ABSTRACT
Zika virus (ZIKV) is an emerging flavivirus that can cause birth defects and neurologic complications. Molecular tests are effective for diagnosing acute ZIKV infection, although the majority of infections produce no symptoms at all or present after the narrow window in which molecular diagnostics are dependable. Serology is a reliable method for detecting infections after the viremic period; however, most serological assays have limited specificity due to cross-reactive antibodies elicited by flavivirus infections. Since ZIKV and dengue virus (DENV) widely cocirculate, distinguishing ZIKV infection from DENV infection is particularly important for diagnosing individual cases or for surveillance to coordinate public health responses. Flaviviruses also elicit type-specific antibodies directed to non-cross-reactive epitopes of the infecting virus; such epitopes are attractive targets for the design of antigens for development of serological tests with greater specificity. Guided by comparative epitope modeling of the ZIKV envelope protein, we designed two recombinant antigens displaying unique antigenic regions on domain I (Z-EDI) and domain III (Z-EDIII) of the ZIKV envelope protein. Both the Z-EDI and Z-EDIII antigens consistently detected ZIKV-specific IgG in ZIKV-immune sera but not cross-reactive IgG in DENV-immune sera in late convalescence (>12 weeks postinfection). In contrast, during early convalescence (2 to 12 weeks postinfection), secondary DENV-immune sera and some primary DENV-immune sera cross-reacted with the Z-EDI and Z-EDIII antigens. Analysis of sequential samples from DENV-immune individuals demonstrated that Z-EDIII cross-reactivity peaked in early convalescence and declined steeply over time. The Z-EDIII antigen has much potential as a diagnostic antigen for population-level surveillance and for detecting past infections in patients.
KEYWORDS: comparative epitope mapping, computational prediction, ELISA, Zika virus, antibody-binding region, cross-reactivity, dengue virus, flavivirus, serological diagnosis, surveillance
INTRODUCTION
Zika virus (ZIKV) is an enveloped, positive-sense, single-stranded RNA virus in the Flavivirus genus, which includes other medically important viruses, such as dengue virus (DENV), West Nile virus, and yellow fever virus (1). ZIKV infection has become a major global health concern because it can disseminate rapidly in naive populations and lead to neurologic sequelae, such as a Guillain-Barré-like syndrome, in otherwise healthy individuals. ZIKV also has the unusual ability among human flaviviruses to be transmitted through sexual contact and from mother to fetus during pregnancy (2). Congenital ZIKV infection can cause developmental abnormalities, including ocular damage, microcephaly, and fetal death (2–5). People at risk of DENV infection are also at risk of ZIKV infection, as both viruses are transmitted by Aedes mosquitoes (3).
Accurate diagnosis is critical to many aspects of the public health response to the Zika disease epidemic (6) but is complicated by multiple factors. Clinically, it is impossible to discern among myriad causes of acute fever and/or rash. Molecular tests are useful for detecting symptomatic flavivirus infections during the brief period immediately following infection (7). However, most individuals with ZIKV infection never seek medical attention because they are asymptomatic or experience only a mild, self-limited illness (8, 9). Beyond this acute period, serological tests are necessary to detect ZIKV infections and to support public health efforts, such as prenatal evaluation and management, risk reduction counseling, and surveillance and outbreak investigations.
Unfortunately, most serological tests lack specificity due to cross-reactive antibodies elicited by flavivirus infections. Neutralization assays, which are more specific but less widely available due to their resource-intensive nature, may or may not clarify IgM results that suggest ZIKV or DENV infection, leaving many weeks of waiting for a diagnosis or giving the ambiguous designation “recent flavivirus infection” (10, 11). Patient serum collected 5 or more days after the onset of symptoms contains a complex mixture of antibody populations against the viral envelope (E) protein, directed to epitopes that are unique to the infecting virus as well as to epitopes that are conserved among flaviviruses (12, 13). Consequently, assays that employ the whole virus or E as antigen do not reliably distinguish infections caused by ZIKV from those caused by DENV (14). Recombinant ZIKV antigens containing epitopes recognized by type-specific but not cross-reactive antibody are needed for the development of serological diagnostic assays with greater specificity for ZIKV infection.
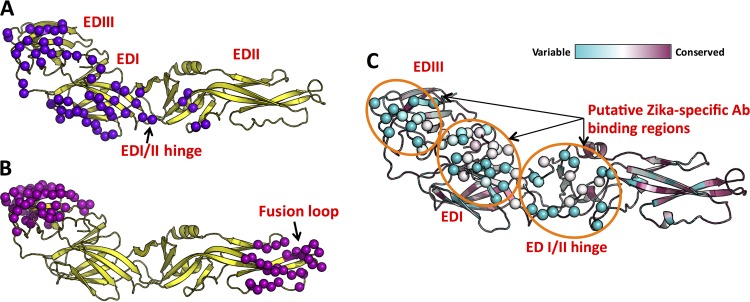
The surface of the ZIKV virion is decorated by 180 copies of E with icosahedral symmetry (12, 15–19). Each E protein monomer is composed of an amino-terminal ectodomain (E80; amino acids [aa] 1 to 403), two amphipathic α-helices, and two carboxy-terminal membrane-spanning α-helices (17–19). The surface-exposed E80 region comprises three distinct domains (EDI, EDII, and EDIII), with EDI in the center. EDI (aa 1 to 49, 136 to 195, and 286 to 302) and EDII are noncontiguous in sequence and are connected by a flexible hinge region (EDI/II hinge), whereas EDIII (aa 303 to 403) is a continuous domain extending from EDI (Fig. 1).
FIG 1.
Identification of putative virus-specific antigenic regions on ZIKV E protein. We performed mapping of type-specific (A) and cross-reactive (B) epitopes on E protein by using experimentally determined antibody complex structures available in the Protein Data Bank. Contact residues observed at the interface between E protein and antibody in the complexes are shown as spheres (purple or magenta). (C) Mapping of the degrees of conservation of amino acid positions among eight clinically relevant flaviviruses. The color scale (cyan, variable region; and maroon, conserved region), as described in ConSurf (33, 51), is shown at the top. Three highly variable regions that overlap type-specific antibody-binding regions in panel A were identified as putative ZIKV-specific antibody-binding regions (orange circles), and the corresponding amino acid residues within this region are shown as spheres.
Here we present the design, production, and evaluation of ZIKV EDI and EDIII antigens (referred to here as Z-EDI and Z-EDIII, respectively) for serological diagnosis of ZIKV by use of well-characterized early- and late-convalescent-phase immune sera from individuals infected by ZIKV, DENV, or both.
MATERIALS AND METHODS
Human subjects and clinical specimens. (i) Samples from North Carolina (15 samples).
Sera were collected from North Carolina residents or visitors with possible or confirmed DENV or ZIKV infection based on self-reported symptoms and travel to or prior residence in areas where flaviviruses are endemic. All specimens were deidentified. All University of North Carolina (UNC) donations were collected in compliance with the Institutional Review Board (IRB) of UNC-Chapel Hill (protocol 08-0895).
(ii) Samples from Nicaragua (24 samples).
