Abstract
Objective
Bipolar II disorder (BD II) is associated with marked morbidity and mortality. Quetiapine, the treatment with greatest evidence for efficacy in BD II depression, is associated with metabolic burden. Psychotherapy, a treatment with few side effects, has not been systematically evaluated in BDII. This study compared psychotherapy plus placebo to psychotherapy plus pharmacotherapy as treatments for BD II depression.
Methods
From 2010-2015, unmedicated adults (n=92) with DSM-IV-TR bipolar II depression were randomly assigned to weekly sessions of Interpersonal and Social Rhythm Therapy (IPSRT) plus placebo or IPSRT plus quetiapine and followed for 20 weeks.
Results
For primary outcomes, IPSRT + quetiapine yielded significantly faster improvement on HRSD-17 (F=3.924, df=1115.4, p=.048) and greater improvement on Young Mania Rating Scale (F=4.242, df= 58.5, p=.044) scores. Both groups, however, improved significantly over time with comparable response rates (≥50% reduction in depression scores): 67.4% (62/92) in the entire sample, with no between-group differences. Those randomized to their preferred treatment were 4.5 times more likely to respond (OR=4.48, 95% CI= 1.20-16.77, p=0.026). IPSRT + quetiapine assignment was associated with significantly higher Body Mass Index over time (F=6.671, df=67.96; p=.012), higher rates of dry mouth (79% v. 58%; χ2=4.0, p=0.046), and trend toward more over-sedation (100% vs. 92%; χ2=3.4, p=.063).
Conclusions
IPSRT plus quetiapine resulted in greater symptomatic improvement but also more side effects than IPSRT alone. A subset of participants improved with IPSRT alone, although absence of an inactive comparator limits interpretation of this finding. Receipt of preferred treatment was associated with better outcomes. Harms, benefits, and preferences should be considered when recommending treatments for bipolar II depression.
Keywords: bipolar disorder, depression, psychotherapy, atypical antipsychotic agents, interpersonal psychotherapy, preferences
Bipolar disorder type II (BD II) affects 1.1% of the population1 and is associated with high levels of morbidity and mortality. Characterized by multiple, protracted depressive episodes,2 BD II is at least as disabling as—some would suggest more disabling than—BD I.3 Although little is known about optimal approaches to pharmacotherapy,4 even less is known about the role of psychotherapy in the management of BD II. Given the large side effect burden associated with medications used to treat BD II5, 6 and limited evidence with respect to efficacy,4 it is important to consider the potential role of psychotherapy, a treatment with few side effects. Many individuals suffering from BD II struggle with issues that lend themselves to psychosocial remediation, including the challenges of differentiating hypomania from well periods, disordered daily routines, and the negative impact of illness on relationships and functioning. 7 Thus, psychotherapy may play an important role in management of BD II.
Definitive studies of psychotherapy for BD II have not been conducted. Trials testing interventions for BD more broadly have included subsets of individuals diagnosed with BD II with results suggesting possible efficacy of combined psychotherapy and mood stabilizing medication for BD II.8-10 Whereas psychotherapy alone is contraindicated for BD I, it may be appropriate for individuals with BD II who, by definition, are at low risk of experiencing fully syndromal manic episodes or psychosis 11 and may wish to avoid risks associated with pharmacotherapy. 7 Efficacy of psychotherapy monotherapy in BD II is unknown, although we have previously demonstrated feasibility of this approach in two small trials.12, 13
The present study sought to compare psychotherapy plus placebo to psychotherapy plus pharmacotherapy as treatments for BD II depression. We evaluated Interpersonal and Social Rhythm Therapy (IPSRT),14 an evidence-based psychotherapy for BD I15 as a treatment for BD II depression. We compared IPSRT plus placebo to IPSRT plus quetiapine, an atypical antipsychotic medication that has established efficacy for BD II depression.16 Quetiapine therapy is associated with high levels of sedation and has documented metabolic risks.17 We hypothesized that individuals who received IPSRT plus quetiapine would have better symptomatic outcomes than those assigned to IPSRT plus placebo but also more side effects. Broader goals of the study were to prospectively identify individuals who can be effectively managed with psychotherapy alone—a potential advantage for individuals who cannot tolerate medication side effects or those who wish to avoid risks associated with medications. Thus, we sought to conduct exploratory moderator analyses to characterize subgroups who fared better with IPSRT plus placebo and those who needed medication to improve.
METHODS
This study (NCT01133821 ClinicalTrials.gov) was conducted from 2010 to 2015 in an urban, academic medical center. All study procedures were approved by the University of Pittsburgh Institutional Review Board. Potential participants provided informed written consent after receiving a complete study description.
Study Design
This was a 20 week, randomized, double blind, placebo-controlled, parallel-group, study in adult outpatients with BD II depression recruited from provider referrals, advertisements, and research registries. Participants were randomly assigned either to Interpersonal and Social Rhythm Therapy plus placebo (IP) or Interpersonal and Social Rhythm Therapy plus quetiapine (IQ).
Participants
Participants were males or females, 18 – 65 years of age, meeting DSM-IV-TR 18 criteria for bipolar II disorder, in a current major depressive episode, confirmed by Structured Clinical Interview for DSM (SCID-I),19 and scoring ≥15 on the 17-item Hamilton Rating Scale for Depression (HRSD-17). 20
Exclusion criteria were 1) any psychotic or organic mental disorder, bipolar I disorder, current alcohol or drug dependence, borderline or antisocial personality disorder; 2) acute suicidal or homicidal ideation or requiring a higher level of psychiatric care; 3) non-fluent in English; 4) current participation individual psychotherapy; 5) prior lack of response to ≥ 12 weeks of IPSRT conducted by a trained therapist; 6) prior lack of response to ≥ 6 weeks of 300 mg of quetiapine; 7) current treatment with psychotropic medications; 8) pregnancy; 9) active medical problem that better explained symptoms.
Psychotropic medications were prohibited except low doses of lorazepam (0.5-2 mg) for insomnia or agitation. Participants who met eligibility criteria but were on psychotropic medications at time of informed consent were gradually tapered off medications and reevaluated to ensure that they still met eligibility criteria following one week off of all medications prior to randomization (Supplementary eTable 1).
