Abstract
Objectives
Recurrent respiratory papillomatosis (RRP) is a chronic disease of the respiratory tract that occurs in both children and adults. It is caused by the human papillomavirus (HPV), in particular low‐risk HPV6 and HPV11, and aggressiveness varies among patients. RRP remains a chronic disease that is difficult to manage. This review provides perspectives on current and future management of RRP.
Results
The current standard of care is surgical excision, with adjuvant therapies as needed. Surgical management of RRP has evolved with the introduction of microdebriders and photoangiolytic lasers; the latter can now be used in the office setting. Numerous adjuvant pharmacologic therapies have been utilized with some success. Also, exciting preliminary data show that HPV vaccines may prolong the time to recurrence in the RRP population. There is also optimism that wide‐spread HPV vaccination could reduce RRP incidence indirectly by preventing vertical HPV transmission to newborns.
Conclusion
To date, the biology of RRP is not well understood, although it has been noted to become more aggressive in the setting of immune suppression. Additional research is needed to better understand immune system dysfunction in RRP such that immunomodulatory approaches may be developed for RRP management.
Level of Evidence
4
Keywords: Recurrent respiratory papillomatosis (RRP), human papillomavirus (HPV), laryngeal papillomatosis, microdebrider, vaccine
INTRODUCTION
Recurrent respiratory papillomatosis (RRP) is a rare disease caused by low‐risk human papillomavirus (HPV) types 6 and 11; it is characterized by recurrent exophytic papillomas of the epithelial mucosa in the respiratory tract (Fig. 1).1, 2 Based on the age of patients, RRP is characterized as juvenile‐onset or adult‐onset. Patients presenting with this disease before 12 years of age are diagnosed with juvenile‐onset recurrent respiratory papillomatosis (JO‐RRP), while patients presenting after 12 years of age are diagnosed with adult–onset recurrent respiratory papillomatosis (AO‐RRP).3 Derkay et al. estimated an incidence rate of 4.3 per 100,000 in JO‐RRP and 1.8 per 100,000 in AO‐RRP.4 However, prevalence of RRP is variable and depends on several factors, including geographic location, age of onset, and socioeconomic condition (Table 1). JO‐RRP occurs via vertical transmission during pregnancy or is acquired at birth from an HPV‐infected mother; it has a more aggressive clinical course.5, 6 Acquisition of AO‐RRP is not well studied. A report showed that AO‐RRP risk is associated with the number of sexual partners; however, this finding was not confirmed in a subsequent study.7, 8
Figure 1.
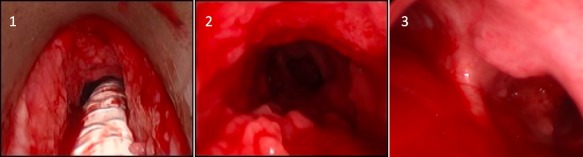
Single patient with tracheal and right mainstem bronchial involvement. This indicates more severe RRP with distal spread. 1. Subglottic papilloma. 2. Distal tracheal papilloma. 3. Two sites of papilloma growth: at the anterior tracheal wall just proximal to carina and just distal to the carina at the right proximal mainstem bronchus.
RRP = Recurrent respiratory papillomatosis.
Table 1.
Summary of Epidemiological Data in Reviewed Studies.
| Study | Study Years | Study Design | Incidence Rate | Prevalence Rate | Conclusion |
|---|---|---|---|---|---|
| Lindeberg and Elbrond107 | 1965–1984 | Retrospective study of 231 patients first presenting with RRP between the years 1965 and 1984 | Overall: 3.84 per 100,000; JO‐RRP: 3.62 per 100,000; AO‐RRP: 3.94 per 100,000 | N/A | The incidence rate in the Danish subpopulation remained constant between 1969 and 1984; the low incidence from 1965–1968 could be real or due to selection bias |
| Derkay4 | 1993–1994 | 315 otolaryngologists completed the survey | JO‐RRP: 4.3 per 100,000; AO‐RRP: 1.8 per 100,000 | N/A | A registry of patients with current RRP would benefit future research protocols and help the long‐term follow‐up of patients |
| Armstrong et al.108 | 1996 | 101 physicians in Atlanta and 139 physicians from Seattle participated | JO‐RRP: 1.11 per 100,000 in Atlanta and 0.36 per 100,000 in Seattle | JO‐RRP: 2.59 per 100,000 in Atlanta and 1.69 per 100,000 in Seattle | Data represents a crude estimate of the national incidence and prevalence rates |
| Armstrong et al.109 | 1997–1998 | 20 tertiary care pediatric otolaryngology centers surveyed | N/A | N/A | Children diagnosed before the age of 3 were more likely to have severe RRP than those diagnosed after the age of 3 |
| Reeves et al.5 | 1996–2002 | 22 tertiary care pediatric otolaryngology centers surveyed | N/A | N/A | Young age was the most important determinant of disease severity |
| Campisi et al.110 | 1994–2007 | Multicenter | JO‐RRP: 0.24 per 100,000 children aged 14 years and younger | JO‐RRP: 1.11 per 100,000 children aged 14 years and younger | Successfully developed Canadian national database for JO‐RRP |
Literature estimates that 5% of the population carries HPV DNA in the larynx, yet only a small percentage develop RRP.9 So, why do some HPV‐infected individuals not develop RRP? It is hypothesized that RRP is a multi‐gene disease that polarizes innate and adaptive immune responses to tolerate HPV6/11 infection and predisposes certain individuals to develop RRP. Studies have shown that early HPV proteins, driven predominately by HPV E6, alter the innate immune response and skew adaptive immunity to a TH2‐like phenotype.10, 11, 12, 13, 14, 15, 16 In addition, certain HLA alleles as well as the absence of specific innate immune receptors may also predispose an individual to RRP development and contribute to disease severity.17, 18 There is also evidence that, of the low‐risk subtypes, HPV11 is associated with a more aggressive clinical course than HPV6, but more research is necessary to understand the differences between these viral proteins.19 Furthermore, a 2% malignant degeneration incidence has been observed in RRP patients.20 Spontaneous degeneration may be due to the fact that low‐risk HPVs can drive gene expression in papilloma similar to that characteristically found in some malignancies.13
Currently, there is no cure for the disease, and treatment is primarily focused on maintaining airway patency and voice quality. Patients often require multiple surgeries in a short amount of time and occasionally adjuvant therapy when surgery is unable to control the disease, making RRP an expensive disease to treat. It has been reported that the average number of surgeries in the first five years of diagnosis is 5.1 per year, dropping to 0.1 per year after 15 years.5, 21 Chesson et al. estimated that the lifetime cost per case of RRP is $198,500, not including drug treatment, with tracheotomy care accounting for approximately 5% of this estimate and the remainder from surgical costs.22
Traditional management of RRP has been surgical excision in the operating room (OR) under general anesthesia, primarily with potassium‐titanyl‐phosphate (KTP) lasers or microdebriders, with some surgeons also using CO2 lasers or cold steel instruments. The advent of the flexible fiber delivery system has made in‐office laser procedures possible, which can save time and health‐care expense and be more convenient for patients.23, 24 RRP remains a difficult disease to manage; this review provides perspectives on current and future means of RRP management.
COUNSELING RRP PATIENTS
HPV is classified as a sexually‐transmitted virus; however, RRP is not. Newly diagnosed AO‐RRP patients often have many questions regarding disease acquisition, course, and transmission, making it important to provide a framework for discussion between the patient and the health‐care provider. In children, vertical transmission from an HPV‐positive mother is presumed to occur in the birth canal and not from caregivers or siblings via horizontal transmission.25, 26 A maternal history of genital papilloma is the leading risk factor for JO‐RRP, and there is conflicting evidence whether birth by caesarian section is protective against RRP incidence in newborns.25, 27, 28 In addition, there is evidence for horizontal transmission of HPV in children with a history of suspected sexual abuse.29 HPV6 and HPV11 are the most common causes of genital papilloma, spreading by direct contact in areas of friction and mucosal disruption.30 Thus, one possible mode of HPV infection related to AO‐RRP is orogenital spread of HPV.31, 32 HPV6 and HPV11 were reported to be present in the oral cavity of less than 0.5% of the non‐RRP population between 14 and 69 years of age.33 On the other hand, 26 of 27 (96%) RRP patients were found to have concurrent oral cavity HPV. Moreover, 67% of long‐term sexual partners of HPV‐positive RRP patients had oral cavity HPV present.34 However, data is conflicting regarding correlation of RRP risk with number of sexual partners, and health‐care providers are not obligated to disclose HPV status with a patient's sexual partner(s). It should be noted that the disclosure of anogenital HPV could result in anxiety and negatively impact interpersonal relationships.35 In general, health‐care providers should convey the up‐to‐date literature regarding HPV to RRP patients; however, this discussion needs to proceed in a careful and sensitive manner.
