Abstract
Background:
Human papilloma viruses (HPVs) are small DNA viruses that have been identified in periodontal pocket as well as gingival sulcus. High risk HPVs are also associated with a subset of head and neck carcinomas. It is thought that the periodontium could be a reservoir for HPV.
Aims:
1. Detection of Human Papilloma virus (HPV) in periodontal pocket as well as gingival of patients having localized chronic periodontitis and gingival sulcus of periodontally healthy subjects. 2. Quantitative estimation of E6 and E7 mRNA in subjects showing presence of HPV3. To assess whether periodontal pocket is a reservoir for HPV.
Settings and Design:
This case-control study included 30 subjects with localized chronic Periodontitis (cases) and 30 periodontally healthy subjects (controls). Two samples were taken from cases, one from periodontal pocket and one from gingival sulcus and one sample was taken from controls.
Methods and Materials:
Samples were collected in the form of pocket scrapings and gingival sulcus scrapings from cases and controls respectively. These samples were sent in storage media for identification and estimation of E6/E7 mRNA of HPV using in situ hybridization and flow cytometry.
Statistical analysis:
Statistical analysis was done by using, mean, percentage and Chi Square test. A statistical package SPSS version 13.0 was used to analyze the data. P value < 0.05 was considered as statistically significant.
Results:
pocket samples as well as sulcus samples for both cases and controls were found to contain HPV E6/E7 mRNAInterpretation and
Conclusion:
Presence of HPV E6/E7 mRNA in periodontium supports the hypothesis that periodontal tissues serve as a reservoir for latent HPV and there may be a synergy between oral cancer, periodontitis and HPV. However prospective studies are required to further explore this link.
Key words: E6/E7 mRNA, humanpapillomavirus, periodontitis
INTRODUCTION
Periodontal disease is characterized by the presence of gingival inflammation, loss of tissue attachment, periodontal pocket formation, and alveolar bone loss around the affected tooth.
It is widely accepted that the initiation and progression of periodontitis are dependent on the presence of Gram-negative and Gram-positive microorganisms capable of causing diseases.[1]
However, the traditional theories that explain the pathophysiology of periodontitis are not able to explain various aspects of the disease including the failure of progression of all cases of gingivitis to periodontitis, site specificity, its episodic nature, etc.[2] Hence, research has branched out in different directions, one of which includes viruses.
At least, three characteristics of periodontal microorganisms have been identified that can contribute to their ability to act as pathogens: the capacity to colonize, i.e., invade and reproduce in host cells, ability to evade antimicrobial host defenses mechanisms and the ability to produce substances that can directly initiate tissue destruction.[3]
Some of these properties have been found in viruses, and of late the role of viruses in the pathogenesis of periodontal disease is being investigated. This includes herpesviruses and human papillomavirus (HPV).[4,5,6]
Of these viruses, the HPV is of particular interest as it has been found in the oral cavity and in particular in the marginal periodontium,[7,8,9] and is also known to be associated with malignancies of the head and neck.[10] HPV associated head and neck carcinomas are of increasing importance and constitute a unique entity among head and neck carcinomas.[11] Some authors state that there may be a synergy between chronic periodontitis, oral HPV infection, and head and neck carcinoma.[11,12] There are several points of evidence for this; the periodontium may act as a reservoir for HPV,[8,9] chronic periodontitis may have a role in the natural history of HPV in patients with base of tongue carcinomas,[13] by facilitating the initial infection and persistence of HPV infection in the periodontal pocket.[14,15]
Second, HPV is known to be the causative agent of epithelial malignancies of the uterine cervix.[16] The high-risk HPV types (most often HPV 16 and occasionally HPV 18) identified in HPV-positive tonsillar squamous cell carcinoma are similar to those found in cervical cancers.[13]
Third, there are similarities between uterine cervical epithelium and junctional epithelium of the periodontium. Both of these have a rapidly dividing basal layer to which HPV has a great affinity.[14]
This indicates that chronic periodontitis (which facilitates the life cycle of HPV) may have a role in increasing the risk of head and neck carcinoma.