Measures
Raters blind to treatment assignment conducted assessments at baseline and 8, 12, and 20-week follow-up except as indicated below. Diagnoses were confirmed with the SCID-I19 and SCID-II. 21 Depressive symptoms were assessed weekly using the 17-item HRSD and the expanded 25-item version that includes reverse neurovegetative symptoms 22 and the Montgomery-Asberg Depression Rating Scale (MADRS). 23 Mania symptoms were rated weekly using the Young Mania Rating Scale (YMRS). 24 Interrater reliability, as measured by intraclass correlations were 0.98, 0.99, and 0.98 for YMRS, HRSD-25, and HRSD-17, respectively.
Panic-Agoraphobic Spectrum Self-Report (PAS-SR) measures lifetime panic-agoraphobic spectrum symptoms.25 Global illness severity was evaluated weekly using the Clinical Global Impressions Scale, Bipolar Version (CGI-BP) which includes separate clinician ratings for depression and mania on two 7-point Likert-type scales. 26 Functional Assessment Short Test (FAST) is a 24-item measure of functioning for BD with higher scores indicating more impairment. 27 Composite Scale of Morningness (CSM), self-reported measure of diurnal preference for activity, 28 ranges from 13 (extreme eveningness) to 55 (extreme morningness).
Multidimensional Assessment of Thymic States (MATHYS), a self-administered visual analog scale, evaluates state-like emotional reactivity in the past week.29 Lower scores indicate inhibition/hyporeactivity; higher scores indicate excitation/hyperreactivity (range 0 to 200).
Treatment Response to Antidepressant Questionnaire (TRAQ) is a semi-structured interview designed to systematically collect information regarding previous antidepressant treatment, adequacy of trials, and nature of response. 30 Individuals were coded as poor or good responders to antidepressants based on prior treatment response.
Height was assessed at baseline and weight was measured at each treatment visit to calculate Body Mass Index (BMI). Side effects were measured weekly with the Patient Rated Inventory of Side Effects (PRISE), a standardized rating measure of somatic symptoms.31 An over-sedation variable was created by combining 3 items from the PRISE (sleeping too much, fatigue, and decreased energy). Participants were asked prior to randomization whether they preferred treatment with psychotherapy alone, psychotherapy and medication, or had no preference. For purposes of analyses, responses were dichotomized (“received preferred treatment” or “other”).
Allocation
As the CONSORT diagram shows (Figure 1), 207 individuals were screened to yield 92 individuals eligible for randomization. Randomization was conducted by an independent data manager not otherwise involved in study procedures. Assignment to IQ (n=47) or IP (n=45) was generated in random blocks. Because of the negative impact of co-occurring manic symptoms during depression32 and comorbid borderline personality disorder on BD outcomes,33, 34 randomization was stratified on baseline YMRS scores (≥10) and number of SCID-II borderline personality disorder traits endorsed (≥3).
Figure 1.
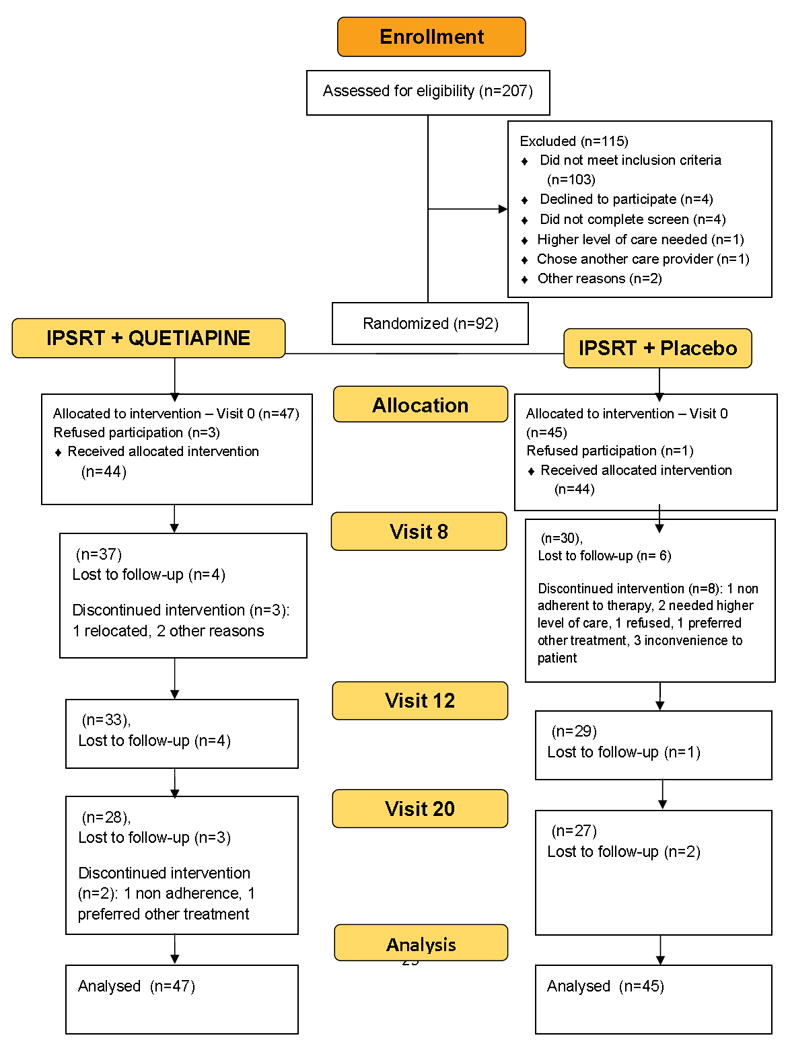
CONSORT Diagram
Interventions
Each participant received 45-minute, individual, psychotherapy sessions from the same therapist. Participants were seen weekly until remission and then biweekly until week 20, thus could receive up to 20 IPSRT sessions. Therapists (master’s or doctoral level professionals with ≥ 3 years of clinical experience) administered IPSRT. Sessions were video- or audio-recorded to monitor fidelity. All therapists participated in weekly supervision with expert supervisor feedback. Therapist adherence to IPSRT was assessed using the 22-item IPSRT Therapy Rating Scale.35 Raters trained to maintain a criterion level of agreement within 1 point (ICC ≥0.80 for each scale item) rated a randomly selected subset (25%) of sessions to ensure fidelity. This instrument has been used in previous studies to evaluate the extent to which core components of IPSRT are present in sessions.
