ABSTRACT
The middle ear epithelium is derived from neural crest and endoderm, which line distinct regions of the middle ear cavity. Here, we investigate the distribution of putative stem cell markers in the middle ear, combined with an analysis of the location of label-retaining cells (LRCs) to create a map of the middle ear mucosa. We show that proliferating cells and LRCs were associated with specific regions of the ear epithelium, concentrated in the hypotympanum at the base of the auditory bulla and around the ear drum. Sox2 was widely expressed in the endodermally derived ciliated pseudostratified epithelium of the hypotympanum. This part of the middle ear showed high levels of Wnt activity, as indicated by the expression of Axin2, a readout of Wnt signalling. Keratin 5 showed a more restricted expression within the basal cells of this region, with very little overlap between the Sox2- and keratin 5-positive epithelium, indicating that these genes mark distinct populations. Little expression of Sox2 or keratin 5 was observed in the neural crest-derived middle ear epithelium that lined the promontory, except in cases of otitis media when this epithelium underwent hyperplasia. This study lays the foundation for furthering our understanding of homeostasis and repair in the middle ear.
KEY WORDS: Endoderm, Neural crest, Sox2, Keratin 5, Label-retaining cells, Otitis media
Summary: Neural crest- and endoderm-derived parts of the middle ear epithelium have distinct patterns of proliferation and distribution of keratin 5 and Sox2 that may impact on response during inflammation.
INTRODUCTION
The middle ear is lined with a diverse epithelium derived from endoderm and neural crest (Thompson and Tucker, 2013). The epithelium can be either simple or pseudostratified (Sade, 1966; Thompson and Tucker, 2013), covers the whole middle ear cavity, including the inner surface of the eardrum, and is continuous with the Eustachian tube and therefore the pharynx. The pseudostratified epithelium has been shown to extend from the opening of the Eustachian tube in tracks and contains ciliated cells and secretory cells (Sade, 1966). This epithelium lines the ventral part of the middle ear cavity known as the hypotympanum. The simple epithelium is found in the epitympanic region (roof or attic) in the dorsal part of the cavity near the ossicles and covering the promontory overlying the cochlea, with slight species-specific variation in the extent the cilia tracks extend up towards the attic (Kuijpers et al., 1984; Lim et al., 1967; Sade, 1966) (Fig. 1A for schematic of middle ear anatomy). The ciliated region corresponds to the cells of endoderm origin whereas the simple unciliated region is derived from neural crest cells, which undergo a mesenchymal-epithelial transition and switch on epithelial markers such as E-cadherin and keratin 14 (Thompson and Tucker, 2013). Some ciliated cells are observed in the dorsal attic region, suggesting that the neural crest might be able to form cilia (Luo et al., 2017).
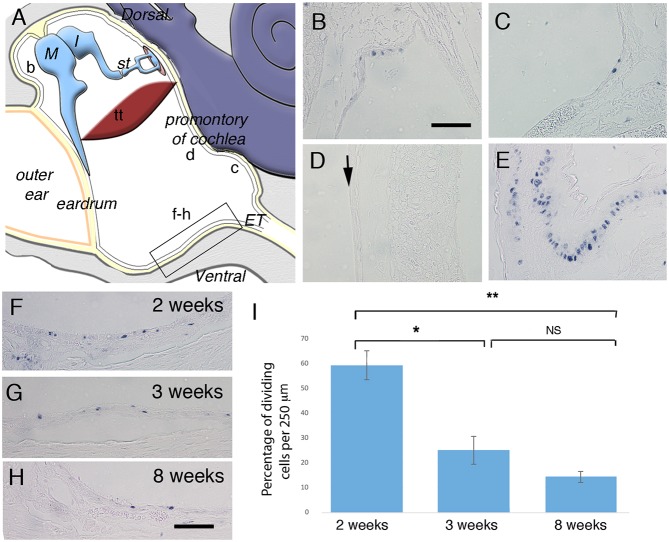
Fig. 1.
Proliferation in distinct parts of the postnatal middle ear epithelium. (A) Schematic illustrating the middle ear cavity with b-d,f-h showing the position of B-D,F-H. The boxed region in A shows the area used for cell counts. (B-H) Frontal sections immunostained for PCNA. (B) Dorsal to eardrum. (C) Close to Eustachian tube. (D) Promontory over cochlea. Arrow indicates negative middle ear epithelium. (E) Proximal trachea epithelium. (F-H) Ventral epithelium showing the position of cell counts at 2 weeks (F), 3 weeks (G) and 8 weeks (H). (I) Graph showing average proliferating cell density in the hypotympanum (per 250 µm) at 2 (n=3 ears), 3 (n=3 ears) and 8 (n=3 ears) weeks. Error bars indicate s.e.m. *P<0.05, **P<0.005; NS, not significant. Student's unpaired t-test. M, malleus; I, Incus; st, stapes; tt, tensor tympani; ET, Eustachian tube. Scale bars: 100 µm in B-H.
The middle ear lining acts as a barrier against infection and chemical damage and must be able to self-renew during adult life in order to maintain normal homeostasis of this tissue. In vivo and in culture, the middle ear epithelium is able to secrete a multitude of innate defence proteins from its apical surface, helping to keep the middle ear cavity sterile (Mulay et al., 2016). Despite this, the middle ear cavity can often become inflamed, known as otitis media. During this time, epithelial changes are observed with an increase in proliferation, a reduction in cilia and an increase in the number of goblet cells (Atef and Ayad, 2004; Lim and Birck, 1971; Fuchs et al., 2013). Thirty-one million cases of chronic otitis media with effusion are reported each year and its complications are important causes of preventable hearing loss, particularly in developing countries (Monasta et al., 2012).
Recently, it has been shown that the middle ear mucosa expresses keratin 5 (K5) in the basal cells of both the ciliated and unciliated middle ear epithelium, with short-term lineage tracing of K5 cells showing that these basal cells can form ciliated cells (Luo et al., 2017). This indicates that adult K5 stem cells can play a role in homeostasis of the ear epithelium. In addition, cells expressing putative stem cell markers, α6-integrin, β1-integrin, p63 and keratin 19, have been located in the ectodermal (outer layer) component of the eardrum (Kim et al., 2015; Knutsson et al., 2011; Wang et al., 2004). These cells appear in potential niches, around the annulus and at the manubrium, where the middle ear ossicles contact the membrane. The middle ear epithelium therefore does appear to have a putative stem cell population.
This paper aims to extend this research particularly focusing on the distribution of putative stem/progenitor cells within the middle ear epithelium in neural crest and endoderm-derived regions. To achieve this, we have investigated the presence of label-retaining cells (LRC), using pulse chase BrdU, analysed the expression of putative stem cells markers and equated their distribution to the embryonic origin of the epithelium. For markers, we have chosen keratin 5 (K5), owing to its recently described expression in the basal epithelium of the middle ear, and the transcription factor Sox2 (sex determining region Y - box 2). Sox2 is a well-established epithelial stem cell marker in a number of adult systems: pituitary (Fauquier et al., 2008), lens epithelium, glandular stomach, testis (Arnold et al., 2011), bronchi (Tompkins et al., 2009) and teeth (Juuri et al., 2012). In many of these systems, Wnt signalling has been shown to be central to the control of stem/progenitor cell activity and may act as a niche factor to maintain stem cells in a self-renewing state (Nusse, 2008). We have therefore also compared the distribution of Wnt activity, using the Axin2 reporter mouse, with the pattern of putative stem cells across the middle ear epithelium.
