ABSTRACT
We investigated the effect of replicative aging on antifungal resistance in Candida glabrata. Our studies demonstrate significantly increased transcription of ABC transporters and efflux pump activity in old versus young C. glabrata cells of a fluconazole-sensitive and -resistant strain. In addition, higher tolerance to killing by micafungin and amphotericin B was noted and is associated with higher transcription of glucan synthase gene FKS1 and lower ergosterol content in older cells.
KEYWORDS: Candida glabrata, aging, antifungal resistance, drug resistance mechanisms, efflux pumps
TEXT
Candida glabrata is a formidable pathogen that, analogous to other yeasts, undergoes replicative aging (1). We have reported that growth of older (defined as more generations lived) C. glabrata cells was more inhibited by fluconazole (FLC) (1). This finding is relevant because, in vivo, the host's neutrophils selectively kill younger cells, which causes old yeast cells to accumulate in the host. Aging C. glabrata mother cells have larger cell bodies and thicker cell walls than those of the younger daughter cells (1).
The specific mechanism that underlies the observed age-associated FLC resistance in old C. glabrata cells is not known. Azole resistance can have different underlying mechanisms, some of which may act in combination (2). Although mutations of ERG11 and PDR1 can mediate azole resistance, these mutations would be inherited by the daughter cells and persist (3–5). Data from Saccharomyces cerevisiae indicate that genomic stability rather than mutation rate changes during replicative aging (6). Other studies have reported that C. glabrata subpopulations originating from a clonal population can develop heteroresistance to FLC through transient upregulation of efflux pumps (7, 8). Active efflux of azoles is accomplished by membrane transporters, which belong to one of the two superfamilies in fungi: the ATP-binding-cassette superfamily (ABC) and the major facilitator superfamily (MFS) (9–11). Several ABC transporters have been linked to drug resistance in C. glabrata, of which Cdr1p and Pdh1p have been extensively investigated (12–16). Previously published transcriptome data comparing young and 14-generation-old C. glabrata cells of the FLC-sensitive BG2 strain demonstrated increased expression of the genes CDR1 (2-fold), PDR16 (8-fold), and YBT1 (10-fold), all of which encode ABC transporters associated with FLC resistance (1).
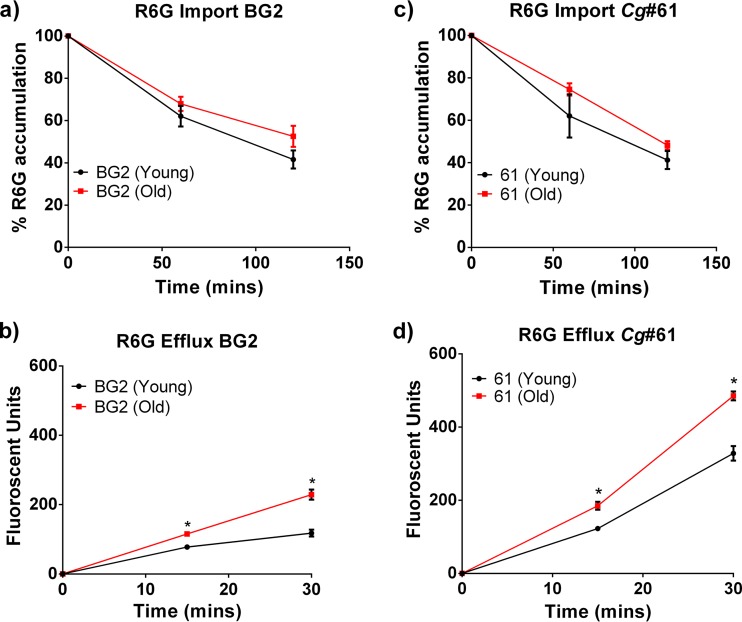
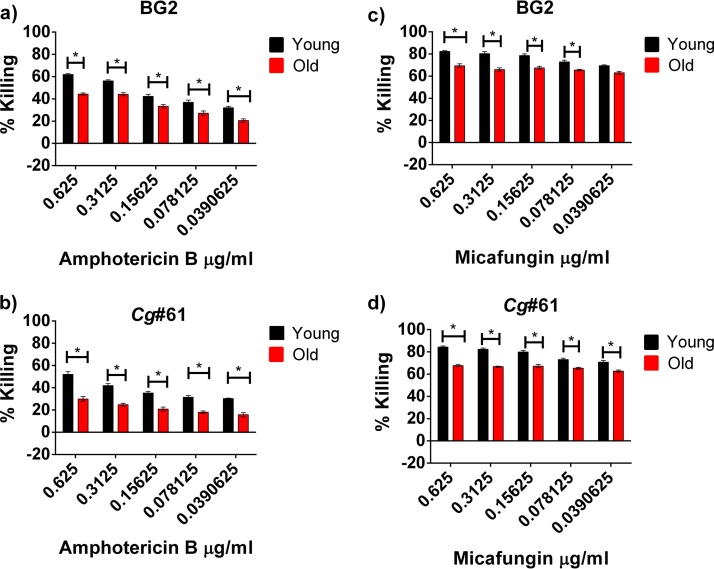
In this study, we investigated these findings further. Young and old C. glabrata cells were isolated by first labeling 108 exponentially growing cells with biotin and then allowing growth for six generations. Biotin-labeled C. glabrata cells were then bound by streptavidin-linked magnetic beads and sorted on a column. A purity of >90% in the samples was verified with fluorescein isothiocyanate-streptavidin biotin stain as described previously (17). The number of generations was calculated from the number of doubling times of the initial population. First, the expression of ABC transporters was quantified in young and old cells of a