SURGICAL MANAGEMENT OF RRP
The current standard of care for the management of RRP is surgical excision. Objectives of surgery are to preserve adequate voice quality and airway patency.36 Complete eradication is not necessarily the goal, as HPV is believed to remain dormant in laryngeal epithelial cells whether active papilloma is visible or not. More extensive excision of papilloma from sites that are not contributing to airway or voice‐related goals has not been shown to reduce recurrence rates.37 In fact, aggressive resection may be counter‐productive, in that injury to the mucosal surface has been associated with increased expression of HPV in nearby HPV‐infected cells.38 Aggressive resection is also contraindicated in the setting of disease involving the anterior or posterior commissure; these sites often require staged or sub‐total removal of the papilloma. This measured surgical approach preserves function by preventing webbing and scarring at the anterior commissure to limit dysphonia and at the posterior commissure to limit airway obstruction. Interestingly, a retrospective study of 29 patients found that the most common sites of recurrence were the anterior commissure, subglottis, and epiglottis, and that these subsites tended to be closely correlated with submucosal glandular density. In this study, recurrence rates at these subsites were controlled by en bloc excision that included underlying submucosal glands and scar tissue from previous procedures.36 In patients with very aggressive disease, an additional goal is to prevent distal spread of papilloma to the lower respiratory tract.37, 39 Tracheotomies are usually reserved for patients with aggressive disease that has the potential to occlude the airway, as studies have shown that a tracheostomy provides an additional site for rapid colonization and distal spread of RRP. If tracheotomy is unavoidable, decannulation is advised as soon as the disease is controlled and patency of the airways is maintained.40, 41
Surgical instruments have evolved in the management of RRP from non‐powered laryngeal instruments to lasers, and more recently to microdebriders (Fig. 2).42, 43, 44, 45, 46 Different types of lasers and cold instruments can be used separately or in combination, and both cold instruments and lasers can offer excellent surgical outcomes. In comparison to lasers, an increase in complication rate and a decrease in voice quality have been reported for cold instruments; however, these differences may be highly dependent on the surgeons' technical skills.47
Figure 2.
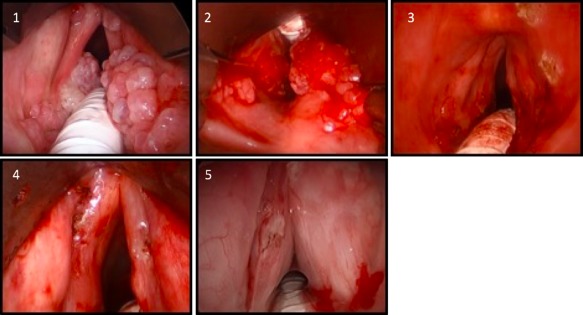
Images 1–4 are from a single patient. 1. Appearance of papilloma with supraglottic and vocal fold involvement. 2. Appearance during debridement. 3. Appearance post‐KTP laser treatment. 4. Magnified view of vocal folds post‐KTP laser treatment. 5. Appearance after cidofovir injection into vocal folds (separate patient).
KTP = potassium‐titanyl‐phosphate; RRP = Recurrent respiratory papillomatosis.
Lasers
Laser surgery offers several advantages and disadvantages in the removal of laryngeal papillomas. Lasers have better hemostatic properties and longer working distances than cold instruments.48 However, laser procedures require more personnel to ensure efficacy and safety and have greater installation and maintenance costs.48 Mechanically, it is important that repeated laser energy is not delivered to the same location because it could result in deep tissue injury. For this reason, the shortest possible pulse and lowest possible power that will effectively accomplish the procedure is recommended.24 Other safety concerns of lasers include tracheal injuries, tracheoesophageal fistula formation, and airway tract burns.24, 49 Determining whether or not laser surgery is the best option should be based on the surgeon's experience and skill, anatomical location of the lesion, and the patient's anatomy.50
There are two broad categories of lasers that differ in their selectivity: cutting/ablating lasers, such as 10,600‐nm CO2 and 2,013‐nm Thulium lasers, which target water, and photoangiolytic lasers, such as 585‐nm pulsed‐dye (PDL) and 532‐nm potassium‐titanyl‐phosphate (KTP) lasers, which target hemoglobin.24 The first laser utilized in managing RRP was the CO2 laser. Multiple surveys report that CO2 lasers are more widely used than photoangiolytic lasers, although KTP lasers are gaining popularity.40, 42, 51 The CO2 laser's cutting ability, along with its ability to cauterize, have made it a popular tool.24, 52 Conversely, photoangiolytic lasers precisely target hemoglobin within the microcirculation of the highly vascularized papillomatous tissue and may have better hemostatic effects than the CO2 laser.50 Photoangiolytic lasers have also shown better preservation of surrounding normal tissue.24, 53 However, one group did report in 2004 that CO2 lasers are less likely to cause deep tissue damage than photoangiolytic lasers.54
Comparing the two different photoangiolytic lasers, hemoglobin absorbs the KTP laser wavelength more strongly than the PDL wavelength, resulting in greater coagulation and less adjacent tissue damage.55 Thus, KTP lasers are more widely used than PDLs for the removal of papillomas. Kuet et al. investigated the effectiveness of both KTP and PDL photoangiolytic surgery in the treatment of RRP in a retrospective case series including 68 KTP cases and 13 PDL cases, and found that both significantly improved voice‐related quality of life, Derkay score, and need for operative intervention under general anesthesia at the 18‐month follow‐up interval.56 The Derkay score is a staging system to classify the severity of RRP; the operating surgeon assigns a score from 0 to 3 (0 = absent, 1 = surface lesion, 2 = raised lesion, and 3 = bulky lesion) to each site in the aerodigestive tract, and these scores are added to obtain the composite severity score.57 Early clinical data with photoangiolytic lasers in RRP have been encouraging and should be further investigated.
Microdebrider
Microdebriders have gained popularity due to the possible risks associated with the use of lasers and the speed they provide when removing bulkier lesions. Microdebriders afford the surgeon simultaneous debridement by the rapidly rotating blade and selective suctioning of the affected tissue.49 In fact, microdebriders are often used in combination with lasers, with microdebriders first removing the bulk of the papilloma, then lasers providing hemostatic ability and more precise treatment of sessile disease. Advantages of microdebriders over lasers and cold instruments include shorter operating time and absence of thermal injury.58
In‐Office Procedures
The advent of awake in‐office laser procedures for RRP has offered an alternative to traditional OR management under general anesthesia. In general, office laser procedures are well‐tolerated in adult patients who have received adequate topical anesthesia, and most patients experience minimal postoperative pain.59, 60 In many cases, patients can drive themselves to and from the appointment. Several studies have shown that both KTP and PDL lasers are safe and effective for in‐office treatment of RRP.61, 62, 63, 64, 65 Serious complications are very rare, with mild discomfort during the procedure being the most common complication.62 Advantages of in‐office procedures over OR management include avoidance of general anesthesia risks, reduced health‐care cost, and shorter procedural times.23, 24 While office procedures decrease the number of surgeries and general anesthetics, it is not an option for every patient; those with bulky or extensive papillomas or inadequate tolerance of the scope are poor candidates. Awake procedures are also not suitable for most children with RRP.23 Literature suggests that adult patients presenting with a new diagnosis of RRP should be treated first in the OR under general anesthesia to allow for disease evaluation and tissue biopsies; however, subsequent procedures can be done in the office depending on time and extent of disease, patient tolerance, and surgeon experience. Furthermore, if there is a significant change in growth pattern, a new biopsy is warranted in the OR.23 A study found that patients were less likely to be managed in the office if they were diagnosed at an earlier age, had greater disease severity, or had diabetes.66 In addition, two pilot studies showed preliminary evidence for post‐excision, office‐based, intralesional administration of the adjuvants bevacizumab and cidofovir improving the outcome of KTP and CO2 laser excisions, respectively.67, 68
ADJUVANT THERAPIES FOR RRP
Surgery is the primary treatment modality for RRP; however, approximately 20% of RRP patients require adjuvant therapy because surgery alone cannot control the disease.42, 69 A survey indicated that surgeons typically consider adjuvant therapy in patients getting surgery more than 3–4 times per year, but actual indications are not well‐defined. In young professionals with high voice demands, for example, adjuvant therapy may be used sooner.4 Current adjuvants have a range of actions including immunomodulation, disruption of HPV replication, control of inflammation, and prevention of angiogenesis; yet, due to the incurable nature of RRP, these therapies can only be considered as adjuvant to surgery. In addition, some of these therapies have only been evaluated in small group or case studies and need more powerful randomized controlled trials to sufficiently evaluate their efficacy in RRP management.