IPSRT, described in greater detail in the manual,14 combines a focus on interpersonal relationships 36 with behavioral interventions to modify social rhythms. Patients develop more regular routines and sleep patterns to regulate underlying biologic abnormalities associated with BD II, thereby reducing symptoms and improving outcomes. Patients completed a weekly self-report assessment, the Social Rhythm Metric (SRM),37 to track and modify their social routines. IPSRT adapted for BD II disorder is described elsewhere.7, 38
Quetiapine and placebo were dispensed in identically appearing capsules. Medication was flexibly dosed with a starting dose of 50 mg per day, increased weekly by 50 mg daily as tolerated to a maximum of 300 mg per day. Participants who could not tolerate 300 mg could remain in the study on the maximally tolerated dose. Participants who could not take any dosage of study medication were retained in the study on no medication but remained in their original allocation.
Analyses
Intent-to-treat analyses were conducted. Change over time on primary outcomes (HRSD, YMRS) and other continuous measures (MADRS, FAST) were evaluated using mixed effect models with maximum likelihood (ML) estimation, and random intercept and slope. This approach creates a two-level hierarchical model that nests time within individual.
To determine whether individual growth trajectories were nonlinear, higher-order polynomial models were tested, adding quadratic and cubic parameters to linear models. Goodness of fit of models were compared using 2 log-likelihood and Schwarz’s Bayesian Criterion indices. To test treatment effect on shapes of individual growth trajectories, treatment was examined as a time-invariant covariate to explore group differences in change over time. Treatment by time interactions were included in the models.
Potential moderators were selected a priori based on clinical relevance and evidence from the literature: age, gender, years of education, marital status, CSM score, number of hypomanic symptoms (baseline YMRS score), being on medication prior to entry, mood reactivity (MATHYS score), family history of bipolar disorders, prior treatment response to antidepressants (TRAQ), reverse neurovegetative symptoms (items 18 to 25 on HRSD-25), insomnia (items 4-6 on HRSD), lifetime anxiety (PAS-SR), and treatment preference.
To explore moderators of treatment outcome,39 we constructed separate models that included treatment (T) as an independent variable, one moderator (M) and their interaction (T X M). When the main effect of M was significant but the interaction was not, the variable was considered a non-specific predictor of outcome. When the interaction was significant, regardless of a significant main effect, the variable was considered a moderator. Continuous moderator variables were centered around the mean and dichotomous variables were coded as -1/2, +1/2. Effect sizes for predictors and moderators were expressed as standardized regression coefficients. Moderator analyses were not corrected for multiple comparisons because they are hypothesis-generating.39
Statistical tests were performed at 2-sided 5% significance level (α= 0.05). Analyses were conducted using SPSS, version 23, and Stata, version 13.
RESULTS
Baseline Demographic and Clinical Characteristics
Baseline demographic and clinical characteristics of participants are provided in Table 1. Treatment groups differed only on number of prior hypomanic episodes: IP had more (median =12) than IQ (median = 6). Although not statistically significant, almost twice as many participants in the IP group (n=11; 24%) tapered off medication prior to entry compared to IQ (n= 6; 13%) (Supplementary eTable 1).
Table 1.
Baseline Demographic and Clinical Variables
| Variable | IPSRT + QUETIAPINE (N = 47) | IPSRT + PLACEBO (N = 45) | P |
|---|---|---|---|
| Gender (n, % male) | 15 (31.9%) | 19 (42.2%) | 0.31 |
| Age (mean±SD) | 30.9±10.3 | 33.9±11.2 | 0.18 |
| Ethnicity (n, %Hispanic) | 1 (2.1%) | 2 (4.4%) | 0.53 |
| Race | |||
| N, % Caucasian | 31 (66.0%) | 35 (77.8%) | |
| N, % African American | 10 (21.3%) | 6 (13.3%) | |
| N, % other | 6 (12.7%) | 4 (8.9%) | |
| Marital Status | 0.80 | ||
| Never Married | 30 (63.8%) | 27 (60%) | |
| Married/Living as Married | 11 (23.4%) | 10 (22.2%) | |
| Separated/Divorced/Widowed | 6 (12.8%) | 8 (17.8%) | |
| Education (highest level attained) | 0.11 | ||
| High School Degree or Less | 4 (8.5%) | 9 (20%) | |
| Some college or Associates Degree | 26 (55.3%) | 22 (48.9%) | |
| Bachelor’s Degree | 11 (23.4%) | 13 (28.9%) | |
| Graduate or Professional Degree | 6 (12.8%) | 1 (2.2%) | |
| Total Income per Year | 0.19 | ||
| <$30,000 | 27 (57.4%) | 21 (46.7%) | |
| $30,000-$74,999 | 19 (40.4%) | 19 (42.2%) | |
| ≥$75,000 | 1 (2.1%) | 5 (11.1%) | |
| Psychotropic medication prior to entering study | 6 (12.8%) | 11 (24.4%) | 0.15 |
| Duration of current depressive episode (weeks) (median with Interquartile range) | 17 (8-114.5) | 17 (8-82-7) | 0.48 |
| Lifetime Diagnosis of Anxiety – DSM IV (%) | 31 (66%) | 29 (64.4%) | 0.88 |
| Current Diagnosis of Anxiety – DSM IV (%) | 28 (59.6%) | 26 (57.8%) | 0.86 |
| # Lifetime Episodes of Depression (median) | 3 | 6 | 0.07 |
| # Lifetime Episodes of Hypomania (median) | 6 | 12 | 0.05 |
| Hamilton Rating Scale for Depression – 17 Item (mean±SD) | 19.6±3.9 | 21.0±4.6 | 0.12 |
| Hamilton Rating Scale for Depression – 25 Item (mean±SD) | 24.7±4.9 | 26.0±5.4 | 0.24 |
| Young Mania Rating Scale (mean±SD) | 6.2±3.5 | 6.2±3.4 | 0.96 |
| BMI (mean±SD) | 27.6±8.0 | 26.4±5.7 | 0.53 |
Outcomes by Treatment
Participants attended on average 11.6 (± 7.2) psychotherapy sessions over 20 weeks, without between-group differences (t=0.17, p=0.865). Mean quetiapine dosage was 172.3±71.3 mg per day, range 50-300 mg. Dropout rates were high (40%; Figure 1) but did not differ by treatment assignment or treatment preference (for both, p >0.05). On primary outcomes, there were significant time effects for HRSD-17 (F=89.7, df=1102.9, p<.001) and YMRS (F=21.1, df=56.5, p<0.001) with a significant time by group interaction favoring IQ [HRSD-17 (F=3.924, df=1115.4, p=.048 for the quadratic term); (see Figure 2) and YMRS (F=4.242, df= 58.5, p=.044 for the linear term)]. There were also significant time by group interactions favoring IQ for MADRS (F=4.060, df=977.3, p=.044) and CGI-severity (F=8.197, df=1054.4, p=.004) scores. There were no group differences in FAST or in SRM scores over time (for both, p > 0.05).
