engraftment defect of Vav1−/− HSPCs.
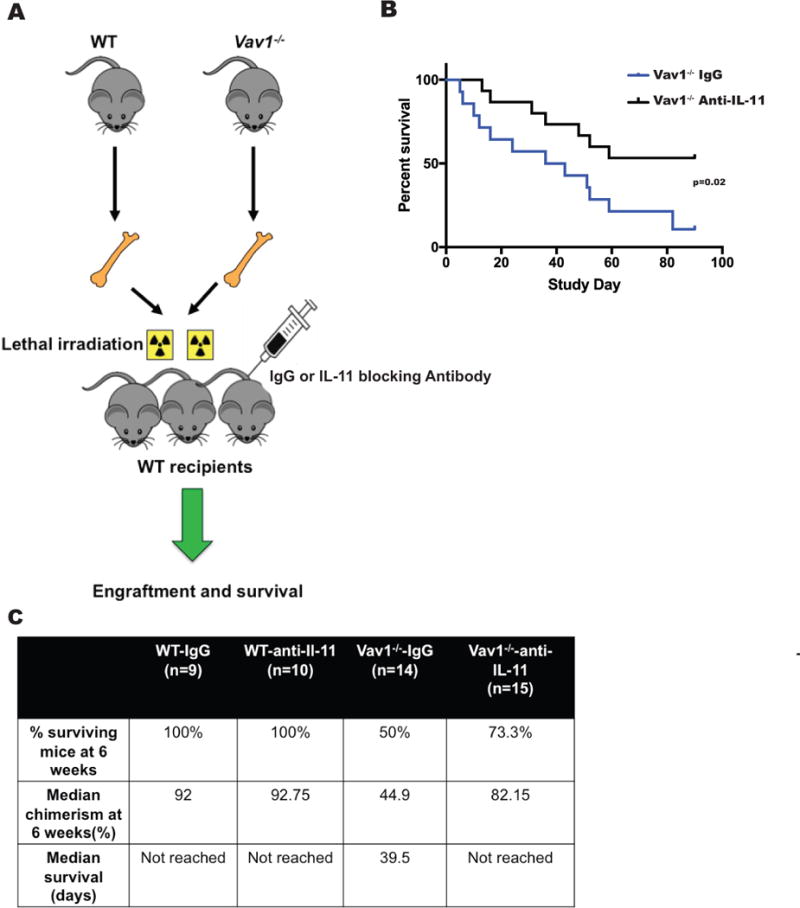
A) Schema of the experimental design for transplantation studies shown in B) and C). Lethally irradiated recipients were treated with an IL-11 blocking antibody or an IgG isotype control, and transplanted with BM cells from WT or Vav1−/− mice. IL-11 blocking antibody or isotype control were injected 6 and 24 h after irradiation at the dose of 1 mg/Kg of body weight. B) Overall survival of lethally irradiated recipients treated with IgG or an IL-11 blocking antibody and transplanted with WT or Vav1−/− adult HSPCs. C) Analysis of mice in the 4 cohorts of the study. Engraftment of WT or Vav1−/− HSPCs into lethally irradiated syngeneic recipients treated with an IL-11 blocking antibody or an isotype control was evaluated by % of donor-derived PB cells 6 weeks after transplantation. Table indicates the median percentage of chimerism in surviving mice at the indicated time point.
Discussion
Vav1 is a hematopoietic-specific activator (GEF) of Rac, a Rho GTPase that mediates HSC engraftment and retention in the BM.[6, 7, 18] We have previously reported that Vav1−/− HSPCs are unable to respond to SDF1α and fail to engraft in lethally irradiated recipient mice.[22] Vav1 knockout mice have been characterized [28] and have an immunologic phenotype restricted to T cells, without evidence of HSC deficiency. Indeed, we have previously noted that Vav1−/− adult mice have normal content of HSCs in the BM. Here, we show that the number of Vav1−/− HSPCs and their function in clonogenic assays appear normal during ontogeny in the FL, fetal blood and in newborn BM. In spite of this, data presented here confirm that fetal Vav1−/− HSPCs poorly engraft in lethally irradiated recipients. The defective engraftment can be mimicked by defect in primitive hematopoietic cell growth in long-term cultures on stroma from irradiated mice and further confirmed in a co-culture system with irradiated but not with non-irradiated stromal cell lines.
