Abstract
The arachidonic acid cascade is arguably the most widely known biologic regulatory pathway. Decades after the seminal discoveries involving its cyclooxygenase and lipoxygenase branches, studies of this cascade remain an active area of research. The third and less widely known branch, the cytochrome P450 pathway leads to highly active oxygenated lipid mediators, epoxy fatty acids (EpFAs) and hydroxyeicosatetraenoic acids (HETEs), which are of similar potency to prostanoids and leukotrienes. Unlike the COX and LOX branches, no pharmaceuticals currently are marketed targeting the P450 branch. However, data support therapeutic benefits from modulating these regulatory lipid mediators. This is being approached by stabilizing or mimicking the EpFAs or even by altering the diet. These approaches lead to predominantly beneficial effects on a wide range of apparently unrelated states resulting in an enigma of how this small group of natural chemical mediators can have such diverse effects. EpFAs are degraded by soluble epoxide hydrolase (sEH) and stabilized by inhibiting this enzyme. In this review, we focus on interconnected aspects of reported mechanisms of action of EpFAs and inhibitors of soluble epoxide hydrolase (sEHI). The sEHI and EpFAs are commonly reported to maintain homeostasis under pathological conditions while remaining neutral under normal physiological conditions. Here we provide a conceptual framework for the unique and broad range of biological activities ascribed to epoxy fatty acids. We argue that their mechanism of action pivots on their ability to prevent mitochondrial dysfunction, to reduce subsequent ROS formation and to block resulting cellular signaling cascades, primarily the endoplasmic reticulum stress. By stabilizing the mitochondrial – ROS – ER stress axis, the range of activity of EpFAs and sEHI display an overlap with the disease conditions including diabetes, fibrosis, chronic pain, cardiovascular and neurodegenerative diseases, for which the above outlined mechanisms play key roles.
Keywords: arachidonic acid, epoxydocosapentaenoic acid, epoxy fatty acids, soluble epoxide hydrolase, mitochondria, endoplasmic reticulum stress
1 Introduction
Eicosanoids generated from cyclooxygenase (COX) and lipoxygenase (LOX) mediated metabolism of arachidonic acid (ARA) are well-known bioactive lipids that promote and maintain inflammatory signaling cascades. Efforts towards understanding the fundamental characteristics of the potent and profound actions of these lipid metabolites in addition yielded a multitude of therapeutic approaches to quell inflammation and pain [1, 2]. There is continued interest in therapeutically targeting the enzymes, receptors and metabolites within these two predominantly pro-inflammatory pathways, while the latest identified branch of the ARA cascade, known as the cytochrome P450 branch offers underexploited features and novel therapeutic targets [3].
The cytochrome P450 branch, yields potent ARA metabolites including hydroxyeicosatetraenoic acids (HETEs) and epoxyeicosatrienoic acids (EETs) [4]. Progress attained within the last decade strongly suggests that EETs and other EpFA are opposing counterparts to the largely pro-inflammatory prostanoids, leukotrienes and HETEs [5, 6]. This P450 branch of the ARA cascade is attracting increasing attention both in fundamental regulatory biology and as a clinical target to treat a variety of diseases [7–9]. It is now clear that unsaturated free fatty acids in general are substrates for the cytochrome P450s generating EpFA. There is considerable structural diversity among bioactive lipids bearing an epoxide functionality (Figure 1). Together with the hepoxilins these molecules are classified as epoxy fatty acids (EpFAs).
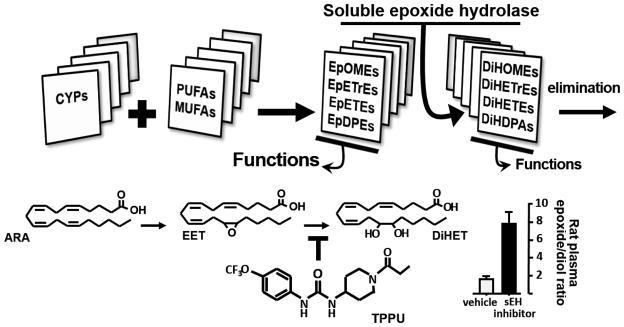
Figure 1. Epoxides of unsaturated and largely polyunsaturated fatty acids (MUFAs and PUFAs) are the major anti-inflammatory and analgesic mediators of the P450 branch of the arachidonic acid cascade.
These epoxides are made largely by cytochrome P450 enzymes and may be stored as phospholipids. The best studied are the EETs (EpETrEs), but epoxides of LA (EpOMEs), EPA (EpETEs), and DHA (EpDPEs) also are chemical mediators with epoxides of the ω-3 DHA or EDPs being particularly more? active. These epoxides are converted at varying rates but generally with high Vmax and low Km to the corresponding diols, for example EETs are converted by sEH to DHETs (DiHETrEs). These diols are generally less bioactive, tend to move out of cells, and are rapidly conjugated and excreted. DHETs and particularly the diols of linoleic acid have been shown to be pro-inflammatory at the high concentrations present in sepsis. Inhibitors of sEH such at TPPU stabilize epoxy fatty acids (EpFA) such as the EETs shown, to increase the lipid epoxide to diol ratios in the blood which are associated with reduced inflammation and pain.
1.1 Epoxide hydrolase mediated degradation rapidly regulates the titers of EpFAs
The epoxide moiety on an EpFA is dissimilar to the reactive epoxide toxins such as those from aflatoxin or polycyclic aromatic hydrocarbons in that it does not react with nucleophiles in biological milieu. In addition, the lipid backbones of EpFA do not intercalate into nucleic acids which would be critical for genotoxicity. The EpFAs are chemically stable at neutral pH although, consistent with their roles in cellular signaling, exhibit short in vivo half-lives due to rapid hydrolysis by epoxide hydration [10]. This property has been a stumbling block that impeded early progress in the field. The breakthrough was contingent on identification of epoxide hydrolase (EH) activity and recognition of the soluble epoxide hydrolase (sEH, EPHX2, E.C.3.3.2.10) as an enzyme that rapidly degrades EpFAs followed by tools to chemically and genetically knock out or induce the expression of this enzyme [8]. While mammals express several different epoxide hydrolases, sEH seems to be the common denominator for the inactivation of most, if not all EpFA species in most tissues. In tissue extracts, if sEH activity is blocked with selective inhibitors, much of EpFA degrading activity is eliminated [11]. Spector estimated that most of the degradation of EpFAs was due to sEH explaining why its inhibition increases titers of EpFAs in vivo [10]. However, these higher levels of EpFAs seem to be cleared from the system by degradation via alternate pathways limiting the EpFA titers achieved by sEHI. Therefore, degradation by sEH seems to be the major regulatory step that, with biosynthesis and release from phospholipid stores, adjusts the EpFA titers. In at least one exceptional case, brain microsomal EH (mEH, EPHX1, E.C.3.3.2.9) may contribute to degradation of EpFAs [12]. The mEH and sEH share a common structure and catalytic mechanism involving the formation and hydrolysis of a covalent intermediate [11]. Even though EpFAs are not among the preferred substrates for mEH, its expression in the brain is much higher than that of sEH suggesting that brain titers of EpFAs are co-regulated by both EHs. In several cases, the efficacy of sEH inhibitors have been enhanced by maintaining animals on a diet enriched with ω-3 and depleted in ω-6, suggesting that epoxides of DHA and EPA are more potent mediators in some systems than the corresponding EETs [13]. Additionally, the ω-terminal epoxides of EPA and DHA are turned over by sEH at much slower rate than most of the other regioisomers [14]. This in vivo stability may contribute to the biological activity of these EpFA and to the broad biological benefits of dietary ω-3 fatty acids.
The discovery of potent and selective inhibitors of sEH beginning early 2000s resulted in broader interest towards EpFAs and the sEH [15]. Consequently, simultaneous progress was attained both in fundamental understanding of these bioactive lipid mediators and in therapeutic applications by way of inhibiting sEH. An increasing number of functions and activities of EpFAs and sEHI continue to be reported. With the use of potent sEH inhibitors, numerous groups published key observations that support clinical development of sEH inhibitors [7, 16].
2 Novel paths to positively modulating EpFA titers
The dynamics of EpFA generation is much less clear with the numerous cytochrome P450 isozymes involved in EpFA synthesis [4]. Cytochrome P450 2C and 2Js appear to dominate the synthesis of EpFAs, under normal conditions, but they carry out multiple oxidative reactions in addition to epoxidation [17]. It appears that synthesis of EpFAs occurs on a continuous basal level and a portion of the de novo EpFA mass is diverted to lipid bilayer membrane fractions for storage. However, the rate of EpFAs synthesis is not constant and seems to be acutely responsive to multiple stimuli, particularly under pathological conditions. A recent report demonstrated a biphasic change in EpFA levels over the course of 0–24h of inflammation [6]. Clearly, there are pools of quantifiable intra- and extra-cellular, free acid EpFAs both of which are stabilized and augmented by sEH inhibitors. Tetrachlorodioxin and other xenobiotics induce cytochrome P450 enzymes usually studied for their role in xenobiotic metabolism, sufficiently that they contribute to titers of EpFAs [18]. Since the 2C and 2J as well as a variety of other cytochrome p450s will oxidize multiple unsaturated fatty acids, it is not surprising that the EpFA produced depends in part on the diet as well as the circulating and stored lipids. The dramatic increase in dietary linoleic acid resulting in formation of bioactive 18 carbon metabolites, often termed leukotoxin and iso-leukotoxin, is an example of deleterious effects of dietary lipids [19]. In contrast, the epoxides of ω-3 unsaturated fatty acids such as DHA and EPA in some fish oil appear to be more powerful and generally more beneficial chemical mediators than the corresponding EETs from ARA [13, 14].
