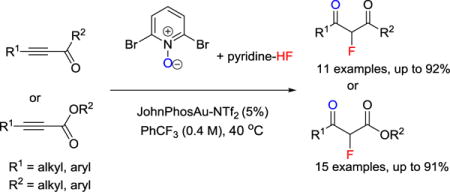
Abstract
We developed an efficient synthesis of 2-fluoro-1,3-dicarbonyl compounds using readily available alkynyl ketones or esters as starting material. The key step is the insertion of hydrogen fluoride (HF) to the gold carbene intermediate generated from cationic gold catalyzed addition of N-oxides to alkynyl ketones or esters. This method gives excellent chemical yields and regioselectivity with good functional group tolerance.
Keywords: Gold Catalysis; Fluorination; 1,3-Dicarbonyl Compounds; Alkynyl Esters and Ketones
Graphical abstract
Due to fluorine’s unique properties such as small size and metabolically resistant C-F bond, the selective substitution of hydrogen by fluorine constitutes a key strategy in pharmaceuticals, agrochemicals and materials research.[1] More specifically, fluorinated 1,3-dicarbonyl compounds are important fluorine building blocks and motifs in medicine and materials.[2] Literature syntheses of fluorinated 1,3-dicarbonyl compounds are generally based on the electrophilic fluorination of 1,3-dicarbonyl compound precursors (Scheme 1, a–e). For examples, Shibata and coworkers reported the fluorination of β-keto-esters using methyl NFSI as fluorination agent under Lewis acid-catalysis (Scheme 1a).[3] Many other electrophilic fluorination systems like Selectfluor in imidazolium ionic liquid (Scheme 1b),[4] Selectfluor in continuous-flow microreactors,[5] Selectfluor/ZnCl2 (Scheme 1c) have been developed.[6] The fluorination of 1,3-dicarbonyl compound precursors can also be achieved using Ar-I/HF-pyridine/mCPBA (Scheme 1d)[7] and HF/PhIO fluorination system (Scheme 1e).[8] In addition, the Hayes and Moody group reported a nucleophilic fluorination of α-diazo-β-ketoesters with HBF4 as a fluorine-carrier (Scheme 1f).[9] These methods do have some drawbacks though, among them: 1) relatively limited scope; 2) non-atom-economic fluorination systems; 3) poor functional group tolerance caused by the use of strong oxidants; and 4) difficulty in controlling overreaction (i.e., difluorination).[6],[10]
Scheme 1.
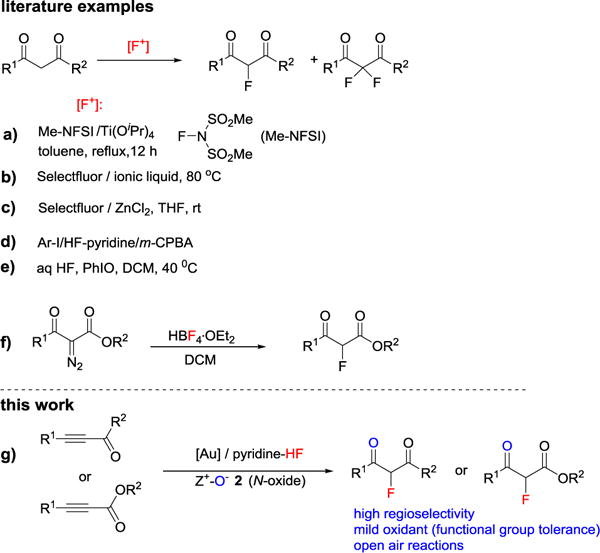
Synthesis of fluorinated 1,3-dicarbonyl compounds.
Because alkynyl ketones or esters are readily available compounds, and because the alkynyl group can be considered as a masked ketone, the task of converting alkynyl ketones or esters to fluorinated 1,3-dicarbonyl compounds is a straightforward synthetic method. Hydrogen fluoride (HF) is one the most atom-economic fluorination reagent. Furthermore, HF can be regarded as compatible with cationic gold catalysts; this was demonstrated by the synthesis of fluoroalkenes through the gold catalyzed addition of HF to alkynes[11] and the synthesis of α-fluoroketone via HF insertion to a gold carbene intermediate.[12] These methods proved efficient when a terminal alkyne were utilized as substrate; internal alkynes showed low or no reactivity, and the regioselectivity depended on steric and electronic biases at either end of the alkyne. We proposed that the ester or ketone group could function as an activator and director in the fluorination of an alkyne. Herein, we report a widely applicable, highly efficient and divergent synthesis of 2-fluoro-1,3-dicarbonyl compounds from readily available alkynyl ketones/esters (Scheme 1g).
