Abstract
In the retina, Müller glia have the potential to become progenitor cells with the ability to proliferate and regenerate neurons. However, the ability of Müller glia-derived progenitor cells (MGPCs) to proliferate and produce neurons is limited in higher vertebrates. Using the chick model system, we investigate how retinoic acid (RA) -signaling influences the proliferation and the formation of MGPCs. We observed an up-regulation of cellular retinoic acid binding proteins (CRABP) in the Müller glia of damaged retinas where the formation of MGPCs is known to occur. Activation of RA-signaling was stimulated, whereas inhibition suppressed the proliferation of MGPCs in damaged retinas and in FGF2-treated undamaged retinas. Furthermore, inhibition of RA-degradation stimulated the proliferation of MGPCs. Levels of Pax6, Klf4, and cFos were up-regulated in MGPCs by RA agonists and down-regulated in MGPCs by RA antagonists. Activation of RA-signaling following MGPC proliferation increased the percentage of progeny that differentiated as neurons. Similarly, the combination of RA and IGF1 significantly increased neurogenesis from retinal progenitors in the circumferential marginal zone (CMZ). In summary, RA-signaling stimulates the formation of proliferating MGPCs and enhances the neurogenic potential of MGPCs and stem cells in the CMZ.
Graphical Abstract
Introduction
The capacity for retinal regeneration varies substantially across vertebrate species. In response to injury, the teleost fish is able to regenerate a functional retina, whereas birds and mammals are unable to mount a significant regenerative response (Lenkowski and Raymond 2014). Despite the wide divergence in the regenerative capacity between species, Müller glia are the cellular source for regenerated neurons in the fish, chick, and mouse retina (Fausett and Goldman 2006; Fischer and Reh 2001; Ueki et al. 2015). In uninjured retina, Müller glia perform a wide variety of support functions to retinal neurons (Reichenbach and Bringmann 2013). However, even in healthy retinas, Müller glia express genes commonly associated with progenitor cells (Blackshaw et al. 2004; Roesch et al. 2008; Ueno et al. 2017). This unique genomic profile may underlie the ability of Müller glia to reprogram into proliferating progenitors. Understanding the signaling pathways responsible for regulating the regenerative potential of MGPCs is important for developing novel therapies to treat sight-threatening diseases of the retina.
A large network of cell-signaling pathways is known to regulate the reprogramming of Müller glia into MGPCs (reviewed by (Gallina et al. 2014a; Goldman 2014; Hamon et al. 2016; Lenkowski and Raymond 2014). MAPK, Jak/Stat, Wnt/β-catenin, PI3K/Akt/mTOR, Hedgehog, BMP/TGFβ/Smad and Notch signaling have been shown to be involved in the reprogramming of Müller glia in both the fish and the chick retina (Conner et al. 2014; Fischer et al. 2009a; Fischer et al. 2009b; Gallina et al. 2015; Ghai et al. 2010; Kassen et al. 2009; Meyers et al. 2012; Nelson et al. 2012; Todd and Fischer 2015; Todd et al. 2017; Todd et al. 2016; Wan et al. 2014; Zelinka et al. 2016; Zhao et al. 2014). Relatively little is known about the signaling pathways that drive the reprogramming of Müller glia in the mammalian retina. MAPK- and Wnt-signaling can stimulate, to a small extent, the proliferation of Müller glia in damaged rodent retinas (Karl et al. 2008; Liu et al. 2012). Virus-mediated gene transfer of β-catenin- to Müller glia has been reported to stimulate the formation of MGPCs in undamaged rodent retinas (Yao et al. 2016). Additionally, forced expression of the proneural bHLH transcription factor Ascl1 has been shown to stimulate neuronal regeneration from Müller glia in the rodent retina (Jorstad et al. 2017; Pollak et al. 2013; Ueki et al. 2015). Ascl1 is required for regeneration of the fish retina (Fausett et al. 2008) and is known to be up-regulated in MGPCs in the retinas of both fish and chicks (Fausett et al. 2008; Fischer and Reh 2001; Hayes et al. 2007).
The retinoic acid (RA)-signaling may play important roles in retinal regeneration since this cell-signaling pathways is known to be essential in neuronal differentiation and patterning during development (Maden 2007). RA-signaling promotes neural differentiation in the developing zebrafish, chick, and rodent retina (Hyatt et al. 1996; Kelley et al. 1994; Kelley et al. 1999; Stenkamp et al. 1993; Valdivia et al. 2016). During embryonic development in the chick retina, interference of RA-signaling through forced expression of a dominant-negative RA receptor resulted in reduced proliferation of progenitors and disruption of dorsal-ventral patterning (Sen et al. 2005). In the context of retinal regeneration in rodents, exogenous RA has been shown to promote the differentiation of bipolar neurons from MGPCs (Ooto et al. 2004), whereas other studies failed to replicate these results (Karl et al. 2008). The purpose of this study was to investigate how RA-signaling impacts the reprogramming of Müller glia into proliferating neurogenic MGPCs in the chick retina in vivo.
Methods and Materials
Animals
The use of animals was according to the guidelines established by the National Institutes of Health and the Ohio State University. Newly hatched chickens (Gallus gallus domesticus; white leghorn strain) were obtained from Meyer Hatchery (Polk, Ohio). Chicks were housed in a stainless steel brooder at 25°C, received water and Purinatm chick starter ad libitum, and kept on 12:12 hour light:dark cycle (lights on at 8 am).
Intraocular injections
Chickens were anesthetized and intraocular injections performed as described previously (Fischer et al., 1999). In short, anesthesia was achieved via inhalation of 2.5% isoflurane in oxygen (Fischer et al., 1999). The right eyes were injected with the “test” compound and the contra-lateral left eyes were injected with vehicle (control). Compounds were injected in 20 μl sterile saline with 0.05 mg/ml bovine serum albumin added as a carrier. Compounds used in these studies included N-methyl-D-aspartate (NMDA; 38.5 or 154 μg/dose), FGF2 (250 ng/dose; R&D systems), liarozole dihydrochloride (4μg/dose; 5-[(3-Chlorophenyl)-1H-imidazol-1-ylmethyl]-1H-benzimidazole dihydrochloride; Tocris), TTNBP (4μg/dose; 4-[(E)-2-(5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid), Retinoic Acid (RA; 5μg/dose; Sigma-Aldrich), BMS493 (4-[(1E)-2-[5,6-Dihydro-5,5-dimethyl-8-(2-phenylethynyl)-2-naphthalenyl]ethenyl]benzoic acid: 2μg/dose; Tocris), IGF1 (400ng/dose; R&D Systems). A two μg dose of EdU (5-ethynyl-2′-deoxyuridine) was injected to label proliferating cells. Injection paradigms are included in each figure.
Fixation, sectioning and immunocytochemistry
Tissues were fixed, sectioned and immunolabeled as described previously (Fischer et al. 2008b; Fischer et al. 2009b). The working dilutions and sources of antibodies are listed in table 1. None of the fluorescence resulted from auto-fluorescence or non-specific binding of secondary antibodies; sections labeled with secondary antibodies alone contain no significant fluorescence. Secondary antibodies included donkey-anti-goat-Alexa488/568, goat-anti-rabbit-Alexa488/568/647, goat-anti-mouse-Alexa488/568/647, goat anti-rat-Alexa488 (Life Technologies) diluted to 1:1000 in PBS plus 0.2% Triton X-100.
Table 1.
Antibodies, sources and working dilutions. Patterns of labeling and stimulus-dependent changes in levels of immunolabeling using these antibodies are consistent with previous reports (Fischer and Omar, 2005; Fischer et al., 2009a, 2009b; Fischer et al., 2014; Todd and Fischer, 2015).
| Antigen | Working dilution | Host | Clone or catalog number | Source |
|---|---|---|---|---|
| Sox2 | 1:1000 | goat | Y-17 | Santa Cruz Immunochemicals |
| Sox9 | 1:2000 | mouse | AB5535 | Chemicon |
| Pax6 | 1:1000 | rabbit | PRB-278P | Covance |
| Klf4 | 1:50 | rabbit | ARP38430 | Aviva Systems Biology |
| cFos | 1:400 | rabbit | K-25 | Santa Cruz Immunochemicals |
| Glutamine Synthetase | 1:2000 | mouse | ab125724 | Abcam |
| CD45 | 1:200 | Mouse | HIS-C7 | Cedi Diagnostic |
| Nkx2.2 | 1:80 | Mouse | 74.5A5 | DSHB |
| CRABP | 1:1000 | Mouse | C1 | Dr. J Saari, University of Washington |
| Lim 1/2 | 1:50 | Mouse | 4F2 | DSHB |
| Lim 3 | 1:100 | Mouse | 67.4E12 | DSHB |
| Otx2 | 1:1000 | Goat | AF1979 | R&D Systems |
| HuD/HuC | 1:600 | Mouse | A21271 | Invitrogen |
Labeling for EdU
Following immunolabeling procedures, sections were fixed in 4% formaldehyde in PBS for 5 minutes at room temperature, and washed twice for 5 minutes in PBS. Sections were incubated for 30 minutes at room temperature in 2M Tris, 50 mM CuSO4, Alexa Fluor 568 Azide (Thermo Fisher Scientific), and 0.5M ascorbic acid in dH2O. Finally, sections were washed in PBS for 5 minutes and coverglass mounted 80% glyercol in water, as described previously (Todd, et al. 2017).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
Dying cells were identified by labeling for fragmented DNA by using the TUNEL method. We used the In Situ Cell Death Kit (TMR red; Roche Applied Science), according to the manufacturer’s instructions.