A recent report further illustrated the feasibility of enhancing the synthesis of EpFAs while impeding their degradation using omeprazole, a strong inducer of several cytochrome P450s, combined with sEH inhibitor [20]. Individually applied, omeprazole and sub-therapeutic dose of sEHI were devoid of activity whereas the combination was sufficient to modulate the EpFA titers to display an anti-nociceptive effect. The dramatic changes in the levels of EpFAs and the resulting biological effects of altering them illustrate how environmental chemicals and pharmaceuticals can alter this regulatory system. An additional approach to regulate the titers of EpFAs appears to be co-administration of inhibitors of phosphodiesterase with sEHI [21]. Elevated levels of cAMP or cGMP are obtained by blocking their degradation by PDE isozymes. This results in release of free fatty acids from membrane stores [22]. Such treatments resulted in a 10 to 30-fold increase in the ratio of circulating EpFAs to their dihydroxy- metabolites and displayed anti-nociceptive effects. Consequently, the use of sEHI enabled significant progress in understanding the conditions under which EpFAs are synthesized, released, stored and metabolized. The use of sEHI along with other probes offers further advantages in gaining mechanistic insight into the EpFA system and as possible synergists for therapeutic applications [5].
2.1 Converging towards an overarching mechanism of action
Among the remaining scientific challenges is the need for a more comprehensive understanding of how EpFAs function. A myriad of biological functions is attributed to EETs and other EpFAs and these are covered in several recent reviews [3, 7–9, 23, 24]. In many cases, these characteristics were either discovered or substantiated with the use of sEH inhibitors. On the other hand, results from the broader community illustrate the functional equivalency of using individual EpFAs, mixtures of EpFA regioisomers, EpFA mimics, sEH inhibitors, sEH−/− mice, cytochrome P450 inducers and inhibitors, P450 −/− mice and combinations thereof.
Like numerous other signaling molecules, the function of EpFAs and sEHI strongly depend on the biological context. This context dependent activity of EpFAs and sEHI increase the overall complexity but could be key for elucidating their mechanism of action. Specifically, the analgesic efficacy of EpFAs and sEHI appear to occur through activity dependent modulation of pain signaling [25]. In the absence of an underlying painful condition, neither EpFAs nor sEHI display quantifiable effects [26]. The absence of measurable effect on normal physiology is consistently observed across different laboratories and studies involving rodent and non-rodent experimental models. This is in stark contrast to their strong efficacy under pathological conditions [27]. Therefore, EpFAs and sEHI seem to function in a way to maintain homeostasis. This argument is best illustrated by the ability of sEHI to shift both hypertension and hypotension towards normotension in rodent models [28, 29].
A receptor molecule that mediates the multifarious effects of EpFAs is yet to be identified. However, a growing body of evidence demonstrate that differences in structure yield changes in functionality. Different regio- and optical isomers can yield different effects K+ channel function [30, 31]. The parent fatty acid of EpFA is also critical. For example, selective effects of DHA vs. ARA derived EpFAs on angiogenesis have been described, while epoxides and particularly diol metabolites of LA have been observed to be pro-inflammatory and toxic [19, 32–34]. EpFA and their corresponding diols can be further metabolized to bioactive tetrahydrofuran diols by P450s and some are substrates for COX and LOX enzymes [35]. Such findings support the argument that EpFA may function through multiple receptors or transduction systems and the possibility that EpFAs are biased ligands of a G-protein coupled receptor.
Here we discuss two recently described molecular mechanisms mediating EpFAs functions (Figure 1). These appear to unify a framework for the diverse actions of EpFAs in that they are far-reaching, fundamental mechanisms. The caveat is that this contemporary view is an evolving concept and may illuminate how EpFAs act under different disease conditions.
3. The enigma of broad efficacy
Potent and metabolically stable sEH inhibitors (sEHI) allowed by inference a variety of biologists to query the action of EpFA on many pathological conditions. In most cases, these observations were followed up by extensive complementary information. sEHI were first observed to reduce blood pressure [36] and then vascular inflammation and downstream effects such as atherosclerosis. A study showing initial resolution of systemic inflammation [37] was followed by a variety of studies demonstrating dramatic reduction of inflammation in a variety of systems. As these inflammatory pathologies were examined unexpected and usually beneficial effects were observed. For example, sEHI not only reduce periodontal inflammation but also enhanced the regrowth of bone through alteration of the RANK - RANKL system [38]. The reduction of inflammation was observed in multiple models of intestinal, renal, pancreatic, brain and even cardiac inflammation, so it was not a surprise that they reduced atrial fibrillation and fibrosis that lead to cardiac hypertrophy [39]. Since inflammation drives a number of disease states it was not a surprise that sEHI reduce hepatic, renal, pancreatic, and asthma-associated pulmonary fibrosis [40–45]. The compounds were even active in a bleomycin model of idiopathic pulmonary fibrosis [40]. Since sEHI were so effective in resolving inflammation it was no surprise that the sEHI also reduce inflammatory pain [5, 26]. However, it was unexpected to observe a reduction in neuropathic pain since these pain states are often refractory to control with NSAIDs and steroids [25]. This indication will be covered in more detail. The broad activity of EpFA supports the hypothesis that the EpFA act as homeostatic modulators returning pathological conditions such as hypertension, hypotension, or inflammation back toward normal physiology. Broadly the compounds reduce the comorbidities associated with diabetes and in the presence of elevated ω-3 lipids seem to ameliorate the actual disease state. However, a biochemical pathway to explain efficacy on these disparate biological processes remains elusive.
The observation that reduction of the unfolded protein response (UPR) and endoplasmic reticulum stress (ER stress) pathways was associated with the beneficial action of sEHI in a variety of pathological conditions in rodent systems raised the possibility of a universal mechanism of action of EpFA [27, 45–47]. It is well established that high blood carbohydrate can alter biology through the ER stress and in severe states can result in diabetes. Thus, the ER stress pathway can explain how sEHI alleviates many of the pathologies associated with diabetes. Another input into the regulation of ER stress is the increase in reactive oxygen species (ROS) production. ROS themselves can be considered an endogenous chemical mediator and high ROS can be formed by a number of conditions. However, a major source of ROS occurs under conditions that cause mitochondrial dysfunction [48]. Thus, in this review we present the hypothesis that the beneficial effects of EpFA in many disease states can be explained, at least in part, through modulation of the ER stress pathway and stabilization of mitochondria.
4. EpFAs, sEHI, ROS and mitochondrial function
4.1. Mitochondrial function and ROS
Since the mitochondria are the primary source of intracellular energy, efficient mitochondrial function is critical. The mitochondria are also important regulators of cell survival and death [49]. ROS can be generated from many other intracellular sources including xanthine oxidase (XO), lipoxygenases, nitric oxide (NO) synthases, cytochrome P450 enzymes, and nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidases [50]. However, mitochondria have been suggested to be the major intracellular site of ROS production [51, 52]. ROS can act as a signaling molecule important in the regulation of cellular events such as cell growth. However, high ROS levels were shown to be associated with cell dysfunction and death [53]. To prevent ROS from causing cellular dysfunction, cells have several systems to reduce ROS levels. However, overproduction of ROS leads to oxidative stress and cellular dysfunction when the antioxidant systems are unable to reduce ROS levels. The mitochondria themselves are susceptible to ROS produced in the mitochondria and elsewhere, resulting in damages to electron transport chain components and mitochondrial DNA [54, 55].
ROS has been shown to cause ARA peroxidation, resulting in the formation of isoprostanes and isofurans [56, 57]. Isoprostanes and isofurans show biological properties that may account for some of the pathophysiological effects associated with oxidative injury [58]. In an animal model of mitochondrial dysfunction (mice treated with doxorubicin), the ratio of kidney isofurans to F2-isoprostanes increased relative to control mice [59]. The ratio of isofurans to F2-isoprostanes was proposed as an indicator of mitochondrial dysfunction since isofurans are preferentially formed under high oxygen tension conditions such as when less oxygen is required by the mitochondria for oxidative phosphorylation [60]. It is possible that the lower ARA levels, due to the formation of isofurans in the presence of high ROS levels, is the result of less EETs being produced leading to mitochondrial dysfunction.
4.2 Ischemia reperfusion (IR), mitochondrial damage, and EETs
Ischemia reperfusion (IR) injury has been shown to cause significant mitochondrial damages including the opening of the mitochondrial permeability transition pore (mPTP) allowing free passage of molecules < 1.5 kDa [49, 61]. The opening of the mPTP is affected by the mitochondrial membrane potential (ΔΨm), which is a measure of the electrochemical gradient formed to generate ATP, with lower ΔΨm enhancing mPTP opening. A few reports suggest that EETs and other EpFAs may have protective intracellular effects by reducing mitochondria damage that occurs during IR [62]. The loss of ΔΨm and mPTP opening caused by laser-induced oxidative stress was significantly reduced in in H9c2 cardiac cells treated with 14, 15-EET (1μM) [62]. The protective effect of EET was prevented by the EET antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (1μM, 14, 15-EEZE) [62]. Improved post ischemic recovery following IR was observed in hearts from wild-type mice treated with 11,12-EET, sEH null mice, and mice overexpressing cardiac myocyte-specific human cytochrome P450 2J2 [62]. Co-administration of 14,15- EET and K+ channel blockers 5-HD (mitochondrial ATP-sensitive K+ channel inhibitor) or paxilline (high-conductance Ca2+-activated K+ channel inhibitor) diminished EET-mediated loss of ΔΨm. These results suggest that EET-mediated protection involved limiting mPTP opening and that EET-mediated effects might involve activating mitochondrial K+ channels that are important for mitochondrial membrane potential [62]. Similar to the studies by Katragadda et al., sarcolemmal ATP-sensitive K+ channels were found to be important for the EET-mediated effects in starved HL-1 cells [63]. Other studies have also suggested that EETs can activate cardiac mitochondrial KATP channels [64]. Additionally, Samokhvalov et al. found that AMP-activated protein kinase (AMPK) was important in conferring the positive effects of EETs on starved HL-1 cells [63]. A summary of how mitochondrial ROS affects other intracellular signaling systems is shown in Figure 2.
