Summary
Objective
To characterize the evolution of behavioral and electrographic seizures in experimental electrical stimulation-based model of status epilepticus (SE) in C57Bl/6 mice; and to relate SE to various outcomes, including death and epileptogenesis.
Methods
SE was induced by continuous hippocampal stimulation and was evaluated by review of EEG recordings, spectral display, and behavior.
Results
Seizures were initially locked to the electrical trains but later became independent of them. Following the end of stimulation, autonomous seizures continued for more than 5 minutes in 85% of the animals. There was ongoing 2–3 Hz rhythmic, high-amplitude, slow spike-wave discharges (HASDs) associated with purposeless, repetitive, continuously circling and exploratory behavior. There were high-amplitude fast discharges (HAFDs) associated with worsening of behavioral seizures and were interspersed with the ongoing HASDs. Death during SE occurred in 23% of the animals, and it was preceded by a stage 5 behavioral seizure. In the waning stage of SE, severe seizures and HAFDs dissipated, HASDs slowed down, and normal behavior was restored in most animals. Epilepsy developed in 33% of the animals monitored after SE.
Significance
The electrical stimulation model of SE can be used to study mechanisms of SE and its adverse consequences, including death and epileptogenesis.
Keywords: Status epilepticus, continuous hippocampal stimulation, death, temporal lobe epilepsy
Introduction
Status epilepticus (SE) is a condition consisting of abnormally prolonged seizures, and it can have long-term consequences, including neuronal death, neuronal injury, and alteration of neuronal networks, depending on the type and duration of seizures 1. The thirty-day all-cause mortality associated with an episode of status epilepticus is reported to be 19% 2. Results of the febrile status epilepticus study suggest that prolonged seizures can result in hippocampal injury and development of epilepsy in children 3;4.
Several chemoconvulsant models of SE have been developed in mice 5–7. These models are important tools in understanding the mechanisms of epileptogenesis 6;8. These models have limitations when used to study the pathophysiology of SE. High mortality during SE observed in some models is challenging to control and use of therapeutic agents such diazepam can further confound study of mechanisms of SE 9–11. In addition, chemoconvulsants, such as kainate, can also exert direct toxic effects 12. Finally, correlation between mouse EEG and behavior during SE has not been described.
Electrical stimulation models offer an alternative method to induce SE that is free of additional actions and with a single predefined seizure focus 13;14. We set out to develop an electrical stimulation model of SE in mice that is modified from the previously developed continuous hippocampal stimulation model in rats 14. The evolution of EEG during SE was studied in detail, and the characteristics of EEG and behavioral seizures were correlated with the outcome.
Materials and methods
All studies were performed in accordance with protocols approved by the Animal Care and Use Committee of the University of Virginia. Adult male and female C57Bl/6 mice (22 g–25 g, 40 to 50 days old) were used in these studies; 4 mice were kept in each cage, mice had ad libitum access to food and water and they were maintained in a normal light/dark cycle. The results were similar between male and female mice; hence, the data were pooled.
Electrode implantation and induction of SE
Animals were stereotaxically implanted with a bipolar insulated stainless-steel electrode in the left hippocampus (3 mm AP, 3 mm ML, 3 mm DV), bilateral supra-dural cortical electrodes, and a cerebellar reference electrode. SE was induced by a modified continuous hippocampal stimulation (CHS) protocol 14. A week after electrode implantation surgery, the animals were connected to a video-EEG monitoring system (Grass ARUA LTM64 using Twin software) via a flexible cable connected to the amplifier through an electrical swivel. EEG data were digitized at 400 Hz and stored on a central file server along with video for subsequent analysis. To determine the after-discharge threshold (ADT), the bipolar hippocampal electrode was connected to a constant current stimulator (A-M systems model 2100). A 0.75 ms biphasic square wave pulse at 50 Hz was applied for 10 s to determine the after-discharge threshold (ADT). The initial stimulation was set at 40 μA, and the pulse amplitude was increased by 20 μA in successive stimulation trains, separated by at least 60 seconds until a seizure, of at least a 5-s duration, was observed on the EEG. If a seizure could not be triggered by a maximum stimulation intensity of 200 μA, the animal was excluded from the study. The current intensity was set to twice the magnitude of ADT, with a minimum at 100 μA, and continuous stimulation was performed by cyclic application of 10 sec, 50 Hz stimulation trains for 10 sec followed by a 5 sec off period for 60 min. Induction of SE was marked by rhythmic and evolving spike-wave discharges for 5 min or more at the end of the stimulation period. Animals were monitored by continuous video-EEG until the end of SE, which was determined based on the drop of spike-wave discharge frequency below 1 Hz, disappearance of thigmotactic behavior, and resumption of feeding and grooming.
Continuous video-EEG monitoring of animals to record spontaneous seizures was initiated 2 weeks after SE. Mice were reattached to the video/EEG system via a cable containing a 4-channel unity gain operational-amplification headstage to eliminate cable artifact. Spontaneous seizures were characterized by evolving spike-wave discharges with >2 Hz frequency and 3 times the baseline amplitude lasting 15 sec or longer; the average duration of the 21 spontaneous seizures recorded was 39 ± 4 sec. Animals were considered to be epileptic when 2 seizures were recorded.
Power spectrum analysis
EEG data were exported to LabChart 7 (ADI Instruments). A referential hippocampal EEG channel was selected to generate a power spectrogram. The analysis consisted of a Fast Fourier Transform using a Cosine-Bell data window with a window size of 1024 data points (2.56 seconds). This method resulted in a frequency resolution of 0.375 Hz. A window overlap of 87.5% was used to help smooth the x-axis of the spectrogram. The power was expressed as μV2.
Behavioral seizure scoring
Behavioral seizures were scored using a modified Racine scale 15. The baseline behavior of animals during SE, which involved continuous exploration of the cage, was classified as stage 1. Facial twitching, head bobbing, or behavioral arrest were classified as stage 2, unilateral forelimb clonus as stage 3, bilateral forelimb clonus as stage 4, rearing and falling as stage 5, and running seizures and involuntary jumping in the cage as stage 6. When behavioral seizures were scored independent of EEG characteristics, then animals were observed for 1 min, and the highest behavioral score during the epoch was scored. Behavior was also scored in conjunction with EEG characteristics, using the modified Racine scale.
