Abstract
Background
Given the relative importance of cognitive impairment, there was considerable interest in identifying the cognitive profile of PD patients, in order to ensure specific and appropriate therapeutic interventions.
Purpose
To determine the effects of physical exercise programs on cognitive function in PD patients, compared with the control group.
Data sources
Medline, Cochrane, Scopus, PEDro and Web of Science (last searched in September 2016).
Study selection
Randomized clinical trials examining the effects of physical exercise programs and cognitive function in PD patients. Nine studies fulfilled the selection criteria and were included in this review.
Data extraction
Characteristics of the publication, characteristics of the participants, test used for cognitive screening, cognitive domain assessed, tools used to assess cognitive function, characteristics of the experimental intervention, characteristics of the control group, mean results and standard deviation of function cognitive. The PEDro score was used to evaluate methodological quality.
Data synthesis
Most eligible studies showed good methodological quality based on the PEDro scale. Studies have shown that adapted tango for PD patients, cognitive training combined with motor training, and treadmill training promote the preservation or improvement of cognitive function in PD patients.
Limitations
The diversity of cognitive tests used to assess cognitive function and the high heterogeneity identified between the physical exercise programs.
Conclusions
Physical exercise programs promote positive and significant effects on global cognitive function, processing speed, sustained attention and mental flexibility in PD patients, at a mild to moderate stage for patients with a 6-year clinical diagnosis of PD. However, treadmill training performed 3 times a week for about 60 minutes and for a period of 24 weeks produced larger improvements in cognition.
Introduction
Parkinson’s disease (PD) is a chronic, progressive and multisystem neurodegenerative disease [1] and has historically been considered specifically as a motor disorder. [2] However, in the past decades, a wide spectrum of non-motor manifestations of this disease has been recognized. [3,4]
In this context, cognitive impairment is one of the most prominent features of PD, [5,6] considering that 25 to 30% of the patients have cognitive deficits at the onset of the disease and 50% of the patients demonstrate significant cognitive decline in the first three to five years of illness. [7,8] Mild cognitive impairment (MCI) affects between 18.9 and 38.2% of patients in the early stages of the disease. [9]
A prospective cohort study with PD patients shows that mild cognitive impairment (MCI) in the first year of PD diagnosis indicates a high risk of dementia. More than 25% of MCI patients diagnosed with PD developed dementia within 3 years of follow-up compared to less than 1% of MCI patients without a diagnosis of PD. Among MCI patients at the onset of the disease and after one year of follow-up, almost half of them evolved into dementia. [10]
The clinical status of PD has been compared to an iceberg: the visible part represents the motor symptoms, and most non-visible part represents the various non-motor manifestations, which are not often recognized in clinical practice. [11] Also, PD diagnosis is often complex and there are no reliable diagnostic biomarkers. Thus, the diagnosis is based on clinical skills and methods. [5] However, mild cognitive impairment (MCI) may even precede motor symptoms. [12]
One of the most feared PD complications for patients and their caregivers is the development of dementia. [13] The prevalence of dementia is higher than 75% in patients who survive 10 years with the diagnosis of PD. [14] It is estimated that 80% of PD patients will progress to dementia associated with PD.14 The term “Parkinson´s disease dementia” (PDD) refers to dementia that develops at least 12 months after the onset of the motor changes. When dementia is developed in the first 12 months of disease progression, the criteria for the diagnosis of Lewy bodies dementia are met. [15] PDD is the most severe manifestation among the cognitive changes and it increases the risk of death. [16]
In a systematic review, the prevalence of PDD was estimated at 0.5% in individuals aged 65 years or older. The percentage of PDD among individuals with dementia was 3.6% (3.1–4.1), with an estimated prevalence of PDD of 0.2% in individuals aged 65 years or older. Despite the methodological variation of the included studies, the authors suggest that 24 to 31% of PD patients show dementia and that 3 to 4% of dementia in the population could be associated with PD. The estimated prevalence of PDD in the general population over 65 years is 0.2 to 0.5%. [17]
Cognitive deficits sometimes occur in the early stages of PD, and in these cases, they may not be clinically apparent, but detectable only by specific tests. [18] In the systematic review conducted by Romann et al. [19], the authors assessed the most commonly used instruments for cognitive evaluation in PD patients undergoing deep brain stimulation and found that there is no consensus about such cognitive instruments. In another systematic review, [20] the authors demonstrated that further research is needed on cognitive function screening tools that can be used to detect early signs of cognitive impairment. Several tools do not cover all fundamental cognitive domains; moreover, there are only a few longitudinal studies with follow-up periods long enough to observe cognitive changes.
