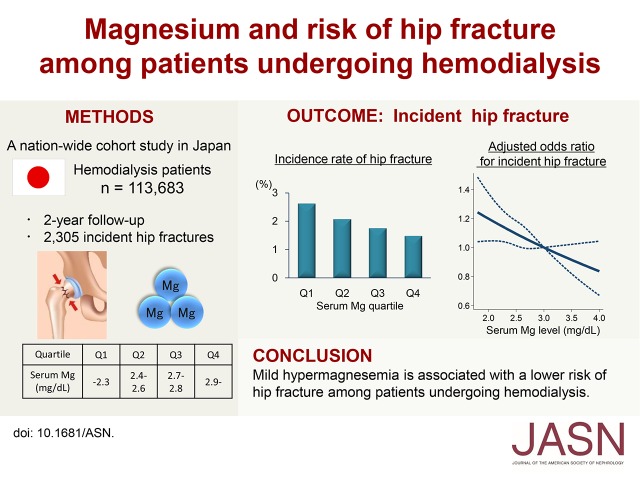
Abstract
Magnesium is an essential mineral for bone metabolism. However, little is known about the relationship between magnesium and the risk of fractures. In this cohort study, we elucidated the association between serum magnesium level and the risk of incident hip fracture among patients undergoing hemodialysis. We identified 113,683 patients undergoing hemodialysis with no history of hip fracture from a nation-wide database of patients undergoing dialysis in Japan. During a 2-year follow-up, a total of 2305 (2%) new hip fractures occurred. The crude incidence rate was significantly higher among patients in the lower quartiles of serum magnesium levels (2.63%, 2.08%, 1.76%, and 1.49% in Q1–Q4, respectively; P<0.001 for trend). The range of serum magnesium levels (in milligrams per deciliter) in each quartile was as follows: Q1, <2.3; Q2, 2.4–2.6; Q3, 2.7–2.8, and Q4, >2.9. After adjustment for demographic and clinical factors, patients in Q1 had a 1.23-fold higher risk for hip fracture than those in Q4 (95% confidence interval, 1.06 to 1.44; P<0.01). Similarly, an inverse probability weighting analysis showed an increased risk of hip fracture among patients in the lower magnesium quartiles. We did not observe significant effect modifications in subgroup analyses. The population-attributable fraction of serum magnesium level for incident hip fractures was 13.7% (95% confidence interval, 3.7% to 22.7%), which was much higher than that of serum calcium, serum phosphate, and parathyroid hormone levels. Thus, mild hypermagnesemia is associated with a lower risk of hip fracture among patients undergoing hemodialysis.
Keywords: magnesium, hip fracture, hemodialysis, mineral and bone disorders
Hip fractures are a life-threatening complication in patients undergoing hemodialysis, resulting in an increased risk of death and hospitalization with substantial economic burden.1–3 The incidence rate of hip fractures among patients undergoing dialysis is four to ten times higher than that in the age- and sex-matched general population.4–11 Factors associated with the increased fracture risk in patients undergoing dialysis include older age, female sex, low body mass index, muscle weakness, and a high tendency for falls.7,12–14 In addition, mineral and bone disorders in CKD, including vitamin D deficiency and altered parathyroid hormone (PTH) level, adversely affect bone metabolism in patients undergoing dialysis.15
Magnesium (Mg), the fourth most abundant cation in the human body, is an essential bone mineral. Nearly half of the total body Mg is present in the bone.16 Several animal studies have shown that a low-Mg diet impairs bone strength.17–23 In a meta-analysis of population-based cohort studies, the amount of dietary Mg intake was positively associated with the bone mineral density of the femoral neck, total hip, and forearm.24 Therefore Mg might affect the risk of major bone fractures. Recently, two cohort studies of primarily non-CKD populations have reported that lower serum Mg levels are associated with an increased risk of fractures, including hip fractures.25,26
To date, studies evaluating the relationship between Mg and the risk of fractures in patients with CKD are lacking. The bone Mg content is highly variable in patients undergoing dialysis compared with patients who are not undergoing dialysis.27 In addition to the potential negative effects of Mg deficiency on bone metabolism, concerns have been raised that excess Mg may impair bone mineralization and provoke osteomalacia-like changes in patients with ESRD.28 In this context, it is important to elucidate the effect of Mg on the fracture risk for a wide range of Mg levels. Herein, we utilized a large, nation-wide database of patients undergoing hemodialysis to analyze whether high and/or low serum Mg levels are associated with an increased risk of incident hip fracture in this population.
Results
The database contained a total of 217,791 patients aged 18 years or older, who received in-center hemodialysis/hemodiafiltration in Japan. The patient selection flowchart is depicted in Figure 1. We identified 167,301 patients without a previous history of hip fracture, who had available data on the incidence of hip fractures during the study period. Among these, 113,683 patients with baseline serum Mg data were included in the analysis. We first compared the baseline characteristics between patients with and without serum Mg data (Supplemental Table 1). The standardized mean differences between these two groups were >0.1 for all covariates, indicating that there was no apparent selection bias with regard to the measurement of serum Mg levels in this cohort.
Figure 1.
Patient selection flowchart. HD/HDF, hemodialysis/hemodiafiltration.