Immunohistochemistry and flurojade labeling
Three days following SE animals were transcardially perfused with 4% paraformaldehyde in 0.1M phosphate buffer. The brains were post-fixed in the 4% paraformaldehyde solution overnight at 4 °C and then in 30% sucrose in PBS solution until the brains had become sunken. The tissue was frozen in isopentane and horizontal 40 μm-thick slices were obtained on a cryostat (Leica CM 1900). The sections were sequentially collected in 4 groups and sections from one group were used for fluorojade labeling. Free-floating sections were first immunolabeled for NeuN as described before 16. Briefly the tissue was permeabilized by incubation in PBS containing 0.01% triton (PBST) overnight at 4 °C followed by incubation in a blocking solution (PBS containing normal goat serum 50 μl/ml and BSA 10 mg/ml). The sections were then incubated in anti-NeuN antibody (1:300 dilution, Millipore, MAB377) for 3 days. Following 3 times washing with PBST to remove unbound primary antibody, the sections were incubated in alexafluor-594 labeled anti-mouse secondary antibody (1:500 diluted, Life Technologies A11032) overnight. The sections were washed with PBST and then mounted on the positive charged plus slides, air-dried, and used for fluorojade labeling.
Fluorojade labeling was performed as described before 16. The slides were immersed in a basic alcohol solution (1% NaOH in 80% ethanol) for 5 minutes, rinsed in 70% ethanol for 2 min followed by 2 min rinse in distilled water. The slides were then incubated in 0.06% KMnO4 solution for 10 minutes and then rinsed in distilled water 2 times for 2 min each. The slides were then incubated in Fluoro-Jade-C (Histo-Chem Inc., Jefferson, AR, 0.0003% solution in 0.1% acetic acid) for 20 min in the dark. Following labeling, the slides were rinsed 3 times with distilled water and dried at 50°C. Finally, the slides were coversliped with DPX nonfluorescent mounting media.
The tissue was imaged under a Zeiss 780 confocal microscope under 20x magnification.
Statistics
Normally distributed data are presented as the means ± SEM, and median values are presented for data that were not normally distributed. A Kaplan-Meier survival analysis was performed to determine the duration of SE and the latency to onset of epilepsy.
Results
Induction of SE
The mean ADT was 74 ± 5 μA (N=54), and the after-discharge duration at this threshold was 17 ± 1 s (N=54). The male and female animals had similar ADTs (males: 79 ± 8 μA, N=29; and females: 69 ± 7 μA, N=25, p>0.05) and after-discharge durations (males: 16.6 ± 1.3 s, N=29; and females: 17 ± 0.9 s, N=25, p>0.05); hence, the data were pooled.
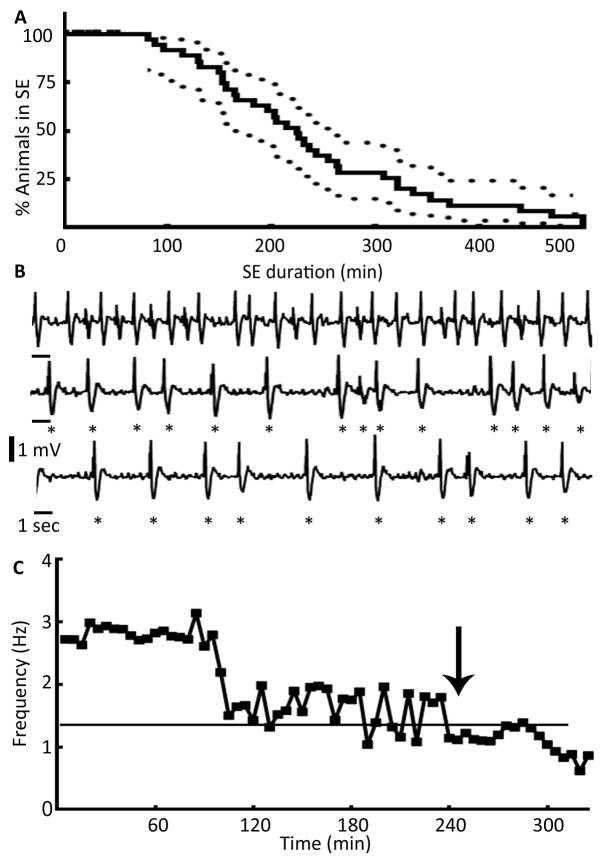
The stimulus intensity was set to twice the magnitude of ADT, at a minimum of 100 μA, for each animal, and hippocampal stimulation was performed by applying 10 sec of pulse train followed by a 5 sec observation period, for 60 min (Fig. 1). Electrographic activity was monitored by the cortical electrodes during the stimulus trains and during the 5 second inter-train intervals. The EEG characteristics during 60 min of stimulation were analyzed in 10 randomly selected animals. The first supra-threshold stimulation elicited a seizure (Fig. 1A, 0 min); however, subsequent stimuli failed to drive a response for a short period lasting 3.06 ± 0.08 min (N=10) (Fig. 1A, panel 5 min). Following this, stimuli evoked ictal discharges; although, occasional periods of refractoriness were observed during the initial and middle phases of stimulation.
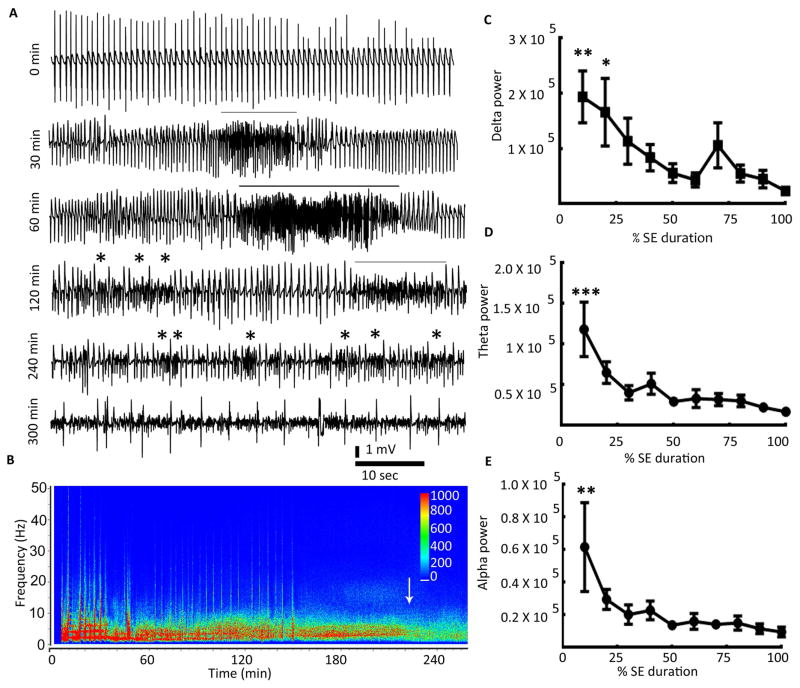
Figure 1. EEG during continuous hippocampal stimulation.
