Abstract
Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is a well-known tumor suppressor that acts as a dual-specificity phosphatase and is frequently mutated in human cancer. Our previous work has demonstrated that PTEN also plays a vital role in type I interferon responses and antiviral innate immunity. Recently, a translational variant of PTEN with a long N-terminal extension (PTEN-L) has been discovered that is secreted into the extracellular environment and enters recipient cells, where it exerts a phosphatase function antagonistic to PI3K/Akt signaling and tumorigenesis. In this study, we demonstrate that PTEN-L promotes type I interferon responses and antiviral innate immunity during viral infection in a phosphatase activity-dependent manner. Compared with canonical PTEN, PTEN-L also exerts its antiviral function when it is applied exogenously in protein form. This finding was confirmed in cell cultures and mouse infection models. Furthermore, PTEN-L enhances the responses of both type I interferon and proinflammatory cytokines, thus suggesting that PTEN-L might possess additional functions compared with those of PTEN. Thus, the antiviral function of PTEN-L may open an avenue for the use of PTEN-L in antiviral therapy, particularly in patients with PTEN-deficient tumors.
Keywords: antiviral responses, innate immunity, PTEN, PTEN-L, type I interferon
Introduction
Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is widely known as a tumor suppressor, and its deficiency results in a variety of human tumors, including brain, breast and prostate tumors.1, 2 PTEN is a phosphatase with 403 amino acids that can dephosphorylate both protein and lipid substrates. The phosphorylation status of the C terminal tail may influence the substrate-specificity of PTEN.3 Phosphatidylinositol 3, 4, 5 triphosphate (PIP3) is the primary substrate of PTEN.4, 5, 6, 7 PTEN antagonizes phosphoinositol-3-kinase (PI3K) via hydrolyzing PIP3 to phosphatidylinositol 4, 5-bisphosphate (PIP2), thus leading to inhibition of the PI3K/Akt/mTOR pathway and restriction of cell growth, proliferation and survival.8, 9, 10 Recently, our group has demonstrated that in addition to its established role in tumor suppression, PTEN plays a critical role in antiviral innate immunity.11, 12 We have demonstrated that the nuclear import of interferon regulatory factor 3 (IRF3), a master transcription factor for interferon (IFN)-β production, is controlled by the phosphorylation status of Ser97. Specifically, phosphorylation results in an inactive protein, whereas dephosphorylation is associated with activation. PTEN dephosphorylates IRF3 at Ser97, thus resulting in release of IRF3 for nuclear import and activation.11
Innate immunity is the first-line defense against antiviral or antimicrobial infection. Innate immunity cooperates with adaptive immunity in eliminating foreign invasion. Initially, pattern recognition receptors (PRRs), including retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), Toll-like receptors (TLRs), NOD-like receptors (NLRs) and DNA sensors recognize pathogen-associated molecular patterns (PAMPs), which are conserved structures in microbes.13, 14 Different PAMPs are sensed by specific PRRs. RLRs, including RIG-I, MDA5 and LGP2, mainly recognize cellular double-stranded RNA or single-stranded RNA from viruses.15, 16, 17 TLRs are located on the cell surface or in endosomes. Most TLRs recruit adaptor MyD88, thereby activating downstream signals and producing proinflammatory cytokines or type-I IFN.18 TLR3 and TLR4 recruit TRIF and consequently activate antiviral responses.19 In antiviral responses, all of these signaling pathways lead to the activation of IRF3 and IRF7 and the establishment of an antiviral status. Our previous finding that PTEN is directly involved in the regulation of antiviral innate immunity indicates cross-talk between anti-tumor and antiviral signaling pathways.
Interestingly, a new form of PTEN, named PTEN-L, has been identified that is translated from an alternative translation initiation codon (CUG) in the 5′ UTR of PTEN messenger RNA (mRNA).20, 21 Compared with PTEN, PTEN-L contains an N-terminal extension of 173 amino acids, which contains a secretion signal for extracellular secretion and a poly-arginine stretch as a cell-penetrating signal sequence similar to the HIV transactivator of transcription (TAT) protein.20 PTEN-L also inhibits the PI3K/Akt/mTOR pathway and suppresses tumorigenesis.20, 22 Given its secretion and cell penetration characteristics, PTEN-L may represent a new horizon for the development of PTEN-based cancer therapy. The N-terminal extension of PTEN-L is intrinsically disordered and contains numerous posttranslational modification sites and protein-interacting motifs, thus indicating that PTEN-L, compared with PTEN, may possess different properties and exhibit additional regulatory functions.23 For example, PTEN-L is in a constitutively active state, whereas PTEN exhibits PIP2-mediated activation.24 PTEN-L also regulates mitochondrial function and energy metabolism.21
Although PTEN-L antagonizes the PI3K/Akt signaling pathway and suppresses tumorigenesis, it is uncertain whether PTEN-L regulates IFN responses and antiviral innate immunity in a manner similar to that of PTEN. In this study, we demonstrated that PTEN-L promotes type I IFN responses and antiviral activity during viral infection in a manner similar to that of PTEN. However, in contrast to PTEN, PTEN-L stimulates the production of proinflammatory cytokines. We further demonstrated that exogenous expression or extracellular administration of secreted or recombinant PTEN-L enhances the antiviral responses of host cells. Collectively, the current study reveals a previously uncharaterized function of PTEN-L and might aid in development of new strategies for the treatment of viral diseases via delivery of PTEN-L, especially in PTEN-deficient patients.