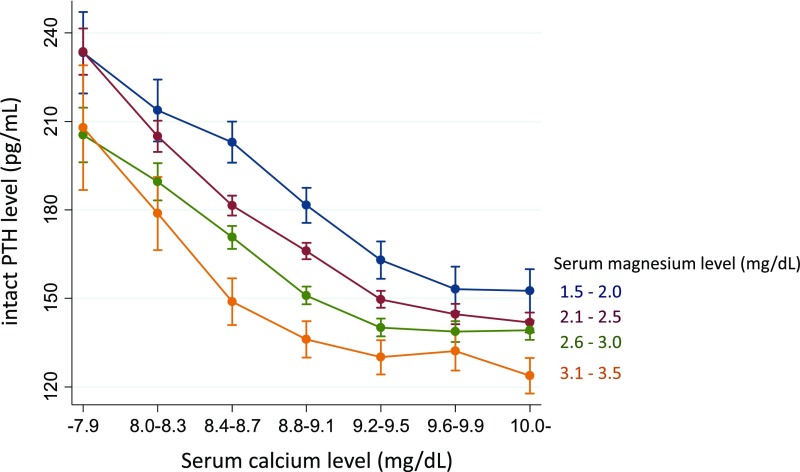
Baseline characteristics according to the quartiles (Q1–Q4) of serum Mg levels are shown in Table 1. Patients in the lower quartiles were older, were more likely to be men, to be physically inactive, and to have a past history of myocardial infarction, cerebral infarction, or limb amputation. Serum calcium and phosphate levels decreased, whereas alkaline phosphatase (ALP) and intact PTH levels increased with decreasing serum Mg quartile. As shown in Figure 2, the calcium–PTH curves shifted to the left as serum Mg levels increased. The negative association between Mg and PTH levels was most evident at low-normal serum calcium levels, but was modest at high and low serum calcium levels (P for interaction <0.001).
Table 1.
Baseline characteristics according to the quartiles of serum magnesium levels
| Serum magnesium level quartiles (Range of serum magnesium levels, mg/dl) | |||||||
|---|---|---|---|---|---|---|---|
| No. (%) of missing values | Total | Q1 | Q2 | Q3 | Q4 | ||
| (≤ 2.3) | (2.4–2.6) | (2.7–2.8) | (≥2.9) | ||||
| Characteristics | n=113,683 | n=30,991 | n=34,011 | n=19,902 | n=28,779 | P for trend | |
| Demographics | |||||||
| Age, yr | 0 (0.0) | 64.9 (12.3) | 68.2 (11.8) | 65.7 (11.9) | 63.4 (12.1) | 61.4 (12.5) | <0.001 |
| Female, % | 0 (0.0) | 37.5 | 35.8 | 36.7 | 38.3 | 39.9 | <0.001 |
| Body mass index, kg/m2 | 12,977 (11.4) | 22.4 (4.2) | 22.4 (4.5) | 22.5 (4.1) | 22.5 (4.0) | 22.3 (4.3) | 0.001 |
| Poor physical activity, % | 994 (0.9) | 8.2 | 12.7 | 7.6 | 5.2 | 6.2 | <0.001 |
| Diabetes mellitus, % | 1 (0.0) | 34.8 | 37.5 | 34.6 | 32.7 | 33.4 | <0.001 |
| Dialysis vintage, yr | 16 (0.0) | 6 [3, 11] | 5 [2, 9] | 6 [3, 11] | 7 [3, 12] | 7 [4, 12] | <0.001 |
| Dialysis time, h/wk | 948 (0.8) | 11.7 (1.8) | 11.5 (2.0) | 11.7 (1.8) | 11.9 (1.6) | 11.9 (1.6) | <0.001 |
| Urea reduction rate, % | 2724 (2.4) | 67.9 (9.9) | 66.8 (10.6) | 68.1 (9.9) | 68.6 (8.6) | 68.6 (9.9) | <0.001 |
| Laboratory data | |||||||
| Albumin, g/dl | 1534 (1.3) | 3.7 (0.4) | 3.6 (0.4) | 3.7 (0.4) | 3.8 (0.3) | 3.8 (0.4) | <0.001 |
| Calcium, mg/dl | 84 (0.0) | 9.3 (0.9) | 9.2 (0.9) | 9.3 (0.9) | 9.3 (0.9) | 9.4 (0.9) | <0.001 |
| Phosphate, mg/dl | 294 (0.3) | 5.1 (1.4) | 4.7 (1.4) | 5.1 (1.4) | 5.3 (1.4) | 5.5 (1.5) | <0.001 |
| Magnesium, mg/dl | 0 (0.0) | 2.62 (0.51) | 2.12 (0.19) | 2.50 (0.08) | 2.75 (0.05) | 3.21 (0.58) | <0.001 |
| CRP, mg/dl | 16,831 (14.8) | 0.1 [0, 0.3] | 0.1 [0, 0.5] | 0.1 [0, 0.3] | 0.1 [0, 0.2] | 0.1 [0, 0.2] | <0.001 |
| Hemoglobin, g/dl | 432 (0.4) | 10.6 (1.2) | 10.4 (1.3) | 10.6 (1.2) | 10.7 (1.2) | 10.8 (1.2) | <0.001 |
| ALP, IU/L | 2,106 (1.9) | 258 (133) | 272 (144) | 258 (132) | 251 (124) | 249 (125) | <0.001 |
| Intact PTH, pg/ml | 7,011 (6.2) | 126 [65, 210] | 129 [68, 211] | 128 [66, 211] | 127 [67, 212] | 122 [61, 205] | <0.001 |
| Past history | |||||||
| Myocardial infarction, % | 412 (0.4) | 6.5 | 8.3 | 6.6 | 5.8 | 5.0 | <0.001 |
| Cerebral infarction, % | 2689 (2.4) | 11.1 | 13.3 | 11.5 | 9.8 | 9.2 | <0.001 |
| Cerebral hemorrhage, % | 276 (0.2) | 4.1 | 4.2 | 4.0 | 4.0 | 4.2 | 0.56 |
| Limb amputation, % | 57 (0.1) | 2.4 | 2.9 | 2.3 | 2.0 | 2.1 | <0.001 |
| Parathyroidectomy, % | 1430 (1.3) | 5.9 | 4.9 | 6.1 | 6.8 | 6.0 | <0.001 |
| Medication | |||||||
| Calcium carbonate, % | 906 (0.8) | 62.0 | 53.6 | 63.7 | 66.4 | 65.9 | <0.001 |
| Sevelamer hydrochloride, % | 936 (0.8) | 29.5 | 17.8 | 27.2 | 34.7 | 41.4 | <0.001 |
| Lanthanum carbonate, % | 1001 (0.9) | 13.8 | 9.9 | 13.4 | 15.6 | 17.1 | <0.001 |
| Vit.D (intravenous), % | 1098 (1.0) | 27.3 | 23.9 | 27.5 | 29.8 | 28.9 | <0.001 |
| Vit.D (oral), % | 973 (0.9) | 39.0 | 41.6 | 40.6 | 38.1 | 35.0 | <0.001 |
| Cinacalcet hydrochloride, % | 1071 (0.9) | 12.2 | 9.2 | 11.7 | 14.2 | 14.8 | <0.001 |
Data presented as mean (SD) or median [interquartile range]. Vit.D, active vitamin D analog.
