Abstract
To understand the effect of woody forages on golden pompano (Trachinotus ovatus) intestinal bacteria diversity and exploit new aquafeed ingredients, the diets of Moringa oleifera Lam (MOL), Broussonetia papyrifera (BP), Neolamarckia cadamba (NC) and Folium mori (FM) formulated with 70% of reference (Ref) diet and 30% of the four woody plants leaves were fed to golden pompano with initial body weight of 34.4 ± 0.5 g for 56 days, respectively. Bacteria diversity of golden pompano intestine and tank water (W) samples were analyzed using high-throughput illumina sequencing and the result indicated that the dominate phyla of golden pompano intestine were Proteobacteria, Bacteroidetes, Firmicutes and Fusobacteria. Proteobacteria in BP was significantly higher than those in NC (P < 0.05). Firmicutes in NC were significantly higher than those in BP and FM (P < 0.05). At genera level, Lactobacillus in NC was significantly higher than those in BP, MOL and FM groups (P < 0.05). The PCoA and heat map analyses showed that the intestinal bacteria community of golden pompano fed with woody forages and Ref diet presented higher similarity and the bacteria community of golden pompano intestine were clearly distinguished from those of W. Phylogenetic investigation of communities by reconstruction of unobserved states showed that the intestinal bacteria dominant metabolism pathways of golden pompano fed with woody forages and Ref diet were biosynthesis of amino acids and carbon metabolism. Overall, the present study first successfully characterized the intestinal bacteria diversity of golden pompano.
Electronic supplementary material
The online version of this article (10.1186/s13568-018-0550-2) contains supplementary material, which is available to authorized users.
Keywords: Golden pompano, Woody forages, Intestinal bacteria, Illumina sequence
Introduction
Golden pompano (Trachinotus ovatus) is a marine water species and mainly distributed in tropical and temperate seas (Niu et al. 2013). It has become a popular cultured species because of its fast growth and high flesh quality. Studies showed that the vertebrate intestine inhabited diverse bacteria which had a mutual relationship with host and played key functions in nutrition (Hassaan et al. 2017) and development (Verner-Jeffreys et al. 2003). Meanwhile, studies also found that Bacteroidetes, Firmicutes and Fusobacteria in intestine could produce digestive enzymes (Becker et al. 2014). However, there is not report about the golden pompano intestinal bacteria diversity.
Aquaculture has become the fastest growing food-producing sector which contributed 50.9% to total global fisheries production (Hamdan et al. 2016). The increased production of intensively reared fish species necessitates the exploitation of new feedstuff resources (Adeoye et al. 2016). Some woody materials with high nutrition and low price may be the most promising new aquatic feed ingredients. Moringa oleifera Lam leaves have moderately high level of protein, amino acid, vitamin A, iron and calcium (Nahid et al. 2003). Meanwhile, studies indicated that the Moringa oleifera Lam could be used to substitute 10% of fishmeal in Nile tilapia diets without significant reduction on growth (Afuang et al. 2003). Broussonetia papyrifera leaves contain lots of biologically active compounds which were benefit to the immunity of organism (Xi et al. 2013). Folium mori leaves and Neolamarckia cadamba leaves have 16.8 and 20.9% crude protein (CP), respectively (Doi et al. 2000).These woody plants with high nutritional value may be ideal new diets sources in aquaculture. However, several studies indicated feedstuff could influence intestinal bacteria diversity, such as, dietary malic acid (Hassaan et al. 2017), thymus vulgaris essential oil (Navarrete et al. 2010) and soybean meal (Merrifield et al. 2009). These findings indicate that further studies on intestine bacteria dynamics of golden pompano fed with new source woody forages is needed.
In the present study, the intestine bacteria of golden pompano was first analyzed by illumina-based high-throughput sequencing, which would contribute to understand the intestinal bacteria diversity and dynamics of golden pompano fed with woody forages and be helpful to the exploitation of woody forges in aquaculture.
Materials and methods
Experimental design and sampling produces
The formulation and proximate composition of Ref diet were showed in Table 1 which contained 34.3% crude protein (CP) and 7.0% crude lipid (EE). Fishmeal and soybean meal were used as major protein sources. Menhaden fish oil, sunflower oil and soybean lecithin were used as lipid sources. The reference (Ref) diet was prepared according to the protocol of Pan et al. (2003). And the mixed wet mash was extruded into pellet diet with 3.5 mm in diameter by twin screw extruders (SLX-80, Guangzhou, China). The diets of Moringa oleifera Lam (MOL), Broussonetia papyrifera (BP), Neolamarckia cadamba (NC) and Folium mori (FM) were formulated with 70% of Ref diet and 30% leafmeals of Moringa oleifera Lam, Broussonetia papyrifera, Neolamarckia cadamba and Folium mori, respectively. The amino acid and nutrients composition of MOL, BP, NC and FM were shown in Table 2. And the nutrients composition of MOL, BP, NC and FM diets were showed in Table 3.
Table 1.
Formulation and proximate composition of Ref diet
| Formulation | % |
|---|---|
| Fishmeal | 31.0 |
| Soybean meal | 21.0 |
| Menhaden fish oil | 2.8 |
| Sunflower oil | 1.0 |
| Soybean lecithin (50%) | 1.5 |
| Wheat flour | 40.2 |
| Ca(H2PO4)2–H2O | 1.5 |
| Taurine | 0.5 |
| Choline chloride (50%) | 0.4 |
| Vitamin premixa | 0.5 |
| Mineral premixb | 0.5 |
| Y2O3/Cr2O3 | 0.1 |
| Nutrients composition analyses | |
| Moisture | 10.1 |
| Crude protein | 34.3 |
| Crude lipid | 7.0 |
| Ash | 7.0 |
| Energy (KJ/g) | 19.5 |
aVitamin premix (g/kg) and b Mineral premix (g/kg) were supplied by Guangzhou Chengyi Aquatic Technology Company
Table 2.