Materials and methods
Viruses, cell culture and transfection
Sendai virus (SeV) and vesicular stomatitis virus (VSV) carrying a GFP reporter gene (VSV-GFP) were kindly provided by Dr Hong-Bing Shu. Wildtype (WT) VSV was provided by Dr Ming-Zhou Chen. Pten−/− and WT mouse embryonic fibroblasts (MEFs) were provided by Dr Yu-Xin Yin and Dr Hong Wu. The PC-3 cell line was purchased from CCTCC. HEK293, HEK293T, Pten−/− and WT MEFs were maintained in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. PC-3 was maintained in RPMI 1640 medium with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. PC-3 cells were transfected with FuGENE HD transfection reagent (Promega, Madison, WI, USA) according to the manufacturer’s instructions. HEK293T cells were transfected with NEOFECT DNA transfection reagent (Neofect Biotech, Beijing) according to the manufacturer’s instructions.
Plasmids
PTEN-L complementary DNA (cDNA) was amplified from total RNA obtained from SeV-stimulated HEK293T cells. PTEN-L was cloned into pEF by using Afl II and Mlu I, the pCDNA3.1 vector by using EcoR I and Xho I, and pHAGE by using Xho I and BamH I. PTEN-L (ATG/ATA) and C297S-L were constructed with a Site-Directed Mutagenesis Kit (TOYOBO), with which the canonical ATG start codon of PTEN was changed to ATA, and the CTG codon was changed to ATG.
Lentiviral packaging and infection
A lentiviral system was utilized to obtain stably transfected cells. PTEN, PTEN-L (ATG/ATA and CTG/ATG) and C297S-L were cloned separately into the lentiviral vector pHAGE. Then, the lentiviral backbone (8 μg), psPAX2 (6 μg) and pMD2.G (3 μg) were transiently transfected into HEK293T cells plated on 100-mm dishes. After 48 h, supernatants were collected and used to infect Pten−/− MEF or PC-3 with polybrene (8 μg/ml). Cells were infected twice to obtain higher transduction efficiency, and puromycin (0.5 μg/ml) was used to screen positive cells.
Western blotting
Cells were washed once with PBS and lysed in RIPA buffer (50 mM Tris, pH 7.6, 1% NP-40, 150 mM NaCl, 0.1% SDS). Protein samples were mixed with 5 × SDS loading buffer and boiled for 5 min. Samples were resolved by SDS–PAGE, transferred to a nitrocellulose membrane (GE Healthcare, Little Chalfont, Buckinghamshire, UK), blocked with TBS containing 0.1% Tween-20 and 5% skim milk and probed with antibodies to PTEN, p-IRF3 (Ser396), IκBα, p-IκBα (CST), Flag (Sigma, St Louis, MO, USA), p65 (Abcam, Cambridge, MA, USA), IRF3, 6 × His tag, LMNB1, β-tubulin, GAPDH and β-actin (Proteintech, Chicago, IL, USA). The antibody to p-IRF3 (Ser97) was obtained in our previous study.11
RNA isolation and quantitative RT-PCR
Total RNA was isolated from Pten−/− MEF and PC-3 cells with TRIzol reagent (Invitrogen, Carlsbad, CA, USA), per the manufacturer’s instructions. mRNA was reverse transcribed into cDNA by M-MLV Reverse Transcriptase (Promega). qRT-PCR was performed with an ABI 7300 Real TIME PCR System. GAPDH was used to normalize the input samples via the ΔΔCt method. Primer sequences were as reported in our previous work.11
VSV plaque assays
The indicated stably transfected cells were plated one day before infection. After infection with VSV-GFP for 2 h, cells were washed three times with PBS, and fresh medium was added. After 24 h, supernatants were collected and diluted to infect VERO cells that were plated on 24-well plates at 90% confluence. After 2 h, supernatants were removed, and methylcellulose was added to plates. Three days later, cells were fixed with formaldehyde (4%) for 20 min and stained with crystal violet (0.2%) for 2 h. Plaques were counted to obtain the viral titer (pfu/ml).
ELISA
Cells were infected with SeV for 8 h. Cytokines were detected by using a human IFN-β ELISA kit (R&D, Minneapolis, MN, USA), human IL-6 ELISA kit (Dakewe Biotech, Shenzhen, China), mouse IFN-β ELISA kit (Biolegend, San Diego, CA, USA), and mouse IL-6 and CXCL1 ELISA kits (Neobioscience Technology, Shenzhen, China).
PTEN-L assays in cell cultures
Briefly, 10 μg of indicated expression plasmids were transfected into HEK293T cells, which were plated on 100 mm-dishes. After 48 h, supernatants were harvested, centrifuged and filtered through a 0.45-micron filter. Pten−/− MEF cells were plated on 12-well plate for 24 h. The culture medium of the 12-well plate was removed, and supernatants collected from HEK293T were added to Pten−/− MEF cultures. After 24 h, supernatants were removed, and VSV-GFP was used to infect the treated cells. Supernatants were also incubated with Pierce Ni-NTA Magnetic Agarose Beads (Thermo Fisher) and then used to treat Pten−/− MEF, as described above. Purified recombinant PTEN-L protein was added into the culture medium of Pten−/− MEFs for 24 h, and then VSV-GFP was used to infect the treated Pten−/− MEF.
Purification of recombinant PTEN and PTEN-L
pRSFDuet-1-PTEN-LΔN21-His or pRSFDuet-1-PTEN-His was transformed into E. coli BL21 grown in Luria broth (LB) medium containing kanamycin at 37 °C. When the OD600 nm value of cell cultures was between 0.6 and 1, isopropyl-β-D-thiogalactopyranoside (IPTG) was added to a final concentration of 0.2 mM, and cell cultures were induced at 20 °C for 18 h. Bacterial cells were harvested by centrifugation and lysed by using a high-pressure homogenizer. His-tagged fusion proteins were purified from cell lysates by affinity chromatography using Ni-NTA His-Bind Resin (Novagen, Madison, WI, USA) according to the manufacturer's instructions.