Five children from the Nicaraguan Pediatric Dengue Cohort Study (PDCS) who were reverse transcription-PCR (RT-PCR) positive for ZIKV and who experienced onset of signs and symptoms of ZIKV infection between 18 January and 16 February 2016 were included. The PDCS is a community-based prospective study of children of 2 to 14 years of age that has been ongoing since August 2004 in Managua, Nicaragua (20). Participants present at the first sign of illness to the Health Center Sócrates Flores Vivas are monitored daily during the acute phase of illness. Acute-phase and convalescent-phase (∼14 to 21 days after onset of symptoms) blood samples are drawn for DENV, chikungunya virus (CHIKV), and ZIKV diagnostic testing from patients meeting the case definition for DENV or ZIKV infection or presenting with undifferentiated febrile illness. All suspected Zika disease cases were confirmed by RT-PCR analysis of serum and/or urine, using triplex assays that simultaneously screen for ZIKV, DENV, and CHIKV infections (ZCD assay [21] or CDC Trioplex assay) or, in some cases, the CDC ZIKV monoplex assay (22) in parallel with a DENV-CHIKV multiplex assay (23). A second set of 19 specimens was obtained from a prospective, hospital-based study of DENV (1998 to present; Nicaraguan Hospital Infantil Manual de Jesús Rivera). Children of 6 months to 14 years of age with suspected flavivirus infection (<7 days of illness) were enrolled (24) and diagnosed by RT-PCR, and blood was obtained at the acute (days 1 to 6) and convalescent (days 14 to 28) phases as well as 3, 6, 12, and 18 months following infection. Only samples obtained prior to the introduction of ZIKV into Nicaragua were used. All studies were approved by the IRBs of the Nicaraguan Ministry of Health and the University of California, Berkeley. Parents or legal guardians of all subjects provided written informed consent, and subjects who were ≥6 years old provided assent.
(iii) Samples from Colombia (6 samples).
Sera were collected in Sincelejo, Colombia, between December 2015 and March 2016, as part of a field investigation of the ZIKV outbreak and an arbovirus surveillance program conducted by the University of Sucre. All participants provided informed consent prior to blood collection, as described in the University of Sucre Bioethics Committee-approved protocol. Samples were collected during the convalescent phase (3 months after symptom onset) from participants who reported ZIKV-related symptoms.
(iv) Samples from Brazil (9 samples).
A cohort of pregnant women with confirmed or suspected ZIKV infection during pregnancy in Vitoria, Espírito Santo State, Brazil, were enrolled in 2016 in a clinical study to follow ZIKV and other related viruses by RT-PCR, serology, and clinical outcomes for the mother-infant pair, under a protocol approved by the national and local IRBs.
(v) Samples from Sri Lanka (13 samples).
Sera were collected in the convalescent phase from patients with confirmed DENV infection. Acute infection was confirmed by detection of DENV RNA and/or the presence of DENV-specific IgM and IgG in the serum. Samples were collected 2 to 12 weeks after infection, as previously described (25). The IRBs of both the La Jolla Institute for Allergy and Immunology and the Medical Faculty, University of Colombo (serving as an NIH-approved IRB for Genetech), approved all protocols described for this study.
Sera were heat inactivated at 56°C for 30 min. The serostatuses of specimens were categorized as primary or secondary infection by use of neutralization assays (see Tables S1 and S4 in the supplemental material) as previously described (26). Fivefold-diluted sera were mixed with 50 to 100 focus-forming units of DENV1, DENV2, DENV3, DENV4, or ZIKV per well in Dulbecco's modified Eagle medium supplemented with 2% fetal bovine serum (FBS). Virus-antibody mixtures were incubated for 1 h at 37°C, transferred to a confluent monolayer of Vero cells, and then overlaid with medium containing 1% methylcellulose. Infected cell foci were detected 48 h after infection, following fixation with 4% paraformaldehyde and incubation with 500 ng/ml of flavivirus-cross-reactive mouse monoclonal antibody E60 (27) for 2 h at room temperature. After incubation for 1 h with a 1:5,000 dilution of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Sigma), foci were detected by addition of TrueBlue substrate (KPL). Foci were analyzed with a CTL Immunospot instrument. Fifty percent inhibitory concentration (IC50) values were calculated using the sigmoidal dose-response (variable slope) equation in Prism 7 (GraphPad Software). Reported values were required to have an R2 value of >0.75, a hill slope of >0.5, and an IC50 within the range of the assay.
Flavivirus infection status was determined by considering the profile of neutralizing activity of each specimen toward each of five flaviviruses (ZIKV and DENV1 to -4) in the epidemiologic context of the donor. Specimens with neutralizing antibodies to any one serotype of DENV or to ZIKV, with minimal cross-neutralizing antibodies, were defined as having primary flavivirus infections (meaning that the IC50 for a single DENV serotype or ZIKV was >4-fold higher than that for any other virus tested). In most cases, the person's travel history corroborated the immune status. Sera that had high levels of neutralizing antibody to >2 flaviviruses were defined as having secondary flavivirus infections. Most secondary infection samples were from persons who had resided in countries where DENV or ZIKV is endemic. The characteristics of all the samples used in the study are presented in Tables S1 to S4 and S6.
Protein production.
A codon-optimized gene encoding Z-EDI or Z-EDIII from ZIKV strain H/PF/2013 (28) was cloned into the pET PPL His6 MBP expression vector (2K-T) by use of a ligation-independent cloning method (29). The 2K-T plasmid was a gift from Scott Gradia (Addgene plasmid 37183). Maltose binding protein (MBP) fused to Z-EDI or Z-EDIII was expressed in Escherichia coli BL21(DE3)pLysS and purified using amylose affinity resin. The ZIKV E80 antigen (aa 1 to 404) was expressed in the Expi293 transient expression system and purified by use of Ni-nitrilotriacetic acid (Ni-NTA) affinity resin as previously described (30, 31).
IgG ELISA.
Human serum IgG binding was measured by enzyme-linked immunosorbent assay (ELISA) as previously described (32). Recombinant ZIKV E80 antigen (500 ng/well) was used to coat the plate, blocked with 3% milk, and incubated with human serum at the indicated dilution at 37°C for 1 h. Z-EDIII and Z-EDI sandwich ELISAs were the same as described above, except that the antigens (200 ng/well) were captured by use of a murine anti-MBP monoclonal antibody (New England BioLabs). Bound IgG was detected with an alkaline phosphatase-conjugated anti-human secondary antibody by incubation with a p-nitrophenyl phosphate substrate (Sigma), and absorbance at 405 nm was measured on an Epoch plate reader (BioTek). The mean binding signal for each serum was calculated from duplicates by subtracting the mean absorbance of the background signal obtained from positive serum with no antigen (for ZIKV E80) or MBP (for Z-EDIII and Z-EDI). Statistical analysis was performed using the Mann-Whitney U test in Prism 7.0b for nonparametric comparison of recombinant antigen reactivities between sera from ZIKV and DENV patients.
Molecular modeling and structural analysis.
For amino acid conservation analysis by ConSurf (33), eight flavivirus E protein sequences (from ZIKV, four serotypes of DENV, St. Louis encephalitis virus, Japanese encephalitis virus, and yellow fever virus) were used. The ConSurf algorithm assigns a relative conservation score to each residue and normalizes the score such that the average is zero and negative and positive deviations denote the degrees of conservation and variation, respectively. The relative conservation scores were then converted to values ranging from 1 to 9 (1 for most variable [cyan], 5 for average [white], and 9 for most conserved [purple]) to generate a heat map that was used to color the molecular surface of the ZIKV E protein structure.