clinical FLC-resistant C. glabrata strain (Cg#61 MIC, 64 μg/ml). RNA was extracted from 14-generation-old Cg#61 cells, which were isolated by magnetic-bead column separation as described previously (1). The cDNA levels of specific ABC transporters were quantified by reverse transcription-quantitative PCR (qRT-PCR) using transporter gene-specific primers (see Table S1 in the supplemental material). Again, significantly increased expression of CDR1 (49-fold), PDR16 (4.5-fold), and YBT1 (76-fold) was documented in older cells (see Fig. S1 in the supplemental material). Next, a functional analysis of efflux activity was performed with the ABC-transporter-specific fluorescent dye rhodamine 6G (R6G) (Sigma-Aldrich) following a previously published protocol (18). Briefly, 106 young and 14-generation-old cells of both BG2 and Cg#61 cells were starved under deenergized conditions for 2 h in 1× phosphate-buffered saline (PBS)-containing 5 mM 2-deoxyglucose (Sigma-Aldrich). After 2 h, R6G was added to the young and old cells at a final concentration of 1 μM, and the samples were collected every 30 mins for 2 h. The samples were spun down, and R6G import was measured as the decrease in fluorescence of the supernatants over time in a spectrometer (excitation of 525 nm and emission at 555 nm). These data showed that old and young cells of both strains accumulated comparable amounts of R6G at a similar rate (Fig. 1), demonstrating that the thicker cell wall of older cells did not interfere with import. After 2 h of import, the cells were washed with PBS, and 2% glucose was added to initiate the efflux. The samples were collected every 15 mins for a total efflux time of 30 mins and spun down, and R6G efflux was measured as the increase in fluorescence of the supernatant over time. In these assays, old cells of FLC-sensitive and -resistant C. glabrata isolates showed significant increases in R6G efflux pump activity compared with that in their younger daughter cells (Fig. 1). As predicted by qRT-PCR data, Cg#61 cells exhibited a higher baseline efflux and more pronounced upregulation of efflux with aging. Last, we explored whether the in vitro activities of echinocandins and amphotericin B were reduced in older C. glabrata cells, because the cellular levels of their specific targets, namely, ergosterol and β-1,3-glucan, were altered by aging (1). However, only the expression of FKS1 and not that of FKS2 was altered between the young and old C. glabrata cells (1). Specifically, in the older cells, ergosterol content was decreased as a result of redirection of the ergosterol pathway to upstream or side pathways (1), whereas β-1,3-glucan content was increased as a result of increased glucan synthase (FKS1) transcription (1), by 29-fold in BG2 and 9-fold in Cg#61 cells (see Fig. S1). Antifungal efficacy was assessed in standard killing assays, where 104 cells/ml of young and 14-generation-old C. glabrata were exposed to decreasing concentrations of amphotericin B and micafungin for 2 h. Higher tolerance of old BG2 and Cg#61 cells was documented for both drugs after plating 100 μl of drug-treated cells on 25 ml yeast extract-peptone-dextrose (YPD) medium (Fig. 2).