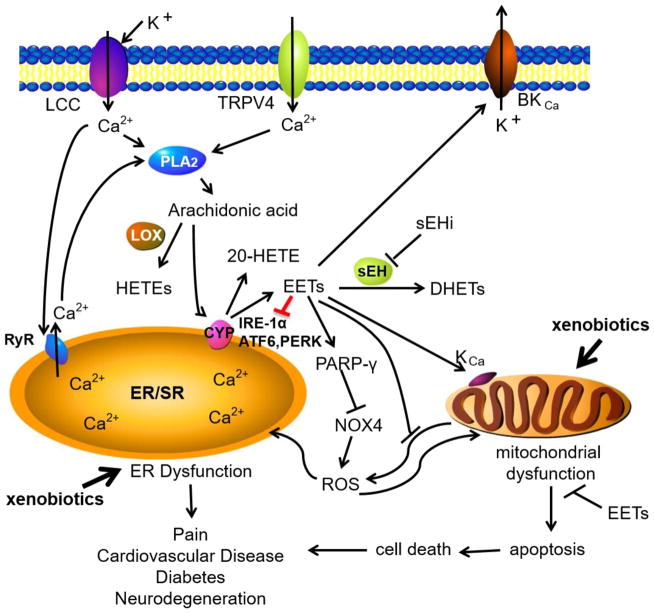
Figure 2. Signaling mechanisms involved in ROS induced ER dysfunction.
In the presence of xenobiotic stress, ROS produced from the mitochondria may induce ER dysfunction. EETs and sEHI reduce the mitochondrial and NADPH oxidase (NOX4). EETs also activate important potassium channels on the mitochondria. Possible independent from these effects EETs and sEHI prevent the activation of the signaling cascade governed by the three major ER resident sensors inositol requiring protein 1α (IRE-1α), protein kinase RNA-like endoplasmic reticulum kinase (PERK) and activating transcription factor 6 (ATF6). RyR, ryanodine receptor; LCC, L-type Ca2+ channel; TRPV4, transient receptor potential cation channel subfamily V member 4; BKCa, calcium activated big potassium channel; KCa, calcium activated potassium channel; PARP-γ, poly(ADP-ribose) polymerase; K+, potassium ions, Ca2+, calcium ions.
Treatment of starved HL-1 cells or neonatal cardiomyocytes with UA-8 (13-(3-propylureido)tridec-8-enoic acid), a dual-acting synthetic analog with both sEHI and EET-mimetic properties, improved enzymatic activities of key proteins involved in mitochondrial respiratory chain (citrate synthase, succinate dehydrogenase, and cytochrome c oxidase) [63]. In another study using HL-1 cells, co-treatment of starved cells with UA-8 or 14,15-EET improved mitochondrial function [65]. Aconitase activity, often used to access mitochondrial oxidative stress [66], was decreased after 6 or more hours of starvation, while treatment of cells with UA-8 and 14,15-EET prevented the reduced activity [65]. In the latter study, the beneficial effect of UA-8 and 14,15-EET was prevented by 14,15-EEZE. Mitochondrial respiration, measured as the ratio between the basal mitochondrial oxygen consumption and ADP-stimulated states is used to calculate the respiratory ratio control (RCR). The mitochondrial RCR, measured as the ratio between the basal mitochondrial oxygen consumption and ADP-stimulated states, is an important indicator of oxidative phosphorylation efficiency [65]. Starvation of HL-1 cells reduced RCR while increasing the ADP/ATP ratio, which were both prevented by UA-8 [65]. In this study, El-Sikhry et al. further demonstrated that EETs were important in preserving mitochondrial function.
Furthermore, Batchu et al. showed that a nicotinamide-based sEHI, BI00611953, provided cardioprotection against IR injury in mice [67]. This cardioprotection was suggested to be partly due to the sEHI maintaining mitochondrial function. BI00611953 reduced the infarct (damaged area) size following IR when compared to control hearts, similar to what was observed in hearts from mice lacking sEH, or hearts perfused with 1μM 11,12-EETs. In H9c2 cells, BI0611953 also delayed the loss of ΔΨm caused by anoxia-reoxygenation and increased hypoxia-inducible factor (HIF)-1α DNA binding [67]. Use of the plasma membrane K(ATP) channel inhibitor (glibenclamide), or the putative EET receptor antagonist 14,15-EEZE, eliminated the cardioprotection conferred by BI00611953[67].
4.3 Mitochondrial damage and EETs in non-cardiovascular cell types
In addition to the cardioprotective effects of EETs on mitochondrial function discussed above, EETs are also protective against pathological conditions induced by non-cardiovascular mitochondrial dysfunction, such as in hippocampal astrocytes [68]. Exposure of cultured neonatal hippocampal astrocytes to amyloid-β (Aβ) induces mitochondrial dysfunction as judged by reduced ΔΨm and fragmentation. Pre-incubation of the cells with 11,12-EET or 14,15-EET prevented the mitochondrial depolarization and fragmentation. Reducing the endogenous EET levels with a P450 inhibitor, MS-PPOH, further reduced the ΔΨm caused by Aβ. Pretreatment with EETs also reduced the decrease in mitochondrial oxygen consumption observed when Aβ is added. A well-established mechanism for Aβ-induced toxicity is through increasing ROS generation [69] which was prevented by EETs [68].
4.4 Potential mechanisms for attenuation of mitochondrial dysfunction by EETs or sEH inhibition
An important aspect of the HL-1 study was that EETs reduced caspase 3 and proteasome activities in starved HL-1 and neonatal cardiomyocytes cells [63]. Another cell-based study using H9c2 cardiac cells showed that BI0611953 decreased ROS generation, proteasome activity and caspase 3 activity [67]. Changes in caspase 3 and proteasome activities were shown to be associated with the intracellular stress response. Proteasomes are proteolytic complexes that are responsible for the degradation of most damaged, misfolded, or unneeded intracellular proteins. Proteasome dysfunction is associated with several major diseases including Parkinson’s disease, Huntington’s disease, cardiac hypertrophy, heart failure, cancer, and autoimmune diseases [70–72]. A recent report suggests that proteasome inhibition is an important contributor to mitochondrial dysfunction and the subsequent increased cytosolic oxidative stress and cell death [51]. How EETs can cause changes in proteasome activity remains to be resolved.
Caspase 3 activation plays an important role in apoptosis [73] and increased or decreased levels of apoptosis occur in diseased cells [74]. Since the release of certain proteins from mitochondria into the cytoplasm is known to activate caspase proteases and cause apoptosis [75], the reduction in caspase 3 is likely to be a consequence of EETs improving mitochondrial function.
One important question is how do EET-mediated effects reach the mitochondria. One possibility is that caveolin-1, and possibly other caveolins, may be important for cardioprotection mediated by EETs [76]. Cardiomyocyte plasma membrane and mitochondria isolated from hearts of wild-type mice subjected to IR showed caveolin-1 loss and lack of caveolae. Plasma membrane and mitochondria isolated from sEH null hearts or hearts from 11,12-EET-treated mice showed increased caveolin-1 and the presence of caveolae when compared to wild-type mice [76].
5. The unfolded protein response
The endoplasmic reticulum (ER) is a dynamic, multifunctional organelle with specialized domains and structures spanning the cell from the nuclear envelope to the plasma membrane [77]. ER functions encompass protein biosynthesis, folding and trafficking as well as calcium homeostasis among others [78]. Hence, the ER plays a critical and elegant role in maintaining cellular and organismic homeostasis [79]. Numerous and fluctuating stressors impact ER function including excessive accumulation of unfolded proteins, hypoxia, oxidative stress, ROS and calcium depletion of ER stores that can lead to ER stress. Cells use potent adaptive mechanisms to counter the deleterious effects of ER stress and to maintain the functional integrity of the ER, collectively known as the unfolded protein response (UPR) [80]. In eukaryotic cells UPR is triggered by transmembrane sensors that detect unfolded proteins in the ER through their luminal domain and convey information through their cytosolic domain. Three canonical branches of the UPR have been well characterized and include the ER transmembrane proteins PKR-like ER-regulated kinase (PERK), inositol requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6) [81, 82]. These initiator proteins respond to changes in protein-folding status and activate distinct and sometimes overlapping pathways. In the absence of stress, these sensors are rendered inactive through binding of their intraluminal domains to the chaperone 78-kDa glucose regulated protein (Grp78, also termed BiP) [83]. Accumulation of unfolded proteins in the ER lumen dislodges BiP from these sensors leading to the oligomerization and activation of IRE1α and PERK, and translocation of ATF6 to the Golgi resulting in a cascade of downstream signaling events [84]. IRE1α activation leads to the unconventional splicing of X-box binding protein 1 (XBP1) mRNA leading to the synthesis of a nuclear XBP1 form which induces the transcription of genes encoding ER chaperones, proteins involved in ER biogenesis and in secretion [85–87]. The second canonical branch includes PERK, which is a serine/threonine kinase whose autophosphorylation at threonine (Thr980) within its activation loop is essential for its activation [88]. PERK phosphorylates the α-subunit of the eukaryotic translation initiation factor 2 (eIF2α) at serine51, a modification that blocks initiation of mRNA translation in response to ER stress [88–90]. Of note, three other kinases can phosphorylate eIF2α at serine 51: double stranded RNA-dependent kinases (PKR), heme-regulated inhibitor kinase and general control nonderepressible protein 2 [91]. Activation of the third canonical branch of the UPR requires ATF6 translocation to the Golgi body and processing to generate an active transcription factor [92, 93]. Active ATF6 translocates to the nucleus and induces the expression of genes that facilitate the degradation and clearance of misfolded proteins [94]. These UPR arms synergize to attenuate stress by increasing the relative folding capacity by attenuating translation, modulating ER biogenesis and ER-associated protein degradation [95]. However, if the compensatory mechanisms fail to facilitate the adaptation of cells to ER stress, induction of UPR can lead to elimination of stressed cells by apoptosis [96, 97].
6 Modulation of ER stress by soluble epoxide hydrolase
Several studies utilizing genetic and pharmacological approaches establish sEH as a physiologically relevant regulator of UPR and a potential therapeutic target for mitigating complications associated with ER stress. Indeed, sEH genetic deficiency or pharmacological inhibition attenuates ER stress and favorably impacts metabolic homeostasis, fibrosis/tissue remodeling and neuropathic pain [41, 42, 45, 46, 98].
