Abstract
Olmsted syndrome (OS) is a rare congenital skin disorder characterized by severe palmoplantar and periorificial keratoderma, alopecia, onychodystrophy, and severe pruritus. Recently, pathogenic ‘gain-of-function‘ mutations of the transient receptor potential vanilloid 3 gene (TRPV3), which encodes a cation channel involved in keratinocyte differentiation and proliferation, hair growth, inflammation, pain and pruritus, have been identified to cause OS. Due to the rarity, the pattern of inheritance of OS is still unclear. We report a case of OS in a 3-year-old Korean girl and its underlying gene mutation. The patient presented with a disabling, bilateral palmoplantar keratoderma with onychodystrophy. She also exhibited pruritic eczematous skin lesions around her eyes, ears and gluteal fold. Genetic analysis identified a heterozygous p.Gly568Val missense mutation in the exon 13 of TRPV3. To our knowledge, this is the first case of OS in the Korean population showing a missense mutation p.Gly573Ser.
Keywords: Olmsted syndrome, TRPV3, missense mutation, keratoderma, pruritus
INTRODUCTION
Olmsted syndrome (OS, OMIM 614594) is a rare genodermatosis characterized by mutilating palmoplantar keratoderma, periorificial keratotic plaques, and severe itching.1,2 Patients suffer also from diffuse alopecia, constricting digital bands, and onychodystrophy.2,3 To date, approximately 70 cases of OS have been reported. Most cases are sporadic; however, familial OS has also been found.1,4,5 Pathogenic mutations in the transient receptor potential vanilloid 3 gene (TRPV3) are the cause of autosomal-dominant OS. TRPV3 is a thermosensitive cation nonselective channel predominantly expressed in keratinocytes and sensory neurons.6 Gain-of-function mutations in TRPV3 lead to constitutive activity in mutant channels, resulting in enhanced keratinocyte apoptosis and hyperkeratosis.4 Herein, we report a first Korean case of Olmsted syndrome harboring a relatively rare missense mutation within TRPV3, p.Gly568Val.
CASE REPORT
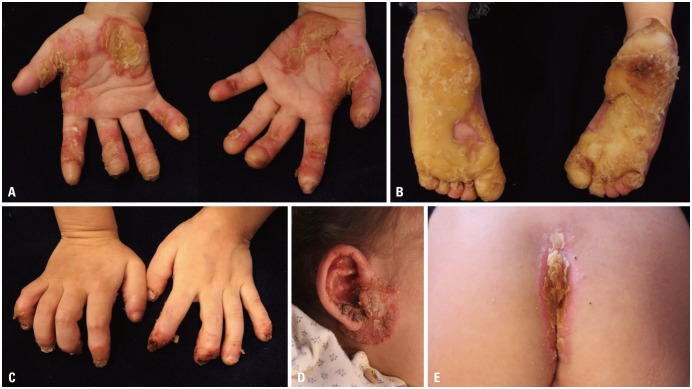
A 3-year-old girl visited our department with severe bilateral palmoplantar hyperkeratosis. Thick, yellowish hyperkeratotic plaques with a foul odor were found on both palms and soles (Fig. 1A and B). She also had thin, brittle nails on all fingers and toes (Fig. 1C), and thin and sparse scalp hairs. The hyperkeratotic lesions initially developed as several punctuate hyperkeratotic plaques, gradually extending to the entire palmoplantar surface. There was yellowish hyperkeratosis on her gluteal fold (Fig. 1D). Multiple hyperkeratotic papules with erythematous, eczematous patches were noted around her eyes, ears and nose (Fig. 1E). She complained of intense pruritus, causing sleep disturbance. She also had walking difficulty due to pain of the plantar lesions.
Fig. 1. Skin lesions at the initial clinical presentation. Diffuse severe keratoderma on (A) the palmar and (B) the plantar surfaces. (C) Onychodystrophy of all fingernails. (D) Periauricular hyperkeratotic papules with eczematous patches. (E) Hyperkeratotic yellowish plaques on the gluteal fold.