Due to the strong impact of cognitive disorders on the quality of life of patients and their caregivers, it is important to find tools needed to manage cognitive decline. [21] Given the relative importance of cognitive impairment, there was considerable interest in identifying the cognitive profile of PD patients, in order to ensure specific and appropriate therapeutic interventions. [22] From this point of view, the literature on this topic is quite comprehensive. [8,23–26]
In the systematic review conducted by Hindle et al. [27] the effectiveness of various types of interventions on cognition in PD patients was assessed. However, the study was limited to the period between 2000 and 2011, and the descriptors related to cognition were restricted. The result of the review produced four clinical studies related to physical exercise and physical therapy and four studies focusing on combined therapy. Likewise, the systematic review conducted by Murray et al. [28], which aimed to analyze the evidence on the effects of exercise on cognition in PD in humans and animal models, showed that cognition-related descriptors were also restricted, and that nine clinical studies in humans demonstrated several modalities of physical exercise.
This study aimed to verify the effects of physical exercise programs on cognitive function in Parkinson’s disease patients, compared with the control group, through a systematic review of randomized clinical trials of the last 10 years. The secondary objectives were to verify the most commonly used tools to assess cognitive screening and cognitive function, as well as to check the cognitive domains evaluated and post-intervention effects (follow-up).
Method
This systematic review was registered under the number CRD42016047788 in the International Prospective Register of Systematic Reviews—PROSPERO and follows the recommendations proposed by the Preferred Reporting Items for Systematic Review and Meta-Analysis: The PRISMA Statement (S1 PRISMA Checklist). [29]
Eligibility criteria
Randomized clinical trials were included in this review. All included studies investigated physical exercise programs and cognitive function in PD patients. They were indexed in previously selected databases with available abstracts, full online access of the last 10 years and no language restrictions. Such restriction has the objective to show a current panorama of the analyzed studies.
Search strategy
MEDLINE (Medical Literature Analysis and Retrieval System on-line) via Pubmed, Cochrane, Scopus (Elsevier), PEDro and Web of Science were the selected electronic databases for this study. The search strategy included the descriptors proposed in the Medical Subject Headings (MeSH) related to the topics (see Table 1), associated with a sensitive list of search terms for randomized controlled trials (RCTs), developed by Robinson and Dickersin. [30] All search operations were performed in September 2016.
Table 1. Descriptors used in search strategy.