Figure 2.
Magnesium modulates the calcium–PTH curves. A total of 110,305 patients were included in this analysis. Patients with a history of parathyroidectomy or prescribed cinacalcet hydrochloride were excluded. Dots represent the predicted mean value of the intact PTH level from a multivariate linear regression model with adjustment for age, sex, serum phosphate levels, and the use of intravenous active vitamin D analogs. Error bars represent 95% CIs. A significant interaction between serum calcium and magnesium levels on PTH level was observed (P<0.001).
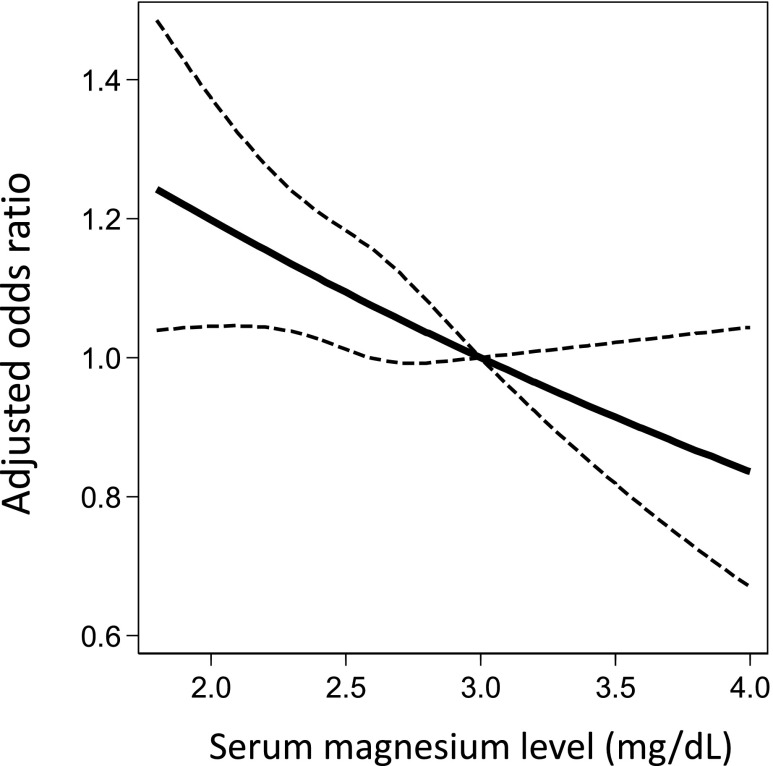
During the 2-year follow-up period, a total of 2305 (2.03%) new hip fractures occurred. The crude incidence rate significantly increased with decreasing serum Mg quartile (2.63%, 2.08%, 1.76%, and 1.49% in Q1–Q4, respectively; P<0.001 for trend). This trend was consistent for both men (1.79%, 1.46%, 1.32%, and 1.03% in Q1–Q4, respectively; P<0.001 for trend) and women (4.14%, 3.16%, 2.47%, and 2.19% in Q1–Q4, respectively; P<0.001 for trend). Multivariate logistic regression analyses revealed a dose-response relationship between Mg quartile and incident hip fracture risk (Table 2). In a fully adjusted model, patients in Q1 had a 1.23-fold higher risk (95% confidence interval [95% CI], 1.06 to 1.44) of incident hip fracture compared with those in Q4 (Table 2; model 4). Similar results were obtained after missing values were imputed using a multiple imputation method (Table 2; model 5). A 1-mg/dl increase in serum Mg level (as a continuous variable) was associated with a 14.3% decrease (95% CI, 3.8 to 23.8; P<0.01) in the risk of incident hip fracture. An adjusted restricted cubic spline curve revealed that the risk of fracture almost linearly decreased as serum Mg levels increased up to 4.0 mg/dl (Figure 3).
Table 2.
Logistic regression analysis for incident hip fracture
| No. (%) of Fractures | Serum Magnesium Level Quartiles | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 n=30,991 816 (2.63) | Q2 n=34,011 709 (2.08) | Q3 n=19,902 350 (1.76) | Q4 n=28,779 430 (1.49) | |||||||||
| OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Model 1 | 1.78 | 1.58 to 2.01 | <0.001 | 1.40 | 1.24 to 1.58 | <0.001 | 1.18 | 1.02 to 1.36 | 0.02 | 1.00 | — | — |
| Model 2 | 1.33 | 1.17 to 1.52 | <0.001 | 1.20 | 1.05 to 1.38 | <0.01 | 1.17 | 1.00 to 1.36 | 0.05 | 1.00 | — | — |
| Model 3 | 1.29 | 1.11 to 1.50 | 0.001 | 1.21 | 1.04 to 1.41 | 0.01 | 1.21 | 1.02 to 1.44 | 0.03 | 1.00 | — | — |
| Model 4 | 1.23 | 1.06 to 1.44 | <0.01 | 1.19 | 1.02 to 1.39 | 0.03 | 1.19 | 1.00 to 1.42 | 0.05 | 1.00 | — | — |
| Model 5 | 1.21 | 1.05 to 1.39 | <0.01 | 1.18 | 1.03 to 1.35 | 0.02 | 1.14 | 0.97 to 1.33 | 0.11 | 1.00 | — | — |
| Model 6 | 1.24 | 1.04 to 1.47 | 0.02 | 1.13 | 0.96 to 1.34 | 0.14 | 1.12 | 0.92 to 1.36 | 0.24 | 1.00 | — | — |
Model 1: unadjusted. Model 2: adjusted for age, sex, body mass index, dialysis duration, urea reduction rate, dialysis vintage, physical activity, and diabetes mellitus. Model 3: adjusted for the covariates in model 2, with the addition of calcium, phosphate, ALP, intact PTH (quartile), albumin, CRP, and hemoglobin. Model 4: adjusted for the covariates in model 3, with the addition of past history of cardiovascular disease (myocardial infarction, cerebral infarction, cerebral hemorrhage, and amputation), medication (calcium carbonate, sevelamer hydrochloride, lanthanum carbonate, active vitamin D analog [intravenous and oral], and cinacalcet hydrochloride), and a history of parathyroidectomy. Model 5: multiple imputation analysis adjusted for the covariates in model 4. Model 6: IPW model adjusted for the covariates in model 4. OR, odds ratio; —, not applicable.
