Abstract
Background
Patients with acute pulmonary embolism (PE) exhibit wide variation in clinical presentation and outcomes. Our understanding of the pathophysiologic mechanisms differentiating low-risk and high-risk PE is limited, so current risk-stratification efforts often fail to predict clinical deterioration and are insufficient to guide management.
Objectives
To improve our understanding of the physiology differentiating low-risk from high-risk PE, we conducted the first-ever high-throughput metabolomics analysis (843 named metabolites) comparing PE patients across risk strata within a nested case-control study.
Patients/Methods
We enrolled 92 patients diagnosed with acute PE and collected plasma within 24 hours of PE diagnosis. We used linear regression and pathway analysis to identify metabolites and pathways associated with PE risk-strata.
Results
When we compared 46 low-risk to 46 intermediate/high-risk PE, 50 metabolites were significantly different after multiple testing correction. These metabolites were enriched in the following pathways: TCA Cycle, Fatty Acid Metabolism (Acyl Carnitine), and Purine Metabolism, (Hypo)Xanthine/Inosine Containing. Additionally, Energy, Nucleotide, and Amino Acid pathways were down-regulated in intermediate/high-risk PE patients. When we compared 28 intermediate-risk to 18 high-risk PE patients, 41 metabolites differed at a nominal p-value level. These metabolites were enriched in Fatty Acid Metabolism (Acyl Cholines), and Hemoglobin and Porphyrin Metabolism.
Conclusion
Our results suggest that high-throughput metabolomics can provide insight into the pathophysiology of PE. Specifically, changes in circulating metabolites reflect compromised energy metabolism in intermediate/high-risk PE patients. These findings demonstrate the important role metabolites play in the pathophysiology of PE and highlight metabolomics as a potential tool for risk stratification of PE.
Keywords: Pulmonary Embolism, Venous Thromboembolism, Metabolomics, Metabolism, Risk
Introduction
Patients with acute pulmonary embolism (PE) exhibit wide variation in both clinical presentation and outcomes. Mortality ranges from <1% for low-risk PE patients to >50% for high-risk/massive PE patients who present with cardiovascular collapse or shock [1]. For intermediate-risk/submassive patients who present with large PE and evidence of right ventricular (RV) dysfunction, but are hemodynamically stable, mortality is estimated at 7.7%–15% [1, 2].
Currently, clinicians risk-stratify patients using a combination of imaging tests (e.g. echocardiography, computed tomography pulmonary angiography [CTPA]) and biomarkers (e.g. troponin and brain natriuretic peptides) to identify RV dysfunction. However, measures of RV dysfunction often fail to predict clinical deterioration and are insufficient to guide management in many cases. Other intuitive measures, such as clot burden, are also inadequate predictors of adverse outcomes after PE [2–4]. Our ability to risk-stratify patients is limited by our understanding of the pathophysiology of clinical deterioration after PE; including the metabolic changes associated with acute thromboembolism. A better understanding of the physiology that differentiates low-risk from high-risk PE is needed to improve our ability to risk-stratify patients.
Using modern, high-throughput metabolomics, we can now assess a broad array of metabolites and metabolic profiles associated with disease risk and changes in physiologic status. However, metabolomics technology has not yet been used to compare patients across PE risk-strata. We performed the first high-throughput metabolomics study comparing PE patients across different risk-strata (low-, intermediate- and high-risk), with the goal of identifying specific metabolites and pathways that are associated with these clinically relevant PE subtypes.
Materials/Methods
Study population
We performed a case-control study nested within a prospective non-interventional cohort study of consecutive adult patients (age ≥18 years) who presented to the Emergency Department of Massachusetts General Hospital and diagnosed with PE between October 2008 and December 2011. Details of enrollment have been described previously [5]. For the current analysis, all enrolled patients had radiographically diagnosed PE, defined as a filling defect in a pulmonary artery on CTPA. Patients were eligible if PE was diagnosed within 24 hours of Emergency Department registration. Patients were excluded if they were <18 years old, did not provide informed consent or were not available for follow up (e.g. homeless, prisoners).
Study staff used a standard data collection form to abstract the following information from medical records: demographic data, comorbid illnesses, vital signs, laboratory results, radiological findings and treatments. The study was approved by the Human Research Committee of Partners HealthCare (protocol 2008-P-002001). All patients provided written informed consent prior to blood sample collection.
Blood samples were obtained from enrolled patients within 24 hours of PE diagnosis (based on the time CTPA was performed). Samples were collected in potassium EDTA tubes, centrifuged, transferred to 500µL vials and stored in a −70°C freezer. Samples were kept frozen until they were shipped for metabolomic analysis on dry ice. For the current analysis, we only included patients with a blood sample that had been processed and frozen within 60 minutes of collection.
To gather data used to classify study patients as to whether they had low/intermediate/high-risk PE, study staff reviewed medical records and interviewed patients and their clinicians every day for five days after enrollment. We also performed follow up using a validated combination of telephone calls and computer medical record review on days five and 30 to identify deaths, recurrent PE or bleeding events after hospital discharge [6].