Mouse experiments
Conditional Pten-knockout mice were obtained in our previous work.11 Six-week-old female mice were used in the experiment. The indicated purified PTEN or PTEN-L protein (5 mg/kg) was injected into mice though the tail vein for 3 days. Then, 1 × 108 VSV were intraperitoneally injected into mice. All animal experiments were performed in accordance with the Regulations of Hubei Province Laboratory Animal Management and approved by Animal Experiment Ethics Committee in Wuhan University, China.
Nuclear/Cytosolic fractionation
Nuclear and cytoplasmic extracts were obtained using a Nuclear-Cytosol Extraction Kit (Applygen Technologies, Beijing, China) according to the manufacturer’s instructions.
Results
PTEN-L promotes type I IFN responses
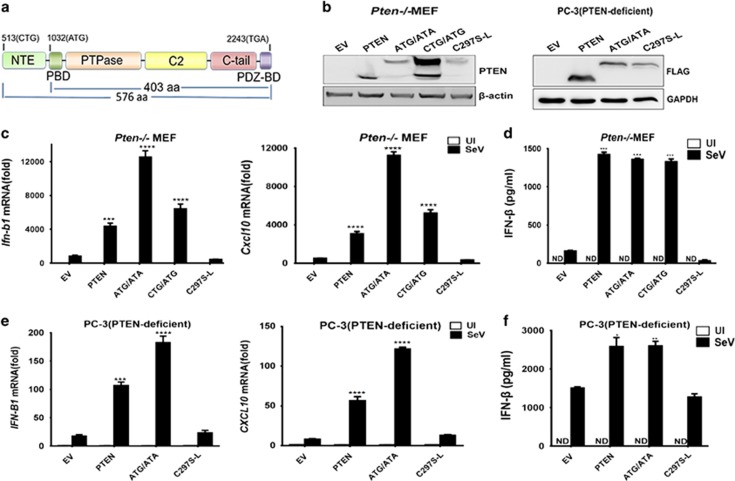
To reveal the roles of PTEN-L in innate immunity and antiviral responses, we first observed whether PTEN-L promoted type I IFN responses during viral infection. We generated three PTEN-L expression constructs in both lentiviral and other expression vectors: PTEN-Lctg/atg (CTG/ATG for short), which contained the sequence of wild-type PTEN gene and expressed both canonical PTEN (from ATG codon) and long isoform PTEN-L (from non-canonical start codon CTG); PTEN-Latg/ata (ATG/ATA for short), in which the CTG codon was changed to ATG and canonical PTEN ATG to ATA so that only PTEN-L but no PTEN was produced; and C297S-L, in which the phosphatase active site cysteine at aa 297 was replaced by serine in the backbone of construct ATG/ATA, thus resulting in a phosphatase-dead PTEN-L mutant that was used as a negative control (Figure 1a).
Figure 1.
PTEN-L promotes type I IFN responses. (a) Domain organization of PTEN and PTEN-L. (b) Detection of PTEN-L and PTEN expression in MEFs and PC-3 cells. PTEN-null cells expressing empty vector (EV), PTEN-Latg/ata (ATG/ATA for short), PTEN-Lctg/atg (CTG/ATG for short) and the phosphatase dead mutant C297S-L, which was derived from ATG/ATA, were lysed and resolved by SDS–PAGE. PTEN expression was analyzed by anti-PTEN immunoblotting. (c and e) Quantitative RT-PCR assays to measure the relative expression levels of IFN-B1 and CXCL10. The stably transfected MEFs or transiently transfected PC-3 cells were uninfected (UI for short) or infected with SeV (50 HA Units/ml) for 8 h. Cells were lysed in TRIzol to isolate RNA that was reverse transcribed to cDNA for q-PCR analysis. (d and f) Cell culture supernatants were harvested for ELISA. The data represent the average of three independent experiments and were analyzed with a two-tailed unpaired t test. Graphs indicate mean±s.d. (n=3) derived from three independent experiments. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
We then transduced or transfected the indicated constructs into PTEN-knockout MEFs (Pten−/− MEF) or PTEN-deficient PC-3 cells so that all the PTEN-L isoforms and mutants were stably expressed in MEFs or transiently expressed in PC-3 (Figure 1b). The lentiviral vector-transduced PTEN-expressing and PTEN-null MEFs were obtained from our previous study.11 As shown in Figure 1c, expression of PTEN-L (both ATG/ATA and CTG/ATG) stimulated the transcription of the IFN-β gene (Ifn-b1) and its downstream gene Cxcl10 during SeV infection, and the levels were comparable to that of PTEN. In contrast, the phosphatase-dead mutant C297S-L and PTEN-null control did not promote the type I IFN responses. In agreement with this observation, the level of secreted IFN-β in the culture supernatant of PTEN-L was significantly higher than that of the phosphatase-dead C297S-L (Figure 1d). These results also suggested that the function of PTEN-L in IFN responses was also phosphatase activity-dependent, similarly to canonical PTEN.
Moreover, we also assessed the effect of PTEN-L in the human prostate cancer cell PC-3, which lacks PTEN. As shown in Figures 1e and f, the results were consistent with those in PTEN-knockout MEFs. Altogether, these results indicated that PTEN-L promoted type I IFN responses during viral infection in a phosphatase-dependent manner.