FIG 1.
R6G import (a, c) and efflux (b, d) were characterized in old (14 generations) and young (0 to 3 generations) cells of strains BG2 (a, b) and Cg#61 (c, d) as described previously (18). The assays were performed in triplicate. Error bars, standard deviations between replicates. All data points were subjected to multiple t tests using the Holm-Sidak method. *, P < 0.05.
FIG 2.
Antifungal killing assay. A total of 104 cells/ml of old (14 generations) and young (0 to 3 generations) cells of both BG2 (a, c), and Cg#61 (b, d) strains were exposed to amphotericin B (a, b) and micafungin (c, d) for 2 h and incubated at 37°C. After incubation, 100 μl cells from each condition were plated in 25 ml YPD medium and incubated at 37°C for 48 h. CFU were then counted, and the percentages of killings were calculated and plotted. Old and young cells with no exposure to drugs were used as controls. Shaded bars, specific concentration of the individual drugs used in these assays. The assays were performed in triplicate. Error bars, standard deviations between replicates. Multiple t tests using the Holm-Sidak method were used to analyze the statistical significance of the data. *, P < 0.05.
Our data indicate that enhanced tolerance to different antifungal drug classes can be observed in two different C. glabrata strains, when aged to 14 generations. Enhanced FLC and amphotericin B resistance was also found in older Cryptococcus neoformans cells, which accumulate in spinal fluid during chronic infection (19). Similarly, enhanced caspofungin resistance of old Candida albicans cells has been described (20).
Although the changes in drug tolerances between young and old Candida cells seem modest (Fig. 2), differential killing or growth inhibition of young and old cells would magnify increased accumulation of older cells over time in drug-treated patients, and such persistence may potentially contribute to therapeutic failure. This may be most relevant when static drugs such as FLC are used. Our data suggest that enhanced FLC resistance in older C. glabrata cells is the result of enhanced efflux pump activities of ABC transporters. MFS transporters were not assessed but may contribute to enhanced FLC efflux in that some were found to be upregulated in aging cells. Why ABC transporters are overexpressed in older C. glabrata cells remains unknown, and no studies have linked increased levels of the transcription factor Pdr1p and increased ABC transporter transcription. Interestingly, in S. cerevisiae, ABC transporter Pdr5p is inherited asymmetrically by the daughter cells, and therefore they exhibit enhanced efflux activity compared with that in old S. cerevisiae cells (21). Gene amplification secondary to age-induced genomic instability must also be taken into account. Aneuploidy by the gain of small chromosomes and segmental aneuploidy have been described in resistant C. glabrata isolates, especially in the left arm of chromosome F, which encodes the ABC transporters AUS1 and PDH1 (22). Whether higher levels of the target enzyme Fks1p contribute to enhanced tolerance to micafungin needs further investigation. Mutations in FKS1 and FKS2 are predominantly implicated in micafungin resistance (23). However, in resistant C. glabrata strains, a decrease in enzyme function was associated with a relative increase in FKS1 expression (24). Additionally, in C. albicans biofilms, overexpression of FKS1 was associated with resistance (25). Last, amphotericin B resistance in Candida lusitaniae is associated with elevated ERG6 levels and lower ergosterol (26), so the observed resistance to killing was expected. Our data are the first to show that efflux pump activity is upregulated by cell aging. Further research is required to determine whether this transient phenotypic resistance as a result of aging followed by selection of old C. glabrata cells contributes to treatment failure. Note that it would not be diagnosed by standard antifungal testing because that is performed with young in vitro-grown Candida populations. This research is important because drug resistance related to ABC transporters is clinically relevant in pathogenic fungi.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Tamas Szekely for helpful discussions.