Forty-six intermediate/high-risk PE patients met the study criteria and had blood samples available that were processed within 60 minutes of blood draw. These patients were 1:1 matched to 46 low-risk patients on age, gender, and cancer status (any vs. no history of malignancy). Intermediate/high-risk PE were defined as PE in a lobar or main pulmonary artery with: 1) hemodynamic instability (hypotension, pulselessness, persistent profound bradycardia, or need for advanced cardiac life support); 2) evidence of RV dysfunction (RV dilatation, hypokinesis or septal bowing on echocardiogram); 3) elevated N-terminal pro-brain natriuretic peptide (NT-proBNP) >500 pg/ml; or 4) myocardial necrosis (troponin t >0.1 ng/ml). Low-risk PE was defined as segmental or smaller PE without markers of adverse prognosis used to define intermediate/high-risk PE. Intermediate/high-risk PE was then divided into intermediate- and high-risk, with high-risk PE defined as PE with persistent hypotension or need for vasopressor therapy, need for advanced cardiac life support, occurrence of an unstable cardiac dysrhythmia, need for thrombolysis or thrombectomy, need for positive pressure ventilation, or death. Among the 46 intermediate- and high-risk patients, 18 (39%) had high-risk PE and 28 (61%) had intermediate-risk PE. Demographics of the analyzed patients are shown in Table 1.
Table 1.
Demographics of Analyzed Patients by PE* Risk Category.
| Low-Risk PE 46 patients |
Intermediate/ High-Risk PE 46 patients |
Intermediate- Risk PE 28 patients |
High-Risk PE 18 patients |
|||||
|---|---|---|---|---|---|---|---|---|
| Age (mean, SD†) | 62 | 15 | 62 | 16 | 61 | 18 | 64 | 12 |
| n | % | n | % | n | % | n | % | |
| Female | 20 | 43% | 20 | 43% | 13 | 46% | 7 | 39% |
| Race: | ||||||||
| White | 43 | 94% | 41 | 89% | 25 | 89% | 16 | 89% |
| Black | 2 | 4% | 5 | 11% | 3 | 11% | 2 | 11% |
| Other | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| Ethnicity: | ||||||||
| Non-Hispanic | 45 | 98% | 46 | 100% | 28 | 100% | 18 | 100% |
| Hispanic | 1 | 2% | 0 | 0% | 0 | 0% | 0% | 0% |
| Insurance: | ||||||||
| Private | 27 | 59% | 32 | 70% | 20 | 72% | 12 | 67% |
| Medicare | 17 | 37% | 11 | 24% | 6 | 21% | 5 | 28% |
| Other | 2 | 4% | 3 | 6% | 2 | 7% | 1 | 5% |
| Comorbidities: | ||||||||
| Coronary Artery Disease‡ | 6 | 13% | 3 | 6% | 3 | 11% | 0 | 0% |
| Congestive Heart Failure | 3 | 6% | 4 | 9% | 1 | 4% | 3 | 17% |
| Lung Disease § | 10 | 22% | 10 | 22% | 6 | 21% | 4 | 22% |
| Asthma | 5 | 11% | 4 | 9% | 2 | 7% | 2 | 11% |
| COPD | 3 | 6% | 2 | 4% | 1 | 4% | 1 | 6% |
| Other | 2 | 4% | 5 | 11% | 5 | 18% | 0 | 0% |
| Malignancy¶ | 19 | 41% | 20 | 43% | 9 | 32% | 11 | 61% |
| Active/Palliative | 8 | 17% | 8 | 17% | 1 | 4% | 7 | 39% |
| Inactive | 10 | 22% | 12 | 26% | 8 | 29% | 4 | 22% |
| Status Unknown | 1 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| Renal Insufficiency or Failure** | 1 | 2% | 2 | 4% | 2 | 7% | 0 | 0% |
| Cerebrovascular Disease†† | 4 | 9% | 4 | 9% | 3 | 11% | 1 | 6% |
Table 1 Notes:
PE = Pulmonary Embolism;
SD = Standard Deviation;
COPD = Coronary Artery Disease includes any history of myocardial infarction, angina, coronary artery stenting or bypass surgery
Asthma, COPD and Other lung disease are not mutually exclusive.
Active malignancy includes any cancer that is being actively treated or palliated, and metastatic disease. Inactive cancer includes a prior history of cancer that is not actively being treated or palliated.
Renal insufficiency or failure includes renal insufficiency, renal failure and hemodialysis.
Cerebrovascular disease includes any history of ischemic or hemorrhagic stroke or transient ischemic attack.