Figure 2.
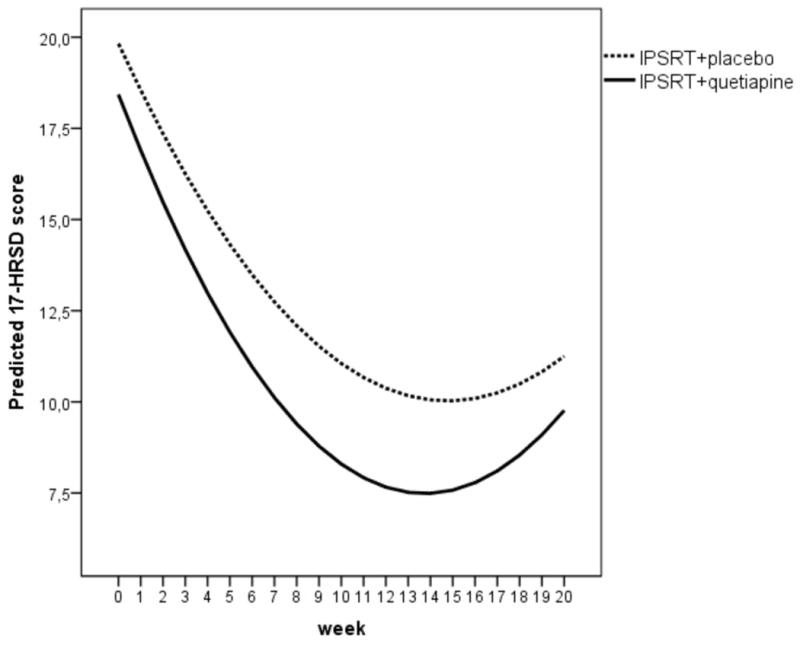
Estimated change in Hamilton Depression Rating Scale -17 item (HDRS-17) Scores Using Mixed-Effect Models (a)
(a) (F=3.924, df=1115.4, p=.048). The curves represent the quadratic growth trajectories in the two groups over time.
Both treatments yielded comparable response rates, defined as ≥50% reduction in HRSD-25 scores from baseline to endpoint: 67.4% (62/92) in the entire sample, with no significant between-group difference [60.0% (27/45) of IP vs. 74.5% (35/47) of IQ; NS]. Overall rates of remission (3 consecutive weeks with HRSD-25 ≤8 and YMRS ≤8) were 31.5% (29/92), with no between-group differences [28.9% (13/45) of IP vs. 34.0% (16/47) of IQ; NS]. In the quetiapine group, mean final dose (mg) was 209.0±75.1 in remitters (n=16) versus 143.15±96.9 non-remitters (n=31) (t=2.37, p<0.05). Significantly more individuals in IP used at least one dose of lorazepam: 60% (27/45) in IP versus 34% (16/47) in IQ (χ2=6.22, p=.013)]. Only 7% (6/92) participants experienced a single YMRS score ≥ 15 over the course of the study, and this did not differ by group. No one experienced an episode of mania. Although study medication was administered double blind, participants receiving quetiapine correctly guessed treatment assignment (at either week 8 or 20; last available guess) numerically more often than those assigned to IP [91% (29/32) versus 75% (21/28)] but this difference was not statistically significant (Fisher exact test, p=0.10)].
Those assigned to IQ experienced a modest estimated linear increase in BMI over time from 28.1 to 28.6 kg/m,2 in contrast to a slight decline in BMI among those assigned to IP from 26.8 to 26.7 kg/m2 (F=6.671, df=67.96; p=.012) . Early weight gain (>5% in first month) occurred in 5% (2/44) of those in IQ and none in IP (NS). In a subset for whom PRISE information was available (n=80), those assigned to IQ reported at least once during the study significantly higher rates of dry mouth (79% v. 58%; χ2=4.0, p=0.046) and a trend toward higher rates of over-sedation (100 % vs. 92 %; χ2=3. 4, p=.063) vs. IP. By contrast, complaints of restlessness were significantly higher in the IP group (95% v. 76%; χ2=5. 4, p=.02). See Table 2 and Supplementary eFigure1.
Table 2.