This research was funded by the National Institutes of Health (NIH 1R01AI127704-01A1 to B.C.F.).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02227-17.
REFERENCES
- 1.Bouklas T, Alonso-Crisostomo L, Szekely T Jr, Diago-Navarro E, Orner EP, Smith K, Munshi MA, Del Poeta M, Balazsi G, Fries BC. 2017. Generational distribution of a Candida glabrata population: resilient old cells prevail, while younger cells dominate in the vulnerable host. PLoS Pathog 13:e1006355. doi: 10.1371/journal.ppat.1006355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whaley SG, Rogers PD. 2016. Azole resistance in Candida glabrata. Curr Infect Dis Rep 18:41. doi: 10.1007/s11908-016-0554-5. [DOI] [PubMed] [Google Scholar]
- 3.Vermitsky JP, Earhart KD, Smith WL, Homayouni R, Edlind TD, Rogers PD. 2006. Pdr1 regulates multidrug resistance in Candida glabrata: gene disruption and genome-wide expression studies. Mol Microbiol 61:704–722. doi: 10.1111/j.1365-2958.2006.05235.x. [DOI] [PubMed] [Google Scholar]
- 4.Vermitsky JP, Edlind TD. 2004. Azole resistance in Candida glabrata: coordinate upregulation of multidrug transporters and evidence for a Pdr1-like transcription factor. Antimicrob Agents Chemother 48:3773–3781. doi: 10.1128/AAC.48.10.3773-3781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva DB, Rodrigues LM, Almeida AA, Oliveira KM, Grisolia AB. 2016. Novel point mutations in the ERG11 gene in clinical isolates of azole resistant Candida species. Mem Inst Oswaldo Cruz 111:192–199. doi: 10.1590/0074-02760150400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaya ALA, Gladyshev VN. 2015. Evidence that mutation accumulation does not cause aging in Saccharomyces cerevisiae. Aging Cell 14:366–371. doi: 10.1111/acel.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Ami R, Zimmerman O, Finn T, Amit S, Novikov A, Wertheimer N, Lurie-Weinberger M, Berman J. 2016. Heteroresistance to fluconazole is a continuously distributed phenotype among Candida glabrata clinical strains associated with in vivo persistence. mBio 7:pii=e00655-16. doi: 10.1128/mBio.00655-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claudino AL, Peixoto Junior RF, Melhem MS, Szeszs MW, Lyon JP, Chavasco JK, Franco MC. 2009. Mutants with heteroresistance to amphotericin B and fluconazole in Candida. Braz J Microbiol 40:943–951. doi: 10.1590/S1517-83822009000400028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sa-Correia I, dos Santos SC, Teixeira MC, Cabrito TR, Mira NP. 2009. Drug:H+ antiporters in chemical stress response in yeast. Trends Microbiol 17:22–31. doi: 10.1016/j.tim.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Cannon RD, Lamping E, Holmes AR, Niimi K, Baret PV, Keniya MV, Tanabe K, Niimi M, Goffeau A, Monk BC. 2009. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev 22:291–321, table of contents. doi: 10.1128/CMR.00051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morschhauser J. 2010. Regulation of multidrug resistance in pathogenic fungi. Fungal Genet Biol 47:94–106. doi: 10.1016/j.fgb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Song JW, Shin JH, Kee SJ, Kim SH, Shin MG, Suh SP, Ryang DW. 2009. Expression of CgCDR1, CgCDR2, and CgERG11 in Candida glabrata biofilms formed by bloodstream isolates. Med Mycol 47:545–548. doi: 10.1080/13693780802210726. [DOI] [PubMed] [Google Scholar]