Metabolomics assays
Metabolomics assays were conducted at Metabolon (Morrisville, NC, USA) using tandem ultra-performance liquid chromatography followed by mass spectrometry (UPLC/MS). UPLC/MS was done using an ACQUITY ultra-performance liquid chromatography (UPLC) (Waters Corporation, MA, USA) coupled to an Q-Exactive high resolution/accurate mass spectrometer (Thermo Scientific Corporation, MA, USA) interfaced with heated electrospray ionization [7] and Orbitrap mass analyzer operated at 35,000 mass resolution. These metabolomics assays have been previously described in detail [8]. Briefly, after sample accession and preparation, each sample was divided into one backup aliquot and four aliquots for the different measurement methods: 1) acidic positive ion conditions optimized for more hydrophilic compounds, 2) acidic positive ion conditions optimized for more hydrophobic compounds, 3) basic negative ion optimized conditions using a separate dedicated C18 column, and 4) negative ionization following elution from a HILIC column. Compounds were identified by comparison to library entries of purified standards or recurrent unknown entities. Peaks were quantified using area-under-the-curve. Several normalization steps were performed including normalization to account for inter-day tuning differences and for volume available/utilized for extraction.
Statistical Analyses
We used multivariable linear regression to identify metabolites that differed between low-risk and intermediate/high-risk PE as well as between intermediate- and high-risk PE. Metabolites were first transformed using the natural logarithm and then scaled to mean = 0 and standard deviation = 1. Each metabolite was modeled as a continuous variable and regressed on risk status (low-risk vs. intermediate/high-risk PE or intermediate- vs. high-risk) adjusting for age, sex, and cancer status. The False Discovery Rate (FDR) procedure by Benjamini and Hochberg [9] was used to account for multiple comparisons. FDR controls the proportion of false discoveries, i.e. the proportion of falsely rejected null hypotheses. Additionally, FDR adjustments represent a preferred alternative to the stringent Bonferroni correction [10] by balancing the power of our study against the ability to avoid false positives. We also used the non-parametric Wilcoxon two-sample test (also known as Mann-Whitney U-Test) to assess the influence of non-normally distributed metabolites in the linear regression analysis.
To further identify specific pathways that were enriched (or depleted) in low-risk PE patients compared with intermediate/high-risk PE patients, we applied Fisher’s Exact test to the metabolites that were associated with PE risk subtypes at FDR-corrected p-value ≤0.05 in the linear regression analysis. Because there were no metabolites that differed between intermediate- and high-risk PE at an FDR-corrected p-value ≤0.05 (possibly due to low statistical power), we instead included all metabolites that showed association at a nominal p-value ≤0.05. To adjust for multiple testing in the pathway analysis, we used an FDR-corrected p-value ≤0.2 to declare significance.
In a post-hoc analysis we used linear regression to calculate how strong the metabolites identified as different (after multiple testing correction) between low-risk PE and intermediate/high-risk PE groups are associated with each of the individual components used to define low-risk and intermediate/high risk PE groups: myocardial necrosis, heart failure, hemodynamic instability and right ventricle (RV) dysfunction. In each individual analysis, intermediate/high-risk was defined as evidence of: myocardial necrosis (troponin levels ≥0.1 ng/ml, 8 participants identified), heart failure (NT-proBNP levels ≥500 pg/ml, 36 participants identified), hemodynamic instability (SBP levels ≤ 90 mm Hg, 18 participants identified), RV dysfunction (RV dilatation or RV hypokinesis or RV bowing septum, 33 participants identified). We acknowledge the low number of cases for myocardial necrosis and hemodynamic instability but we include the corresponding FDR-corrected p-values and think that showing all results creates a more detailed picture of the associations with the individual PE components.
Results
Metabolomics assays of serum samples from 92 patients (see Table 1 for demographics) using four methods resulted in 843 named metabolites measured: 367 with LC/MS negative, 77 with LC/MS Polar, 205 with LC/MS Positive Early, and 207 with LC/MS Positive Late. The metabolites were structured in eight super-pathways (Amino Acid, Carbohydrate, Cofactors and Vitamins, Energy, Lipid, Nucleotide, Peptide and Xenobiotics) and 91 sub-pathways such as tricarboxylic acid (TCA) cycle, Fatty Acid Metabolism and Sphingolipid Metabolism. The assignment of each metabolite to super- and sub-pathways is shown in the supplementary data.