For type-specific epitope mapping, structures of monoclonal antibody complexes with E or E fragments (Protein Data Bank [PDB] IDs 4UIF [34], 5A1Z [34], 4UIH [34], 3IYW [35], 4C2I [36], 3J05 [37], 3J6U [36], 3UAJ [38], 3UC0 [38], and 1ZTX [39]) were aligned to the reference E protein structure by use of PyMol (The PyMOL Molecular Graphics System, version 1.8; Schrödinger, LLC). For cross-reactive epitope mapping, antibody structure complexes with E or E fragments (PDB IDs 4UT9 [40], 4UT6 [40], 4UTA [40], 3I50 [41], 2R29 [42], 3UZQ [43], 4FFY [44], 5AAM [45], 4L5F, 4BZ2, 4AL8 [46], 3UYP [43], 3UZE [43], and 3UZV [43]) were aligned to the reference E protein structure by use of PyMOL. Contact residues in the E protein-antibody interface were then identified by a 5.0-Å cutoff distance between any atoms in E and any atom in the antibody. All molecular figures were drawn with PyMOL.
RESULTS
Computational prediction of ZIKV-specific antibody-binding regions.
ZIKV E protein shares 55 to 58% sequence identity with DENV E proteins and contains highly conserved epitopes that are responsible for extensive cross-reactivity with polyclonal serum antibodies (47). However, people infected with ZIKV develop some antibodies that neutralize ZIKV but not DENV, demonstrating the presence of epitopes that are unique to ZIKV (26, 48, 49). To identify E protein antigenic regions that may be targets for ZIKV-specific antibodies, we generated and compared surface maps of known DENV antibody epitopes and a map of surface amino acid conservation between different flaviviruses, including ZIKV and the 4 DENV serotypes (Fig. 1). Surface amino acid sequence conservation analysis has been used to identify conserved and variable regions between proteins (50). Our rationale is that such conservation analysis combined with the knowledge of conformational epitopes of E protein can guide prediction of ZIKV-specific antigenic regions.
To perform comparative epitope mapping of E protein, we superimposed experimentally determined structures for type-specific and cross-reactive antibody-E protein complexes onto a reference E structure. Analyzing the residues at the interface between the E protein and the antibody showed that there are two possible cross-reactive antibody-binding sites on the surface of E protein: one site is at the tip of EDII, which contains the fusion loop, and the other is located on the EDIII surface formed by β-strands A, B, E, and G (Fig. 1B). Next, we used the ConSurf algorithm (33, 51) to obtain a conservation score for each amino acid position across 8 different E proteins from clinically relevant flaviviruses (Fig. 1C). Projecting the ConSurf conservation scores onto the molecular surface of the ZIKV E structure showed that most of the solvent-exposed outer surface is variable between flaviviruses, whereas the surface adjacent to the stem region, the transmembrane helices, and the regions contributing to intermolecular assembly are largely conserved. The correlations between cross-reactive epitopes and the conserved regions and between virus-specific epitopes and variable regions were evident across the maps. Accordingly, we identified three regions that we predicted would be recognized by ZIKV type-specific antibodies: a region around the solvent-exposed “glycosylation loop” on EDI and the edge of EDI, a region on the outer surface of the flexible hinge region formed between EDI and EDII, and a region on the “lateral ridge” of EDIII (Fig. 1C).
Expression of ZIKV recombinant antigens.
Following our prediction that epitopes recognized by ZIKV type-specific antibodies are located mainly on EDI and EDIII, we designed two constructs of Z-EDI and Z-EDIII fused to maltose binding protein (MBP) for periplasmic expression in E. coli. Soluble recombinant Z-EDI and Z-EDIII were readily purified by amylose affinity chromatography, with yields of ∼3 mg of purified protein from 1 liter of bacterial culture (Fig. 2A and B). Size exclusion chromatography (SEC) analysis showed that the recombinant antigens behaved as monomeric proteins in solution (Fig. 2C), and the Ellman assay (52) confirmed the presence of intact intramolecular disulfide bonds in the Z-EDI and Z-EDIII antigens. Moreover, Z-EDIII was able to bind to the mouse monoclonal antibodies ZV-2, ZV-48, and ZV-67, which recognize conformational epitopes (48). We also expressed the entire ectodomain of ZIKV E protein (Z-E80) to use as a reference antigen to evaluate the performances of Z-EDI and Z-EDIII.
FIG 2.
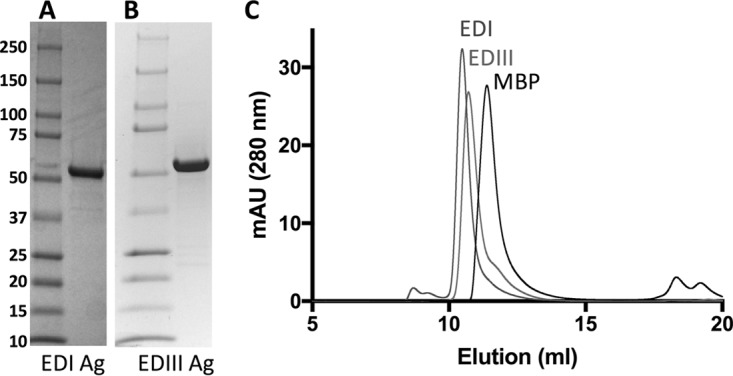
Analysis of purified recombinant antigens by SDS-PAGE and size exclusion chromatography (SEC). Purified Z-EDI (A) and Z-EDIII (B) antigens (6 μg/lane) were subjected to SDS-PAGE under reducing conditions and then stained with Coomassie brilliant blue. Molecular size markers and their apparent masses are shown on the left. (C) SEC overlays of purified EDI, EDIII, and MBP antigens. Protein samples in PBS were subjected to SEC on a Superdex75 10/300GL column. mAU, milli-absorbance units.
Immune sera from people exposed to DENV and ZIKV.
To evaluate recombinant antigens for serological detection of ZIKV infection, we assembled panels of 22 late-convalescent-phase samples (collected >12 weeks after infection) and 43 early-convalescent-phase samples (collected 2 to 12 weeks after infection) from individuals who were exposed to ZIKV, DENV, or both through travel or residence in areas of endemicity (see Tables S1 to S4 in the supplemental material). We categorized the serostatus of each sample in the panels as primary flavivirus immune (evidence of only one serotype of DENV or ZIKV), secondary flavivirus immune (evidence of more than one serotype of DENV or both ZIKV and DENV), or naive (no evidence of DENV or ZIKV) by using a combination of neutralizing activity, RT-PCR, and/or IgG seroconversion as described in Materials and Methods.
Evaluation of ZIKV E80, EDI, and EDIII antigens for serological detection of remote infections (>12 weeks postinfection).