6.1 Metabolic homeostasis
The ER is highly responsive to nutrient status of the cell and impacts responses such as inflammation and stress-signaling pathways that are linked to metabolic regulation and diseases such as obesity and type 2 diabetes [99, 100]. Accordingly, the UPR is a critical determinant of metabolic homeostasis and ER dysfunction is a contributor to the pathogenesis of metabolic diseases. Chronic ER stress in adipose and liver, which significantly impacts their functions, has been described in obese rodents and humans [101, 102]. In addition, up-regulation of ER stress markers such as BiP, phosphorylated PERK/eIF2α in adipose and liver of Ob/Ob mice are linked to insulin resistance and hepatic steatosis [103]. Moreover, activation of eIF2α/ATF4 pathway alters glucose metabolism by decreasing insulin sensitivity in peripheral insulin-responsive tissues [104]. Notably, attenuation of ER stress using chemical chaperones restores systemic insulin sensitivity and enhances insulin action in insulin-responsive tissues of obese and diabetic mice [105], prevents high fat diet (HFD)-induced body weight gain in mice [106], and improves hepatic and muscle insulin sensitivity in obese human subjects in mice while treatment of obese human subjects [107].
A growing body of evidence identifies sEH as a regulator of metabolic homeostasis, at least in large part, by modulating ER stress. sEH deficiency yields a favorable metabolic outcome, prevents HFD-induced insulin resistance and improves glucose tolerance in mice [98]. In addition, sEH deficiency and pharmacological inhibition in vivo associate with enhanced hepatic and adipose insulin signaling [98]. Bettaieb et al reported that sEH expression increases in liver and adipose tissue of mice fed HFD and in adipose tissue of overweight human subjects concomitant with increased ER stress [46]. In addition, they demonstrated that sEH deficiency and pharmacological inhibition in vivo attenuate HFD-induced ER stress in liver and adipose tissue. Moreover, pharmacological inhibition of sEH in HepG2 cells and 3T3-L1 adipocytes mitigates chemical-induced ER stress consistent with being cell autonomous. Importantly, sEH pharmacological inhibition enhances insulin signaling in HepG2 cells [46]. In line with this observation, cells treated with epoxy fatty acids (sEH substrates) exhibit increased insulin signaling while cells treated with diols (sEH products) exhibit blunted insulin signaling that is mitigated by a chemical chaperone that attenuates ER stress [46]. These findings were confirmed and extended by Lopez-Vicario et al who demonstrate that sEH inhibition improves hepatic function through attenuation of ER stress and enhanced autophagy [47]. Together, these studies establish sEH as a regulator of metabolic homeostasis through modulation of ER stress.
sEH through its metabolism of EpFA is also emerging as a significant regulator of pancreas endocrine and exocrine functions. In a type 1 diabetes model (streptozotocin-treated mice), sEH deficiency and pharmacological inhibition attenuate hyperglycemia and promote insulin secretion [108, 109]. In addition, in a HFD-induced model of type 2 diabetes sEH deficiency and pharmacological inhibition increase pancreatic islet mass and vascularization [98]. The molecular mechanisms underlying sEH action in pancreatic β-cells and whether they encompass modulation of ER stress remain to be determined. However, it is worth noting that the UPR (in particular the PERK/eIF2α branch) is indispensable for proper functioning and survival of pancreatic β-cells in response to metabolic stresses such as increased demand for insulin [110] [80, 110, 111]. sEH is also a contributor to pancreas exocrine functions and is implicated in chronic and acute pancreatitis. Indeed, simultaneous pharmacological inhibition of sEH and RAF1 proto-oncogene serine/threonine kinase (c-RAF) reduces the severity of chronic pancreatitis [112]. Moreover, sEH deficient mice exhibit attenuated chemical-induced (cerulein- and arginine-induced) acute pancreatitis [42]. Importantly, sEH pharmacological inhibition, before and after induction of acute pancreatitis, mitigates the severity of disease in mice suggesting that sEH inhibition may be of therapeutic value in this disease [41]. Moreover, sEH pharmacological inhibition was associated with decreased cerulein- and arginine-induced pancreatic inflammatory response and ER stress. Of note, activation of ER stress is associated with acute pancreatitis [113] and treatment with ER chaperones mitigates the disease severity in animal models [114, 115]. Collectively, these studies open the possibility that modulation of EETs and other EpFA to reduce ER stress may be a potential approach to prevent perturbations of pancreatic exocrine functions and treat pancreatitis. Yet, more studies are required to determine whether this is indeed a possibility.
6.2 Fibrosis and tissue remodeling
Activation of the canonical branches of the UPR is established in models of fibrotic diseases including heart, lung, hepatic and renal fibrosis [116–119]. For example, activation of IRE1α or ATF6 promotes TGF-β-induced myofibroblastic differentiation of lung fibroblasts and inflammation of epithelial cells and their mesenchymal transition [120]. On the other hand, the chemical chaperone 4-phenylbutyric acid (4-PBA) attenuates cardiac fibrosis [121] as well as TGF-β1 expression and Smad signaling in the lung [122] and liver [123].
Several studies establish that sEH deficiency/inhibition attenuates the development of fibrosis in multiple organs. sEH deficiency and pharmacological inhibition (using 1-trifluoromethoxy-phenyl-3-(1-propionylpiperidin-4-yl) urea; TPPU) attenuate hepatic fibrosis and ER stress induced by carbon tetrachloride in mice [45]. Further, pharmacological inhibition of sEH using two additional inhibitors (1- trifluoromethoxy-phenyl-3 (1-methylsulfonyl)piperidin-4-yl) urea; TUPS and trans-4-{4-[3-(4-trifluoromethoxyphenyl)ureido]cyclohexyloxy}benzoic acid; t-TUCB) yields comparable effects on ER stress and fibrosis indicating that these effects are not unique to a particular probe but a characteristic of inhibition of sEH [45]. In addition, EETs analogs reduce renal fibrosis in a mouse model of unilateral ureteral obstruction. These protective effects are mediated, at least in part, through downregulation of NF-κB, TGF-β1/Smad3 and inflammatory signaling pathways [124, 125]. Moreover, sEH pharmacological inhibition using TPPU decreases bleomycin-induced inflammation and deposition of collagen in lungs of mice [40]. Similarly, administration of 14,15-EET suppresses cigarette smoke extract-induced inflammation and apoptotic cell death of lung epithelial cells through attenuation of ER stress [126]. Collectively, these studies establish a role for sEH in fibrotic diseases and suggest that attenuation of ER stress through sEH inhibition may serve as a potential therapeutic target for these diseases either alone or with other inhibitors of mitochondrial dysfunction, ER stress or aspects of the UPR response.
6.3 ER stress is a key determinant of neuropathic pain
Although the role of ER stress is well established for a number of neurological disorders such as Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis and prion disease, these conditions are invariably chronic and degenerative diseases. In contrast, we demonstrated a key role for the ER stress pathway using models of both chronic and acute pain [27]. Studies investigating the in vivo involvement of ER stress pathways in various disorders consistently utilized models of chronic diseases since it was thought that ER stress occurs over a long period of time such as weeks to months. Therefore, it was surprising to observe rapid and robust pain responses when two structurally and mechanistically distinct ER stress inducers were administered to the glaborous skin of the hind paw in rats. Although injection of tunicamycin and dimethyl celecoxib both resulted in nocifensive behavior and drastically reduced withdrawal responses within minutes, the two inducers also had significant phenotypical differences. Both ER stress inducers increased sensitivity to tactile stimulation, while tunicamycin led to decreased sensitivity to thermal stimuli. In contrast, dimethyl celecoxib did not induce changes on thermal withdrawal threshold. Remarkably, a small molecule blocker of ER stress response, 4-PBA reversed the rapid pain-related behavior in these models and other models of chronic pain along with preventing the activation of the UPR. Since the publication of these key results several other studies corroborated the role of sub-arms of the UPR, in particular, phosphorylation and activation of eIF2α, in nociceptive physiology [127, 128].
Although the role of ER stress in regulating pain responses continues to emerge, we demonstrated the causal role of activated ER stress pathways in diabetic neuropathy [27]. The mechanistic understanding of diabetes induced neuropathy is still incomplete and multiple pathological mechanisms seem to be responsible for the progressive degeneration of nerve fibers, starting from distal sites. However, the activation and sustained presence of the ER stress response in peripheral nerves seem to be a uniting feature that could cooperate with and encompass other mechanisms and cause peripheral neuropathy. High levels of glucose have been demonstrated to lead to the activation of UPR branches [105]. In peripheral nerves, ER components are distributed across and juxtaposed with mitochondria along the axons [129]. The protein synthetic machinery including ribosomes also travel across the axons to the nerve endings. Excess glucose and active ER stress signaling seems to predispose peripheral nerves to aberrant firing and activation of apoptotic signaling. These anatomic and physiologic constraints seem to be in line with earlier occurrence of neuropathic pain in type I vs type II diabetic patients. Furthermore, the importance of mitochondria generated reactive oxygen species and the contribution of numerous endglycation products to both ER stress and pain have been demonstrated independently. Therefore, in peripheral nerves of diabetic patients the unique spatial disposition of mitochondria and ER components may underlie the susceptibility of these neurons to ROS generation and simultaneous activation of ER stress. These events could then maintain the conditions which ultimately lead to neuropathy. In summary, work on sEH and EpFA resulted in identification of the key role of ER stress responses in chronic pain. Even though this concept and related findings are new and limited in scope, they argue that therapeutic targeting of the ER stress pathway or its components is a potential approach for the treatment of diverse painful conditions.