Surprisingly, we found that both fetal and adult Vav1−/− HSPCs can engraft in non-conditioned immunologically deficient mice. In this experimental setting, recipient mice do not receive any irradiation and have a defective endogenous compartment of HSPCs due to a genetic mutation in c-Kit, the transmembrane kinase receptor for stem cell factor (SCF) and an important survival factor for HSPCs.[43, 44] Given the absence of an irradiation-induced cytokine storm in this strain, the difference in engraftment of Vav1−/− HSPCs appears to be due to an increased sensitivity to inhibitory signals amplified in the irradiation setting or a blunted response to positive regulators unique to the post-irradiation HM in the BM.
In an unbiased screen for cytokines markedly increased in the BM after irradiation, we noted the induction of IL-11, a cytokine our laboratory originally cloned from a primate bone marrow cell line.[35] IL-11 is a member of the gp130 family of cytokines, which also includes IL-6, LIF and OSM, all factors with well-characterized or emerging roles in blood cells.[45] IL-11 has previously been shown to affect either directly or indirectly HSC and progenitor functions.[46–49] Recombinant human IL-11 (Neumega®) was developed as a treatment for chemotherapy-induced thrombocytopenia [50] but has significant pro-inflammatory side effects. We validated the upregulation of IL-11 after irradiation and demonstrated that treatment of HSPCs with IL-11 (with or without SCF) directly activates Vav1 in vitro and the absence of Vav1 leads to defective Rac activation upon stimulation with IL-11. The defective IL-11 signaling in the absence of Vav1 also includes abnormal baseline and attenuated agonist-induced activation of STAT3 and JNK, a molecular signature previously implicated with reduced engraftment of HSPCs.[7, 41, 42] Furthermore, we demonstrate that irradiation-induced upregulation of IL-11 in vivo specifically affects the engraftment potential of Vav1−/− HSPCs, without altering the reconstitution potential of WT HSPCs. Depletion of IL-11 after irradiation partially rescued the engraftment defect of Vav1−/− HSPCs. The partial rescue of the engraftment phenotype could be due to the fact that the blocking antibody might not be completely efficient in reducing IL-11 to levels compatible with normal engraftment of Vav1−/− HSPCs. Moreover, the fact that even mice displaying donor-derived chimerism have a short-term engraftment in our experiments suggest that IL-11 may have a specific detrimental effect on Vav1−/− progenitors rather than true long-term repopulating stem cells.
Conclusion
The detrimental effect of IL-11 on Vav1−/− HSPC engraftment is particularly interesting for at least two reasons. First, it clearly defines a central role for the Vav-Rac axis in overcoming the cytokine milieu produced during irradiation to allow HSCs to engraft. In this respect, our data underscore the need for further investigating the role of the Vav-Rac pathway in response to cytokines produced following alternative preparative regimens (e.g., chemotherapy) and disease states (e.g., infections, injury). Second, we describe here that increased level of IL-11 in the absence of appropriate signal transduction downstream of the gp130 receptor can have a negative impact on HSPCs. It is well known that depending on the specific in vivo context, the same cytokine can elicit different outputs in the same cell type, based on the repertoire of different signaling pathways it activates. This phenomenon (the so-called “signal orchestration model”) has been extensively documented for IL-6,[51] another cytokine of the gp130 family known to play both pro- and anti-inflammatory effects depending on the in vivo environmental circumstances. It is possible that in absence of Vav1, IL-11 induces compensatory pathways in