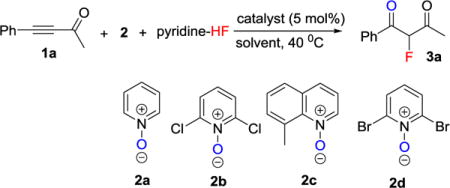
We carried out the fluorination of alkynyl ketone 1a as our model reaction (Table 1). The oxidant we use was an N-oxide, which is mild,[13], [14] hence, we expected good functional group tolerance. We recently demonstrated that excess amounts of silver salt in gold catalysis almost always had a deleterious effect on the reactivity of gold catalysts and that a preformed gold catalyst like L-Au-NTf2, usually exhibited the best reactivity.[15] So, initially we used the preformed gold catalyst JohnPhos-AuNTf2.[16] We screened the effects of N-oxides 2 (Table 1, entries 1–4), and found that N-oxide 2d gave the best result (Table 1, entry 4). The screening of various solvents indicated that PhCF3 was the optimal solvent (Table 1, entries 4–7). We proceeded to investigate the effects of the ligands (Table 1, entries 6–10).[17] All the ligands tested gave reasonably good results, but, among them, JohnPhos was slightly better (Table 1, entries 6–10). We also confirmed that a silver salt alone was not able to catalyze this reaction (Table 1, entry 11).
Table 1.
Optimization of fluorination of alkynyl ketone.[a]
| ||||
|---|---|---|---|---|
|
| ||||
| Entry | Catalyst | N-oxide 2 | Solvent | Yield[b] (%) |
| 1 | JohnPhosAuNTf2 | 2a | DCM | 58 |
| 2 | JohnPhosAuNTf2 | 2b | DCM | 73 |
| 3 | JohnPhosAuNTf2 | 2c | DCM | 60 |
| 4 | JohnPhosAuNTf2 | 2d | DCM | 80 |
| 5 | JohnPhosAuNTf2 | 2d | PhF | 86 |
| 6 | JohnPhosAuNTf2 | 2d | PhCF3 | 93 |
| 7 | XPhosAuCI+AgNTf2 | 2d | PhCF3 | 91 |
| 8 | Dppf(AuCI)2+AgNTf2 | 2d | PhCF3 | 89 |
| 9 | IPrAuCI+AgNTf2 | 2d | PhCF3 | 84 |
| 10 | PPh3AuCI+AgNTf2 | 2d | PhCF3 | 90 |
| 11 | AgNTf2 | 2d | PhCF3 | 0 |
| 12[c] | JohnPhosAuNTf2 | 2d | PhCF3 | 80 |
| 13[d] | JohnPhosAuNTf2 | 2d | PhCF3 | 85 |
| 14[e] | JohnPhosAuNTf2 | 2d | PhCF3 | 89 |
Conditions: 1a (0.2 mmol), N-Oxide 2 (0.28 mmol), pyridine-HF (0.8 mmol HF, 4 equiv), catalyst (5 mol%), activator (5 mol%), solvent (0.4 mL), 40 °C in a sealed polypropylene tube for 8 h.
Determined by GC-MS analysis.
Pyridine-HF (2 equiv) was used.
Pyridine-HF (2.5 equiv) was used.
pyridine-HF JohnPhosAuNTf2 (1 mol%) was used.