Interferon
Interferon (IFN) therapy is one of the first systemic adjuvant treatments used to manage RRP.49 Interferons are proteins released from leukocytes in response to a variety of stimuli, including viral infection, to upregulate antigen production and activate immune cells.70 The clinical efficacy of IFN therapy in the treatment of RRP is controversial.70, 71, 72 One group reported that 117 of 160 (73.1%) of patients treated with adjuvant IFN‐alpha‐2b had complete or partial response measured by extent of recurrence.72 Conversely, another group showed that initial growth rate reduction of papillomas from IFN‐alpha treatment in the first six months post‐treatment was not durable and became insignificant in the second six months post‐treatment.70 Unmodified recombinant IFN‐alpha is no longer on the market and has been replaced by pegylated‐IFN‐alpha‐2a (peg‐IFN‐alpha‐2a). One study treated 11 AO‐RRP patients with peg‐IFN‐alpha‐2a in combination with granulocyte monocyte–colony‐stimulating factor (GM‐CSF) and found that 11/11 (100%) showed no relapse at 12 months' follow‐up.73 Side effects for IFN therapy include neurologic disorders, mental disturbances, thrombocytopenia, leukopenia, hair loss, and fever.71 Despite some positive evidence for adjuvant IFN therapy, it is rarely used due to the emergence of intralesional adjuvants, such as cidofovir and bevacizumab, which have fewer local and systemic side effects.
Cidofovir
Cidofovir is a cytosine nucleotide analog that blocks the replication of DNA viruses by inhibiting viral DNA polymerase.74 Its mechanism of action against HPV is not well understood, although it has been hypothesized that it acts by augmenting the immune system or inducing apoptosis.75 Intralesional administration of cidofovir has been fairly well‐tolerated, with limited systemic toxicity.76, 77, 78, 79, 80 Prospective trials in patients treated with intralesional cidofovir have shown marked papilloma regression as well as complete disease remission in both the JO‐RRP and AO‐RRP populations (Table 2). A group investigated the efficacy of intralesional cidofovir following surgical excision in 16 JO‐RRP patients and found a 75% complete remission rate, with stable disease to a mean of 33.6 months.81, 82, 83 In a separate cohort by the same group, intralesional cidofovir with surgery in 19 AO‐RRP patients induced an 89% complete remission rate, with stable disease to a mean of 24 months.81, 82, 84 Interestingly, Broekema and Dikkers reported that 5 of 188 (2.7%) patients receiving intralesional cidofovir developed dysplasia. However, it is important to note that cidofovir is most likely not the cause of dysplasia in this cohort since the incidence of spontaneous malignant degeneration in RRP is 2–3%.76 According to a 2013 RRP task force survey of 74 laryngeal surgeons that have used cidofovir to treat RRP, cidofovir may be initiated when surgical debulking is required every two to three months, and dosing should remain below established safe levels (3 mg/kg) and volume.85
Table 2.
Summary of Cidofovir in Reviewed Studies.
| Study | Patients (N) | Mean No. of Injections | Mean Concentration (mg/mL) | Treatment (mo.) | Mean Follow‐up (mo.) | Results | Conclusion |
|---|---|---|---|---|---|---|---|
| Snoeck et al.80 | 17 (16 AO/1 JO) | 7.0 | 2.5 | 5 | 15 | Complete remission in 14 (82.4%) patients, 13 AO and 1 JO | Treatment was well‐tolerated and no immediate side effects were observed |
| Bielamowicz et al.111 | 14 AO | 6.0 | 4.17‐6.25 | 12 | Up to 3 years | Complete remission in all 14 (100%) patients | Intralesional cidofovir is a good treatment option with limited local and systemic effects |
| Akst et al.112 | 11 JO | 4, if recurred an additional 4 | 5, if recurred an additional 10 | 4, if recurred an additional 4 | 1 | Derkay severity score decreased in all patients from a mean ± SD of 13.7 ± 6.0 to 2.1 ± 3.4 | Intralesional cidofovir reduced burden of disease in children with RRP; recurrent disease may be treated with increased dosage |
| Lee et al.113 | 16 (12 AO/4 JO) | 3.5 | 2.5‐5 | 3 weeks | 25.4 | Complete remission in 10 (77%) patients, 8 AO and 2 JO | Found to be efficacious in treating RRP; more follow up is needed to analyze long‐term effectiveness |
| Naiman et al. and Coulombeau et al.81, 82, 83 | 16 JO | 8.9 | 5–7.5 | 2–4 weeks | 33.6 | Complete remission obtained in 12 (75%) patients; remission stable to a mean of 33.6 months follow‐up | Surgical excision in combination with intralesional cidofovir is efficacious; relapse associated with long delay in initiating cidofovir treatment |
| Naiman et al. and Coulombeau et al.81, 82, 84 | 19 AO | 4.5 | 5–7.5 | 2–4 weeks | 24 | Complete remission was obtained in 17 (89%) patients; remission stable to a mean of 24 months follow‐up | Surgical excision in combination with intralesional cidofovir is efficacious in AO‐RRP; concentration and interval between injections influenced the number of injections needed to achieve remission |
AO = adult‐onset; JO = juvenile‐onset; RRP = Recurrent respiratory papillomatosis
Bevacizumab
Bevacizumab is a recombinant monoclonal humanized antibody that blocks angiogenesis by inhibiting the human vascular endothelial growth factor A (VEGF‐A).49 Approved by the FDA in 2004, it was the first angiogenesis inhibitor available in the US and was used as an adjuvant to chemotherapy in metastatic cancers.86 Rahbar et al. conducted a retrospective study to determine the role of VEGF‐A in the pathogenesis of RRP patients.87 Strong expression of VEGF‐A mRNA was noted in the squamous epithelium of all 12 RRP patients, and strong expression of VEGFR‐1 and VEGFR‐2 were noted in the endothelial cells of the papillomas' blood vessels.87 These observations provided the rationale for assessing the use of bevacizumab in the context of RRP. Several studies have shown that bevacizumab is relatively safe and active in JO‐RRP and AO‐RRP (Table 3). Sidell et al. treated eight JO‐RRP patients by debulking the papillomas with a KTP laser, then administering intralesional bevacizumab at 14.25 mg doses at four‐ to six‐week intervals.88 The median Derkay scores decreased by 58% post‐treatment and, moreover, the time between procedures more than doubled.88 Another group studied the efficacy of adjunct bevacizumab with KTP laser excision on 20 adult patients with bilateral vocal fold RRP.89 They reported that 19/20 (95%) patients had better disease control in the bevacizumab/KTP laser‐treated vocal fold than on the KTP laser‐only‐treated vocal fold, despite selecting the vocal fold with more extensive disease to receive the bevacizumab/KTP laser treatment.89 Consistent with these studies, other groups have reported promising results using the combination of KTP laser and bevacizumab, with minimal complications.88, 90, 91, 92
Table 3.
Summary of Bevacizumab in Reviewed Studies.