Low-risk PE vs. intermediate/high-risk PE
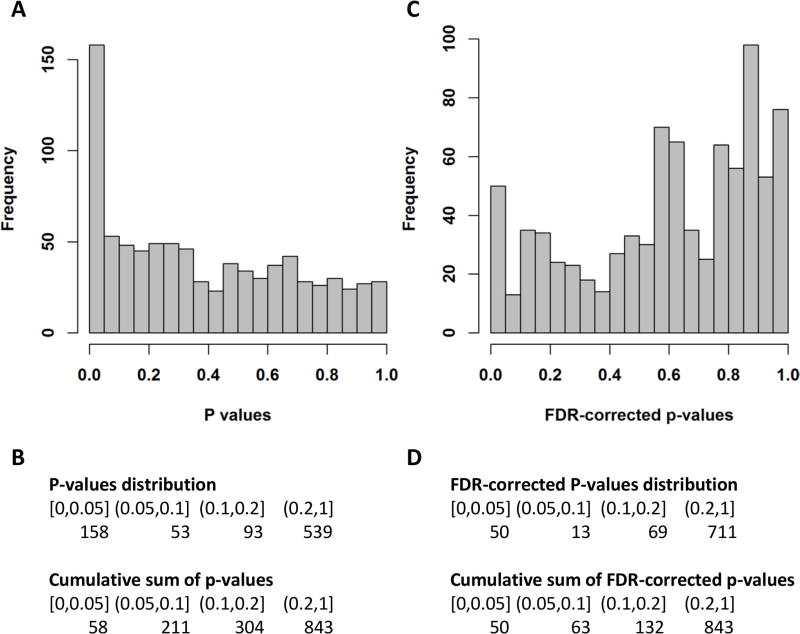
We identified 158 metabolites that differed between low-risk PE and intermediate/high-risk PE at a nominal p-value level of 0.05 (Figure 1, A and B). After multiple testing correction, 50 metabolites were significant at FDR-corrected p-value ≤0.05 (Figure 1, C and D). A heat map of these metabolites and a heat map of the correlations between them are shown in Figure S1 and Figure S2, respectively. Results for all metabolites, including calculated p-values and FDR-corrected p-values, can be found in supplementary Table S1. The top three (p-value ≤9×10−5, FDR-corrected p-value ≤0.017) identified metabolites were: xanthosine, alpha-ketoglutarate, and arachidoylcarnitine (C20). Similar results were found with the Wilcoxon two sample test (data not shown).
Figure 1.
Results of the linear regression analysis comparing intermediate/high-risk PE to low-risk PE. Each metabolite was regressed on the PE severity subtype (intermediate/high vs. low) adjusting for age, gender and cancer diagnosis. A Histogram of the p-values of the low-risk vs. intermediate/high-risk association analysis for 843 metabolites. B Table view of the distribution of p-values and their cumulative sum. C Histogram of the FDR-corrected p-values of the low-risk vs. intermediate/high-risk association analysis for 843 metabolites. D Table view of the distribution of FDR-corrected p-values and their cumulative sum.
Pathway analysis identified three sub-pathways (TCA Cycle, Fatty Acid Metabolism (Acyl Carnitine), and Purine Metabolism, (Hypo)Xanthine/Inosine Containing) and three super-pathways (Energy, Nucleotide, and Amino Acid) as significantly enriched (FDR-corrected p-value ≤0.2) in the 50 metabolites that were significantly different (FDR-corrected p-value ≤0.05) between low-risk and intermediate/high-risk PE patients (Table 2). The complete results of the pathway analysis are shown in supplementary Table S2.
Table 2.
Pathways enrichment (FDR-corrected p-values ≤0.2) in the group of metabolites that differ significantly between intermediate/high-risk PE and low-risk PE. A complete list of the analysed metabolites is available in supplementary Table S1. The complete results of the pathway analysis is available in supplementary Table S3.
| NMiP* | NSDMiP† | P-value | FDR-corrected p-value | |
|---|---|---|---|---|
| Super - Pathways | ||||
| Energy | 10 | 5 | 0.00012 | 0.00097 |
| Nucleotide | 34 | 6 | 0.01202 | 0.03204 |
| Amino Acid | 168 | 15 | 0.07002 | 0.14005 |
| Sub - Pathways | ||||
| TCA Cycle | 9 | 5 | 0.00006 | 0.00289 |
| Fatty Acid Metabolism (Acyl Carnitine) | 31 | 9 | 0.00003 | 0.00289 |
| Purine Metabolism, (Hypo)Xanthine/Inosine containing | 7 | 3 | 0.00581 | 0.17629 |
Table 2 Notes:
NMiP = Number of metabolites in pathway
NSDMiP = Number of significantly different metabolites in pathway
The most significant enrichment (FDR-corrected p-value = 0.002) was found for TCA Cycle, and Fatty Acid Metabolism (Acyl Carnitine) pathways. Five of the nine metabolites in the TCA Cycle pathway and nine of the 31 metabolites in the Fatty Acid Metabolism (Acyl Carnitine) pathway were depleted in the intermediate/high-risk group compared to the low-risk group. Interestingly, when considering all measured metabolites belonging to the TCA Cycle pathway, all but one of them had higher values in the intermediate/high-risk PE group compared with the low-risk PE group. The five significantly different metabolites driving the enrichment of the TCA Cycle pathway were: alpha-ketoglutarate, malate, isocitrate, fumarate and cis-aconitate. Enrichment of the Fatty Acid Metabolism (Acyl Carnitine) pathway was driven by: arachidoylcarnitine (C20), stearoylcarnitine (C18), linoleoylcarnitine (C18:2), 3-hydroxybutyrylcarnitine (2 isomers), myristoylcarnitine (C14), linolenoylcarnitine (C18:3), suberoylcarnitine (C8-DC) and oleoylcarnitine (C18:1).