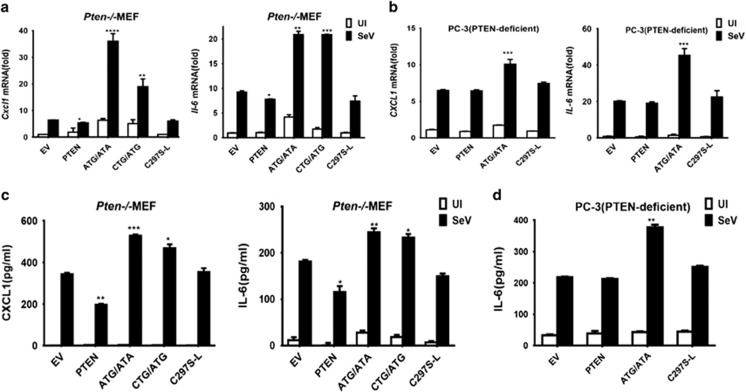
PTEN-L promotes production of proinflammatory factors
In innate immune responses, both antiviral type I IFN and inflammatory cytokines are commonly induced. However, our previous study has demonstrated that canonical PTEN mainly contributes to the stimulation of type I IFN production but not proinflammatory cytokines.11 Given that PTEN-L, compared with PTEN, contains a long N-terminal extension, it may acquire additional functions. Therefore, we further investigated whether PTEN-L might promote the proinflammatory cytokine response. We used PTEN-knockout MEFs (Pten−/− MEF) and human PC-3 cells that were reconstituted with different variants of PTEN-L, as described above. Expression of proinflammatory cytokines CXCL1 and IL-6 was detected after challenge with SeV. PTEN-L expression markedly enhanced the mRNA levels of the proinflammatory cytokines in both types of cells infected with SeV, whereas PTEN did not (Figures 2a and b). Furthermore, the levels of secreted IL-6 and CXCL1 in the culture supernatants were significantly higher than those in PTEN-knockout MEFs expressing PTEN or PTEN-L dead control (Figure 2c) and PC-3 (Figure 2d). Collectively, these data suggested that PTEN-L stimulates production of proinflammatory cytokines and type I IFN. This finding is in clear contrast to the notion that PTEN that activates only IFN responses.
Figure 2.
PTEN-L stimulates proinflammatory factors. (a and b) Quantitative RT-PCR was conducted to measure the relative expression levels of CXCL1 and IL-6. Stably transfected MEFs or transiently transfected PC-3 cells were uninfected (UI for short) or infected with SeV (50 HA Units/ml) for 8 h. Cells were lysed in TRIzol to isolate RNA, which was reverse transcribed to cDNA for further qPCR analysis. (c and d) Analysis of proinflammatory cytokines from the cell culture supernatants, as assessed by ELISA. The data represent the average of three independent experiments and were analyzed by two-tailed unpaired t test. Graphs indicate mean±s.d. (n=3) derived from three independent experiments. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
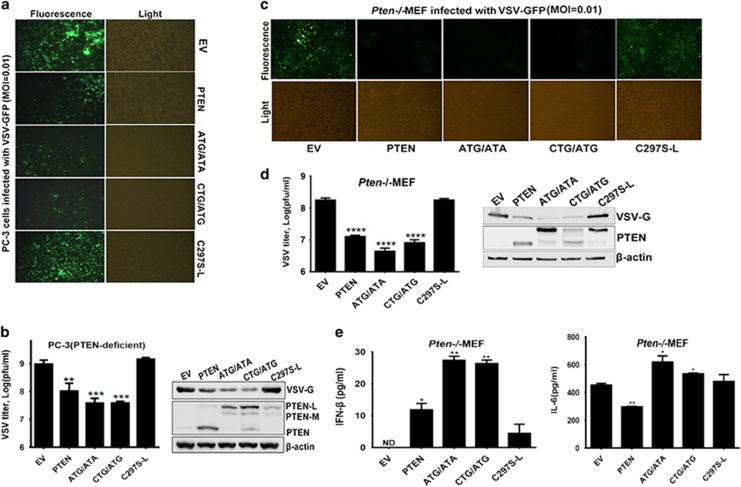
PTEN-L enhances cellular antiviral responses
Given that PTEN-L is involved in the type I IFN and inflammatory responses, we next assessed whether PTEN-L affected antiviral activity. We infected indicated types of PC-3 cells with IFN-sensitive VSV-GFP and detected virus propagation. In this commonly used system, the intensity of green fluorescence correlates with the amount of viral replication. As shown in Figure 3a, expression of PTEN-L (ATG/ATA or CTG/ATG) and PTEN remarkably decreased the fluorescence compared with expression of C297S-L or empty vector control. Accordingly, VSV titers and the level of VSV-encoded glycoprotein G (VSV-G) were lower in the PTEN- or PTEN-L-expressing cells (Figure 3b). One band smaller than PTEN-L but larger than PTEN was also observed, which might represent another isoform of PTEN (PTEN-M by size)25 or a degradation product of PTEN-L. Similar results were observed in MEFs (Figures 3c and d). We also detected the levels of secreted IFN-β and IL-6 in the culture supernatants (Figure 3e), and the results were consistent with those after SeV infection, as shown in Figure 2. Altogether, the results showed that PTEN-L substantially promotes antiviral activity in infected cells.
Figure 3.