Figure 3.
Adjusted odds ratio for hip fracture decreases linearly as serum magnesium level increases. Model was adjusted for age, sex, body mass index, dialysis duration, urea reduction rate, dialysis vintage, physical activity, diabetes mellitus, calcium, phosphate, ALP, intact PTH (quartile), albumin, CRP, hemoglobin, past history of cardiovascular disease (myocardial infarction, cerebral infarction, cerebral hemorrhage, and amputation), medication (calcium carbonate, sevelamer hydrochloride, lanthanum carbonate, active vitamin D analog [intravenous and oral], and cinacalcet hydrochloride), and a history of parathyroidectomy. The dashed line represents the 95% CI. The reference serum magnesium value is 3.0 mg/dl.
To examine the influence of low Mg levels on the fracture risk, we divided the patients in Q1 into two groups according to the lower fifth percentile of serum Mg levels (Q1a [≤1.9 mg/dl] and Q1b [>1.9 mg/dl]). In the fully adjusted model, the odds ratio in Q1a and Q1b versus Q4 was 1.34 (95% CI, 1.05 to 1.70; P=0.02) and 1.21 (95% CI, 1.03 to 1.43; P=0.02), respectively.
To further control for potential confounders, an inverse probability weighting (IPW) approach was utilized. All of the baseline covariates were well balanced across the Mg quartiles after IPW (Supplemental Table 2). The IPW model with full multivariate adjustment confirmed that patients in Q1 had a significantly higher risk of hip fracture compared with those in Q4 (Table 2; model 6).
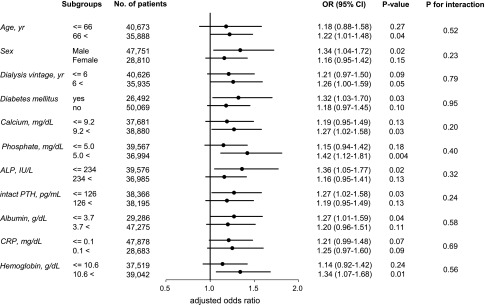
In subgroup analyses for several prespecified covariates, we found no significant interactions. As shown in Figure 4, patients in Q1 had an elevated risk of fracture compared with those in Q4 across all subgroups, although this difference did not reach statistical significance in several subgroups because of insufficient statistical power in those subgroups.
Figure 4.
Adjusted odds ratio for hip fracture in the lowest magnesium quartile is elevated across the subgroups. Dots represent the adjusted odds ratios (ORs) for incident hip fractures in the lowest versus the highest quartile of serum magnesium levels. Bars represent 95% CIs.
A multinomial logistic regression analysis was used to account for death as an alternative risk event. Death occurred in 9398 patients (8.27%) during the follow-up period. Crude mortality rates significantly increased with decreasing Mg quartile (11.99%, 7.87%, 6.08%, and 6.20% in Q1–Q4, respectively; P<0.001 for trend). In this analysis, lower serum Mg levels were again associated with a significantly higher risk of fracture (Table 3).
Table 3.
Multinomial logistic regression analysis for incident hip fracture with death treated as an alternative risk event
| Serum Magnesium Level Quartiles | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) of fractures | Q1 n=30,991 816 (2.63) | Q2 n=34,011 709 (2.08) | Q3 n=19,902 350 (1.76) | Q4 n=28,779 430 (1.49) | ||||||||
| RR | 95% CI | P Value | RR | 95% CI | P Value | RR | 95% CI | P Value | RR | 95% CI | P Value | |
| 1.24 | 1.06 to 1.45 | <0.01 | 1.17 | 1.00 to 1.37 | 0.05 | 1.17 | 0.98 to 1.40 | 0.08 | 1.00 | — | — | |
Model adjusted for age, sex, body mass index, dialysis duration, urea reduction rate, dialysis vintage, physical activity, diabetes mellitus, calcium, phosphate, ALP, intact PTH (quartile), albumin, CRP, hemoglobin, past history of cardiovascular disease (myocardial infarction, cerebral infarction, cerebral hemorrhage, and amputation), medication (calcium carbonate, sevelamer hydrochloride, lanthanum carbonate, active vitamin D analog [intravenous and oral], and cinacalcet hydrochloride), and a history of parathyroidectomy. RR, relative risk ratio; —, not applicable.
We conducted two additional analyses. First, we performed a time-to-event analysis. In a competing risks regression model, accounting for death as a competing event, the subhazard ratio for incident hip fracture in Q1–Q4 was 1.23 (95% CI, 1.05 to 1.44; P<0.01), 1.19 (95% CI, 1.02 to 1.38; P=0.03), 1.19 (95% CI, 1.00 to 1.42; P=0.05), and 1.00 (reference), respectively. Second, in a Poisson regression analysis, incidence rate ratio for incident hip fracture in Q1–Q4 was 1.22 (95% CI, 1.05 to 1.43; P=0.01), 1.18 (95% CI, 1.01 to 1.37; P=0.04), 1.18 (95% CI, 1.00 to 1.41; P=0.06), and 1.00 (reference), respectively.