Purine Metabolism, (Hypo)Xanthine/Inosine Containing is the third pathway that was enriched in the metabolites associated with intermediate/high-risk PE patients. This pathway was driven by xanthosine and hypoxanthine (FDR-corrected p-value ≤0.05), which were both higher in the intermediate/high risk PE groups compared with the low-risk PE group.
Pearson correlations among all measured acyl carnitines are shown in supplementary Figure S3. All acyl carnitines were positively correlated to each other.
Metabolites identified as different between low-risk and intermediate/high-risk PE groups were also associated with the individual PE components heart failure and RV dysfunction. Most metabolites had similar effects and similar significance levels (Figure S4). These associations were different when analyzing the individual PE components hemodynamic instability and myocardial necrosis. We want to remind the reader the small sample sizes in the case of these two analyses: 8 cases of myocardial necrosis and 18 cases of hemodynamic instability.
Intermediate-risk PE vs. high-risk PE
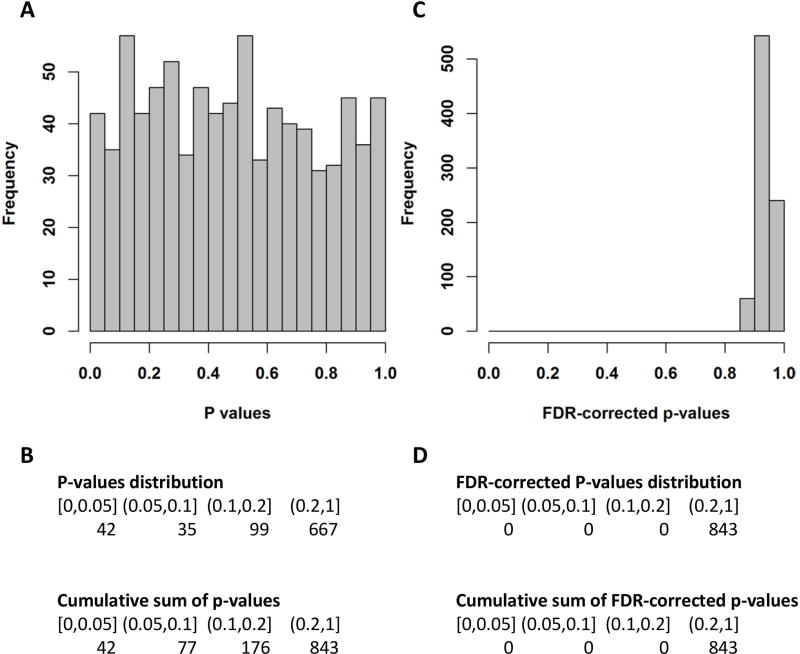
We identified 41 metabolites that differed between intermediate-risk PE and high-risk PE at a nominal p-value level of 0.05 (Figure 2, A and B). After multiple testing correction, none of the metabolites were significant at an FDR-corrected p-value ≤0.05 (Figure 2, C and D). All tested metabolites, including calculated p-values and FDR-corrected p-values, are presented in supplementary Table S3. The top three (nominal p-value ≤0.008) identified metabolites were all assigned to the super-pathway Lipid: 1-palmitoyl-2-arachidonoyl-GPC (16:0/20:4n6), 1-stearoyl-GPI (18:0) and arachidonoylcholine. Similar results were observed with the Wilcoxon two sample test (data not shown).
Figure 2.
Results of the linear regression analysis comparing high-risk to intermediate-risk PE. Each metabolite was regressed on the PE severity subtype (high-risk vs. intermediate-risk) adjusting for age, gender and cancer diagnosis. A Histogram of the p-values of the high-risk vs. intermediate-risk association analysis for 843 metabolites. B Table view of the distribution of p-values and their cumulative sum. C Histogram of the FDR-corrected p-values of the high-risk vs. intermediate-risk association analysis for 843 metabolites. D Table view of the distribution of FDR-corrected p-values and their cumulative sum.
Two sub-pathways (Fatty Acid Metabolism (Acyl Choline), and Hemoglobin and Porphyrin Metabolism) were identified as enriched (FDR-corrected p-value ≤ 0.2, Table 3), among the 41 metabolites that were nominally significantly different (p-value ≤0.05) between intermediate- and high-risk PE patients. The complete results of the enrichment analysis are shown in supplementary Table S4.
Table 3.
Pathways enrichment (FDR-corrected p-values ≤0.2) in the group of metabolites that differ on a nominal scale between intermediate-risk and high-risk PE. A complete list of the analyzed metabolites is available in supplementary Table S2. The complete results of the pathway analysis is available in supplementary Table S4.
| NMiP* | NSDMiP† | P-values | FDR-corrected p-values | |
|---|---|---|---|---|
| Sub – Pathways | ||||
| Fatty Acid Metabolism (Acyl Choline) | 7 | 5 | 4.7×10−6 | 0.00043 |
| Hemoglobin and Porphyrin Metabolism | 7 | 3 | 0.00351 | 0.15952 |
Table 3 Notes:
NMiP = Number of metabolites in pathway
NSDMiP = Number of significantly different metabolites in pathway
Fatty Acid Metabolism (Acyl Choline) showed the highest enrichment (FDR-corrected p-value = 0.00043) with five out of seven metabolites in the pathway having higher levels in the high-risk PE group compared with the intermediate-risk PE group: arachidonoylcholine, oleoylcholine, palmitoylcholine, docosahexaenoylcholine and dihomo-linolenoyl-choline. The remaining two metabolites showed the same trend at p-value ≤0.1.