HSPCs that ultimately lead to their exhaustion and lack of engraftment. The abnormally high baseline activation of STAT3 and JNK (both known to regulate proliferation and quiescence of hematopoietic stem cells) observed in Vav1−/− HSPCs might be important in this respect. An alternative explanation to our data is that given the lack of appropriate signal transduction downstream of the gp130 receptor in Vav1-deficient cells, IL-11 levels might be too high in the HM and reach a critical threshold that is toxic to HSPCs. Further studies are ongoing to test these two hypotheses and to clarify this point. In summary, our data confirm a central role of the Vav1-Rac axis in integrating a wide range of extracellular stimuli and regulating multiple, independent and agonist-specific functions.[10, 52]
Finally, using a novel cellular barcoding system Sun et al. have suggested that large numbers of progenitors rather than HSCs functionally defined by transplantation assays sustain native hematopoiesis.[26] Our finding that HSC migration during fetal development and adult steady state hematopoiesis are not affected by the lack of Vav1, but Vav1−/− HSC display a severe phenotype during engraftment into an irradiated microenvironment could be interpreted based on this new view of hematopoiesis. Indeed, the observation that the role of Vav1 may be different in ‘transplanted’ HSCs vs primitive cells maintaining steady-state hematopoiesis could be explained by having specific biochemical pathways that are required for functions of “traditional stem cells” involved in engraftment in a conditioned recipient that are distinct from those utilized in homeostatic conditions, including during ontogeny-associated migration of HSCs from one tissue to another. In this view, the vulnerability of Vav1-deficient HSCs during transplantation, which is not evident during steady-state hematopoiesis can be explained by a reliance of HSPCs on ‘alternatively utilized’ biochemical pathways that are perturbed by deletion of this crucial protein. At the moment, this is a provocative but speculative interpretation of our findings. Subsequent studies will focus on a more detailed genetic analysis of pathways that might be activated or downregulated upon deletion of Vav1 during transplantation.
In conclusion, our study suggests that identifying specific responses of HSPCs to individual cytokines is crucial to deepening our understanding of the BM engraftment process and will ultimately lead to a clearer knowledge of pathways that could be exploited as therapeutic targets to improve the efficiency of this process.
Supplementary Material
Significance Statement.
De Vita et al. dissect the mechanism underlying the lack of engraftment of Vav1−/− hematopoietic stem and progenitor cells and describe a novel, unexpected role for the Vav1-Rac pathway in mitigating the proinflammatory cytokine IL-11 induced by irradiation.
Acknowledgments
The authors acknowledge the helpful discussions and comments from members of the Williams laboratory and wish to thank the Flow Lab HSCI Core at Boston Children’s Hospital, particularly Ronald Mathieu. We also thank Leslie Silberstein, David Scadden, and Xosé R. Bustelo for helpful discussions. We thank Maria Suarez for administrative assistance. This work was supported by NIH grants 5R01DK062757 (DAW) and 5T32HL007574 (SDV).
Footnotes
Authorship Contributions
Serena De Vita: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing
Yanhua Li: Collection and assembly of data, data analysis and interpretation
Chad E. Harris: Collection and assembly of data
Meaghan K. McGuinness: Collection and assembly of data
Clement Ma: Data analysis and interpretation
David A. Williams: Conception and design, data analysis and interpretation, manuscript writing
All authors approved the final version of the manuscript.