- 13.Shahrokhi S, Noorbakhsh F, Rezaie S. 2017. Quantification of CDR1 gene expression in fluconazole resistant Candida glabrata strains using real-time PCR. Iran J Public Health 46:1118–1122. [PMC free article] [PubMed] [Google Scholar]
- 14.Sanglard D, Ischer F, Calabrese D, Majcherczyk PA, Bille J. 1999. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob Agents Chemother 43:2753–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Culakova H, Dzugasova V, Valencikova R, Gbelska Y, Subik J. 2015. Stress response and expression of fluconazole resistance associated genes in the pathogenic yeast Candida glabrata deleted in the CgPDR16 gene. Microbiol Res 174:17–23. doi: 10.1016/j.micres.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Kaur R, Castano I, Cormack BP. 2004. Functional genomic analysis of fluconazole susceptibility in the pathogenic yeast Candida glabrata: roles of calcium signaling and mitochondria. Antimicrob Agents Chemother 48:1600–1613. doi: 10.1128/AAC.48.5.1600-1613.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain N, Cook E, Xess I, Hasan F, Fries D, Fries BC. 2009. Isolation and characterization of senescent Cryptococcus neoformans and implications for phenotypic switching and pathogenesis in chronic cryptococcosis. Eukaryot Cell 8:858–866. doi: 10.1128/EC.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharya S, Sobel JD, White TC. 2016. A combination fluorescence assay demonstrates increased efflux pump activity as a resistance mechanism in azole-resistant vaginal Candida albicans isolates. Antimicrob Agents Chemother 60:5858–5866. doi: 10.1128/AAC.01252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouklas T, Pechuan X, Goldman DL, Edelman B, Bergman A, Fries BC. 2013. Old Cryptococcus neoformans cells contribute to virulence in chronic cryptococcosis. mBio 4:pii=e00455-13. doi: 10.1128/mBio.00455-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seneviratne CJ, Jin LJ, Samaranayake YH, Samaranayake LP. 2008. Cell density and cell aging as factors modulating antifungal resistance of Candida albicans biofilms. Antimicrob Agents Chemother 52:3259–3266. doi: 10.1128/AAC.00541-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azbarova AV, Galkina KV, Sorokin MI, Severin FF, Knorre DA. 2017. The contribution of Saccharomyces cerevisiae replicative age to the variations in the levels of Trx2p, Pdr5p, Can1p and Idh isoforms. Sci Rep 7:13220. doi: 10.1038/s41598-017-13576-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polakova S, Blume C, Zarate JA, Mentel M, Jorck-Ramberg D, Stenderup J, Piskur J. 2009. Formation of new chromosomes as a virulence mechanism in yeast Candida glabrata. Proc Natl Acad Sci U S A 106:2688–2693. doi: 10.1073/pnas.0809793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Effron G, Lee S, Park S, Cleary JD, Perlin DS. 2009. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-beta-d-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob Agents Chemother 53:3690–3699. doi: 10.1128/AAC.00443-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nett JE, Crawford K, Marchillo K, Andes DR. 2010. Role of Fks1p and matrix glucan in Candida albicans biofilm resistance to an echinocandin, pyrimidine, and polyene. Antimicrob Agents Chemother 54:3505–3508. doi: 10.1128/AAC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young LY, Hull CM, Heitman J. 2003. Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob Agents Chemother 47:2717–2724. doi: 10.1128/AAC.47.9.2717-2724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.