6.4 Other modes of action of EETs
In addition to the mode of actions mentioned above, other cellular events may mediate the beneficial effects of EpFAs. Exogenous EET supplementation in vitro attenuates the rise in intracellular Ca2+ and increased SERCA2a protein levels and activity. These protective effects are thought to be mediated through the role of EETs in increasing the expression of antioxidant enzymes resulting in reduced levels of reactive oxygen species, thus preventing SERCA oxidation [130]. Likewise, the overexpression of the CYP2J2, a predominant epoxygenases responsible for the biosynthesis of EETs in heart and endothelial cells suppressed ER stress in mice cardiomyocytes and protected against cardiac failure by maintaining intracellular Ca2+ homeostasis and SERCA2a expression and activity [131]. In line with these findings, overexpression of the rat homologue CYP2J3 prevented high fat diet-induced ER stress in adipose tissue of rats and inhibited thapsigargin-induced ER stress in differentiated 3T3-L1 mouse adipocytes [132]. In agreement with these observations, exogenous administration of EETs to differentiated 3T3-L1 adipocytes was protective against ER stress and resulted in increased adiponectin expression and secretion [132]. Moreover, pretreatment of lung epithelial cells with 14,15-EET decreased cigarette smoke extracts-induced expression of ER stress markers and was protective against apoptotic cell death [126]. The protective effects of epoxy fatty acids against ER stress seem to be accompanied with a reduction in mitochondrial dysfunction, inflammation and production of ROS, which are closely linked with ER stress [133]. In agreement with these observations, exogenous EETs, or CYP2J2 overexpression, inhibited mitochondrial dysfunction and decreased the levels of oxidative stress and cell apoptosis in lung tissues induced by ischemia/reperfusion in a human pulmonary artery endothelial cells [134]. Additionally, exposure to cigarette smoke extracts increased ROS generation and the phosphorylation of p38 and JNK, which were all attenuated by pretreatment with 14,15-EET. Collectively, these findings establish EpFAs as a significant contributor to ER stress and mitochondrial dysfunction and identify sEH as a novel promising target to prevent ER/mitochondria stress and their associated diseases.
6.5 Potential mechanisms for attenuation of ER stress by sEH deficiency
The molecular mechanisms that underlie attenuation of ER stress by sEH deficiency and pharmacological inhibition remain to be determined. A potential mechanism involves regulation of calcium homeostasis by EETs. Often, elevation of intracellular free calcium results from inhibition of sarco/endoplasmic reticulum calcium ATPase (SERCA) activity [135]. Exogenous supplementation with EETs in vitro attenuates the rise in intracellular calcium and increases SERCA2a expression and activity [136]. Likewise, overexpression of the CYP2J2, a predominant oxygenase responsible for EETs biosynthesis suppresses ER stress in mice cardiomyocytes and protects against cardiac failure by maintaining intracellular calcium homeostasis and SERCA2a expression and activity [130]. These mechanisms would reduce the response of ER stress to agents such as glucose, xenobiotics, and ROS. Another potential mechanism through which EETs can alleviate ER stress is decreased ROS production. EETs reduce ROS production and preserve mitochondrial function [137] and recent studies suggest that mitochondrial ROS production and oxidative stress can contribute to ER stress [138, 139]. This suggests that increased expression of antioxidant enzymes and reduction in ROS production mediated by EETs [137, 140] may lead to decreased ER stress. Thus, additional studies are needed to decipher the precise molecular mechanisms underlying regulation of ER stress by sEH and the contribution of ER stress to the beneficial effects of sEH deficiency and inhibition [141].
7 Conclusions
Overall, the data strongly suggest that significant reductions in EETs and other EpFA are likely to result in mitochondrial dysfunction, endoplasmic reticulum dysfunction, and subsequent metabolic impairment while increasing EpFA may ameliorate these pathologies. Although experimental data are lacking, it is plausible that EETs could directly prevent the activation of the signaling cascade governed by the three major ER resident sensors IRE-1α, protein kinase RNA-like endoplasmic reticulum kinase (PERK), and ATF6. ROS seems to be important contributor to mitochondrial and endoplasmic reticulum dysfunction. However, the source of the ROS production under most conditions is unclear. While the mitochondria have been suggested to be an important intracellular ROS producer [48], several other sources of ROS exist. It is also possible that some cell types produce the ROS which then acts on other cell types (Figure 3). Yet it increasingly appears that modulation of the mitochondria – ROS – ER stress axis by increasing key EpFAs provides a unifying hypothetical mechanism of action for sEHI and EpFA mimics that explain their positive biological effects on what at first appear to be quite diverse diseases.
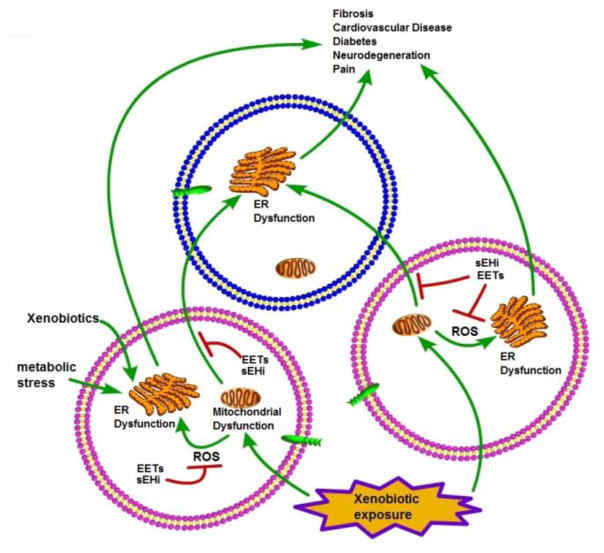
Figure 3. Schematic diagram showing the relationship among mitochondrial dysfunction, ER stress and EpFA.
ER dysfunction is associated with numerous diseases including diabetes, cardiovascular, and neuronal diseases. sEH inhibitors (sEHI) and by implication the stabilized EpFAs are unique in improving symptoms in a variety of apparently unrelated disease states ranging from neuropathic pain and atrial fibrillation to pathological fibrosis and diabetes while sEHI have little if any effect on normal animals. This enigma is explained in part by EpFA stabilizing the mitochondrial →ROS → ER stress axis. The unfolded protein response and ER stress, working in part together with the cellular proteasome act to maintain cellular homeostasis. This homeostasis can be disrupted by a number of factors such as glucose associated with diabetes, xenobiotics such as paraquat or MPTP, and particularly reactive oxygen species (ROS) from many sources. ROS can be dramatically increased by mitochondrial dysfunction in turn associated both with disease states or exposure to xenobiotics including NSAIDs and triclosan. EpFA stabilized by sEHI protect the mitochondria, reduce the downstream activation of ER stress by ROS, hyperglycemia and other factors and thus ameliorate disease symptoms.
Highlights.
The mechanism of action of epoxy fatty acids and inhibitors of the soluble epoxide hydrolase pivots on their ability to prevent mitochondrial dysfunction, to reduce subsequent ROS formation and to block resulting cellular signaling cascades, primarily the endoplasmic reticulum stress. By stabilizing the mitochondrial – ROS – ER stress axis, the range of activity of EpFAs and sEHI display an overlap with the disease conditions including diabetes, fibrosis, chronic pain, cardiovascular and neurodegenerative diseases, for which the above outlined mechanisms play key roles.
Acknowledgments
Funding
Research supported by the NIH/NIEHS R01 ES002710, NIH/NIDDK R01 DK103616, and the Superfund Research Program P42 ES004699) to BDH, (R01DK090492 and R01DK095359) to FGH, (R00DK100736) to AB, American Heart Association (16GRNT31350040) to AVG. Dr Haj is Co-Leader of the Endocrinology and Metabolism Core of UC, Davis Mouse Metabolic Phenotyping Center (MMPC) which is funded by U24DK092993.
Abbreviations
- 4-PBA
4-phenylbutyric acid
- ARA
arachidonic acid
- ATF6
activating transcription factor 6
- BiP or Grp78
78-kDa glucose regulated protein
- COX
cyclooxygenase
- CYP
cytochrome P450
- DHA
docosahexaenoic acid
- eIF2α
eukaryotic translation initiation factor 2 alpha
- EPA
eicosapentaenoic acid
- DHET
dihydroxyeicosatrienoic acid
- EpDPE
epoxydocosapentaenoic acid
- EET
epoxyeicosatrienoic acid
- EpFA
epoxy fatty acid
- ER
endoplasmic reticulum
- HEPE
hydroxyeicosapentaenoic acid
- HETE
hydroxyeicosatetraenoic acid
- HFD
high fat diet
- IRE1α
inositol-requiring enzyme 1 alpha
- LOX
lipoxygenase
- mEH
microsomal epoxide hydrolase
- mPTP
mitochondrial permeability transition pore
- ΔΨm
mitochondrial membrane potential
- NSAID
non-steroidal anti-inflammatory drug
- PERK
PKR-like ER-regulated kinase
- RANK and RANKL
Receptor Activator of Nuclear Factor κ B and RANK ligand
- ROS
reactive oxygen species
- sEH
soluble epoxide hydrolase
- sEHI
soluble epoxide hydrolase inhibitor
- TPPU
1-trifluoromethoxy-phenyl-3-(1-propionylpiperidin-4-yl) urea
- t-TUCB
trans-4-{4-[3-(4-trifluoromethoxyphenyl)ureido]cyclohexyloxy}benzoic acid
- TUPS
1- trifluoromethoxy-phenyl-3 (1-methylsulfonyl)piperidin-4-yl) urea
- UA-8
13-(3-propylureido)tridec-8-enoic acid
- UPR
unfolded protein response
- XBP1
X-box binding protein 1
Footnotes
Conflicts of Interest
BI, AB, FH and BH are all inventors on University of California Patents related to this work. BI and BH are founders of EicOsis which is developing sEHI as therapeutics.