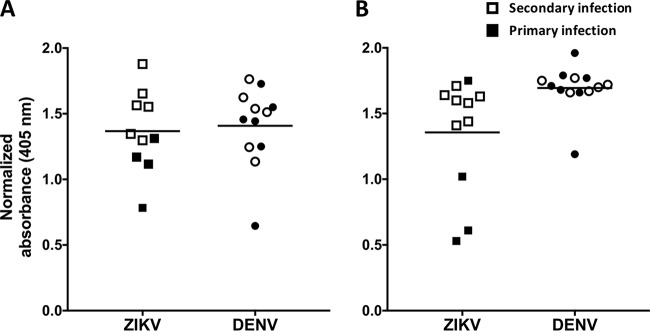
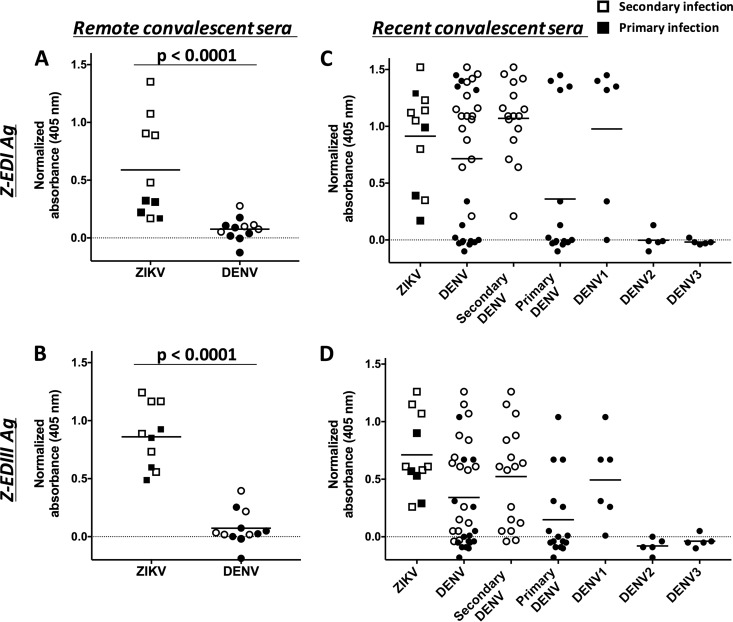
Although ZIKV-immune sera reacted strongly with ZIKV E80, immune sera from individuals infected with DENV consistently showed high levels of cross-reactivity with recombinant ZIKV E80 antigen in a standard IgG ELISA (Fig. 3A). Using an anti-MBP monoclonal antibody to capture MBP fusion proteins, we developed a sandwich ELISA to measure serum IgG levels to Z-EDI and Z-EDIII (Fig. 4A and B). At late convalescence, ZIKV-immune sera recognized Z-EDIII and Z-EDI antigens significantly better than DENV-immune sera (P < 0.0001 by the Mann-Whitney test). Consequently, the Z-EDI and Z-EDIII antigens may be useful for specific detection of remote (>12 weeks) ZIKV infections in areas with endemic DENV transmission.
FIG 3.
Binding of recombinant E80 antigen to sera from patients with remote (A) and recent (B) ZIKV and/or DENV infection. Sera from primary (filled symbols) and secondary (unfilled symbols) ZIKV- and DENV-infected patients were diluted 1:20, and the IgG antibodies bound to recombinant E80 antigen were measured by ELISA. Sera collected ≥12 weeks after infection were defined as remote infections, and sera collected within the first 12 weeks were considered to represent recent infections. The horizontal lines represent the means.
FIG 4.
Binding of Z-EDI and Z-EDIII with remote (A and B) and recent (C and D) convalescent-phase sera from patients infected with ZIKV and/or DENV. Primary (filled symbols) and secondary (unfilled symbols) human serum samples were diluted 1:20, and the IgG antibodies bound to Z-EDI (A and C) or Z-EDIII (B and D) were measured using a sandwich ELISA. Sera collected ≥12 weeks after infection were defined as remote infections, and sera collected within the first 12 weeks were considered to represent recent infections. Statistical significances are indicated at the top of the graphs (Mann-Whitney U test). P values of <0.0001 were considered statistically significant. The horizontal lines represent the means.
Evaluation of ZIKV E80, EDI, and EDIII antigens for serological detection of recent infections (2 to 12 weeks postinfection).
At early convalescence, immune sera collected from ZIKV-infected individuals had high levels of IgG that bound to Z-E80, Z-EDI, and Z-EDIII (Fig. 3B and 4C and D). However, DENV-immune sera collected during the early convalescent phase also reacted strongly with the Z-EDI and Z-EDIII antigens. To dissect Z-EDI and Z-EDIII cross-reactivities during early convalescence, the data were regrouped and the IgG binding activities compared between ZIKV-immune sera and primary or secondary DENV-immune sera (Fig. 4C and D). The IgG reactivities of Z-EDI and Z-EDIII were practically indistinguishable between ZIKV- and DENV-immune sera in secondary cases. While the reactivities to naive and primary DENV2 or -3 sera were mostly at the baseline level, five of the six primary DENV samples collected during the DENV1 epidemic in Sri Lanka showed high reactivity to Z-EDI and Z-EDIII. Recently, individuals with prior DENV1 infection were shown to produce high levels of Z-EDIII-cross-reactive antibodies in early convalescence (53, 54). A conserved lysine residue (K394) on the lateral ridge of ZIKV and DENV1 EDIII was suggested to be responsible for a common mode of binding to DENV1 antibody. However, introducing an alanine at this site (K394A mutation) did not change the reactivity of Z-EDIII against DENV1-immune sera from Sri Lanka (Table S5). As our initial IgG assays were performed using a 1:20 dilution of serum, we further diluted the early-convalescent-phase samples in an attempt to improve specificity. Dilution of early-convalescent-phase serum to dilutions of up to 1:180 was not adequate to improve the specificity of Z-EDI and Z-EDIII against secondary DENV- or DENV1-immune sera (Fig. S1). One major difference in the compositions of antigen-specific antibody populations in early versus late convalescence may be the presence of high levels of IgM. However, depleting total IgM from early-convalescent-phase primary ZIKV samples did not increase IgG binding to Z-EDI or Z-EDIII (data not shown), indicating that IgM does not outcompete IgG for antigen binding in our assay. Taken together, the results showed that IgG cross-reactivity with the Z-EDI and Z-EDIII antigens in DENV-immune sera was pronounced in early-convalescent-phase samples (<12 weeks) from secondary DENV and primary DENV1 infections but not at the late convalescent (>12 weeks) phase.
Longitudinal analysis of ZIKV EDI and EDIII cross-reactivities in DENV-immune samples.
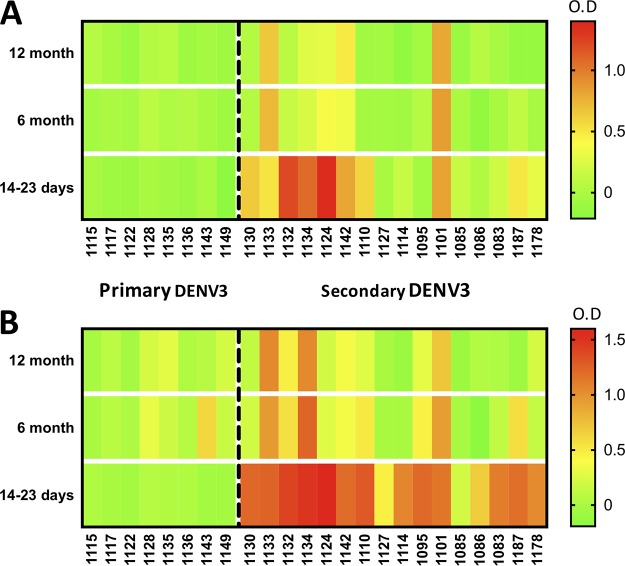
Next, we evaluated sequential serum samples collected as part of a hospital-based study in Nicaragua from 24 children with laboratory-confirmed primary and secondary DENV3 infections (collected before the introduction of ZIKV to the region) (Table S6). This study allowed us to define the time course of cross-reactivity with the Z-EDI and Z-EDIII antigens as well as to compare cross-reactivity with Z-EDI and Z-EDIII in primary DENV infections with that in secondary DENV infections in individuals infected with the same serotype of DENV. All eight primary DENV3 cases showed minimal to no cross-reactivity to Z-EDI and Z-EDIII <1, 6, and 12 months after DENV infection (Fig. 5A and B). However, most secondary DENV-immune samples showed reactivity to Z-EDI and Z-EDIII 14 to 23 days after DENV3 infection. By 6 months after infection, only 4 of 16 subjects still maintained cross-reactive antibodies to Z-EDIII (Fig. 5A), based on a stringent cutoff of an optical density (OD) of 0.3 (the lowest OD observed for the Z-EDIII antigen with any ZIKV-immune sample used in this study). While a trend of declining cross-reactivity was also observed for Z-EDI antibodies, 7 of 16 subjects exhibited cross-reactivity to the secondary DENV3 cases even at 12 months postinfection (Fig. 5B). We concluded that among people exposed to secondary DENV infections, cross-reactive Z-EDIII antibodies typically decline to background levels by 6 months postinfection.