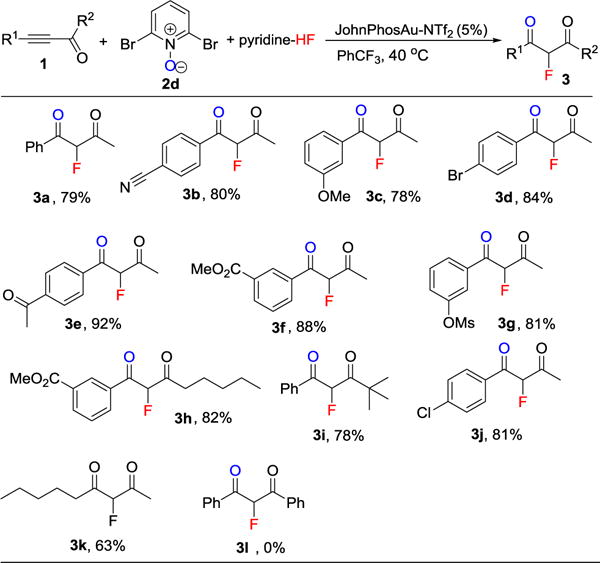
With the optimized conditions in hand, we explored the substrate scope and functional group tolerance for the fluorination of alkynyl ketones (Table 2). We evaluated the effect of different [R1, R2] combinations in the alkynyl ketones 1 (Table 2). The substitution pattern (meta, para) and electronic properties of aromatic substituents (electron deficient or rich) of R1 played a small role; good yields were obtained regardless (Table 2, 3a–3f). Various functional groups such as nitrile (Table 2, 3b), ether (Table 2, 3c), halide (Table 2, 3d), ketone (Table 2, 3e), ester (Table 2, 3f, 3h), and sulfonate (Table 2, 3g) were well tolerated. It should be noted that when both R1, R2 were aromatics, no reaction took place (Table 2, 3i). Lack of reactivity in this case may be caused by the fact that the triple bond and two phenyl rings form a highly delocalized system which weaken the polarization of triple bond.
Table 2.
Scope for the synthesis of 2-fluoro-1,3-diketone 3.[a]
|
Reaction conditions: alkyne 1 (0.2 mmol), N-Oxide 2a (0.28 mmol), pyridine-HF (0.8 mmol HF), JohnPhosAuNTf2 (5 mol %), PhCF3 (0.4 mL), 40 °C in a sealed polypropylene tube for 4 h or overnight. All yields are isolated yields; 3 exists as a mixture of ketone form and enol form.
Table 3.
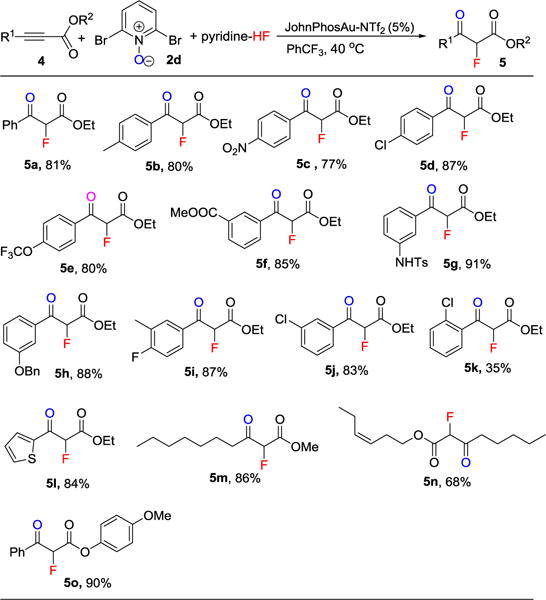
Scope for the synthesis of 2-fluoro-3-keto-ester 5.[a]
|
Reaction conditions: alkyne 4 (0.2 mmol), N-Oxide 2a (0.28 mmol), pyridine-HF (0.8 mmol HF), JohnPhosAuNTf2 (5 mol %), PhCF3 (0.4 mL), 40 °C in a sealed polypropylene tube for 4 h or overnight; rt. All yields are isolated yields.
Our reaction conditions could also be applied to the fluorination of alkynyl esters 4, leading to the synthesis of 2-fluoro-3-ketoester 5 (Table 3). First, we evaluated the effect of R1 substitution of the 3-ketoester 4: electron rich and electron deficient substituents only had a very small effect on the chemical yields (Table 3, 5a–5l). The substitution pattern (ortho, meta, para) of R1 only played a small role; good yields were obtained regardless (Table 3, 5a–5l). And various functional groups such as nitro group (Table 2, 5c), chloride (Table 3, 5d, 5j, 5k), ester (Table 3, 5f), sulfonamide (Table 3, 5g), benzyl ether (Table 3, 5h), fluoride (Table 3, 5i) and alkene (Table 3, 5n) were well tolerated. We also evaluated more [R1, R2] combinations, such as [R1 = alkyl, R2 = alkyl] (Table 3, 5m–5n), [R1 = alkyl, R2 = aryl] (Table 3, 5o). These combinations gave good yields of product 5. It should be noted that an activated ester (phenol ester, 5o) survived our conditions. Activated esters like 5o can be used for further transformations like amide formation.