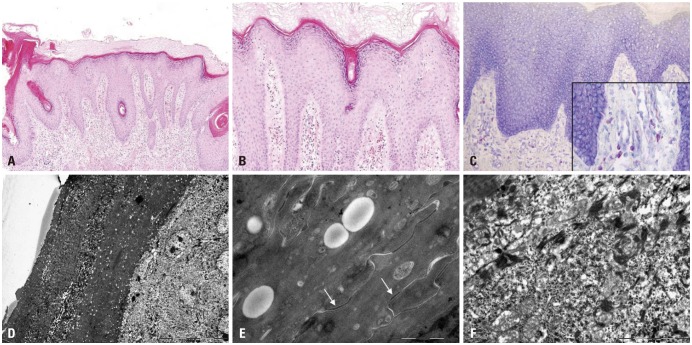
Her height and weight were below the 3rd percentile for her age. Complete blood counts and routine chemistry panels revealed no abnormalities. Her parents were unaffected. A skin biopsy from the coccygeal lesion revealed hyperkeratosis, occasional parakeratosis, hypergranulosis, acanthosis, papillomatosis and follicular plugging with dilated vessels and inflammatory infiltrates of lymphocytes, histiocytes and eosinophils (Fig. 2A and B) in the upper dermis. Toluidine blue stains showed increased numbers of mast cells in the dermis (Fig. 2C). Electron microscopy showed multiple, variable-sized electron-lucent lipid vacuoles in the hyperkeratotic horny layer (Fig. 2D and E), and numerous polysome-like structures in the upper spinous layer (Fig. 2F).
Fig. 2. Skin biopsy features. (A and B) Hyper- and parakeratosis, hypergranulosis, irregular acanthosis and follicular plugging. Dilated vessels with inflammatory cellular infiltrates of lymphocytes, histiocytes and eosinophils in the upper dermis (hematoxylin-eosin, ×100 and ×200, respectively). (C) Increased number of mast cells in the upper dermis (toluidine blue, ×400). (D) Electron microscopy shows numerous lipid droplets in the corneocytes. Parakeratotic corneocytes are also noted (×2K). (E) Electron-lucent lipid droplets are observed, but corneodsmosomes are well preserved (arrows) (×40K). (F) Increased number of polysomes in the keratinocytes of the upper spinous layer (×20K).
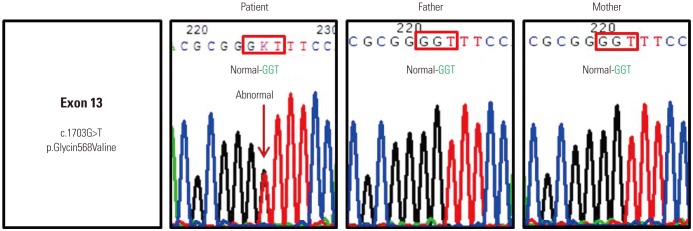
Genomic DNA was extracted from the peripheral blood of the patient and her parents. Sanger sequencing using primers that spanned all exons and exon-intron boundaries (NM_001258205.1) identified a heterozygous G>T transversion at position c.1703 in exon 13 of TRPV3, which leads to the conversion of a glycine residue to valine (p.Gly568Val) (Fig. 3) (primers are listed in Supplementary Table 1, only online). This mutation was not identified in the unaffected parents, indicating a de novo mutation. We did not discover this mutation on 50 healthy Korean controls. The patient showed moderate improvement after treatment with a systemic acitretin, antihistamine, topical antibacterial, steroid ointment and moisturizer.
Fig. 3. Genetic analysis identified a heterozygous missense mutation in c.1703G>T of exon 13 of transient receptor potential vanilloid-3 (TRPV3) (p.Gly-568Val) in the patient's DNA, but not in either parent.