FIG 5.
Patterns of cross-reactivity of Z-EDIII (A) and ZED-I (B) antigens with longitudinal DENV samples. Intensities of serum binding in sandwich ELISAs for Z-EDIII and Z-EDI are shown as heat maps. Longitudinal samples (collected 14 to 23 days, 6 months, and 12 months postinfection) from patients with primary (left of the dashed line) or secondary (right of the dashed line) DENV3 infection were diluted 1:20, and the IgG antibodies bound to Z-EDI or Z-EDIII were measured using a sandwich ELISA. The resulting normalized OD values are represented by a color scale (green, lowest values; yellow, middle values; and red, highest values).
DISCUSSION
As ZIKV is emerging in areas with intense DENV transmission and, more recently, clinical trials of DENV vaccines, there is an urgent need for simple serological assays to distinguish ZIKV infections from DENV infections. Our comparative analysis of surface amino acid conservation among flavivirus E proteins and homology epitope mapping pointed to three regions on ZIKV E protein as potential targets of ZIKV type-specific antibodies. Here we evaluated the utility of recombinant Z-EDI and Z-EDIII antigens, which display two of the three predicted ZIKV-specific antigenic regions. Our results demonstrate that Z-EDIII and, to a lesser extent, Z-EDI are strong candidate antigens for serological tests to differentiate ZIKV infections from DENV infections when samples are collected >12 weeks after infection. The recombinant antigens performed equally well for both primary and secondary infection samples, indicating that specificity was not reduced by high levels of cross-reactive antibodies characteristic of secondary flavivirus infection.
In contrast to that in late convalescence, we observed a high level of cross-reactivity in early-convalescent-phase DENV samples (2 to 12 weeks after infection). Early-convalescent-phase cross-reactivity was more pronounced in secondary than in primary DENV cases. Among individuals exposed to primary DENV infections, we observed low to undetectable levels of antibodies that cross-reacted with Z-EDI and Z-EDIII, except in the case of primary DENV1 infections. Recent studies defined an epitope on EDIII that is conserved between DENV1 and ZIKV (55). A single point mutation at this epitope failed to eliminate the cross-reactivity, indicating the need for additional mutations to ablate the epitope as well as the possibility of other conserved epitopes between ZIKV and DENV1. In secondary DENV cases, we consistently observed high levels of cross-reactivity at early convalescence, irrespective of serotype or geographic location of sample collection.
Our longitudinal analysis of Z-EDI and Z-EDIII reactivities, spanning from early to late convalescent phase, showed that flavivirus-cross-reactive IgG antibodies comprise a transient population that is produced early after infection and declines thereafter, whereas ZIKV-specific responses are more stable over time (26). While the cellular mechanisms responsible for the differential decline of cross-reactive and type-specific serum antibodies are not known, one possible explanation is that many of the cross-reactive antibodies are derived from early plasmablasts or extrafollicular B cells that are not maintained as long-lived plasma cells or memory B cells.
Development of serological tests for diagnosing ZIKV infection in the context of prior flavivirus infection is a challenging and complex problem that remains a major unmet need. To date, there are only three serological assays for ZIKV approved by the U.S. Food and Drug Administration, under an emergency use authorization (56), and a few other commercial tests are available in countries outside the United States or for research purposes. These assays use either NS1, recombinant E, or another, unspecified ZIKV antigen (57). The Centers for Disease Control and Prevention MAC (IgM) ELISA exhibits well-publicized limitations, including false-negative results (58), false-positive results due to cross-reactive antibody from DENV infection (59), and persistence of ZIKV IgM beyond the previously presumed 12-week window (60). Our findings of cross-reactive IgG binding in early convalescence indicate that this period will be the most challenging for optimization of assay specificity. Thus, there is roughly a 10-week period (weeks 2 to 12) following infection when current and next-generation serodiagnostic results may remain ambiguous. One important step forward is found in a recent report evaluating an NS1-based blockade-of-binding assay for ZIKV diagnosis (61). This assay leverages a ZIKV type-specific monoclonal antibody recognizing a nonconserved epitope on ZIKV NS1 (62). Again, a certain secondary DENV group displayed reduced specificity in this NS1-based assay during early convalescence. It may be that a combination of antigens is required to achieve optimal sensitivity and specificity for serum antibody detection, particularly during early convalescence.
Additional issues preclude optimal implementation of many currently available serological assays. First, the serum panels used to evaluate these assays come from remnant clinical specimens or archived sera not collected systematically and specifically for analysis of clinical performance in diagnosing individuals with multiple flavivirus exposures. Second, sera from individuals with a single flavivirus infection history and residing in regions where flavivirus infection is not endemic are not representative of the populations for whom improved diagnostics are most critical, namely, those residing in the tropics, where individuals experience multiple and frequent flavivirus exposures. We are involved with ongoing studies designed to address this shortcoming. Third, sensitivity in different IgM assays can be less than 80%, particularly outside the range of 6 to 60 days, when IgM assays perform best. Lastly, not only have false-positive ZIKV test results been reported due to current or previous DENV infection, but DENV tests may also be positive following confirmed ZIKV infections. The cumulative experience with ZIKV serodiagnosis, to date, clearly indicates that novel approaches will be required.
There are a few notable limitations to our study. Our goal was to explore recombinant ZIKV E antigens for development of improved serodiagnostics. The moderate sample size to which we had access allowed us to achieve that goal; however, a larger sample size will be necessary to define more precise cutoff values and to fully evaluate sensitivity and specificity. Ideally, a candidate diagnostic test would be evaluated in a large cohort of patients with PCR-confirmed infection status, representing multiple serotypes of DENV and other flavivirus exposures, and with availability of longitudinal specimens collected at early and late times after infection.
Diversity in infecting strains of ZIKV may elicit antibodies that target different epitopes or different permutations of the same antigenic region of E protein. While we evaluated only a single construct for each of the Z-EDI and Z-EDIII antigens, we believe that these antigens (from a ZIKV isolate from French Polynesia) are likely to be representative of the vast majority of ZIKV strains in circulation. In fact, E protein amino acid sequences from ZIKV isolates from several different times and places vary by only ≤1%, and both African and Asian lineage strains perform similarly in binding and neutralization assays, suggesting that ZIKV exists as a single serotype (26, 63).