RESULTS
Proliferation is not uniform throughout the middle ear epithelium
As homeostasis within the epithelium of the middle ear has not yet been studied, an antibody against proliferative cell nuclear antigen (PCNA) was used to label dividing cells at three different stages: P (postnatal day) 14, P21 and 8 weeks (n=3 ears for each stage). At 2 weeks, the ear is still developing and maturation of the middle ear mucosa is occurring, whereas already by 3 weeks the adult morphology is evident (Thompson and Tucker, 2013). At each stage, the location of dividing cells was not uniform throughout the middle ear epithelium; instead, there were discrete regions with many dividing cells and larger regions with few or no proliferative cells. At all three ages, the pattern was very similar with, proliferation found close to the developing ear drum (Fig. 1B) and the Eustachian tube extending from the hypotympanum part of the cavity (Fig. 1C,F-H). Other regions of the middle ear cavity, such as overlying the promontory of the cochlea and within the attic, largely had no proliferating cells within the epithelium (Fig. 1D). As a positive control, the tracheal epithelium can be viewed in the same sections as the middle ear. Like the middle ear epithelium near the Eustachian tube, the trachea has a ciliated pseudostratified epithelium, but the number of proliferating cells was higher, indicating a faster turnover in this tissue (Fig. 1E).
At 2 weeks, many more proliferating cells were observed in the middle ear epithelium, as would be expected because the ear, though functional at this stage, is still maturing (Fig. 1F). Cell counts were then performed on the epithelium of the hypotympanum (counted region shown by the boxed area in Fig. 1A). The percentage of dividing cells decreased dramatically between 2 weeks and 3 weeks of age (P=0.013); however, only a slight decrease was observed between 3 and 8 weeks, which was non-significant (P=0.153) (Fig. 1I). The difference between the percentage of proliferating cells at 2 weeks and 8 weeks was highly significant (P=0.002) (Fig. 1I).
Label-retaining cells are concentrated in distinct regions of the middle ear
Having established which areas are proliferating, we then turned to identification of label-retaining cells (LRC) in the middle ear. For this we used BrdU pulse chase, in which BrdU was given to a pregnant mother via the drinking water from embryonic day 9 of gestation (E9) to E16, in order to label all the dividing cells within her embryos. All the littermates therefore had the same dose of BrdU and should be labelled uniformly. The pups were then collected at three stages: postnatal day 1 (P1) to confirm all cells within the tissues of the middle ear were labelled; at 3 weeks (P21), to locate slow cycling cells when the middle ear has fully formed; and at 8 weeks postnatally, to locate LRCs in the adult mouse middle ear epithelium (n=3).
At P1, BrdU immunohistochemistry revealed all cells within the mouse middle ear were positive for BrdU (Fig. 2A,A′), confirming saturated labelling of this tissue at this time point. By P21, BrdU-retaining cells were dramatically reduced in number compared with P1; from this point, and continuing in the adult (8 weeks), the distribution of positive cells was restricted to certain regions of the epithelium (illustrated schematically in Fig. 2B).
Fig. 2.
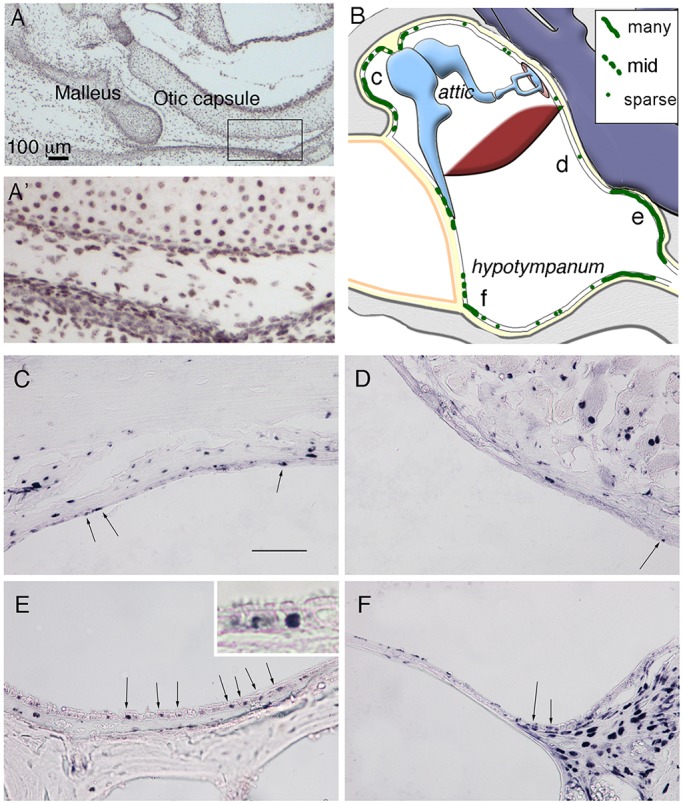
Label-retaining cells are concentrated in specific parts of the middle ear. (A,A′) Immunostained frontal section of a P1 mouse middle ear, following treatment of BrdU in the mother’s drinking water for 7 days using an antibody against BrdU. All cells are labelled in the ear. The boxed region in A is enlarged in A′. (B) Schematic highlighting distribution of BrdU-positive cells (green) within an example 8-week-old postnatal middle ear. Letters c-f represent regions shown in C-F. (C-F) Frontal sections of 8-week-old postnatal middle ear immunostained against BrdU, in the epithelium of the attic close to the ear drum (C), overlying the cochlea (D), in the hypotympanum close to the Eustachian tube (E) and near the ear drum on the ventral side (F). Arrows in C-F indicate positive epithelial cells. Inset in E shows labeling in basal cells. Scale bars: 100 µm in A; 100 µm in C-F.
At 8 weeks postnatally, BrdU-retaining cells were found scattered within the pseudostratified endodermally derived epithelium. These regions of the middle ear cavity included the ventral regions close to the Eustachian tube (Fig. 2E) and in the epithelium surrounding the eardrum on the ventral side (Fig. 2F). Here, the BrdU-positive cells were associated with the basal lamina (see inset Fig. 2E). Positive cells were also observed in the simpler epithelium in the attic near to the ear drum (Fig. 2C). In contrast, these LRC were generally absent from the neural crest-derived epithelium overlying the cochlea (Fig. 2D).