The enrichment of Hemoglobin and Porphyrin Metabolism (FDR-corrected p-value =0.16) was driven by three of the seven metabolites in the pathway: I-urobilinogen, bilirubin (E,E) and bilirubin (Z,Z). Interestingly, all seven metabolites belonging to this pathway showed lower levels in the high-risk PE group compared with the intermediate-risk PE group.
Discussion
We performed the first high-throughput metabolomics study to examine differences associated with various strata of PE severity. We identified individual metabolites and pathways that differed significantly between low-risk and intermediate/high-risk PE as well as between intermediate- and high-risk PE. These findings highlight the important role metabolites play in the pathophysiology of PE and the role metabolomics may play in helping to further define the pathophysiology of PE and in risk-stratifying PE patients.
The super-pathways Energy, Nucleotide, and Amino Acid were enriched in metabolites that differed between low-risk and intermediate/high-risk PE patients. All metabolites driving this enrichment showed higher values in the intermediate/high-risk PE group, suggesting an up-regulation of these pathways in these patients. It is possible that high-risk PE decreases circulation sufficiently to compromise energy metabolism. This is consistent with previous studies that showed that anaerobic metabolism, measured by serum lactate is associated with adverse outcomes after PE [11, 12]. The role of the Nucleotide and Amino Acid super-pathways in PE has not been previously described and should be studied further.
Acyl carnitines were identified as significantly different between low-risk and intermediate/high-risk PE patients, while acyl cholines were different between intermediate and high-risk PE patients. Among the 50 metabolites identified as significantly different between low-risk and intermediate/high-risk PE, nine were acyl carnitines. These metabolites drove the observed enrichment of Fatty Acid Metabolism. These findings are consistent with a published study of venous thromboembolism (VTE) metabolomics, which reported that acyl carnitines levels were reduced in VTE cases compared with controls [13]. The authors note that patients with low levels of acyl carnitines C12:2 and C18:2 had an increased risk of incident VTE. We also observed an association with acyl carnitines C18:2 (FDR-corrected p-value = 0.03) and C12:0 (FDR-corrected p-value = 0.2), where the intermediate/high-risk PE groups had higher levels compared with the low-risk group. We compared subjects with low-risk PE to those with intermediate/high-risk PE, while Deguchi et al. compared subjects with and without VTE. Thus, acyl carnitines may be associated with both risk of incident VTE and PE severity. The exact role of these metabolites in VTE pathophysiology warrants further exploration.
We also found that short and medium chain acyl carnitines (≤ 20 carbon atoms), which were identified as different between low-risk PE and intermediate/high-risk PE, are highly correlated with each other and less correlated with long chain acyl carnitines (>20 carbon atoms), which were not significantly different between low-risk and intermediate/high-risk PE (supplementary Figure S1). This suggests that short and medium chain (but not long chain) acyl carnitines are tightly regulated and play a role in PE severity.
When we compared intermediate with high-risk PE patients, we found that 5 acyl cholines differed between the two groups, driving the enrichment of Fatty Acid Metabolism (Acyl Cholines). Interestingly, acyl cholines had higher in high-risk PE patients than in intermediate-risk PE patients. The literature is very sparse on acyl cholines. Nevertheless, experiments on the embryos and larvae of sea urchins, sensitive to cholinergic compounds, showed that synthetically created arachidonoylcholine exhibited cholinomimetic activity similar to that of nicotine [14]. Smoking is an established risk factor for VTE, but whether this is related to the association between acyl cholines and PE severity in our study remains to be determined [15, 16].
Additionally, we observed the Hemoglobin and Porphyrin Metabolism pathway to be enriched when comparing high-risk PE group with the intermediate-risk PE group. This is interesting, and consistent with proteomics studies that observed lower levels of haptoglobin in high-risk PE subjects compared to less severe PE, including a study by Insenser and colleagues, who observed lower circulating haptoglobin concentrations in high-risk PE patients compared to less severe PE patients [17]. This finding was also seen in animal studies, using lung and serum samples from rats [18–20]. Lower levels of haptoglobin have also been observed in PE patients with severe pulmonary hypertension [21]. Haptoglobin has the capacity to bind to hemoglobin during hemolysis, which may affect vasoactive mediators and inflammatory processes [22, 23]. Our results, together with the existing literature, suggest hemoglobin metabolism down-regulation together with the down-regulation of haptoglobin may play an important role in the physiology of severe PE and may help identify patients at risk for adverse outcomes.