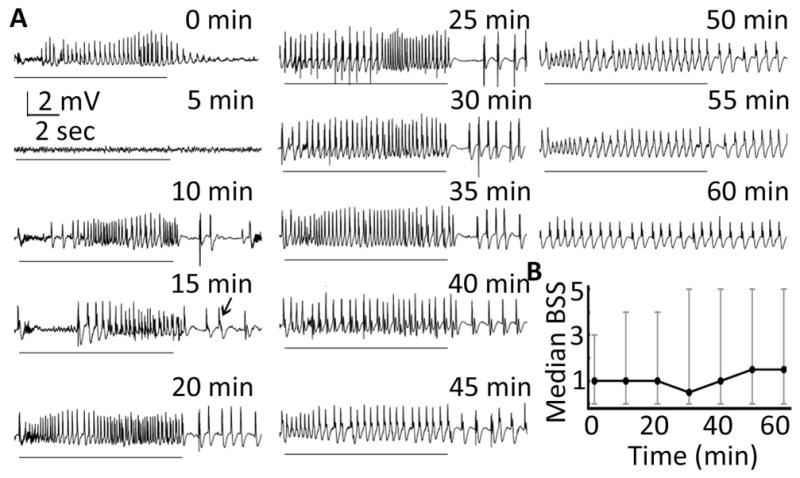
(A) Traces illustrating EEG activity recorded from cortical electrodes during the 10-sec period when 0.75 msec biphasic square wave pulses at 50 Hz were applied to the left hippocampus and during the 5-sec observation period in between consecutive stimuli. The black line below each EEG trace marks the 10-sec stimulation period. Please note the refractoriness marked by failure to evoke discharges during the stimulation period in the 5-min trace. The appearance of spikes during the 5-sec observation period is marked by arrows in the 15-min trace. (B) Median behavioral seizure score (BSS) along with the range during 60 min of stimulation (N=10). The behavioral seizures were scored for 1 min every 10 min.
As stimulation continued, spike-wave discharges appeared during the 5-sec observation period with a latency of 18.53 ± 0.99 min (N=10, Fig. 1A, 15 min). With the progression of stimulation, the spike-wave discharges observed during the inter-stimulus period became frequent and rhythmic (Fig. 1A, 30 min). Rhythmic discharges with a threshold frequency greater than 1 Hz were observed during the inter-stim interval starting at 33.33 ± 0.99 min (N=10) from the start of stimulation. The frequency of evoked-spikes during the stimulation period diminished as the inter-stimulus spike-wave discharges became rhythmic, and eventually the evoked- and inter-stimulus seizures became morphologically indistinguishable (Fig. 1A, panel 60 min). Thus, during stimulation, seizures transited from discrete events to continuous rhythmic spike-wave activity.
Video recordings of behavioral seizures were scored at 10 min intervals 15 (Fig. 1B). The animals explored and moved about the cage periodically during the stimulation period. Seizures were initially less intense (stage 1 to 3), but they progressively became more intense. Some animals experienced stage 4 or 5 seizures towards the end of stimulation, and the median behavioral seizure score increased. Two animals died during stimulation following a stage 5 or 4 seizure.
Autonomous phase of SE
In 7 of the 52 surviving animals, seizures stopped when stimulus trains ended, and in one animal, seizures stopped 3 min after the end of stimulation. In all other animals, seizures continued beyond 5 minutes, lasting for at least 1 hour (ranging from 81 minutes to 588 minutes, N=44). Thus, 85% of the animals that underwent stimulation experienced autonomous SE (Fig. 2A).
Figure 2. Status epilepticus induced by continuous hippocampal stimulation.
(A) EEG recorded from the hippocampal electrode in a representative animal with self-sustaining SE. The 0-min point corresponds to the end of hippocampal stimulation. The lines above EEG traces mark the intermittent presence of fast discharges (HAFDs/LAFDs) within the continuous rhythmic spike-wave activity. Stars mark the instances of increased frequency, but these were not counted as HAFDs/LAFDs since their duration was less than 8 sec. (B) Total power of EEG recorded from the hippocampal electrode was plotted against time. The power in the 0–20 Hz range was high during the autonomous phase of SE (first 3 quarters of SE duration) and declined during the waning phase (the 4th quarter of SE duration). The arrow marks the end of SE. The scale bar represents power in μV2. (C, D, E) The graphs demonstrate EEG power in the delta, theta, and alpha frequencies during SE. The EEG power was determined for 1-min intervals from the end of stimulation to the end of SE. Since the duration of SE varied between animals, the power was expressed as a percentage of the SE duration (N=10), * p<0.05, ** p<0.005, and *** p<0.0001, one-way ANOVA with Dunnett’s multiple comparison test vs the power at 100% (end of SE).
The EEG power was displayed on a compressed time scale encompassing the end of stimulation to the end of SE (defined in the methods above) using spectral colors (red high, blue low); it revealed distinct patterns that varied with time. Power was high (red) within 1–10 Hz frequencies for 2–3 hours following the end of stimulation (Fig. 2B). This effect faded over time as the end of SE approached. The power in the delta (1–4 Hz), theta (4–8 Hz), and alpha (8–12 Hz) ranges was quantified for 10-minute epochs from the end of stimulation to the end of SE (Fig. 2C–E). The SE duration (end of SE) varied from animal to animal and to describe SE in the population studied, the time period from the end of electrical stimulation to the end of SE was divided into four quarters. The lowest power was present in the last quarter of the SE episode in each of the frequency bands. To further describe electrical stimulation-induced SE, we refer to the last quarter of SE as the waning phase and the remaining three quarters as autonomous phases.
In a majority of the animals, visual examination of EEG revealed rhythmic high-amplitude slow discharges (HASDs, 2.05 ± 0.045 Hz, N=10) that persisted for the duration of SE. The animals demonstrated a basal hyperexploratory, thigmotactic behavior (stage 1, supplementary video 1) throughout the course of SE. The animals continuously explored the periphery of the cage with unidirectional head turning. Briefly, the animals would start to explore in the opposite direction, only to resume the clock-wise (or counter-clock-wise) manner of turning and exploration after one or two turns around the cage. This background was punctuated by severe seizures.
HASDs were interrupted by intermittent high- or low-amplitude fast discharges (HAFDs and LAFDs, respectively) during which power of the EEG dramatically increased (Fig. 3A, B). The evolution of these HAFDs resembled that of an electrographic seizure (Fig. 3D); spike frequency increased at the onset (Fig. 3D(ii)), followed by the increase in amplitude in combination with further increase in the spike frequency (Fig. 3D(iii)). The spike-wave discharges slowed down, and the EEG was finally suppressed for 1–3 sec (Fig. 3D(iv)) before the rhythmic spikes (HASDs) resumed. Although a number of fast discharges shared some of these characteristics, there were several instances where different kind of fast discharges, LAFDs, were observed (Fig. 3F); these events showed no evolution, ended abruptly and were diminished in amplitude compared to the ongoing activity.