Sox2 cells predominate in the adult endodermally derived epithelium
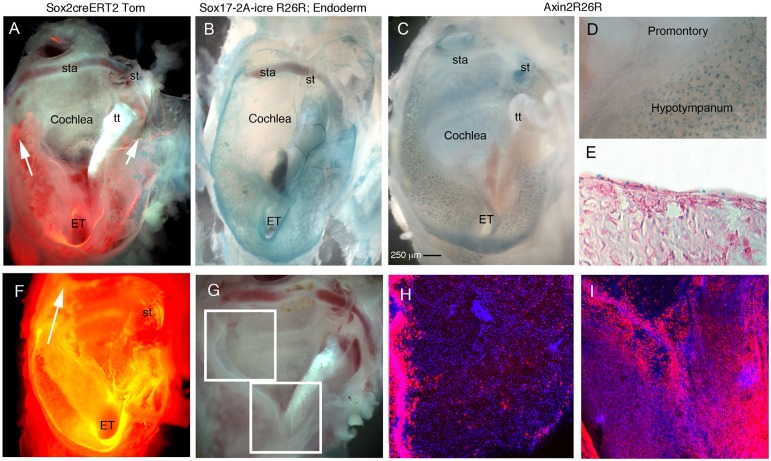
The transcription factor Sox2 has been shown to be expressed by adult stem progenitor cells in other tissues (Arnold et al., 2011; Fauquier et al., 2008; Juuri et al., 2012; Tompkins et al., 2009) and therefore was examined in the middle ear. For this, the Sox2creERT2Tom mouse line (Andoniadou et al., 2013) was used to investigate Sox2 expression. Tamoxifen was injected at P21 and the samples were collected 2 days after (P23) to allow for cre recombination to occur. The middle ear was viewed in wholemount, with the ear drum and associated lateral wall removed to allow imaging of the ventral and dorsal part of the cavity. In such preparations, the connection to the pharynx via the Eustachian tube, the promontory and the stapes with stapedial artery was visible (Fig. 3). A large number of Sox2-positive cells were observed in the ventral region of the middle ear cavity, close to the Eustachian tube (Fig. 3A). Expression also extended in tracks around the promontory of the cochlea, but no expression was observed overlying the cochlea. Expression dorsally around the stapes was also absent. The pattern of Sox2 expression mimicked that of the endoderm, as highlighted using the Sox17-2A-icreR26R mouse line (Engert et al., 2009; Soriano, 1999) (Fig. 3B). As Wnt activity has been associated with the control of stem cell niches, we looked at Wnt activity using an Axin2LacZ reporter line at P21 (Lohi et al., 2010). Intriguingly, large numbers of LacZ-positive cells were associated with the endoderm-derived region expressing Sox2, with intense blue spots of Axin2 observed in the epithelium (Fig. 3C,D). Positively stained cells lined the area around the opening to the Eustachian tube (hypotympanum) but were absent from the promontory overlying the cochlea and the epithelium in the attic region (Fig. 3C,D). In section, Axin2-positive cells were observed scattered throughout the epithelium of the hypotympanum, localized to the nucleus, which is expected given that these mice have a nuclear localised (NL) LacZ (Fig. 3E) (Lustig et al., 2002). To follow the fate of the Sox2 cells and their progeny, the Sox2creERT2tdTom were left for up to 6 months prior to culling (n=5). Similar to the expression of Sox2 2 days post-tamoxifen (Fig. 3A), after 6 months the majority of Tomato-positive cells were in the hypotympanum and generally excluded from the promontory over the cochlea (Fig. 3F). However, stripes of expression in the inner ear itself were evident (Fig. 3F). Using confocal microscopy, scattered cells were evident in the attic region (Fig. 3H), with a few isolated cells labelled over the promontory (Fig. 3H,I). The general distribution and number of Tomato-positive cells was very similar to that after 2 days, suggesting that these cells may not be the stem cells of the middle ear. The main difference between 2 days and 6 months post-tamoxifen was in the extent of the expression around the sides of the cochlea, with the Tomato-labelled cells extending towards the dorsal region towards the stapedial artery.
Fig. 3.
High levels of Sox2 expression and Wnt activity restricted to the endodermally derived part of the middle ear. (A-D) Whole-mount preparation of auditory bullae (AB) at P21 after ear drum removal showing the Eustachian tube (ET) ventrally (bottom) and stapes (st) dorsally (top). (E) Section of AB shown in C. (F-I) Whole-mount preparation of auditory bullae at 6 months. (A) Sox2creERTom mouse, tamoxifen injected 48 h prior to cull. Sox2-positive cells (and any progeny formed in the last 48 h) labelled in red. Arrows indicate tracts of red cells around the promontory. (B) Sox17-2A-icreR26R mouse, endoderm-derived cells and blood vessels stained blue. (C-E) Axin2R26R middle ears. Active Wnt signalling stains blue. (C) Whole bulla. (D) Positive scattered cells in hypotympanum near the ET, but not in the promontory overlying the cochlea. (E) Cryosection through the hypotympanum showing scattered blue cells in the epithelium, counterstained with Eosin. (F) AB; dark-field view showing Sox2-positive cells and progeny in orange. Tomato expression is also observed in the cochlea underlying the middle ear in stripes at 6 months. Arrow indicates Tomato-positive cells extending up to the attic around the promontory. (G) Dissected AB, boxed regions show areas highlighted by confocal microscopy in H,I. (H) Promontory and attic region with sparse isolated Tomato-positive cells. Large numbers of positive cells are observed at the margin of the promontory reaching up to the dorsal part of the middle ear. (I) Hypotympanum covered in Sox2-positive cells as at 2 days post-tamoxifen. The promontory (V-shape at top of image) only has a few positive cells in comparison. tt, tensor tympani muscle; st, stapes; ET, Eustachian tube; sta, stapedial artery running through stapes. Scale bar: 250 µm in A-C,F.
Sox2 and keratin 5 mark distinct populations in the middle ear epithelium
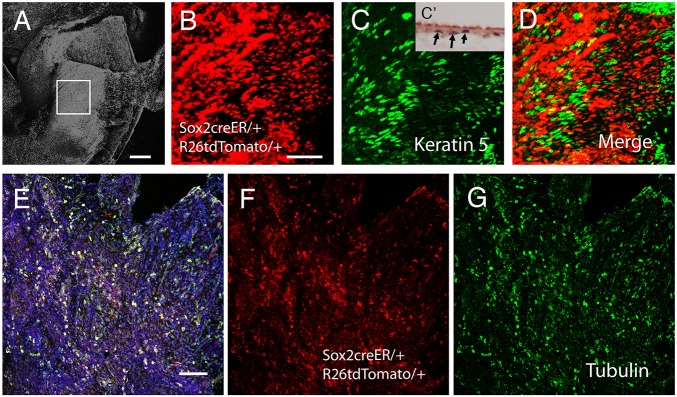
To understand how Sox2 expression related to other markers in the middle ear epithelium, Sox2 expression (Sox2creERT2tdTom mice 2 days post-tamoxifen injection) was compared with expression of another potential stem cell marker, keratin 5. Tamoxifen was injected at 3 weeks, once the ear had fully formed. Keratin 5 has been reported to be expressed in the middle ear epithelium in both the ciliated and non-ciliated epithelium (Luo et al., 2017). Like Sox2, keratin 5 was expressed at high levels in the hypotympanum (Fig. 4C) with only occasional positive cells in the simple neural crest-derived epithelium over the promontory at 3 weeks. Importantly, however, very little overlap was observed between Sox2 and keratin 5 in the epithelium, with the majority of keratin 5-positive cells not expressing Sox2 (Fig. 4A-D). These two genes therefore mark distinct populations of cells in the bulla epithelium. In section, keratin 5 was restricted to the basal epithelial cells, as observed for the LRCs identified by BrdU (Fig. 4C inset). Keratin 5 therefore represents a good potential marker of epithelial stem cells in the ear. Given the large number of ciliated cells in the hypotympanum, expression of Sox2 was compared with that of acetylated α-tubulin, a marker for ciliated epithelial cells (Fig. 4E-G). Labelling for acetylated α-tubulin almost completely overlapped with expression of Sox2, with only a very few Sox2 cells not expressing α-tubulin.