| Topics | Descriptors |
|---|---|
| Physical exercise programs | Physical Therapy Modalities"[Mesh], "Physical Therapy Modalities", “Modalities, Physical Therapy”, “Modality, Physical Therapy”, “Physical Therapy Modality”, “Physiotherapy (Techniques)”, “Physiotherapies (Techniques)”, “Physical Therapy Techniques”, “Physical Therapy Technique”, “Techniques, Physical Therapy”, "Exercise Movement Techniques"[Mesh], "Exercise Movement Techniques", “Movement Techniques, Exercise”, “Exercise Movement Technics”, "Exercise Therapy"[Mesh], "Exercise Therapy", “Therapy, Exercise”, “Exercise Therapies”, “Therapies, Exercise”, "Exercise"[Mesh], "Exercise", “Exercises”, “Exercise, Physical”, “Exercises, Physical”, “Physical Exercise”, “Physical Exercises”, “Exercise, Isometric”, “Exercises, Isometric”, “Isometric Exercises”, “Isometric Exercise”, “Exercise, Aerobic”, “Aerobic Exercises”, “Exercises, Aerobic”, “Aerobic Exercise”, "Resistance Training"[Mesh], "Resistance Training", “Training, Resistance”, “Strength Training”, “Training, Strength”, “Weight-Lifting Strengthening Program”, “Strengthening Program, Weight-Lifting”, “Strengthening Programs, Weight-Lifting”, “Weight Lifting Strengthening Program”, “Weight-Lifting Strengthening Programs”, “Weight-Lifting Exercise Program”, “Exercise Program, Weight-Lifting”, “Exercise Programs, Weight-Lifting”, “Weight Lifting Exercise Program”, “Weight-Lifting Exercise Programs”, “Weight-Bearing Strengthening Program”, “Strengthening Program, Weight-Bearing”, “Strengthening Programs, Weight-Bearing”, “Weight Bearing Strengthening Program”, “Weight-Bearing Strengthening Programs”, “Weight-Bearing Exercise Program”, “Exercise Program, Weight-Bearing”, “Exercise Programs, Weight-Bearing”, “Weight Bearing Exercise Program”, “Weight-Bearing Exercise Programs” |
| Cognitive function | "Cognition"[Mesh], “Cognitions”, “Cognitive Function”, “Cognitive Functions”, “Function, Cognitive”, “Functions, Cognitive”, "Executive Function"[Mesh], “Executive Functions”, “Function, Executive”, “Functions, Executive”, “Executive Control”, “Executive Controls”, "Problem Solving"[Mesh], "Perception"[Mesh], “Perceptions", "Memory"[Mesh], "Attention"[Mesh], “Attentions”, “Concentration”, “Concentrations”, "Learning"[Mesh] |
| Population | "Parkinson Disease"[Mesh], "Parkinson Disease", “Idiopathic Parkinson’s Disease”, “Lewy Body Parkinson Disease”, “Lewy Body Parkinson’s Disease”, “Primary Parkinsonism”, “Parkinsonism, Primary”, “Parkinson Disease, Idiopathic”, “Parkinson’s Disease”, “Parkinson’s Disease, Idiopathic”, “Parkinson’s Disease, Lewy Body”, “Idiopathic Parkinson Disease”, “Paralysis Agitans” |
Source: Author’s own production
EndNote 3.4 was used to manage reference material during searches.
Selection of studies and data extraction
Two independent reviewers initially evaluated the studies according to titles and abstracts, identified by the search strategy. Then, the reviewers evaluated the complete articles and selected studies according to the specified eligibility criteria. Disagreements between the reviewers were resolved by consensus.
The following data was extracted from the selected studies: identification of the publication, location (country) of the study, sample size, characteristics of the participants (gender, mean age, years of education, stage of the disease and mean duration of disease), test used for cognitive screening, cognitive domain assessed, tools used to assess cognitive function, characteristics of the experimental intervention, characteristics of the control group, duration of interventions, duration of follow-up, mean results and standard deviation of function cognitive, the information about the professional who supervised the physical exercises.
Effect size calculation
The effect size calculation was performed according to Cohen’s d, where d = M1—M2/s (mean difference between, M1—M2, divided by the standard deviation, s, in each group).
The guidelines to interpret effect sizes are given in points and classified as small, medium or large effect sizes (i.e., d = 0.2 small, d = 0.5 medium, d = 0.8 large). The effect size analysis included the studies grouped by the same assessment tool of cognitive function. Studies that did not observe significant differences for the variable of interest (function cognitive) were removed from the effect size analysis.