PTEN-L enhances cellular antiviral responses. (a and c) PTEN-null cells reconstituted with empty vector (EV), PTEN, PTEN-Latg/ata (ATG/ATA), PTEN-Lctg/atg (CTG/ATG) and the phosphatase dead mutant C297S-L were exposed to VSV-GFP (MOI=0.01) infection for 36 h. The replication of VSV-GFP was assessed by microscopy. (b and d) Supernatants were subjected to VSV plaque assays. Cells were lysed and then subjected to SDS–PAGE. VSV replication was analyzed by anti-VSV-G and β-actin immunoblotting. PTEN expression was analyzed by anti-PTEN immunoblotting. (e) Pten−/− MEF supernatants were also collected for ELISA. Graphs represent mean±s.d. (n=3) derived from three independent experiments. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
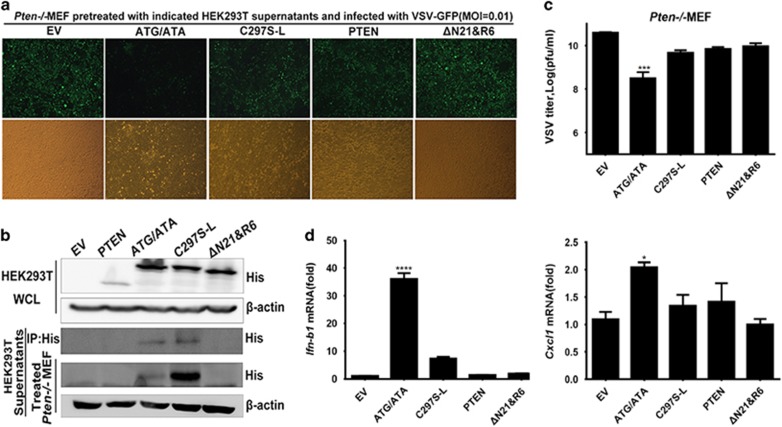
Secreted PTEN-L mediates type I IFN responses and antiviral immunity
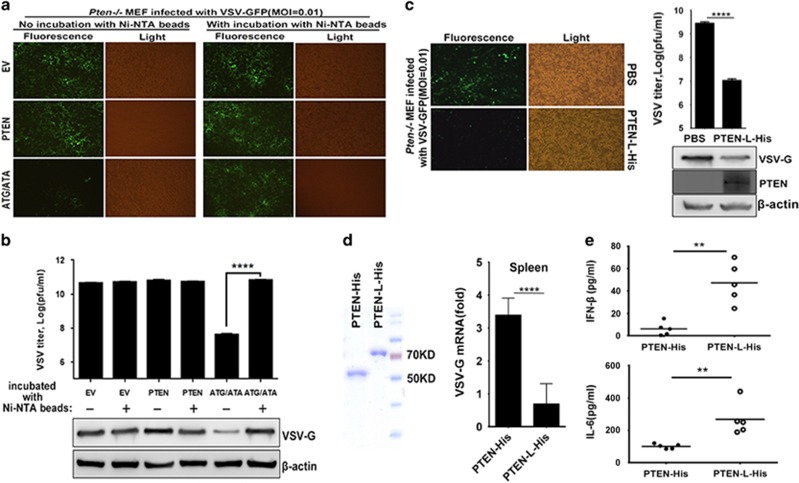
PTEN-L has been identified as a secreted and cell-penetrating protein;20 thus, PTEN-L protein might potentially be developed into an antiviral agent for direct application. We overexpressed His-tagged PTEN, PTEN-L, phosphatase-dead mutant C297S-L and PTEN-LΔN21&R6 (ΔN21&R6 for short) in HEK293T cells. The PTEN-L ΔN21&R6 mutant contained a deletion of both the secretion and penetration signal and was incapable of secreting and entering cells. Cell culture supernatants that might have contained the secreted PTEN-L were then collected and used to treat PTEN-knockout MEFs. As shown in Figure 4a, the PTEN-knockout MEFs treated with secreted PTEN-L but not the phosphatase-dead mutant displayed less virus propagation. Treatment with supernatants from PTEN- and ΔN21&R6-expressing cell cultures did not exert antiviral effects on PTEN-knockout MEFs (Figure 4a), but this lack of activity might have resulted from the inability to secrete PTEN and ΔN21&R6. To demonstrate that overexpressed PTEN-L was secreted from HEK293T cells and penetrated Pten−/− MEF, the supernatants were incubated with Ni-NTA magnetic agarose beads that bound to the 6x-histidine-tagged PTEN-L. PTEN-L and C297S-L were secreted from HEK293T and penetrated Pten−/−MEFs but not PTEN and ΔN21&R6 (Figure 4b). Thus, phosphatase activity is important for the role of PTEN-L in antiviral activity. In agreement with fluorescence assay results, the virus titer was decreased (Figure 4c), and type I IFN and CXCL1 mRNA levels were increased in the cells treated with PTEN-L compared with control cells (Figure 4d).
Figure 4.
Secreted PTEN-L mediates type I IFN responses and antiviral immunity. (a) Pten−/− MEFs were treated with the supernatants of HEK293T cell cultures transfected with the following PTEN variants: PTEN-Latg/ata (ATG/ATA), C297S-L, PTEN and PTEN-LΔN21&R6 (ΔN21&R6) or empty vector (EV). Then, cells were infected with VSV-GFP. VSV-GFP replication was observed by microscopy. (b) HEK293T supernatants were collected. Cells were lysed and then subjected to SDS–PAGE. Collected supernatants were incubated with Ni-NTA magnetic agarose beads to pull down His-tagged protein. Treated MEFs were lysed and subjected to SDS–PAGE analysis of secreting and cell-penetrating of PTEN-L. (c) Pten−/− MEF supernatants were subjected to VSV plaque assays. (d) Pten−/− MEF cells were lysed in TRIzol to isolate RNA, which was reverse transcribed to cDNA. Quantitative RT-PCR was conducted to measure the relative expression levels of IFN-B1 and CXCL1. The data represent the average of three independent experiments and were analyzed by two-tailed unpaired t test. Graphs show the mean±s.d. (n=3) derived from three independent experiments. *P<0.05, ***P<0.001, ****P<0.0001.
