ABSTRACT
Although KPC enzymes are most common among carbapenemases produced by Enterobacter cloacae complex globally, the epidemiology varies from one country to another. While previous studies have suggested that IMP enzymes are most common in Japan, detailed analysis has been scarce thus far. Here, we carried out a molecular epidemiological study and plasmid analysis of IMP-1-producing E. cloacae complex isolates collected from three hospitals in central Tokyo using whole-genome sequencing. Seventy-one isolates were classified into several sequence types (STs), and 49 isolates were identified as Enterobacter hormaechei ST78. Isolates of ST78 were divided into three clades by core-genome single nucleotide polymorphism (SNP)-based phylogenetic analysis. Whereas isolates of clade 3 were isolated from only one hospital, isolates of clade 1 and 2 were identified from multiple hospitals. Ten of 12 clade 1 isolates and 1 of 4 clade 2 isolates carried blaIMP-1 on IncHI2 plasmids, with high similarity of genetic structures. In addition, these plasmids shared backbone structures with IncHI2 plasmids carrying blaIMP reported from other countries of the Asia-Pacific region. All isolates of clade 3 except one carried blaIMP-1 in In1426 on IncW plasmids. An isolate of clade 3, which lacked IncW plasmids, carried blaIMP-1 in In1426 on an IncFIB plasmid. These observations suggest that IMP-producing E. cloacae complex isolates with a diversity of host genomic backgrounds have spread in central Tokyo, and they indicate the possible contribution of IncHI2 plasmids toward this phenomenon.
KEYWORDS: carbapenemase-producing Enterobacteriaceae, Enterobacter cloacae complex, IMP-1, metallo-β-lactamase, whole-genome sequencing, plasmid
INTRODUCTION
Carbapenemase production is the most important mechanism contributing to carbapenem resistance in Enterobacteriaceae, and previous reports have suggested that carbapenemase-producing Enterobacteriaceae (CPE) have higher potential to cause lethal infection and nosocomial spread than non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae (CRE) (1, 2). While KPC enzymes are the most common carbapenemases among those produced by Enterobacteriaceae on a global basis, the epidemiology varies from one country to another. Although information about the epidemiology of CPE in Japan has been limited, several studies have suggested that IMP enzymes are the most common among carbapenemases produced by Enterobacteriaceae (3–5).
Molecular analysis revealed that several high-risk clones have contributed to the global spread of antimicrobial resistance genes in Enterobacteriaceae. For instance, Escherichia coli sequence type 131 (ST131) and Klebsiella pneumoniae ST258 have played crucial roles in the global spread of blaCTX-M and blaKPC, respectively (6). Although the presence of several high-risk clones such as ST78 and ST66 among Enterobacter cloacae complex (ECC) isolates has been suggested, information about the relationship between these high-risk clones and carbapenemase genes has been limited until now (7, 8).
Plasmids carrying resistance genes play an important role in the spread of resistance genes among different clones and different species. In fact, institutional and regional outbreaks of CPE involving multiple clones and/or species mediated by specific plasmids carrying carbapenemase genes have been reported repeatedly (9–11). This underscores the importance of detailed analysis of resistance plasmids in molecular epidemiological studies of CPE. Although complete nucleotide sequences of numerous plasmids carrying carbapenemase genes, such as blaKPC, blaNDM, and blaOXA-48-like, have been determined (12–15), detailed information about the plasmids carrying blaIMP has been scarce partly because CPE producing IMP enzymes have been rare in most parts of the world (8).
In this study, we performed molecular epidemiological analysis of metallo-β-lactamase (MBL)-producing ECC isolates collected from three hospitals in central Tokyo and characterized plasmids harboring blaIMP, with a special emphasis on the isolates belonging to ST78.
RESULTS
Identification of MBL genes and whole-genome sequencing of MBL-producing ECC isolates.
A total of 71 frozen stored MBL-producing ECC clinical isolates, consisting of 20 isolates from hospital A, 40 isolates from hospital B, and 11 isolates from hospital C, were collected (Table 1). All isolates were positive for blaIMP-1-group by PCR. We sequenced the whole genomes of all MBL-producing ECC isolates. A total of 71 genome sequences were obtained at an average depth of 149.3 (standard deviation [SD], 48.1) (see Data Set S1 in the supplemental material). Assembled genomes had an average number of 179.1 (SD, 70.4) contigs and an N50 value of 90,921 bp (SD, 44,765.7 bp).
TABLE 1.
ST and species of 71 IMP-1-producing E. cloacae complex isolates from three hospitals
| ST | Species identificationa | No. of isolates |
|||
|---|---|---|---|---|---|
| Hospital A | Hospital B | Hospital C | Total | ||
| ST78 | E. hormaechei | 11 | 36 | 2 | 49 |
| ST90 | E. hormaechei subsp. steigerwaltii | 8 | 0 | 0 | 8 |
| ST175 | E. hormaechei subsp. steigerwaltii | 0 | 0 | 8 | 8 |
| ST233 | E. hormaechei | 1 | 0 | 0 | 1 |
| ST234 | E. hormaechei subsp. steigerwaltii | 0 | 1 | 0 | 1 |
| ST242 | E. asburiae | 0 | 3 | 0 | 3 |
| ST243 | E. kobei | 0 | 0 | 1 | 1 |
Species identification was performed with ANI.
MLST and species identification.
Seventy-one ECC isolates were classified into 7 STs by multilocus sequence typing (MLST) (Table 1). Isolates belonging to ST78 were most common, accounting for 69.0% (n = 49) of all isolates, followed by those belonging to ST90 (n = 8) and ST175 (n = 8). The isolates belonging to ST90, ST175, and ST234 were identified as Enterobacter hormaechei subsp. steigerwaltii, and the isolates belonging to ST242 and ST243 were identified as belonging to Enterobacter asburiae and Enterobacter kobei, respectively, by average nucleotide identity (ANI). The isolates belonging to ST78 and ST233 were identified as E. hormaechei. While the isolate belonging to ST78 was detected in all three hospitals, the isolates belonging to other STs were collected only from a specific hospital: ST90 and ST233 from hospital A, ST242 and ST234 from hospital B, and ST175 and ST243 from hospital C.
Phylogenetic analysis and estimation of evolutionary rate.