| Study | Therapy Techniques | Patients (N) | Mean Age (yr. ± SD) | Mean Dose (mg ± SD) | Treatment Interval | Results | Conclusion |
|---|---|---|---|---|---|---|---|
| Maturo et al.90 | Surgical debridement with microdebrider, KTP laser debulking, and intralesional bevacizumab injection | 3 | 4.66 | 1.25 | 1–6 mo. | Derkay scores lowered significantly and PVRQOL scores improved in two patients; time between surgeries increased in all patients | Bevacizumab appears to show efficacy in increasing time between surgeries for children with severe RRP |
| Zietels et al.89 | KTP laser debulking followed by bevacizumab injection into vocal folds | 20 | 18–60 | 10 | Once every six weeks for six months | 19/20 (95%) patients showed less disease in the bevacizumab‐treated vocal cord despite starting with more disease | Bevacizumab has a synergistic effect with KTP laser in RRP without systemic or local complications |
| Best et al.91 | KTP laser (63/100 procedures) then intralesional bevacizumab injection | 43 | 48 ± 14 | 30 ± 13 | Mean 2.3 treatment sessions | No local or systemic side effects measured by physiologic data | Higher doses of bevacizumab are relatively safe in adult patients with laryngeal RRP |
| Rogers et al.92 | Surgical debridement with microdebrider, KTP laser debulking, and intralesional bevacizumab injection | 10 | 3.55 | 2.5 | 6–9 weeks | Time between surgeries increased, number of procedures per year decreased, Derkay scores decreased, and PVRQOL scores improved | Intralesional bevacizumab treatment may increase duration of time between surgical procedures; can improve voice QOL |
| Sidell et al.88 | KTP laser debulking followed by intralesional bevacizumab injection | 8 | 9.25 | 14.25 | 4–6 weeks | Median Derkay scores decreased by 58% post‐treatment and time between procedures increased by a median of 2.05x | High‐dose bevacizumab appears to yield positive results for pediatric patients with RRP |
KTP = potassium‐titanyl‐phosphate; RRP = Recurrent respiratory papillomatosis; PVRQOL = pediatric voice‐related quality of life; QOL = quality of life
Celecoxib
Celecoxib is a COX‐2‐selective non‐steroidal anti‐inflammatory drug used to manage pain and inflammation associated with osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, painful menstruation, and other acute and chronic pain indications.49 Overexpression of COX‐2 was observed in the papillomas of RRP patients, and this increase was proposed to be a consequence of epithelial growth factor receptor (EGFR) and phosphatidylinositol 3‐kinase (PI‐3K) signaling.93 In 2009, Limsukon et al. showed success in treating an RRP patient with a combination of celecoxib and erlotinib (a tyrosine kinase inhibitor) at doses of 400 mg per day and 150 mg per day, respectively.94 This patient had several surgical procedures and received IV cidofovir, but her recurrence rate accelerated and her disease began to involve the mainstem and segmental bronchi.94 The patient underwent a 3‐month surveillance bronchoscopy following erlotinib/celecoxib therapy and surprisingly, there was no evidence of disease recurrence.94 A randomized double‐blind controlled study to determine the safety and efficacy of celecoxib in both pediatric and adult RRP patients was recently completed (NCT 00571701). The primary objective of this trial is to determine the efficacy of celecoxib response in comparison to conventional endoscopy and surgical treatment. All the patients in this study were evaluated under general anesthesia for disease severity at three‐month intervals for 30 months; any papillomas present at the time of evaluation were surgically excised. Patients were randomly divided into early and delayed treatment arms. Patients in the early treatment arm received 12 months of 400 mg (adults), 200 mg (pediatric weight greater than 25 kg), or 100 mg (pediatric weight between 12 and 25 kg) of celecoxib daily, then 12 months of placebo daily. The late treatment arm received daily placebo for the first 12 months, then daily celecoxib for the second 12 months. Primary endpoint data showed that celecoxib treatment did not affect the mean percent change in papilloma growth rate at the 12‐month measurement compared to baseline (p = 0.57). Analysis of secondary outcomes showed no reduction in papilloma growth rate when comparing age (juvenile‐ vs. adult‐onset, p = 1.00), gender (male vs. female, p > 0.3), or HPV subtype (HPV6 vs. HPV11, p > 0.5).
PD‐1 Inhibitor
Programmed cell death protein 1 (PD‐1), which is present on the surface of leukocytes, negatively regulates the immune system when it binds to ligands PD‐L1 and PD‐L2 on antigen‐presenting cells (APCs); PD‐L1 has been shown to be highly expressed in HPV‐associated head and neck squamous cell carcinoma (HNSCC).95, 96 PD‐1 inhibitors, such as pembrolizumab, block the interaction between PD‐1 and its ligands and have clinical efficacy in numerous advanced solid tumors, including HPV‐associated HNSCC.97 The activity against HPV‐associated HNSCC has prompted investigators to initiate a Phase II clinical trial to assess the efficacy of pembrolizumab in RRP (NCT02632344). RRP patients will be administered 200 mg pembrolizumab as a 30‐minute IV infusion every three weeks on day 1 of each cycle, after all procedures and assessments have been completed. This is consistent with current dosing standards in treatment of recurrent or metastatic HNSCC.
HPV Vaccine
Perhaps the most exciting development in the management of RRP is prevention through HPV vaccination. The quadrivalent HPV vaccine, Gardasil, has activity against both low‐risk HPV types 6 and 11 and high‐risk HPV types 16 and 18.49 Since HPV6 and HPV11 are the predominant etiologic factors for RRP, the quadrivalent vaccine has been used to manage RRP.98 Forster et al. used the quadrivalent vaccine to treat a two‐year‐old boy with severe JO‐RRP.99 The boy's condition was stabilized after the third immunization and no surgery was needed for 10 months.99 Young et al. conducted a retrospective chart review of 20 RRP patients treated with Gardasil and reported that 13/20 (65%) patients had complete remission or partial remission, with a 3.1‐month increase in the time between surgical interventions.100 Another study of 11 AO‐RRP patients reported an increase in the mean time between surgical interventions from 271 to 537 days (p = 0.03) and a decrease in mean surgeries per year from 2.16 to 0.93 (p = 0.02) after quadrivalent vaccination.101 A recent systematic review of seven studies investigated the role of quadrivalent HPV vaccination for secondary prevention of RRP. All seven case reports or cohort studies treating active RRP with quadrivalent HPV vaccination reported an increased interval between surgeries or decreased recurrence.102 Furthermore, studies have shown that quadrivalent vaccination in RRP patients with HPV DNA‐positivity and zero or low anti‐HPV antibodies increased both anti‐HPV6 and anti‐HPV11 antibodies.98, 103, 104 These case reports and small study series show encouraging results; however, multi‐center randomized controlled trials are needed to fully assess the efficacy of the HPV vaccination as a therapeutic vaccine in the RRP population. Currently, the Centers for Disease Control and Prevention (CDC) recommends the new nonavalent vaccine, Gardasil‐9. Current trends indicate that wide‐spread vaccination of pre‐adolescent females will further decrease HPV genital wart acquisition. This is expected to reduce the incidence of secondary laryngeal infections to newborns via vertical HPV transmission and, in turn, reduce JO‐RRP and overall RRP incidence.105
Other Adjuvants
Other novel adjuvant approaches have been attempted for the management of RRP, with some clinical success (Table 4). Gefitinib, an EGFR tyrosine kinase inhibitor, was used in a life‐threatening RRP case when all other treatments were exhausted. This particular case was a 14‐year‐old black male born with fetal alcohol syndrome. He underwent a tracheostomy at three‐months‐old due to extensive HPV11‐associated RRP and subsequently, disease recurred with complete airway stenosis that extended to the trachea and mainstem bronchi. He was treated with IFN‐alpha‐2a until he developed hypertension, nephrotic syndrome, and renal failure at age eight. Attempts to control disease with surgical resection, local debulking, oral and inhaled ribavirin (antiviral), indole‐3‐carbinol, and PDT all failed, and he was not a candidate for cidofovir due to renal failure. After being treated surgically for a life‐threatening airway obstruction, gefitinib was administered at a dosage of 250 mg twice daily for 11 months because his papillomas overexpressed EGFR and were becoming increasingly life‐threatening. Debulking procedures were significantly reduced from 15 procedures per three months before gefitinib treatment to five procedures per three months during gefitinib treatment, with acceptable toxicity.106 This exciting case report suggests that EGFR inhibitors may be offered as second‐line therapy in EGFR‐positive RRP.
Table 4.
Other Adjuvant Therapies.
| Treatment | Rationale | Study Type | Treatment Type | Administration | No. of Patients | Follow‐Up | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Acyclovir114 | Antiviral drug that targets thymidine kinase expressed by herpes simplex virus‐1 and Epstein‐Barr virus, which are occasional concurrent and co‐infections of HPV in RRP | Case series | Antiviral agent | PO | 3 adults | 1 year | Complete remission with no residual disease after 1 year follow‐up in 2 patients | Oral acyclovir as an adjuvant to surgery may reduce recurrence in RRP; larger cohort studies are needed to assess efficacy |
| Ribavirin115 | Antiviral drug that is used to treat respiratory syncytial virus pneumonia in infants and has shown some promise in treating aggressive RRP | Uncontrolled clinical trial | Antiviral agent | PO | 4 (1 child; 3 adults) | 4 months | 2 adults achieved minimal recurrence; the other adult and child achieved increased intervals between surgeries | Ribavirin may be an effective adjuvant to laser surgery, but needs a larger controlled clinical trial to assess efficacy |
| Indole‐3‐carbinol (I3C)116 | RRP lesions exhibit increased estrogen binding, and a study in mice showed that inhibition of estrogen metabolism with I3C reduced HPV‐induced papilloma tumor formation by 75%117 | Prospective, open label, multicenter | Dietary supplement (cruciferous vegetables) | PO | 33 (9 children; 24 adults) | Mean 4.8 years | After 8 months or more of treatment, 11 (33%) patients had cessation of papilloma growth and did not require further surgery, 10 (30%) had reduced papilloma growth rate, and 12 (36%) had no evident response | There is potential for I3C as an adjuvant to surgery, but larger blinded, controlled studies need to be performed |
| Cis‐retinoic acid118 | In the aerodigestive tract, vitamin A deficiency has shown increased hyperkeratinization and squamous metaplasia, while excess has shown to suppress squamous differentiation and cause mucous metaplasia | Double‐blind, randomized pilot | Retinoid | PO | 9 | 18–34 months | 4/6 (67%) treated patients experienced recurrence, and all experienced toxicity | Cis‐retinoic acid appears ineffective as an adjuvant to surgery in RRP and further studies do not seem warranted |
HPV = human papilloma virus; RRP = Recurrent respiratory papillomatosis
CONCLUSION
RRP is a chronic disease that is difficult to manage due to the unpredictability of its recurrence and aggressiveness. Literature supports that low‐risk HPV 6/11 enters the basal epithelium and drives local immune dysfunction, resulting in benign, exophytic papillomas. Evidence points toward the polarization of the adaptive immune system to a TH2‐like or T‐reg phenotype by low‐risk HPV E6, which suppresses clearance of HPV infection.10, 16 It is still unclear, however, why certain RRP patients experience a more severe disease course than others. There is evidence that frequency of certain HLA alleles as well as the absence of certain innate immune receptors may predispose people to develop RRP, and also may be linked to disease aggressiveness.11, 18 Research is building to support the notion that the papilloma microenvironment is immunocompromised, and thus, regaining TH1 cell function may be a tractable approach to prevent persistent infection.