Photography, measurements, cell counts and statistics
Wide-field photomicrographs were obtained using a Leica DM5000B microscope and Leica DC500 digital camera. Confocal photomicrographs were obtained using a Leica SP8 imaging system at the Hunt-Curtis Imaging Facility in the Department of Neuroscience at The Ohio State University. Images were adjusted and figures constructed by using Adobe Photoshop.
Cell counts were performed on images that were sampled from different regions of the retina. To avoid region-specific differences within the retina, cell counts were consistently made from 5,400 μm2 from central or peripheral regions of retina for each data set. Central retina was defined as the region within a 3mm radius of the posterior pole of the eye, and peripheral retina was defined as an annular region between 3mm and 0.5mm from the far peripheral edge of the retina. The cell-type identity of EdU-labeled cells was determined based on findings that 100% of the proliferating cells in the chick retina are comprised of Sox2/9+ Müller glia in the INL/ONL, Nkx2.2+ Non-astrocytic Inner Retinal Glial (NIRG) cells in the IPL, GCL and NFL, and CD45+ microglia (Fischer et al. 2010; Zelinka et al. 2012). Sox2/9+ nuclei in the INL/ONL were identified as Müller glia based on their relatively large size and fusiform shape.
Similar to previous reports (Fischer et al. 2009a; Fischer et al. 2009b; Fischer et al. 2010; Ghai et al. 2009), immunofluorescence was quantified by using ImagePro6.2 (Media Cybernetics, Bethesda, MD, USA). Images to be using for quantification were obtained using identical illumination, microscope, and camera settings. Retinal areas were randomly sampled over the INL and ONL. Measurement for content in the nuclei of Müller glia/MGPCs were made by selecting the total area of pixels with values ≥70 (0 = black and 255 = saturated) for Sox2 or Sox9 (in the red channel), and copying Klf4 or Pax6 (in the green channel). These copied data were pasted into a separate file for quantification or onto 70% grayscale background to produce figures. The density sum was calculated as the sum of values for all pixels within thresholded regions. These calculations were determined for at least 5 different retinas for each experimental condition.
GraphPad Prism 6 was used for statistical analyses. A two-tailed, paired t-test was used to determine significance of difference between two treatment groups accounting for inter-individual variability (means of treated-control values). A two-tailed, unpaired t-test was used to determine significance of difference between two treatment groups.
Results
Up-regulation of CRABP in Müller glia after NMDA damage
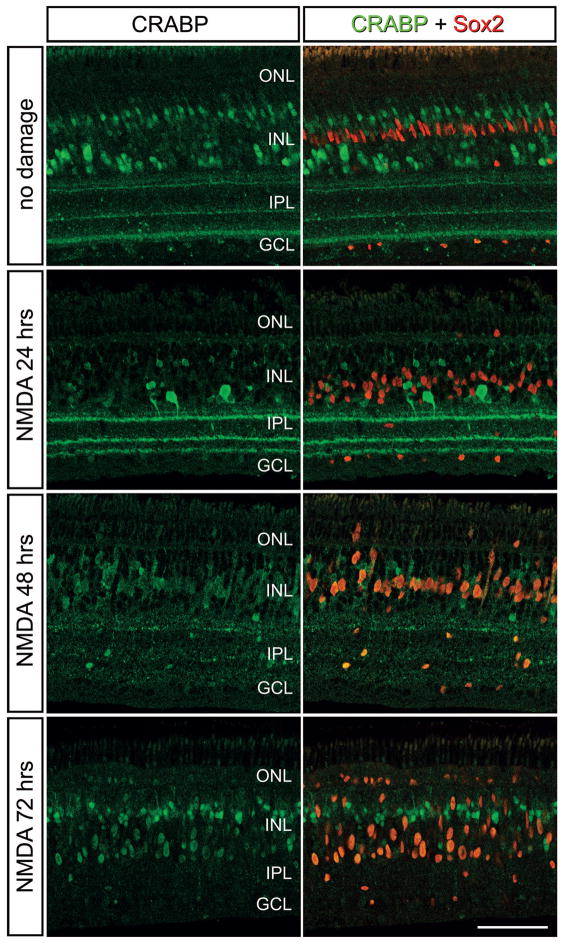
RA binds to cellular RA-binding proteins (CRABP1/2) which allows RA to shuttle through the cytoplasm and enter the nucleus (Cvekl and Wang 2009). In the nucleus, RA dissociates from CRABP and interacts with receptors that can bind to RA-target genes and activate transcription (Cvekl and Wang 2009). Accordingly, we probed for the expression of CRABP in NMDA-damaged retinas where proliferating MGPCs are known to form (Fischer and Reh 2001). Consistent with previous reports (Fischer et al. 1999), immunoreactivity for CRABP was detected in presumptive bipolar and amacrine cells (Fig. 1). Many of these cells were destroyed or down-regulated CRABP by 1 day after NMDA-treatment (Fig. 1). At 2 days after NMDA-treatment, when MGPCs are known to re-enter the cell cycle (Fischer and Reh 2001), there was an increase in the levels of CRABP in Müller glia/MGPCs cytoplasm and nuclei (Fig. 1). By 3 days after NMDA-treatment, CRABP was detected in the nuclei of Sox2+ MGPCs (Fig. 1). Collectively, these findings suggest that Müller glia and/or MGPCs respond to retinal damage by up-regulating RA-signaling.
Figure 1.
Patterns of expression of CRABP in damaged retinas. Retinas were obtained from eyes that were injected with saline at P7 or 1 μmol of NMDA, and tissues harvested at 1, 2, and 3 days later. Sections of the retina were labeled with antibodies to CRABP (green) and Sox2 (red). The calibration bar (50 μm) in the bottom right panel applies to all panels. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, ONL – outer nuclear layer.
RA-signaling stimulates MGPC proliferation after retinal damage
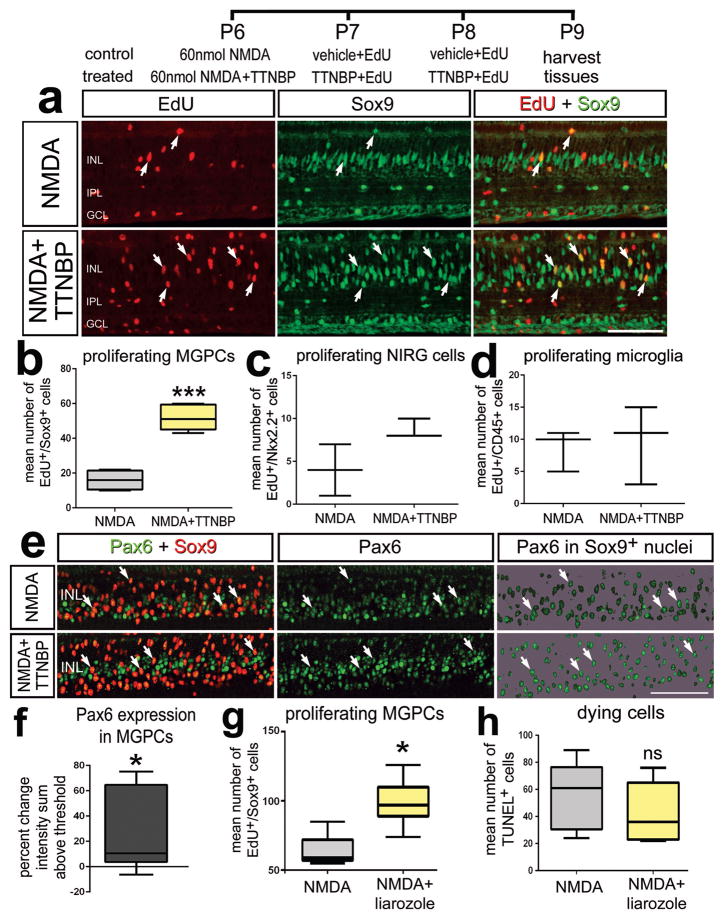
RA has been reported to regulate the proliferation of neural progenitor cells in different regions of the developing CNS (Hyatt et al. 1992; Marsh-Armstrong et al. 1994; Sen et al. 2005). Accordingly, we tested whether application of TTNPB, a potent analog of RA (Astrom et al. 1990), influenced the proliferation of MGPCs following low-levels of NMDA-induced damage where relatively few MGPCs are known to form (Fischer et al. 2004). Treatment of damaged retinas with TTNPB resulted in a significant increase in the numbers of proliferating Müller glia/MGPCs (Figs. 2a–b). By comparison, TTNPB did not affect the proliferation of Non-astrocytic Inner Retinal Glial (NIRG) cells and microglia (Figs. 2c,d). NIRG cells are a distinct type of glial cell that has been described in the retinas of birds (Fischer et al. 2010; Rompani and Cepko 2010) and possibly snakes and turtles (Todd et al. 2015a). Treatment with TTNPB also increased levels of Pax6-expression in Müller glia/MGPCs (Figs. 2e,f). Pax6 has been shown to be required for Müller glia-mediated regeneration in the fish retina (Thummel et al. 2010). By comparison, levels of the stem cell factor Klf4 were unaffected by treatment with TTNPB (not shown). To further examine whether RA-signaling is involved in the formation of MGPCs we examined whether proliferation was influenced by inhibition of RA-degradation. We applied the compound liarozole-dihydrochloride, an inhibitor of cytochrome P450 (Van Wauwe et al. 1992); cytochrome P450 metabolizes RA making it inactive, therefore, the inhibition of cytochrome P450 leads to elevated levels of RA (Thatcher and Isoherranen 2009). We found that intraocular injections of liarozole significantly increased the number of proliferating Müller glia/MGPCs in damaged retinas (Fig. 2g).