Contributors
BI, AB, FGH, AVG, BDH all contributed to writing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vane J. The mechanism of action of anti-inflammatory drugs. Int J Clin Pract Suppl. 2003;(135):2. [PubMed] [Google Scholar]
- 2.Marnett LJ. The COXIB experience: a look in the rearview mirror. Annu Rev Pharmacol Toxicol. 2009;49:265–90. doi: 10.1146/annurev.pharmtox.011008.145638. [DOI] [PubMed] [Google Scholar]
- 3.Spector AA, Kim HY. Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim Biophys Acta. 2015;1851(4):356–65. doi: 10.1016/j.bbalip.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50(Suppl):S52–6. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, Eiserich JP, Hammock BD. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103(37):13646–51. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilroy DW, Edin ML, De Maeyer RP, Bystrom J, Newson J, Lih FB, Stables M, Zeldin DC, Bishop-Bailey D. CYP450-derived oxylipins mediate inflammatory resolution. Proc Natl Acad Sci U S A. 2016;113(23):E3240–9. doi: 10.1073/pnas.1521453113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8(10):794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kodani SD, Hammock BD. The 2014 Bernard B. Brodie award lecture-epoxide hydrolases: drug metabolism to therapeutics for chronic pain. Drug Metab Dispos. 2015;43(5):788–802. doi: 10.1124/dmd.115.063339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. American journal of physiology. Cell physiology. 2007;292(3):C996–1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 11.Morisseau C, Hammock BD. Gerry Brooks and epoxide hydrolases: four decades to a pharmaceutical. Pest Manag Sci. 2008;64(6):594–609. doi: 10.1002/ps.1583. [DOI] [PubMed] [Google Scholar]
- 12.Marowsky A, Burgener J, Falck JR, Fritschy JM, Arand M. Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience. 2009;163(2):646–61. doi: 10.1016/j.neuroscience.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Wagner K, Vito S, Inceoglu B, Hammock BD. The role of long chain fatty acids and their epoxide metabolites in nociceptive signaling. Prostaglandins Other Lipid Mediat. 2014;113–115:2–12. doi: 10.1016/j.prostaglandins.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51(12):3481–90. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, Sanborn JR, Hammock BD. Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci U S A. 1999;96(16):8849–54. doi: 10.1073/pnas.96.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazaar AL, Yang L, Boardley RL, Goyal NS, Robertson J, Baldwin SJ, Newby DE, Wilkinson IB, Tal-Singer R, Mayer RJ, Cheriyan J. Pharmacokinetics, pharmacodynamics and adverse event profile of GSK2256294, a novel soluble epoxide hydrolase inhibitor. Br J Clin Pharmacol. 2016;81(5):971–9. doi: 10.1111/bcp.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401(6752):493–7. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 18.Diani-Moore S, Ma Y, Gross SS, Rifkind AB. Increases in levels of epoxyeicosatrienoic and dihydroxyeicosatrienoic acids (EETs and DHETs) in liver and heart in vivo by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and in hepatic EET:DHET ratios by cotreatment with TCDD and the soluble epoxide hydrolase inhibitor AUDA. Drug Metab Dispos. 2014;42(2):294–300. doi: 10.1124/dmd.113.055368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng J, Plopper CG, Lakritz J, Storms DH, Hammock BD. Leukotoxin-diol: a putative toxic mediator involved in acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2001;25(4):434–8. doi: 10.1165/ajrcmb.25.4.4104. [DOI] [PubMed] [Google Scholar]
- 20.Goswami SK, Inceoglu B, Yang J, Wan D, Kodani SD, da Silva CA, Morisseau C, Hammock BD. Omeprazole increases the efficacy of a soluble epoxide hydrolase inhibitor in a PGE(2) induced pain model. Toxicol Appl Pharmacol. 2015;289(3):419–27. doi: 10.1016/j.taap.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inceoglu B, Wagner K, Schebb NH, Morisseau C, Jinks SL, Ulu A, Hegedus C, Rose T, Brosnan R, Hammock BD. Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proc Natl Acad Sci U S A. 2011;108(12):5093–7. doi: 10.1073/pnas.1101073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egan JJ, Greenberg AS, Chang MK, Wek SA, Moos MC, Jr, Londos C. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc Natl Acad Sci U S A. 1992;89(18):8537–41. doi: 10.1073/pnas.89.18.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiological reviews. 2012;92(1):101–30. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imig JD. Epoxyeicosatrienoic acids, hypertension, and kidney injury. Hypertension. 2015;65(3):476–82. doi: 10.1161/HYPERTENSIONAHA.114.03585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, Wagner K, Jones PD, Morisseau C, Hammock BD. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci U S A. 2008;105(48):18901–6. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79(24):2311–9. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inceoglu B, Bettaieb A, Trindade da Silva CA, Lee KS, Haj FG, Hammock BD. Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc Natl Acad Sci U S A. 2015;112(29):9082–7. doi: 10.1073/pnas.1510137112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulu A, Inceoglu B, Yang J, Singh V, Vito S, Wulff H, Hammock BD. Inhibition of soluble epoxide hydrolase as a novel approach to high dose diazepam induced hypotension. Journal of Clinical toxicology. 2017;6(3):10000300. doi: 10.4172/2161-0495.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275(51):40504–10. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- 30.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K(+)-channel activity. Am J Physiol. 1996;270(5 Pt 2):F822–32. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]
- 31.Lu T, VanRollins M, Lee HC. Stereospecific activation of cardiac ATP-sensitive K(+) channels by epoxyeicosatrienoic acids: a structural determinant study. Mol Pharmacol. 2002;62(5):1076–83. doi: 10.1124/mol.62.5.1076. [DOI] [PubMed] [Google Scholar]
- 32.Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med. 1997;3(5):562–6. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Zhu J, Lyu F, Panigrahy D, Ferrara KW, Hammock B, Zhang G. omega-3 polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat. 2014;113–115:13–20. doi: 10.1016/j.prostaglandins.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang G, Kodani S, Hammock BD. Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Prog Lipid Res. 2014;53:108–23. doi: 10.1016/j.plipres.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moghaddam M, Motoba K, Borhan B, Pinot F, Hammock BD. Novel metabolic pathways for linoleic and arachidonic acid metabolism. Biochim Biophys Acta. 1996;1290(3):327–39. doi: 10.1016/0304-4165(96)00037-2. [DOI] [PubMed] [Google Scholar]
- 36.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circulation research. 2000;87(11):992–8. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 37.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A. 2005;102(28):9772–7. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trindade-da-Silva CA, Bettaieb A, Napimoga MH, Lee KSS, Inceoglu B, Ueira-Vieira C, Bruun D, Goswami SK, Haj FG, Hammock BD. Soluble Epoxide Hydrolase Pharmacological Inhibition Decreases Alveolar Bone Loss by Modulating Host Inflammatory Response, RANK-Related Signaling, Endoplasmic Reticulum Stress, and Apoptosis. The Journal of pharmacology and experimental therapeutics. 2017;361(3):408–416. doi: 10.1124/jpet.116.238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, Kim IH, Tuteja D, Mateo RK, Singapuri A, Davis BB, Low R, Hammock BD, Chiamvimonvat N. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103(49):18733–8. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y, Sun GY, Liu T, Duan JX, Zhou HF, Lee KS, Hammock BD, Fang X, Jiang JX, Guan CX. Soluble epoxide hydrolase inhibitor 1-trifluoromethoxyphenyl-3- (1-propionylpiperidin-4-yl) urea attenuates bleomycin-induced pulmonary fibrosis in mice. Cell Tissue Res. 2016;363(2):399–409. doi: 10.1007/s00441-015-2262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bettaieb A, Chahed S, Bachaalany S, Griffey S, Hammock BD, Haj FG. Soluble Epoxide Hydrolase Pharmacological Inhibition Ameliorates Experimental Acute Pancreatitis in Mice. Mol Pharmacol. 2015 doi: 10.1124/mol.114.097501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bettaieb A, Chahed S, Tabet G, Yang J, Morisseau C, Griffey S, Hammock BD, Haj FG. Effects of soluble epoxide hydrolase deficiency on acute pancreatitis in mice. PloS one. 2014;9(11):e113019. doi: 10.1371/journal.pone.0113019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J, Bratt J, Franzi L, Liu JY, Zhang G, Zeki AA, Vogel CF, Williams K, Dong H, Lin Y, Hwang SH, Kenyon NJ, Hammock BD. Soluble epoxide hydrolase inhibitor attenuates inflammation and airway hyperresponsiveness in mice. Am J Respir Cell Mol Biol. 2015;52(1):46–55. doi: 10.1165/rcmb.2013-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kujal P, Certikova Chabova V, Skaroupkova P, Huskova Z, Vernerova Z, Kramer HJ, Walkowska A, Kompanowska-Jezierska E, Sadowski J, Kitada K, Nishiyama A, Hwang SH, Hammock BD, Imig JD, Cervenka L. Inhibition of soluble epoxide hydrolase is renoprotective in 5/6 nephrectomized Ren-2 transgenic hypertensive rats. Clinical and experimental pharmacology & physiology. 2014;41(3):227–37. doi: 10.1111/1440-1681.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris TR, Bettaieb A, Kodani S, Dong H, Myers R, Chiamvimonvat N, Haj FG, Hammock BD. Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. 2015 doi: 10.1016/j.taap.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bettaieb A, Nagata N, AbouBechara D, Chahed S, Morisseau C, Hammock BD, Haj FG. Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J Biol Chem. 2013;288(20):14189–99. doi: 10.1074/jbc.M113.458414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez-Vicario C, Alcaraz-Quiles J, Garcia-Alonso V, Rius B, Hwang SH, Titos E, Lopategi A, Hammock BD, Arroyo V, Claria J. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides. Proc Natl Acad Sci U S A. 2015;112(2):536–41. doi: 10.1073/pnas.1422590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy MP. How mitochondria produce reactive oxygen species. The Biochemical journal. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao X, Imig JD. Kidney CYP450 enzymes: biological actions beyond drug metabolism. Current drug metabolism. 2003;4(1):73–84. doi: 10.2174/1389200033336892. [DOI] [PubMed] [Google Scholar]