In the light of literature indicating a link between periodontitis, HPV, and oral head and neck carcinoma, it is advantageous to use diverse methods of HPV detection in the periodontium. Previously, HPV DNA has been detected using polymerase chain reaction among other methods.[8,17,18] However, there is evidence that E6/E7mRNA testing is more specific than DNA testing for HPV detection.[19] (The HPV genome is composed of three major regions: the long control region, the early genes E1–E8 and late genes L1–L2.[20] The viral E6 and E7 oncoproteins are necessary for malignant conversion).[21]
This study detects presence or absence of HPV in periodontium by detection of E6/E7 mRNA using in situ hybridization and flow cytometry.
MATERIALS AND METHODS
Sources of data
A total of 60 individuals were included in the study based on inclusion and exclusion criteria. It was made clear to the potential individuals that participation was voluntary and written informed consent was obtained from those who agreed to participate.
Patients diagnosed with localized chronic periodontitis according to the 1999 classification of periodontal diseases (American Academy of Periodontology) with clinical attachment loss >5 mm involving less than 30% of total sites, clinically manifested as a periodontal pocket, with ages ranging from 21 to 70 years were included in this study.
Patients with signs of aggressive periodontitis; arc shaped bone loss, familial history of aggressive periodontitis were excluded. Patients who presented with a history of carcinoma of head and neck or a visible growth on the base of the tongue or pharynx area were excluded. Patients on antiviral drugs were also excluded from the study.
Methods of collection of data
Patients were divided into two groups of 30 patients each and a total of 90 samples were taken:
Controls were defined as patients with no attachment loss and patients were defined as those diagnosed with localized chronic periodontitis: two samples were collected from patients, one each from periodontal pocket and gingival sulcus.
Among the 30 cases, 9 (30%) were female and 21 (70%) were male. Among the controls, 18 (60%) were female and 12 (40%) were males [Figure 1].
Figure 1.

Gender distribution for cases and controls
The samples were in the form of periodontal pocket scrapings and sulcus scrapings, respectively, the samples were collected from buccal aspects of teeth. One sample each was collected from the controls, in the form of gingival sulcus scrapings [Figure 2].
Figure 2.

The study design
Samples were collected by scraping pockets and gingival sulci using area-specific curets after administering the adequate local anesthetic. The sample obtained was stored in carrier medium and sent to: Curehealth Diagnostics (ISO 9001 2009). Address: 234-A, Chand Plaza, Sant Nagar, East of Kailash, New Delhi-110065, India.
Method of analysis
The sample was analyzed at Curehealth Diagnostics using the standardized detection method for HPV:
The sample is first centrifuged at 3500 rpm (1000 gauge) for 5 min to obtain a pellet and a supernatant. The supernatant is discarded. The sample is then washed with phosphate buffered saline followed by a second centrifugation at 3500 rpm for 5 min. Eight hundred microliter of a permeabilizing reagent (Reagent 1) is added to allow the entry of the probe and vortexed, followed by incubation for 1 h at room temperature. Now, a prehybridization buffer (Reagent 2) is added and the solution is vortexed followed by centrifugation at 3500 rpm for 5 min. This process removes debris from the sample. The solution is incubated for 30 min at 43°C [Figure 3].
Figure 3.
Prehybridization buffer: Reagent 2
The probe is now added which consists of a probe diluent (Reagent 4) and concentrated probe (Reagent 5). Hundred and three microliter is added to each sample (100 μl Reagent 4 and 3 μl Reagent 5).
One milliliter posthybridization buffer (Reagent 6) is then added to the solution to remove the unwanted binding between two strands of mRNA and between probe and mRNA. Centrifugation is carried out again to obtain a pellet and a supernatant of which the supernatant is discarded. Treatment is carried out with Reagent 7, also a posthybridization buffer and the solution is incubated at 43°C for 15 min.