Analyzed amino acid and nutrients composition of MOL, FM, BP and NC
| Items | MOL | FM | BP | NC |
|---|---|---|---|---|
| Aspartic acid (%) | 2.4 | 1.7 | 2.0 | 1.2 |
| Threonine | 1.2 | 0.8 | 0.8 | 0.6 |
| Serine | 1.1 | 0.8 | 0.8 | 0.6 |
| Glutamic acid (%) | 3.2 | 1.9 | 1.9 | 1.6 |
| Proline | 1.2 | 0.9 | 1.0 | 0.6 |
| Glycine | 1.4 | 0.9 | 1.0 | 0.7 |
| Alanine | 1.7 | 1.1 | 1.1 | 0.7 |
| Valine | 1.4 | 1.0 | 1.0 | 0.7 |
| Methionine (%) | 0.1 | 0.1 | 0.1 | 0.1 |
| Isoleucine (%) | 1.2 | 0.8 | 0.8 | 0.6 |
| Leucine | 2.3 | 1.4 | 1.5 | 1.1 |
| Tyrosine | 0.8 | 0.5 | 0.5 | 0.4 |
| Phenylalanine (%) | 1.6 | 0.9 | 1.0 | 0.7 |
| Lysine | 1.6 | 1.1 | 1.1 | 0.7 |
| Histidine | 0.6 | 0.3 | 0.4 | 0.3 |
| Arginine | 1.5 | 0.9 | 1.0 | 0.7 |
| Total amino acid (%) | 23.1 | 15.1 | 15.8 | 11.1 |
| Moisture (%) | 1.9 | 0.9 | 1.4 | 1.5 |
| CP (%) | 26.6 | 17.9 | 18.3 | 13.4 |
| EE (%) | 4.3 | 4.4 | 3.5 | 2.7 |
| Ash (%) | 10.8 | 11.6 | 16.0 | 7.6 |
| Energy (KJ/g) | 17.8 | 16.9 | 15.4 | 18.7 |
Table 3.
Analyzed nutrients composition of MOL, BP, NC and FM diets
| Nutrients composition analysis | MOL | BP | NC | FM |
|---|---|---|---|---|
| Moisture (%) | 10.6 ± 0.0 | 10.4 ± 0.1 | 11.0 ± 0.0 | 11.0 ± 0.1 |
| Crude protein (%) | 33.1 ± 0.1 | 30.7 ± 0.1 | 29.2 ± 0.1 | 31.0 ± 0.1 |
| Crude lipid (%) | 6.4 ± 0.0 | 7.3 ± 0.0 | 6.8 ± 0.0 | 6.4 ± 0.0 |
| Ash (%) | 8.3 ± 0.1 | 8.7 ± 0.1 | 6.9 ± 0.3 | 9.6 ± 0.0 |
| Energy (KJ/g) | 19.4 ± 0.0 | 19.2 ± 0.1 | 19.4 ± 0.2 | 19.0 ± 0.2 |
The experimental golden pompano were obtained from Shenzhen Base of South China Sea Fisheries Research Institute and fed with Ref diet and woody forages for 1 week to acclimatize experimental conditions and feed. Then 300 healthy fish with initial weight of 34.4 ± 0.5 g were randomly stocked in 15 tanks with 20 fishes in each aquariums (500 L water capacity). Each test diet was fed to fish in three parallel aquariums. All golden pompano were fed with diet at 07:30, 12:30 and 18:00 daily by hand to apparent satiation for 56 days and the woody forages did not influence the feeding in the present study. 1/4–1/3 of the water in aquariums was changed with the filterable sea water every day. During the feeding trial, water temperature ranged at 24–26 °C, pH at 7.6–7.8, salinity at 15–17 g/L, N–NH4− < 0.1 mg/L and DO > 5 mg/L.
After the feeding trial, golden pompano were fasted 7 h. Three fish were randomly picked up from each aquarium and sacrificed with tricaine methanesulfonate (MS-222). Then the fish were dissected with sterile scissors and intestines were filled with chyme. Intestinal contents were carefully collected to 1.5 mL sterile centrifuge tube. In addition, 300 mL tank sea water (W) sample was collected and filtered by 0.22 μm pore size hydrophilic polyethersulfone membrane filter. All samples were stored at − 80 °C. In addition, the proximate composition analyses of five diets were based on the Official Analytical Chemists (AOAC 1995).
DNA extraction and PCR amplification
The microbial DNA of golden pompano intestine and tank water was extracted using EZNA Stool DNA Kit (Omega Bio-tek). The V3–V4 region of bacteria 16S ribosomal RNA gene was amplified by PCR using the primers V338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and V806R (5′-GGACTACHVGGGTWTCTAAT-3′). All PCR amplifications were performed in triplicate at 20 μL reactions containing 4 μL of 5× FastPfu buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL (1 unit) of FastPfu polymerase (TransGen AP221-02: TransStart™ FastPfu DNA polymerase, TransGen Biotech, Beijing, China) and 10 ng of template DNA. The thermal cycling program was performed as followings: 95 °C for 5 min, followed by 25 cycles at 95 °C for 60 s, 50 °C for 60 s, 72 °C for 60 s and a final extension at 72 °C for 7 min. The PCR products were examined using 1.8% agarose gel and excised and purified using the QIAquick Gel extraction kit (Qiagen, Hilden, Germany) according to the manufacture protocol. Purified amplicons were pooled in equimolar quantities and sequenced with Illumina HiSeq 2500 platform.