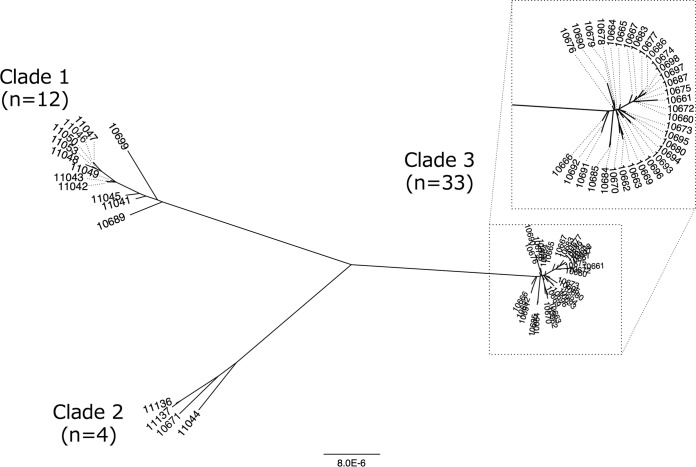
Core-genome single nucleotide polymorphism (SNP)-based phylogenetic analysis revealed the presence of three major clades of MBL-producing E. hormaechei ST78 isolates. Clades 1, 2, and 3 consisted of 12, 4, and 33 isolates, respectively (Fig. 1). While isolates of clade 3 were isolated only from hospital B, isolates belonging to clade 1 and clade 2 were isolated from multiple hospitals (Table 2). The average substitution rate in the core genome was estimated to be 4.53 SNPs (95% highest posterior density interval, 1.40 to 7.74 SNPs) per genome per year, and the time of divergence of clade 1 and clade 2 was estimated to be around 120 years ago (Fig. S1).
FIG 1.
Phylogenetic tree of IMP-1-producing Enterobacter hormaechei ST78 isolates (n = 49; TUM prefixes have been removed for clarity) constructed with maximum-likelihood phylogenetic analysis based on single nucleotide polymorphisms (SNPs) in the core genome and excluding homologous recombination sequences. The core-genome region was 86.9% (4,027,037/4,633,407 bp) of the genome of the reference strain, E. cloacae ECNIH3 ST97. The scale distance corresponds to the number of substitutions per site.
TABLE 2.
Characteristics of IMP-1-producing Enterobacter hormaechei ST78
| Clade and isolate no. | Hospital | Isolation date (mo/day/yr) | Antimicrobial resistance genes | Plasmid replicon(s) | MIC (μg/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Imipenem | Cefotaxime | Cefepime | Aztreonam | Piperacillin-tazobactam | Piperacillin | Moxalactam | Amikacin | Ciprofloxacin | |||||
| Clade 1 | |||||||||||||
| TUM10689 | B | 8/24/2010 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | 0.5 | 512 | 32 | 128 | 256/4 | 256 | >512 | 1 | 0.25 |
| TUM10699 | B | 9/16/2010 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | 1 | 512 | 32 | 128 | >512/4 | >512 | >512 | 2 | 0.25 |
| TUM11041 | A | 1/19/2007 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | ≥0.25 | 512 | 64 | 64 | 128/4 | 128 | >512 | 2 | 0.125 |
| TUM11042 | A | 2/24/2007 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | ≥0.25 | >512 | 64 | 256 | 512/4 | 512 | >512 | 2 | 0.125 |
| TUM11043 | A | 3/23/2007 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | ≥0.25 | >512 | 64 | 256 | 256/4 | 256 | >512 | 1 | 0.25 |
| TUM11045 | A | 9/18/2007 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | 4 | >512 | 256 | 128 | 512/4 | 512 | >512 | 2 | 0.25 |
| TUM11046 | A | 7/7/2009 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | 2 | >512 | 64 | 256 | >512/4 | >512 | >512 | 2 | 0.25 |
| TUM11047 | A | 7/9/2009 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | 2 | >512 | 64 | 256 | 512/4 | 512 | >512 | 2 | 0.25 |
| TUM11048 | A | 7/21/2009 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | 2 | >512 | 64 | 256 | 512/4 | 512 | >512 | 2 | 0.5 |
| TUM11049 | A | 7/24/2009 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | 1 | >512 | 64 | 128 | 64/4 | 64 | >512 | 2 | 0.25 |
| TUM11050 | A | 9/9/2009 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | 2 | >512 | 128 | 256 | 512/4 | 512 | >512 | 2 | 0.25 |
| TUM11053 | A | 5/26/2010 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | 4 | >512 | 128 | 256 | 256/4 | 256 | >512 | 2 | 0.25 |
| Clade 2 | |||||||||||||
| TUM10671 | B | 7/23/2010 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | 4 | 256 | 32 | 32 | 64/4 | 64 | >512 | 2 | 1 |
| TUM11044 | A | 8/10/2007 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | 2 | 256 | 128 | 32 | 32/4 | 32 | >512 | 2 | 2 |
| TUM11136 | C | 9/22/2009 | blaIMP-1, aac(6′)-IIc, sul1, tet(B) | IncHI2 | 4 | 512 | 32 | 32 | 128/4 | 128 | >512 | 1 | 1 |
| TUM11137 | C | 10/25/2009 | blaIMP-1, aac(6′)-IIc, sul1, tet(B), dfrA25 | IncHI2, IncN | 64 | 128 | 512 | 8 | 32/4 | 32 | >512 | 1 | 2 |
| Clade 3 | |||||||||||||
| TUM10660 | B | 6/24/2010 | blaIMP-1, aac(6′)-Ib, dfrA15 | IncW, IncFII, IncFIB | 4 | 256 | 64 | 0.5 | 16/4 | 16 | >512 | 4 | 2 |
| TUM10661 | B | 6/24/2010 | blaIMP-1, aac(6′)-Ib | IncW, IncFII, IncFIB | 16 | >512 | 64 | 16 | 64/4 | 64 | >512 | 4 | 16 |
| TUM10662 | B | 7/5/2010 | blaIMP-1, aac(6′)-Ib, strAB, qnrS1 | IncW, IncFII | 1 | 64 | 8 | 64 | 16/4 | 16 | 256 | 2 | 32 |
| TUM10663 | B | 7/5/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB | IncW, IncFII | 0.5 | 64 | 8 | < = 0.25 | 16/4 | 16 | 256 | 2 | 64 |
| TUM10664 | B | 7/6/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB, qnrS1 | IncW, IncFII | 2 | >512 | 128 | 64 | 64/4 | 64 | >512 | 4 | 8 |
| TUM10665 | B | 7/7/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB | IncW | 0.5 | 512 | 8 | 64 | 128/4 | 128 | >512 | 2 | 64 |
| TUM10666 | B | 7/9/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB, qnrS1 | IncW, IncFII | 1 | 256 | 16 | 1 | 16/4 | 16 | 512 | 8 | 32 |