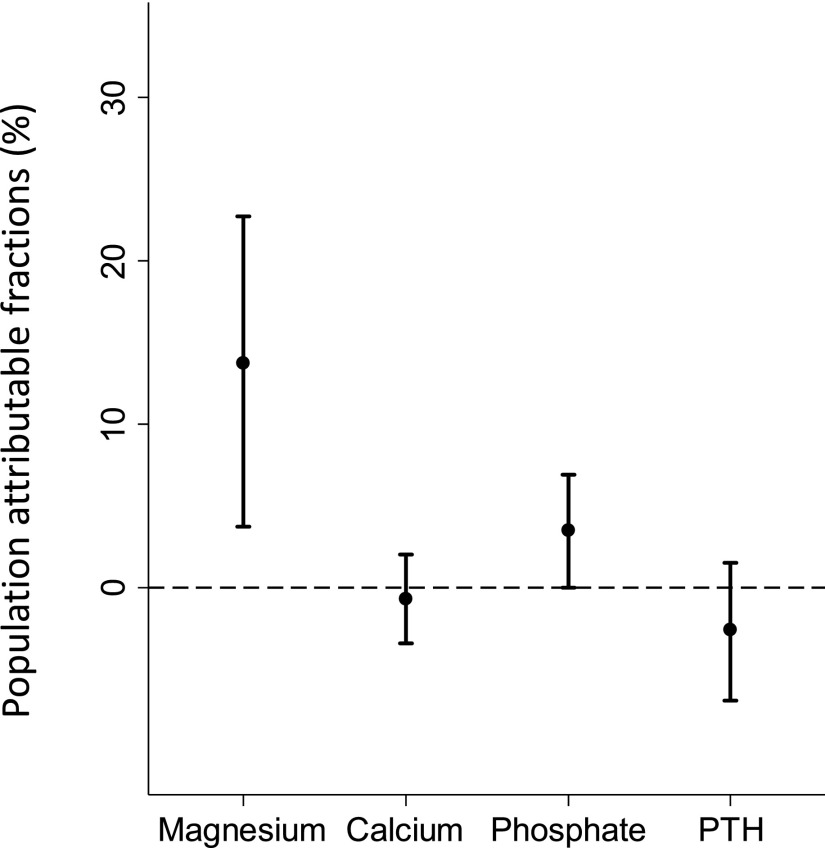
Finally, we estimated the population-attributable risk fraction (PAF) for the incidence of hip fracture. The adjusted PAF for lower serum Mg levels (i.e., Q1–Q3) was 13.7% (95% CI, 3.7% to 22.7%). This result implies that 13.7% of all new hip fractures could have been prevented if the serum Mg levels in Q1–Q3 range (≤2.8 mg/dl) had been corrected to those in Q4 (>2.8 mg/dl). Notably, the PAF for Mg was much higher than that for other components of the mineral and bone disorders (Figure 5); on the basis of the target ranges in the Japanese guidelines,29 the PAFs for calcium, phosphate, and intact PTH were −0.7% (95% CI, −3.4% to 2.0%), 3.5% (95% CI, 0.0% to 6.9%), and −2.6% (95% CI, −6.9% to 1.5%), respectively.
Figure 5.
PAF for incident hip fractures is much greater for magnesium than that for calcium, phosphate, and intact PTH. Bars represent 95% CIs. The dashed line represents 0% in PAF.
Discussion
In this large-scale cohort study of patients undergoing hemodialysis, we found that patients with higher serum Mg levels had a lower risk of hip fracture. This association was robustly demonstrated after extensive adjustment for relevant clinical factors, including the components of mineral and bone disorders in CKD. Further controlling for baseline confounders using the IPW method provided similar results. The fracture risk linearly decreased with increasing serum Mg level, even when the level exceeded the upper limit of the reference range. In addition, the Mg level had a high PAF, suggesting its substantial clinical impact on the risk of hip fracture. To our knowledge, this is the first study to show a relationship between Mg and the risk of hip fracture among patients with kidney disease. Although we have previously reported that mild hypermagnesemia is linked to improved survival in patients undergoing hemodialysis,30 these results suggest that higher Mg levels may also be beneficial in reducing the fracture risk.
Experimental studies have found the deleterious effects of Mg deficiency on bone metabolism. In vitro studies have demonstrated that a low extracellular Mg concentration stimulates osteoclastogenesis and inhibits osteoblasts proliferation through the upregulation of inducible nitric oxide synthase.31,32 In animal studies, a Mg-deficient diet has been shown to activate osteoclasts and cause excessive bone mineralization, leading to impairment of bone strength.17–23 Consistent with these experimental data, our study showed that a lower serum Mg level increases the risk of hip fracture. On the other hand, there is concern that excess Mg accumulation in the bone might also be harmful because of its anticalcification property.28 Nevertheless, the use of Mg-containing phosphate binders has been reported not to affect bone mineralization in nephrectomized mice.33 Our findings provide further evidence that hypermagnesemia, at least within the clinically relevant range (≤4.0 mg/dl), decreases the risk of hip fracture. The 2-year follow-up period, however, might be insufficient to detect an adverse effect of excess Mg. Because the relationship between serum Mg levels and mortality may be U-shaped,30 future intervention studies should carefully decide the target range of serum Mg levels in patients undergoing hemodialysis.
Mg is an agonist of the calcium-sensing receptor.34 A previous in vitro study has shown that Mg could suppress PTH secretion from the parathyroid glands, particularly at moderately low extracellular calcium levels, whereas this inhibitory effect was largely diminished at high and low calcium levels.35 Our study is the first to clinically confirm this experimental observation, finding that Mg may have a suppressive effect on PTH secretion especially at low-normal serum calcium levels, whereas this effect may become weaker at high and low serum calcium levels. Given that the calcium load for patients undergoing dialysis may be unfavorable in terms of the progression of vascular calcification and mortality,36 increasing Mg level might be a useful strategy to control PTH levels, especially among patients with low-normal serum calcium levels. Although Mg may reduce the fracture risk partly through its effect on PTH, the association between Mg and fracture risk observed in our study remained significant after adjustment for PTH levels.