The sample is then subjected to flow cytometry (Accuri C6 flow cytometer, manufactured by BD, USA) and analyzed [Figure 4].
Figure 4.

Accuri 6 flow cytometer
RESULTS
A 2% cutoff is used as screening marker for early prediction of active infection in samples of cervical epithelium as per standard procedure by cure health laboratory.
Among the cases, of the 30 pocket samples examined, 15 contained E6/E7 mRNA. Of these, 13 contained <2% mRNA and 2 of the samples contained >2% mRNA.
Among the sulcus samples obtained from cases, of the 30 samples taken, 19 did not contain E6/E7 mRNA, 9 contained <2% mRNA, and 2 contained >2% mRNA.
Among the sulcus samples obtained from controls, 18 did not contain E6/E7 mRNA,11 contained <2% mRNA, and 1 contained >2% mRNA.
Among the cases, 54.5% of maxillary pocket sites examined showed absence of E6/E7 mRNA and 45.5%of sites showed presence of E6/E7 mRNA, while 47.4% of mandibular pockets showed absence of E6/E7 while 52.6% showed presence of E6/E7 mRNA.
On examining the sulcus samples of cases, it was found that 60% of maxillary sites did not contain E6/E7 mRNA and 40% contained E6/E7 mRNA.65% of mandibular sites showed no E6/E7 mRNA while 35% contained E6/E7 mRNA [Figure 5].
Figure 5.

The graph depicts presence of E6/E7 mRNA among samples cases and controls
Results are expressed in the form of dot blots. Each dot blot shows cells (recorded as signal “events") of periodontal pocket scraping or gingival sulcus scraping origin representing forward and side scatter characteristics of squamous epithelial cells. The accompanying histogram shows cells with overexpression of E6/E7 mRNA in the form of a second peak in the histogram [Figures 6 and 7].
Figure 6.

The flow cytometry analysis is expressed as a dot blot, representing forward and side scatter for epithelial cells
Figure 7.
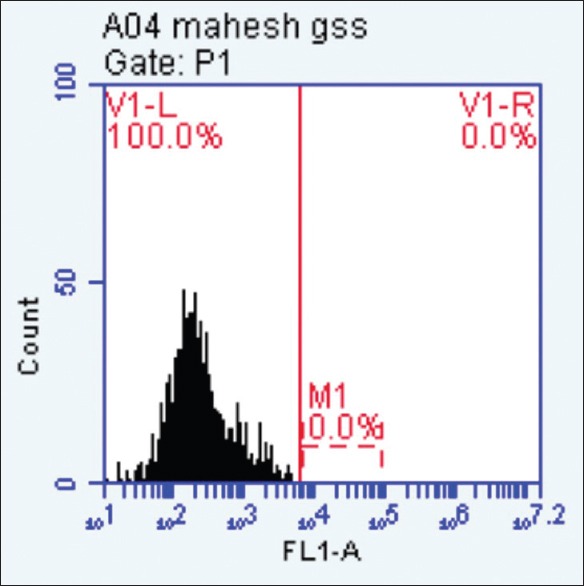
The cells showing overexpression of E6/E7 mRNA in the histogram are shown in the form of a “second peak”
DISCUSSION
Periodontitis is an inflammatory disease of the supporting tissues of the teeth. It has a multifactorial etiology, involving a complex interaction among specific bacteria, the host response, and risk factors. Causative agents include Gram-positive and Gram-negative anaerobic bacteria associated with an oral biofilm.[22]
However, theories proposed so far to explain the etiopathogenesis of periodontitis have not been able to account for several clinical features of the disease including the failure of progression of all cases of gingivitis to periodontitis, site specificity, its episodic nature, etc.[3]
Hence, research on periodontitis has taken various different directions. Recently, the role of viruses in the pathogenesis of chronic periodontitis is being investigated, including herpes virus and HPV.[4,5]
HPV has been shown to have a positive association with periodontitis. A number of studies have been performed, identifying the presence of HPV in periodontium: A polymerase chain reaction (PCR) study was done in which gingival biopsies were taken from 38 individuals with clinically diagnosed periodontal disease were examined. The presence of HPV DNA was studied using nested PCR, followed by subsequent hybridization with a cocktail of 12 high-risk HPV oligoprobes and in situ hybridization with probes for HPV screening and the HPV subtype.[17] High-risk HPV types were detected in 26% (8/31) of the gingival biopsies with PCR. By using in situ hybridization, the viral DNA was localized to the coronal part of the junctional epithelium in the periodontal pocket.[8] However, in this study HPV DNA was detected.