Quality assessment
Two reviewers (FCS and RRI) independently assessed the methodological quality of randomized controlled trials (RCTs) using the PEDro scale, which is based on the Delphi list, developed by Verhagen and colleagues. [31] The PEDro score ranges from 3 to 9 points. We used a cut-off of 4 points on the PEDro scale to indicate good quality (equal to or greater than 5 points) and poor quality (equal to or greater than 4 points) studies. Disagreements were resolved by discussion between the reviewers.
Results
Literature search
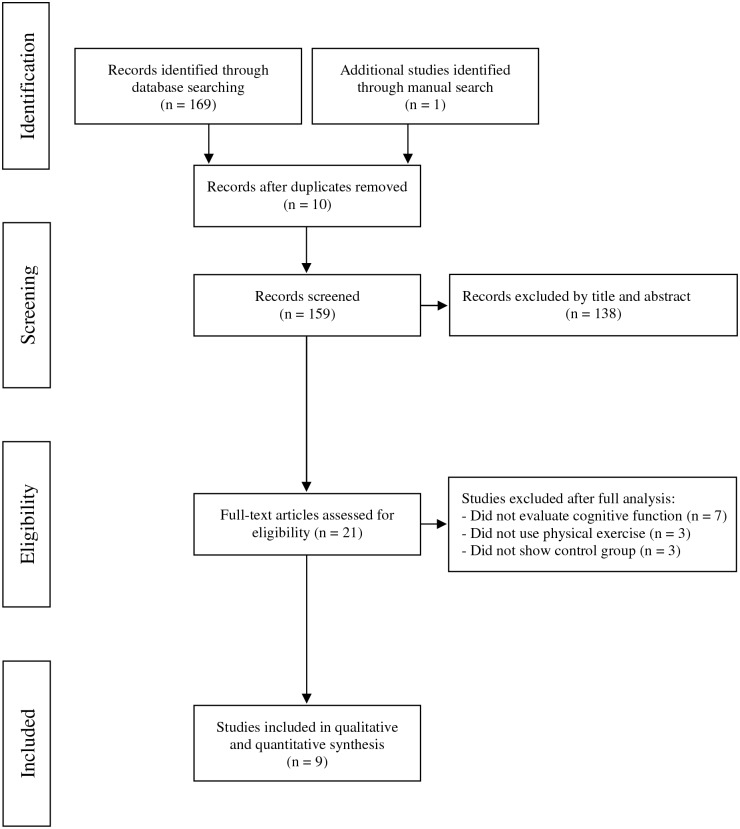
The literature search resulted in the identification of 169 articles. Ten studies were excluded after checking for duplicate studies, and 138 studies were excluded because their titles and abstracts did not address the theme of the present study. One article was included as a result of the manual search. Our detailed review revealed that nine studies were potentially relevant, and therefore, these studies were included. The flowchart summarizes the search strategy (Fig 1).
Fig 1. Flow diagram of 09 studies included in this review.
Study characteristics
The main characteristics of the included studies are described in Table 2. Three studies were conducted in Canada, [32–34] two studies in the United States of America, [35,36] two studies in Brazil, [37,38] one study in Italy [39] and one in Australia. [40] The sample size ranged from 17 [39] to 39 participants. [32] Most patients were male (n = 151). The mean age of the participants in the intervention groups ranged from 59 [32] to 71.2 years old [39] and in the control groups, it ranged from 60.6 [40] to 74.4 years old. [35] The stage of Parkinson’s disease (Hoehn and Yahr) varied from mild to moderate, and the mean duration of the disease ranged from 4.7 [37] to 11.2 years. [39]
Table 2. Main characteristics of the studies included in this review.