Figure 2.
Activation of RA-signaling stimulates the proliferation of MGPCs in damaged retinas. Eyes were injected with a relatively low dose (60 nmol) of NMDA (control) or NMDA + RAR agonist (TTNBP; treated) at P6, vehicle + EdU (control) or TTNBP + EdU (treated) at P7 and P8, and tissue harvested at P9. Sections of the retina were labeled for EdU-incorporation (red) and antibodies to Sox9 (green; a), or Pax6 (green) and Sox9 (red; c). Arrows indicate the nuclei of Müller glia/MGPCs. The box plots in illustrate the mean, upper extreme, lower extreme, upper quartile and lower quartile (n≥6 animals). Significance of difference (***p<0.0001) was determined by using a t-test (b,c,d,g,h) or (*p<0.05) was determined by using a Mann-Whitney U test (f). Arrows indicate the nuclei of MGPCs. The calibration bar panels a and e represents 50 μm. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
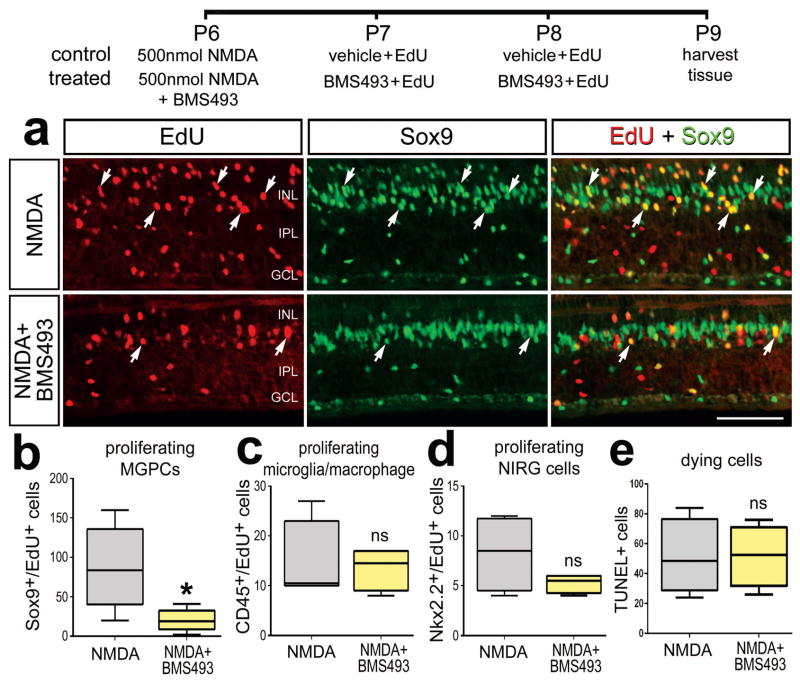
Since activation of RA-signaling enhanced the formation of proliferating MGPCs, we tested whether inhibition of RA-signaling suppressed the formation of MGPCs in damaged retinas. We tested whether the small-molecule Retinoic Acid Receptor (RAR) antagonist BMS493 (Germain et al. 2009) influenced the formation of proliferating MGPCs in retinas damaged by a relatively high dose of NMDA. We found that inhibition of RAR significantly decreased numbers of EdU-labeled MGPCs (Figs. 3a,b). This effect was specific to Müller glia/MGPCs as inhibition of RAR had no effect on the proliferation of NIRG cells or microglia/macrophages (Figs. 3c,d). Collectively, these findings suggest that RA-signaling is mitogenic to Müller glia/MGPCs in damaged retinas.
Figure 3.
Inhibition of RA-signaling suppresses the proliferation of MGPCs in damaged retinas. Eyes were injected with a relatively high dose (500 nmol) of NMDA alone (control) or NMDA+RAR antagonist (BMS493) at P7, vehicle+EdU or RAR antagonist + EdU at P8 and P9, and tissues harvested at P10. Sections of the retina were labeled for EdU-incorporation and antibodies to Sox9 (green; a). The box plots illustrate the mean, upper extreme, lower extreme, upper quartile and lower quartile (n=7 animals). Significance of difference (*p<0.05) was determined by using a t-test. Arrows indicate the nuclei of MGPCs. The calibration bar (50 μm) in panel a applies to a alone. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, ns – not significant.
The formation of proliferating MGPCs is known to be increased with elevated levels of retinal damage (Fischer et al. 2004) and reactive microglia (Fischer et al. 2014b). We found that treatment of damaged retinas with TTNBP, liarozole, or BMS493 had no effect upon numbers of dying TUNEL+ cells (Fig 2h, 3d) or levels of CD45 in microglia (data not shown) which is diagnostic of microglia reactivity (Fischer et al. 2014b; Gallina 2015). Thus, the effects of RA-agonists and –antagonists on the proliferation of MGPCs occurred independent of levels of retinal damage or microglial reactivity.
Activation of RA-signaling in FGF2-treated retinas stimulates MGPC-formation in the absence of retinal damage
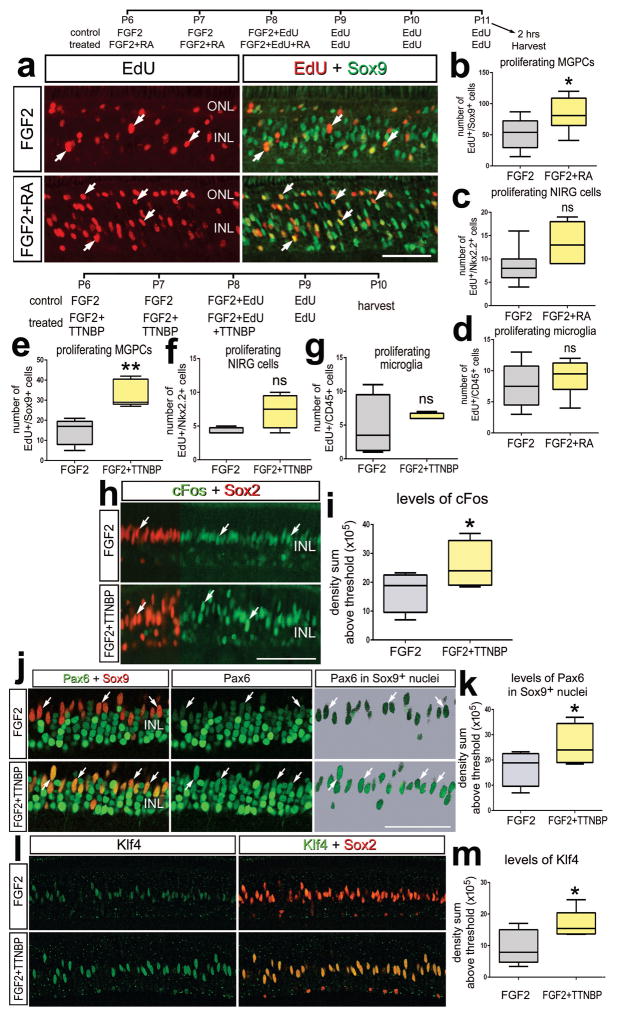
We tested whether activation of RA-signaling in undamaged retinas stimulated the formation of proliferating MGPCs. Four consecutive daily intraocular injections of TTNBP or exogenous RA had no influence on the proliferation of Müller glia in the absence of damage (not shown). By comparison, treatment with four consecutive daily doses of FGF2 is sufficient to result in the formation of numerous proliferating MGPCs in healthy retinas (Fischer et al. 2014b). However, three doses of FGF2 in combination with other mitogens such as Sonic Hedgehog (Todd and Fischer 2015), insulin, IGF1 (Fischer et al. 2002; Fischer and Reh 2000), CNTF (Todd et al. 2016), or in combination with inhibitors of glucocorticoid- (Gallina et al. 2014b) or TGFβ/Smad2-signaling (Todd et al. 2017) have been shown to potentiate the formation of MGPCs. We found that three consecutive daily doses of FGF2 in combination with RA or TTNPB stimulated the formation of proliferating MGPCs (Figs. 4a–c). The potentiating effects of RA and TTNPB were specific to Müller glia as microglia and NIRG cell proliferation was unaffected (Figs. 4c,d,f,g). TTNPB increased levels of cFos-expression in the nuclei of FGF2-treated Müller glia/MGPCs (Figs. 4h,i). RA combined with FGF2 also stimulated cFos expression in Sox2+ Müller glia/MGPCs (not shown). We have previously found that expression levels of cFos are correlated to the proliferation of MGPCs (Fischer et al. 2009b; Todd and Fischer 2015). Consistent with the notion that activation of RA-signaling promotes the reprogramming of Müller glia into MGPCs, we found increased expression of the stem cell-associated transcription factors Pax6 and Klf4 in Müller glia treated with FGF2 and TTNBP compared to levels seen in Müller glia treated with FGF2 alone (Figs.4j–m).