Percentage of participants experiencing self-reported side effects on the Patient Rated Inventory of Side Effects (PRISE)
| treatment assignment | ||||||
|---|---|---|---|---|---|---|
| IPSRT+placebo (N=38) | IPSRT+quetiapine (N=42) | |||||
| N | % | N | % | χ2-test, p-value | ||
| Diarrhea | no | 21 | 55.3% | 22 | 52.4% | .796 |
| yes | 17 | 44.7% | 20 | 47.6% | ||
| Constipation | no | 24 | 63.2% | 18 | 42.9% | .069 |
| yes | 14 | 36.8% | 24 | 57.1% | ||
| Dry mouth | no | 16 | 42.1% | 9 | 21.4% | .046 |
| yes | 22 | 57.9% | 33 | 78.6% | ||
| Nausea/vomiting | no | 16 | 42.1% | 18 | 42.9% | .946 |
| yes | 22 | 57.9% | 24 | 57.1% | ||
| Palpitations | no | 20 | 52.6% | 20 | 47.6% | .654 |
| yes | 18 | 47.4% | 22 | 52.4% | ||
| Dizziness on standing | no | 17 | 44.7% | 17 | 40.5% | .700 |
| yes | 21 | 55.3% | 25 | 59.5% | ||
| Chest pain | no | 23 | 60.5% | 28 | 66.7% | .568 |
| yes | 15 | 39.5% | 14 | 33.3% | ||
| Rash | no | 28 | 73.7% | 36 | 85.7% | .179 |
| yes | 10 | 26.3% | 6 | 14.3% | ||
| Increased perspiration | no | 23 | 60.5% | 28 | 66.7% | .568 |
| yes | 15 | 39.5% | 14 | 33.3% | ||
| Itching | no | 20 | 52.6% | 24 | 57.1% | .685 |
| yes | 18 | 47.4% | 18 | 42.9% | ||
| Dry skin | no | 15 | 39.5% | 13 | 31.0% | .425 |
| yes | 23 | 60.5% | 29 | 69.0% | ||
| Headache | no | 5 | 13.2% | 6 | 14.3% | .884 |
| yes | 33 | 86.8% | 36 | 85.7% | ||
| Tremors | no | 27 | 71.1% | 30 | 71.4% | .970 |
| yes | 11 | 28.9% | 12 | 28.6% | ||
| Poor coordination | no | 21 | 55.3% | 16 | 38.1% | .124 |
| yes | 17 | 44.7% | 26 | 61.9% | ||
| Dizziness | no | 19 | 50.0% | 16 | 38.1% | .284 |
| yes | 19 | 50.0% | 26 | 61.9% | ||
| Blurred vision | no | 24 | 63.2% | 22 | 52.4% | .330 |
| yes | 14 | 36.8% | 20 | 47.6% | ||
| Ringing in ears | no | 28 | 73.7% | 24 | 57.1% | .121 |
| yes | 10 | 26.3% | 18 | 42.9% | ||
| Difficulty urinating | no | 31 | 81.6% | 36 | 85.7% | .617 |
| yes | 7 | 18.4% | 6 | 14.3% | ||
| Painful urination | no | 36 | 94.7% | 35 | 83.3% | .107 |
| yes | 2 | 5.3% | 7 | 16.7% | ||
| Frequent urination | no | 22 | 57.9% | 21 | 50.0% | .479 |
| yes | 16 | 42.1% | 21 | 50.0% | ||
| Menstrual irregularity | no | 31 | 81.6% | 30 | 71.4% | .287 |
| yes | 7 | 18.4% | 12 | 28.6% | ||
| Difficulty sleeping | no | 5 | 13.2% | 9 | 21.4% | .331 |
| yes | 33 | 86.8% | 33 | 78.6% | ||
| Sleeping too much | no | 9 | 23.7% | 4 | 9.5% | .086 |
| yes | 29 | 76.3% | 38 | 90.5% | ||
| Loss of sexual desire | no | 15 | 39.5% | 14 | 33.3% | .568 |
| yes | 23 | 60.5% | 28 | 66.7% | ||
| Trouble achieving orgasm | no | 23 | 60.5% | 21 | 50.0% | .345 |
| yes | 15 | 39.5% | 21 | 50.0% | ||
| Trouble with erections | no | 32 | 84.2% | 35 | 83.3% | .915 |
| yes | 6 | 15.8% | 7 | 16.7% | ||
| Anxiety | no | 2 | 5.3% | 6 | 14.3% | .179 |
| yes | 36 | 94.7% | 36 | 85.7% | ||
| Poor concentration | no | 4 | 10.5% | 6 | 14.3% | .612 |
| yes | 34 | 89.5% | 36 | 85.7% | ||
| General malaise | no | 12 | 31.6% | 12 | 28.6% | .769 |
| yes | 26 | 68.4% | 30 | 71.4% | ||
| Restlessness | no | 2 | 5.3% | 10 | 23.8% | .020 |
| yes | 36 | 94.7% | 32 | 76.2% | ||
| Fatigue | no | 3 | 7.9% | 5 | 11.9% | .550 |
| yes | 35 | 92.1% | 37 | 88.1% | ||
| Decreased energy | no | 3 | 7.9% | 3 | 7.1% | .899 |
| yes | 35 | 92.1% | 39 | 92.9% | ||
| Other (specify) | no | 27 | 71.1% | 27 | 64.3% | .519 |
| yes | 11 | 28.9% | 15 | 35.7% | ||
Moderators of Treatment Outcomes
Baseline YMRS scores moderated treatment response with those experiencing > 9 hypomanic symptoms less likely to respond to IQ (OR=0.872, 95% CI= 0.760-0.999, p=.048) than to IP (see Supplementary eFigure 2). MATHYS total score was a moderator of functional outcomes (change in FAST scores) with those scoring above the mean (more hyperreactivity) more likely to improve with IQ (F=5.947, df=94.1, p=.017) than IP. CSM scores moderated outcomes such that higher scores (more morningness) was associated with greater improvement in HRSD-25 scores when randomized to IQ (F=7.219, df=69.2, p=.009). See Table 3 for a summary of effect sizes for significant moderators.
Table 3.