Sequence analyses
Paired-end reads were merged with FLASH v1.2.7 according to overlap more than 10 bps. Raw Tags were filtered by Trimmomatic v0.33 and chimeric sequences were removed by UCHIME v4.2. Effective Tags were clustered at a 97% sequence identity into operational taxonomic units (OTUs) using UCLUST in QIIME (version 1.8.0) software package (Edgar 2010; Caporaso et al. 2010). Each OTU were aligned to SILVA bacteria database using PyNAST (Koetschan et al. 2014). Taxonomic OTU assignments were accomplished by Ribosomal Database Project (RDP) Classifier with a minimum confidence of 80% (Caporaso et al. 2010).
Heatmap was analyzed through “R vegan package” (Kang et al. 2013). Abundance-based coverage estimator (ACE), Chao 1, Shannon, Simpson and Good’s coverage indices were used to analyze the richness and diversity. Rarefaction curves were analyzed with MOTHUR (version v.1.30). Principal coordinate analyses (PCoA) were based on the binary Jaccard distances (Lozupone and Knight 2005). Gene prediction was used with the phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) and Greengenes database v13.5 (de Oliveira et al. 2013; Parks et al. 2014).
Statistical analyses
The results were analyzed by One-way ANOVA at 5% significance level using SPSS version 20.0 (SPSSInc, Chicago, IL) and shown as mean ± SE.
Nucleotide sequence accession number
The raw reads were deposited to the NCBI Sequence Read Archive (SRA) database under accession number SRP115358.
Results
Statistical analysis of sequences
1,086,123 effective tags were obtained from 18 samples. The average lengths of effective tags were 422 bps. A total of 5092 OTUs at 97% sequence similarity were obtained, with average of 282 OTUs in each sample. Rarefaction curves indicated that the obtained sequence could reflect majority of bacteria diversity in each sample (Fig. 1).
Fig. 1.
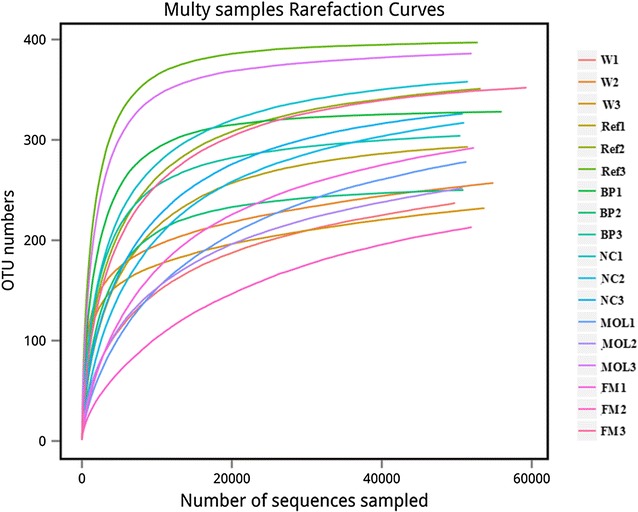
Rarefaction analyses of all samples. Rarefaction curves represented the number of operational taxonomic unit (OTU) detected in W (W1, W2, W3), Ref (Ref1, Ref2, Ref3), BP (BP1, BP2, BP3), NC (NC1, NC2, NC3), MOL (MOL1, MOL2, MOL3) and FM (FM1, FM2, FM3). Sequences were clustered at 97% sequence similarity
Table 4 presented the alpha diversity of W, Ref, BP, NC, MOL and FM. The ACE and Chao 1 indices of W, Ref, BP, NC, MOL and FM ranged from 279.0 ± 29.1 to 340.0 ± 28.5, 280.0 ± 29.3 to 342.0 ± 29.3, respectively. The Simpson and Shannon indices of W, Ref, BP, NC, MOL and FM ranged from 0.1 ± 0.0 to 0.3 ± 0.1, 2.7 ± 0.3 to 3.7 ± 0.3, respectively. The bacteria diversity and richness had not significant difference in W, Ref, BP, NC, MOL and FM (P > 0.05).
Table 4.
OTUs, ACE, Chao 1, Simpson, Shannon and Good’s coverage for 16s rRNA libraries of all samples
| Sample ID | W | Ref | BP | NC | MOL | FM |
|---|---|---|---|---|---|---|
| OTUs | 229.0 ± 7.7 | 333.0 ± 29.7 | 276.0 ± 29.5 | 319.0 ± 11.7 | 279.0 ± 44.3 | 259.0 ± 44.8 |
| ACE | 281.0 ± 8.0 | 340.0 ± 28.5 | 279.0 ± 29.1 | 334.0 ± 13.7 | 313.0 ± 29.6 | 294.0 ± 31.7 |
| Chao1 | 289.0 ± 13.8 | 342.0 ± 29.3 | 280.0 ± 29.3 | 339.0 ± 6.3 | 328.0 ± 20.0 | 290.0 ± 34.4 |
| Simpson | 0.3 ± 0.2 | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.1 |
| Shannon | 2.7 ± 0.7 | 3.3 ± 0.5 | 3.6 ± 0.0 | 2.7 ± 0.3 | 3.2 ± 0.7 | 3.7 ± 0.3 |
| Good’s coverage | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.1 | 1.0 ± 0.0 |
In the same row, values with different letter superscripts mean significant differences (P < 0.05)
Taxonomic composition
A total of 15 phyla were detected in W, Ref, BP, NC, MOL and FM and the most abundant phyla were Proteobacteria, Bacteroidetes, Firmicutes and Fusobacteria (Fig. 2). The most abundant phyla in Ref were Fusobacteria (30.2%), Firmicutes (29.7%), Proteobacteria (27.3%) and Bacteroidetes (9.5%). Proteobacteria (39.1, 23.8, 16.4 and 46.1%), Firmicutes (17.5, 46.7, 55.9 and 18.2%), Fusobacteria (26.7, 14.8, 17.1 and 14.9%) and Bacteroidetes (8.2, 10.7, 9.0 and 7.7%) were the dominate phyla in FM, MOL, NC and BP, respectively. In W, the dominate phyla were Proteobacteria (79.6%), Firmicutes (2.8%), Bacteroidetes (10.1%). Proteobacteria in BP group was significantly higher than those in NC (P < 0.05). Firmicutes in NC were higher than those in BP and FM (P < 0.05). Proteobacteria in Ref, BP, NC, MOL and FM were significantly lower than those in W (P < 0.05). Fusobacteria in Ref, BP, NC, MOL and FM groups were significantly higher than those in W (P < 0.05).