Currently, RRP is managed by surgical excision primarily, both in the operating room and in the office setting. Tools including the microdebrider, useful for removing bulky disease, and photoangiolytic lasers such as the KTP laser, useful for more precise targeting of pathologic tissue, are both frequently employed in surgery for RRP. The advent of the flexible laser system and good topical anesthesia techniques have allowed for office‐based procedures to replace OR procedures in many RRP cases. This is an advantage for both the patient and the surgeon, as it has been shown to decrease procedural time and costs, minimize risks of anesthesia, and allow patients to take less time off work. Patient selection for in‐office procedures is dependent upon a variety of factors, including extent of disease, patient tolerance, and surgeon experience.
When surgery is insufficient to control the disease course, adjuvant pharmacologic therapies are administered. IFN‐alpha was the earliest treatment strategy to augment the immune system in RRP patients; however, systemic side effects of IFN‐alpha have limited its use, and adjuvant therapies have moved toward intralesional bevacizumab and cidofovir. Immunomodulatory agents, such as the anti‐PD‐1 antibody pembrolizumab, have shown activity against HPV‐associated HNSCC, and an ongoing Phase II clinical trial has been initiated to assess the efficacy of pembrolizumab in RRP. Furthermore, HPV vaccines, including the newly developed nonavalent Gardasil‐9, have shown therapeutic benefit, but randomized clinical trials are needed to evaluate the efficacy of HPV vaccines to reduce the recurrence rate of RRP. Due to the low incidence of RRP, acquiring a sufficient number of patients may be difficult for a single institutional study; thus, large, multi‐center clinical trials are required to assess the efficacy of the HPV vaccines. Routine HPV vaccination in pre‐adolescent children is also expected to secondarily reduce RRP incidence by decreasing the incidence of genital warts caused by HPV, thus reducing vertical transmission of HPV to newborns.
In conclusion, RRP is incurable with current treatment modalities. Adjuvant therapies are utilized when surgery cannot control disease, and the efficacy of adjuvants is limited to increasing the time interval between surgical procedures. Additional research on the interplay between low‐risk HPV and the immune system is critical and may lead to the development of novel immunomodulatory approaches to better manage RRP patients.
Conflicts of interest: The authors whose names are listed above certify that they have no affiliations with or involvement in any organization that has financial or non‐financial interest in the material discussed in this manuscript.
Footnotes
JO‐RRP = juvenile‐onset recurrent respiratory papillomatosis; AO‐RRP = adult‐onset recurrent respiratory papillomatosis
N/A indicates that incidence rates were not evaluated in that study.
N/A indicates that prevalence rates were not evaluated in that study.
Contributor Information
Quintin Pan, Email: Quintin.Pan@osumc.edu.
Laura Matrka, Email: Laura.Matrka@osumc.edu.
BIBLIOGRAPHY
- 1. Duggan MA, Lim M, Gill MJ, Inoue M. HPV DNA typing of adult‐onset respiratory papillomatosis. Laryngoscope 1990;100(6):639–642. [DOI] [PubMed] [Google Scholar]
- 2. Derkay CS, Wiatrak B. Recurrent respiratory papillomatosis: a review. Laryngoscope 2008;118(7):1236–1247. [DOI] [PubMed] [Google Scholar]
- 3. Larson DA, Derkay CS. Epidemiology of recurrent respiratory papillomatosis. APMIS 2010;118(6–7):450–454. [DOI] [PubMed] [Google Scholar]
- 4. Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg 1995;121(12):1386–1391. [DOI] [PubMed] [Google Scholar]
- 5. Reeves WC, Ruparelia SS, Swanson KI, Derkay CS, Marcus A, Unger ER. National registry for juvenile‐onset recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg 2003;129(9):976–982. [DOI] [PubMed] [Google Scholar]
- 6. Rodier C, Lapointe A, Coutlee F, et al. Juvenile respiratory papillomatosis: risk factors for severity. J Med Virol 2013;85(8):1447–1458. [DOI] [PubMed] [Google Scholar]
- 7. Kashima HK, Shah F, Lyles A, et al. A comparison of risk factors in juvenile‐onset and adult‐onset recurrent respiratory papillomatosis. Laryngoscope 1992;102(1):9–13. [DOI] [PubMed] [Google Scholar]
- 8. Ruiz R, Achlatis S, Verma A, et al. Risk factors for adult‐onset recurrent respiratory papillomatosis. Laryngoscope 2014;124(10):2338–2344. [DOI] [PubMed] [Google Scholar]
- 9. Abramson AL, Steinberg BM, Winkler B. Laryngeal papillomatosis: clinical, histopathologic and molecular studies. Laryngoscope 1987;97(6):678–685. [DOI] [PubMed] [Google Scholar]
- 10. Bonagura VR, Hatam LJ, Rosenthal DW, et al. Recurrent respiratory papillomatosis: a complex defect in immune responsiveness to human papillomavirus‐6 and ‐11. APMIS 2010;118(6–7):455–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonagura VR, Vambutas A, DeVoti JA, et al. HLA alleles, IFN‐gamma responses to HPV‐11 E6, and disease severity in patients with recurrent respiratory papillomatosis. Hum Immunol 2004;65(8):773–782. [DOI] [PubMed] [Google Scholar]
- 12. DeVoti J, Hatam L, Lucs A, et al. Decreased Langerhans cell responses to IL‐36gamma: altered innate immunity in patients with recurrent respiratory papillomatosis. Mol Med 2014;20:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeVoti JA, Rosenthal DW, Wu R, Abramson AL, Steinberg BM, Bonagura VR. Immune dysregulation and tumor‐associated gene changes in recurrent respiratory papillomatosis: a paired microarray analysis. Mol Med 2008;14(9–10):608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenthal DW, DeVoti JA, Steinberg BM, Abramson AL, Bonagura VR. T(H)2‐like chemokine patterns correlate with disease severity in patients with recurrent respiratory papillomatosis. Mol Med 2012;18:1338–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosenthal DW, Schmidtmayerova H, Steinberg BM, et al. Recurrent respiratory papillomatosis (RRP): Disease severity associates with enhanced TH2‐like dendritic cell chemokine (DC‐CK1) plasma expression. J Allergy Clin Immunol 115(2):S81. [Google Scholar]
- 16. DeVoti JA, Steinberg BM, Rosenthal DW, et al. Failure of gamma interferon but not interleukin‐10 expression in response to human papillomavirus type 11 E6 protein in respiratory papillomatosis. Clin Diagn Lab Immunol 2004;11(3):538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonagura VR, Siegal FP, Abramson AL, et al. Enriched HLA‐DQ3 phenotype and decreased class I major histocompatibility complex antigen expression in recurrent respiratory papillomatosis. Clin Diagn Lab Immunol 1994;1(3):357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bonagura VR, Du Z, Luo L, et al. KIR3DS1, KIR2DS1, and KIR2DS5 protect against the development of severe recurrent respiratory papillomatosis (RRP) in HPV‐6/11‐infected patients. J Allergy Clin Immunol 2009;123(2):S165–S165. [Google Scholar]
- 19. Rabah R, Lancaster WD, Thomas R, Gregoire L. Human papillomavirus‐11‐associated recurrent respiratory papillomatosis is more aggressive than human papillomavirus‐6‐associated disease. Pediatr Dev Pathol 2001;4(1):68–72. [DOI] [PubMed] [Google Scholar]