DISCUSSION
To our knowledge, this report describes the first Korean case of OS with a rare pathogenic mutation, p.Gly568Val, in the TRPV3 gene. Mutations in TRPV3 causing OS have been identified in five different amino acid residues, comprising p.Gly568, p.Gly573, p.Leu673, p.Trp692, and p.Asn415_Arg416.3,4,5,6,7,8,9 TRPV3 contains six transmembrane domains (S1–S6), and p.Gly573 which is within the linker region between S4–S5 is the most commonly reported mutation to date in TRPV3.3 Mutations at codon 568, like our case, have been reported in only four OS cases so far; the heterozygous missense mutations at p.Gly568Val and p.Gly568Asp and a splice site mutation at p.Gly568Cys.9,10,11 The amino acid Gly568 also resides within the linker between S4–S5, but near the boundary of S4 and is highly conserved across several species.10 Previous in silico analysis confirmed that the same amino acid substitution in TRPV3 identified in our patient renders the selectivity filter of this ion channel more hyperpermeable.11 Therefore, this variant can be classified as pathogenic (PS1).12
No consistent genotype-phenotype correlations for mutations in the TRPV3 gene are known yet. Notably, a substantial phenotypic diversity in TRPV3-related OS has been reported, even in families whose members share the same mutation, in terms of the severity and extent of the palmoplantar keratoderma and the presence or absence of other hyperkeratotic lesions.10 Indeed, a brazillian OS patient with p.Gly568Val showed mild phenotype such as focal and mild keratodermas, whereas a Japanese case and our patient harboring the same mutation presented diffuse and severe symptoms.10,11 These findings suggest a possibility that additional genetic modifiers or environmental factors may affect the phenotype.
In addition to its role as a thermosensor, TRPV3 plays a significant role in mediating itch and pain sensation and regulating keratinocyte proliferation and differentiation, hair growth, wound healing, and inflammation.13 Several ‘gain-of-function’ mutations in the TRPV3 gene in OS patients were confirmed to exaggerate calcium influx, epidermal turnover, and apoptosis in keratinocytes. Many polysome-like structures that are revealed by electron microscopy may reflect a stimulation of keratinocytes proliferation, which results in the massive acanthosis and hyperkeratosis. In addition, numerous lipid droplets in the corneocytes that are similar to the structural abnormalities in the stratum corneum (SC) of genodermatoses such as Netherton syndrome, lamellar ichthyosis, congenital ichthyosiform erythroderma, and other erythrodermic disorders such as erythrodermic psoriasis may suggest the disturbed cornification with severe perturbation of SC barrier function in OS.14,15 Similar to the TRPV3 transgenic mice, the lesional skin of our patient showed increased mast cells which may trigger cutaneous inflammation and severe itch.
Currently, there are no effective treatments for OS. Topical treatments including emollients, keratolytics, retinoids, corticosteroids, systemic retinoids, and methotrexate provide only temporary symptom relief.3 Our case was moderately improved by low-dose systemic retinoids and topical corticosteroid. Recently, fatty acid-derived pro-resolvents have been found to modulate the activities of transient receptor potential ion channels. Bang, et al.16 reported 17(R)-resolvin D1, a member of docosahexaenoic acid-derived series of resolvins, can specifically suppress TRPV3-mediated activity. Specific inhibitors of TRPV3, such as 17(R)-resolvin D1, could be a promising, biocompatible therapeutic approach for OS in the future. Although TRPV3 mutations were identified as a cause of OS, the exact pathomechanism is still poorly understood. Further studies on the pathogenesis of this unique disease might lead to the development of more effective treatments.
Footnotes
The authors have no financial conflicts of interest.