Fig. 2.
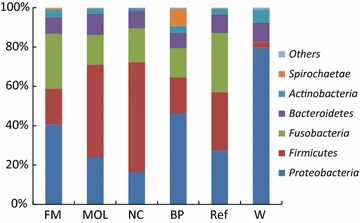
The intestinal bacteria communities at phyla level. The color-coded bar plot showed the percentages of intestinal bacteria communities in W, Ref, BP, NC, MOL and FM groups at phyla level. “Others” meant the relative abundance were less than 1%
At genera level, 224 bacteria genera were detected from all samples (Fig. 3). Cetobacterium (30.1%), Lactobacillus (18.4%), Buchnera (4.9%) and Escherichia–Shigella (4.9%) were the dominate genera in Ref group. Cetobacterium (26.6, 14.8, 17.1 and 14.8%), Lactobacillus (7.5, 20.8, 47.7 and 3.3%), Buchnera (4.3, 5.9, 2.6 and 14.6%) and Escherichia–Shigella (6.3, 5.9, 2.6 and 14.6%) were the dominate genera in FM, MOL, NC and BP groups. In W, Pseudoalteromonas (0.6%) and Lactobacillus (0.5%) were the dominate genera. Lactobacillus in NC were significantly higher than those in BP, MOL and FM groups (P < 0.05). Escherichia–Shigella and Buchnera in BP were significant higher than those in NC (P < 0.05). Prevotella_9 in NC were significant higher than those in FM. Meanwhile, Cetobacterium in Ref and FM were significantly higher than those in W (P < 0.05).
Fig. 3.
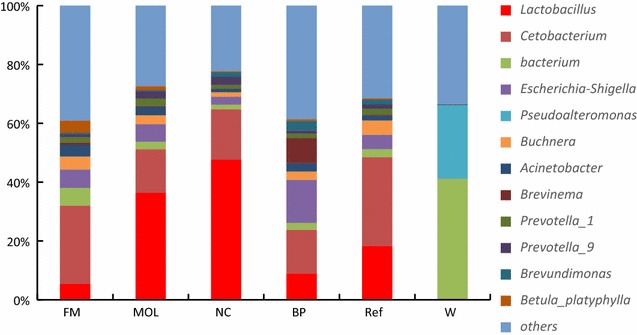
The intestinal bacteria communities at genera level. The color-coded bar plot showed the percentages of intestinal bacteria communities in W, Ref, BP, NC, MOL and FM groups at genera level. Others meant the relative abundance were less than 1%
Clustering dissimilarities
The principal coordinates analysis (PCoA) indicated that the bacteria community in Ref, BP, NC, MOL and FM clustered together and the bacterial community in Ref, BP, NC, MOL and FM was vaster difference with that in W (Fig. 4). In addition, the heatmap using binary Jaccard distances also showed the similar trend with that of PCoA (Fig. 5). These result showed that the intestinal bacteria community of golden pompano fed with woody forages and reference diet presented higher similarity. The bacteria community in golden pompano intestine was vaster difference from that in W.
Fig. 4.
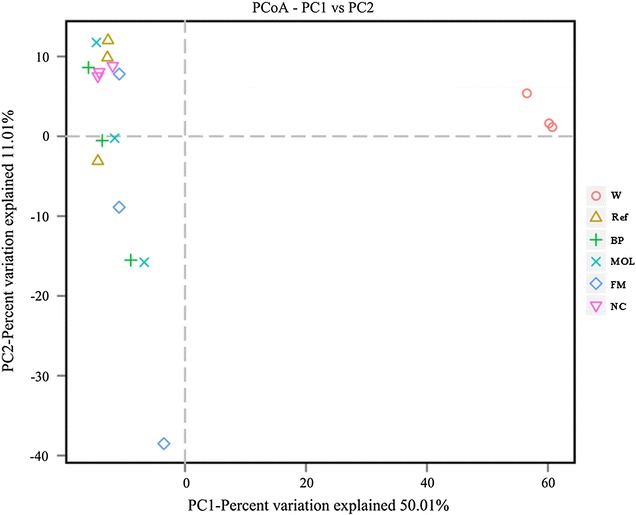
PCoA analyzed the bacteria community. The colored circles represented the bacteria from W, Ref, BP, NC, MOL and FM. PCoA analyzed by with binary Jaccard distances
Fig. 5.