| TUM10667 | B | 7/10/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB, qnrS1 | IncW, IncFII, IncFIB | 0.5 | 32 | 4 | ≥0.25 | 8/4 | 8 | 256 | 4 | 16 |
| TUM10669 | B | 5/6/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB | IncW, IncFII | 0.5 | 64 | 4 | ≥0.25 | 8/4 | 8 | >512 | 2 | 32 |
| TUM10670 | B | 7/14/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB, qnrS1 | IncW, IncFII | 1 | 64 | 8 | 2 | 8/4 | 8 | 128 | 2 | 32 |
| TUM10672 | B | 7/26/2010 | blaIMP-1, aac(6′)-Ib, dfrA15 | IncW, IncFII, IncFIB | 0.5 | 64 | 4 | 16 | 4/4 | 4 | 128 | 4 | 2 |
| TUM10673 | B | 7/31/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB, qnrS1 | IncW, IncFII | 2 | 128 | 16 | 1 | 16/4 | 16 | >512 | 8 | 32 |
| TUM10674 | B | 8/9/2010 | blaIMP-1, aac(6′)-Ib, dfrA15 | IncW, IncFII, IncFIB | 0.5 | 32 | 8 | 4 | 4/4 | 4 | 128 | 4 | 2 |
| TUM10675 | B | 8/10/2010 | blaIMP-1, aac(6′)-Ib, dfrA15 | IncW, IncFII, IncFIB | 0.5 | 32 | 4 | < = 0.25 | 4/4 | 4 | 128 | 4 | 4 |
| TUM10676 | B | 8/11/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB, qnrS1 | IncW, IncFII | 2 | 64 | 8 | 1 | 8/4 | 8 | >512 | 8 | 64 |
| TUM10677 | B | 8/12/2010 | blaIMP-1, aac(6′)-Ib, dfrA15 | IncW, IncFII, IncFIB | 2 | 128 | 32 | 0.5 | 8/4 | 8 | >512 | 8 | 2 |
| TUM10678 | B | 8/12/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB, qnrS1 | IncW, IncFII | 1 | 128 | 4 | 0.5 | 4/4 | 4 | 128 | 2 | 32 |
| TUM10679 | B | 8/12/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB, qnrS1 | IncW | 1 | 64 | 8 | 4 | 8/4 | 8 | 256 | 2 | 64 |
| TUM10680 | B | 8/17/2010 | blaIMP-1, aac(6′)-Ib, strAB, qnrS1 | IncW, IncFII | 2 | 256 | 64 | < = 0.25 | 8/4 | 8 | >512 | 8 | 16 |
| TUM10683 | B | 8/18/2010 | blaIMP-1, aac(6′)-Ib, dfrA15 | IncW, IncFII, IncFIB | 0.5 | 32 | 8 | 0.5 | 4/4 | 4 | 128 | 8 | 4 |
| TUM10684 | B | 8/19/2010 | blaIMP-1, aac(6′)-Ib, sul1, dfrA15, strAB, qnrS1 | IncW, IncFII | 0.5 | 64 | 16 | 1 | 16/4 | 16 | >512 | 8 | 64 |
| TUM10685 | B | 8/19/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB, qnrS1 | IncW, IncFII | 1 | 128 | 8 | 64 | 8/4 | 8 | 256 | 2 | 64 |
| TUM10686 | B | 8/23/2010 | blaIMP-1, aac(6′)-Ib, dfrA15 | IncW, IncFII, IncFIB | 0.5 | 128 | 4 | 16 | 4/4 | 4 | 256 | 2 | 4 |
| TUM10687 | B | 8/23/2010 | blaIMP-1, aac(6′)-Ib, dfrA15 | IncW, IncFII, IncFIB | 0.5 | 256 | 4 | 32 | 4/4 | 4 | 128 | 2 | 2 |
| TUM10690 | B | 8/24/2010 | blaIMP-1, aac(6′)-Ib, sul1, dfrA15, strAB, qnrS1 | IncW, IncFII | 0.5 | 64 | 4 | 2 | 8/4 | 8 | 256 | 4 | 16 |
| TUM10691 | B | 8/24/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB, qnrS1 | IncW | 1 | 32 | 8 | 0.5 | 8/4 | 8 | 256 | 2 | 64 |
| TUM10692 | B | 8/24/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB, qnrS1 | IncW | 1 | 64 | 16 | 0.5 | 8/4 | 8 | >512 | 4 | 64 |
| TUM10693 | B | 8/30/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB | IncW | 4 | 256 | 128 | 1 | 32/4 | 32 | >512 | 8 | 4 |
| TUM10694 | B | 8/30/2010 | blaIMP-1, aac(6′)-Ib, strAB | IncW | 4 | 256 | 128 | 0.5 | 32/4 | 32 | >512 | 8 | 8 |
| TUM10695 | B | 9/1/2010 | blaIMP-1, aac(6′)-Ib, strAB, qnrS1 | IncFII, IncFIB | 0.5 | 32 | 2 | < = 0.25 | 8/4 | 8 | 64 | 4 | 32 |
| TUM10696 | B | 9/7/2010 | blaIMP-1, aac(6′)-Ib, dfrA15, strAB, qnrS1 | IncW, IncFII | 0.5 | 128 | 4 | 64 | 16/4 | 16 | 128 | 2 | 2 |
| TUM10697 | B | 9/11/2010 | blaIMP-1, aac(6′)-Ib, dfrA15 | IncW, IncFII, IncFIB | 1 | 64 | 16 | 8 | 64/4 | 64 | >512 | 4 | 32 |
| TUM10698 | B | 9/11/2010 | blaIMP-1, aac(6′)-Ib, dfrA15 | IncW, IncFII, IncFIB | 2 | 128 | 16 | 64 | 8/4 | 8 | >512 | 4 | 4 |
Antimicrobial susceptibility and resistance genes.
All MBL-producing E. hormaechei ST78 isolates were resistant to cefotaxime and moxalactam and susceptible to amikacin. The rates of resistance to piperacillin, piperacillin-tazobactam, cefepime, aztreonam, imipenem, and ciprofloxacin were 26.5% (n = 13), 26.5% (n = 13), 59.2% (n = 29), 51.0% (n = 25), 18.4% (n = 9), and 55.1% (n = 28), respectively (Table 2).
All isolates of clade 1 and clade 2 harbored blaIMP-1, aac(6′)-IIc, sul1, and tet(B). An isolate of clade 2 (TUM11137) also carried dfrA15. All isolates of clade 3 harbored blaIMP-1 and aac(6′)-Ib-cr. In addition, dfrA, strA, strB, qnaS1, and sul1 were carried by 29, 22, 22, 17, and 2 isolates of clade 3, respectively (Table 2).
Characteristics of the plasmids harboring blaIMP-1.
While replicon typing with PlasmidFinder confirmed that all isolates of clades 1 and 2 carried IncHI2 plasmids, all isolates of clade 3 except one (TUM10695) carried IncW plasmids (Table 2). blaIMP-1 and rep genes of IncW were found on the same contig in 17 isolates of clade 3. Most of the isolates of clade 3 also carried an IncFIB and/or IncFII plasmid. TUM10695 carried an IncFIB plasmid and IncFII plasmid, and blaIMP-1 and rep genes of IncFIB were found on the same contig.