Figure 4.
In the absence of retinal damage, activation of RA-signaling stimulates the formation of proliferating MGPCs in FGF2-treated retinas. (a–d) Eyes were injected with FGF2 alone (control) or FGF2+RA (treated) at P6 and P7, FGF2+EdU or FGF2+EdU+RA at P8, EdU alone at P9, P10 and P11, and tissues harvested 2 hrs after the last injection. (e–m) Eye were injections with FGF2 alone (control) or FGF2+TTNBP at P6 and P7, FGF2+EdU or FGF2+EdU+TTNBP at P8, EdU alone at P9 and tissues harvested at P10. Sections of the retina were labeled for EdU-incorporation and antibodies to Sox9 (green; a), cFos (green) and Sox2 (red; h), Pax6 (green) and Sox9 (red; j), or Klf4 (green) and Sox2 (red; l). Arrows indicate the nuclei of MGPCs. (b–g,I,k,m) The box plots illustrate the mean, upper extreme, lower extreme, upper quartile and lower quartile (n≥6 animals). Significance of difference (*p<0.05) was determined by using a t-test. The calibration bar in panels a,h,j and l represents 50 μm. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, ONL – outer nuclear layer, ns – not significant.
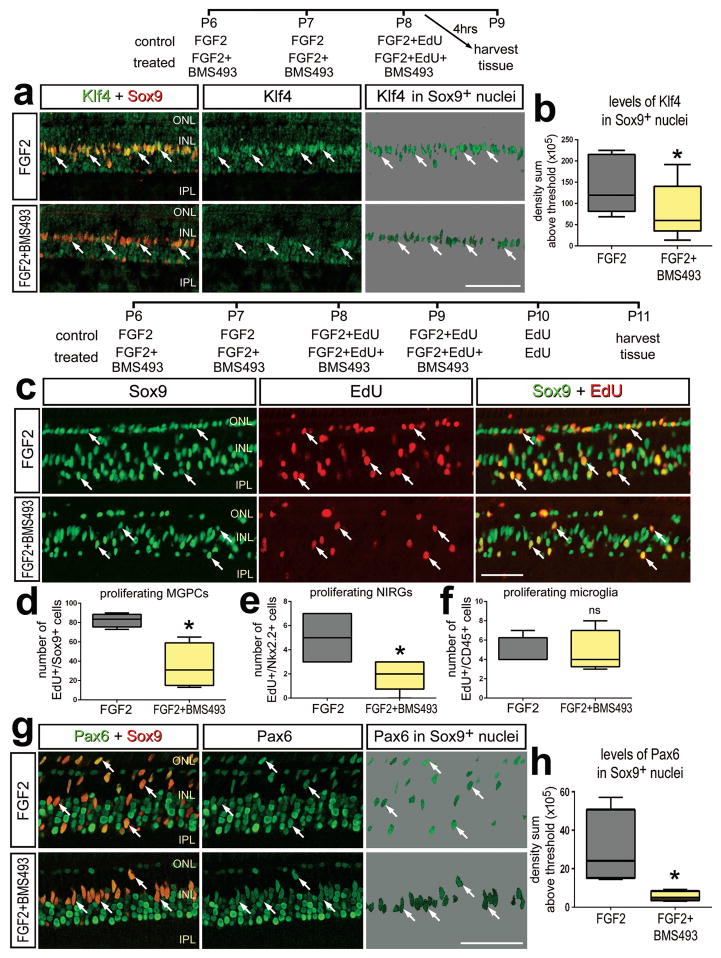
In accordance with findings that RA-agonists stimulate the formation of MGPCs, we found that inhibition of RA-signaling in FGF2-treated retinas suppresses the formation of proliferating MGPCs. When BMS483 was combined with three consecutive daily injections of FGF2, we found a significant decrease in the levels of Klf4 that were expressed by Müller glia compared to levels seen in Müller glia treated with FGF2 alone (Figs. 5a,b). These findings suggest that inhibition of RA-signaling in FGF2-treated Müller glia suppresses the acquisition of progenitor phenotype.
Figure 5.
In the absence of retinal damage, inhibition of RA-signaling with BMS493 suppresses the proliferation of MGPCs in FGF2-treated retinas. a,b: Eyes were injected with FGF2 alone (control) or FGF2+BMS493 (treated) at P6, P7 and P8, and tissues harvested at 4 hrs after the last injection. c–h: Eye were injected with FGF2 alone (control) or FGF2+BMS493 (treated) at P6 and P7, FGF2+EdU or FGF2+BMS439+EdU at P8 and P9, EdU alone at P10, and tissues harvested at P11. Sections of the retina were labeled for Sox9 (red) and Klf4 (green;a); EdU-incorporation (red) and Sox9 (green; c), or Pax6 (green) and Sox9 (red; g). Arrows indicate the nuclei of MGPCs (b, d–f, h). The box plots illustrate the mean, upper extreme, lower extreme, upper quartile and lower quartile (n=7 animals). Significance of difference (*p<0.05) was determined by using a t-test. The calibration bar in panels a, c and g represent 50 μm. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, ONL – outer nuclear layer.
When BMS493 was combined with four consecutive daily injections of FGF2, we found a significant decrease in the number of EdU-labeled Müller glia/MGPCs (Figs. 5c,d). In addition, we found that BMS493 inhibited the proliferation of NIRG cells, whereas the proliferation of microglia was not affected (Figs. 5c,d). Inhibition of RA-signaling in FGF2-treated retinas resulted in a significant decrease in levels of Pax6 in the nuclei of Müller glia/MGPCs (Figs. 5e,f), consistent with the notion that RA-signaling promotes the reprogramming of Müller glia into MGPCs.
RA-treatment increases neuronal differentiation from MGPC-progeny
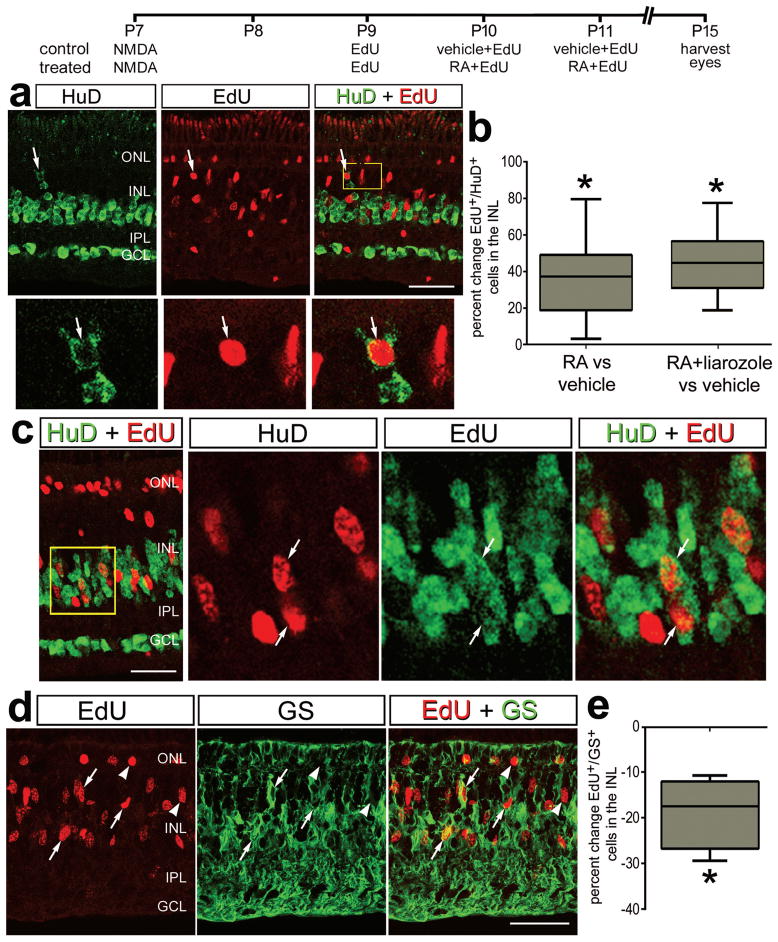
RA-signaling is known to promote neuronal and glial differentiation during neural development (reviewed by (Janesick et al. 2015)). Thus, we tested whether application of RA following the proliferation of MGPCs influence the differentiation of the progeny. We applied RA at 3 and 4 days after NMDA-treatment, starting 24 hours after an application of EdU to label proliferating MGPCs which are known re-enter the cell cycle at 48 hours after NMDA-treatment (Fischer and Reh 2001). We found that RA-treatment increased the percentage of MGPC-progeny that differentiated into HuC/D-expressing neurons by nearly 40% (Figs. 6a,b). In addition, RA-treatment resulted in a decrease in the percentage of MGPC-progeny that differentiated into GS-expressing Müller glia by nearly 20% (Figs. 6c,d). Since exogenous RA may be degraded by endogenous CYP26, we tested whether RA combined with liarozole influenced neurogenesis from MGPCs. No further increase in neurogenesis resulted from the combination of liarozole and RA compared to RA alone (Fig. 6b). This suggests that the effects of exogenous RA were not diminished by degradation. We failed to find evidence of differentiation of MGPC progeny into Lim1/2+ horizontal cells or Lim3+ bipolar cells/immature photoreceptors (data not shown). In the chick retina, Lim1/2 is expressed by GABAergic horizontal cells (Fischer et al. 2007), and Lim3 is expressed by a subset of mature bipolar cells and is transiently expressed by immature photoreceptors (Fischer et al. 2008a). Since these newly generated Edu+ cells colocalize with HuD, a marker found in amacrine cells and because they are found within the INL we presume that these are regenerated amacrine cells (Fischer and Reh, 2001).