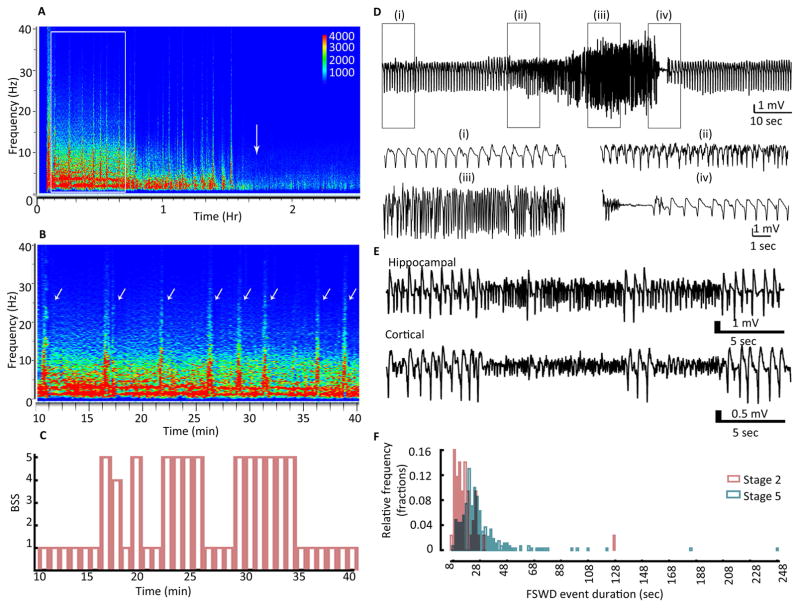
Figure 3. Fast discharges during SE and their correlation with behavioral seizures.
(A) Spectrogram illustrating the power of EEG recorded from the hippocampus during SE in a representative animal. The white arrow marks the end of SE. The white box marks the spectrum that is magnified in panel B. (B) The spectrogram illustrates EEG power during a period of 10 to 40 min following the end of hippocampal stimulation. The power in the 0–10 Hz frequency range was high. Arrows mark the HAFDs/LAFDs. (C) The behavioral seizure score over the 30-min duration corresponding to the spectrogram is illustrated in panel B. Behavioral seizures were scored every minute. The animals were exploratory during SE (stage 1), and the instances of fast discharges correlated with behavior ranging from head bobbing (stage 2) to rearing and falling (stage 5). Time 0 corresponds to 10 min from the end of stimulation. (D) Hippocampal EEG showing a HAFD. Traces below show magnified EEG from boxed regions (i), (ii), (iii), and (iv) demonstrating the baseline activity (i), onset of fast discharge event marked by increase in the frequency (ii), a further increase in the frequency of spike-wave discharges and enhancement of amplitude (iii) and the end of the HAFD marked by suppression of EEG followed by resumption of baseline activity (iv). (E) EEG from hippocampal and cortical electrodes showing a LAFD, characterized by an abrupt increase in the frequency along with suppression of amplitude. (F) Frequency distribution histogram showing the duration of fast discharges accompanied by stage 5 or stage 2 behavioral seizures, N=43 events accompanied by BSS 2 and N=266 events accompanied by BSS 5.
We determined whether the occurrence of fast discharges and their duration changed over the course of self-sustaining SE. The fast discharges with spike frequency at least 3 times that of the background seizure activity and durations of 8 sec or longer were considered and counted (Fig. 4A, B). These events were more frequent (43 ± 7% of all the events) during the first quarter of the autonomous phase and became rare during the waning phase (Fig. 4B, 6 ± 2%). The duration of these events remained stable over the course of SE (data not shown).
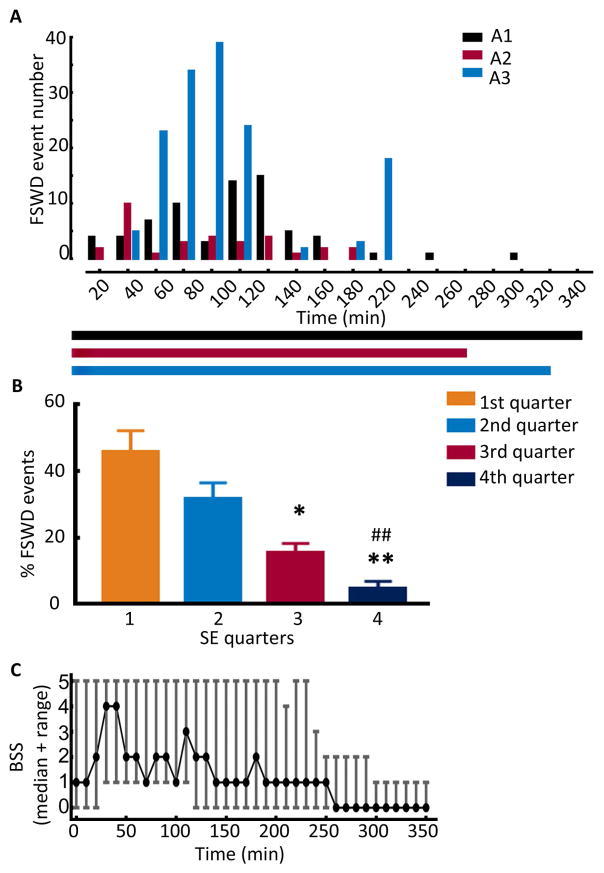
Figure 4. Changes in the characteristics of fast discharges over the course of SE.
(A) The occurrence of fast discharges over the course of SE in 3 representative animals (A1, A2, and A3). The events were counted in 20-min time periods over the entire length of SE. The horizontal bars below the graph show the duration of SE in each animal. (B) The percentage of fast discharges in the four quarters of SE. The events in each quarter of SE were counted and expressed as a percentage of the total number of fast discharges in each animal, N=13, * p<0.05 vs 1st quarter, ** and ## p<0.005 vs 1st and 2nd quarter. (C) Distribution of behavioral seizures over the length of SE, N=13. The graph represents the median BSS along with the range. Seizures were scored over a period of 1 min every 10 min, and time 0 corresponds to the end of hippocampal stimulation.
The behavior of animals altered transiently during HAFDs (Fig. 3C), ranging from unilateral forelimb clonus (stage 3) to forelimb clonus with rearing and falling (stage 5, supplementary video 2) or occasional running and jumping fit (stage 6). In contrast, during LAFDs, the behavior of animals did not change, or the animals demonstrated only behavioral arrest or head bobbing (stage 2). 46% of the fast discharges were accompanied by stage 5 behavioral seizures, whereas behavior did not change during 18% of the events. Furthermore, LAFDs associated with stage 2 behavioral seizures were of shorter duration (Fig. 3F, ranging from 8 to 127 sec, median 17 sec, N=43) than those of the HAFDs accompanied by stage 5 behavioral seizures (Fig. 3F, ranging from 8 to 248 sec, median 23 sec, N=266). The animals resumed thigmotactic behavior after termination of HAFDs (Fig. 3C).