Plasmids carrying blaIMP-1 were successfully transferred by conjugation in 10 of 12 clade 1 isolates (except TUM10689 and TUM10699), 2 of 4 clade 2 isolates (TUM11136 and TUM11137), and 31 of 33 clade 3 isolates (except TUM10695 and 10698). All transconjugants of clade 1 isolates and one of two transconjugants of clade 2 isolates (TUM11136) were positive for IncHI2 by PCR-based Inc/Rep typing. Transconjugants of TUM11137 were positive for IncN. All transconjugants of clade 3 isolates were positive for IncW.
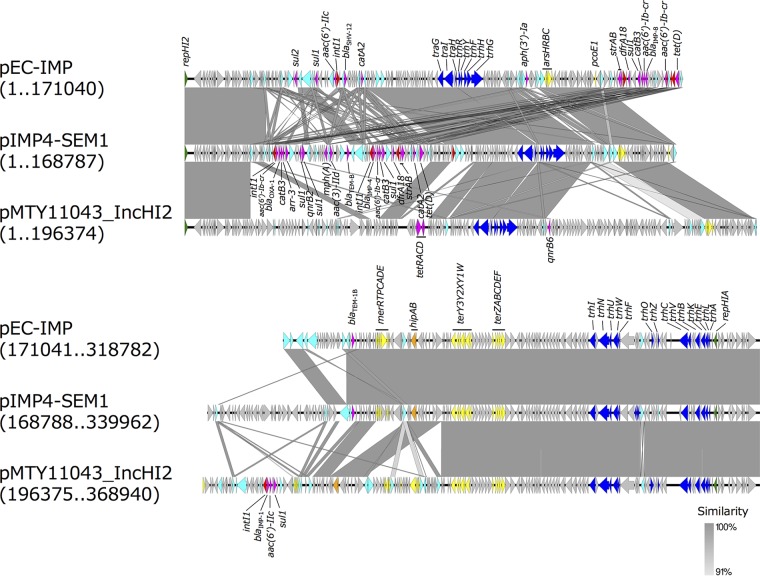
A representative plasmid of IncHI2, pMTY11043_IncHI2, from TUM11043 was 368,940 bp in length, exhibited a GC content of 47.3%, and had 425 predicted open reading frames (ORFs) (GenBank accession number AP018352.1). It carried a class 1 integron containing blaIMP-1 [In316 with blaIMP-1, aac(6′)-IIc, and sul1] and qnrB6. The backbone structure of pMTY11043_IncHI2 was highly similar to that of pIMP4-SEM1 of IMP-4-producing Salmonella enterica serovar Typhimurium (GenBank accession number KX810825) and pEC-IMP of IMP-8-producing E. cloacae (EU855787) (Fig. 2) (16, 17). pMTY11043_IncHI2, pIMP4-SEM1, and pEC-IMP carried genes for the HipBA toxin-antitoxin system (hipBA) and heavy metal ion resistance genes for arsenic (arsHRBC operon) and tellurium (terABCDEFWXYZ). Of the 11 IncHI2 plasmids carrying blaIMP-1, which were successfully transferred by conjugation, 7 were determined to be ST1 by in silico plasmid MLST (pMLST) (Table 3). The nucleotide sequences of conjugative IncHI2 plasmids carrying blaIMP-1 from isolates of clade 1 and 2 had high similarity (Fig. S2).
FIG 2.
Comparison of pMTY11043_IncHI2 (GenBank accession number AP018352.1) carrying blaIMP-1 with pEC-IMP (GenBank accession number EU855787) and pIMP4-SEM (KX810825) drawn with EasyFig, version 2.1. These IncHI2 plasmids belong to pMLST-ST1. Block arrows indicate confirmed or putative open reading frames (ORFs) and their orientations. Arrow size is proportional to the predicted ORF length. The color code is as follows: green, replication initiation protein genes; blue, conjugal transfer genes; cyan, transposase genes; yellow, heavy metal resistance genes; red, integrase genes; magenta, antibiotic resistance genes; orange, toxin-antitoxin system genes. Putative, hypothetical, and unknown genes are represented by gray arrows.
TABLE 3.
Results of pMLST of conjugative IncHI2 plasmids harboring blaIMP-1 carried by the isolates of clades 1 and 2
| Clade and plasmid | Host | Allele profile |
pMLST result | |
|---|---|---|---|---|
| smr0199 | smr0018 | |||
| Clade 1 | ||||
| pMTY11041_IncHI2 | TUM11041 | 1 | 1 | ST1 |
| pMTY11042_IncHI2 | TUM11042 | 1 | 1 | ST1 |
| pMTY11043_IncHI2 | TUM11043 | 1 | 1 | ST1 |
| pMTY11045_IncHI2 | TUM11045 | 1 | 1 | ST1 |
| pMTY11046_IncHI2 | TUM11046 | 1 | 1 | ST1 |
| pMTY11047_IncHI2 | TUM11047 | 1 | 1 | ST1 |
| pMTY11048_IncHI2 | TUM11048 | 1 | One nucleotide substitution compared with allele 1b | NDa |
| pMTY11049_IncHI2 | TUM11049 | 1 | Seven nucleotide substitutions compared with allele 5c | ND |
| pMTY11050_IncHI2 | TUM11050 | 1 | 1 | ST1 |
| pMTY11053_IncHI2 | TUM11053 | 1 | Complete nucleotide sequence of allele 1 disrupted by ISVsa5 | ND |
| Clade 2 | ||||
| pMTY11136_IncHI2 | TUM11136 | 1 | 1 | ST1 |
ND, not determined.
smr0018 sequence of pMTY11048_IncHI2 is the closest match to allele 1.
smr0018 sequence of pMTY11049_IncHI2 is the closest match to allele 5.
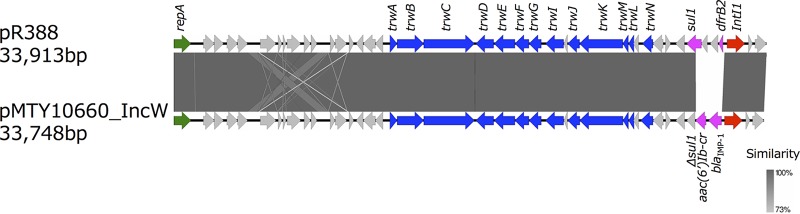
A representative plasmid of IncW, pMTY10660_IncW (GenBank accession number AP018350.1), was 33,748 bp in length, exhibited a GC content of 57.2%, and had 40 predicted ORFs. It carried a class 1 integron containing blaIMP-1 [In1426 with blaIMP-1 and aac(6′)-Ib-cr]. The nucleotide sequence of pMTY10660_IncW highly resembled that of pR388 from E. coli with trimethoprim-sulfonamide resistance (GenBank accession number BR000038) except for the contents of gene cassettes of a class 1 integron (Fig. 3).