Fig. 4.
Keratin 5 and Sox2 mark distinct populations of cells in the middle ear epithelium. (A-G) Confocal images of the auditory bulla (AB). (A) DAPI image showing region of the hypotympanum imaged. Boxed area is enlarged in B-D. (B) Sox2creERTom mouse, tamoxifen injected 48 h prior to cull. (C) Keratin 5 immunostaining (green) of Sox2creERTom bulla. C′ shows a section through the hypotympanum with keratin 5-labelled cells in black (arrows). (D) Merged image of B and C showing only a small overlap between Sox2 and keratin 5. Cells expressing both show as yellow. (E-G) Sox2creERTom mouse tamoxifen injected 48 h prior to cull, labelled with acetylated α-tubulin to highlight ciliated cells. (E) Merged image; double-labelled cells are yellow. Blue indicates nuclei (DAPI). (F) Sox2creERTom (red). (G) Acetylated α-tubulin (green). There is a large overlap between these two markers. Scale bars: 200 µm in A; 50 µm in B-D; 100 µm in E-G.
Dual origin of ciliated cells in the middle ear
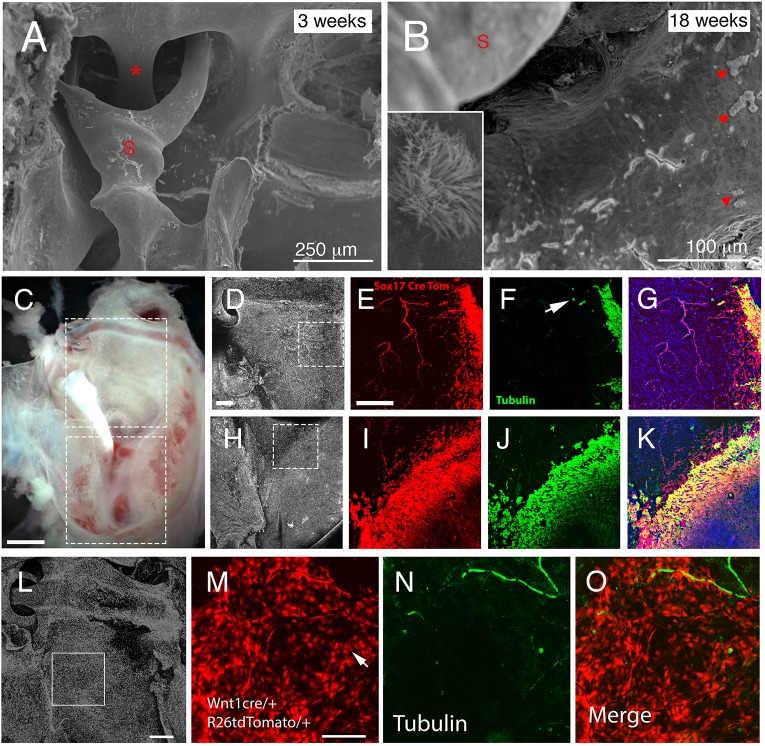
Cilia have recently been described in the dorsal part of the ear near to the tympanic membrane, which might be predicted to derive from the neural crest (Luo et al., 2017). Pseudostratified ciliated cells are found in the ventral cavity close to the Eustachian tube and extend in tracks around the promontory of the cochlea reaching around the tympanic membrane. At 2 weeks, cilia are not observed in the dorsal part of the middle ear cavity in mice (Thompson and Tucker, 2013); however, at 18 weeks some ciliated cells were present in patches near to the stapes and oval window (Fig. 5A,B). This suggests that either the neural crest cells start forming cilia, or that the endoderm cells migrate further up the ear into the dorsal regions over time. The extension of Sox2-positive cells towards the dorsal parts of the middle ear suggest that some migration of cells does occur (Fig. 3). To clarify this situation, cilia distribution and endoderm origin was investigated using dual labelling of Sox172Aicre/tdTom (red) and α-acetylated tubulin (green) at 5 months. Endodermally derived (red) cells could be observed spreading out from the hypotympanum and lateral edges of the bulla (Fig. 5C-E,H,I). Blood vessels that line the bulla were also labelled using this mouse (see Fig. 5E). The large majority of ciliated cells (green) were located within the endodermal domain and therefore were labelled yellow (Fig. 5F,G,J,K), a few single tubulin-positive cells, however, were observed at a slight distance from the endodermally derived epithelium, suggesting a potential neural crest origin. To confirm this, acetylated α-tubulin staining was performed on Wnt1cre/tdTom mice at 3 months (Fig. 5L-O). In these mice, the epithelium near the ossicles is derived from the neural crest (red) (Fig. 5M). Very few ciliated cells were observed in this region, as would be expected, whereas neurons were visible (green) (Fig. 5N). The lone ciliated cells, however, were labelled as neural crest, indicating that the neural crest derived epithelium was able to develop into ciliated cells over time (Fig. 5O).
Fig. 5.
Dual origin of ciliated cells in the ear. (A,B) Scanning electron microscopy images of the middle ear showing the attic region with the stapedial artery (asterisk) and stapes (S) inserting into the oval window in control mice. (A) In 3-week-old animals, no cilia were observed adjacent to the stapes. (B) In contrast, at 18 weeks, control mice displayed patches of ciliated cells (arrowheads). Inset shows magnification of the cilia patch. (C) Dissected auditory bulla of Sox17creERT/tdTom mouse. Boxes highlight region imaged by confocal microscopy. (D,H) DAPI staining highlighting nuclei. Boxes highlight the regions shown in E-G and I-K. (D-G) Lateral edges of attic. (H-K) Border of hypotympanum and promontory. (E,I) Some endoderm cells (red) appear to spread into the neural crest-derived regions of the attic and promontory. (F,J) The ciliated cells (acetylated α-tubulin, green) are mainly located overlapping with the endoderm but a few cells do not co-express the endoderm marker (arrow in F). (G,K) Merged image. Endoderm-derived ciliated cells are in yellow. (L-O) Dissected auditory bulla of Wnt1cre/tdTom mouse. DAPI staining highlighting nuclei in the attic region. Box highlights the region imaged in M-O. (M) Neural crest-derived epithelial cells show up as red. Arrow highlights a red cell. (N) Acetylated α-tubulin (green) highlights nerves and a few lone ciliated cells. (O) Merged image showing ciliated cell is of neural crest origin. Scale bars: 250 µm in A; 100 µm in B; 500 µm in C; 200 µm in D-K; 200 µm in L; 100 µm in M-O.