Effect Sizes (Outcomes) for Statistically Significant Predictors and Moderators (a)
| Variable | Non Specific Predictor | Moderator favoring IPSRT + Quetiapine | Moderator favoring IPSRT + Placebo | Comment |
|---|---|---|---|---|
| Young Mania Rating Scale (YMRS) | 0.11 (Change in Functional Assessment Short Test) | -0.08 (Treatment Response; ≥50% reduction in HRSD-25 scores from baseline to endpoint) | Higher YMRS score predicted more rapid improvement in functioning (F=6.037, p=0.016) and was associated with increased likelihood of response if assigned to IPSRT + placebo (OR=0.872, p=0.048) | |
| Multidimensional Assessment of Thymic States (MATHYS) | -0.01 (Change in Hamilton Rating Scale for Depression; HRSD-25) | -0.04 (Change in Functional Assessment Short Test) | Hyperreactivity predicted faster improvement in depression (F=5.243, p=0.025) and more rapid improvement in functioning in those assigned to IPSRT + quetiapine (F=5.947, p=0.017) | |
| Reverse Neurovegetative symptoms | 0.11 (Change in HRSD-25) | Fewer atypical depression symptoms was associated with faster improvement in depression (F=7.087, p=0.009) | ||
| Panic-Agoraphobic Spectrum Self-Report (PAS-SR) | 0.02 (Change in HRSD-25) | Lower PAS-SR scores were associated with more rapid improvement in depression (F=10.738, p=0.002) | ||
| Composite Scale of Morningness (CSM) | 0.57 (Change in Body Mass Index; BMI) | -0.06 (Change in HRSD-25) | Eveningness showed a trend toward predicting greater BMI reductions (F=3.572, p=0.060) and morningness was associated with faster improvement in depression in those assigned to IPSRT + quetiapine (F=7.219, p=0.009) | |
| Treatment Preference | 0.49 (Treatment Response; ≥50% reduction in HRSD-25 scores from baseline to endpoint) | Receiving preferred treatment was associated with increased likelihood of response (OR=4.48, p=0.026) |
(a) Effect sizes are expressed as standardized regression coefficients. Effect sizes for moderators are derived from the variable X treatment interaction term and those for predictors from the main effect of the variable.
Family history of bipolar disorder, prior treatment response to antidepressant, reverse neurovegetative symptoms, insomnia, PAS-SR scores, and treatment preference were not moderators of outcomes.
Predictors of Treatment Outcomes
Total MATHYS score was a non-specific predictor of outcome, with those scoring above the mean (hyperreactivity) having greater improvement on HRSD-25 than those scoring below the mean (F=5.243, df=71.8, p=.025). Fewer reverse neurovegetative symptoms (F=7.087, df=110.9, p=.009) and low PAS-SR scores (F=10.738, df=70.2, p=.002) predicted more rapid improvement on the HRSD-25. Treatment preference was a significant predictor of response, such that individuals randomized to their preferred treatment were 4.5 times more likely to respond, regardless of assignment (OR=4.48, 95% CI= 1.20-16.77, p=0.026). YMRS scores were positive predictors of FAST scores, with higher scores predicting greater change in functioning (F=6.037, df=100.5, p=.016).
Family history of bipolar disorder, prior treatment response to antidepressant, insomnia, and CSM scores were not predictors of outcomes.
DISCUSSION
Treatment with IPSRT and quetiapine yielded better symptomatic outcomes, however IQ was also associated with more side effects, including a statistically significant increased risk for weight gain (increased BMI). Absolute weight gain was small, and only 5% of the IQ group met criteria for early weight gain (>5% in first month), a strong predictor of subsequent weight gain.40 Given the growing global burden of obesity41 and likelihood that obesity negatively affects the course of bipolar disorder,42 this modest difference constitutes a non-trivial treatment consideration. Because of the chronicity of BD II and need for maintenance treatment, a relatively small increase in BMI observed over 20 weeks could be even greater if exposure to quetiapine were to continue over years or even decades. In other studies, more than 50% of quetiapine-exposed patients gained significant weight over a year of treatment.43, 44
All participants improved on primary symptom measures (HRSD, YMRS), including those who received IPSRT alone. Sixty percent of those assigned to IP responded to treatment, rates comparable to those seen with pharmacotherapy alone.16 This suggests that, for those who do not wish to incur the risks of weight gain, dry mouth, or sedation, IPSRT alone is a viable option. Interestingly, those with higher YMRS scores (>9) at baseline did better with IP than IQ. Within the very truncated distribution observed in this trial (by definition, no episodes of mania), higher YMRS scores may be a proxy for increased energy, allowing individuals with higher energy to take steps required to make optimal use of psychotherapy. This result should be interpreted with caution, however, because only 8 individuals in the IP group and 10 individuals in the IQ group had YMRS scores >9. More IP participants complained of restlessness on the PRISE, suggesting that addition of quetiapine mitigated intolerable agitation/activation.
Those with low CSM scores (below the median value of 30) fared better with IPSRT plus quetiapine, suggesting that morningness traits may enable those who are somewhat phase advanced to better tolerate quetiapine-induced sedation. Individuals whose clinical presentation was characterized hypersomnia, anergia, hyperphagia, hyporeactivity, and high lifetime anxiety symptoms improved more slowly, regardless of intervention received, suggesting that these individuals may require longer or different courses of treatment.
Perhaps informed by prior treatment experiences or self-knowledge, treatment preference was a potent global predictor of response, showing that those who got the treatment they preferred did better and suggesting that patient preference—including the option of psychotherapy-- should be considered when managing BD II depression.
The mean dose of quetiapine in this study (172 mg) was lower than the 300-600 mg used in trials with forced titration schedules,16 raising the intriguing possibility that combining medication with a bipolar-specific psychotherapy many enable individuals to be managed with lower-than-usual doses of medications, thus potentially mitigating some side effects. This hypothesis, although not formally tested in this trial, is perhaps supported by the fact that weight gain, though present, was somewhat lower than other quetiapine studies that used higher doses.43, 44 An alternate hypothesis is that individuals with BD II may require lower doses of quetiapine than those with BD I.
Because maintenance treatment is recommended for BD II,45 evaluation of both interventions needs to be considered in light of implications for long-term follow up. Whether psychotherapy alone will suffice as a maintenance treatment for BD II is unknown. Risk of metabolic dysregulation in BD46 and burdens of long-term exposure to medications like quetiapine,47, 48 however, are well documented. Thus, low side-effect treatment options like IPSRT monotherapy are appealing, but longer term research is needed before it can be recommended.
Limitations of the current study include absence of an inactive psychotherapy comparator, high dropouts, and overall poor remission rates. Medication withdrawal phenomena may have disadvantaged a subset of participants. Effect sizes for moderators and predictors were small and uncorrected for multiple comparisons, suggesting that although findings were statistically significant, they require confirmation in a larger trial. Power was limited for some comparisons, potentially leading to Type II errors. Correct guessing of treatment assignment indicates failure of blinding for participants, although raters remained blinded. Our sample was predominantly white and relatively well educated which may limit generalizability of findings to other groups.