While the present work provides the platform for incorporating Z-EDI and Z-EDIII into a suitable antigen-antibody binding assay for the purposes of surveillance, vaccine efficacy studies, and risk reduction counseling, further modification of Z-EDI and Z-EDIII may improve their utility in the early convalescent phase of ZIKV infection. Cross-reactive antibodies may be depleted using recombinant DENV antigens, but depletion techniques are tedious and time-consuming (26). Introducing amino acid variation through protein engineering is an attractive strategy to eliminate cross-reactive antibody-binding sites while preserving unique epitopes within Z-EDIII and Z-EDI antigens. The high signal we observed for IgG binding to Z-EDIII with a simple ELISA format is encouraging, although a combination of Z-EDI and Z-EDIII as well as fusion of antigens to protein scaffolds may also be tested for improvement of the sensitivity of the assay. Finally, we observed that some individuals are strongly IgG seropositive for only one of the Z-EDI or Z-EDIII antigen, raising the possibility that a multiplex platform employing a panel of antigens may improve sensitivity (64). This approach also has the advantage of allowing the design of expanded antigen panels to detect antibodies specific for additional pathogens that cause clinical presentations similar to those for DENV and ZIKV.
In conclusion, we have demonstrated that Z-EDI and Z-EDIII contain important epitopes that can be used to resolve current serodiagnostic limitations. Ultimately, this work can lead to development of crucial point-of-care ZIKV diagnostics amenable to field use in resource-limited settings. In the process, much can be learned about the epitopes targeted by durable type-specific and cross-reactive human antibodies generated upon ZIKV exposure, which is important for the design of highly efficacious DENV and ZIKV vaccines.
Supplementary Material
ACKNOWLEDGMENTS
These studies were supported by NIAID, NIH, grants R01AI107731 (A.M.D.S.), R21AI134073 (A.M.D.S. and L.P.), R01AI099631 (A.B.), R21/R33AI100186 (A.B.), P01AI106695 (E.H.), and U19AI118610 (E.H.) and by CDC contract 200-2017-93142 (A.M.D.S.). Further support was provided by ZIKAPLAN, which received funding from the European Union's Horizon 2020 research and innovation program under grant agreement 734584. The Brazil pregnancy cohort was funded by FAPES grant 306/2016-74910132/16 (S.R.P.). The Sri Lankan cohort study was funded by NIH contracts HHSN272200900042C and HHSN27220140045C (A.S.).
The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Gabriel Hoesktra for his technical assistance with protein expression and purification. We also thank Magelda Montoya for assistance with this study, as well as past and present members of the study teams based at the Centro de Salud Sócrates Flores Vivas, the Hospital Infantil Manuel de Jesús Rivera, the National Virology Laboratory in the Centro Nacional de Diagnóstico y Referencia, and the Sustainable Sciences Institute in Nicaragua for their dedication and high-quality work, and we are grateful to the study participants and their families.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01504-17.
REFERENCES
- 1.Musso D, Baud D, Gubler DJ. 2016. Zika virus: what do we know? Clin Microbiol Infect 22:494–496. doi: 10.1016/j.cmi.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 2.Lazear HM, Diamond MS. 2016. Zika virus: new clinical syndromes and its emergence in the Western Hemisphere. J Virol 90:4864–4875. doi: 10.1128/JVI.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazear HM, Stringer EM, de Silva AM. 2016. The emerging Zika virus epidemic in the Americas: research priorities. JAMA 315:1945–1946. doi: 10.1001/jama.2016.2899. [DOI] [PubMed] [Google Scholar]
- 4.Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S, Polonio CM, Cunha I, Freitas CL, Brandao WN, Rossato C, Andrade DG, Faria DP, Garcez AT, Buchpigel CA, Braconi CT, Mendes E, Sall AA, Zanotto PM, Peron JP, Muotri AR, Beltrao-Braga PC. 2016. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK. 2016. Zika virus impairs growth in human neurospheres and brain organoids. Science 352:816–818. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- 6.The National Academies of Sciences, Engineering, and Medicine. 2016. Potential research priorities to inform public health and medical practice for domestic Zika virus: workshop in brief. The National Academies Press, Washington, DC. doi: 10.17226/23404. [DOI] [PubMed] [Google Scholar]
- 7.Bingham AM, Cone M, Mock V, Heberlein-Larson L, Stanek D, Blackmore C, Likos A. 2016. Comparison of test results for Zika virus RNA in urine, serum, and saliva specimens from persons with travel-associated Zika virus disease—Florida, 2016. MMWR Morb Mortal Wkly Rep 65:475–478. doi: 10.15585/mmwr.mm6518e2. [DOI] [PubMed] [Google Scholar]
- 8.Duffy MR, Chen T-H, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. 2009. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 9.Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, Shan Yan A, Cao-Lormeau VM, Broult J. 2014. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill 19:20761. doi: 10.2807/1560-7917.ES2014.19.14.20761. [DOI] [PubMed] [Google Scholar]
- 10.Rabe IB, Staples JE, Villanueva J, Hummel KB, Johnson JA, Rose L, MTS, Hills S, Wasley A, Fischer M, Powers AM. 2016. Interim guidance for interpretation of Zika virus antibody test results. MMWR Morb Mortal Wkly Rep 65:543–546. doi: 10.15585/mmwr.mm6521e1. [DOI] [PubMed] [Google Scholar]
- 11.Granger D, Hilgart H, Misner L, Christensen J, Bistodeau S, Palm J, Strain AK, Konstantinovski M, Liu D, Tran A, Theel ES. 2017. Serologic testing for Zika virus: comparison of three Zika virus IgM-screening enzyme-linked immunosorbent assays and initial laboratory experiences. J Clin Microbiol 55:2127–2136. doi: 10.1128/JCM.00580-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wahala WM, Silva AM. 2011. The human antibody response to dengue virus infection. Viruses 3:2374–2395. doi: 10.3390/v3122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, Ahmed R, Suthar MS, Wrammert J. 2016. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A 113:7852–7857. doi: 10.1073/pnas.1607931113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison AC, Minnick SL, Rocha C, Forshey BM, Stoddard ST, Getis A, Focks DA, Russell KL, Olson JG, Blair PJ, Watts DM, Sihuincha M, Scott TW, Kochel TJ. 2010. Epidemiology of dengue virus in Iquitos, Peru 1999 to 2005: interepidemic and epidemic patterns of transmission. PLoS Negl Trop Dis 4:e670. doi: 10.1371/journal.pntd.0000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crill WD, Chang GJ. 2004. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J Virol 78:13975–13986. doi: 10.1128/JVI.78.24.13975-13986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothman AL. 2011. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol 11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 17.Dai L, Song J, Lu X, Deng YQ, Musyoki AM, Cheng H, Zhang Y, Yuan Y, Song H, Haywood J, Xiao H, Yan J, Shi Y, Qin CF, Qi J, Gao GF. 2016. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe 19:696–704. doi: 10.1016/j.chom.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Ng TS, Ooi JS, Shi J, Lok SM. 2016. Structure of the thermally stable Zika virus. Nature 533:425–428. doi: 10.1038/nature17994. [DOI] [PubMed] [Google Scholar]