| First Author, year | Location of the study (country) | Sample (n) | Gender | Mean age (years) | Years of Education | Stage of the disease (Hoehn and Yahr) | Mean duration of the disease (years) | Cognitive screening | Cognitive domain | Tool used to assess cognitive function |
|---|---|---|---|---|---|---|---|---|---|---|
| Picelli, 2016 [39] | Italy | 17 | Intervention group–treadmill: M = 5/F = 4 Control: M = 4/F = 4 |
Intervention group–treadmill: 71.2 Control: 71.6 |
NR | Mild to moderate | Intervention group–treadmill: 11.2 Control: 10.8 |
MMSE > 24 | Executive function, global cognitive function, processing speed, sustained attention, cognitive flexibility, operational memory | FAB-it; MoCA; Trail Making Test A and B; MI test |
| Rios Romenets, 2015 [34] | Canada | 33 | Tango: M = 12/F = 6 Control: M = 7/F = 8 |
Tango: 63.2 Control: 64.3 |
NR | Tango = 2 Control = 1.7 |
Tango = 5.5 Control = 7.7 |
Movement Disorder Study—criteria for the clinical diagnosis of Parkinon´s disease dementia (PDD) | Global Cognitive Function—Visual- spatial abilities—Attention | MoCA |
| Duchesne, 2015 [32] | Canada | 39 | PD: M = 13/F = 6 HC: M = 8/F = 12 |
PD: 59 HC: 64 |
PD: 15.05 HC: 15.7 |
PD: 2 | PD: 8.1 | MMSE and MoCA > 24 | Executive function | Stroop test; Trail Making Test A and B |
| Nadeau, 2014 [33] | Canada | 34 | Control: M = 9/F = 2 Speed TT: M = 8/F = 4 Mixed TT: M = 10/F = 1 |
Speed TT: 64.0 Mixed TT: 60.1 Control:64.3 |
NR | Speed TT: 1.92 Mixed TT: 1.95 Control:1.86 |
NR | MMSE > 24 | Global Cognitive Function Cognition |
MMSE; PDQ-39 |
| Nocera, 2013 [36] | USA | 21 | Tai-Chi: M = 7/F = 8 Control group: M = 4/F = 2 |
Tai-Chi: 66 Control: 65 |
Tai-Chi: 16 Control group: 15 |
NR | Tai-Chi: 8.08 Control group: 6.83 |
MMSE > 26 | Cognition Attention Executive function Language Memory |
PDQ-39; Trail Making Test (A-B); Stroop Color Word test; Letter Verbal Fluency; Category Verbal Fluency; Digits Span Backwards subtest of Wechsler Memory Scale |
| McKee, 2013 [35] | USA | 33 | Tango: 12M/12F Lecture Series:8M/1F |
Tango: 68.45 Lecture Series: 74.4 |
Tango: 16.5 Lecture Series: 16.4 |
NR | Tango: 7.0±5.5 Lecture Series: 7.2±4.9 |
MoCA | Global Cognitive Function Executive function Visual- spatial function |
MoCA; Brooks Spatial Task; Reverse Corsi Blocks |
| Pompeu, 2012 [37] | Brazil | 32 | M:17 F:15 |
Experimental group: 68.6 Control group: 66.2 |
5–15 | 1,7 | Control group: 5.2±3.4 Experimental group: 4.7±5.4 |
MMSE > 23 | Global Cognitive Function | MoCA |
| Cruise, 2011 [40] | Australia | 28 | Experimental group: M = 9/F = 6 Control group: M = 9/F = 4 |
Experimental group: 59.47 Control group: 60.6 |
NR | NR | Experimental group: 5.87±3.18 Control group: 5.46±3.63 |
MMSE > 24 | Memory Executive function Language | Verbal Fluency (F,A,S); Spatial Working Memory (SWM); Stockings of Cambridge (SOC); Spatial Recognition Memory (SRM); Pattern Recognition Memory (PRM); Semantic Fluency for Animals |
| Tanaka, 2009 [38] | Brazil | 20 | Training group: M = 5/F = 5 Control group: M = 4/F = 6 |
Training group: 64.80 Control group: 64.6 |
NR | Training group: 1.40 Control group: 1.75 |
NR | MMSE | Executive function Memory | Wisconsin Card Sorting Test; Wechsler Adult Intelligence Scale III |
Source: Author’s own production
In relation to the interventions developed, several forms of physical exercises can be observed, such as treadmill training, dance (Argentine Tango and Adapted Tango for PD patients), stationary bicycle training, Tai-Chi, cognitive training (Wii FitTM) combined with motor training (stretching, strengthening and exercises of axial mobility), and multimodal exercise (stretching, strengthening, exercises for coordination and balance) (Table 3). In most studies (n = 7), participants in the control group exercised without supervision, performed mild to moderate intensity physical activities, attended lectures with structured activities, and joined discussions on "physical, mental and social well-being and scientific advances related to the Parkinson’s disease”, or were instructed to maintain their usual activities. The interventions were performed two to three times a week during 40 to 90 minutes in each session. The length of the programs varied from 7 [37] to 24 weeks [38] and only two studies presented follow-up ranging from 2 [37] to 3 months. [35] Generally, the studies used moderate and high intensities controlled by calculating maximal repetition, maximal heart rate, subjective perception of exertion and VO2max.