We also scored behavioral seizures in 10 animals every 10 min, irrespective of the EEG characteristics. As seen in Figure 4C, stage 5 behavioral seizures involving rearing and falling were observed at least once in 9 out of the 10 animals, and the median latency to the first stage 5 seizure from the beginning of stimulation was 66 min (N=10). The behavioral seizures were most intense in the first quarter of the autonomous phase of SE and then gradually became less intense. Furthermore, severe seizures interrupted less intense grade seizures intermittently throughout the autonomous phase.
Death during SE
Ten animals died during the autonomous phase of SE. Termination of electrocerebral activity accompanied by immobility and loss of tone were used to mark death. Death occurred within the first hour of the autonomous phase of SE in 9 out of 10 animals, and the median time to death was 30 min, whereas SE was abnormally prolonged (8.2 hr) in the 10th animal. In these animals, the EEG power was high and convulsive seizures were frequent (Fig. 5A, D). Review of EEG demonstrated almost continuous HAFDs in animals that died (Fig. 5A), whereas HAFDs were intermittent in the survivors (Fig. 2B, Fig. 3A). The frequency of basal EEG activity was also higher in the animals that died compared to that in the animals that survived (4.3 ± 0.7 Hz vs 3.0 ± 0.3 Hz, N=10, p<0.05). We then compared behavior during the first 60 min of the autonomous phase of SE to that of the animals that died during SE and the survivors (Fig. 5D, E). Severe behavioral seizures were more common in the animals that died; the median behavioral seizure score was 5 in these animals compared to 2 in the survivors. We noticed that 3 out of 10 animals died during a stage 6 convulsion (supplementary video 3), whereas one animal died during a stage 5 seizure. Therefore, we studied the relationship between a stage 5 or 6 seizure and death. The time interval between a stage 5 or 6 seizure and death ranged from 0 to 90 sec, and the median time to death from the last stage 5 or 6 seizure was 15 sec (N=10, Fig. 5C). Thirty four out of 44 animals (77%) survived self-sustaining SE. The average duration of SE in the surviving animals was 236 ± 20 min (N=34, Fig. 6A).
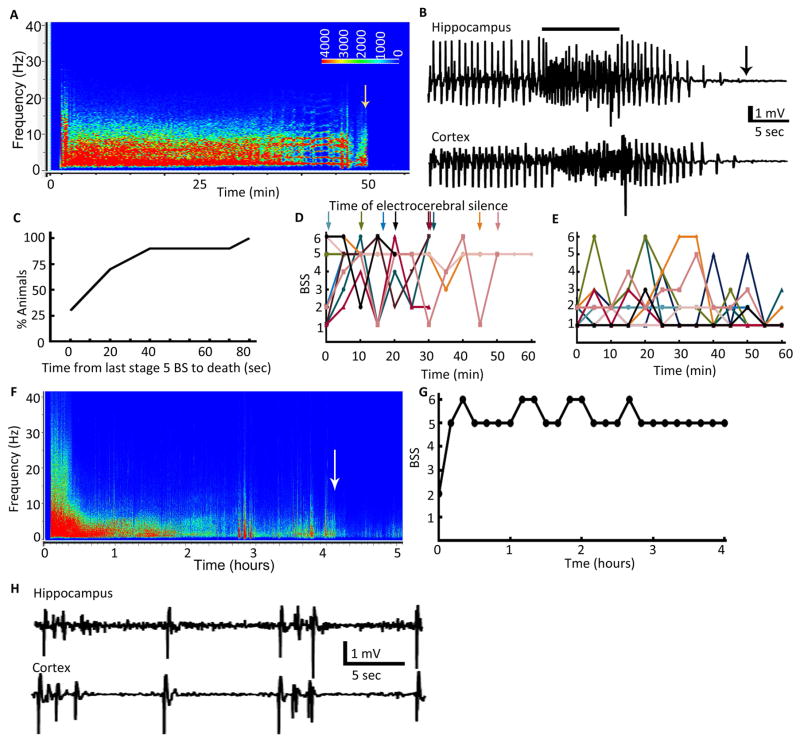
Figure 5. EEG and behavioral seizure characteristics associated with mortality during SE.
(A) EEG power spectrum of an animal that died during SE. The time of death is marked by an arrow. The power of EEG between 0–20 Hz was high for the entire duration. (B) EEG recorded from hippocampal and cortical electrodes showing a fast discharge after which the EEG became flat within a few seconds marking the death of the animal. The line above the EEG trace marks the fast discharge. The arrow marks the time of death. (C) Cumulative distribution plot showing duration between the last convulsive seizure and the time of death, N=10. (D) BSS in animals that died during SE and (E) BSS in 10 randomly selected animals during the first 60 min of self-sustaining SE. These animals experienced stage 1 or 2 behavioral seizures most of the time with intermittent intense seizures of stage 4 to6. (F) EEG power spectrum of an animal that survived SE but died 2 days later. Arrow marks the end of SE. The power of EEG was high during the first 1 h of SE and there were HAFDs. (G) BSS over the course of SE. Behavioral seizures were scored every 10 min in the same animal as in A. (H) EEG showing the burst suppression pattern during the waning phase of SE seen in some of the animals that died during or soon after SE.
Figure 6. EEG characteristics marking the end of SE.
(A) A Kaplan-Meyer survival plot illustrating the duration of SE. Percentage of animals in SE along with 95% confidence intervals were plotted against time, N= 34. (B) An EEG illustrating the end of SE marked by loss of rhythmicity and decline in the frequency of spike-wave discharges below 1 Hz. Highly rhythmic activity higher than 1 Hz (top trace) slowly transitioned to arrhythmic activity (middle trace), which finally dropped to a frequency below 1 Hz (bottom trace). Individual spikes are marked by stars to highlight transition of rhythmic to arrhythmic activity and a drop in the frequency below 1 Hz. (C) The graph shows frequency of spike-wave discharges during the autonomous and waning phases of SE and the drop in the frequency below 1 Hz that marked the end of SE, and time 0 marks the end of hippocampal stimulation.
Some of the animals survived SE, but subsequently died. The power of EEG was also higher during the initial period of SE, and the EEG demonstrated frequent HAFDs in these animals (Fig. 5F). These animals also consistently experienced generalized tonic-clonic seizures (Fig. 5G). Some of these animals demonstrated a burst suppression pattern in EEG during the waning phase of SE, which was more prominent in cortical EEGs than in hippocampal EEGs (Fig. 5H).