- 50.Droge W. Free radicals in the physiological control of cell function. Physiological reviews. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 51.Maharjan S, Oku M, Tsuda M, Hoseki J, Sakai Y. Mitochondrial impairment triggers cytosolic oxidative stress and cell death following proteasome inhibition. Scientific reports. 2014;4:5896. doi: 10.1038/srep05896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ines D, Jukić I, Mihaljević Z, Ćosić A, Kibel A. The Metabolites of Arachidonic Acid in Microvascular Function. In: Lenasi H, editor. Microcirculation Revisited - From Molecules to Clinical Practice. InTechOpen; 2016. [Google Scholar]
- 53.Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: cell survival and cell death. International journal of cell biology. 2010;2010:214074. doi: 10.1155/2010/214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–95. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 55.Sohal RS, Sohal BH, Orr WC. Mitochondrial superoxide and hydrogen peroxide generation, protein oxidative damage, and longevity in different species of flies. Free radical biology & medicine. 1995;19(4):499–504. doi: 10.1016/0891-5849(95)00037-x. [DOI] [PubMed] [Google Scholar]
- 56.Milne GL, Yin H, Hardy KD, Davies SS, Roberts LJ., 2nd Isoprostane generation and function. Chemical reviews. 2011;111(10):5973–96. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, 2nd, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free radical biology & medicine. 2005;38(6):698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 58.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clinical chemistry. 2006;52(4):601–23. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 59.Gamboa JL, Billings FTt, Bojanowski MT, Gilliam LA, Yu C, Roshanravan B, Roberts LJ, 2nd, Himmelfarb J, Ikizler TA, Brown NJ. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiological reports. 2016;4(9) doi: 10.14814/phy2.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fessel JP, Hulette C, Powell S, Roberts LJ, 2nd, Zhang J. Isofurans, but not F2-isoprostanes, are increased in the substantia nigra of patients with Parkinson’s disease and with dementia with Lewy body disease. Journal of neurochemistry. 2003;85(3):645–50. doi: 10.1046/j.1471-4159.2003.01709.x. [DOI] [PubMed] [Google Scholar]
- 61.Neckar J, Kopkan L, Huskova Z, Kolar F, Papousek F, Kramer HJ, Hwang SH, Hammock BD, Imig JD, Maly J, Netuka I, Ostadal B, Cervenka L. Inhibition of soluble epoxide hydrolase by cis-4-[4-(3-adamantan-1-ylureido)cyclohexyl-oxy]benzoic acid exhibits antihypertensive and cardioprotective actions in transgenic rats with angiotensin II-dependent hypertension. Clin Sci (Lond) 2012;122(11):513–25. doi: 10.1042/CS20110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katragadda D, Batchu SN, Cho WJ, Chaudhary KR, Falck JR, Seubert JM. Epoxyeicosatrienoic acids limit damage to mitochondrial function following stress in cardiac cells. Journal of molecular and cellular cardiology. 2009;46(6):867–75. doi: 10.1016/j.yjmcc.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 63.Samokhvalov V, Alsaleh N, El-Sikhry HE, Jamieson KL, Chen CB, Lopaschuk DG, Carter C, Light PE, Manne R, Falck JR, Seubert JM. Epoxyeicosatrienoic acids protect cardiac cells during starvation by modulating an autophagic response. Cell death & disease. 2013;4:e885. doi: 10.1038/cddis.2013.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, Gooch R, Foley J, Newman J, Mao L, Rockman HA, Hammock BD, Murphy E, Zeldin DC. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circulation research. 2004;95(5):506–14. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- 65.El-Sikhry HE, Alsaleh N, Dakarapu R, Falck JR, Seubert JM. Novel Roles of Epoxyeicosanoids in Regulating Cardiac Mitochondria. PloS one. 2016;11(8):e0160380. doi: 10.1371/journal.pone.0160380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bota DA, Van Remmen H, Davies KJ. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS letters. 2002;532(1–2):103–6. doi: 10.1016/s0014-5793(02)03638-4. [DOI] [PubMed] [Google Scholar]
- 67.Batchu SN, Lee SB, Samokhvalov V, Chaudhary KR, El-Sikhry H, Weldon SM, Seubert JM. Novel soluble epoxide hydrolase inhibitor protects mitochondrial function following stress. Canadian journal of physiology and pharmacology. 2012;90(6):811–23. doi: 10.1139/y2012-082. [DOI] [PubMed] [Google Scholar]
- 68.Sarkar P, Zaja I, Bienengraeber M, Rarick KR, Terashvili M, Canfield S, Falck JR, Harder DR. Epoxyeicosatrienoic acids pretreatment improves amyloid beta-induced mitochondrial dysfunction in cultured rat hippocampal astrocytes. American journal of physiology. Heart and circulatory physiology. 2014;306(4):H475–84. doi: 10.1152/ajpheart.00001.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77(6):817–27. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 70.Gomes AV. Genetics of proteasome diseases. Scientifica. 2013;2013:637629. doi: 10.1155/2013/637629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui Z, Scruggs SB, Gilda JE, Ping P, Gomes AV. Regulation of cardiac proteasomes by ubiquitination, SUMOylation, and beyond. Journal of molecular and cellular cardiology. 2014;71:32–42. doi: 10.1016/j.yjmcc.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bett JS. Proteostasis regulation by the ubiquitin system. Essays in biochemistry. 2016;60(2):143–151. doi: 10.1042/EBC20160001. [DOI] [PubMed] [Google Scholar]
- 73.Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell death and differentiation. 2015;22(4):526–39. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. Role of apoptosis in disease. Aging. 2012;4(5):330–49. doi: 10.18632/aging.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annual review of genetics. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chaudhary KR, Cho WJ, Yang F, Samokhvalov V, El-Sikhry HE, Daniel EE, Seubert JM. Effect of ischemia reperfusion injury and epoxyeicosatrienoic acids on caveolin expression in mouse myocardium. Journal of cardiovascular pharmacology. 2013;61(3):258–63. doi: 10.1097/FJC.0b013e31827afcee. [DOI] [PubMed] [Google Scholar]
- 77.Lynes EM, Simmen T. Urban planning of the endoplasmic reticulum (ER): how diverse mechanisms segregate the many functions of the ER. Biochim Biophys Acta. 2011;1813(10):1893–905. doi: 10.1016/j.bbamcr.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song BJ, Suh SK, Moon KH. A simple method to systematically study oxidatively modified proteins in biological samples and its applications. Methods Enzymol. 2010;473:251–64. doi: 10.1016/S0076-6879(10)73013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 80.Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, Arnold SM. The unfolded protein response in nutrient sensing and differentiation. Nature reviews. 2002;3(6):411–21. doi: 10.1038/nrm829. [DOI] [PubMed] [Google Scholar]
- 81.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature reviews. 2007;8(7):519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 82.Hummasti S, Hotamisligil GS. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circulation research. 107(5):579–91. doi: 10.1161/CIRCRESAHA.110.225698. [DOI] [PubMed] [Google Scholar]
- 83.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3(1):99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 84.Benjamin IJ. Viewing a stressful episode of ER: is ATF6 the triage nurse? Circulation research. 2006;98(9):1120–2. doi: 10.1161/01.RES.0000223522.47948.16. [DOI] [PubMed] [Google Scholar]
- 85.Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90(6):1031–9. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- 86.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–91. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 87.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415(6867):92–6. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 88.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–4. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 89.Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Molecular and cellular biology. 1998;18(12):7499–509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D. Stress-induced gene expression requires programmed recovery from translational repression. The EMBO journal. 2003;22(5):1180–7. doi: 10.1093/emboj/cdg112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han AP, Fleming MD, Chen JJ. Heme-regulated eIF2alpha kinase modifies the phenotypic severity of murine models of erythropoietic protoporphyria and beta-thalassemia. J Clin Invest. 2005;115(6):1562–70. doi: 10.1172/JCI24141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Molecular cell. 2000;6(6):1355–64. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 93.Okada T, Yoshida H, Akazawa R, Negishi M, Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. The Biochemical journal. 2002;366(Pt 2):585–94. doi: 10.1042/BJ20020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adachi Y, Yamamoto K, Okada T, Yoshida H, Harada A, Mori K. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct Funct. 2008;33(1):75–89. doi: 10.1247/csf.07044. [DOI] [PubMed] [Google Scholar]
- 95.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annual review of biochemistry. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 96.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes & development. 1998;12(7):982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes & development. 2002;16(11):1345–55. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luria A, Bettaieb A, Xi Y, Shieh GJ, Liu HC, Inoue H, Tsai HJ, Imig JD, Haj FG, Hammock BD. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci U S A. 2011;108(22):9038–43. doi: 10.1073/pnas.1103482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rozpedek W, Markiewicz L, Diehl JA, Pytel D, Majsterek I. The role of the adaptive stress response in the pathogenesis of neurodegenerative diseases, cancer and diabetes mellitus type 2. Pol Merkur Lekarski. 2015;39(234):393–7. [PubMed] [Google Scholar]
- 100.Dandekar A, Mendez R, Zhang K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol Biol. 2015;1292:205–14. doi: 10.1007/978-1-4939-2522-3_15. [DOI] [PubMed] [Google Scholar]