Figure 6.
Activation of RA-signaling in damaged retinas stimulated the neuronal differentiation and suppressed the glial differentiation of MGPC-progeny. Eyes were injected with 500 nmol NMDA at P7, EdU at P9, vehicle+EdU (control) or RA+EdU or RA+liarozole+EdU (treated) at P10 and P11, and tissues harvested at P15.
Sections of the retina were labeled for EdU-incorporation (red) and antibodies to HuC/D (green) or GS (green). Arrows indicate the nuclei of EdU-labeled neurons or glia.
The box plots illustrate the mean, upper extreme, lower extreme, upper quartile and lower quartile (n=6 animals). Significance of difference (*p<0.05) was determined by using a Mann-Whitney U test. Arrows indicate the nuclei of MGPCs. The calibration bar in panels b, c,d represents 50 μm. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, ONL – outer nuclear layer.
Neuronal differentiation is increased by RA-treatment of retinal progenitors in the CMZ
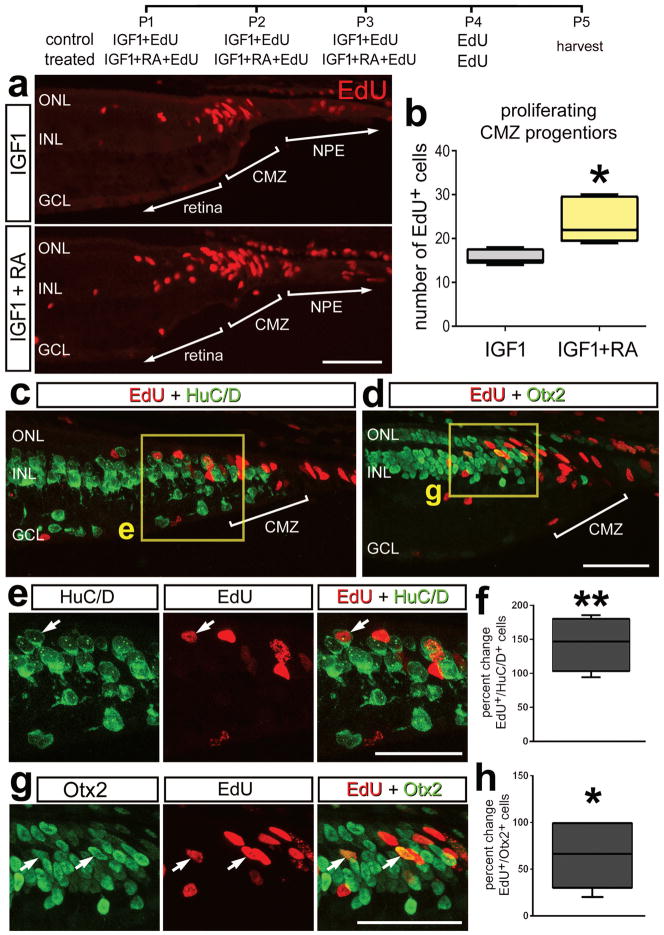
Progenitor cells are known to be organized into a CMZ at the far peripheral edge of the retina in the eyes of different vertebrate species including chicks (Fischer and Reh 2000; Ghai et al. 2008). The progenitors in the CMZ are relatively quiescent and capable of proliferating at increased rates and differentiating as neurons when treated with exogenous growth factors (Fischer et al. 2002). Accordingly, we tested whether RA influenced the proliferation of CMZ progenitors at posthatch day 1 when the CMZ is most active (Fischer and Reh, 2000. We found that 3 consecutive daily intraocular injections of RA alone had no effect upon the proliferation of CMZ progenitors (not shown). We next tested application of RA in combination with IGF1, a factor known to prime proliferation of CMZ progenitors (Todd & Fischer, 2015). The combination of RA with IGF1 significantly increased the proliferation of retinal progenitors in the CMZ compared to IGF1 alone (Figs. 7a,b.). In addition, the combination of RA and IGF1 resulted in a significant increase in the percentage of cells at the retinal margin that differentiated as neurons. We found that the percentage of cells that differentiated as HuC/D+ neurons was increased nearly 150% (Figs. 7c,e,f), and the percentage of cells that differentiated as Otx2+ neurons was increased by nearly 60% (Figs. 7d,g,h).
Figure 7.
Activation of RA-signaling stimulates the neuronal differentiation of progeny of CMZ progenitor cells. Eyes were injected with IGF1+EdU (control) or IGF1+EdU+RA (treated) at P1, P2 and P3, EdU alone at P4, and tissues harvested at P5. Sections of the peripheral retina and CMZ were labeled for EdU-incorporation (red; a,c,d,e,g) and antibodies to HuC/D (green; c and e) or Otx2 (green; d and g). Arrows indicate the nuclei of EdU-labeled neurons. The areas boxed-out in yellow in panels c and d are enlarged 1.5-fold and split into red and green channels in panels e and g. The box plots illustrate the mean, upper extreme, lower extreme, upper quartile and lower quartile (n≥4 animals). Significance of difference (*p<0.05) was determined by using a t-test (b) or (*p<0.05, **p<0.01) by using a Mann Whitney U test. Arrows indicate the nuclei of newly generated cells. The calibration bar (50 μm) in panel a applies to a alone, and the bar in d applies to d, e and g. Abbreviations: INL – inner nuclear layer, GCL – ganglion cell layer, ONL – outer nuclear layer, CMZ – circumferential marginal zone, NPE – non-pigmented epithelium.
Discussion
Our findings implicate RA-signaling as an important player in the complex network of signaling pathways that controls MGPC-formation. Components of the RA-signaling pathway are up-regulated during retinal regeneration in the goldfish (Nagashima et al. 2009) and frog retinas (Duprey-Diaz et al. 2016) in response to optic nerve injury. We found that CRABP is up-regulated in Müller glia in response to retinal damage when MGPCs are forming in the chick retina. This suggests that the involvement of the RA-signaling pathway may be conserved across species. It is likely that RA-signaling is manifested in Müller glia and MGPCs. There are many RA-pathway and RA-target genes expressed by normal Müller glia in the rodent retina (Roesch et al. 2008). Normal Müller glia appear to express very low levels of RARγ, RARα, and RARβ (Roesch et al. 2008) and the levels of expression are increased in response to retinal damage (Roesch et al. 2012). In the frog retina, RALDH and CRABP1 were detected in Müller glia processes (Duprey-Diaz et al. 2016). Müller glia are known to regulate local RA-metabolism with respect to photopigment regeneration. Cone photoreceptors recycle their chromophores via Müller glia. In this pathway, all-trans retinol is transported from cones to Müller glia, retinol is converted into 11-cis retinol by all-trans retinol isomerase and then stored as retinyl esters within Müller glia or transported back to the cones (Wang and Kefalov 2011). Thus, components of the RA-signaling pathway are in place for Müller glia to respond and provide signals.
Glial and neural progenitor cells are known to contribute to RA-signaling in different contexts. Cultured astrocytes express the enzymes for RA biosynthesis and produce active RA and antagonism of RAR prevents glia-induced neuronal differentiation from stem cells (Kornyei et al. 2007). Additionally, during development, radial glia and slowly dividing astrocytes in the postnatal sub-ventricular zone (SVZ) respond to RA-signaling by proliferating at increased rates (Haskell and LaMantia 2005). The cell-signaling and metabolic pathways that are involved in RA-signaling appear to be in place for glia and neural progenitors to respond by proliferating and/or modifying their phenotype. However, further studies are required to better determine the effects of RA on mature glia in the central nervous system.
RA-agonists failed to stimulate the formation of proliferating MGPCs in the absence of damage, suggesting that co-activation of additional signaling pathways or up-regulation of receptors is required to render Müller glia responsive. In support of this notion, RA-agonists stimulated the proliferation of MGPCs in NMDA-damaged retinas or undamaged retinas treated with FGF2. These findings are reminiscent of previous findings wherein activation of Hedgehog-, Smad1/5/8-, mTor or Jak/Stat-signaling in Müller glia is not sufficient to stimulate the formation of proliferating MGPCs in undamaged retinas, whereas activation of these pathways stimulated the proliferation of MGPCs in damaged retinas or undamaged FGF2-treated retinas (Todd and Fischer 2015; Todd et al. 2017; Todd et al. 2016; Zelinka et al. 2016). Interestingly, in undamaged retinas, Müller glia readily activate the second messengers that are part of BMP/Smad, CNTF/Jak/Stat, IGF/PI3K/mTor cell-signaling pathways (Todd et al. 2017; Todd et al. 2016; Zelinka et al. 2016). However, the mitogenic effects of these pathways manifest only when combined with neuronal damage or FGF/MAPK-signaling and in the presence of reactive microglia (Fischer et al. 2014b). Alternatively, FGF/MAPK-signaling and NMDA-mediated damage may lead to changes in pathways, such as the Notch- and β-catenin pathways, that enable the reprogramming of Müller glia into proliferating MGPCs (Gallina et al. 2015; Ghai et al. 2010; Hayes et al. 2007).