Our study has several strengths and some limitations. All of our subjects had radiographically confirmed PE, and blood samples drawn within 24 hours of PE diagnosis. Samples were all processed within 60 minutes of collection, minimizing the risk of pre-processing effects. Moreover, our study was nested within a prospective cohort with highly granular outcome assessment. The risk of ascertainment or recall bias is therefore minimal. One limitation of our analysis is the number (n = 92) of study participants. Thus, results need to be interpreted with caution, in particular for the intermediate- to high-risk PE comparison (n = 46) where we used nominal p-values to assign statistical significance of individual metabolites. Another limitation of our study is that the metabolites were quantified as peak areas and not concentrations. In order to use metabolites in a clinical setting, new metabolomics assays need to be designed to measure their concentrations. However, our approach represents the state of the art in high-throughput metabolomics experiments. Additionally, due to the unique nature of our study (blood draw at the time of diagnosis and processing within one hour) it was not possible to replicate these findings in another population. Although the results of our study are consistent with the currently only other VTE metabolomics study [13], replication of our results in another study is desired. However, to the best of our knowledge, this is the largest study to date of VTE metabolomics, and the first study relating metabolomics to PE severity. Our results are statistically significant for the low-risk to intermediate/high-risk PE comparison, suggestive in case of the intermediate to high-risk PE, and the fact that our findings are consistent with previous research supports the veracity of our results.
We performed the first study to examine metabolites and metabolic pathways in patients of varying PE severity. We identified individual metabolites as well as sub- and super-pathways that were significantly different between low-risk and intermediate/high-risk PE as well as between intermediate-risk and high-risk PE. These findings suggest that metabolites play an important role in the pathophysiology of PE. Larger studies are needed to confirm our results and to assess the potential use of metabolites as biomarkers for PE severity.
Supplementary Material
Essentials.
Risk-stratification often fails to predict clinical deterioration in pulmonary embolism (PE).
First-ever high-throughput metabolomics analysis of risk-stratified PE patients.
Changes in circulating metabolites reflect a compromised energy metabolism in PE.
Metabolites play a key role in the pathophysiology and risk stratification of PE.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute (HL116854), by The Harvard Milton Foundation, and by the National Institutes of Health (HL098048).
C. Kabrhel has received grants to his institution from Janssen Pharmaceuticals, Siemens Healthcare Diagnostics, Diagnostics Stago, NIH/NHLBI, and has consulting agreements between his institution and Janssen Pharmaceuticals and Siemens Healthcare Diagnostics. J. A. Lasky-Su reports personal fees from Metabolon, Inc., outside the submitted work.
P. Kraft and L. B. Harrington report grants from National Institutes of Health, during the conduct of the study.
Footnotes
Presentation: International Society for Thrombosis and Haemostasis, Berlin, 2017
Authorship Contributions: O. A. Zeleznik, E. M. Poole, S. Lindstrom, A. Van Hylckama Vlieg, and C. Kabrhel designed the study. B. A. Parry, N. Giordano, and C. Kabrhel collected the data. O. A. Zeleznik, E. M. Poole, and P. Kraft performed the analyses. C. Kabrhel obtained funding. O. A. Zeleznik and C. Kabrhel drafted the manuscript. O. A. Zeleznik, E. M. Poole, S. Lindstrom, P. Kraft, A. Van Hylckama Vlieg, J. A. Lasky-Su, L. B. Harrington, K. Hagan, J. Kim, B.A. Parry, N. Giordano, and C. Kabrhel edited the manuscript.
Disclosure of Conflicts of Interest: All other authors declare no competing financial interests.