End of self-sustaining SE
The waning phase of SE was characterized by several changes in the EEG and behavior of the animals. The frequency of HASDs started to decline and eventually dropped below 1 Hz (Fig. 6B, C), which was the primary criteria used to mark the end of SE. Oftentimes, the spikes also lost rhythmicity before their frequency dropped below 1 Hz (Fig. 6B); however, in a fraction of animals, the rhythmicity was maintained despite the decrease in spike frequency below 1 Hz. The occurrence of fast discharges also declined significantly during the waning phase of SE (Fig. 4A, B). Animals were considered out of SE if the frequency of spike-wave discharges declined below 1 Hz and behavioral and electrographic seizures did not recur for 1 hour.
Behavior of 20 animals was studied following the end of electrographic SE. The animals became quieter; they would initiate thigmotactic behavior, but then they would stop, sniff the food, groom, or sit quietly. Even when the animals explored the cage, the pattern was not repetitive. Occasional head bobbing was observed in some of the animals. The animals also started to spend time at the center of the cage. The stereotypic thigmotactic behavior ended within 11 min (ranging from 0 to 81 min) in 15 out of 20 animals. These animals also resumed grooming and feeding. In 1 animal hyperexploratory behavior continued for 8 hours after the end of electrographic seizure activity. The remaining 4 animals that had undergone frequent convulsive seizures during SE were unresponsive following the end of SE, and they demonstrated a burst suppression pattern on their EEG. These animals were removed from video EEG monitoring for supportive care.
Recurrent spontaneous seizures
Since SE often triggers epileptogenesis, we determined whether the animals developed recurrent spontaneous seizures. Twenty-nine animals were saved to determine the onset of spontaneous seizures; however, 4 animals died 2 to 4 days after SE, and one animal lost recording electrodes. Re-monitoring of the remaining 24 animals was started 2 weeks after SE and was continued until two spontaneous seizures were detected or for 60 days, as described in the methods. Frequent spikes were detected in all animals except 1 (96%) (supplementary figure 1B). Two or more spontaneous seizures lasting for 37 ± 4 sec (N=23, supplementary figure 1A, supplementary video 4) were detected in 8 of the 24 animals (33%), whereas a single seizure was detected during 60 days of monitoring in 4 of the 24 (17%) animals.
Since we had performed an in-depth analysis of EEG and behavioral characteristics of animals during self-sustaining SE, we retrospectively determined whether these factors could be correlated with epileptogenesis. The SE duration was similar in animals that experienced one or more spontaneous seizures (seizure group: 234 ± 28 min, N=12) and animals that experienced frequent spikes but not spontaneous seizures (spike group: 236 ± 51 min, N=11). A similar number of fast discharges occurred in the seizure and spike groups (27 ± 4, N=11 vs 22 ± 5, N=12, respectively). However, a larger fraction of fast discharges was associated with convulsive seizures in the seizure group (66 ± 6%, N=12) than those in the spike group (35 ± 7%, N=11, p<0.002).
Neurodegeneration following SE
SE causes neurodegeneration 17. In order to determine whether CHS-induced SE caused neuronal death, 5 animals were perfused 3 days after SE and degenerating neurons were labeled using fluorojade-C. Extensive fluorojade labeling was present in the CA1 and CA3 regions (supplementary figure 2A, supplementary table 1). In addition sparse labeling was also observed in the dentate hilus.
Discussion
The key findings of this study are as follows: 1) the electrical stimulation of the hippocampus in mice caused prolonged, self-sustaining seizures with variable outcomes, including death, epileptogenesis, or no epilepsy but persistent spiking during the study period; 2) the background seizure activity during SE consisted of ongoing HASDs, which were associated with distinctive thigmotactic behavior; 3) severe behavioral seizures were associated with HAFDs; 4) SE waned over a period of time, with diminishing frequency of HAFDs/LAFDs and severe seizures, slowing of HASDs and a gradual return of normal behavior; and 5) death during SE was preceded by a stage 5 or 6 seizure. In addition, this retrospective analysis suggested that SE with frequent HAFDs and severe behavioral seizures was associated with death or epileptogenesis.
Previously, a detailed characterization of EEG during SE was described by Treiman and colleagues 18. They faithfully described the evolution of EEG in animal models and in humans with generalized convulsive SE. Seizures evolved from discrete seizures as well as from continuous seizures, waxing and waning seizures, seizures with suppression, and periodic discharges. This EEG classification predicted the response to therapy 19. The current study adds to this description by describing the relationship between EEG, behavior and outcomes. We observed that convulsive stage 5 or 6 seizures were more likely to occur during longer HAFDs. SE induced by electrical stimulation of the basolateral amygdala also demonstrates a similar pattern of HASDs interrupted by HAFDs associated with convulsions 20. In patients undergoing continuous EEG monitoring for seizures, brief bursts of increased power on compressed spectral displays can be used to identify seizures 21.
Although we pre-defined a specific endpoint for SE (see methods), the current study suggested that the end of SE is a slow process, which includes waning frequency of severe seizures and HAFDs/LAFDs, diminishing frequency of HASDs, and, ultimately, the restoration of normal behavior. A combination of electrographic and behavioral characteristics may better define the end of SE than either behavior or EEG features alone. For example, the end of SE may be defined as the time when normal behavior is restored and EEG spike-wave discharges fall below a specific frequency. Interestingly, several clinical trials have used absence of clinically apparent seizures and improving consciousness to define the end of SE 22–24. Consideration of a patient’s behavioral state may help determine the end of SE in clinical settings where the ictal-interictal continuum is ambiguous 25.
Death occurred during or shortly following a convulsive (stage 5 or 6) seizure in this cohort suggesting that generalized tonic-clonic seizures pose a danger to life. There is growing recognition that episodes of SE vary in severity. In clinical settings, generalized tonic-clonic seizures, age, underlying etiology, history of epilepsy, and the type of epileptiform discharges have been related to prognosis for survival 26;27. Generalized convulsive SE is difficult to treat and is associated with unfavorable outcomes 2;28. In a recent study, high frequency of spike-wave discharges in EEG in animals with kainate-induced seizures were associated with central apnea 29. Recent studies using the kainic acid model have emphasized that the frequency of generalized tonic-clonic seizures during SE determines its severity and outcome 6;8;30. In the future, carefully designed studies to monitor cardiac and respiratory functions during experimental SE are needed to clarify the relationship between convulsive seizures and death and to elucidate the underlying neurobiological mechanisms. Convulsive seizures also increase the risk of sudden unexpected death in epilepsy (SUDEP), which is a major contributor of mortality in epilepsy. In 25 to 80% of the cases of SUDEP, death is preceded by a seizure 31;32.