Although bone Mg content can be evaluated by bone biopsy, this procedure is highly invasive and is not feasible for large-scale studies. Serum Mg level has been reported to correlate well with bone Mg content,37 as Mg that exists in the bone hydration shell and hydroxyapatite crystal surface is readily exchangeable and is in equilibrium with the extracellular Mg.16 Therefore, serum Mg level is considered a practical and easy-to-measure maker of bone Mg content. We believe that the observed association between Mg and fracture risk was detectable because the serum Mg level reflects the bone Mg content.
The estimated PAF for Mg was high (13.7%), indicating that Mg may be involved in a considerable proportion of hip fracture cases in patients undergoing hemodialysis. In addition, the PAF for Mg was much higher than that for other components of mineral and bone disorders in CKD (i.e., calcium, phosphate, and PTH). This may be because the latter three factors are routinely monitored and managed in current clinical practice for patients undergoing dialysis. Consequently, the number of patients with abnormal calcium, phosphate, and PTH levels conferring an increased risk of fractures is very small. In contrast, Mg is generally not yet regarded as a treatment target. Here, we found that a large proportion of patients undergoing hemodialysis (i.e., Q1–Q3) had serum Mg levels that were significantly associated with an increased risk of hip fracture. Therefore, nephrologists should be more aware of the clinical importance of Mg on the fracture risk. Interventions for Mg may produce a substantial benefit in the prevention of fractures in patients undergoing dialysis.
The limitations of our study include its observational design, which hampers causal inference between Mg and fracture risk. In particular, patients in the lower Mg quartiles were older and had a higher prevalence of cardiovascular comorbidities, which may confound the relationship between Mg and fracture risk. However, we performed an extensive adjustment for related factors in the multivariate models. In addition, the association was observed in a variety of statistical models, including the IPW model. We confirmed that a potential selection bias arising from the measurement of serum Mg levels was minimal, as there were no considerable differences in the baseline characteristics between patients with and without missing serum Mg data. The dataset contained missing data other than Mg; thus, we performed an imputation method which did not substantially alter the results. We did not have data on the use of proton pump inhibitors (PPIs). Although the causal relationship has not been clarified, it has been reported that the use of PPIs is associated with lower serum Mg levels,38 as well as an increased risk of fractures39 in patients undergoing hemodialysis. Therefore, although it is tempting to speculate that Mg is an intervening factor between the use of PPIs and the fracture risk, PPIs might confound the association between Mg and fracture risk. We could not test the sensitivity and specificity of hip fracture diagnoses by questionnaire-based survey. The data were limited to the first occurrence of hip fracture and thus cannot be extrapolated to the risk of repeated fractures. Because the dialysate Mg concentration in Japan is 1.0 mEq/L, we may not be able to extrapolate our findings to patients dialyzed with different Mg concentrations. It is worth investigating whether high Mg dialysates provide benefits for reducing the risk of fracture. Finally, because the follow-up period was 2 years, longer-term effects of hypermagnesemia on fracture risk could not be determined.
In conclusion, we found that mild hypermagnesemia is independently associated with a lower risk of hip fracture in patients undergoing hemodialysis. Compared with that for calcium, phosphate, and PTH, Mg had a substantially high PAF, indicating the clinical importance of low Mg level for the fracture risk in patients undergoing hemodialysis. Although Mg has long been under-recognized in the field of mineral and bone disorders in CKD, our findings provide strong motivation for future interventional studies examining the therapeutic efficacy of Mg for the prevention of fractures.
Concise Methods
Database and Patient Selection
Data were extracted from the Japanese Society for Dialysis Therapy-Renal Data Registry, which has been described elsewhere in detail.40 Briefly, the Japanese Society for Dialysis Therapy has implemented annual questionnaire-based surveys of dialysis facilities throughout Japan since 1968. The questionnaire was filled out by medical staff in each facility. As the response rate has exceeded 95% every year, the database covers nearly all patients undergoing dialysis in Japan. Because data on serum Mg levels were collected for the first time in 2009, the datasets of 2009, 2010, and 2011 were combined and analyzed in our study. We included patients who (1) were aged 18 years and older, (2) received in-center hemodialysis/hemodiafiltration, (3) had no previous history of hip fracture, and (4) had serum Mg data.
The study protocol was approved by the Ethics Committee of the Japanese Society for Dialysis Therapy.
Baseline Covariates
The following data were collected: demographics (age, sex, body mass index, physical activity, the primary kidney disease [diabetes or nondiabetes], hemodialysis vintage, and duration of dialysis treatment [hours per week]); laboratory measurements (predialysis albumin, urea nitrogen, calcium, phosphate, Mg, C-reactive protein [CRP], hemoglobin, ALP, and intact and whole PTH levels); prescriptions (phosphate binders [calcium carbonate, sevelamer hydrochloride, and lanthanum carbonate], cinacalcet hydrochloride, and active vitamin D analogs [intravenous and oral]); and past history of parathyroidectomy, cardiovascular diseases (myocardial infarction, cerebral infarction, cerebral hemorrhage, and amputation of the extremities), and hip fracture. Poor physical activity was defined for patients who spend more than a half of a day in bed. Whole PTH levels were multiplied by 1.7 to obtain an equivalent value for intact PTH. The dialysate Mg concentration in Japan is 1.0 mEq/L.
Outcomes
We detected an occurrence of a new hip fracture during the study period if a patient’s past history of hip fracture was “no” in 2009 and “yes” in 2010 or 2011. We also obtained information about month of death to account for death as an alternative risk event.
Although we did not have the exact date of fracture occurrence, we applied the following method to additionally perform a time-to-event analysis9: if a patient did not suffer a hip fracture, he or she was censored at death or the end of the study period, whichever came first. If a patient suffered a hip fracture, the fracture was assumed to occur at the middle of his or her survival time during the study period.