References
- 1.Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation. 2006;113:577–82. doi: 10.1161/CIRCULATIONAHA.105.592592. [DOI] [PubMed] [Google Scholar]
- 2.Becattini C, Agnelli G. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. European Respiratory Journal. 2017;49:1601732. doi: 10.1183/13993003.01732-2016. [DOI] [PubMed] [Google Scholar]
- 3.Hariharan P, Dudzinski DM, Rosovsky R, Haddad F, MacMahon P, Parry B, Chang Y, Kabrhel C. Relation Among Clot Burden, Right-Sided Heart Strain, and Adverse Events After Acute Pulmonary Embolism. The American Journal of Cardiology. 2016;118:1568–73. doi: 10.1016/j.amjcard.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 4.Jain CC, Chang Y, Kabrhel C, Giri J, Channick R, Rodriguez-Lopez J, Rosovsky RP, Fogerty A, Rosenfield K, Jaff MR. Impact of Pulmonary Arterial Clot Location on Pulmonary Embolism Treatment and Outcomes (90 Days) The American Journal of Cardiology. 2017;119:802–7. doi: 10.1016/j.amjcard.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Kabrhel C, Okechukwu I, Hariharan P, Takayesu JK, MacMahon P, Haddad F, Chang Y. Factors associated with clinical deterioration shortly after PE. Thorax. 2014 doi: 10.1136/thoraxjnl-2013-204762. thoraxjnl-2013-204762. [DOI] [PubMed] [Google Scholar]
- 6.Kline JA, Mitchell AM, Runyon MS, Jones AE, Webb WB. Electronic medical record review as a surrogate to telephone follow-up to establish outcome for diagnostic research studies in the emergency department. Acad Emerg Med. 2005;12:1127–33. doi: 10.1197/j.aem.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Katajamaa M, Oresic M. Processing methods for differential analysis of LC/MS profile data. BMC Bioinformatics. 2005;6:179. doi: 10.1186/1471-2105-6-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long T, Hicks M, Yu H-C, Biggs WH, Kirkness EF, Menni C, Zierer J, Small KS, Mangino M, Messier H. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nature Genetics. 2017;49:568–78. doi: 10.1038/ng.3809. [DOI] [PubMed] [Google Scholar]
- 9.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society Series B (Methodological) 1995:289–300. [Google Scholar]
- 10.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. Journal of clinical epidemiology. 2014;67:850–7. doi: 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Vanni S, Nazerian P, Bova C, Bondi E, Morello F, Pepe G, Paladini B, Liedl G, Cangioli E, Grifoni S. Comparison of clinical scores for identification of patients with pulmonary embolism at intermediate–high risk of adverse clinical outcome: the prognostic role of plasma lactate. Internal and Emergency Medicine. 2016:1–9. doi: 10.1007/s11739-016-1487-6. [DOI] [PubMed] [Google Scholar]
- 12.Vanni S, Viviani G, Baioni M, Pepe G, Nazerian P, Socci F, Bartolucci M, Bartolini M, Grifoni S. Prognostic value of plasma lactate levels among patients with acute pulmonary embolism: the thrombo-embolism lactate outcome study. Annals of emergency medicine. 2013;61:330–8. doi: 10.1016/j.annemergmed.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Deguchi H, Banerjee Y, Trauger S, Siuzdak G, Kalisiak E, Fernández JA, Hoang L, Tran M, Yegneswaran S, Elias DJ. Acylcarnitines are anticoagulants that inhibit factor Xa and are reduced in venous thrombosis, based on metabolomics data. Blood. 2015;126:1595–600. doi: 10.1182/blood-2015-03-636761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bezuglov V, Zinchenko G, Nikitina L, Buznikov G. Arachidonoylcholine and N, N-dimethylaminoethyl arachidonate, new cholinergic compounds. Russian Journal of Bioorganic Chemistry. 2001;27:200–3. doi: 10.1023/a:1011341623358. [DOI] [PubMed] [Google Scholar]
- 15.Hansson P-O, Eriksson H, Welin L, Svärdsudd K, Wilhelmsen L. Smoking and abdominal obesity: risk factors for venous thromboembolism among middle-aged men: the study of men born in 1913. Archives of internal medicine. 1999;159:1886–90. doi: 10.1001/archinte.159.16.1886. [DOI] [PubMed] [Google Scholar]
- 16.Tapson VF. The role of smoking in coagulation and thromboembolism in chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society. 2005;2:71–7. doi: 10.1513/pats.200407-038MS. [DOI] [PubMed] [Google Scholar]
- 17.Insenser M, Montes-Nieto R, Martínez-García MÁ, Duran EF, Santiuste C, Gomez V, Kline JA, Escobar-Morreale HF, Jimenez D. Identification of reduced circulating haptoglobin concentration as a biomarker of the severity of pulmonary embolism: a nontargeted proteomic study. PloS one. 2014;9:e100902. doi: 10.1371/journal.pone.0100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Sq, Qi Hw, Wu Cg, Zhang Xj, Yang Sg, Zhao X, Wu Z, Wang Y, Que Hp, Liu Sj. Comparative proteomic study of acute pulmonary embolism in a rat model. Proteomics. 2007;7:2287–99. doi: 10.1002/pmic.200500665. [DOI] [PubMed] [Google Scholar]
- 19.Li S-q, Yun J, Xue F-b, Yang S-g, Que H-p, Zhao X, Wu Z, Wang Y, Liu S-j. Comparative proteome analysis of serum from acute pulmonary embolism rat model for biomarker discovery. Journal of proteome research. 2007;6:150–9. doi: 10.1021/pr0603102. [DOI] [PubMed] [Google Scholar]
- 20.Watts JA, Lee Y-Y, Gellar MA, Fulkerson M-BK, Hwang S-I, Kline JA. Proteomics of microparticles after experimental pulmonary embolism. Thrombosis research. 2012;130:122–8. doi: 10.1016/j.thromres.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Kline JA, Marchick M, Hogg M. Reduction in plasma haptoglobin in humans with acute pulmonary embolism causing tricuspid regurgitation. Journal of Thrombosis and Haemostasis. 2009;7:1597–9. doi: 10.1111/j.1538-7836.2009.03535.x. [DOI] [PubMed] [Google Scholar]
- 22.Giblett E. The haptoglobin system. Ser Haematol. 1968;1:3–20. [Google Scholar]
- 23.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clinical chemistry. 1996;42:1589–600. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.