Consistent with our findings, RA-signaling has been found to be mitogenic to neural progenitors in different regions of the developing nervous system. In the fish retina, exogenous addition of RA during the optic primordia stage causes proliferation in ventral retina and a duplication of the entire retina (Hyatt et al. 1992), while inhibition of RA-signaling leads to reduced proliferation of progenitors and retinas that lack a ventral region (Marsh-Armstrong et al. 1994). In the embryonic chick retina, inhibition of RA-signaling by a dominant-negative RA receptor resulted in reduced proliferation of retinal progenitors (Sen et al. 2005). Similarly, disulfram, an inhibitor of RA synthesis attenuates the proliferation of SVZ progenitors in vivo (Wang et al. 2005). However, other reports have found that RA has no effect or suppresses the proliferation of neural progenitor cells (Hyatt et al. 1996; Jacobs et al. 2006; Valdivia et al. 2016), possibly due to the pro-differentiation effects of RA (Janesick et al. 2015). Taken together, these findings suggest that the effects of RA-signaling on proliferation and neuronal differentiation are context dependent.
We find that RA-signaling is recruited into the network of pathways that regulate the formation of proliferating MGPCs. This is evident by the findings that inhibition of RAR in FGF2-treated retinas reduced numbers of proliferating MGPCs. In the undamaged avian retina, consecutive daily application of FGF2 is sufficient to stimulate the formation of proliferating MGPCs by activating a network of cell signaling pathways that includes MAPK-, glucocorticoid-, Hedgehog-, Wnt/β-catenin-, Hedgehog, Jak/Stat- and BMP/Smad-signaling (Fischer et al. 2009b; Gallina et al. 2015; Gallina et al. 2014b; Todd and Fischer 2015; Todd et al. 2017; Todd et al. 2016). A similar network of signaling pathways regulates the regenerative potential of MGPCs in the zebrafish retina (reviewed by (Goldman 2014; Lenkowski and Raymond 2014). Relatively little is known about the cell signaling events underlying MGPC formation in the mammalian retina. However, common between fish, bird, and rodent model systems, activation of MAPK-, Hedgehog- and Wnt/β-catenin-signaling in damaged retinas promotes the formation of MGPCs (reviewed by (Hamon et al. 2016)). Interactions between FGF/MAPK and RA-signaling are known to occur during neural development where these signaling pathways drive neural patterning and proliferation of progenitor cells (Diez del Corral et al. 2003; Liu et al. 2001). Furthermore, RA can activate MAPK effectors in hippocampal neurons and neuroblastoma cells (Chen et al. 2008; Masia et al. 2007). The precise mechanisms by which RA-signaling interacts with MAPK-signaling during the formation of MGPCs requires further investigation. It is possible that crosstalk between MAPK and RA-signaling occurs at the level GATA transcription factors which can be phosphorylated by MAPKs and then form complexes with ligand-bound RARα (Tsuzuki et al. 2004).
The potential of MGPCs to produce neurons in the retinas of birds and mammals is very limited. Thus, to harness the regenerative potential of MGPCs methods to enhance the neuronal differentiation of the progeny of MGPCs is required. Activation of RA-signaling increased the percentage of neuronal progeny from MGPCs in damaged retinas. This data adds RA-signaling to the small list of pathways, including Notch-, glucocorticoid-, and gp130/Jak/Stat-signaling, that are known to influence neuronal differentiation from MGPCs in the higher vertebrate (Gallina et al. 2014b; Hayes et al. 2007; Todd et al. 2016). Previously, RA was reported to increase the amount of bipolar cell differentiation from MGPCs in the rat retina (Ooto et al. 2004), however another study in the mouse failed to replicate this result (Karl et al. 2008). RA is a key determinant of neuronal differentiation in a variety of systems (reviewed by (Janesick et al. 2015). During development, RA-signaling promotes photoreceptor differentiation in zebrafish, chick and rodent retinas (Hyatt et al. 1996; Kelley et al. 1999; Stenkamp et al. 1993). RA-signaling has recently been implicated in the regulation of photoreceptor patterning in the high-acuity area of the chick retina and potentially to human fovea patterning (da Silva and Cepko 2017). In rodent, monkey, and human, exogenous RA promotes rod photoreceptor differentiation from embryonic stem cells (Osakada et al. 2008). In adult mammals, RA-signaling promotes neurogenesis from stem cells. Mice fed a diet that is retinoid-depleted have decreased neurogenesis in the dentate gyrus (Jacobs et al. 2006). By comparison, exogenous RA increases neuronal differentiation in explant cultures of SVZ progenitors (Jacobs et al. 2006; Wang et al. 2005). RA-signaling may promote neuronal differentiation through interactions with Notch-signaling and Ascl1-mediated transcription (Jacob et al. 2013; Johnson et al. 1992). Interestingly, forced expression of Ascl1 is sufficient to reprogram Müller glia into neurogenic MGPCs in the mammalian retina (Jorstad et al. 2017; Ueki et al. 2015). Taken together, these data suggest RA-signaling is important for adult neurogenesis and is active in adult stem cells niches that support neurogenesis.
In the retina, a discrete stem cell niche is found within the CMZ. In juvenile and adult fish, retinal growth occurs by the addition of concentric rings of cells produced by CMZ progenitors (Fischer et al. 2014a). CMZ progenitors have also been described in the embryonic and posthatch chick retina, where these progenitors proliferate and express a variety of progenitor-associated markers (Fischer and Reh 2000; Ghai et al. 2008). Recent reports have described a population of CMZ progenitors that give rise to neurons in the mouse retina (Belanger et al. 2017; Marcucci et al. 2016). IGF1 is known to stimulate the proliferation of CMZ progenitors in the chick (Fischer and Reh 2000). Here we report that exogenous RA combined with IGF1 increases the neurogenic capacity of CMZ progenitors in the chick retina. Interestingly, IGF1 is also known to prime CMZ progenitors and non-pigmented epithlial cells, adjacent to the CMZ, to become receptive or respond differentially to factors such FGF2, EGF, HB-EGF and Sonic Hedghog (Fischer et al. 2002; Fischer and Reh 2003; Ritchey et al. 2012; Todd and Fischer 2015; Todd et al. 2015b). We provide novel data that CMZ progenitors in the chick retina are capable of producing Otx2+ neurons. Presumably, these Otx2+/EdU+ cells are newly born bipolar cells or photoreceptors (Nishida et al. 2003). We failed to find newly born Otx2+ neurons derived from RA-treated MGPCs, suggesting that the CMZ progenitors have a broader neurogenic potential than MGPCs, and/or the neurogenic micro-environment is more permissive near the CMZ compared to more central regions of the retina. By comparison, elevated RA-signaling increases the neurogenesis from CMZ progenitors in the zebrafish (Valdivia et al. 2016). Collectively, these findings implicate RA-signaling in the regulation of CMZ progenitor function and neurogenesis.
Conclusions
We conclude that activation of RA-signaling stimulates both the proliferation and neurogenic potential of MGPCs in the avian retina. We find that RA-signaling is included in the network of cell-signaling pathways that are activated in response to neuronal damage and FGF2-mediated stimulation. Although activators and inhibitors of RA-signaling had little effect upon Müller glia in normal retinas, RA-signaling promoted progenitor phenotype and proliferation of MGPCs in damaged and FGF2-treated retinas. Importantly, activation of RA-signaling following proliferation of MGPCs enhanced neuronal differentiation at the expense of glial differentiation. We conclude that RA-signaling is a promising target to enhance the formation of neurogenic MGPCs.
Acknowledgments
The antibody to Nkx2.2 (developed by Drs. T.M Jessell and S. Brenner-Morton) were obtained from the Developmental Studies Hybridoma Bank developed under auspices of the NICHD and maintained by the University of Iowa, Depart ment of Biological Sciences, Iowa City, IA 52242. This work was supported by a grant (EY022030-4) from the National Eye Institute, National Institutes of Health.
Footnotes
Author Contributions:
LT designed and executed experiments, gathered data, constructed figures and contributed to writing the manuscript. LS and CQ executed experiments, gathered data, constructed figures and contributed to writing the manuscript. AJF designed experiments, constructed figures and contributed to writing the manuscript. No competing interests declared by any of the authors.