The model presented here is a less intense SE model as the frequency of discharges during SE is lower compared to that seen in chemoconvulsant models, such as pilocarpine, in which the basal frequency of discharges is around 6–7 Hz 5. The rhythmic discharges persisted in the majority of animals following the end of electrical stimulation. It is known that GABAA receptor-mediated inhibition of DGCs is reduced during SE 33–35, which results in the breakdown of dentate gating function 36;37. The recruitment of a reentrant circuit involving hippocampus proper, parahippocampal structures and entorhinal cortex then sustains the activity 38.
Frequent spikes were detected on the EEGs of all the animals except for one following SE. These spikes were recorded 2 weeks after SE and persisted for the duration of the recording, suggesting a persistent increase in excitability in these animals. One third of the animals that survived SE and were monitored developed recurrent spontaneous seizures, whereas only one seizure was recorded in 16% of animals. The animals in which only spikes or one seizure were detected during the recording period may also become epileptic. If the frequency of recurrent spontaneous seizures is low, longer recordings may be needed to detect two or more seizures. However, the seizure-free latent period ranging from 3 to 7 weeks seen in this model is considerably longer than that observed in the pilocarpine or KA models of SE, which ranges from one day to a few weeks 6;9;10;39;40. The animals were not recorded after 2 spontaneous seizures were detected; thus, the frequency of spontaneous seizures was not ascertained.
Many models of SE have been described in mice; these include seizures induced by systemic administration of pilocarpine or kainic acid 5–8. Pilocarpine-induced SE appears to be quite severe, with high mortality (ranging from 50–96%). Despite pretreatment with scopolamine and administration of diazepam, 2–3 hr after the onset of SE, a large fraction of surviving animals become epileptic with a short seizure-free period 9–11. In comparison with the existing models, the model presented here is less severe, with lower mortality, lower rate of epileptogenesis and a longer latent period.
Neurodegeneration is a hall-mark of SE 17. Extensive fluorojade labeling of CA3 and CA1 neurons and sparse labeling in the dentate hilus seen in this study indicates that electrical stimulation-induced SE is also associated with cell death as seen in chemoconvulsant models of SE 7;16;41–43.
In conclusion, we have developed a mouse model of SE and TLE that is associated with high survival without drug treatment. We have explored the association between EEG and behavioral characteristics and demonstrate a correlation between HAFDs accompanied by convulsive seizures with the subsequent onset of recurrent spontaneous seizures. Future prospective studies are needed to determine the correlation between characteristics of SE and its outcomes, including epileptogenesis and death.
Supplementary Material
(A) A seizure in an animal that became epileptic following SE. The traces below show magnified boxed areas (i) to (v). (B) EEG illustrating the presence of frequent spikes following SE.
(A) Fluorojade-C (green) labeling in the hippocampus 3 days following CHS-induced SE. The neurons were identified using expression of NeuN, a neuronal marker protein (red). The images were acquired under 20x magnification. The marked areas containing (B) CA1, (C) CA3 and (D) dentate hilus are magnified.
The animals showed thigmotactic, hyperexploratory behavior during basal SE.
A stage 5 behavioral seizure that was associated with HAFD on the EEG during SE.
Death of an animal during SE following a stage 6 seizure.
A spontaneous seizure recorded during night in an epileptic animal.
Key points.
Status epilepticus (SE) was induced in mice by electrical stimulation of the hippocampus.
EEG demonstrated high-amplitude slow discharges (HASDs) associated with repetitive exploratory behaviors.
Brief high-amplitude fast discharges (HAFDs) associated with more severe behavioral seizures occurred throughout SE.
Mice having severe behavioral seizures were more likely to die or develop epilepsy
Acknowledgments
This study was supported by NIH grants RO1 NS 040337 and RO1 NS 044370 to JK.
Footnotes
Conflict of Interest:
None of the authors has any conflict of interest to disclose.
References
- 1.Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus: Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56:1515–23. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 2.DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–35. doi: 10.1212/wnl.46.4.1029. [DOI] [PubMed] [Google Scholar]
- 3.Lewis DV, Shinnar S, Hesdorffer DC, et al. Hippocampal sclerosis after febrile status epilepticus: The FEBSTAT study. Ann Neurol. 2014;75:178–85. doi: 10.1002/ana.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shinnar S, Bello JA, Chan S, et al. MRI abnormalities following febrile status epilepticus in children: the FEBSTAT study. Neurology. 2012;79:871–7. doi: 10.1212/WNL.0b013e318266fcc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turski WA, Cavalheiro EA, Bortolotto ZA, Mello LM, Schwarz M, Turski L. Seizures produced by pilocarpine in mice: A behavioral, electroencephalographic and morphological analysis. Brain Res. 1984;321:237–53. doi: 10.1016/0006-8993(84)90177-x. [DOI] [PubMed] [Google Scholar]
- 6.Puttachary S, Sharma S, Tse K, et al. Immediate epileptogenesis after kainate-induced status epilepticus in C57BL/6J mice: Evidence from long term continuous video-EEG telemetry. PLoS ONE. 2015;10:e0131705. doi: 10.1371/journal.pone.0131705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLin JP, Steward O. Comparison of seizure phenotype and neurodegeneration induced by systemic kainic acid in inbred, outbred, and hybrid mouse strains. Eur J Neuroscience. 2006;24:2191–202. doi: 10.1111/j.1460-9568.2006.05111.x. [DOI] [PubMed] [Google Scholar]
- 8.Puttachary S, Sharma S, Thippeswamy A, Thippeswamy T. Immediate epileptogenesis: Impact on brain in C57BL/6J mouse kainate model. Front Biosci (Elite Ed) 2016;8:390–411. doi: 10.2741/e775. [DOI] [PubMed] [Google Scholar]
- 9.Cavalheiro EA, Santos NF, Priel MR. The pilocarpine model of epilepsy in mice. Epilepsia. 1996;37:1015–9. doi: 10.1111/j.1528-1157.1996.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann G, Balgooyen L, Mattis J, Deisseroth K, Buckmaster PS. Hilar somatostatin interneuron loss reduces dentate gyrus inhibition in a mouse model of temporal lobe epilepsy. Epilepsia. 2016;57:977–83. doi: 10.1111/epi.13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzuferi M, Kumar G, Rospo C, Kaminski RM. Rapid epileptogenesis in the mouse pilocarpine model: Video-EEG, pharmacokinetic and histopathological characterization. Exp Neurol. 2012;238:156–67. doi: 10.1016/j.expneurol.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Meldrum BS. Concept of activity-induced cell death in epilepsy: historical and contemporary perspectives. Prog Brain Res. 2002;135:3–11. doi: 10.1016/S0079-6123(02)35003-9. [DOI] [PubMed] [Google Scholar]