FIG 3.
Comparison of the nucleotide sequences of pMTY10660_IncW harboring blaIMP-1 (GenBank accession number AP018350.1) and pR388 (BR000038). The color code is the same as that described in the legend of Fig. 2.
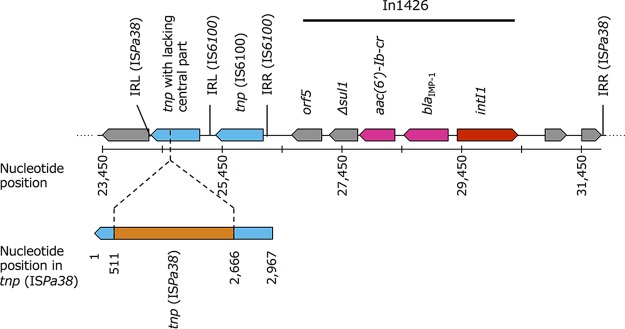
pMTY10695_IncFIB (GenBank accession number AP018351.1) was 112,622 bp in length, exhibited a GC content of 51.8%, and had 134 predicted ORFs. Large numbers of coding sequences (CDSs) were annotated as hypothetical proteins of unknown function using the DFAST automatic annotation pipeline. pMTY10695_IncFIB carried In1426-like pMTY10660_IncW. In1426 was located between the inverted repeat, left (IRL), and inverted repeat, right (IRR), of ISPa38 together with a curtailed tnpA of ISPa38 lacking the central part of the gene in pMTY10695_IncFIB (Fig. 4). The backbone structure of IncFIB plasmids from isolates of clade 3 had high similarity, except that In1426 and its surrounding structure were inserted only in pMTY10695_IncFIB (Fig. S3).
FIG 4.
Structural features of In1426, a class 1 integron containing blaIMP-1, and surrounding nucleotide sequences in pMTY10695_IncFIB (GenBank accession number AP018351.1). The insertion sequence IS6100 was located downstream of In1426. The central part of the transposase gene of ISPa38 (2,154 bp/2,967 bp) was lacking. The missing region is in orange. IRR, inverted repeat, right; IRL, inverted repeat, left.
DISCUSSION
In this study, we analyzed 71 MBL-producing ECC isolates collected from three hospitals in central Tokyo, Japan. Although all isolates carried blaIMP-1, MLST revealed that isolates of various STs contributed to the regional spread of IMP-1-producing ECC. ST78 was most common and the only clone isolated from all three hospitals. Isolates of E. hormaechei ST78 were classified into three clades by core-genome SNP-based phylogenetic analysis. Plasmids of four Inc types, IncHI2, IncW, IncN, and IncFIB, were involved in the spread of blaIMP-1.
The MLST scheme for ECC was developed by Miyoshi-Akiyama et al. (18). In that study, 101 ECC isolates collected from a hospital in Tokyo and a commercial clinical laboratory in Japan were classified by MLST, and isolates of ST78 were most common (11.9%). While criteria for the collection of the isolates in the study were not described in detail, it is possible that E. hormaechei ST78 is dominant among ECC clinical isolates in Japan regardless of their antibiotic susceptibilities. Our study showed that the proportion of the isolates of E. hormaechei ST78 was extremely high among IMP-1-producing ECC isolates collected in central Tokyo. ST78 was also one of the most common clones among the expanded-spectrum cephalosporin-resistant ECC isolates collected across Europe and Israel in a previous study (7). Isolates of E. hormaechei ST78 may have high potential to acquire genes for resistance to extended-spectrum β-lactams, as has been observed in E. coli ST131 and K. pneumoniae ST258.
While isolates of clade 3 of E. hormaechei ST78 were detected only from hospital B, isolates of clades 1 and 2 were detected from multiple hospitals. It would be unusual for a patient to visit more than one of the three hospitals participating in this study because they cover different populations. In addition, calculation of the nucleotide substitution rate revealed that clade 1 and clade 2 branched clearly in advance of the period of the isolation of the IMP-1-producing ECC isolates in this study. Therefore, the isolation of IMP-1-producing ECC isolates of the same clade in different hospitals may suggest the endemicity of clades 1 and 2 in this area. It appears that clonal spread of isolates of clade 3 was occurring in hospital B. On the other hand, isolates of clades 1 and 2 were also detected in hospital B during the 5-month period for the collection of the isolates. A similar situation was observed in an institution-wide outbreak of KPC-producing K. pneumoniae (9). Nosocomial spread of a specific outbreak strain (K. pneumoniae ST941) and sporadic influx of an endemic strain (K. pneumoniae ST258) took place simultaneously. In addition, groups of IMP-1-producing ECC isolates belonging to the same ST were recovered in all three hospitals: 8 isolates of E. hormaechei subsp. steigerwaltii ST90 in hospital A, 3 isolates of E. asburiae ST242 in hospital B, and 8 isolates of E. hormaechei subsp. steigerwaltii ST175 from hospital C together with isolates of E. hormaechei ST78. While isolates of E. hormaechei subsp. steigerwaltii ST175 from hospital C were divided into 2 groups by pulsed-field gel electrophoresis (PFGE), isolates of E. hormaechei subsp. steigerwaltii ST90 from hospital A were indistinguishable, and the same applied to isolates of E. asburiae ST242 from hospital B (data not shown). A polyclonal outbreak of KPC-producing ECC was also reported in another study (19). These findings may reflect the complexity of the molecular epidemiology of outbreaks caused by CPE.
All isolates of clades 1 and 2 of E. hormaechei ST78 carried highly similar IncHI2 plasmids harboring blaIMP-1 that were successfully transferred by conjugation even though clades 1 and 2 appeared to have diverged long ago, as mentioned above. We applied the PacBio RS system to obtain the complete sequence of a representative IncHI2 plasmid (pMTY11043_IncHI2). Detailed plasmid analysis using technologies which generate long-read data, including the PacBio RS system, has provided important information, such as the evidence for transfer of plasmids carrying carbapenemase genes in the hospital environment and complex plasmid rearrangement during long-term human colonization of CPE in previous studies (12, 20). The whole nucleotide sequence of pMTY11043_IncHI2 demonstrated that the backbone structure of the plasmid had high similarity with that of pEC-IMP harboring blaIMP-8 of an ECC clinical isolate from Taiwan and pIMP4-SEM1 harboring blaIMP-4 of S. Typhimurium from cats in Australia (16, 17). Furthermore, these IncHI2 plasmids have common genes for toxin-antitoxin systems and heavy metal resistance. Carriage of these genes may aid the consequential maintenance of the plasmids in diverse environments (21–23). Interestingly, it has been demonstrated that transfer of IncHI2 plasmids was involved in the spread of blaIMP-4 between different species of Enterobacteriaceae in previous studies from Queensland in Australia (10). These observations, together with our findings, suggest that IncHI2 may play a key role in the spread of blaIMP in Enterobacteriaceae although it remains to be elucidated whether the impact is regional or global.