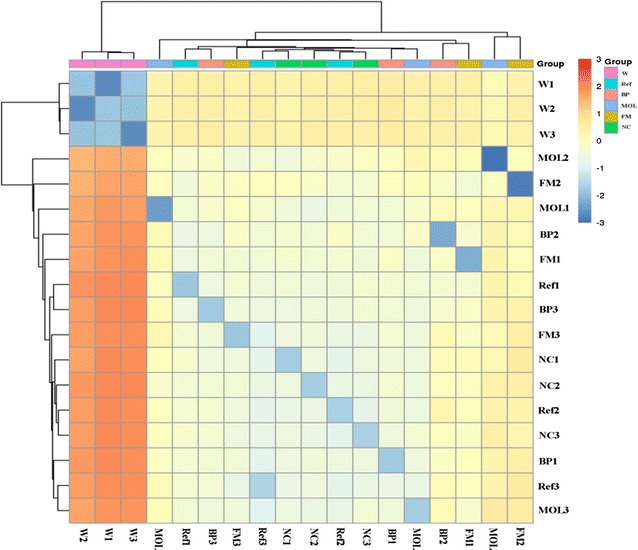
The heatmap of all samples. The different color intensities represented the relative bacteria abundance in W, Ref, BP, NC, MOL and FM groups. Figure was constructed using binary Jaccard distances. The distance of samples gradually increased with the color from blue to red
Predicted metabolic pathways and functions
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) was used to predict the intestine bacterial functional composition of golden pompano fed with woody forages and reference diet. A total of 338 KEGG orthology (KO) pathways were detected in FM, MOL, NC, BP and Ref groups. Among the 338 pathways, 148, 31, 20 and 18 KEGG pathways were related with metabolism, environmental information processing, genetic information processing and cellular process, respectively. Further analysis the 20 most abundant pathways, four pathways (“arginine and proline metabolism”, “glycine, serine and threonine metabolism”, “valine, leucine and isoleucine degradation”, “alanine, aspartate and glutamate metabolism”) were related with the amino acid metabolism and the glycine, serine and threonine metabolism in FM were significantly higher than those in the Ref, BP, NC and MOL (P < 0.05). Six pathways (“butanoate metabolism”, “pyruvate metabolism”, “propanoate metabolism”, “glyoxylate and dicarboxylate metabolism”, “glycolysis/gluconeogenesis”, “carbon metabolism”) were related with carbon metabolism. Two pathways (“purine metabolism”, “pyrimidine metabolism”) were related with nucleotide metabolism. Meanwhile, the two-component system related with signal transduction in BP was significantly higher than those in NC and MOL (P < 0.05). ABC transporters related with membrane transport in BP was significantly higher than that in Ref and FM (P < 0.05). Oxidative phosphorylation related with energy metabolism in FM was significantly higher than those in NC (P < 0.05) (Additional file 1: Table S1).
Discussion
Intestinal bacteria were involved indigestion, immunity and physiology (Huyben et al. 2017). This study provided the first characterization of intestinal bacteria diversity of golden pompano with illumina-based high-throughput sequencing. And the result indicated that the most phyla of golden pompano intestinal bacteria were Proteobacteria, Firmicutes, Fusobacteria and Bacteroidetes. The result was similar with the studies in rainbow trout (Lyons et al. 2015), Eastern African cichlid (Baldo et al. 2015) and Asian carp (Ye et al. 2014). Meanwhile, at the present study, bacterial diversity presented vastly difference between golden pompano intestine and water sample. The result was similar with Johnson et al. (2008) and the phenomenon may be related with the host intestinal environment exerts selective pressure on intestine microbial community establishment (Rungrassamee et al. 2014). The relationship between intestinal bacteria and habitat environmental bacteria was needed further research.
To further analyses the intestinal bacteria dynamic, the abundance of intestinal bacteria of golden pompano fed with woody forages and reference diet were further analyzed. At the present study, the Proteobacteria and Firmicutes were the dominate phylum. Proteobacteria in Broussonetia papyrifera group was significantly higher than those in Neolamarckia cadamba group and Firmicutes in Neolamarckia cadamba group were higher than those in Folium mori group and Broussonetia papyrifera group. The result indicated that the Broussonetia papyrifera may be benefit for the Proteobacteria and Neolamarckia cadamba may be benefit for the Firmicutes in golden pompano intestine. Meanwhile, the study indicated that Proteobacteria could catabolize feedstuff components (Jumpertz et al. 2011). And the Firmicutes were the dominate phylum in the intestine of Eastern African cichlid (Baldo et al. 2015) and Nile tilapia (Jumpertz et al. 2011). The Bacteroidetes and Fusobacteria presented no significant difference in the woody forages groups and reference groups. Studies indicated that Bacteroidetes is and Fusobacteria were related with the nutrition metabolism and absorb (Spence et al. 2006). Meanwhile, Fusobacteria was found in the intestine of Easter African cichild (Baldo et al. 2015) and grass carp (Ni et al. 2014).
At genera level, Lactobacillus was the dominate bacteria of Firmicutes. At the present study, the Lactobacillus in Neolamarckia cadamba group was significantly higher than those in Folium mori group and Broussonetia papyrifera group. Research indicated that Lactobacillus could produce lactic acid which benefit to the health of intestinal tract (Corsetti et al. 1998). Cetobacterium were the dominate genera of Fusobacteria and study showed that Cetobacterium could be helpful for protein digestion and vitamin B12 produce (Finegold et al. 2003). Meanwhile, Cetobacterium was also been found in the intestine of grass carp (Hao et al. 2017). Escherichia–Shigella and Buchnera were the dominate genera of Proteobacteria. Prevotella_9 were the dominate genera of Bacteroidetes. Studies indicated that Escherichia–Shigella, Buchnera and Prevotella_9 were belonged to conditional pathogen (Peleg et al. 2008; Hamilton et al. 2013; Scher et al. 2013). These conditional pathogens were also found in yellow catfish and grass carp (Wu et al. 2010; Hao et al. 2017). Meanwhile, Escherichia–Shigella and Buchnera in Broussonetia papyrifera group were significant higher than those in Neolamarckia cadamba group. And the Prevotella_9 in Neolamarckia cadamba group were significant higher than those in Folium mori groups. The phenomenon indicated that the woody forages influence the intestine bacteria abundance and the intestine bacteria were related with the nutrition metabolism and immunity of golden pompano. The further study would be focus on the contribution of intestine bacteria to the physiology and immunity of host.