Putative stem/progenitor cell expression alters with otitis media
Otitis media (OM) is an inflammation of the middle ear mucosa, which is incredibly common, especially during childhood. Chronic otitis media with effusion (OME) can result in tissue remodelling, including hyperplasia of the epithelium. Given that putative stem cells were found predominately in the ciliated pseudostratified endodermally derived epithelium and minimally in the simple neural crest-derived epithelium, we moved to investigate the response of these two epithelial tissues to otitis media. For this we used the Tbx1+/− mouse model (Liao et al., 2004; Fuchs et al., 2013), which develops OM due to Eustachian tube dysfunction (Fuchs et al., 2015). As expected, the Tbx1 heterozygous mice showed signs of otitis media, with thickening of the mucosa and infiltration of cells within the middle ear cavity (Fig. 6A,B). Hyperplasia of the epithelium occurred throughout the middle ear, increasing with the degree of severity of the OM, as highlighted by increased expression of E-cadherin (Fig. S1) at P28. The underlying mesenchymal tissue under the epithelium also underwent hyperplasia, this being more extreme in the hypotympanum, with expansion/invasion of blood vessels (Fig. S1), whereas the tissue over the cochlea was less visibly affected (see border highlighted in Fig. 6A,B). As might be predicted given this level of hyperplasia, increased levels of proliferation were observed across the middle ear epithelium, agreeing with earlier reports (Lim and Birck, 1971). This change in the epithelium was particularly marked on the promontory where normally very little (or no) proliferation is observed in a healthy ear (Fig. 6C,D), suggesting that this epithelium undergoes major remodelling during injury. To investigate the effect on putative stem cells, the expression of keratin 5 was analysed in these mice (n=2). The expression of keratin 5 in the pseudostratifed endodermally derived epithelium was similar to the wild type at P28 (Fig. 6E,F); however, the epithelium overlying the cochlea in mice with otitis media appeared drastically different, with many cells expressing keratin 5 (Fig. 6G,H). In all cases, the keratin 5-positive cells were located close to the basal lamina. As an observation, we had one Sox2creERTom sample, which had naturally occurring otitis media at P23. This sample had been injected with tamoxifen at P21 and collected 2 days later, as a base level of Sox2 expression. In this mouse, Sox2-positive cells were observed overlying the cochlea (Fig. 6I), a region normally devoid of Sox2-positive cells and their progeny (see Fig. 3A). Keratin 5 and Sox2 are therefore both upregulated in the neural crest-derived parts of the ear in response to injury.
Fig. 6.
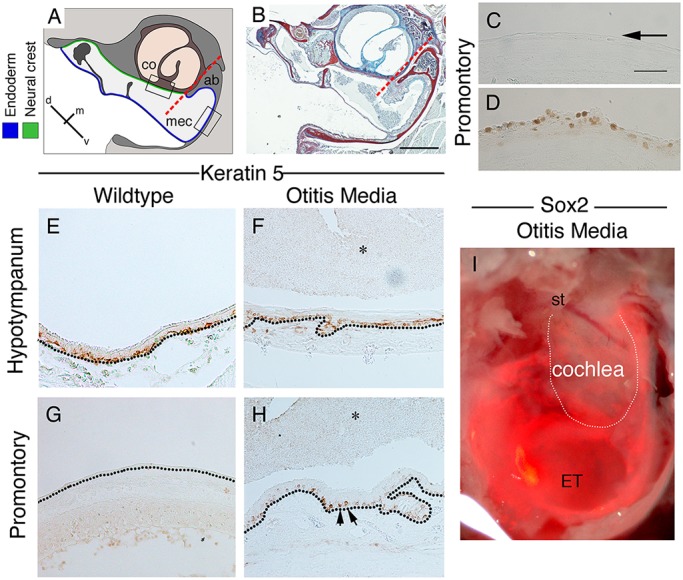
Changes to keratin 5, PCNA and Sox2 expression with otitis media. (A) Schematic of mouse middle ear in frontal section, highlighting the border between the endoderm and neural crest. (B) Trichrome stain of a Tbx1/+ middle ear with otitis media at 11.5 weeks. The ear is full of effusion and infiltrated cells, and the endodermally lined hypotympanum shows signs of hyperplasia. Dashed red lines in A,B indicate the border between neural crest and endoderm. (C-H) Frontal sections of mouse middle ear in Tbx1/+mice (D,F,H) and control littermates (C,E,G) at P28. (C,D) PCNA (brown) on promontory. Arrow in C highlights negative control epithelium. (E-H) Keratin 5 immunohistochemistry. In wild-type mice (E,G), keratin 5 is expressed by basal stem cells in the pseudostratified epithelium (E). (G) Only a few keratin 5-positive cells are observed in the simple epithelium overlying the cochlea. (F,H) In Tbx1/+ mice with otitis media, keratin 5 expression in the ventral middle ear looks similar to the wild type (F). (H) In contrast, overlying the cochlea, many keratin 5-positive cells were observed (arrows). (E-H) Dotted black lines indicate the basal lamina underlying the epithelium. (I) Whole-mount image of a Sox2creERT/tdTom auditory bulla with OM. Mouse injected 2 days prior to P21 to activate cre. Fluorescent image overlying bright-field image. Positive cells overlie the cochlea. st, stapes; ET, Eustachian tube. White line indicates the cochlea. Scale bars: 200 µm in B; in C, 100 µm for C-H.
DISCUSSION
The mouse middle ear epithelium lines the middle ear cavity and is composed of regions of ciliated pseudostratified epithelium and regions of simple epithelium with a dual origin, derived from endoderm and neural crest (Thompson and Tucker, 2013). The middle ear epithelium functions to keep the cavity clear of debris and to act as a barrier against infection. As with the majority of adult tissues, the middle ear epithelium must undergo continual cycles of self-renewal in order to maintain homeostasis of the cells and to combat injury. This study has highlighted that the diverse epithelium of the middle ear cavity contains distinct regions. Some regions are populated with many putative stem progenitor cells, whereas other regions hold very few such cells – this pattern reflecting the embryonic origin of the tissue.
Proliferation of the epithelium is not uniform across the mouse middle ear
Analysis of proliferation patterns across the ear highlighted that levels of proliferation in the adult epithelium were not uniform, with distinct patches of higher proliferation around the ear drum and near the Eustachian tube, which suggests that these areas have a higher turnover rate when compared with adjacent regions lining the cochlea and in the attic. Overall proliferation of the epithelium was highest in the mouse 2 weeks postnatally, but decreased significantly by 3 weeks. This pattern of proliferation fits with the growth of the middle ear, which increases rapidly up to P15 (2 weeks) and reaches close to its adult size by 3 weeks (Richter et al., 2010). Proliferation from 3 weeks onwards is therefore likely to be for replacement of cells rather than due to growth.