Clade 3 of E. hormaechei ST78 carrying IncW plasmids harboring blaIMP-1 has been identified as an institutional outbreak clone of hospital B. Interestingly, an isolate of clade 3 from hospital B (TUM10695) lacked an IncW plasmid and carried an IncFIB plasmid harboring In1426, possibly transferred from IncW plasmids by ISPa38-mediated transposition.
Our study has several limitations. First, the number of hospitals participating in the collection of the isolates was limited, and the hospitals represented only the central area of Tokyo. Whether the epidemiology of these hospitals reflects that throughout Japan is unknown. Nevertheless, the complexity of the molecular epidemiology of the hospital-wide outbreak and the regional spread of MBL-producing ECC were demonstrated. Second, we did not collect clinical information of the patients from whom the MBL-producing ECC isolates were isolated. Active surveillance cultures might have been taken in outbreak settings together with cultures from the patients with infections. In addition, the duration of the collection of the isolates was different in each hospital. Therefore, interpretation of the significance of the number of isolates belonging to each clone is difficult.
In this study, we demonstrated that E. hormaechei ST78 was the major clone among IMP-1-producing ECC in central Tokyo. While IMP-1-producing E. hormaechei ST78 was divided into three clades by core-genome SNP-based phylogenetic analysis, isolates of clades 1 and 2 were isolated from multiple hospitals. Four types of plasmids were involved in the spread of blaIMP-1 in central Tokyo, and IncHI2 plasmids carried by isolates of clades 1 and 2 had backbone structures similar to the structure of an IncHI2 plasmid carrying blaIMP reported from other countries of the Asian-Pacific region.
MATERIALS AND METHODS
Collection of bacterial isolates.
Frozen stored MBL-producing ECC clinical isolates were sent to the Department of Microbiology and Infectious Diseases, School of Medicine, Toho University, from three university hospitals with >900 beds engaged in tertiary medical care in central Tokyo, Japan. Primary identification of bacterial species was performed with a Microscan WalkAway (Beckman Coulter, CA, USA) or Vitek2 (bioMérieux, France) instrument, and MICs were determined with Microscan WalkAway or the broth dilution method, according to CLSI guidelines, with Eiken dry plates (Eiken Chemical Co., Ltd. Tokyo, Japan) in each case (24). ECC isolates showing MICs of 4 μg/ml or higher for ceftazidime or ceftizoxime were judged as possible MBL producers, and confirmation testing was performed with ceftazidime 30-μg disks (Eiken Chemical, Tokyo, Japan) and sodium mercaptoacetate (SMA) 3-mg disks (Eiken Chemical, Tokyo, Japan). ECC isolates showing enlargement of inhibitory zone diameters around the ceftazidime disk by >5 mm when it was located adjacent to an SMA disk were determined to be MBL-producing ECC (24). These isolates were stored frozen from January 2007 to February 2011 in hospital A, from May 2010 to September 2010 in hospital B, and from July 2008 to October 2010 in hospital C.
This study was conducted with approval from the Research Ethics Board of the Toho University School of Medicine (no. 25068).
Screening of blaIMP-1-group genes by PCR.
PCR screening of blaIMP-1-group genes of MBL-producing ECC isolates was performed as reported previously (5).
Whole-genome sequencing, identification of bacterial species, and MLST.
To determine the draft whole-genome sequence of MBL-producing ECC isolates, DNA was extracted from bacteria by phenol-chloroform treatment. We used a Nextera XT DNA library preparation kit (Illumina, Inc., CA, USA) to prepare DNA libraries for sequencing. Libraries were sequenced on a MiSeq system for 600 cycles (300-bp paired-end reads). Draft genomes (contigs) were obtained using the CLC Genomics Workbench (Qiagen). Species identification was performed using average nucleotide identity (ANI) and the EzBioCloud database (25, 26). We used cutoff values of 96% (27, 28) or more and 98% or more of the ANI value compared with the genomic sequences of the type strain for species and subspecies identification, respectively. The sequence type was confirmed using BLASTn-based in silico MLST with de novo-assembled whole-genome sequencing data using E. cloacae MLST databases in PubMLST.org (http://pubmlst.org/ecloacae/) (29).
Forty-nine isolates identified as IMP-1-producing Enterobacter hormaechei ST78 were used for additional analysis as described below.
Phylogenetic analysis and estimation of evolutionary rate.
Core-genome single nucleotide polymorphism (SNP)-based phylogenetic analysis was performed with whole-genome sequencing data. The MiSeq sequencing data were aligned to the genomic sequence of the reference isolate, E. cloacae ECNIH3 ST93 (GenBank accession number CP008897), using the Burrows-Wheeler Aligner (BWA) with “MEM” option (30). The E. cloacae ECNIH3 ST97 isolate was selected as the reference isolate for mapping because it was closest to ST78 by the phylogenetic analysis based on seven MLST allele sequences (3,511 bp) among isolates with available whole-genome data sets. We constructed a core-genome alignment using SAMtools (version 1.1) mpileup (31) and VarScan (version 2.3.7) mpileup2cns (32) and then a maximum-likelihood tree using PhyML (33). Using this as the starting tree, we inferred homologous recombination events that imported DNA fragments from outside the ST and constructed a clonal phylogeny with corrected branch lengths using ClonalFrameML (34). We also conducted this analysis after excluding all of clade 3 (Fig. 1) consisting of an institutional outbreak clone in a single hospital in 2010 that seemed to evolve differently from the other strains. After excluding the recombined sequences, we estimated the average substitution rate across all sites in the core genome and the divergence time of clade 1 and clade 2 identified by the phylogenetic analysis using BEAST (version 2.4.7) (35). BEAST was run for 20 million generations, sampling every 200 states, using the general time-reversible (GTR) substitution model.
Antibiotic susceptibility testing and identification of antimicrobial resistance genes.
Antibiotic susceptibility testing was performed with the broth dilution method according to CLSI guidelines (M7-A7) (24). The following antimicrobial agents were used for antibiotic susceptibility testing: piperacillin, cefotaxime, cefepime, imipenem (all, Sigma Chemical, St. Louis, MO, USA); ciprofloxacin (LKT Laboratories, MN, USA); aztreonam (Tokyo Chemical Industry Co. Ltd., Tokyo, Japan); tazobactam (Toyama Chemical Co., Ltd., Toyama, Japan); moxalactam (Shionogi & Co., Ltd., Osaka, Japan); amikacin (Wako Pure Chemical Industries, Ltd., Tokyo, Japan). Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as the quality control strains for antibiotic susceptibility testing. The results were interpreted according to CLSI guidelines (36).