Statistical Analyses
To examine the relationship between Mg and PTH–calcium curves, we performed a multivariate linear regression analysis with adjustment for age, sex, serum phosphate level, and intravenous active vitamin D analog use. Patients with a history of parathyroidectomy or who were prescribed cinacalcet hydrochloride were excluded.
Logistic regression analyses were performed to estimate the odds ratios and 95% CIs for incident hip fracture. We constructed several multivariate models: model 1, unadjusted; model 2, adjusted for age, sex, body mass index, dialysis duration, urea reduction rate, dialysis vintage, physical activity, and diabetes mellitus; model 3, adjusted for the covariates in model 2, with the addition of calcium, phosphate, ALP, intact PTH, albumin, CRP, and hemoglobin levels; and model 4, adjusted for the covariates in model 3, with the addition of a past history of cardiovascular diseases, prescriptions (phosphate binders, active vitamin D analog [intravenous and oral], and cinacalcet hydrochloride), and a history of parathyroidectomy. An adjusted restricted cubic spline curve was depicted with three knots to show a potentially nonlinear relationship between serum Mg level and fracture risk. The association between fracture risk and serum Mg as a continuous variable was also examined using a fully adjusted model. Additionally, we performed a multinomial logistic regression analysis to account for death as an alternative risk event.41
To further control for baseline confounding, we conducted a fully adjusted multivariate logistic regression analysis with IPW. The propensity score was estimated using a multinomial logistic regression analysis with Mg quartile as the dependent variable and all baseline covariates as independent variables.
A multiple imputation analysis was performed to fill in the missing values of the baseline covariates by creating five imputed datasets. Intact PTH levels were log-transformed to normalize the distribution. We did not impute dialysis vintage and CRP data because of strong departures from normality.
Subgroup analyses were conducted for several prespecified variables. We entered a product term between Mg and each variable into the fully adjusted multivariate logistic model to evaluate potential interactions affecting the risk of hip fracture.
In the time-to-event analysis, we used a competing risks regression model to estimate a subhazard ratio for incident hip fracture, accounting for death as a competing risk event. We also performed a multivariate Poisson regression analysis in which a follow-up duration was defined as survival time. These models were adjusted for all covariates in model 4.
All reported P values are two-sided, and P values <0.05 were considered statistically significant. All statistical analyses were performed using Stata 13 statistical software (StataCorp, College Station, TX).
Disclosures
None.
Supplementary Material
Acknowledgments
The authors wish to express heartfelt appreciation to the Japanese Society for Dialysis Therapy, the principal investigators of all prefectures, and all of the personnel and patients at the institutions participating in this survey.
Parts of this article were presented in abstract form at the annual meeting of the American Society of Nephrology Kidney Week, November 18, 2016, Chicago, IL.
The contents and opinions in this paper are those of the authors only and do not reflect those of the Japanese Society for Dialysis Therapy.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017080849/-/DCSupplemental.
References
- 1.Mittalhenkle A, Gillen DL, Stehman-Breen CO: Increased risk of mortality associated with hip fracture in the dialysis population. Am J Kidney Dis 44: 672–679, 2004 [PubMed] [Google Scholar]
- 2.Tentori F, McCullough K, Kilpatrick RD, Bradbury BD, Robinson BM, Kerr PG, Pisoni RL: High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int 85: 166–173, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM: PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis 47: 149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, Wong C, Stehman-Breen C: Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 58: 396–399, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Coco M, Rush H: Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis 36: 1115–1121, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Ball AM, Gillen DL, Sherrard D, Weiss NS, Emerson SS, Seliger SL, Kestenbaum BR, Stehman-Breen C: Risk of hip fracture among dialysis and renal transplant recipients. JAMA 288: 3014–3018, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, Mason N, Prutz KG, Young EW, Pisoni RL: Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 70: 1358–1366, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Arneson TJ, Li S, Liu J, Kilpatrick RD, Newsome BB, St Peter WL: Trends in hip fracture rates in US hemodialysis patients, 1993-2010. Am J Kidney Dis 62: 747–754, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Wakasugi M, Kazama JJ, Taniguchi M, Wada A, Iseki K, Tsubakihara Y, Narita I: Increased risk of hip fracture among Japanese hemodialysis patients. J Bone Miner Metab 31: 315–321, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Maravic M, Ostertag A, Torres PU, Cohen-Solal M: Incidence and risk factors for hip fractures in dialysis patients. Osteoporos Int 25: 159–165, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Kim SM, Long J, Montez-Rath M, Leonard M, Chertow GM: Hip fracture in patients with non-dialysis-requiring chronic kidney disease. J Bone Miner Res 31: 1803–1809, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Leinau L, Perazella MA: Hip fractures in end-stage renal disease patients: Incidence, risk factors, and prevention. Semin Dial 19: 75–79, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Stehman-Breen CO, Sherrard DJ, Alem AM, Gillen DL, Heckbert SR, Wong CS, Ball A, Weiss NS: Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int 58: 2200–2205, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Jamal SA, Leiter RE, Jassal V, Hamilton CJ, Bauer DC: Impaired muscle strength is associated with fractures in hemodialysis patients. Osteoporos Int 17: 1390–1397, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Babayev R, Nickolas TL: Bone disorders in chronic kidney disease: An update in diagnosis and management. Semin Dial 28: 645–653, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Alfrey AC, Miller NL: Bone magnesium pools in uremia. J Clin Invest 52: 3019–3027, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creedon