Stem/progenitor cells cluster in the ciliated part of the middle ear
Interestingly, the areas with higher proliferation overlapped with the regions with label-retaining cells. These slow cycling cells were located at high levels within defined regions of the endodermal pseudostratified epithelium and were closely associated with the basement membrane. This location suggests that they are likely to be basal stem cells, similar to those observed in the trachea (Rock et al., 2010). It is interesting to note that some regions of the middle ear, such as around the ear drum and near the Eustachian tube, had both high levels of LRCs and proliferating cells, suggesting that these two populations are intermingled and may regulate each other. To further support the presence of stem cells within the middle ear epithelium, we investigated expression of two well-used stem cell markers: keratin 5 and Sox2 (Arnold et al., 2011; Fauquier et al., 2008; Hong et al., 2004; Juuri et al., 2012; Rock et al., 2009; Tompkins et al., 2009). Both markers were expressed in the hypotympanum close to the Eustachian tube and surrounding the eardrum, with almost no positive cells in the unciliated epithelium lining the attic and cochlea.
In previous publications, Sox2 has been shown to be expressed by a subset of cells in the adult, such as the immature basal cells of squamous epithelium (Arnold et al., 2011). In contrast, in the middle ear, many cells in the endoderm-derived epithelium expressed Sox2, together with acetylated α-tubulin, a marker of ciliated cells. This wide expression pattern in differentiated cells suggests Sox2 is not a stem cell marker in the middle ear epithelium. Sox2 has also been shown to be expressed by a large number of cells in the adult trachea. In the trachea, the Sox2 cells have been shown to be important for maintaining proliferation and differentiation during normal homeostasis and following injury (Que et al., 2009). When Sox2 progeny were tracked over a period of up to 6 months, the expression pattern remained fairly constant, with sparse Tomato expression in the neural crest-derived tissues, suggesting that Sox2 cells do not contribute substantially to homeostasis in this region.
In the epithelium of the trachea and middle ear, basal cells have been shown to express keratin 5 and have the potential during normal homeostasis to form differentiated ciliated cells (Rock et al., 2009; Luo et al., 2017). A similar basal expression of keratin 5 was observed in the hypotympanum of the middle ear. Interestingly, the keratin 5 cells showed very little overlap with the abundant Sox2-positive cells, highlighting that these genes are expressed by distinct populations in the middle ear. The small overlap between the two markers suggests that the keratin 5-positive cells differentiate into ciliated Sox2-positive cells during normal homeostasis. Our expression analysis of keratin 5 in the ventral ciliated epithelium agrees with the recent findings by Luo et al.; however, we have a very different expression in the non-ciliated neural crest-derived epithelium. In this part of the ear, we observed very few keratin 5-labelled cells, in keeping with sparse numbers of LRCs in this region, while Luo et al. reported that all the cells were keratin 5 positive, as shown using immunofluorescence. Intriguingly, however, in the same paper, use of a Keratin5creERT2 line induced by tamoxifen, led to only sporadic labelling of keratin 5 in the neural crest-derived epithelium, more in keeping with our results.
The expression domain of Sox2 and keratin 5 was found in a region of high Wnt activity, as highlighted by Axin2 reporter mice. This concentration of Wnt signalling in the hypotympanum suggests that Wnt may play a role in regulating expression of Sox2 and keratin 5 in the ear, potentially by providing a niche for stem/progenitor cell maintenance (Nusse, 2008).
Endoderm cells move over time to populate some parts of the dorsal middle ear
As the ear matured, the boundary between the endoderm- and neural-derived epithelium became less well defined. Endodermal cells were observed scattered among neural crest cells extending out from the hypotympanum. This coincided with increased numbers of ciliated cells in the dorsal part of the middle ear, as viewed by an extension of the Tomato expression domain in Sox2ert2cre/Tom mice tracked for 6 months after induction. Ciliated cells have also been reported in the dorsal part of the middle ear, close to the ear drum (Luo et al., 2017), and a few single ciliated cells have been reported overlying the cochlea (Sade, 1966). Our data suggest that at least some of these dorsally located ciliated cells are formed by neural crest-derived cells taking on a ciliated morphology, in addition to a contribution by endodermally derived cells that have moved around the middle ear cavity. The neural crest-derived epithelium therefore does appear to have the potential to form complex epithelial cell types.
Epithelial changes during middle ear disease
Keratin 5- and Sox2-positive cells were found to be restricted to the endodermally derived pseudostratified epithelium in healthy ears. Interestingly, during periods of otitis media, the epithelium underwent extensive hyperplasia, and the expression of keratin 5 and Sox2 was upregulated in the epithelium overlying the cochlea and in the attic, which normally have sparse expression of these markers. The increase in keratin 5 and Sox2 was associated with an increase in proliferation of the epithelium in response to the disease, which was particularly pronounced on the neural crest-derived promontory, as this tissue usually has an extremely low rate of proliferation. The way the middle ear epithelium repairs itself after infection/inflammation may therefore rely on a different mechanism than that normally used during homeostasis, with the endoderm and neural crest-derived tissue potentially having a different reaction to injury.
This study investigates the different properties of the neural crest and endodermally derived epithelium of the middle ear, highlighting dramatic differences in the expression of putative stem progenitor cells, the activity of signalling pathways, and the location of proliferating and label-retaining cells. Otitis media can affect the distribution of keratin 5- and Sox2-expressing cells, which may play a central role in the epithelial hyperplasia observed during inflammation.
MATERIALS AND METHODS
Mice
Heterozygous Sox17-2A-iCre (Engert et al., 2009), Sox2creERT2 (Andoniadou et al., 2013) and Wnt1-Cre males (Danielian et al., 1998) were mated overnight with homozygous R26R (Soriano, 1999) or homozygous tdTomato [Gt(ROSA)26 Sor tm14(CAG-tdTomato); Hze JAX labs] females. Tbx1 heterozygous males (Lindsay et al., 2001) and Axin2LacZ+/− males (Lustig et al., 2002) (Jackson Labs 009120) were mated to C57Bl6 and CD1 wild-type females, respectively. Pups from these crosses were taken at a range of pre- and post-weaning stages with all animals culled using a schedule 1 method as approved by the Home Office and King's College London. Prior to culling, Sox2creERT2/tdTom mice were injected with a single dose of tamoxifen to induce cre recombination. The Axin2 mice have a nuclear localised (NL) LacZ inserted into the start codon of the Axin2 gene. Axin2-positive pups were selected using a quick X-gal staining procedure on the embryonic bodies/lungs (20 min in X-Gal solution at 37°C), by imaging with a Leica dissecting fluorescence microscope or by genotyping. Negative pups were used for controls and immunohistochemistry. Samples were either dissected for whole bulla imaging or fixed in paraformaldehyde and processed through sucrose into OCT (VWR) for cryosectioning, or decalcified in an ethylenediaminetetraacetic acid (EDTA) solution or 10% formic acid solution and dehydrated through ethanol or methanol for wax processing. Mice with otitis media showed effusion in the middle ear and their tympanic membranes bulged outwards and were slightly opaque.