Extracellular application of PTEN-L protein mediates antiviral responses in cell cultures and in vivo
To demonstrate that the antiviral effect was caused by secreted PTEN-L instead of other antiviral molecules, supernatants of indicated transfected HEK293T cells were incubated with Ni-NTA magnetic agarose beads to deplete the His-tagged PTEN-L and then used to treat Pten−/− MEFs. As shown in Figure 5a, after depletion of PTEN-L, the inhibition of virus propagation by the PTEN-L supernatant was no longer evident. However, regardless of incubation with beads, the supernatants of empty vector and PTEN did not exhibit any antiviral effects. The results of VSV titers and the levels of VSV-G were consistent with the fluorescence results (Figure 5b). We also prepared recombinant PTEN-L protein (PTEN-L-His) from an E. coli expression system. The purified PTEN-L-His or PBS control was added into the culture medium of Pten−/− MEFs. As shown in Figure 5c, the PTEN-knockout MEFs treated with PTEN-L-His exhibited less virus propagation. Moreover, VSV titers and VSV-G levels were lower in PTEN-L-His-treated cells than in cells treated with PBS (Figure 5c). Altogether, these results indicated that PTEN-L inhibits virus replication when it is applied extracellularly in cell cultures.
Figure 5.
Extracellular application of PTEN-L protein mediates antiviral responses in cell cultures and in vivo. (a) The supernatants of indicated transfected HEK293T cells were incubated with or without Ni-NTA magnetic agarose beads and used to treat Pten−/− MEF. VSV-GFP (MOI=0.01) was used to infect the treated Pten−/− MEF, and VSV-GFP replication was observed by microscopy. (b) Supernatants were subjected to VSV plaque assays. Cells were lysed and then subjected to SDS-PAGE. VSV replication was analyzed by anti-VSV-G and β-actin immunoblotting. (c) Pten−/− MEF cells were treated with PBS or 100 nM purified proteins from pRSFDuet-1-PTEN-LΔN21-His (PTEN-L-His) and infected by VSV-GFP (MOI=0.01). Supernatants were subjected to VSV plaque assays. Cells were lysed and then subjected to SDS-PAGE. VSV replication was analyzed by anti-VSV-G and β-actin immunoblotting. PTEN-L-His penetration into Pten−/− MEF cells was confirmed by anti-PTEN immunoblotting. (d) Recombinant purified PTEN-His and PTEN-L-His used for mice experiments were analyzed by SDS-PAGE and Coomassie BB staining. PTEN mutant mice were injected with purified PTEN or PTEN-L protein (5 mg/kg) for three days and then infected intraperitoneally with VSV at 1 × 108 pfu per mouse (n⩾5; 6 weeks old). Quantitative RT-PCR analysis of mRNA encoding VSV-G protein in the spleen in the indicated treated mice after infected with VSV for 24 h. (e) ELISA analysis of IFN-β and IL-6 in serum from PTEN- or PTEN-L-treated mice was also performed. The data represent average of three independent experiments and were analyzed by two-tailed unpaired t test. Graphs indicate mean±s.d. (n=3) derived from three independent experiments. **P<0.01, ****P<0.0001.
We also observed the antiviral effect of recombinant PTEN-L protein in vivo by using a mouse model. Purified PTEN-L-His or PTEN-His was injected into PTEN-mutant mice though the tail vein. VSV was then used to infect mice. In accordance with the observations in the cell culture model, we found that VSV titers in mice injected with PTEN-L-His were noticeably lower than those of mice injected with PTEN-His (Figure 5d). Moreover, we also assessed IFN-β and IL-6 serum levels in mice injected with indicated proteins after VSV challenge. In agreement with results from the cell culture model, injection of the long isoform PTEN-L caused PTEN-mutant mice to acquire higher antiviral innate immune responses (Figure 5e). Altogether, treatment of cells or animals with PTEN-L protein promoted antiviral responses and suppressed viral infection.