References
- 1.Alshemmari S, Ameen R, Gaziev J. Haploidentical hematopoietic stem-cell transplantation in adults. Bone Marrow Res. 2011;2011:303487. doi: 10.1155/2011/303487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gyurkocza B, Gutman J, Nemecek ER, et al. Treosulfan, fludarabine, and 2-Gy total body irradiation followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome and acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20:549–555. doi: 10.1016/j.bbmt.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Negrin RS. Introduction to the review series on “Advances in hematopoietic cell transplantation”. Blood. 2014;124:307. doi: 10.1182/blood-2014-05-566679. [DOI] [PubMed] [Google Scholar]
- 4.Shizuru JA, Negrin RS, Weissman IL. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annual review of medicine. 2005;56:509–538. doi: 10.1146/annurev.med.54.101601.152334. [DOI] [PubMed] [Google Scholar]
- 5.Yu VW, Scadden DT. Hematopoietic Stem Cell and Its Bone Marrow Niche. Current topics in developmental biology. 2016;118:21–44. doi: 10.1016/bs.ctdb.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu Y, Filippi MD, Cancelas JA, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 7.Cancelas JA, Lee AW, Prabhakar R, et al. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- 8.Nayak RC, Chang KH, Vaitinadin NS, et al. Rho GTPases control specific cytoskeleton-dependent functions of hematopoietic stem cells. Immunol Rev. 2013;256:255–268. doi: 10.1111/imr.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 10.Troeger A, Williams DA. Hematopoietic-specific Rho GTPases Rac2 and RhoH and human blood disorders. Exp Cell Res. 2013;319:2375–2383. doi: 10.1016/j.yexcr.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 12.Vigil D, Cherfils J, Rossman KL, et al. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10:842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Mata R, Boulter E, Burridge K. The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12:493–504. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- 15.Boulter E, Garcia-Mata R, Guilluy C, et al. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nature cell biology. 2010;12:477–483. doi: 10.1038/ncb2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol. 2011;21:718–726. doi: 10.1016/j.tcb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aspenstrom P. Effectors for the Rho GTPases. Curr Opin Cell Biol. 1999;11:95–102. doi: 10.1016/s0955-0674(99)80011-8. [DOI] [PubMed] [Google Scholar]
- 18.Cancelas JA. On how Rac controls hematopoietic stem cell activity. Transfusion. 2011;51(Suppl 4):153S–159S. doi: 10.1111/j.1537-2995.2011.03378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Movilla N, Dosil M, Zheng Y, et al. How Vav proteins discriminate the GTPases Rac1 and RhoA from Cdc42. Oncogene. 2001;20:8057–8065. doi: 10.1038/sj.onc.1205000. [DOI] [PubMed] [Google Scholar]
- 20.Rapley J, Tybulewicz VL, Rittinger K. Crucial structural role for the PH and C1 domains of the Vav1 exchange factor. EMBO reports. 2008;9:655–661. doi: 10.1038/embor.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katzav S. Vav1: A Dr. Jekyll and Mr. Hyde protein--good for the hematopoietic system, bad for cancer. Oncotarget. 2015;6:28731–28742. doi: 10.18632/oncotarget.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Aguilera A, Lee YJ, Lo Celso C, et al. Guanine nucleotide exchange factor Vav1 regulates perivascular homing and bone marrow retention of hematopoietic stem and progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9607–9612. doi: 10.1073/pnas.1102018108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou YR, Kottmann AH, Kuroda M, et al. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development [see comments] Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 24.Ara T, Tokoyoda K, Sugiyama T, et al. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19:257–267. doi: 10.1016/s1074-7613(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 25.Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Ramos A, Chapman B, et al. Clonal dynamics of native haematopoiesis. Nature. 2014;514:322–327. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busch K, Klapproth K, Barile M, et al. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015;518:542–546. doi: 10.1038/nature14242. [DOI] [PubMed] [Google Scholar]
- 28.Turner M, Mee PJ, Walters AE, et al. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity. 1997;7:451–460. doi: 10.1016/s1074-7613(00)80367-2. [DOI] [PubMed] [Google Scholar]
- 29.Waskow C, Madan V, Bartels S, et al. Hematopoietic stem cell transplantation without irradiation. Nature methods. 2009;6:267–269. doi: 10.1038/nmeth.1309. [DOI] [PubMed] [Google Scholar]