The proposed mechanism is illustrated in Scheme 2. First, addition of N-oxides 2 to the cationic gold activated alkyne starting material gives vinyl gold intermediate A, which then transforms into the reactive gold carbene intermediate B.[18] The hydrogen bonding interaction between HF and A may facilitate the formation of gold carbene B. Finally, insertion of HF gold carbene B gives products 3 or 5.[12]
 |
(eq-1) |
Scheme 2.
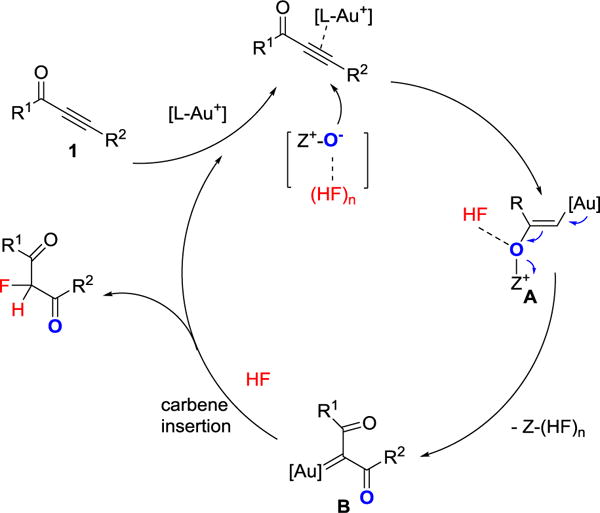
Proposed mechanism for the formation of 3 and 5.
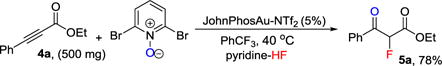
Our protocol could be easily scaled up without affecting the chemical yield (eq 1). The versatility of fluorinated 1,3-dicarbonyl compounds 3 and 5 in organic synthesis is clearly underscored in Scheme 3. For examples, reaction of 3a with hydrazine leads to a facile synthesis of fluorinated pyrazole 6.[19] Iridium-catalyzed asymmetric allylic alkylation of 5a produces the highly functionalized fluoro-building block 7.[20] And the guanidine-catalyzed Michael addition of 5a gives 8 in good yield and selectivity.[21] [3+3] Cyclization of 3a with 1,3-bis(silyl enol ethers leads to an efficient synthesis of fluorinated phenols 9.[22] Asymmetric Mannich reaction of 5a with a bifunctional thiourea catalyst furnishes 10.[23]
Scheme 3.
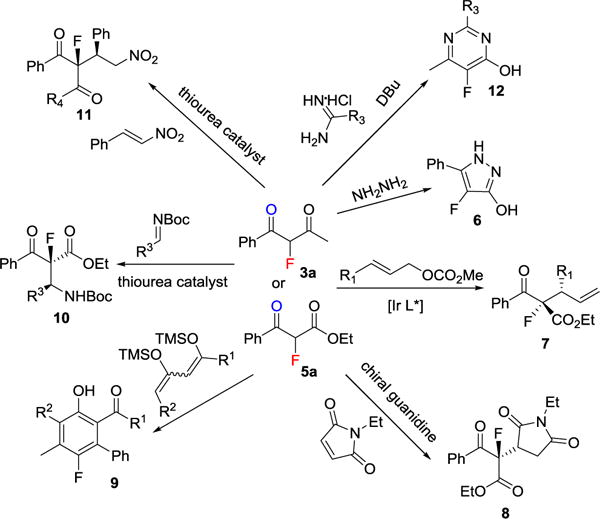
Divergent syntheses from 3 or 5 from literature reports.
Similarly, a highly enantioselective Michael addition of 3a to nitrostyrene yields 11 with tertiary stereocenters.[24] 3 can also be used in the synthesis of fluorinated pyrimidine scaffold 12.[25]
In summary, we have developed a widely applicable synthesis of 2-fluoro-1,3-dicarbonyl compounds starting with readily available alkynyl ketones or esters. This method gave excellent chemical yields and regioselectivity with good functional group tolerance. And the reaction can be conducted under ambient atmosphere. Other fluorination systems based on HF/gold combinations are currently being investigated in our laboratories.