Mechanism of PTEN-L-mediated antiviral innate immunity
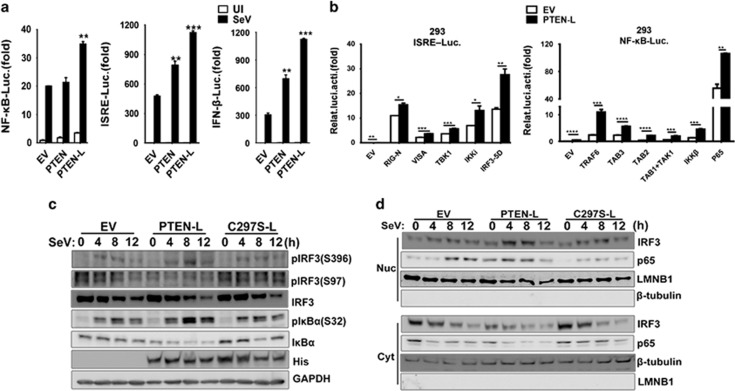
In the previous results, we found that PTEN-L promoted both type I IFN and proinflammatory responses. However, the specific mechanism was unclear. Thus, we sought to investigate how PTEN-L promoted these two pathways. Using reporter assays, we first confirmed that PTEN-L enhanced the activation of the IFNB1 promoter, interferon-stimulating regulatory element (ISRE) promoter containing IRF3-responsive positive regulatory domains (PRD) III and I and NF-κB responsive promoter, whereas PTEN enhanced the activation of the IRF3-responsive element (Figure 6a). Then, we next observed the level at which PTEN-L functioned. As shown in Figure 6b, expression of PTEN-L markedly promoted the IRF3-responsive promoter ISRE reporter induced by upstream activators, including RIG-I, MAVS, TBK1 and IKKε, and the constitutively active phosphorylation mimetic IRF3-5D. These results suggested that PTEN-L might adopt a mechanism similar to that of PTEN for its antiviral function. In addition, TRAF6-, TAB3-, TAB2-, TAK1-, IKKβ- and p65-induced activation of NF-κB was enhanced when PTEN-L was overexpressed (Figure 6b right panel). We then transiently transfected PTEN-deficient PC-3 cells with empty vector, PTEN-L and C297S-L and detected the active state of IRF3 and NF-κB. The phosphorylation state of IRF3 at Ser97 was maintained at a low level in the presence of PTEN-L compared with empty vector and C297S-L (Figure 6c). In contrast, the phosphorylation of Ser396 of IRF3 was not decreased. This observation further confirmed that PTEN-L adopted a mechanism in phosphatase-dependent IRF3 activation similar to that of canonical PTEN. In the NF-κB pathway, PTEN-L significantly promoted the phosphorylation of IκBα at Ser32, a result consistent with the low level of IκBα (Figure 6c). Given that the phosphorylation of IRF3 and IκBα influenced nuclear translocation of IRF3 and p65, respectively, we then analyzed the nuclear import of IRF3 and p65 regulated by PTEN-L. After transfection with indicated plasmids into PC-3 cells, we found that reconstitution with PTEN-L facilitated the nuclear import of both IRF3 and p65, whereas the empty vector and C297S-L did not have this effect after stimulation by SeV (Figure 6d). These data suggested that PTEN-L promoted antiviral innate responses by positively regulating the activation and nuclear import of IRF3 and p65, thus enhancing the expression of IFN-β and proinflammatory cytokines.
Figure 6.
PTEN-L enhanced antiviral innate immunity by promoting IRF3 and p65 nuclear translocation. (a) HEK293 cells were transfected with PTEN, PTEN-L or empty vector (EV) together with a luciferase (Luc) reporter of the NF-κB promoter, ISRE promoter and IFN-β promoter. Twenty-four hours after transfection, cells were or infected with SeV for 10 h or left uninfected, and luciferase assays were performed. (b) HEK293 cells were transfected with the IRF3-responsive ISRE reporter together with the indicated RIG-N, VISA, TBK1, IKKε and IRF3-5D expression plasmids (left panel) or the NF-κB reporter together with the indicated TRAF6, RIP1, TAB3, TAB2, TAB1/TAK1, IKKβ and p65 expression plasmids (right panel). Luciferase assays were performed 24 h after transfection. The data represent the average of three independent experiments and were analyzed by two-tailed unpaired t test. Graphs indicate mean±s.d. (n=3) derived from three independent experiments. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. (c) PC-3 cells were transfected with empty vector, PTEN-L (ATG/ATA) and C297S-L for 24 h and then infected with SeV for the indicated times. Cells were lysed and then subjected to SDS-PAGE and analysis by the indicated antibodies. (d) PC-3 cells were transfected with empty vector, PTEN-L (ATG/ATA) and C297S-L for 24 h and then infected with SeV for the indicated times. Cells were collected and subjected to nucleus-cytoplasm extraction. Then, 5% of cytoplasmic extracts and 10% of nuclear extracts were used for SDS–PAGE and analysis with the indicated antibodies.
Discussion
PTEN has been known to be a tumor suppressor for approximately two decades. Earlier studies have demonstrated that PTEN resides in the cytosol and nucleus, where it antagonizes PI3K/Akt signaling and protects genome stability.26 Recently, a variant of PTEN, PTEN-L, with a large N-terminal extension has been discovered. PTEN-L is secreted into the extracellular milieu and transported into neighboring recipient cells.20 PTEN-L possesses anti-tumor activity by antagonizing PI3K/Akt signaling in a manner similar to canonical PTEN.20 In addition, PTEN-L also plays a regulatory role in mitochondrial function and energy metabolism.21 In this study, we found that PTEN-L promotes type I IFN responses and antiviral immunity in virus-infected cells. The greatest interest in PTEN-L lies in its secretion and autonomous entry into cells. Thus, PTEN-L has the potential to be developed into a protein drug. Recent studies have indicated that PTEN-L may be used in antiviral treatment and anti-tumor therapy, particularly in PTEN-deficient cancer patients.
Very recently, we have demonstrated that canonical PTEN plays a pivotal role in IFN responses and antiviral innate immunity.11 Here, we demonstrated that PTEN-L and PTEN exhibit both similarities and differences in antiviral activity. First, PTEN-L possesses similar potency to that of PTEN in promoting type I IFN responses and antiviral activity. Second, the function of PTEN-L in antiviral immunity is IRF3 mediated and phosphatase activity dependent, thus suggesting that PTEN-L may adopt a similar mechanism in its antiviral function as demonstrated for PTEN. IRF3 activation and nuclear import are commonly regulated during viral infection.27 Third, PTEN-L differs from PTEN in that it can be applied extracellularly, given that it possesses the ability to enter recipient cells. Fourth, unlike PTEN, PTEN-L stimulates proinflammatory responses (Figure 2). Such differences may result from the differences in protein structure between PTEN-L and PTEN, given that PTEN-L has a large N-terminal extension (NTE) with an additional 173 amino acids. On the basis of computational analyses, the NTE region may have potential to interact with a large array of proteins.23 Therefore, we hypothesized that the function of PTEN-L in activation of proinflammatory responses might depend on its interactions with some other known proteins involved in the related signaling pathways. In this work, we demonstrated that PTEN-L promotes the activation and nuclear import of NF-κB. The detailed molecular mechanism remains to be clarified in future studies.