- 20. Nebesio CL, Mirowski GW, Chuang TY. Human papillomavirus: clinical significance and malignant potential. Int J Dermatol 2001;40(6):373–379. [DOI] [PubMed] [Google Scholar]
- 21. Silverberg MJ, Thorsen P, Lindeberg H, Ahdieh‐Grant L, Shah KV. Clinical course of recurrent respiratory papillomatosis in danish children. Arch Otolaryngol Head Neck Surg 2004;130(6):711–716. [DOI] [PubMed] [Google Scholar]
- 22. Chesson HW, Forhan SE, Gottlieb SL, Markowitz LE. The potential health and economic benefits of preventing recurrent respiratory papillomatosis through quadrivalent human papillomavirus vaccination. Vaccine 2008;26(35):4513–4518. [DOI] [PubMed] [Google Scholar]
- 23. Shah MD, Johns MM, 3rd Office‐based laryngeal procedures. Otolaryngol Clin North Am 2013;46(1):75–84. [DOI] [PubMed] [Google Scholar]
- 24. Yan Y, Olszewski AE, Hoffman MR, et al. Use of lasers in laryngeal surgery. J Voice 2010;24(1):102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kosko JR, Derkay CS. Role of cesarean section in prevention of recurrent respiratory papillomatosis–is there one? Int J Pediatr Otorhinolaryngol 1996;35(1):31–38. [DOI] [PubMed] [Google Scholar]
- 26. Sun JD, Weatherly RA, Koopmann CF, Jr , Carey TE. Mucosal swabs detect HPV in laryngeal papillomatosis patients but not family members. Int J Pediatr Otorhinolaryngol 2000;53(2):95–103. [DOI] [PubMed] [Google Scholar]
- 27. Silverberg MJ, Thorsen P, Lindeberg H, Grant LA, Shah KV. Condyloma in pregnancy is strongly predictive of juvenile‐onset recurrent respiratory papillomatosis. Obstet Gynecol 2003;101(4):645–652. [DOI] [PubMed] [Google Scholar]
- 28. Shah K, Kashima H, Polk BF, Shah F, Abbey H, Abramson A. Rarity of cesarean delivery in cases of juvenile‐onset respiratory papillomatosis. Obstet Gynecol 1986;68(6):795–799. [PubMed] [Google Scholar]
- 29. Sinclair KA, Woods CR, Kirse DJ, Sinal SH. Anogenital and respiratory tract human papillomavirus infections among children: age, gender, and potential transmission through sexual abuse. Pediatrics 2005;116(4):815–825. [DOI] [PubMed] [Google Scholar]
- 30. Taliercio S, Cespedes M, Born H, et al. Adult‐onset recurrent respiratory papillomatosis: a review of disease pathogenesis and implications for patient counseling. JAMA Otolaryngol Head Neck Surg 2015;141(1):78–83. [DOI] [PubMed] [Google Scholar]
- 31. Aaltonen LM, Rihkanen H, Vaheri A. Human papillomavirus in larynx. Laryngoscope 2002;112(4):700–707. [DOI] [PubMed] [Google Scholar]
- 32. Partridge JM, Koutsky LA. Genital human papillomavirus infection in men. Lancet Infect Dis 2006;6(1):21–31. [DOI] [PubMed] [Google Scholar]
- 33. Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012;307(7):693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Born H, Ruiz R, Verma A, et al. Concurrent oral human papilloma virus infection in patients with recurrent respiratory papillomatosis: a preliminary study. Laryngoscope 2014;124(12):2785–2790. [DOI] [PubMed] [Google Scholar]
- 35. Diaz ML. Counseling the patient with HPV disease. Obstet Gynecol Clin North Am 2013;40(2):391–402. [DOI] [PubMed] [Google Scholar]
- 36. Kim HT, Baizhumanova AS. Is recurrent respiratory papillomatosis a manageable or curable disease? Laryngoscope 2016;126(6):1359–1364. [DOI] [PubMed] [Google Scholar]
- 37. Siegel B, Smith LP. Management of complex glottic stenosis in children with recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol 2013;77(10):1729–1733. [DOI] [PubMed] [Google Scholar]
- 38. Chow LT, Broker TR, Steinberg BM. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS 2010;118(6–7):422–449. [DOI] [PubMed] [Google Scholar]
- 39. Hartnick CJ, Boseley ME, Franco RA, Jr , Cunningham MJ, Pransky S. Efficacy of treating children with anterior commissure and true vocal fold respiratory papilloma with the 585‐nm pulsed‐dye laser. Arch Otolaryngol Head Neck Surg 2007;133(2):127–130. [DOI] [PubMed] [Google Scholar]
- 40. Tasca RA, Clarke RW. Recurrent respiratory papillomatosis. Arch Dis Child 2006;91(8):689–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cole RR, Myer CM, 3rd, Cotton RT. Tracheotomy in children with recurrent respiratory papillomatosis. Head Neck 1989;11(3):226–230. [DOI] [PubMed] [Google Scholar]
- 42. Schraff S, Derkay CS, Burke B, Lawson L. American Society of Pediatric Otolaryngology members' experience with recurrent respiratory papillomatosis and the use of adjuvant therapy. Arch Otolaryngol Head Neck Surg 2004;130(9):1039–1042. [DOI] [PubMed] [Google Scholar]
- 43. El‐Bitar MA, Zalzal GH. Powered instrumentation in the treatment of recurrent respiratory papillomatosis: an alternative to the carbon dioxide laser. Arch Otolaryngol Head Neck Surg 2002;128(4):425–428. [DOI] [PubMed] [Google Scholar]
- 44. Patel N, Rowe M, Tunkel D. Treatment of recurrent respiratory papillomatosis in children with the microdebrider. Ann Otol Rhinol Laryngol 2003;112(1):7–10. [DOI] [PubMed] [Google Scholar]
- 45. Pasquale K, Wiatrak B, Woolley A, Lewis L. Microdebrider versus CO2 laser removal of recurrent respiratory papillomas: a prospective analysis. Laryngoscope 2003;113(1):139–143. [DOI] [PubMed] [Google Scholar]
- 46. Dedo HH, Yu KC. CO(2) laser treatment in 244 patients with respiratory papillomas. Laryngoscope 2001;111(9):1639–1644. [DOI] [PubMed] [Google Scholar]
- 47. Xu W, Han D, Hou L, Zhang L, Yu Z, Huang Z. Voice function following CO2 laser microsurgery for precancerous and early‐stage glottic carcinoma. Acta Otolaryngol 2007;127(6):637–641. [DOI] [PubMed] [Google Scholar]
- 48. Zeitels SM, Burns JA. Laser applications in laryngology: past, present, and future. Otolaryngol Clin North Am 2006;39(1):159–172. [DOI] [PubMed] [Google Scholar]
- 49. Carifi M, Napolitano D, Morandi M, Dall'Olio D. Recurrent respiratory papillomatosis: current and future perspectives. Ther Clin Risk Manag 2015;11:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ossoff RH, Coleman JA, Courey MS, Duncavage JA, Werkhaven JA, Reinisch L. Clinical applications of lasers in otolaryngology–head and neck surgery. Lasers Surg Med 1994;15(3):217–248. [DOI] [PubMed] [Google Scholar]
- 51. Murono S, Nakanishi Y, Tsuji A, et al. Trends in the management of recurrent respiratory papillomatosis in Japan. Auris Nasus Larynx 2015;42(3):218–220. [DOI] [PubMed] [Google Scholar]
- 52. Remacle M, Lawson G, Watelet JB. Carbon dioxide laser microsurgery of benign vocal fold lesions: indications, techniques, and results in 251 patients. Ann Otol Rhinol Laryngol 1999;108(2):156–164. [DOI] [PubMed] [Google Scholar]
- 53. Franco RA, Jr , Zeitels SM, Farinelli WA, Faquin W, Anderson RR. 585‐nm pulsed dye laser treatment of glottal dysplasia. Ann Otol Rhinol Laryngol 2003;112(9 Pt 1):751–758. [DOI] [PubMed] [Google Scholar]
- 54. Karamzadeh AM, Wong BJ, Crumley RL, Ahuja G. Lasers in pediatric airway surgery: current and future clinical applications. Lasers Surg Med 2004;35(2):128–134. [DOI] [PubMed] [Google Scholar]
- 55. Broadhurst MS, Akst LM, Burns JA, et al. Effects of 532 nm pulsed‐KTP laser parameters on vessel ablation in the avian chorioallantoic membrane: implications for vocal fold mucosa. Laryngoscope 2007;117(2):220–225. [DOI] [PubMed] [Google Scholar]