To further analysis the intestinal bacteria community of golden pompano, the PCoA and heatmap analyses indicated that the woody forages produced less influence on the intestinal bacteria community in this study. However, previous studies indicated that the diet composition could influence the intestinal bacteria community (Schmidt et al. 2016; Yu et al. 2014). The phenomena may because the diets component played the key role in regulating intestinal bacteria community (Huyben et al. 2017).
To further understand the intestinal bacteria metabolism diversity, the intestinal bacteria metabolic function of golden pompano fed with woody forages and reference diet were analyzed and result indicated that biosynthesis of amino acids and carbon metabolism were the dominate intestinal bacteria metabolism pathways of golden pompano. Meanwhile, Ni et al. (2014) also found that the carbon metabolism were also the dominant metabolism pathways of the grass carp intestinal bacteria. ABC transporters in Broussonetia papyrifera group were significantly higher than those in the groups of reference and Folium mori and study showed that ABC transporters were benefit for the uptake of nutrition (Yan et al. 2016). Meanwhile, in the present study, glycine, serine and threonine metabolism pathways in Folium mori group were significantly higher than those in the groups of reference, Broussonetia papyrifera, Neolamarckia cadamba and Moringa oleifera Lam (P < 0.05). Meanwhile, amino acid metabolism were also found in the intestinal bacteria of turbot (Xing et al. 2013) and grass carp (Ni et al. 2014). And study showed that the amino acid metabolism of the intestinal bacteria were linearly increased with dietary nutrition levels (Wang et al. 2017). These results indicated that the woody forages influenced on the golden pompano intestinal bacteria metabolism functions and the intestinal bacteria of golden pompano may take participate in the nutrition metabolism. The further relationship between intestinal bacteria and diet digestion and absorb was needed further research.
Therefore, the predominate composition of golden pompano intestine bacteria were Proteobacteria, Firmicutes, Fusobacteria and Bacteroidetes. The 30% leafmeals of Moringa oleifera Lam, Broussonetia papyrifera, Folium mori, Neolamarckia cadamba influenced the abundance of golden pompano intestinal bacteria in this study. The dominate intestinal bacteria metabolism pathways of golden pompano fed with woody forages and reference diet were biosynthesis of amino acids and carbon metabolism. Meanwhile, the Broussonetia papyrifera may be benefit for the nutrition uptake with the ABC transporters metabolism pathway and the Folium mori may improve the amino acid metabolism with glycine, serine and threonine metabolism pathway in the intestinal bacteria of golden pompano.
In summary, we first successfully characterized the intestinal bacteria diversity of golden pompano using illumina-based high-throughput sequencing. The further study would be focus on the contributions of intestine bacteria to the physiology and immunity of host. These conclusions are great importance to understand the golden pompano intestinal bacteria diversity and exploit new diets source in aquaculture.
Additional file
Authors’ contributions
BC contributed to the sample collection, data analysis and manuscript writing; LG contributed to fish feeding and samples collection; QP contributed to experimental design and manuscript writing. All authors read and approved the final manuscript.
Acknowledgements
We are very grateful for financial support from Department of Ocean and Fishery of Guangdong Province (A201701C03) and Dr. Heizhao Lin and Zhong Huang for their material supports and kind helps in many aspects.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
The study does not contain any individual person’s data.
Ethics approval and consent to participate
All animal experiment procedures were conducted in accordance with the guidelines of the Animal Welfare Act and protocols were approved by the Institutional Animal Care and Use Committee of the South China Agriculture University.
Funding
Financial support was from the Department of Ocean and Fisheries of Guangdong Province (A201701C03), China.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- MOL
Moringa oleifera Lam leaves
- BP
Broussonetia papyrifera leaves
- FM
Folium mori leaves
- NC
Neolamarckia cadamba leaves
- Ref
reference diet
- CP
crude protein
- EE
crude lipid
- W
water
- QIIME
quantitative insights into microbial ecology
- OUTs
operational taxonomic units
- RDP
Ribosomal Database Project
- ACE
abundance-based coverage estimator
- PCoA
principal coordinate analysis
- PICRUSt
phylogenetic investigation of communities by reconstruction of unobserved states
- SRA
Sequence Read Archive
- KO
KEGG orthology
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13568-018-0550-2) contains supplementary material, which is available to authorized users.
Contributor Information
Biao Chen, Email: chenbiao11@mails.ucas.ac.cn.
Liu-ling Gao, Email: 914949489@qq.com.
Qing Pan, Email: qpan@scau.edu.cn.