To demonstrate that PTEN-L protein can be administered extracellularly for antiviral treatment, we adopted two strategies: collection of secreted PTEN-L in culture supernatant (Figure 4) and generation of recombinant PTEN-L protein (Figure 5). As expected, both forms of PTEN-L suppressed virus replication. The antiviral function of recombinant PTEN-L was tested in a mouse infection model (Figure 5d). In the future, pure PTEN-L should be prepared in sufficient quantities and tested in more animal models infected with different viruses. One study has demonstrated that PTEN is exported into exosomes and accepted by recipient cells, in which PTEN exhibits phosphatase activity.28, 29 However, we did not observe the antiviral activity when we treated cells with the culture supernatant of PTEN-expressing cells (Figure 4). This result might be tentatively explained as follows. The recruitment of PTEN into exosomes is Ndfip1 dependent, whereas HEK293T lacks endogenous Ndfip1. Indeed, PTEN is exported in exosomes only when Ndfip1 is overexpressed in HEK293T cells.28 In summary, PTEN-L exhibits potential for exogenous use in antiviral and antitumor therapies.
Acknowledgments
We thank Dr Hong Wu for providing Pten−/− MEFs and Dr Hongliang Li for Ptenflox/flox mice and Dr Mingzhou Chen for providing VSV as a gift. This work was supported by the National Nature Science Foundation of China (grant 81620108020), the China ‘973’ Basic Research Program (#2013CB911101) and Hubei Provincial Science & Technology Innovation Team grant (#2015CFA009).
Footnotes
The authors declare no conflict of interest.
References
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997; 275: 1943–1947. [DOI] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997; 15: 356–362. [DOI] [PubMed] [Google Scholar]
- Masson GR, Perisic O, Burke JE, Williams RL. The intrinsically disordered tails of PTEN and PTEN-L have distinct roles in regulating substrate specificity and membrane activity. Biochem J 2016; 473: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 1998; 273: 13375–13378. [DOI] [PubMed] [Google Scholar]
- Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA et al. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci USA 1998; 95: 13513–13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science 2002; 296: 1655–1657. [DOI] [PubMed] [Google Scholar]
- Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer 2006; 6: 184–192. [DOI] [PubMed] [Google Scholar]
- Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J et al. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA 1999; 96: 6199–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 1998; 95: 29–39. [DOI] [PubMed] [Google Scholar]
- Furnari FB, Lin H, Huang HS, Cavenee WK. Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proc Natl Acad Sci USA 1997; 94: 12479–12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhu M, Pan R, Fang T, Cao YY, Chen S et al. The tumor suppressor PTEN has a critical role in antiviral innate immunity. Nat Immunol 2016; 17: 241–249. [DOI] [PubMed] [Google Scholar]
- Chen L, Guo D. The functions of tumor suppressor PTEN in innate and adaptive immunity. Cell Mol Immunol 2017; 14: 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006; 124: 783–801. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011; 34: 637–650. [DOI] [PubMed] [Google Scholar]
- Kang DC, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci USA 2002; 99: 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 2004; 5: 730–737. [DOI] [PubMed] [Google Scholar]
- Rothenfusser S, Goutagny N, DiPerna G, Gong M, Monks BG, Schoenemeyer A et al. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J Immunol 2005; 175: 5260–5268. [DOI] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol 2011; 30: 16–34. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003; 301: 640–643. [DOI] [PubMed] [Google Scholar]
- Hopkins BD, Fine B, Steinbach N, Dendy M, Rapp Z, Shaw J et al. A secreted PTEN phosphatase that enters cells to alter signaling and survival. Science 2013; 341: 399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, He S, Yang J, Jia X, Wang P, Chen X et al. PTENalpha, a PTEN isoform translated through alternative initiation, regulates mitochondrial function and energy metabolism. Cell Metab 2014; 19: 836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang P, Lin C, Yu Q, Wu J, Wang L et al. Relevance and therapeutic possibility of PTEN-long in renal cell carcinoma. PLoS One 2015; 10: e114250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaney P, Uversky VN, Dave V. The PTEN Long N-tail is intrinsically disordered: increased viability for PTEN therapy. Mol bioSystems 2013; 9: 2877–2888. [DOI] [PubMed] [Google Scholar]
- Johnston SB, Raines RT. Catalysis by the tumor-suppressor enzymes PTEN and PTEN-L. PLoS One 2015; 10: e0116898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzani I, Ivanov IP, Andreev DE, Dmitriev RI, Dean KA, Baranov PV et al. Systematic analysis of the PTEN 5' leader identifies a major AUU initiated proteoform. Open Biol 2016; 6: 150203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol 2009; 4: 127–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Cao W, Han T, Ren S, Feng J, Chen T et al. Inducible Rubicon facilitates viral replication by antagonizing interferon production. Cell Mol Immunol 2017; 14: 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putz U, Howitt J, Doan A, Goh CP, Low LH, Silke J et al. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signaling 2012; 5: ra70. [DOI] [PubMed] [Google Scholar]
- Putz U, Mah S, Goh CP, Low LH, Howitt J, Tan SS. PTEN secretion in exosomes. Methods 2015; 77-78: 157–163. [DOI] [PubMed] [Google Scholar]