- 13.Mazarati AM, Wasterlain CG, Sankar R, Shin D. Self-sustaining status epilepticus after brief electrical stimulation of the perforant path. Brain Res. 1998;801:251–3. doi: 10.1016/s0006-8993(98)00606-4. [DOI] [PubMed] [Google Scholar]
- 14.Lothman EW, Bertram EH, Bekenstein JW, Perlin JB. Self-sustaining limbic status epilepticus induced by 'continuous' hippocampal stimulation: electrographic and behavioral characteristics. Epilepsy Res. 1989;3:107–19. doi: 10.1016/0920-1211(89)90038-7. [DOI] [PubMed] [Google Scholar]
- 15.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 16.Sun C, Mtchedlishvili Z, Bertram EH, Erisir A, Kapur J. Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulation-based animal model of temporal lobe epilepsy. J Comp Neurol. 2007;500:876–93. doi: 10.1002/cne.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujikawa DG. Prolonged seizures and cellular injury: understanding the connection. Epilepsy & behavior : E&B. 2005;7(Suppl 3):S3–11. doi: 10.1016/j.yebeh.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Treiman DM, Walton NY, Kendrick C. A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Res. 1990;5:49–60. doi: 10.1016/0920-1211(90)90065-4. [DOI] [PubMed] [Google Scholar]
- 19.Wang NC, Good LB, Marsh ST, Treiman DM. EEG stages predict treatment response in experimental status epilepticus. Epilepsia. 2009;50:949–52. doi: 10.1111/j.1528-1167.2008.01911.x. [DOI] [PubMed] [Google Scholar]
- 20.Brandt C, Glien M, Potschka H, Volk H, Löscher W. Epileptogenesis and neuropathology after different types of status epilepticus induced by prolonged electrical stimulation of the basolateral amygdala in rats. Epilepsy Res. 2003;55:83–103. doi: 10.1016/s0920-1211(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 21.Moura LM, Shafi MM, Ng M, et al. Spectrogram screening of adult EEGs is sensitive and efficient. Neurology. 2014;83:56–64. doi: 10.1212/WNL.0000000000000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345:631–7. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- 23.Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus Intravenous Therapy for Prehospital Status Epilepticus. N Engl J Med. 2012;366:591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bleck T, Cock H, Chamberlain J, et al. The Established Status Epilepticus Trial 2013. Epilepsia. 2013;54:89–92. doi: 10.1111/epi.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaspard N, Hirsch LJ. Pitfalls in ictal EEG interpretation: critical care and intracranial recordings. Neurology. 2013;80:S26–S42. doi: 10.1212/WNL.0b013e31827974f8. [DOI] [PubMed] [Google Scholar]
- 26.Rossetti AO, Logroscino G, Milligan TA, Michaelides C, Ruffieux C, Bromfield EB. Status Epilepticus Severity Score (STESS): a tool to orient early treatment strategy. J Neurol. 2008;255:1561–6. doi: 10.1007/s00415-008-0989-1. [DOI] [PubMed] [Google Scholar]
- 27.Giovannini G, Monti G, Tondelli M, et al. Mortality, morbidity and refractoriness prediction in status epilepticus: Comparison of STESS and EMSE scores. Seizure. 2017;46:31–7. doi: 10.1016/j.seizure.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339:792–8. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 29.Villiere SM, Nakase K, Kollmar R, Silverman J, Sundaram K, Stewart M. Seizure-associated central apnea in a rat model: Evidence for resetting the respiratory rhythm and activation of the diving reflex. Neurobiol Dis. 2017;101:8–15. doi: 10.1016/j.nbd.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tse K, Puttachary S, Beamer E, Sills GJ, Thippeswamy T. Advantages of repeated low dose against single high dose of kainate in C57BL/6J mouse model of status epilepticus: behavioral and electroencephalographic studies. PLoS ONE. 2014;9:e96622. doi: 10.1371/journal.pone.0096622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology. 2005;64:1131–3. doi: 10.1212/01.WNL.0000156352.61328.CB. [DOI] [PubMed] [Google Scholar]
- 32.Walczak TS, Leppik IE, D’Amelio M, et al. Incidence and risk factors in sudden unexpected death in epilepsy: A prospective cohort study. Neurology. 2001;56:519–25. doi: 10.1212/wnl.56.4.519. [DOI] [PubMed] [Google Scholar]
- 33.Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci. 2008;28:2527–38. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724–33. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terunuma M, Xu J, Vithlani M, et al. Deficits in phosphorylation of GABAA receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28:376–84. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res Suppl. 1992;7:273–80. [PubMed] [Google Scholar]
- 37.Lothman EW, Stringer JL, Bertram EH. The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res Suppl. 1992;7:301–13. [PubMed] [Google Scholar]
- 38.Stringer JL, Lothman EW. Maximal dentate gyrus activation: characteristics and alterations after repeated seizures. J Neurophysiol. 1989;62:136–43. doi: 10.1152/jn.1989.62.1.136. [DOI] [PubMed] [Google Scholar]
- 39.Mouri G, Jimenez-Mateos E, Engel T, et al. Unilateral hippocampal CA3-predominant damage and short latency epileptogenesis after intra-amygdala microinjection of kainic acid in mice. Brain Res. 2008;1213:140–51. doi: 10.1016/j.brainres.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 40.Shinoda S, Araki T, Lan JQ, et al. Development of a model of seizure-induced hippocampal injury with features of programmed cell death in the BALB/c mouse. J Neurosci Res. 2004;76:121–8. doi: 10.1002/jnr.20064. [DOI] [PubMed] [Google Scholar]
- 41.Schauwecker PE. Strain differences in seizure-induced cell death following pilocarpine-induced status epilepticus. Neurobiol Dis. 2012;45:297–304. doi: 10.1016/j.nbd.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia. 1991;32:778–82. doi: 10.1111/j.1528-1157.1991.tb05533.x. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–52. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) A seizure in an animal that became epileptic following SE. The traces below show magnified boxed areas (i) to (v). (B) EEG illustrating the presence of frequent spikes following SE.
(A) Fluorojade-C (green) labeling in the hippocampus 3 days following CHS-induced SE. The neurons were identified using expression of NeuN, a neuronal marker protein (red). The images were acquired under 20x magnification. The marked areas containing (B) CA1, (C) CA3 and (D) dentate hilus are magnified.
The animals showed thigmotactic, hyperexploratory behavior during basal SE.
A stage 5 behavioral seizure that was associated with HAFD on the EEG during SE.
Death of an animal during SE following a stage 6 seizure.
A spontaneous seizure recorded during night in an epileptic animal.