Experimental Section
General procedure for fluorination of 1 or 4
A polypropylene vial was charged with alkynone 1 or alkynoate 4 (0.2 mmol, 1 equiv), 2,6-dibromopyridine N-oxide 2d (0.28 mmol, 70 mg), and HF-pyridine (HF content 70 wt/wt%, 0.8 mmol, 27 mg, 4 equiv) and PhCF3 (0.4 mL). The reaction was commenced by the addition of JohnPhosAuNTf2 (5 mol%, 7.75 mg) and the reaction mixture was heated to 40 °C. The progress of the reaction was monitored by TLC. The reaction typically took 4 h or overnight to complete. Upon completion, the mixture was quenched with saturated sodium bicarbonate. The mixture was extracted with DCM and washed with 1N HCl. The organic layers were collected, dried over anhydrous MgSO4 and filtered. The solvent was removed under reduced pressure and the residue was subjected to flash column chromatography purification (eluent: hexanes/ethyl acetate) to give 2-fluoro-1,3-dicarbonyl compounds 3 or 5.
Supplementary Material
Acknowledgments
We are grateful to the National Science Foundation of China for financial support (NSFC-21472018) and China Recruitment Program of Global Experts for financial support. G.B.H. is grateful to the National Institutes of Health (1R01GM121660-01). XZ is grateful to the China Scholarship Council.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/adsc.201######.
References
- 1.a) Chambers RD. Fluorine in organic chemistry. Blackwell Publishing Ltd./CRC Press; Boca Raton, Fl: 2004. [Google Scholar]; b) Hiyama T. Organofluorine compounds, chemistry and applications. Springer-Verlag; Berlin: 2000. [Google Scholar]; c) Kirsch P. Modern fluoroorganic chemistry. Wiley-VCH; Weinheim: 2004. [Google Scholar]; d) Muller K, Faeh C, Diederich F. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]; e) Schlosser M. Angew Chem Int Ed. 1998;37:1496. doi: 10.1002/(SICI)1521-3773(19980619)37:11<1496::AID-ANIE1496>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]; f) Soloshonok VA. Fluorine-containing synthons, ACS symposium series 911. Oxford University Press; Washington, D.C: 2005. [Google Scholar]; g) Uneyama K. Organofluorine Chemistry. Blackwell publishing; Oxford: 2006. [Google Scholar]
- 2.a) Guo Y, Twamley B, Shreeve J n M. Org Biomol Chem. 2009;7:1716–1722. doi: 10.1039/b900311h. [DOI] [PubMed] [Google Scholar]; b) Kim K-Y, Kim BC, Lee HB, Shin H. J Org Chem. 2008;73:8106–8108. doi: 10.1021/jo8015659. [DOI] [PubMed] [Google Scholar]; c) Prakash GKS, Beier P. Angew Chem Int Ed. 2006;45:2172–2174. doi: 10.1002/anie.200503783. [DOI] [PubMed] [Google Scholar]; d) Burger EC, Barron BR, Tunge JA. Synlett. 2006:2824–2826. [Google Scholar]; e) Davis FA, Han W, Murphy CK. J Org Chem. 1995;60:4730–4737. [Google Scholar]; f) Dolbier WR, Jr, Lee SK, Phanstiel OIV. Tetrahedron. 1991;47:2065–2072. [Google Scholar]
- 3.Fukushi K, Suzuki S, Kamo T, Tokunaga E, Sumii Y, Kagawa T, Kawada K, Shibata N. Green Chem. 2016;18:1864–1868. [Google Scholar]
- 4.Reddy AS, Laali KK. Tetrahedron Lett. 2015;56:5495–5499. [Google Scholar]
- 5.Baumann M, Baxendale IR, Martin LJ, Ley SV. Tetrahedron. 2009;65:6611–6625. [Google Scholar]
- 6.Jiang F, Zhao Y, Hu J. Organic Chemistry Frontiers. 2014;1:625–629. [Google Scholar]