In conclusion, symptomatic benefits were greater when patients were treated with IPSRT plus quetiapine, but this additional improvement came with a risk of more side effects. A subset of patients did well with IPSRT plus placebo, especially those who preferred this modality. Although it is not typically offered to those with BD II, IPSRT alone appears to be a reasonable treatment option, especially for those who prefer it, individuals motivated to implement psychotherapeutic recommendations, and those for whom medication is relatively contraindicated. Future studies should look at combined moderators of outcomes to develop personalized treatment algorithms for BD II.
Supplementary Material
CLINICAL POINTS.
Little is known about the role of psychotherapy in managing bipolar II depression.
Bipolar II depression treatment with psychotherapy plus medication results in more symptomatic improvement than psychotherapy alone but also more side effects.
Psychotherapy alone is a reasonable treatment option for bipolar II depression, especially for those who prefer it and those for whom medication is relatively contraindicated.
Acknowledgments
This study was funded by the National Institute of Mental Health R01 MH084831 (Swartz, PI). The authors would like to thank the individuals who participated in this research project and the clinicians and staff who contributed to the conduct of this trial.
ROLE OF SPONSOR: NIMH had no role in data collection, data management, data analysis, interpretation of data, or in the preparation, review or approval of the manuscript. NIMH approved the study design and provided oversight of the conduct of the study (accrual of subjects, yearly monitoring of study progress).
Footnotes
DISCLOSURES: Dr. Swartz receives royalties from UpToDate. Dr. Frank receives royalties from the American Psychological Association and Guilford Press; she and her spouse serve on an advisory board to Servier International; she and her spouse have equity in Minerva Neuroscience, HealthRhythms, Inc. and Psychiatric Assessments, Inc.; and her spouse has equity in Aliphcom and Minerva N and receives royalties from the Pittsburgh Sleep Quality Index. During the past three years, Dr. Thase has been a consultant to: Alkermes, Allergan (including Actavis, Forest and Naurex), AstraZeneca, Avenir, Aventis, Bristol-Myers Squibb, Cerecor, Eli Lilly & Co., Gerson Lehman Group, Guidepoint Global, Janssen (includes Johnson & Johnson), H. Lundbeck A/S, MedAvante, Inc., Merck, Neuronetics Inc., Novartis, Otsuka, Nestle (includes PamLab), Pfizer, Roche Inc, Shire US Inc., Sunovion, Takeda, and Teva. During the same time frame, Dr. Thase has received research grants from: Agency for Healthcare Research and Quality, Alkermes, AstraZeneca, Avenir, Eli Lilly & Co., Forest Pharmaceuticals, GlaxoSmithKline, Janssen (Johnson & Johnson), the National Institute of Mental Health, Otsuka Pharmaceuticals, Pharmaneuroboost, Roche, and Takeda. Drs. Rucci, Wallace, Carretta, and Ms. Celedonia report no financial relationships with commercial interests.
Previously presented at the ASCP Annual Meeting in Phoenix, Arizona, May 30-June 3, 2016 and at the ISBD Annual Meeting in Amsterdam, Netherlands July 13-16, 2016.
(NCT01133821 ClinicalTrials.gov)
References
- 1.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Archives of General Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Judd LL, Akiskal HS, Schettler PJ, et al. The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders? Journal of Affective Disorders. 2003;73(1-2):19–32. doi: 10.1016/s0165-0327(02)00324-5. [DOI] [PubMed] [Google Scholar]
- 3.Maina G, Albert U, Bellodi L, et al. Health-related quality of life in euthymic bipolar disorder patients: differences between bipolar I and II subtypes. Journal of Clinical Psychiatry. 2007;68(2):207–212. doi: 10.4088/jcp.v68n0205. [DOI] [PubMed] [Google Scholar]
- 4.Swartz HA, Thase ME. Pharmacotherapy for the treatment of acute bipolar II depression: Current evidence. Journal of Clinical Psychiatry. 2011;72(3):356–366. doi: 10.4088/JCP.09r05192gre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correll CU, Detraux J, De Lepeleire J, De Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015 Jun;14(2):119–136. doi: 10.1002/wps.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(Suppl 1):1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- 7.Swartz HA, Levenson JC, Frank E. Psychotherapy for bipolar II disorder: The role of interpersonal and social rhythm therapy. Prof Psychol Res Pr. 2012;43(2):145–153. doi: 10.1037/a0027671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colom F, Vieta E, Sanchez-Moreno J, et al. Psychoeducation for bipolar II disorder: an exploratory, 5-year outcome subanalysis. Journal of Affective Disorders. 2009;112(1-3):30–35. doi: 10.1016/j.jad.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: A 1-Year randomized trial from the Systematic Treatment Enhancement Program. Archives of General Psychiatry. 2007;64:419–427. doi: 10.1001/archpsyc.64.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh SV, Zaretsky A, Beaulieu S, et al. A randomized controlled trial of psychoeducation or cognitive-behavioral therapy in bipolar disorder: a Canadian Network for Mood and Anxiety treatments (CANMAT) study [CME] Journal of Clinical Psychiatry. 2012 Jun;73(6):803–810. doi: 10.4088/JCP.11m07343. [DOI] [PubMed] [Google Scholar]
- 11.Coryell W, Keller M, Endicott J, Andreasen N, Clayton P, Hirschfeld R. Bipolar II illness: course and outcome over a five-year period. Psychological Medicine. 1989;19(1):129–141. doi: 10.1017/s0033291700011090. [DOI] [PubMed] [Google Scholar]
- 12.Swartz HA, Frank E, Cheng Y. A randomized pilot study of psychotherapy and quetiapine for the acute treatment of bipolar II depression. Bipolar Disorders. 2012 Mar;14(2):211–216. doi: 10.1111/j.1399-5618.2012.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swartz HA, Frank E, Frankel DR, Novick D, Houck PR. Psychotherapy as monotherapy for the treatment of bipolar II depression: A proof of concept study. Bipolar Disorders. 2009;11:89–94. doi: 10.1111/j.1399-5618.2008.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank E. Treating bipolar disorder: A clinician’s guide to interpersonal and social rhythm therapy. New York, NY: Guilford Press; 2005. [Google Scholar]