A study was conducted which used Southern blot DNA detection method.32 P-radiolabeled DNA probes were applied under stringent conditions to 20 interproximal gingival papilla specimens revealed homologous viral sequences in one of six cases of adult periodontitis (HPV 16), 1 of 2 cases of rapidly progressive periodontitis (HPV 6/HPV 11), 2 of 2 cases of acute gingivitis in psychiatric institutionalized patients (HPV 6; HPV 6/HPV 11), and 2 of 10 cases of acute gingivitis in AIDS patients (HPV 6/HPV 11/HPV 16; HPV 6). The detection of HPV 6, 11, 16 DNAs or related-DNAs in periodontal tissues without obvious clinical signs of viral infection suggests that the gingival epithelium may act as a reservoir.[9]
A study was conducted wherein 56 gingival samples from subjects with chronic periodontitis, 26 samples from subjects with gingivitis, and 22 samples from subjects with healthy periodontium were analyzed. Total DNA was extracted, and the presence of HPV-16 was assessed using a real-time polymerase chain reaction.[23]
The present study detects E6/E7 mRNA using in situ hybridization and flow cytometry. Detection of E6/E7 transcripts has been shown to be associated with increased sensitivity and specificity as compared to the mere detection of viral DNA when used for detecting precancerous cervical lesions.[19,21]
The viral genome is organized into three segments: early region (E) which comprises of E1, E2, E4, E5, E6, and E7 and represents 50% of the genome, the late region (L) consisting of L1 and L2 which represents 40% of the genome and a genomic regulatory region which represents 10% of the genome.
The viral E6 and E7 oncoproteins are necessary for malignant conversion. The abilities of high-risk HPV E6 and E7 proteins to associate with the tumor suppressors p53 and pRB, respectively, have been suggested as a mechanism by which these viral proteins induce tumor formation.[21]
In this study, healthy sites in the oral cavity of cases and controls (23 samples of the total of all sulcus samples, of which 3 samples was found to have E6/7mRNA above the significant 2% mark) were both found to contain HPV in the form of E6/E7 mRNA. These findings are consistent with those stated by Hormia et al.,[8] according to whom HPV can be detected in normal oral mucosa in 10%–15% of the population.
In the present study, it was found that there was a statistically significant positive correlation between E6/E7 mRNA values and gingival index values, this is consistent with Hormia et al.[8] HPV's cell cycles are found to be closely associated with differentiation of the epithelial cells it infects.[6]
These viruses are known to infect basal and suprabasal cells. Since the gingival pocket is the only site in the oral mucosa where basal cells, known to be targets of HPV at other mucosal sites, are normally exposed to the environment, it is hypothesized that this could be the site of latent HPV.[8]
The junctional epithelium of the periodontium is a rapidly differentiating epithelium whose cells are exfoliated throughout the gingival sulcus before they can differentiate.[24] During periodontal disease, pocket formation occurs which is typically associated with a rapid proliferation and migration of the basal and suprabasal cells of the junctional epithelium. Therefore, pocket epithelium offers a favorable site for replication of HPVs.[25]
Two samples from the case group and one sample from the control group showed E6/7 mRNA above 2%.
In this study, of the total of 90 samples, 38 samples (42.3%) of the samples were found to contain HPV E6/E7 mRNA (0%–2% or >2%), this is in contrast to study by Jacob et al.,[18] the results obtained showed no HPV was detected in the 102 samples analyzed, where the method of analysis used was PCR.