Whole-mount X-gal staining
Sox17-2A-iCre/R26R- and Axin2R26R-positive heads were dissected, opening up the ear canal to reveal the inner surface of the auditory bulla. Tissue was fixed in 4% PFA for 15 min, washed in PBS and stained in X-Gal staining solution for 24 h at room temperature. Pictures were taken with a dissection microscope (Leica MZFiii) and Leica DFC300 camera.
Immunohistochemistry on sections
Wax sections were dewaxed, rehydrated, treated with DAKO antigen retrieval solution at 95°C for 20 min followed by 10 min at room temperature and endogenous peroxidase treated in 3% hydrogen peroxidase. Cryosections were defrosted and washed in PBS. The slides were then incubated with rabbit keratin 5 (1:200; Covance, 905501) or rabbit E-cadherin (1:250; Abcam, ab76319) overnight at 4°C (Thompson and Tucker, 2013). The sections were then washed and incubated in goat anti-rabbit biotin (1:500; Invitrogen) or goat anti-mouse biotin (1:500). Amplification was performed using an ABC kit (Vector) then developed using DAB with Nickel (Vector), counterstained with Menapath green (Menarini Diagnostics) or NovaRed (Vector).
Immunofluorescence in whole mount
Dissected auditory bullas from Sox2Ert2cre/tdTom, Sox17-2A-iCre/tdTom and Wnt1cre/tdTom mice were fixed, washed in PBS and permeabilised in 1% Triton X PBS for several hours. Bulla were blocked in immunohistochemistry blocking solution (10% goat serum, 1% BSA in PBT) and incubated with primary antibody in block (mouse acetylated α-tubulin, 1:200; Sigma, T6793) (rabbit keratin 5, 1:200; Covance, 905501) for 2-3 days (Thompson and Tucker, 2013). The tissue was then washed three times in immunohistochemistry blocking solution and incubated in secondary antibody (Alexa Fluor, mouse/rabbit, 1:500, Invitrogen) before being washed and prepared for confocal imaging (TCS SP5 Leica).
PCNA staining and cell counts
Wax sections were dewaxed, rehydrated through ethanol, digested with 20 µm/ml proteinase K for 15 min at room temperature and endogenous peroxidases blocked with 3% H2O2 for 10 min. The tissue was then pretreated with 0.01 M (pH 6) citric acid for 30 min at 90°C in a water bath before cooling for 10 min and treating with 50 mM NH4Cl for 5 min. Detection of proliferative cell nuclear antigen (PCNA) was achieved using the Invitrogen PCNA colorimetric kit and developed using the Vector DAB staining kit. For cell counts, three samples of each age group were collected and the same 250 µm region of epithelium was analysed over 8-10 µm sections. The number of cells in this region was counted and the average number of cells was calculated along with the s.e.m. The slides were then placed in xylene to remove the coverslips and counterstained with DAPI. Recoverslipped slides were then used to count the total number of cells/nuclei in the same region to provide a final percentage of proliferating cells. Student's t-tests were used to show significant differences between two ages of mice.
BrdU pulse, chase and staining
All dividing cells were BrdU pulse labelled, by replacing the drinking water of a pregnant CD1 mouse with 1 mg/ml BrdU in water, between embryonic days 9 and 16. The pups were collected at P1, P21 and 8 weeks postnatally. The samples were decalcified in EDTA before processing into wax through methanol and tetrahydronaphthalene. Sections were dewaxed, rehydrated and pre-treated with 0.01 M (pH 6) citric acid for 30 min at 95°C in a water bath before cooling for 10 min and treated with 2 M HCl at 40°C for 30 min. Endogenous peroxidases were inactivated with 3% H2O2 for 20 min. Slides were incubated in rat anti-BrdU (1:200; Abcam, ab6326) overnight at 4°C. The sections were then washed and incubated in goat anti-rat biotin (1:500; Life Technologies, 31830). Amplification was performed using an ABC kit (Vector) then developed using DAB with Nickel (Vector).
Scanning electron microscopy
The temporal bones of postnatal mice were dissected and the middle ear epithelium revealed by removing the outer ear, eardrum, tympanic ring, and the malleus and incus. These were then fixed in 2.5% gluteraldehyde in 0.15 M cacodylate buffer (pH 7.2) overnight at 4°C, washed and post-fixed in 1% osmium tetroxide. Next, specimens were dehydrated in ethanol and dried using a Polaron E3000 critical point dryer. After mounting and coating with gold (Emitech K550X sputter coater), the surface of the epithelium was examined and images recorded using a Hitachi S-3500N scanning electron microscope operated at 10 kV in high vacuum mode.
Supplementary Material
Acknowledgements
We thank Cynthia Andoniadou for the Sox2creERT2 mice.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.S.T., H.T.; Methodology: A.S.T., C.J.D., J.M.F., T.H.T., J.C.F., H.T.; Validation: J.M.F., J.C.F.; Formal analysis: A.S.T., C.J.D., J.M.F., J.C.F., H.T.; Investigation: A.S.T., C.J.D., J.M.F., T.H.T., J.C.F., H.T.; Resources: A.S.T.; Writing - original draft: A.S.T., H.T.; Writing - review & editing: A.S.T., J.M.F., T.H.T., J.C.F., H.T.; Supervision: A.S.T., H.T.; Project administration: A.S.T., H.T.; Funding acquisition: A.S.T., H.T.