- 19.Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, Kuhn RJ. 2016. The 3.8 A resolution cryo-EM structure of Zika virus. Science 352:467–470. doi: 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuan G, Gordon A, Aviles W, Ortega O, Hammond SN, Elizondo D, Nunez A, Coloma J, Balmaseda A, Harris E. 2009. The Nicaraguan pediatric dengue cohort study: study design, methods, use of information technology, and extension to other infectious diseases. Am J Epidemiol 170:120–129. doi: 10.1093/aje/kwp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waggoner JJ, Gresh L, Mohamed-Hadley A, Ballesteros G, Davila MJ, Tellez Y, Sahoo MK, Balmaseda A, Harris E, Pinsky BA. 2016. Single-reaction multiplex reverse transcription PCR for detection of Zika, chikungunya, and dengue viruses. Emerg Infect Dis 22:1295–1297. doi: 10.3201/eid2207.160326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waggoner JJ, Ballesteros G, Gresh L, Mohamed-Hadley A, Tellez Y, Sahoo MK, Abeynayake J, Balmaseda A, Harris E, Pinsky BA. 2016. Clinical evaluation of a single-reaction real-time RT-PCR for pan-dengue and chikungunya virus detection. J Clin Virol 78:57–61. doi: 10.1016/j.jcv.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narvaez F, Gutierrez G, Perez MA, Elizondo D, Nunez A, Balmaseda A, Harris E. 2011. Evaluation of the traditional and revised WHO classifications of dengue disease severity. PLoS Negl Trop Dis 5:e1397. doi: 10.1371/journal.pntd.0001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiskopf D, Angelo MA, Grifoni A, O'Rourke PH, Sidney J, Paul S, De Silva AD, Phillips E, Mallal S, Premawansa S, Premawansa G, Wijewickrama A, Peters B, Sette A. 2016. HLA-DRB1 alleles are associated with different magnitudes of dengue virus-specific CD4+ T-cell responses. J Infect Dis 214:1117–1124. doi: 10.1093/infdis/jiw309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins MH, McGowan E, Jadi R, Young E, Lopez CA, Baric RS, Lazear HM, de Silva AM. 2017. Lack of durable cross-neutralizing antibodies against Zika virus from dengue virus infection. Emerg Infect Dis 23:773–781. doi: 10.3201/eid2305.161630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, Pierson TC, Diamond MS. 2006. Antibody recognition and neutralization determinants on domains I and II of West Nile virus envelope protein. J Virol 80:12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baronti C, Piorkowski G, Charrel RN, Boubis L, Leparc-Goffart I, de Lamballerie X. 2014. Complete coding sequence of Zika virus from a French Polynesia outbreak in 2013. Genome Announc 2:e00500-14. doi: 10.1128/genomeA.00500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aslanidis C, de Jong PJ. 1990. Ligation-independent cloning of PCR products (LIC-PCR). Nucleic Acids Res 18:6069–6074. doi: 10.1093/nar/18.20.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metz SW, Gallichotte EN, Brackbill A, Premkumar L, Miley MJ, Baric R, de Silva AM. 2017. In vitro assembly and stabilization of dengue and Zika virus envelope protein homo-dimers. Sci Rep 7:4524. doi: 10.1038/s41598-017-04767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metz SW, Tian S, Hoekstra G, Yi X, Stone M, Horvath K, Miley MJ, DeSimone J, Luft CJ, de Silva AM. 2016. Precisely molded nanoparticle displaying DENV-E proteins induces robust serotype-specific neutralizing antibody responses. PLoS Negl Trop Dis 10:e0005071. doi: 10.1371/journal.pntd.0005071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE Jr, de Silva AM. 2012. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A 109:7439–7444. doi: 10.1073/pnas.1200566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashkenazy H, Abadi S, Martz E, Chay O, Mayrose I, Pupko T, Ben-Tal N. 2016. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res 44:W344–W350. doi: 10.1093/nar/gkw408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fibriansah G, Ibarra KD, Ng TS, Smith SA, Tan JL, Lim XN, Ooi JS, Kostyuchenko VA, Wang J, de Silva AM, Harris E, Crowe JE Jr, Lok SM. 2015. Dengue virus. Cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers. Science 349:88–91. doi: 10.1126/science.aaa8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufmann B, Vogt MR, Goudsmit J, Holdaway HA, Aksyuk AA, Chipman PR, Kuhn RJ, Diamond MS, Rossmann MG. 2010. Neutralization of West Nile virus by cross-linking of its surface proteins with Fab fragments of the human monoclonal antibody CR4354. Proc Natl Acad Sci U S A 107:18950–18955. doi: 10.1073/pnas.1011036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fibriansah G, Tan JL, Smith SA, de Alwis AR, Ng TS, Kostyuchenko VA, Ibarra KD, Wang J, Harris E, de Silva A, Crowe JE Jr, Lok SM. 2014. A potent anti-dengue human antibody preferentially recognizes the conformation of E protein monomers assembled on the virus surface. EMBO Mol Med 6:358–371. doi: 10.1002/emmm.201303404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teoh EP, Kukkaro P, Teo EW, Lim AP, Tan TT, Yip A, Schul W, Aung M, Kostyuchenko VA, Leo YS, Chan SH, Smith KG, Chan AH, Zou G, Ooi EE, Kemeny DM, Tan GK, Ng JK, Ng ML, Alonso S, Fisher D, Shi PY, Hanson BJ, Lok SM, MacAry PA. 2012. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med 4:139ra83. doi: 10.1126/scitranslmed.3003888. [DOI] [PubMed] [Google Scholar]
- 38.Cockburn JJ, Navarro Sanchez ME, Goncalvez AP, Zaitseva E, Stura EA, Kikuti CM, Duquerroy S, Dussart P, Chernomordik LV, Lai CJ, Rey FA. 2012. Structural insights into the neutralization mechanism of a higher primate antibody against dengue virus. EMBO J 31:767–779. doi: 10.1038/emboj.2011.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nybakken GE, Oliphant T, Johnson S, Burke S, Diamond MS, Fremont DH. 2005. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature 437:764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rouvinski A, Guardado-Calvo P, Barba-Spaeth G, Duquerroy S, Vaney MC, Kikuti CM, Navarro Sanchez ME, Dejnirattisai W, Wongwiwat W, Haouz A, Girard-Blanc C, Petres S, Shepard WE, Despres P, Arenzana-Seisdedos F, Dussart P, Mongkolsapaya J, Screaton GR, Rey FA. 2015. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 520:109–113. doi: 10.1038/nature14130. [DOI] [PubMed] [Google Scholar]
- 41.Cherrier MV, Kaufmann B, Nybakken GE, Lok SM, Warren JT, Chen BR, Nelson CA, Kostyuchenko VA, Holdaway HA, Chipman PR, Kuhn RJ, Diamond MS, Rossmann MG, Fremont DH. 2009. Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. EMBO J 28:3269–3276. doi: 10.1038/emboj.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lok SM, Kostyuchenko V, Nybakken GE, Holdaway HA, Battisti AJ, Sukupolvi-Petty S, Sedlak D, Fremont DH, Chipman PR, Roehrig JT, Diamond MS, Kuhn RJ, Rossmann MG. 2008. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nat Struct Mol Biol 15:312–317. doi: 10.1038/nsmb.1382. [DOI] [PubMed] [Google Scholar]