The presence of HPV E6/E7 mRNA in periodontal tissues supports the hypothesis that there may be a synergy between periodontitis, HPV and cancer of the head and neck,[13] with the periodontium acting as a reservoir for latent HPV.[8,9] This is supported by several studies which have indicated that patients with a history of periodontitis have a higher risk for head and neck carcinomas, especially of tongue and oropharynx. Loss of bone around teeth is found to be an independent risk factor for the development of oral carcinoma.[26,27,28]
In this study, in pocket samples, HPV E6/E7 mRNA was found to have a positive, though statistically not significant correlation with sample site (mandibular teeth), this may be because these sites are closer to the tongue. This finding supports the hypothesis that high-risk HPV which are involved in head and neck squamous cell carcinoma of tongue and larynx[10] may be found in sites in close to tongue.[8]
Limitations have been the undesired companion of every study and by that very virtue, they open gateways for further research. Variables such as socioeconomic conditions, gender were not matched, also, matching was not done for samples of mandibular and maxillary teeth. Stress and lifestyle, food habits were not matched.
CONCLUSION
In the light of the observations of this study, it can be concluded that the periodontium may serve as a reservoir for high-risk HPV. High-risk HPV can be detected in periodontal pockets as well as in healthy gingival sulci. The presence of high-risk HPV E6/E7 mRNA above the 2% significant mark may support the hypothesis that there may be synergy between head and neck carcinoma, periodontitis and HPV. Long-term prospective studies are required to further explore this link.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given his their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Listgarten MA. Pathogenesis of periodontitis. J Clin Periodontol. 1986;13:418–30. doi: 10.1111/j.1600-051x.1986.tb01485.x. [DOI] [PubMed] [Google Scholar]
- 2.Schenkein H, Cochran DL, Dyke TV, Blieden T, Cohen RE, Hallmon WW. The pathogenesis of periodontal diseases. J Periodontol. 1999;70:457–70. doi: 10.1902/jop.1999.70.4.457. [DOI] [PubMed] [Google Scholar]
- 3.Slots J. Human viruses in periodontitis. Periodontol 2000. 2010;53:89–110. doi: 10.1111/j.1600-0757.2009.00325.x. [DOI] [PubMed] [Google Scholar]
- 4.Contreras A, Slots J. Active cytomegalovirus infection in human periodontitis. Oral Microbiol Immunol. 1998;13:225–30. doi: 10.1111/j.1399-302x.1998.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 5.Contreras A, Slots J. Herpesviruses in human periodontal disease. J Periodontal Res. 2000;35:3–16. doi: 10.1034/j.1600-0765.2000.035001003.x. [DOI] [PubMed] [Google Scholar]
- 6.Cortez-Lopez Maria E, Sanoval-Rivas L, Ortega-Kermedy Z, Cerdaflores RM. Detection HPV in Mexicans with periodontitis. Asian Acad Res J Multidiscip. 2014;1:307, 15. [Google Scholar]
- 7.Maitland NJ, Cox MF, Lynas C, Prime SS, Meanwell CA, Scully C, et al. Detection of human papillomavirus DNA in biopsies of human oral tissue. Br J Cancer. 1987;56:245–50. doi: 10.1038/bjc.1987.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hormia M, Willberg J, Ruokonen H, Syrjänen S. Marginal periodontium as a potential reservoir of human papillomavirus in oral mucosa. J Periodontol. 2005;76:358–63. doi: 10.1902/jop.2005.76.3.358. [DOI] [PubMed] [Google Scholar]
- 9.Madinier I, Doglio A, Cagnon L, Lefèbvre JC, Monteil RA. Southern blot detection of human papillomaviruses (HPVs) DNA sequences in gingival tissues. J Periodontol. 1992;63:667–73. doi: 10.1902/jop.1992.63.8.667. [DOI] [PubMed] [Google Scholar]