- 30.Milsom MD, Lee AW, Zheng Y, et al. Fanca−/− hematopoietic stem cells demonstrate a mobilization defect which can be overcome by administration of the Rac inhibitor NSC23766. Haematologica. 2009;94:1011–1015. doi: 10.3324/haematol.2008.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JY, Kim M, Ham A, et al. IL-11 is required for A1 adenosine receptor-mediated protection against ischemic AKI. J Am Soc Nephrol. 2013;24:1558–1570. doi: 10.1681/ASN.2013010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ploemacher RE, van der Sluijs JP, Voerman JS, et al. An in vitro limiting-dilution assay of long-term repopulating hematopoietic stem cells in the mouse. Blood. 1989;74:2755–2763. [PubMed] [Google Scholar]
- 33.Nadri S, Soleimani M, Hosseni RH, et al. An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int J Dev Biol. 2007;51:723–729. doi: 10.1387/ijdb.072352ns. [DOI] [PubMed] [Google Scholar]
- 34.Team RDC. R: A language and environment for statistical computing. Vienna, Austria: 2015. [Google Scholar]
- 35.Paul SR, Bennett F, Calvetti JA, et al. Molecular cloning of a cDNA encoding interleukin 11, a novel stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci USA. 1990;87:7512–7516. doi: 10.1073/pnas.87.19.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schibler KR, Yang Y-C, Christensen RD. Effect of interleukin-11 on cycling status and clonogenic maturation of fetal and adult hematopoietic protenitors. Blood. 1992;80:900–903. [PubMed] [Google Scholar]
- 37.Keller DC, Du XX, Srour EF, et al. Interleukin-11 inhibits adipogenesis and stimulates myelopoiesis in human long-term marrow cultures. Blood. 1993;82:1428–1435. [PubMed] [Google Scholar]
- 38.Du XX, Keller DC, Maze R, et al. Comparative effects of in vivo treatment using interleukin-11 and stem cell factor on reconstitution in mice after bone marrow transplantation. Blood. 1993;82:1016–1022. [PubMed] [Google Scholar]
- 39.Lemoli RM, Fogli M, Fortuna A, et al. Interleukin-11 stimulates the proliferation of human hematopoietic CD34+ and CD34+CD33-DR- cells and synergizes with stem cell factor, interleukin-3, and granulocyte-macrophage colony-stimulating factor. J Exp Hematol. 1993;21:1668–1672. [PubMed] [Google Scholar]
- 40.Du XX, Williams DA. Interleukin-11: review of molecular, cell biology and clinical use. Blood. 1997;89:3897–3908. [PubMed] [Google Scholar]
- 41.Geest CR, Coffer PJ. MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol. 2009;86:237–250. doi: 10.1189/jlb.0209097. [DOI] [PubMed] [Google Scholar]
- 42.Mantel C, Messina-Graham S, Moh A, et al. Mouse hematopoietic cell-targeted STAT3 deletion: stem/progenitor cell defects, mitochondrial dysfunction, ROS overproduction, and a rapid aging-like phenotype. Blood. 2012;120:2589–2599. doi: 10.1182/blood-2012-01-404004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zsebo KM, Williams DA, Geissler EN, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 44.Nocka K, Tan JC, Chiu E, et al. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. The EMBO journal. 1990;9:1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annual review of immunology. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 46.Holyoake TL, Freshney MG, McNair L, et al. Ex vivo expansion with stem cell factor and interleukin-11 augments both short-term recovery posttransplant and the ability to serially transplant marrow. Blood. 1996;87:4589–4595. [PubMed] [Google Scholar]
- 47.Miller CL, Eaves CJ. Expansion in vitro of adult murine hematopoietic stem cells with transplantable lympho-myeloid reconstituting ability. Proc Natl Acad Sci. 1997;92:13648–13653. doi: 10.1073/pnas.94.25.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Audet J, Miller CL, Rose-John S, et al. Distinct role of gp130 activation in promoting self-renewal divisions by mitogenically stimulated murine hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1757–1762. doi: 10.1073/pnas.98.4.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakayama A, Matsui H, Fukushima T, et al. Murine serum obtained from bone marrow-transplanted mice promotes the proliferation of hematopoietic stem cells by co-culture with MS-5 murine stromal cells. Growth Factors. 2006;24:55–65. doi: 10.1080/08977190500361762. [DOI] [PubMed] [Google Scholar]
- 50.Kaye JA. The clinical development of recombinant human interleukin 11 (NEUMEGA rhIL-11 growth factor) Stem cells (Dayton, Ohio) 1996;14(Suppl 1):256–260. doi: 10.1002/stem.5530140733. [DOI] [PubMed] [Google Scholar]
- 51.Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 52.Reddy PN, Radu M, Xu K, et al. p21-activated kinase 2 regulates HSPC cytoskeleton, migration, and homing via CDC42 activation and interaction with beta-Pix. Blood. 2016;127:1967–1975. doi: 10.1182/blood-2016-01-693572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