- 7.Kitamura T, Kuriki S, Morshed MH, Hori Y. Org Lett. 2011;13:2392–2394. doi: 10.1021/ol200632d. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki S, Kamo T, Fukushi K, Hiramatsu T, Tokunaga E, Dohi T, Kita Y, Shibata N. Chem Sci. 2014;5:2754–2760. [Google Scholar]
- 9.Pasceri R, Bartrum HE, Hayes CJ, Moody CJ. Chem Commun. 2012;48:12077–12079. doi: 10.1039/c2cc37284c. [DOI] [PubMed] [Google Scholar]
- 10.Leng DJ, Black CM, Pattison G. Org Biomol Chem. 2016;14:1531–1535. doi: 10.1039/c5ob02468d. [DOI] [PubMed] [Google Scholar]
- 11.a) Akana JA, Bhattacharyya KX, Mueller P, Sadighi JP. J Am Chem Soc. 2007;129:7736–7737. doi: 10.1021/ja0723784. [DOI] [PubMed] [Google Scholar]; b) Gorske BC, Mbofana CT, Miller SJ. Org Lett. 2009;11:4318–4321. doi: 10.1021/ol9016782. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) He G, Qiu S, Huang H, Zhu G, Zhang D, Zhang R, Zhu H. Org Lett. 2016;18:1856–1859. doi: 10.1021/acs.orglett.6b00615. [DOI] [PubMed] [Google Scholar]; d) Nahra F, Patrick SR, Bello D, Brill M, Obled A, Cordes DB, Slawin AMZ, O’Hagan D, Nolan SP. ChemCatChem. 2015;7:240–244. doi: 10.1002/cctc.201402891. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Okoromoba OE, Han J, Hammond GB, Xu B. J Am Chem Soc. 2014;136:14381–14384. doi: 10.1021/ja508369z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng X, Liu S, Shi Z, Liu G, Xu B. Angew Chem Int Ed. 2016;55:10032–10036. doi: 10.1002/anie.201603914. [DOI] [PubMed] [Google Scholar]
- 13.a) Wang Y, Muratore ME, Echavarren AM. Chem Eur J. 2015;21:7332–7339. doi: 10.1002/chem.201406318. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Xi Y, Su Y, Yu Z, Dong B, McClain EJ, Lan Y, Shi X. Angew Chem Int Ed. 2014;53:9817–9821. doi: 10.1002/anie.201404946. [DOI] [PubMed] [Google Scholar]; c) Liu Y, Yu Z, Zhang JZ, Liu L, Xia F, Zhang J. Chem Sci. 2016;7:1988–1995. doi: 10.1039/c5sc04319k. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Liu L, Zhang J. Chem Soc Rev. 2016;45:506–516. doi: 10.1039/c5cs00821b. [DOI] [PubMed] [Google Scholar]; e) Nunes dos Santos Comprido L, Klein JEMN, Knizia G, Kästner J, Hashmi ASK. Angew Chem Int Ed. 2015;54:10336–10340. doi: 10.1002/anie.201412401. [DOI] [PubMed] [Google Scholar]; f) Hashmi AS, Toste FD. Modern gold catalyzed synthesis. Wiley-VCH; Weinheim, Germany: 2012. [Google Scholar]; g) Toste FD, Michelet V. Gold catalysis: an homogeneous approach. Imperial College Press; London: 2014. [Google Scholar]
- 14.a) Luo Y, Ji K, Li Y, Zhang L. J Am Chem Soc. 2012;134:17412–17415. doi: 10.1021/ja307948m. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) He W, Li C, Zhang L. J Am Chem Soc. 2011;133:8482–8485. doi: 10.1021/ja2029188. [DOI] [PubMed] [Google Scholar]; c) Ye L, He W, Zhang L. J Am Chem Soc. 2010;132:8550–8551. doi: 10.1021/ja1033952. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Ye L, Cui L, Zhang G, Zhang L. J Am Chem Soc. 2010;132:3258–3259. doi: 10.1021/ja100041e. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Lu B, Li C, Zhang L. J Am Chem Soc. 2010;132:14070–14072. doi: 10.1021/ja1072614. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) He W, Xie L, Xu Y, Xiang J, Zhang L. Org Biomol Chem. 2012;10:3168–3171. doi: 10.1039/c2ob25235j. [DOI] [PubMed] [Google Scholar]; g) Wang Y, Ji K, Lan S, Zhang L. Angew Chem Int Ed. 2012;51:1915–1918. doi: 10.1002/anie.201107561. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Chen H, Zhang L. Angew Chem Int Ed. 2015;54:11775–11779. doi: 10.1002/anie.201504511. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Wang Y, Zheng Z, Zhang L. J Am Chem Soc. 2015;137:5316–5319. doi: 10.1021/jacs.5b02280. [DOI] [PubMed] [Google Scholar]; j) Kawade RK, Liu R-S. Org Lett. 2013;15:4094–4097. doi: 10.1021/ol4020199. [DOI] [PubMed] [Google Scholar]; k) Hung H-H, Liao Y-C, Liu R-S. J Org Chem. 2013;78:7970–7976. doi: 10.1021/jo401161h. [DOI] [PubMed] [Google Scholar]; l) Ghorpade S, Su M-D, Liu R-S. Angew Chem Int Ed. 2013;52:4229–4234. doi: 10.1002/anie.201210313. [DOI] [PubMed] [Google Scholar]; m) Talbot EPA, Richardson M, McKenna JM, Toste FD. Adv Synth Catal. 2014;356:687–691. doi: 10.1002/adsc.201300996. [DOI] [PMC free article] [PubMed] [Google Scholar]; n) Wang L, Xie X, Liu Y. Angew Chem Int Ed. 2013;52:13302–13306. doi: 10.1002/anie.201304700. [DOI] [PubMed] [Google Scholar]; o) Qian D, Hu H, Liu F, Tang B, Ye W, Wang Y, Zhang J. Angew Chem Int Ed. 2014;53:13751–13755. doi: 10.1002/anie.201407717. [DOI] [PubMed] [Google Scholar]; p) Zhao J, Liu J, Xie X, Li S, Liu Y. Org Lett. 2015;17:5926–5929. doi: 10.1021/acs.orglett.5b03160. [DOI] [PubMed] [Google Scholar]; q) Xu M, Ren T-T, Li C-Y. Org Lett. 2012;14:4902–4905. doi: 10.1021/ol302238t. [DOI] [PubMed] [Google Scholar]; r) Xie L, Liang Z, Yan D, He W, Xiang J. Synlett. 2013;24:1809–1812. [Google Scholar]
- 15.Lu Z, Han J, Hammond GB, Xu B. Org Lett. 2015;17:4534–4537. doi: 10.1021/acs.orglett.5b02224. [DOI] [PubMed] [Google Scholar]
- 16.Mézailles N, Ricard L, Gagosz F. Org Lett. 2005;7:4133–4136. doi: 10.1021/ol0515917. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Hammond GB, Xu B. J Am Chem Soc. 2012;134:5697–5705. doi: 10.1021/ja3011397. [DOI] [PubMed] [Google Scholar]
- 18.Xu Z, Chen H, Wang Z, Ying A, Zhang L. J Am Chem Soc. 2016;138:5515–5518. doi: 10.1021/jacs.6b02533. [DOI] [PubMed] [Google Scholar]
- 19.Surmont R, Verniest G, Kimpe N De. Org Lett. 2010;12:4648–4651. doi: 10.1021/ol1019713. [DOI] [PubMed] [Google Scholar]
- 20.Liu W-B, Reeves CM, Stoltz BM. J Am Chem Soc. 2013;135:17298–17301. doi: 10.1021/ja4097829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue H, Jiang D, Jiang H, Kee CW, Hirao H, Nishimura T, Wong MW, Tan C-H. J Org Chem. 2015;80:5745–5752. doi: 10.1021/acs.joc.5b00709. [DOI] [PubMed] [Google Scholar]
- 22.Hussain I, Yawer MA, Lau M, Pundt T, Fischer C, Görls H, Langer P. Eur J Org Chem. 2008;2008:503–518. [Google Scholar]
- 23.Han X, Kwiatkowski J, Xue F, Huang K-W, Lu Y. Angew Chem Int Ed. 2009;48:7604–7607. doi: 10.1002/anie.200903635. [DOI] [PubMed] [Google Scholar]
- 24.a) Kwiatkowski J, Lu Y. Eur J Org Chem. 2015;2015:320–324. [Google Scholar]; b) Han X, Luo J, Liu C, Lu Y. Chem Commun. 2009:2044–2046. doi: 10.1039/b823184b. [DOI] [PubMed] [Google Scholar]
- 25.Lan Y, Chen Y, Cao X, Zhang J, Wang J, Xu X, Qiu Y, Zhang T, Liu X, Liu B-F, Zhang G. J Med Chem. 2014;57:10404–10423. doi: 10.1021/jm501207r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