References
- Astrom A, Pettersson U, Krust A, Chambon P, Voorhees JJ. Retinoic acid and synthetic analogs differentially activate retinoic acid receptor dependent transcription. Biochem Biophys Res Commun. 1990;173:339–45. doi: 10.1016/s0006-291x(05)81062-9. [DOI] [PubMed] [Google Scholar]
- Belanger MC, Robert B, Cayouette M. Msx1-Positive Progenitors in the Retinal Ciliary Margin Give Rise to Both Neural and Non-neural Progenies in Mammals. Dev Cell. 2017;40:137–150. doi: 10.1016/j.devcel.2016.11.020. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Harpavat S, Trimarchi J, Cai L, Huang H, Kuo WP, Weber G, Lee K, Fraioli RE, Cho SH, et al. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2:E247. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Onisko B, Napoli JL. The nuclear transcription factor RARalpha associates with neuronal RNA granules and suppresses translation. J Biol Chem. 2008;283:20841–7. doi: 10.1074/jbc.M802314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner C, Ackerman KM, Lahne M, Hobgood JS, Hyde DR. Repressing Notch Signaling and Expressing TNFalpha Are Sufficient to Mimic Retinal Regeneration by Inducing Müller Glial Proliferation to Generate Committed Progenitor Cells. J Neurosci. 2014;34:14403–19. doi: 10.1523/JNEUROSCI.0498-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Wang WL. Retinoic acid signaling in mammalian eye development. Exp Eye Res. 2009;89:280–91. doi: 10.1016/j.exer.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva S, Cepko CL. Fgf8 Expression and Degradation of Retinoic Acid Are Required for Patterning a High-Acuity Area in the Retina. Dev Cell. 2017;42:68–81. e6. doi: 10.1016/j.devcel.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Duprey-Diaz MV, Blagburn JM, Blanco RE. Optic nerve injury upregulates retinoic acid signaling in the adult frog visual system. J Chem Neuroanat. 2016;77:80–92. doi: 10.1016/j.jchemneu.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26:6303–13. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008;28:1109–17. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Bosse JL, El-Hodiri HM. The ciliary marginal zone (CMZ) in development and regeneration of the vertebrate eye. Exp Eye Res. 2014a;116:199–204. doi: 10.1016/j.exer.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002;129:2283–91. doi: 10.1242/dev.129.9.2283. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Foster S, Scott MA, Sherwood P. Transient expression of LIM-domain transcription factors is coincident with delayed maturation of photoreceptors in the chicken retina. J Comp Neurol. 2008a;506:584–603. doi: 10.1002/cne.21578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220:197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–52. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Growth factors induce neurogenesis in the ciliary body. Dev Biol. 2003;259:225–40. doi: 10.1016/s0012-1606(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Ritchey ER, Scott MA, Wynne A. Bullwhip neurons in the retina regulate the size and shape of the eye. Dev Biol. 2008b;317:196–212. doi: 10.1016/j.ydbio.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Schmidt M, Omar G, Reh TA. BMP4 and CNTF are neuroprotective and suppress damage-induced proliferation of Müller glia in the retina. Mol Cell Neurosci. 2004;27:531–42. doi: 10.1016/j.mcn.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Ritchey ER, Sherwood P. Mitogen-activated protein kinase-signaling regulates the ability of Müller glia to proliferate and protect retinal neurons against excitotoxicity. Glia. 2009a;57:1538–1552. doi: 10.1002/glia.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Tuten W. Mitogen-activated protein kinase-signaling stimulates Müller glia to proliferate in acutely damaged chicken retina. Glia. 2009b;57:166–81. doi: 10.1002/glia.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Zelinka C, Sherwood P. A novel type of glial cell in the retina is stimulated by insulin-like growth factor 1 and may exacerbate damage to neurons and Müller glia. Glia. 2010;58:633–49. doi: 10.1002/glia.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Stanke JJ, Aloisio G, Hoy H, Stell WK. Heterogeneity of horizontal cells in the chicken retina. J Comp Neurol. 2007;500:1154–71. doi: 10.1002/cne.21236. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Wallman J, Mertz JR, Stell WK. Localization of retinoid binding proteins, retinoid receptors, and retinaldehyde dehydrogenase in the chick eye. J Neurocytol. 1999;28:597–609. doi: 10.1023/a:1007071406746. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Zelinka C, Gallina D, Scott MA, Todd L. Reactive microglia and macrophage facilitate the formation of Müller glia-derived retinal progenitors. Glia. 2014b;62:1608–28. doi: 10.1002/glia.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Palazzo I, Steffenson L, Todd L, Fischer AJ. Wnt/betacatenin-signaling and the formation of Müller glia-derived progenitors in the chick retina. Dev Neurobiol. 2015 doi: 10.1002/dneu.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Todd L, Fischer AJ. A comparative analysis of Müller glia-mediated regeneration in the vertebrate retina. Exp Eye Res. 2014a;123:121–130. doi: 10.1016/j.exer.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Zelinka C, Fischer AJ. Glucocorticoid receptors in the retina, Müller glia and the formation of Müller glia-derived progenitors. Development. 2014b;141:3340–51. doi: 10.1242/dev.109835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Zelinka CP, Cebulla CM, Fischer AJ. Activation of glucocorticoid receptors in Müller glia is protective to retinal neurons and suppresses microglial reactivity. Exp Neurol. 2015 doi: 10.1016/j.expneurol.2015.08.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain P, Gaudon C, Pogenberg V, Sanglier S, Van Dorsselaer A, Royer CA, Lazar MA, Bourguet W, Gronemeyer H. Differential action on coregulator interaction defines inverse retinoid agonists and neutral antagonists. Chem Biol. 2009;16:479–89. doi: 10.1016/j.chembiol.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Ghai K, Stanke JJ, Fischer AJ. Patterning of the circumferential marginal zone of progenitors in the chicken retina. Brain Res. 2008;1192:76–89. doi: 10.1016/j.brainres.2007.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai K, Zelinka C, Fischer AJ. Serotonin released from amacrine neurons is scavenged and degraded in bipolar neurons in the retina. J Neurochem. 2009;111:1–14. doi: 10.1111/j.1471-4159.2009.06270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai K, Zelinka C, Fischer AJ. Notch signaling influences neuroprotective and proliferative properties of mature Müller glia. J Neurosci. 2010;30:3101–12. doi: 10.1523/JNEUROSCI.4919-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15:431–42. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon A, Roger JE, Yang XJ, Perron M. Müller glial cell-dependent regeneration of the neural retina: An overview across vertebrate model systems. Dev Dyn. 2016;245:727–38. doi: 10.1002/dvdy.24375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell GT, LaMantia AS. Retinoic acid signaling identifies a distinct precursor population in the developing and adult forebrain. J Neurosci. 2005;25:7636–47. doi: 10.1523/JNEUROSCI.0485-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Nelson BR, Buckingham B, Reh TA. Notch signaling regulates regeneration in the avian retina. Dev Biol. 2007;312:300–11. doi: 10.1016/j.ydbio.2007.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt GA, Schmitt EA, Fadool JM, Dowling JE. Retinoic acid alters photoreceptor development in vivo. Proc Natl Acad Sci U S A. 1996;93:13298–303. doi: 10.1073/pnas.93.23.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt GA, Schmitt EA, Marsh-Armstrong NR, Dowling JE. Retinoic acid-induced duplication of the zebrafish retina. Proc Natl Acad Sci U S A. 1992;89:8293–7. doi: 10.1073/pnas.89.17.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Kong J, Moore S, Milton C, Sasai N, Gonzalez-Quevedo R, Terriente J, Imayoshi I, Kageyama R, Wilkinson DG, et al. Retinoid acid specifies neuronal identity through graded expression of Ascl1. Curr Biol. 2013;23:412–8. doi: 10.1016/j.cub.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Lie DC, DeCicco KL, Shi Y, DeLuca LM, Gage FH, Evans RM. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:3902–7. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janesick A, Wu SC, Blumberg B. Retinoic acid signaling and neuronal differentiation. Cell Mol Life Sci. 2015;72:1559–76. doi: 10.1007/s00018-014-1815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Zimmerman K, Saito T, Anderson DJ. Induction and repression of mammalian achaete-scute homologue (MASH) gene expression during neuronal differentiation of P19 embryonal carcinoma cells. Development. 1992;114:75–87. doi: 10.1242/dev.114.1.75. [DOI] [PubMed] [Google Scholar]
- Jorstad NL, Wilken MS, Grimes WN, Wohl SG, VandenBosch LS, Yoshimatsu T, Wong RO, Rieke F, Reh TA. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature. 2017 doi: 10.1038/nature23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B, Reh TA. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci U S A. 2008;105:19508–13. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassen SC, Thummel R, Campochiaro LA, Harding MJ, Bennett NA, Hyde DR. CNTF induces photoreceptor neuroprotection and Müller glial cell proliferation through two different signaling pathways in the adult zebrafish retina. Exp Eye Res. 2009;88:1051–64. doi: 10.1016/j.exer.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Kelley MW, Turner JK, Reh TA. Retinoic acid promotes differentiation of photoreceptors in vitro. Development. 1994;120:2091–102. doi: 10.1242/dev.120.8.2091. [DOI] [PubMed] [Google Scholar]