A, Flynn A, Cashman K: The effect of moderately and severely restricted dietary magnesium intakes on bone composition and bone metabolism in the rat. Br J Nutr 82: 63–71, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Rude RK, Gruber HE, Wei LY, Frausto A, Mills BG: Magnesium deficiency: Effect on bone and mineral metabolism in the mouse. Calcif Tissue Int 72: 32–41, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Rude RK, Gruber HE, Norton HJ, Wei LY, Frausto A, Kilburn J: Dietary magnesium reduction to 25% of nutrient requirement disrupts bone and mineral metabolism in the rat. Bone 37: 211–219, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Rude RK, Gruber HE, Norton HJ, Wei LY, Frausto A, Kilburn J: Reduction of dietary magnesium by only 50% in the rat disrupts bone and mineral metabolism. Osteoporos Int 17: 1022–1032, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Rude RK, Singer FR, Gruber HE: Skeletal and hormonal effects of magnesium deficiency. J Am Coll Nutr 28: 131–141, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi M, Hara K, Akiyama Y: Effects of vitamin K2 (menatetrenone) and alendronate on bone mineral density and bone strength in rats fed a low-magnesium diet. Bone 35: 1136–1143, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Amizuka N, Li M, Kobayashi M, Hara K, Akahane S, Takeuchi K, Freitas PH, Ozawa H, Maeda T, Akiyama Y: Vitamin K2, a gamma-carboxylating factor of gla-proteins, normalizes the bone crystal nucleation impaired by Mg-insufficiency. Histol Histopathol 23: 1353–1366, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Farsinejad-Marj M, Saneei P, Esmaillzadeh A: Dietary magnesium intake, bone mineral density and risk of fracture: a systematic review and meta-analysis. Osteoporos Int 27: 1389–1399, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Hayhoe RP, Lentjes MA, Luben RN, Khaw KT, Welch AA: Dietary magnesium and potassium intakes and circulating magnesium are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the EPIC-Norfolk cohort study. Am J Clin Nutr 102: 376–384, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Kunutsor SK, Whitehouse MR, Blom AW, Laukkanen JA: Low serum magnesium levels are associated with increased risk of fractures: A long-term prospective cohort study [published online ahead of print April 12, 2017]. Eur J Epidemiol 10.1007/s10654-017-0242-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Haese PC, Couttenye MM, Lamberts LV, Elseviers MM, Goodman WG, Schrooten I, Cabrera WE, De Broe ME: Aluminum, iron, lead, cadmium, copper, zinc, chromium, magnesium, strontium, and calcium content in bone of end-stage renal failure patients. Clin Chem 45: 1548–1556, 1999 [PubMed] [Google Scholar]
- 28.Cunningham J, Rodríguez M, Messa P: Magnesium in chronic kidney disease Stages 3 and 4 and in dialysis patients. Clin Kidney J 5[Suppl 1]: i39–i51, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukagawa M, Yokoyama K, Koiwa F, Taniguchi M, Shoji T, Kazama JJ, Komaba H, Ando R, Kakuta T, Fujii H, Nakayama M, Shibagaki Y, Fukumoto S, Fujii N, Hattori M, Ashida A, Iseki K, Shigematsu T, Tsukamoto Y, Tsubakihara Y, Tomo T, Hirakata H, Akizawa T; CKD-MBD Guideline Working Group; Japanese Society for Dialysis Therapy : Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial 17: 247–288, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Isaka Y: Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int 85: 174–181, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Leidi M, Dellera F, Mariotti M, Banfi G, Crapanzano C, Albisetti W, Maier JA: Nitric oxide mediates low magnesium inhibition of osteoblast-like cell proliferation. J Nutr Biochem 23: 1224–1229, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Belluci MM, Schoenmaker T, Rossa-Junior C, Orrico SR, de Vries TJ, Everts V: Magnesium deficiency results in an increased formation of osteoclasts. J Nutr Biochem 24: 1488–1498, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Neven E, De Schutter TM, Dams G, Gundlach K, Steppan S, Büchel J, Passlick-Deetjen J, D’Haese PC, Behets GJ: A magnesium based phosphate binder reduces vascular calcification without affecting bone in chronic renal failure rats. PLoS One 9: e107067, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quitterer U, Hoffmann M, Freichel M, Lohse MJ: Paradoxical block of parathormone secretion is mediated by increased activity of G alpha subunits. J Biol Chem 276: 6763–6769, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Ortiz ME, Canalejo A, Herencia C, Martínez-Moreno JM, Peralta-Ramírez A, Perez-Martinez P, Navarro-González JF, Rodríguez M, Peter M, Gundlach K, Steppan S, Passlick-Deetjen J, Muñoz-Castañeda JR, Almaden Y: Magnesium modulates parathyroid hormone secretion and upregulates parathyroid receptor expression at moderately low calcium concentration. Nephrol Dial Transplant 29: 282–289, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jamal SA, Vandermeer B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M, Lok CE, Fitchett D, Tsuyuki RT: Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet 382: 1268–1277, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Alfrey AC, Miller NL, Trow R: Effect of age and magnesium depletion on bone magnesium pools in rats. J Clin Invest 54: 1074–1081, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakashima A, Ohkido I, Yokoyama K, Mafune A, Urashima M, Yokoo T: Proton pump inhibitor use and magnesium concentrations in hemodialysis patients: A Cross-Sectional Study. PLoS One 10: e0143656, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen D, Olesen JB, Gislason GH, Abrahamsen B, Hommel K: Risk of fracture in adults on renal replacement therapy: A Danish national cohort study. Nephrol Dial Transplant 31: 1654–1662, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Nakai S, Iseki K, Itami N, Ogata S, Kazama JJ, Kimata N, Shigematsu T, Shinoda T, Shoji T, Suzuki K, Taniguchi M, Tsuchida K, Nakamoto H, Nishi H, Hashimoto S, Hasegawa T, Hanafusa N, Hamano T, Fujii N, Masakane I, Marubayashi S, Morita O, Yamagata K, Wakai K, Wada A, Watanabe Y, Tsubakihara Y: Overview of regular dialysis treatment in Japan (as of 31 December 2009). Ther Apher Dial 16: 11–53, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Dunkler D, Gao P, Lee SF, Heinze G, Clase CM, Tobe S, Teo KK, Gerstein H, Mann JF, Oberbauer R; ONTARGET and ORIGIN Investigators : Risk prediction for early CKD in type 2 diabetes. Clin J Am Soc Nephrol 10: 1371–1379, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.