Funding
This research was funded by Action on Hearing Loss by a Pauline Ashley Award to H.T. (PA13) and by the Wellcome Trust (102889/Z/13/Z). Deposited in PMC for release after 6 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.154393.supplemental
References
- Andoniadou C. L., Matsushima D., Mousavy Gharavy S. N., Signore M., Mackintosh A. I., Schaeffer M., Gaston-Massuet C., Mollard P., Jacques T. S., Le Tissier P. et al. (2013). Sox2+ stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell 13, 433-445. 10.1016/j.stem.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Arnold K., Sarkar A., Yram M. A., Polo J. M., Bronson R., Sengupta S., Seandel M., Geijsen N. and Hochedlinger K. (2011). Sox2+ adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 9, 317-329. 10.1016/j.stem.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atef A. and Ayad E. E. (2004). Ciliary count in chronic suppurative otitis media: comparative quantitative study between mucosal and squamous types using scanning electron microscopy and image analysis. J. Laryngol. Otol. 118, 343-347. 10.1258/002221504323086516 [DOI] [PubMed] [Google Scholar]
- Danielian P. S., Muccino D. and Rowitch D. H. (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8, 1323-1326. 10.1016/S0960-9822(07)00562-3 [DOI] [PubMed] [Google Scholar]
- Engert S., Liao W. P., Burtscher I. and Lickert H. (2009). Sox17-2A-iCre: a knock-in mouse line expressing Cre recombinase in endoderm and vascular endothelial cells. Genesis 47, 603-610. 10.1002/dvg.20540 [DOI] [PubMed] [Google Scholar]
- Fauquier T., Rizzoti K., Dattani M., Lovell-Badge R. and Robinson I. C. A. F. (2008). SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc. Natl. Acad. Sci. USA 105, 2907-2912. 10.1073/pnas.0707886105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. C., Zinnamon F. A., Taylor R. R., Ivins S., Scambler P. J., Forge A., Tucker A. S. and Linden J. F. (2013). Hearing loss in a mouse model of 22q11 deletion syndrome. PLoS ONE 8, e80104 10.1371/journal.pone.0080104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. C., Linden J. F., Baldini A. and Tucker A. S. (2015). A defect in early myogenesis causes Otitis media in two mouse models of 22q11.2 deletion syndrome. Hum. Mol. Genet. 24, 1869-1882. 10.1093/hmg/ddu604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K. U., Reynolds S. D., Watkins S., Fuchs E. and Stripp B. R. (2004). In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L643-L649. 10.1152/ajplung.00155.2003 [DOI] [PubMed] [Google Scholar]
- Juuri E., Saito K., Ahtiainen L., Seidel K., Tummers M., Hochedlinger K., Klein O. D., Thesleff I. and Michon F. (2012). Sox2+ Stem cells contribute to all epithelial lineages of the tooth via Sfrp5+ progenitors. Dev. Cell 23, 317-328. 10.1016/j.devcel.2012.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. W., Kim J., Seonwoo H., Jang K.-J., Kim Y. J., Lim H. J., Lim K.-T., Tian C., Chung J. H. and Choung Y.-H. (2015). Latent progenitor cells as potential regulators for tympanic membrane regeneration. Sci. Rep. 5, 11542 10.1038/srep11542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsson J., von Unge M. and Rask-Andersen H. (2011). Localization of progenitor/stem cells in the human tympanic membrane. Audiol. Neurotol. 16, 263-269. 10.1159/000320612 [DOI] [PubMed] [Google Scholar]
- Kuijpers W., van der Beek J. M. H., Jap P. H. and Tonnaer E. L. G., (1984). The structure of the middle ear epithelium of the rat and the effect of Eustachian tube obstruction. Histochem. J. 807-818. 10.1007/BF01002787 [DOI] [PubMed] [Google Scholar]
- Liao J., Kochilas L., Nowotschin S., Arnold J. S., Aggarwal V. S., Epstein J. A., Brown M. C., Adams J. and Morrow B. E., (2004). Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum. Mol. Genet. 13, 1577-1585. 10.1093/hmg/ddh176 [DOI] [PubMed] [Google Scholar]
- Lim D. J. and Birck H. (1971). Ultrastructural pathology of the middle ear mucosa in serous otitis media. Ann. Otol. Rhinol. Laryngol. 80, 838-853. 10.1177/000348947108000611 [DOI] [PubMed] [Google Scholar]
- Lim D. J., Paparella M. M. and Kimura R. (1967). Ultrastructure of the Eustachian tube and middle ear mucosa in the guinea pig. Acta Otolaryngol. 63, 425-444. 10.3109/00016486709128777 [DOI] [PubMed] [Google Scholar]
- Lindsay E. A. Vitelli F., Su H., Morishima M., Huynh T., Pramparo T., Jerecic V., Ogunrinu G., Sutherland H. F., Scambler P. J. et al. (2001). Tbx1 haploinsufficiency in the DiGeorge syndrome region causes aortic arch defects in mice. Nature 410, 97-101. 10.1038/35065105 [DOI] [PubMed] [Google Scholar]
- Lohi M., Tucker A. S. and Sharpe P. T. (2010). Expression of Axin2 indicates a role for canonical Wnt signalling in development of the crown and the root during pre and postnatal tooth development. Dev. Dyn. 129, 160-167. 10.1002/dvdy.22047 [DOI] [PubMed] [Google Scholar]
- Lustig B., Jerchow B., Sachs M., Weiler S., Pietsch T., Karsten U., van der Wetering M., Clevers H., Schlag P. M., Birchmeier et al. (2002). Negative feedback loop of Wnt signalling through upregulation of conductin/Axin2 in colorectal and liver tumours. Mol. Cell. Biol. 22, 1184-1193. 10.1128/MCB.22.4.1184-1193.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Yi H., Taylor J., Li J.-D., Chi F., Todd N. W., Lin X., Ren D. and Chen P. (2017). Cilia distribution and polarity in the epithelial lining of the middle ear cavity. Sci. Rep. 7, 45870 10.1038/srep45870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monasta L., Ronfani L., Marchetti F., Montico M., Vecchi Brumatti L., Bavcar A., Grasso D., Barbiero C. and Tamburlini G., (2012). Burden of disease caused by otitis media: systematic review and global estimates. PLoS ONE 7, e36226 10.1371/journal.pone.0036226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulay A., Akram K. M., Williams D., Armes H., Russell C., Hood D., Armstrong S., Stewart J. P., Brown S. D. M., Bingle L. et al. (2016). An in vitro model of muring middle ear epithelium. Dis. Models Mech. 9, 1405-1417. 10.1242/dmm.026658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. (2008). Wnt signaling and stem cell control. Cell Res. 18, 523-527. 10.1038/cr.2008.47 [DOI] [PubMed] [Google Scholar]
- Que J., Luo X., Schwartz R. J. and Hogan B. L. M. (2009). Multiple roles for Sox2 in the developing and adult mouse trachea. Development 136, 1899-1907. 10.1242/dev.034629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C. A., Amin S., Linden J., Dixon J., Dixon M. J. and Tucker A. S., (2010). Defects in middle ear cavitation cause conductive hearing loss in the Tcof1 mutant mouse. Hum. Mol. Genet. 19, 1551-1560. 10.1093/hmg/ddq028 [DOI] [PubMed] [Google Scholar]
- Rock J. R., Onaitis M. W., Rawlins E. L., Lu Y., Clark C. P., Xue Y., Randell S. H. and Hogan B. L. (2009). Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 106, 12771-12775. 10.1073/pnas.0906850106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J. R., Randell S. H. and Hogan B. L. M. (2010). Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis. Model. Mech. 3, 545-556. 10.1242/dmm.006031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade J. (1966). Middle ear mucosa. Arch. Otolaryngol. 84, 137-143. 10.1001/archotol.1966.00760030139005 [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71. 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Thompson H. and Tucker A. S. (2013). Dual origin of the epithelium of the mammalian middle ear. Science 339, 1453-1456. 10.1126/science.1232862 [DOI] [PubMed] [Google Scholar]
- Tompkins D. H., Besnard V., Lange A. W., Wert S. E., Keiser A. R., Smith A. N., Lang R. and Whitsett J. A. (2009). Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS ONE 4, e8248 10.1371/journal.pone.0008248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang Z. and Tian J. (2004). [Epidermal stem cells in the tympanic membrane]. Zhonghua Er Bi Yan Hou Ke Za Zhi [Chinese journal of otorhinolaryngology head and neck surgery] 39, 712-716. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.