- 15.Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Archives of General Psychiatry. 2005;62(9):996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- 16.Young AH, Calabrese JR, Gustafsson U, et al. Quetiapine monotherapy in bipolar II depression: combined data from four large, randomized studies. Int J Bipolar Disord. 2013;1:10. doi: 10.1186/2194-7511-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes A, American Psychiatric A, American Association of Clinical E, North American Association for the Study of O. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC, USA: American Psychiatric Association; 1994. [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clincal Interview for DSM-IV Axis I Disorders (SCID) New York: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 20.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;25:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV personality disorders, (SCID-II) Washington, D.C: American Psychiatric Press, Inc; 1997. [Google Scholar]
- 22.Thase ME, Carpenter L, Kupfer DJ, Frank EF. Clinical significance of reversed vegetative subtypes of recurrent major depression. Psychopharmacology Bulletin. 1991;27(1):17–22. [PubMed] [Google Scholar]
- 23.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 24.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. The British journal of psychiatry : the journal of mental science. 1978 Nov;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 25.Shear MK, Frank E, Rucci P, et al. Panic-agoraphobic spectrum: reliability and validity of assessment instruments. Journal of Psychiatric Research. 2001;35(1):59–66. doi: 10.1016/s0022-3956(01)00002-4. [DOI] [PubMed] [Google Scholar]
- 26.Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Research. 1997;73(3):159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 27.Rosa AR, Sanchez-Moreno J, Martinez-Aran A, et al. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin Pract Epidemiol Ment Health. 2007;3:5. doi: 10.1186/1745-0179-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. Journal of Applied Psychology. 1989 Oct;74(5):728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- 29.Henry C, Luquiens A, Lancon C, et al. Inhibition/activation in bipolar disorder: validation of the Multidimensional Assessment of Thymic States scale (MAThyS) BMC Psychiatry. 2013;13:79. doi: 10.1186/1471-244X-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Posternak MA, Young D, Sheeran T, Chelminski I, Franklin CL, Zimmerman M. Assessing past treatment history: test-retest reliability of the Treatment Response to Antidepressant Questionnaire. Journal of Nervous & Mental Disease. 2004;192(2):95–102. doi: 10.1097/01.nmd.0000110280.19284.47. [DOI] [PubMed] [Google Scholar]
- 31.Rush AJ, O’Neill BL. Patient Rated Inventory of Side Effects (PRISE) Dallas, TX: University of Texas Southwestern Medical Center; 1999. [Google Scholar]
- 32.Goldberg JF, Perlis RH, Bowden CL, et al. Manic symptoms during depressive episodes in 1,380 patients with bipolar disorder: findings from the STEP-BD. American Journal of Psychiatry. 2009 Feb;166(2):173–181. doi: 10.1176/appi.ajp.2008.08050746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunayevich E, Sax KW, Keck PE, Jr, et al. Twelve-month outcome in bipolar patients with and without personality disorders. Journal of Clinical Psychiatry. 2000;61(2):134–139. [PubMed] [Google Scholar]
- 34.Swartz HA, Pilkonis PA, Frank E, Proietti JM, Scott J. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disorders. 2005;7(2):192–197. doi: 10.1111/j.1399-5618.2005.00179.x. [DOI] [PubMed] [Google Scholar]
- 35.Wagner EF, Frank E, Steiner S. Discriminating maintenance treatments for recurrent depression: Development and implementation of a rating scale. Journal of Psychotherapy Practice and Research. 1992;1:280–290. [PMC free article] [PubMed] [Google Scholar]
- 36.Klerman GL, Weissman MM, Rounsaville BJ, Chevron ES. Interpersonal Psychotherapy of Depression. New York: Basic Books; 1984. [Google Scholar]
- 37.Monk TH, Flaherty JF, Frank E, Hoskinson K, Kupfer DJ. The Social Rhythm Metric: An instrument to quantify the daily rhythms of life. Journal of Nervous & Mental Disease. 1990;178(2):120–126. doi: 10.1097/00005053-199002000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Swartz HA, Frank E, Frankel D. Psychothérapie interpersonnelle et des rythmes sociaux (PTIRS) dans le trouble bipolaire II : structure du traitement et exemples cliniques. Sante Mentale au Quebec. 2008;33(2):151–184. doi: 10.7202/019673ar. [DOI] [PubMed] [Google Scholar]
- 39.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002 Oct;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 40.Vandenberghe F, Gholam-Rezaee M, Saigi-Morgui N, et al. Importance of early weight changes to predict long-term weight gain during psychotropic drug treatment. J Clin Psychiatry. 2015 Nov;76(11):e1417–1423. doi: 10.4088/JCP.14m09358. [DOI] [PubMed] [Google Scholar]
- 41.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 Aug 30;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McElroy SL, Kemp DE, Friedman ES, et al. Obesity, but not metabolic syndrome, negatively affects outcome in bipolar disorder. Acta Psychiatr Scand. 2015 Jun 26;133(2):144–153. doi: 10.1111/acps.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007 Jul;164(7):1050–1060. doi: 10.1176/ajp.2007.164.7.1050. [DOI] [PubMed] [Google Scholar]
- 44.Kahn RS, Fleischhacker WW, Boter H, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008 Mar 29;371(9618):1085–1097. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- 45.American Psychiatric Association. Practice Guideline for the Treatment of Patients with Bipolar Disorder (Revision) American Journal of Psychiatry. 2002;159(April suppl) [PubMed] [Google Scholar]
- 46.Swartz HA, Fagiolini A. Cardiovascular disease and bipolar disorder: risk and clinical implications. Journal of Clinical Psychiatry. 2012 Dec;73(12):1563–1565. doi: 10.4088/JCP.12ac08227. [DOI] [PubMed] [Google Scholar]
- 47.Vancampfort D, Vansteelandt K, Correll CU, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. American Journal of Psychiatry. 2013 Mar 1;170(3):265–274. doi: 10.1176/appi.ajp.2012.12050620. [DOI] [PubMed] [Google Scholar]
- 48.Correll CU, Frederickson AM, Kane JM, Manu P. Equally increased risk for metabolic syndrome in patients with bipolar disorder and schizophrenia treated with second-generation antipsychotics. Bipolar Disorders. 2008 Nov;10(7):788–797. doi: 10.1111/j.1399-5618.2008.00625.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