- 10.Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12:418–24. doi: 10.1016/s0300-9785(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 11.Pullos AN, Castilho RM, Squarize CH. HPV infection of the head and neck region and its stem cells. J Dent Res. 2015;94:1532–43. doi: 10.1177/0022034515605456. [DOI] [PubMed] [Google Scholar]
- 12.Psyrri A, DiMaio D. Human papillomavirus in cervical and head-and-neck cancer. Nat Clin Pract Oncol. 2008;5:24–31. doi: 10.1038/ncponc0984. [DOI] [PubMed] [Google Scholar]
- 13.Tezal M, Sullivan Nasca M, Stoler DL, Melendy T, Hyland A, Smaldino PJ, et al. Chronic periodontitis-human papillomavirus synergy in base of tongue cancers. Arch Otolaryngol Head Neck Surg. 2009;135:391–6. doi: 10.1001/archoto.2009.6. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen FL, editor. Progress in Oral Cancer Research. New York: Nova Medical Books; 2008. p. 98. [Google Scholar]
- 15.Wiener RC, Sambamoorthi U, Jurevic RJ. Association of periodontitis and human papillomavirus in oral rinse specimens: Results from the national health and nutrition survey 2009-2012. J Am Dent Assoc. 2015;146:382–9. doi: 10.1016/j.adaj.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parra B, Slots J. Detection of human viruses in periodontal pockets using polymerase chain reaction. Oral Microbiol Immunol. 1996;11:289–93. doi: 10.1111/j.1399-302x.1996.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 18.Jacob A, Janam P, Babu Vijayamma JM. Prevalence of human papilloma virus in marginal periodontium and its association with periodontitis: A cross sectional study. J Indian Soc Periodontol. 2014;18:447–50. doi: 10.4103/0972-124X.138682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benevolo M, Vocaturo A, Caraceni D, French D, Rosini S, Zappacosta R, et al. Sensitivity, specificity, and clinical value of human papillomavirus (HPV) E6/E7 mRNA assay as a triage test for cervical cytology and HPV DNA test. J Clin Microbiol. 2011;49:2643–50. doi: 10.1128/JCM.02570-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris BJ. Cervical human papillomavirus screening by PCR: Advantages of targeting the E6/E7 region. Clin Chem Lab Med. 2005;43:1171–7. doi: 10.1515/CCLM.2005.203. [DOI] [PubMed] [Google Scholar]
- 21.Yim EK, Park JS. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat. 2005;37:319–24. doi: 10.4143/crt.2005.37.6.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: Summary of developments, clinical implications and future directions. Periodontol 2000. 1997;14:216–48. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 23.Horewicz VV, Feres M, Rapp GE, Yasuda V, Cury PR. Human papillomavirus-16 prevalence in gingival tissue and its association with periodontal destruction: A case-control study. J Periodontol. 2010;81:562–8. doi: 10.1902/jop.2009.090571. [DOI] [PubMed] [Google Scholar]
- 24.Bosshardt DD, Lang NP. The junctional epithelium: From health to disease. J Dent Res. 2005;84:9–20. doi: 10.1177/154405910508400102. [DOI] [PubMed] [Google Scholar]
- 25.Stubenrauch F, Laimins LA. Human papillomavirus life cycle: Active and latent phases. Semin Cancer Biol. 1999;9:379–86. doi: 10.1006/scbi.1999.0141. [DOI] [PubMed] [Google Scholar]
- 26.Tezal M, Sullivan MA, Reid ME, Marshall JR, Hyland A, Loree T, et al. Chronic periodontitis and the risk of tongue cancer. Arch Otolaryngol Head Neck Surg. 2007;133:450–4. doi: 10.1001/archotol.133.5.450. [DOI] [PubMed] [Google Scholar]
- 27.Meyer MS, Joshipura K, Giovannucci E, Michaud DS. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 2008;19:895–907. doi: 10.1007/s10552-008-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tezal M, Sullivan MA, Hyland A, Marshall JR, Stoler D, Reid ME, et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:2406–12. doi: 10.1158/1055-9965.EPI-09-0334. [DOI] [PubMed] [Google Scholar]