Acquired antimicrobial resistance genes were identified using the ResFinder, version 2.1, database (https://cge.cbs.dtu.dk/services/ResFinder/).
Plasmid analysis.
Plasmid incompatibility replicon typing was performed using PlasmidFinder. Conjugation experiments of blaIMP-1-carrying plasmids were performed with filter mating methods using a sodium azide-resistant mutant of the non-lactose-fermenting E. coli ML4909 strain (a rifampin-resistant mutant of E. coli K-12) as a recipient. Transconjugants were selected on Drigalski lactose agar supplied with moxalactam (16 μg/ml) and sodium azide (150 μg/ml). Carriage of blaIMP-1 by transconjugants was confirmed by PCR, and the Inc type of blaIMP-1-carrying plasmids was determined by PCR-based Inc/Rep typing (37). Conjugation was repeated up to three times if initial attempts were unsuccessful.
Complete nucleotide sequences of representative plasmids of IncW and IncFIB (pMTY10660_IncW and pMTY10695_IncFIB, respectively) were determined manually by connecting the edges of relevant contigs with PCR and Sanger sequencing. To obtain the complete sequence of a representative IncHI2 plasmid (pMTY11043_IncHI2), we used the PacBio RS system (Pacific Biosciences, Menlo Park, CA). The library was prepared using DNA Template Prep kit, version 1.0, and DNA/Polymerase Binding Kit P5 and then bound to MagBeads according to the manufacturer's instructions. Sequencing was performed with DNA Sequencing Kit C3 (Pacific Biosciences) by taking one 180-min movie for a single cell using SMRT (single-molecule, real-time) analysis. PacBio sequence reads were assembled de novo using a hierarchical genome assembly process (HGAP, version 3.0) in SMRT Pipe, version 1.85. We used the Circlator tool for automated circularization of plasmid genome assemblies using PacBio long sequencing reads (38). The annotation of the plasmid sequences was conducted by DFAST (39) and edited manually. Integrons were classified according to the Integrall database (http://integrall.bio.ua.pt/). In silico plasmid multilocus sequence typing of conjugative IncHI2 plasmids carrying blaIMP-1 was performed with whole-genome sequencing data (40). Comparison of conjugative IncHI2 plasmids carrying blaIMP-1 and IncFIB plasmids of clade 3 isolates was performed with BLAST Ring Image Generator (BRIG), version 0.95 (41). Comparison of the sequences of representative plasmids of each Inc group in this study with those of similar plasmids reported previously was performed and visualized with EasyFig, version 2.1 (42).
Accession number(s).
The results of this study were deposited in the NCBI database under BioProject number PRJDB6127. The draft genome sequences of 71 ECC isolates were deposited in DDBJ and the NCBI BioSample database under accession numbers SAMD00089454 to SAMD00089524.
Supplementary Material
ACKNOWLEDGMENTS
We express our deep appreciation to Tse Hsien Koh for his critical reading of the manuscript.
This study was supported by a grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (no. 22591113 to Y.I.).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02091-17.
REFERENCES
- 1.Tamma PD, Goodman KE, Harris AD, Tekle T, Roberts A, Taiwo A, Simner PJ. 2017. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis 64:257–264. doi: 10.1093/cid/ciw741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman ND, Carmeli Y, Walton AL, Schwaber MJ. 2017. Carbapenem-resistant Enterobacteriaceae: a strategic roadmap for infection control. Infect Control Hosp Epidemiol 38:580–594. doi: 10.1017/ice.2017.42. [DOI] [PubMed] [Google Scholar]
- 3.Yano H, Ogawa M, Endo S, Kakuta R, Kanamori H, Inomata S, Ishibashi N, Aoyagi T, Hatta M, Gu Y, Yamada M, Tokuda K, Kunishima H, Kitagawa M, Hirakata Y, Kaku M. 2012. High frequency of IMP-6 among clinical isolates of metallo-β-lactamase-producing Escherichia coli in Japan. Antimicrob Agents Chemother 56:4554–4555. doi: 10.1128/AAC.00617-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shigemoto N, Kuwahara R, Kayama S, Shimizu W, Onodera M, Yokozaki M, Hisatsune J, Kato F, Ohge H, Sugai M. 2012. Emergence in Japan of an imipenem-susceptible, meropenem-resistant Klebsiella pneumoniae carrying blaIMP-6. Diagn Microbiol Infect Dis 72:109–112. doi: 10.1016/j.diagmicrobio.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Hayakawa K, Miyoshi-Akiyama T, Kirikae T, Nagamatsu M, Shimada K, Mezaki K, Sugiki Y, Kuroda E, Kubota S, Takeshita N, Kutsuna S, Tojo M, Ohmagari N. 2014. Molecular and epidemiological characterization of IMP-type metallo-β-lactamase-producing Enterobacter cloacae in a large tertiary care hospital in Japan. Antimicrob Agents Chemother 58:3441–3450. doi: 10.1128/AAC.02652-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathers AJ, Peirano G, Pitout JDD. 2015. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev 28:565–591. doi: 10.1128/CMR.00116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izdebski R, Baraniak A, Herda M, Fiett J, Bonten MJM, Carmeli Y, Goossens H, Hryniewicz W, Brun-Buisson C, Gniadkowski M, MOSAR WP2, WP3 and WP5 Study Groups. 2015. MLST reveals potentially high-risk international clones of Enterobacter cloacae. J Antimicrob Chemother 70:48–56. doi: 10.1093/jac/dku359. [DOI] [PubMed] [Google Scholar]
- 8.Matsumura Y, Peirano G, Motyl MR, Adams MD, Chen L, Kreiswirth B, DeVinney R, Pitout JDD. 2017. Global molecular epidemiology of IMP-producing Enterobacteriaceae. Antimicrob Agents Chemother 61:e02729-16. doi: 10.1128/AAC.02729-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathers AJ, Stoesser N, Sheppard AE, Pankhurst L, Giess A, Yeh AJ, Didelot X, Turner SD, Sebra R, Kasarskis A, Peto T, Crook D, Sifri CD. 2015. Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae at a single institution: insights into endemicity from whole-genome sequencing. Antimicrob Agents Chemother 59:1656–1663. doi: 10.1128/AAC.04292-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sidjabat HE, Heney C, George NM, Nimmo GR, Paterson DL. 2014. Interspecies transfer of blaIMP-4 in a patient with prolonged colonization by IMP-4-producing Enterobacteriaceae. J Clin Microbiol 52:3816–3818. doi: 10.1128/JCM.01491-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerqueira GC, Earl AM, Ernst CM, Grad YH, Dekker JP, Feldgarden M, Chapman SB, Reis-Cunha JL, Shea TP, Young S, Zeng Q, Delaney ML, Kim D, Peterson EM, O'Brien TF, Ferraro MJ, Hooper DC, Huang SS, Kirby JE, Onderdonk AB, Birren BW, Hung DT, Cosimi LA, Wortman JR, Murphy CI, Hanage WP. 2017. Multi-institute analysis of carbapenem resistance reveals remarkable diversity, unexplained mechanisms, and limited clonal outbreaks. Proc Natl Acad Sci U S A 114:1135–1140. doi: 10.1073/pnas.1616248114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conlan S, Thomas PJ, Deming C, Park M, Lau AF, Dekker JP, Snitkin ES, Clark TA, Luong K, Song Y, Tsai Y-C, Boitano M, Dayal J, Brooks SY, Schmidt B, Young AC, Thomas JW, Bouffard GG, Blakesley RW, NISC Comparative Sequencing Program, Mullikin JC, Korlach J, Henderson DK, Frank KM, Palmore TN, Segre JA. 2014. Single-molecule sequencing to track plasmid diversity of hospital-associated carbapenemase-producing Enterobacteriaceae. Sci Transl Med 6:254ra12. doi: 10.1126/scitranslmed.3009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wailan AM, Paterson DL, Kennedy K, Ingram PR, Bursle E, Sidjabat HE. 2015. Genomic characteristics of NDM-producing Enterobacteriaceae isolates in Australia and their blaNDM genetic contexts. Antimicrob Agents Chemother 60:136–141. doi: 10.1128/AAC.01243-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poirel L, Bonnin RA, Nordmann P. 2012. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother 56:559–562. doi: 10.1128/AAC.05289-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoesser N, Sheppard AE, Peirano G, Sebra R, Lynch T, Anson L, Kasarskis A, Motyl MR, Crook DW, Pitout JD. 2016. Complete sequencing of plasmids containing blaOXA-163 and blaOXA-48 in Escherichia coli sequence type 131. Antimicrob Agents Chemother 60:6948–6951. doi: 10.1128/AAC.01130-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y-T, Liao T-L, Liu Y-M, Lauderdale T-L, Yan J-J, Tsai S-F. 2009. Mobilization of qnrB2 and ISCR1 in plasmids. Antimicrob Agents Chemother 53:1235–1237. doi: 10.1128/AAC.00970-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraham S, O'Dea M, Trott DJ, Abraham RJ, Hughes D, Pang S, McKew G, Cheong EYL, Merlino J, Saputra S, Malik R, Gottlieb T. 2016. Isolation and plasmid characterization of carbapenemase (IMP-4) producing Salmonella enterica Typhimurium from cats. Sci Rep 6:35527. doi: 10.1038/srep35527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyoshi-Akiyama T, Hayakawa K, Ohmagari N, Shimojima M, Kirikae T. 2013. Multilocus sequence typing (MLST) for characterization of Enterobacter cloacae. PLoS One 8:e66358. doi: 10.1371/journal.pone.0066358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haraoui LP, Levesque S, Lefebvre B, Blanchette R, Tomkinson M, Mataseje L, Mulvey MR, Miller MA. 2013. Polyclonal outbreak of KPC-3-producing Enterobacter cloacae at a single hospital in Montreal, Quebec, Canada. J Clin Microbiol 51:2406–2408. doi: 10.1128/JCM.02480-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conlan S, Park M, Deming C, Thomas PJ, Young AC, Coleman H, Sison C, NISC Comparative Sequencing Program, Weingarten RA, Lau AF, Dekker JP, Palmore TN, Frank KM, Segre JA. 2016. Plasmid dynamics in KPC-positive Klebsiella pneumoniae during long-term patient colonization. mBio 7:e00742-16. doi: 10.1128/mBio.00742-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maisonneuve E, Gerdes K. 2014. Molecular mechanisms underlying bacterial persisters. Cell 157:539–548. doi: 10.1016/j.cell.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 22.Mourão J, Marçal S, Ramos P, Campos J, Machado J, Peixe L, Novais C, Antunes P. 2016. Tolerance to multiple metal stressors in emerging non-typhoidal MDR Salmonella serotypes: a relevant role for copper in anaerobic conditions. J Antimicrob Chemother 71:2147–2157. doi: 10.1093/jac/dkw120. [DOI] [PubMed] [Google Scholar]
- 23.Gullberg E, Albrecht LM, Karlsson C, Sandegren L, Andersson DI. 2014. Selection of a multidrug resistance plasmid by sublethal levels of antibiotics and heavy metals. mBio 5:e01918–14. doi: 10.1128/mBio.01918-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2006. Methods for Dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—seventh edition. CLSI document M7-A7. Clinical and Laboratory Standards Institute, Wayne, Pa. [Google Scholar]
- 25.Yoon S-H, Ha S-M, Kwon S, Lim J, Kim Y, Seo H, Chun J. 2017. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol 67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter M, Rosselló-Móra R. 2009. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A 106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim M, Oh H-S, Park S-C, Chun J. 2014. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol 64:346–351. doi: 10.1099/ijs.0.059774-0. [DOI] [PubMed] [Google Scholar]
- 28.Chavda KD, Chen L, Fouts DE, Sutton G, Brinkac L, Jenkins SG, Bonomo RA, Adams MD, Kreiswirth BN. 2016. Comprehensive genome analysis of carbapenemase-producing Enterobacter spp.: new insights into phylogeny, population structure, and resistance mechanisms. mBio 7:e02093-16. doi: 10.1128/mBio.02093-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK. 2012. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 34.Didelot X, Wilson DJ. 2015. ClonalFrameML: Efficient inference of recombination in whole bacterial genomes. PLoS Comp Biol 11:e1004041. doi: 10.1371/journal.pcbi.1004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu C-H, Xie D, Suchard MA, Rambaut A, Drummond AJ. 2014. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comp Biol 10:e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CLSI, Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 37.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Hunt M, Silva ND, Otto TD, Parkhill J, Keane JA, Harris SR. 2015. Circlator: automated circularization of genome assemblies using long sequencing reads. Genome Biol 16:294. doi: 10.1186/s13059-015-0849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanizawa Y, Fujisawa T, Kaminuma E, Nakamura Y, Arita M. 2016. DFAST and DAGA: web-based integrated genome annotation tools and resources. Biosci Microbiota Food Health 35:173–184. doi: 10.12938/bmfh.16-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, Møller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alikhan N-F, Petty NK, Ben Zakour NL, Beatson SA. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.