- Kelley MW, Williams RC, Turner JK, Creech-Kraft JM, Reh TA. Retinoic acid promotes rod photoreceptor differentiation in rat retina in vivo. Neuroreport. 1999;10:2389–94. doi: 10.1097/00001756-199908020-00031. [DOI] [PubMed] [Google Scholar]
- Kornyei Z, Gocza E, Ruhl R, Orsolits B, Voros E, Szabo B, Vagovits B, Madarasz E. Astroglia-derived retinoic acid is a key factor in glia-induced neurogenesis. FASEB J. 2007;21:2496–509. doi: 10.1096/fj.06-7756com. [DOI] [PubMed] [Google Scholar]
- Lenkowski JR, Raymond PA. Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res. 2014:94–123. doi: 10.1016/j.preteyeres.2013.12.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hunter DJ, Rooker S, Chan A, Paulus YM, Leucht P, Nusse Y, Nomoto H, Helms JA. Wnt signaling promotes Müller cell proliferation and survival after injury. Invest Ophthalmol Vis Sci. 2012;54:444–53. doi: 10.1167/iovs.12-10774. [DOI] [PubMed] [Google Scholar]
- Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–65. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Marcucci F, Murcia-Belmonte V, Wang Q, Coca Y, Ferreiro-Galve S, Kuwajima T, Khalid S, Ross ME, Mason C, Herrera E. The Ciliary Margin Zone of the Mammalian Retina Generates Retinal Ganglion Cells. Cell Rep. 2016;17:3153–3164. doi: 10.1016/j.celrep.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh-Armstrong N, McCaffery P, Gilbert W, Dowling JE, Drager UC. Retinoic acid is necessary for development of the ventral retina in zebrafish. Proc Natl Acad Sci U S A. 1994;91:7286–90. doi: 10.1073/pnas.91.15.7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masia S, Alvarez S, de Lera AR, Barettino D. Rapid, nongenomic actions of retinoic acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic acid receptor. Mol Endocrinol. 2007;21:2391–402. doi: 10.1210/me.2007-0062. [DOI] [PubMed] [Google Scholar]
- Meyers JR, Hu L, Moses A, Kaboli K, Papandrea A, Raymond PA. beta-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Dev. 2012;7:30. doi: 10.1186/1749-8104-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima M, Sakurai H, Mawatari K, Koriyama Y, Matsukawa T, Kato S. Involvement of retinoic acid signaling in goldfish optic nerve regeneration. Neurochem Int. 2009;54:229–36. doi: 10.1016/j.neuint.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Gorsuch RA, Bailey TJ, Ackerman KM, Kassen SC, Hyde DR. Stat3 defines three populations of Müller glia and is required for initiating maximal Müller glia proliferation in the regenerating zebrafish retina. J Comp Neurol. 2012;520:4294–311. doi: 10.1002/cne.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I, Furukawa T. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci. 2003;6:1255–63. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101:13654–9. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–24. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- Pollak J, Wilken MS, Ueki Y, Cox KE, Sullivan JM, Taylor RJ, Levine EM, Reh TA. Ascl1 reprograms mouse Müller glia into neurogenic retinal progenitors. Development. 2013;140:2619–2631. doi: 10.1242/dev.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013;61:651–78. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- Ritchey ER, Zelinka CP, Tang J, Liu J, Fischer AJ. The combination of IGF1 and FGF2 and the induction of excessive ocular growth and extreme myopia. Exp Eye Res. 2012;99:1–16. doi: 10.1016/j.exer.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch K, Jadhav AP, Trimarchi JM, Stadler MB, Roska B, Sun BB, Cepko CL. The transcriptome of retinal Müller glial cells. J Comp Neurol. 2008;509:225–38. doi: 10.1002/cne.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch K, Stadler MB, Cepko CL. Gene expression changes within Müller glial cells in retinitis pigmentosa. Mol Vis. 2012;18:1197–214. [PMC free article] [PubMed] [Google Scholar]
- Rompani SB, Cepko CL. A common progenitor for retinal astrocytes and oligodendrocytes. J Neurosci. 2010;30:4970–80. doi: 10.1523/JNEUROSCI.3456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen J, Harpavat S, Peters MA, Cepko CL. Retinoic acid regulates the expression of dorsoventral topographic guidance molecules in the chick retina. Development. 2005;132:5147–59. doi: 10.1242/dev.02100. [DOI] [PubMed] [Google Scholar]
- Stenkamp DL, Gregory JK, Adler R. Retinoid effects in purified cultures of chick embryo retina neurons and photoreceptors. Invest Ophthalmol Vis Sci. 1993;34:2425–36. [PubMed] [Google Scholar]
- Thatcher JE, Isoherranen N. The role of CYP26 enzymes in retinoic acid clearance. Expert Opin Drug Metab Toxicol. 2009;5:875–86. doi: 10.1517/17425250903032681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Enright JM, Kassen SC, Montgomery JE, Bailey TJ, Hyde DR. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp Eye Res. 2010;90:572–82. doi: 10.1016/j.exer.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L, Fischer AJ. Hedgehog-signaling stimulates the formation of proliferating Müller glia-derived progenitor cells in the retina. Development. 2015;142:2610–2622. doi: 10.1242/dev.121616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L, Palazzo I, Squires N, Mendonca N, Fischer AJ. BMP- and TGFbeta-signaling regulate the formation of Müller glia-derived progenitor cells in the avian retina. Glia. 2017;65(10):1640–1655. doi: 10.1002/glia.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L, Squires N, Suarez L, Fischer AJ. Jak/Stat signaling regulates the proliferation and neurogenic potential of Müller glia-derived progenitor cells in the avian retina. Sci Rep. 2016;6:35703. doi: 10.1038/srep35703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L, Suarez L, Squires N, Zelinka CP, Gribbins K, Fischer AJ. Comparative analysis of glucagonergic cells, glia and the circumferential marginal zone in the reptilian retina. J Comp Neurol. 2015a doi: 10.1002/cne.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L, Volkov LI, Zelinka C, Squires N, Fischer AJ. Heparin-binding EGF-like growth factor (HB-EGF) stimulates the proliferation of Müller glia-derived progenitor cells in avian and murine retinas. Mol Cell Neurosci. 2015b;69:54–64. doi: 10.1016/j.mcn.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki S, Kitajima K, Nakano T, Glasow A, Zelent A, Enver T. Cross talk between retinoic acid signaling and transcription factor GATA-2. Mol Cell Biol. 2004;24:6824–36. doi: 10.1128/MCB.24.15.6824-6836.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y, Wilken MS, Cox KE, Chipman L, Jorstad N, Sternhagen K, Simic M, Ullom K, Nakafuku M, Reh TA. Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice. Proc Natl Acad Sci U S A. 2015;112:13717–22. doi: 10.1073/pnas.1510595112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K, Iwagawa T, Ochiai G, Koso H, Nakauchi H, Nagasaki M, Suzuki Y, Watanabe S. Analysis of Müller glia specific genes and their histone modification using Hes1-promoter driven EGFP expressing mouse. Sci Rep. 2017;7:3578. doi: 10.1038/s41598-017-03874-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia LE, Lamb DB, Horner W, Wierzbicki C, Tafessu A, Williams AM, Gestri G, Krasnow AM, Vleeshouwer-Neumann TS, Givens M, et al. Antagonism between Gdf6a and retinoic acid pathways controls timing of retinal neurogenesis and growth of the eye in zebrafish. Development. 2016;143:1087–98. doi: 10.1242/dev.130922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wauwe J, Van Nyen G, Coene MC, Stoppie P, Cools W, Goossens J, Borghgraef P, Janssen PA. Liarozole, an inhibitor of retinoic acid metabolism, exerts retinoid-mimetic effects in vivo. J Pharmacol Exp Ther. 1992;261:773–9. [PubMed] [Google Scholar]
- Wan J, Zhao XF, Vojtek A, Goldman D. Retinal Injury, Growth Factors, and Cytokines Converge on beta-Catenin and pStat3 Signaling to Stimulate Retina Regeneration. Cell Rep. 2014;9:285–97. doi: 10.1016/j.celrep.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, Kefalov VJ. The cone-specific visual cycle. Prog Retin Eye Res. 2011;30:115–28. doi: 10.1016/j.preteyeres.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TW, Zhang H, Parent JM. Retinoic acid regulates postnatal neurogenesis in the murine subventricular zone-olfactory bulb pathway. Development. 2005;132:2721–32. doi: 10.1242/dev.01867. [DOI] [PubMed] [Google Scholar]
- Yao K, Qiu S, Tian L, Snider WD, Flannery JG, Schaffer DV, Chen B. Wnt Regulates Proliferation and Neurogenic Potential of Müller Glial Cells via a Lin28/let-7 miRNA-Dependent Pathway in Adult Mammalian Retinas. Cell Rep. 2016;17:165–78. doi: 10.1016/j.celrep.2016.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinka CP, Scott MA, Volkov L, Fischer AJ. The Reactivity, Distribution and Abundance of Non-Astrocytic Inner Retinal Glial (NIRG) Cells Are Regulated by Microglia, Acute Damage, and IGF1. PLoS One. 2012;7:e44477. doi: 10.1371/journal.pone.0044477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinka CP, Volkov L, Goodman ZA, Todd L, Palazzo I, Bishop WA, Fischer AJ. mTor signaling is required for the formation of proliferating Müller glia-derived progenitor cells in the chick retina. Development. 2016;143:1859–73. doi: 10.1242/dev.133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XF, Wan J, Powell C, Ramachandran R, Myers MG, Jr, Goldman D. Leptin and IL-6 family cytokines synergize to stimulate Müller glia reprogramming and retina regeneration. Cell Rep. 2014;9:272–84. doi: 10.1016/j.celrep.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]