References
- Adeoye AA, Jaramillo-Torres A, Fox SW, Merrifield DL, Davies SJ. Supplementation of formulated diets for tilapia (Oreochromis niloticus) with selected exogenous enzymes: overall performance and effects on intestinal histology and microbiota. Anim Feed Sci Tech. 2016;215:133–143. doi: 10.1016/j.anifeedsci.2016.03.002. [DOI] [Google Scholar]
- Afuang W, Siddhuraju P, Becker K. Comparative nutritional evaluation of raw, methanol extracted residues and methanol extracts of moringa (Moringa oleifera Lam.) leaves on growth performance and feed utilization in Nile tilapia (Oreochromis niloticus L.) Aquacult Res. 2003;34(13):1147–1159. doi: 10.1046/j.1365-2109.2003.00920.x. [DOI] [Google Scholar]
- AOAC . In: Official methods of analysis of the AOAC International. 16. Cunniff PA, editor. Arlingaton: AOAC International; 1995. [Google Scholar]
- Baldo L, Riera JL, Tooming-klunderud A, Albà MM, Salzburger W. Gut microbiota dynamics during dietary shift in eastern African cichlid fishes. PLoS ONE. 2015;10(5):e0127462. doi: 10.1371/journal.pone.0127462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AA, Hesta M, Hollants J, Janssens GP, Huys G. Phylogenetic analysis of faecal microbiota from captive cheetahs reveals underrepresentation of Bacteroidetes and Bifidobacteriaceae. BMC Microbiol. 2014;14:43. doi: 10.1186/1471-2180-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttely GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, Mcdonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsetti A, Gobbetti M, Rossi J, Damiani P. Antimould activity of sourdough lactic acid bacteria: identification of a mixture of organic acids produced by Lactobacillus sanfrancisco CB1. Appl Microbiol Biotechnol. 1998;50:253. doi: 10.1007/s002530051285. [DOI] [PubMed] [Google Scholar]
- De Oliveira MNV, Jewell KA, Freitas FS, Benjamin LA, Tótola MR, Borges AC, Moraes CA, Suen G. Characterizing the microbiota across the gastrointestinal tract of a Brazilian Nelore steer. Vet Microbiol. 2013;164(3–4):307–314. doi: 10.1016/j.vetmic.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Doi K, Kojima T, Fujimoto Y. Mulberry leaf extract inhibits the oxidative modification of rabbit and human low density lipoprotein. Biol Pharm Bull. 2000;23(9):1066–1071. doi: 10.1248/bpb.23.1066. [DOI] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Finegold SM, Vaisanen M-L, Molitoris DR, Tomzynski TJ, Song Y, Liu C, Collins MD, Lawson PA. Cetobacterium somerae sp. nov. from human feces and emended description of the genus Cetobacterium. Syst Appl Microbiol. 2003;26(2):177–181. doi: 10.1078/072320203322346010. [DOI] [PubMed] [Google Scholar]
- Hamdan AM, El-Sayed AFM, Mahmoud MM. Effects of a novel marine probiotic, Lactobacillus plantarum AH 78, on growth performance and immune response of Nile tilapia (Oreochromis niloticus) J Appl Microbiol. 2016;120(4):1061–1073. doi: 10.1111/jam.13081. [DOI] [PubMed] [Google Scholar]
- Hamilton MJ, Weingarden AR, Unno T, Khoruts A, Sadowsky MJ. High-throughput DNA sequence analysis reveals stable engraftment of gut microbiota following transplantation of previously frozen fecal bacteria. Gut Microbes. 2013;4(2):125–135. doi: 10.4161/gmic.23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao YT, Wu SG, Jakovlić I, Zou H, Li WX, Wang GT. Impacts of diet on hindgut microbiota and short-chain fatty acids in grass carp (Ctenopharyngodon idellus) Aquacult Res. 2017;48(11):5595–5605. doi: 10.1111/are.13381. [DOI] [Google Scholar]
- Hassaan MS, Soltan MA, Jarmołowicz S, Abdo HS. Combined effects of dietary malic acid and Bacillus subtilis on growth, gut microbiota and blood parameters of Nile tilapia (Oreochromis niloticus) Aquacult Nutr. 2017;24(1):83–93. doi: 10.1111/anu.12536. [DOI] [Google Scholar]
- Huyben D, Nyman A, Vidaković A, Passoth V, Moccia R, Kiessling A, Dicksved J, Lundh T. Effects of dietary inclusion of the yeasts Saccharomyces cerevisiae and Wickerhamomyces anomalus on gut microbiota of rainbow trout. Aquaculture. 2017;473:528–537. doi: 10.1016/j.aquaculture.2017.03.024. [DOI] [Google Scholar]
- Johnson CN, Barnes S, Ogle J, Grimes DJ, Chang YJ, Peacock AD, Kline L. Microbial community analysis of water foregut and hindgut during growth of pacific white shrimp, Litopenaeus vannamei, in closed-system aquaculture. J Word Aquacult Soc. 2008;39(2):251–258. doi: 10.1111/j.1749-7345.2008.00155.x. [DOI] [Google Scholar]
- Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94(1):58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang XL, Liu G, Liu YF, Xu QQ, Zhang M, Fang MY. Transcriptome profile at different physiological stages reveals potential mode for curly fleece in Chinese tan sheep. PLoS ONE. 2013;8(8):e71763. doi: 10.1371/journal.pone.0071763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koetschan C, Kittelmann S, Lu JL, Al-Halbouni D, Jarvis GN, Müller T, Wolf M, Janssen PH. Internal transcribed spacer 1 secondary structure analysis reveals a common core throughout the anaerobic fungi (Neocallimastigomycota) PLoS ONE. 2014;9(3):e91928. doi: 10.1371/journal.pone.0091928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71(12):8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons PP, Turnbull JF, Dawson KA, Crumlish M. Exploring the microbial diversity of the distal intestinal lumen and mucosa of farmed rainbow trout Oncorhynchus mykiss (Walbaum) using next generation sequencing (NGS) Aquacult Res. 2015;48(1):77–91. doi: 10.1111/are.12863. [DOI] [Google Scholar]