- 56. Kuet ML, Pitman MJ. Photoangiolytic laser treatment of recurrent respiratory papillomatosis: a scaled assessment. J Voice 2013;27(1):124–128. [DOI] [PubMed] [Google Scholar]
- 57. Derkay CS, Malis DJ, Zalzal G, Wiatrak BJ, Kashima HK, Coltrera MD. A staging system for assessing severity of disease and response to therapy in recurrent respiratory papillomatosis. Laryngoscope 1998;108(6):935–937. [DOI] [PubMed] [Google Scholar]
- 58. Holler T, Allegro J, Chadha NK, et al. Voice outcomes following repeated surgical resection of laryngeal papillomata in children. Otolaryngol Head Neck Surg 2009;141(4):522–526. [DOI] [PubMed] [Google Scholar]
- 59. Young VN, Smith LJ, Sulica L, Krishna P, Rosen CA. Patient tolerance of awake, in‐office laryngeal procedures: a multi‐institutional perspective. Laryngoscope 2012;122(2):315–321. [DOI] [PubMed] [Google Scholar]
- 60. Rees CJ, Halum SL, Wijewickrama RC, Koufman JA, Postma GN. Patient tolerance of in‐office pulsed dye laser treatments to the upper aerodigestive tract. Otolaryngol Head Neck Surg 2006;134(6):1023–1027. [DOI] [PubMed] [Google Scholar]
- 61. Burns JA, Friedman AD, Lutch MJ, Hillman RE, Zeitels SM. Value and utility of 532 nanometre pulsed potassium‐titanyl‐phosphate laser in endoscopic laryngeal surgery. J Laryngol Otol 2010;124(4):407–411. [DOI] [PubMed] [Google Scholar]
- 62. Koufman JA, Rees CJ, Frazier WD, et al. Office‐based laryngeal laser surgery: a review of 443 cases using three wavelengths. Otolaryngol Head Neck Surg 2007;137(1):146–151. [DOI] [PubMed] [Google Scholar]
- 63. Mouadeb DA, Belafsky PC. In‐office laryngeal surgery with the 585nm pulsed dye laser (PDL). Otolaryngol Head Neck Surg 2007;137(3):477–481. [DOI] [PubMed] [Google Scholar]
- 64. Zeitels SM, Franco RA, Jr , Dailey SH, Burns JA, Hillman RE, Anderson RR. Office‐based treatment of glottal dysplasia and papillomatosis with the 585‐nm pulsed dye laser and local anesthesia. Ann Otol Rhinol Laryngol 2004;113(4):265–276. [DOI] [PubMed] [Google Scholar]
- 65. Centric A, Hu A, Heman‐Ackah YD, Divi V, Sataloff RT. Office‐based pulsed‐dye laser surgery for laryngeal lesions: a retrospective review. J Voice 2014;28(2):262 e269–262 e212. [DOI] [PubMed] [Google Scholar]
- 66. Tatar EC, Kupfer RA, Barry JY, Allen CT, Merati AL. Office‐Based vs Traditional Operating Room Management of Recurrent Respiratory Papillomatosis: Impact of Patient Characteristics and Disease Severity. JAMA Otolaryngol Head Neck Surg. 2017;143(1):55–59. [DOI] [PubMed] [Google Scholar]
- 67. Zeitels SM, Lopez‐Guerra G, Burns JA, Lutch M, Friedman AM, Hillman RE. Microlaryngoscopic and office‐based injection of bevacizumab (Avastin) to enhance 532‐nm pulsed KTP laser treatment of glottal papillomatosis. Ann Otol Rhinol Laryngol Suppl 2009;201:1–13. [DOI] [PubMed] [Google Scholar]
- 68. Co J, Woo P. Serial office‐based intralesional injection of cidofovir in adult‐onset recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol 2004;113(11):859–862. [DOI] [PubMed] [Google Scholar]
- 69. Katsenos S, Becker HD. Recurrent respiratory papillomatosis: a rare chronic disease, difficult to treat, with potential to lung cancer transformation: apropos of two cases and a brief literature review. Case Rep Oncol 2011;4(1):162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Healy GB, Gelber RD, Trowbridge AL, Grundfast KM, Ruben RJ, Price KN. Treatment of recurrent respiratory papillomatosis with human leukocyte interferon. Results of a multicenter randomized clinical trial. N Engl J Med 1988;319(7):401–407. [DOI] [PubMed] [Google Scholar]
- 71. Gerein V, Rastorguev E, Gerein J, Jecker P, Pfister H. Use of interferon‐alpha in recurrent respiratory papillomatosis: 20‐year follow‐up. Ann Otol Rhinol Laryngol 2005;114(6):463–471. [DOI] [PubMed] [Google Scholar]
- 72. Nodarse‐Cuni H, Iznaga‐Marin N, Viera‐Alvarez D, et al. Interferon alpha‐2b as adjuvant treatment of recurrent respiratory papillomatosis in Cuba: National Programme (1994–1999 report). J Laryngol Otol 2004;118(9):681–687. [DOI] [PubMed] [Google Scholar]
- 73. Suter‐Montano T, Montano E, Martinez C, Plascencia T, Sepulveda MT, Rodriguez M. Adult recurrent respirator papillomatosis: a new therapeutic approach with pegylated interferon alpha 2a (Peg‐IFNalpha‐2a) and GM‐CSF. Otolaryngol Head Neck Surg 2013;148(2):253–260. [DOI] [PubMed] [Google Scholar]
- 74. De Clercq E, Descamps J, De Somer P, Holy A. (S)‐9‐(2,3‐Dihydroxypropyl)adenine: an aliphatic nucleoside analog with broad‐spectrum antiviral activity. Science. 1978;200(4341):563–565. [DOI] [PubMed] [Google Scholar]
- 75. Shehab N, Sweet BV, Hogikyan ND. Cidofovir for the treatment of recurrent respiratory papillomatosis: a review of the literature. Pharmacotherapy 2005;25(7):977–989. [DOI] [PubMed] [Google Scholar]
- 76. Broekema FI, Dikkers FG. Side‐effects of cidofovir in the treatment of recurrent respiratory papillomatosis. Eur Arch Otorhinolaryngol 2008;265(8):871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dikkers FG. Treatment of recurrent respiratory papillomatosis with microsurgery in combination with intralesional cidofovir–a prospective study. Eur Arch Otorhinolaryngol 2006;263(5):440–443. [DOI] [PubMed] [Google Scholar]
- 78. Wemer RD, Lee JH, Hoffman HT, Robinson RA, Smith RJH. Case of progressive dysplasia concomitant with intralesional cidofovir administration for recurrent respiratory papillomatosis. Annals of Otology Rhinology and Laryngology 2005;114(11):836–839. [DOI] [PubMed] [Google Scholar]
- 79. Pudszuhn A, Welzel C, Bloching M, Neumann K. Intralesional Cidofovir application in recurrent laryngeal papillomatosis. Eur Arch Otorhinolaryngol 2007;264(1):63–70. [DOI] [PubMed] [Google Scholar]
- 80. Snoeck R, Wellens W, Desloovere C, et al. Treatment of severe laryngeal papillomatosis with intralesional injections of cidofovir [(S)‐1‐(3‐hydroxy‐2‐phosphonylmethoxypropyl)cytosine]. J Med Virol 1998;54(3):219–225. [DOI] [PubMed] [Google Scholar]
- 81. Coulombeau B, Nusa Naiman A, Ceruse P, Froehlich P. [Anti‐viral injectable treatment (cidofovir) in laryngeal papillomatosis]. Rev Laryngol Otol Rhinol (Bord) 2002;123(5):315–320. [PubMed] [Google Scholar]
- 82. Naiman AN, Ayari S, Nicollas R, Landry G, Colombeau B, Froehlich P. Intermediate‐term and long‐term results after treatment by cidofovir and excision in juvenile laryngeal papillomatosis. Ann Otol Rhinol Laryngol 2006;115(9):667–672. [DOI] [PubMed] [Google Scholar]
- 83. Naiman AN, Ceruse P, Coulombeau B, Froehlich P. Intralesional cidofovir and surgical excision for laryngeal papillomatosis. Laryngoscope 2003;113(12):2174–2181. [DOI] [PubMed] [Google Scholar]
- 84. Naiman AN, Abedipour D, Ayari S, et al. Natural history of adult‐onset laryngeal papillomatosis following multiple cidofovir injections. Ann Otol Rhinol Laryngol 2006;115(3):175–181. [DOI] [PubMed] [Google Scholar]
- 85. Derkay CS, Volsky PG, Rosen CA, et al. Current use of intralesional cidofovir for recurrent respiratory papillomatosis. Laryngoscope 2013;123(3):705–712. [DOI] [PubMed] [Google Scholar]
- 86. Shih T, Lindley C. Bevacizumab: An angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther 2006;28(11):1779–1802. [DOI] [PubMed] [Google Scholar]