- 101.Kim RS, Hasegawa D, Goossens N, Tsuchida T, Athwal V, Sun X, Robinson CL, Bhattacharya D, Chou HI, Zhang DY, Fuchs BC, Lee Y, Hoshida Y, Friedman SL. The XBP1 Arm of the Unfolded Protein Response Induces Fibrogenic Activity in Hepatic Stellate Cells Through Autophagy. Scientific reports. 2016;6:39342. doi: 10.1038/srep39342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khadir A, Kavalakatt S, Abubaker J, Cherian P, Madhu D, Al-Khairi I, Abu-Farha M, Warsame S, Elkum N, Dehbi M, Tiss A. Physical exercise alleviates ER stress in obese humans through reduction in the expression and release of GRP78 chaperone. Metabolism. 2016;65(9):1409–20. doi: 10.1016/j.metabol.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 103.Dara L, Ji C, Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 2011;53(5):1752–63. doi: 10.1002/hep.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoshizawa T, Hinoi E, Jung DY, Kajimura D, Ferron M, Seo J, Graff JM, Kim JK, Karsenty G. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J Clin Invest. 2009;119(9):2807–17. doi: 10.1172/JCI39366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Basseri S, Lhotak S, Sharma AM, Austin RC. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. J Lipid Res. 2009;50(12):2486–501. doi: 10.1194/jlr.M900216-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, Klein S. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59(8):1899–905. doi: 10.2337/db10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luo P, Chang HH, Zhou Y, Zhang S, Hwang SH, Morisseau C, Wang CY, Inscho EW, Hammock BD, Wang MH. Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. The Journal of pharmacology and experimental therapeutics. 334(2):430–8. doi: 10.1124/jpet.110.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen L, Fan C, Zhang Y, Bakri M, Dong H, Morisseau C, Maddipati KR, Luo P, Wang CY, Hammock BD, Wang MH. Beneficial effects of inhibition of soluble epoxide hydrolase on glucose homeostasis and islet damage in a streptozotocin-induced diabetic mouse model. Prostaglandins Other Lipid Mediat. doi: 10.1016/j.prostaglandins.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Molecular cell. 2001;7(6):1153–63. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 111.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Molecular cell. 2001;7(6):1165–76. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 112.Liao J, Hwang SH, Li H, Yang Y, Yang J, Wecksler AT, Liu JY, Hammock BD, Yang GY. Inhibition of mutant KrasG12D-initiated murine pancreatic carcinoma growth by a dual c-Raf and soluble epoxide hydrolase inhibitor t-CUPM. Cancer Lett. 2016;371(2):187–93. doi: 10.1016/j.canlet.2015.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kubisch CH, Sans MD, Arumugam T, Ernst SA, Williams JA, Logsdon CD. Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. American journal of physiology. Gastrointestinal and liver physiology. 2006;291(2):G238–45. doi: 10.1152/ajpgi.00471.2005. [DOI] [PubMed] [Google Scholar]
- 114.Seyhun E, Malo A, Schafer C, Moskaluk CA, Hoffmann RT, Goke B, Kubisch CH. Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, acinar cell damage, and systemic inflammation in acute pancreatitis. American journal of physiology. Gastrointestinal and liver physiology. 2011;301(5):G773–82. doi: 10.1152/ajpgi.00483.2010. [DOI] [PubMed] [Google Scholar]
- 115.Malo A, Kruger B, Goke B, Kubisch CH. 4-Phenylbutyric acid reduces endoplasmic reticulum stress, trypsin activation, and acinar cell apoptosis while increasing secretion in rat pancreatic acini. Pancreas. 2013;42(1):92–101. doi: 10.1097/MPA.0b013e318259f6ca. [DOI] [PubMed] [Google Scholar]
- 116.Baiceanu A, Mesdom P, Lagouge M, Foufelle F. Endoplasmic reticulum proteostasis in hepatic steatosis. Nat Rev Endocrinol. 2016;12(12):710–722. doi: 10.1038/nrendo.2016.124. [DOI] [PubMed] [Google Scholar]
- 117.Taniguchi M, Yoshida H. Endoplasmic reticulum stress in kidney function and disease. Curr Opin Nephrol Hypertens. 2015;24(4):345–50. doi: 10.1097/MNH.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 118.Lenna S, Trojanowska M. The role of endoplasmic reticulum stress and the unfolded protein response in fibrosis. Curr Opin Rheumatol. 2012;24(6):663–8. doi: 10.1097/BOR.0b013e3283588dbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ribeiro CM, Boucher RC. Role of endoplasmic reticulum stress in cystic fibrosis-related airway inflammatory responses. Proc Am Thorac Soc. 2010;7(6):387–94. doi: 10.1513/pats.201001-017AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schuliga M, Javeed A, Harris T, Xia Y, Qin C, Wang Z, Zhang X, Lee PV, Camoretti-Mercado B, Stewart AG. Transforming growth factor-beta-induced differentiation of airway smooth muscle cells is inhibited by fibroblast growth factor-2. Am J Respir Cell Mol Biol. 2013;48(3):346–53. doi: 10.1165/rcmb.2012-0151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baek HA, Kim DS, Park HS, Jang KY, Kang MJ, Lee DG, Moon WS, Chae HJ, Chung MJ. Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am J Respir Cell Mol Biol. 2012;46(6):731–9. doi: 10.1165/rcmb.2011-0121OC. [DOI] [PubMed] [Google Scholar]
- 122.Omura T, Asari M, Yamamoto J, Oka K, Hoshina C, Maseda C, Awaya T, Tasaki Y, Shiono H, Yonezawa A, Masuda S, Matsubara K, Shimizu K. Sodium tauroursodeoxycholate prevents paraquat-induced cell death by suppressing endoplasmic reticulum stress responses in human lung epithelial A549 cells. Biochem Biophys Res Commun. 2013;432(4):689–94. doi: 10.1016/j.bbrc.2013.01.131. [DOI] [PubMed] [Google Scholar]
- 123.Wang JQ, Chen X, Zhang C, Tao L, Zhang ZH, Liu XQ, Xu YB, Wang H, Li J, Xu DX. Phenylbutyric acid protects against carbon tetrachloride-induced hepatic fibrogenesis in mice. Toxicol Appl Pharmacol. 2013;266(2):307–16. doi: 10.1016/j.taap.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 124.Kim J, Imig JD, Yang J, Hammock BD, Padanilam BJ. Inhibition of soluble epoxide hydrolase prevents renal interstitial fibrosis and inflammation. Am J Physiol Renal Physiol. 2014;307(8):F971–80. doi: 10.1152/ajprenal.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim J, Yoon SP, Toews ML, Imig JD, Hwang SH, Hammock BD, Padanilam BJ. Pharmacological inhibition of soluble epoxide hydrolase prevents renal interstitial fibrogenesis in obstructive nephropathy. Am J Physiol Renal Physiol. 2015;308(2):F131–9. doi: 10.1152/ajprenal.00531.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu G, Zeng X, Wang H, Hou Q, Tan C, Xu Q, Wang H. 14,15-epoxyeicosatrienoic Acid suppresses cigarette smoke extract-induced apoptosis in lung epithelial cells by inhibiting endoplasmic reticulum stress. Cell Physiol Biochem. 2015;36(2):474–86. doi: 10.1159/000430113. [DOI] [PubMed] [Google Scholar]
- 127.Sharma D, Singh JN, Sharma SS. Effects of 4-phenyl butyric acid on high glucose-induced alterations in dorsal root ganglion neurons. Neuroscience letters. 2016;635:83–89. doi: 10.1016/j.neulet.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 128.Khoutorsky A, Sorge RE, Prager-Khoutorsky M, Pawlowski SA, Longo G, Jafarnejad SM, Tahmasebi S, Martin LJ, Pitcher MH, Gkogkas CG, Sharif-Naeini R, Ribeiro-da-Silva A, Bourque CW, Cervero F, Mogil JS, Sonenberg N. eIF2alpha phosphorylation controls thermal nociception. Proc Natl Acad Sci U S A. 2016;113(42):11949–11954. doi: 10.1073/pnas.1614047113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yoo S, van Niekerk EA, Merianda TT, Twiss JL. Dynamics of axonal mRNA transport and implications for peripheral nerve regeneration. Experimental neurology. 2010;223(1):19–27. doi: 10.1016/j.expneurol.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang X, Ni L, Yang L, Duan Q, Chen C, Edin ML, Zeldin DC, Wang DW. CYP2J2-derived epoxyeicosatrienoic acids suppress endoplasmic reticulum stress in heart failure. Mol Pharmacol. 2014;85(1):105–15. doi: 10.1124/mol.113.087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.You WT, Zhou T, Ma ZC, Liang QD, Xiao CR, Tang XL, Tan HL, Zhang BL, Wang YG, Gao Y. Ophiopogonin D maintains Ca2+ homeostasis in rat cardiomyocytes in vitro by upregulating CYP2J3/EETs and suppressing ER stress. Acta Pharmacol Sin. 2016;37(3):368–81. doi: 10.1038/aps.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu X, Tu L, Feng W, Ma B, Li R, Zheng C, Li G, Wang DW. CYP2J3 gene delivery up-regulated adiponectin expression via reduced endoplasmic reticulum stress in adipocytes. Endocrinology. 2013;154(5):1743–53. doi: 10.1210/en.2012-2012. [DOI] [PubMed] [Google Scholar]
- 133.Tagawa Y, Hiramatsu N, Kato H, Sakoh T, Nakajima S, Hayakawa K, Saito Y, Johno H, Takahashi S, Gu L, Yao J, Kitamura M. Induction of CCAAT/enhancer-binding protein-homologous protein by cigarette smoke through the superoxide anion-triggered PERK-eIF2alpha pathway. Toxicology. 2011;287(1–3):105–12. doi: 10.1016/j.tox.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 134.Chen W, Zheng G, Yang S, Ping W, Fu X, Zhang N, Wang DW, Wang J. CYP2J2 and EETs Protect against Oxidative Stress and Apoptosis in Vivo and in Vitro Following Lung Ischemia/Reperfusion. Cell Physiol Biochem. 2014;33(6):1663–80. doi: 10.1159/000362950. [DOI] [PubMed] [Google Scholar]
- 135.Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54(2):452–61. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- 136.Wang YX, Zeng XJ, Lu LQ, Ma LQ, Jiang DQ, Mu J, Wang XY, Zhang LK, Tang CS, Hao G. Effects of 11, 12-epoxyeicosatrienoic acid preconditioning and postconditioning on Ca(2+)- handling proteins in myocardial ischemia/reperfusion injury in rats. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2007;29(6):787–91. [PubMed] [Google Scholar]
- 137.Liu L, Chen C, Gong W, Li Y, Edin ML, Zeldin DC, Wang DW. Epoxyeicosatrienoic acids attenuate reactive oxygen species level, mitochondrial dysfunction, caspase activation, and apoptosis in carcinoma cells treated with arsenic trioxide. The Journal of pharmacology and experimental therapeutics. 2011;339(2):451–63. doi: 10.1124/jpet.111.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Eletto D, Chevet E, Argon Y, Appenzeller-Herzog C. Redox controls UPR to control redox. J Cell Sci. 2014;127(Pt 17):3649–58. doi: 10.1242/jcs.153643. [DOI] [PubMed] [Google Scholar]
- 139.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105(47):18525–30. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu WJ, Wang T, Wang B, Liu XT, He XW, Liu YJ, Li ZX, Tan R, Zeng HS. CYP2C8-derived epoxyeicosatrienoic acids decrease oxidative stress-induced endothelial apoptosis in development of atherosclerosis: Role of Nrf2 activation. J Huazhong Univ Sci Technolog Med Sci. 2015;35(5):640–5. doi: 10.1007/s11596-015-1483-5. [DOI] [PubMed] [Google Scholar]
- 141.Wagner KM, McReynolds CB, Schmidt WK, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases. Pharmacol Ther. 2017 doi: 10.1016/j.pharmthera.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]