- Merrifield DL, Dimitroglou A, Bradley G, Baker RTM, Davies SJ. Soybean meal alters autochthonous microbial populations, microvilli morphology and compromises intestinal enterocyte integrity of rainbow trout, Oncorhynchus mykiss (Walbaum) J Fish Dis. 2009;32(9):755–766. doi: 10.1111/j.1365-2761.2009.01052.x. [DOI] [PubMed] [Google Scholar]
- Nahid R, Perumal S, Klaus B. Evaluation of nutritional quality of moringa (Moringa oleifera Lam.) leaves as an alternative protein source for Nile tilapia (Oreochromis niloticus L.) Aquaculture. 2003;217(1–4):599–611. [Google Scholar]
- Navarrete P, Toledo I, Mardones P, Opazo R, Espejo R, Romero J. Effect of Thymus vulgaris essential oil on intestinal bacterial microbiota of rainbow trout, Oncorhynchus mykiss (Walbaum) and bacterial isolates. Aquacult Res. 2010;41(10):e667–e678. [Google Scholar]
- Ni JJ, Yan QY, Yu YH, Zhang TL. Factors influencing the grass carp gut microbiome and its effect on metabolism. FEMS Microbiol Ecol. 2014;87(3):704–714. doi: 10.1111/1574-6941.12256. [DOI] [PubMed] [Google Scholar]
- Niu J, Du Q, Lin HZ, Cheng YQ, Huang Z, Wang Y, Wang J, Chen YF. Quantitative dietary methionine requirement of juvenile golden pompano Trachinotus ovatus at a constant dietary cystine level. Aquacult Nutr. 2013;19(5):677–686. doi: 10.1111/anu.12015. [DOI] [Google Scholar]
- Pan Q, Liu S, Tan YG, Bi YZ. The effect of chromium picolinate on growth and carbohydrate utilization in tilapia, Oreochromis niloticus × Oreochromis aureus. Aquaculture. 2003;225(1):421–429. doi: 10.1016/S0044-8486(03)00306-5. [DOI] [Google Scholar]
- Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30(21):3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21(3):538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungrassamee W, Klanchui A, Maibunkaew S, Chaiyapechara S, Jiravanichaisal P, Karoonuthaisiri N. Characterization of intestinal bacteria in wild and domesticated adult black tiger shrimp (Penaeus monodon) PLoS ONE. 2014;9(3):e91853. doi: 10.1371/journal.pone.0091853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, Huttenhower C, Littman DR. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt V, Amaral-Zettler L, Davidson J, Summerfelt S, Good C. The influence of fishmeal-free diets on microbial communities in Atlantic salmon Salmo salar recirculation aquaculture systems. Appl Environ Microbiol. 2016;82:4470–4481. doi: 10.1128/AEM.00902-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence C, Wells WG, Smith CJ. Characterization of the primary starch utilization operon in the obligate anaerobe Bacteroides fragilis: regulation by carbon source and oxygen. J Bacteriol. 2006;188(13):4663–4672. doi: 10.1128/JB.00125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner-Jeffreys DW, Shields RJ, Bricknell IR, Birkbeck TH. Changes in the gut-associated microflora during the development of Atlantic halibut (Hippoglossus hippoglossus L.) larvae in three British hatcheries. Aquaculture. 2003;219(1–4):21–42. doi: 10.1016/S0044-8486(02)00348-4. [DOI] [Google Scholar]
- Wang YY, Gao PH, Wang L, Zhao ZZ, Chen YL, Yang YX. Bacterial community diversity associated with different levels of dietary in the rumen of sheep. Appl Microbiol Biotechnol. 2017;101:3717–3728. doi: 10.1007/s00253-017-8144-5. [DOI] [PubMed] [Google Scholar]
- Wu SG, Gao TH, Zheng YZ, Wang WW, Cheng YY, Wang GT. Microbial diversity of intestinal contents and mucus in yellow catfish (Pelteobagrus fulvidraco) Aquaculture. 2010;303(1–4):1–7. doi: 10.1016/j.aquaculture.2009.12.025. [DOI] [Google Scholar]
- Xi D, Li J, Kuang YW, Xu YM, Zhu XM. Influence of traffic exhausts on elements and polycyclic aromatic hydrocarbons in leaves of medicinal plant Broussonetia papyrifera. Atmos Pollut Res. 2013;4(4):370–375. doi: 10.5094/APR.2013.042. [DOI] [Google Scholar]
- Xing MX, Hou ZH, Yuan JB, Yuan L, Qu YM, Liu B. Taxonomic and functional metagenomic profiling of gastrointestinal tract microbiome of the farmede adult turbot (Scophthalmus maximus) Fems Microbiol Ecol. 2013;86(3):432–443. doi: 10.1111/1574-6941.12174. [DOI] [PubMed] [Google Scholar]
- Yan X, Luo XG, Zhao M. Metagenomic analysis of microbial community in uranium-contaminated soil. Appl Microbiol Biot. 2016;100(1):299–310. doi: 10.1007/s00253-015-7003-5. [DOI] [PubMed] [Google Scholar]
- Ye L, Amberg J, Chapman D, Gaikowski M, Liu WT. Fish gut microbiota analysis differentiates physiology and behavior of invasive Asian carp and indigenous American fish. ISME J. 2014;8(3):541–551. doi: 10.1038/ismej.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HN, Zhu J, Pan WS, Shen SR, Shan WG, Das UN. Effects of fish oil with a high content of n–3 polyunsaturated fatty acids on mouse gut microbiota. Arch Med Res. 2014;45(3):195–202. doi: 10.1016/j.arcmed.2014.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