- 87. Rahbar R, Vargas SO, Folkman J, et al. Role of vascular endothelial growth factor‐A in recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol 2005;114(4):289–295. [DOI] [PubMed] [Google Scholar]
- 88. Sidell DR, Nassar M, Cotton RT, Zeitels SM, de Alarcon A. High‐dose sublesional bevacizumab (avastin) for pediatric recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol 2014;123(3):214–221. [DOI] [PubMed] [Google Scholar]
- 89. Zeitels SM, Barbu AM, Landau‐Zemer T, et al. Local injection of bevacizumab (Avastin) and angiolytic KTP laser treatment of recurrent respiratory papillomatosis of the vocal folds: a prospective study. Ann Otol Rhinol Laryngol. 2011;120(10):627–634. [DOI] [PubMed] [Google Scholar]
- 90. Maturo S, Hartnick CJ. Use of 532‐nm pulsed potassium titanyl phosphate laser and adjuvant intralesional bevacizumab for aggressive respiratory papillomatosis in children: initial experience. Arch Otolaryngol Head Neck Surg 2010;136(6):561–565. [DOI] [PubMed] [Google Scholar]
- 91. Best SR, Friedman AD, Landau‐Zemer T, et al. Safety and dosing of bevacizumab (avastin) for the treatment of recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol 2012;121(9):587–593. [DOI] [PubMed] [Google Scholar]
- 92. Rogers DJ, Ojha S, Maurer R, Hartnick CJ. Use of adjuvant intralesional bevacizumab for aggressive respiratory papillomatosis in children. JAMA Otolaryngol Head Neck Surg 2013;139(5):496–501. [DOI] [PubMed] [Google Scholar]
- 93. Wu R, Abramson AL, Shikowitz MJ, Dannenberg AJ, Steinberg BM. Epidermal growth factor‐induced cyclooxygenase‐2 expression is mediated through phosphatidylinositol‐3 kinase, not mitogen‐activated protein/extracellular signal‐regulated kinase kinase, in recurrent respiratory papillomas. Clin Cancer Res 2005;11(17):6155–6161. [DOI] [PubMed] [Google Scholar]
- 94. Limsukon A, Susanto I, Hoo GW, Dubinett SM, Batra RK. Regression of recurrent respiratory papillomatosis with celecoxib and erlotinib combination therapy. Chest 2009;136(3):924–926. [DOI] [PubMed] [Google Scholar]
- 95. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192(7):1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lyford‐Pike S, Peng S, Young GD, et al. Evidence for a role of the PD‐1:PD‐L1 pathway in immune resistance of HPV‐associated head and neck squamous cell carcinoma. Cancer Res 2013;73(6):1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single‐agent anti‐programmed death‐1 (MDX‐1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28(19):3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Makiyama K, Hirai R, Matsuzaki H. Gardasil vaccination for recurrent laryngeal papillomatosis in adult men: first report: changes in HPV antibody titer. J Voice 2017;31(1):104–106. [DOI] [PubMed] [Google Scholar]
- 99. Forster G, Boltze C, Seidel J, Pawlita M, Muller A. Juvenile laryngeal papillomatosis ‐ immunisation with the polyvalent vaccine Gardasil (R). Laryngo‐Rhino‐Otologie 2008;87(11):796–799. [DOI] [PubMed] [Google Scholar]
- 100. Young DL, Moore MM, Halstead LA. The use of the quadrivalent human papillomavirus vaccine (Gardasil) as adjuvant therapy in the treatment of recurrent respiratory papilloma. J Voice. 2015;29(2):223–229. [DOI] [PubMed] [Google Scholar]
- 101. Hocevar‐Boltezar I, Maticic M, Sereg‐Bahar M, et al. Human papilloma virus vaccination in patients with an aggressive course of recurrent respiratory papillomatosis. Eur Arch Otorhinolaryngol 2014;271(12):3255–3262. [DOI] [PubMed] [Google Scholar]
- 102. Dion GR, Teng S, Boyd LR, et al. Adjuvant human papillomavirus vaccination for secondary prevention: a systematic review. JAMA Otolaryngol Head Neck Surg 2017;143(6):614–622. [DOI] [PubMed] [Google Scholar]
- 103. Meszner Z, Jankovics I, Nagy A, Gerlinger I, Katona G. Recurrent laryngeal papillomatosis with oesophageal involvement in a 2 year old boy: successful treatment with the quadrivalent human papillomatosis vaccine. Int J Pediatr Otorhinolaryngol 2015;79(2):262–266. [DOI] [PubMed] [Google Scholar]
- 104. Mudry P, Vavrina M, Mazanek P, Machalova M, Litzman J, Sterba J. Recurrent laryngeal papillomatosis: successful treatment with human papillomavirus vaccination. Arch Dis Child 2011;96(5):476–477. [DOI] [PubMed] [Google Scholar]
- 105. Wangu Z, Hsu KK. Impact of HPV vaccination on anogenital warts and respiratory papillomatosis. Hum Vaccin Immunother 2016;12(6):1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bostrom B, Sidman J, Marker S, Lander T, Drehner D. Gefitinib therapy for life‐threatening laryngeal papillomatosis. Arch Otolaryngol Head Neck Surg 2005;131(1):64–67. [DOI] [PubMed] [Google Scholar]
- 107. Lindeberg H, Elbrond O. Laryngeal papillomas: the epidemiology in a Danish subpopulation 1965–1984. Clin Otolaryngol Allied Sci 1990;15(2):125–131. [DOI] [PubMed] [Google Scholar]
- 108. Armstrong LR, Preston EJ, Reichert M, et al. Incidence and prevalence of recurrent respiratory papillomatosis among children in Atlanta and Seattle. Clin Infect Dis 2000;31(1):107–109. [DOI] [PubMed] [Google Scholar]
- 109. Armstrong LR, Derkay CS, Reeves WC. Initial results from the national registry for juvenile‐onset recurrent respiratory papillomatosis. RRP Task Force. Arch Otolaryngol Head Neck Surg 1999;125(7):743–748. [DOI] [PubMed] [Google Scholar]
- 110. Campisi P, Hawkes M, Simpson K, Canadian Juvenile Onset Recurrent Respiratory Papillomatosis Working G . The epidemiology of juvenile onset recurrent respiratory papillomatosis derived from a population level national database. Laryngoscope 2010;120(6):1233–1245. [DOI] [PubMed] [Google Scholar]
- 111. Bielamowicz S, Villagomez V, Stager SV, Wilson WR. Intralesional cidofovir therapy for laryngeal papilloma in an adult cohort. Laryngoscope 2002;112(4):696–699. [DOI] [PubMed] [Google Scholar]
- 112. Akst LM, Lee W, Discolo C, Knott D, Younes A, Koltai PJ. Stepped‐dose protocol of cidofovir therapy in recurrent respiratory papillomatosis in children. Arch Otolaryngol Head Neck Surg 2003;129(8):841–846. [DOI] [PubMed] [Google Scholar]
- 113. Lee AS, Rosen CA. Efficacy of cidofovir injection for the treatment of recurrent respiratory papillomatosis. J Voice 2004;18(4):551–556. [DOI] [PubMed] [Google Scholar]
- 114. Chaturvedi J, Sreenivas V, Hemanth V, Nandakumar R. Management of adult recurrent respiratory papillomatosis with oral acyclovir following micro laryngeal surgery: a case series. Indian J Otolaryngol Head Neck Surg 2014;66(Suppl 1):359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. McGlennen RC, Adams GL, Lewis CM, Faras AJ, Ostrow RS. Pilot trial of ribavirin for the treatment of laryngeal papillomatosis. Head Neck 1993;15(6):504–512; discussion 512–513. [DOI] [PubMed] [Google Scholar]
- 116. Rosen CA, Bryson PC. Indole‐3‐carbinol for recurrent respiratory papillomatosis: long‐term results. J Voice 2004;18(2):248–253. [DOI] [PubMed] [Google Scholar]
- 117. Newfield L, Goldsmith A, Bradlow HL, Auborn K. Estrogen metabolism and human papillomavirus‐induced tumors of the larynx: chemo‐prophylaxis with indole‐3‐carbinol. Anticancer Res 1993;13(2):337–341. [PubMed] [Google Scholar]
- 118. Bell R, Hong WK, Itri LM, McDonald G, Strong MS. The use of cis‐retinoic acid in recurrent respiratory papillomatosis of the larynx: a randomized pilot study. Am J Otolaryngol 1988;9(4):161–164. [DOI] [PubMed] [Google Scholar]