- 43.Cockburn JJ, Navarro Sanchez ME, Fretes N, Urvoas A, Staropoli I, Kikuti CM, Coffey LL, Arenzana Seisdedos F, Bedouelle H, Rey FA. 2012. Mechanism of dengue virus broad cross-neutralization by a monoclonal antibody. Structure 20:303–314. doi: 10.1016/j.str.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Austin SK, Dowd KA, Shrestha B, Nelson CA, Edeling MA, Johnson S, Pierson TC, Diamond MS, Fremont DH. 2012. Structural basis of differential neutralization of DENV-1 genotypes by an antibody that recognizes a cryptic epitope. PLoS Pathog 8:e1002930. doi: 10.1371/journal.ppat.1002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson LN, Tharakaraman K, Rowley KJ, Costa VV, Chan KR, Wong YH, Ong LC, Tan HC, Koch T, Cain D, Kirloskar R, Viswanathan K, Liew CW, Tissire H, Ramakrishnan B, Myette JR, Babcock GJ, Sasisekharan V, Alonso S, Chen J, Lescar J, Shriver Z, Ooi EE, Sasisekharan R. 2015. Structure-guided design of an anti-dengue antibody directed to a non-immunodominant epitope. Cell 162:493–504. doi: 10.1016/j.cell.2015.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Midgley CM, Flanagan A, Tran HB, Dejnirattisai W, Chawansuntati K, Jumnainsong A, Wongwiwat W, Duangchinda T, Mongkolsapaya J, Grimes JM, Screaton GR. 2012. Structural analysis of a dengue cross-reactive antibody complexed with envelope domain III reveals the molecular basis of cross-reactivity. J Immunol 188:4971–4979. doi: 10.4049/jimmunol.1200227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Loriere E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, England P, Stiasny K, Mongkolsapaya J, Heinz FX, Screaton GR, Rey FA. 2016. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 536:48–53. doi: 10.1038/nature18938. [DOI] [PubMed] [Google Scholar]
- 48.Zhao H, Fernandez E, Dowd KA, Speer SD, Platt DJ, Gorman MJ, Govero J, Nelson CA, Pierson TC, Diamond MS, Fremont DH. 2016. Structural basis of Zika virus-specific antibody protection. Cell 166:1016–1027. doi: 10.1016/j.cell.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. 2009. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology 392:103–113. doi: 10.1016/j.virol.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lichtarge O, Bourne HR, Cohen FE. 1996. An evolutionary trace method defines binding surfaces common to protein families. J Mol Biol 257:342–358. doi: 10.1006/jmbi.1996.0167. [DOI] [PubMed] [Google Scholar]
- 51.Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. 2010. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res 38:W529–W533. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ellman GL. 1959. Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 53.Rogers TF, Goodwin EC, Briney B, Sok D, Beutler N, Strubel A, Nedellec R, Le K, Brown ME, Burton DR, Walker LM. 2017. Zika virus activates de novo and cross-reactive memory B cell responses in dengue-experienced donors. Sci Immunol 2:eaan6809. doi: 10.1126/sciimmunol.aan6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robbiani DF, Bozzacco L, Keeffe JR, Khouri R, Olsen PC, Gazumyan A, Schaefer-Babajew D, Avila-Rios S, Nogueira L, Patel R, Azzopardi SA, Uhl LFK, Saeed M, Sevilla-Reyes EE, Agudelo M, Yao KH, Golijanin J, Gristick HB, Lee YE, Hurley A, Caskey M, Pai J, Oliveira T, Wunder EA Jr, Sacramento G, Nery N Jr, Orge C, Costa F, Reis MG, Thomas NM, Eisenreich T, Weinberger DM, de Almeida ARP, West AP Jr, Rice CM, Bjorkman PJ, Reyes-Teran G, Ko AI, MacDonald MR, Nussenzweig MC. 2017. Recurrent potent human neutralizing antibodies to Zika virus in Brazil and Mexico. Cell 169:597.e11–609.e11. doi: 10.1016/j.cell.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolze A, Byun M, McDonald D, Morgan NV, Abhyankar A, Premkumar L, Puel A, Bacon CM, Rieux-Laucat F, Pang K, Britland A, Abel L, Cant A, Maher ER, Riedl SJ, Hambleton S, Casanova J-L. 2010. Whole-exome-sequencing-based discovery of human FADD deficiency. Am J Hum Genet 87:873–881. doi: 10.1016/j.ajhg.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.US FDA. 2016. Zika virus emergency use authorization. https://www.fda.gov/MedicalDevices/Safety/EmergencySituations/ucm161496.htm#zika.
- 57.Safronetz D, Sloan A, Stein DR, Mendoza E, Barairo N, Ranadheera C, Scharikow L, Holloway K, Robinson A, Traykova-Andonova M, Makowski K, Dimitrova K, Giles E, Hiebert J, Mogk R, Beddome S, Drebot M. 2017. Evaluation of 5 commercially available Zika virus immunoassays. Emerg Infect Dis 23:1577–1580. doi: 10.3201/eid2309.162043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis AC. 2017. D.C.'s botched Zika testing leaves dozens of families monitoring for symptoms. https://www.washingtonpost.com/local/dc-politics/dcs-botched-zika-testing-leaves-dozens-of-families-monitoring-for-symptoms/2017/05/09/3ab24958-34db-11e7-b373-418f6849a004_story.html?utm_term=.fcd651095232.
- 59.Education CsDoICa. 2016. FDA warns health care providers against relying solely on Zika virus serological IgM assay results; reminds them to wait for confirmatory test results before making patient management decisions: FDA safety communication. http://www.firstwordmedtech.com/node/992281.
- 60.Oduyebo T, Polen KD, Walke HT, Reagan-Steiner S, Lathrop E, Rabe IB, Kuhnert-Tallman WL, Martin SW, Walker AT, Gregory CJ, Ades EW, Carroll DS, Rivera M, Perez-Padilla J, Gould C, Nemhauser JB, Ben Beard C, Harcourt JL, Viens L, Johansson M, Ellington SR, Petersen E, Smith LA, Reichard J, Munoz-Jordan J, Beach MJ, Rose DA, Barzilay E, Noonan-Smith M, Jamieson DJ, Zaki SR, Petersen LR, Honein MA, Meaney-Delman D. 2017. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure—United States (including U.S. territories), July 2017. MMWR Morb Mortal Wkly Rep 66:781–793. doi: 10.15585/mmwr.mm6629e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balmaseda A, Stettler K, Medialdea-Carrera R, Collado D, Jin X, Zambrana JV, Jaconi S, Cameroni E, Saborio S, Rovida F, Percivalle E, Ijaz S, Dicks S, Ushiro-Lumb I, Barzon L, Siqueira P, Brown DWG, Baldanti F, Tedder R, Zambon M, de Filippis AMB, Harris E, Corti D. 2017. Antibody-based assay discriminates Zika virus infection from other flaviviruses. Proc Natl Acad Sci U S A 114:8384–8389. doi: 10.1073/pnas.1704984114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F, Cameroni E, Hewson R, Harris E, Lanzavecchia A, Sallusto F, Corti D. 2016. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 63.Dowd KA, DeMaso CR, Pelc RS, Speer SD, Smith AR, Goo L, Platt DJ, Mascola JR, Graham BS, Mulligan MJ, Diamond MS, Ledgerwood JE, Pierson TC. 2016. Broadly neutralizing activity of Zika virus-immune sera identifies a single viral serotype. Cell Rep 16:1485–1491. doi: 10.1016/j.celrep.2016.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fonseca BP, Marques CF, Nascimento LD, Mello MB, Silva LB, Rubim NM, Foti L, Silva ED, Ferreira AG, Krieger MA. 2011. Development of a multiplex bead-based assay for detection of hepatitis C virus. Clin Vaccine Immunol 18:802–806. doi: 10.1128/CVI.00265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.