SUPPLEMENTARY MATERIAL
Coding exons (red) sense and antisense primers blue) for mutation screening of TRPV3 gene
References
- 1.Olmsted HC. Keratodermia palmaris et plantaris congenitalis: report of a case showing associated lesions of unusual location. Am J Dis Child. 1927;33:757–764. [Google Scholar]
- 2.Atherton DJ, Sutton C, Jones BM. Mutilating palmoplantar keratoderma with periorificial keratotic plaques (Olmsted’s syndrome) Br J Dermatol. 1990;122:245–252. doi: 10.1111/j.1365-2133.1990.tb08271.x. [DOI] [PubMed] [Google Scholar]
- 3.Duchatelet S, Hovnanian A. Olmsted syndrome: clinical, molecular and therapeutic aspects. Orphanet J Rare Dis. 2015;10:33. doi: 10.1186/s13023-015-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Z, Chen Q, Lee M, Cao X, Zhang J, Ma D, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. 2012;90:558–564. doi: 10.1016/j.ajhg.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Y, Zeng K, Zhang X, Chen Q, Wu J, Li H, et al. A gain-of-function mutation in TRPV3 causes focal palmoplantar keratoderma in a Chinese family. J Invest Dermatol. 2015;135:907–909. doi: 10.1038/jid.2014.429. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 7.Eytan O, Fuchs-Telem D, Mevorach B, Indelman M, Bergman R, Sarig O, et al. Olmsted syndrome caused by a homozygous recessive mutation in TRPV3. J Invest Dermatol. 2014;134:1752–1754. doi: 10.1038/jid.2014.37. [DOI] [PubMed] [Google Scholar]
- 8.Duchatelet S, Guibbal L, de Veer S, Fraitag S, Nitschké P, Zarhrate M, et al. Olmsted syndrome with erythromelalgia caused by recessive transient receptor potential vanilloid 3 mutations. Br J Dermatol. 2014;171:675–678. doi: 10.1111/bjd.12951. [DOI] [PubMed] [Google Scholar]
- 9.Agarwala MK, George R, Pramanik R, McGrath JA. Olmsted syndrome in an Indian male with a new de novo mutation in TRPV3. Br J Dermatol. 2016;174:209–211. doi: 10.1111/bjd.13910. [DOI] [PubMed] [Google Scholar]
- 10.Wilson NJ, Cole C, Milstone LM, Kiszewski AE, Hansen CD, O'Toole EA, et al. Expanding the phenotypic spectrum of Olmsted syndrome. J Invest Dermatol. 2015;135:2879–2883. doi: 10.1038/jid.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagai H, Takaoka Y, Sugano A, Nakamachi Y, Kawano S, Nishigori C. Identification of a heterozygous p.Gly568Val missense mutation in the TRPV3 gene in a Japanese patient with Olmsted syndrome: in silico analysis of TRPV3. J Dermatol. 2017;44:1059–1062. doi: 10.1111/1346-8138.13844. [DOI] [PubMed] [Google Scholar]
- 12.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borbíró I, Lisztes E, Tóth BI, Czifra G, Oláh A, Szöllosi AG, et al. Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J Invest Dermatol. 2011;131:1605–1614. doi: 10.1038/jid.2011.122. [DOI] [PubMed] [Google Scholar]
- 14.Oji V, Tadini G, Akiyama M, Blanchet Bardon C, Bodemer C, Bourrat E, et al. Revised nomenclature and classification of inherited ichthyoses: results of the First Ichthyosis Consensus Conference in Sorèze 2009. J Am Acad Dermatol. 2010;63:607–641. doi: 10.1016/j.jaad.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Fartasch M, Williams ML, Elias PM. Altered lamellar body secretion and stratum corneum membrane structure in Netherton syndrome: differentiation from other infantile erythrodermas and pathogenic implications. Arch Dermatol. 1999;135:823–832. doi: 10.1001/archderm.135.7.823. [DOI] [PubMed] [Google Scholar]
- 16.Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. Br J Pharmacol. 2012;165:683–692. doi: 10.1111/j.1476-5381.2011.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coding exons (red) sense and antisense primers blue) for mutation screening of TRPV3 gene