Table 3. Main intervention characteristics included in this review.
| First Author, Year | Experimental intervention group | Control Group | Duration of interventions (weeks) | Follow-up (months) | Professionals responsible for the intervention |
|---|---|---|---|---|---|
| Picelli, 2016 [39] | Training on treadmill. 1 session a day during 45 minutes, 3 sessions per week. The intervention consisted of 3 stages, with a 5-minute rest between each one: 10 minutes at 1.0Km / h, 10 minutes at 1.5Km/h and 10 minutes at 2.0Km/h | There was no physical training; however, they were instructed to have some type of social interaction at the same frequency and duration of the intervention group training | 4 | - | NR |
| Rios Romenets, 2015 [34] | 24 Argentine tango classes as a couple, during 60 minutes, 2 sessions per week | 24 sessions of physical exercises to be performed alone (specified in a booklet that was handed out to the participants) | 12 | - | Instructors of professional tango dancing |
| Duchesne, 2015 [32] | 3 weekly sessions of high intensity stationary bicycle. In the beginning, the training lasted 20 minutes at 60% of VO2max. Each session added 5 minutes and 5% intensity until reaching 40 minutes of training and 80% of VO2max | 3 weekly sessions of high intensity stationary cycling. In the beginning, the training lasted 20 minutes at 60% of VO2max. Each session added 5 minutes and 5% intensity until reaching 40 minutes of training and 80% of VO2max | 12 | - | Specialized physiotherapist |
| Nadeau, 2014 [33] | Treadmill training, 3 sessions per week, 60 minutes. Each participant performed 80% of the preferred speed during the first week. In the second and third weeks, all participants were encouraged to reach 90 and 100% of their walking speed, respectively. The speed was increased by 0.2 km/h in the next session after reaching 100% of its preferred speed. For the Speed TT group, the speed was increased in the following session by 0.2km/h when the participants perceived their physical exertion as moderate (4 on the Modified Borg Scale) and when the heart rate was below 75% of HRmax. For the Mixed TT group, the inclination of the treadmill was increased by 1%in the following session, when the same progression criteria based on the modified Borg scale and the heart rate were met. Next, the speed of the treadmill (0.2km/h) and the inclination of the treadmill (+ 1%) were increased alternately when the progression criteria were met. | 2 sessions per week and one at home with prescribed exercises. Light and moderate intensity activities: elements of Tai-Chi, Latin dance, resistance band exercises, motor coordination movements. The intensity was adjusted according to the range of movements, duration and speed of exercise. | 24 | - | NR |
| Nocera, 2013 [36] | Tai-Chi sessions during 60 minutes, 3 sessions per week | No intervention | 16 | - | Tai-Chi Master with more than 20 years of experience |
| McKee, 2013 [35] | Adapted Tango lessons. 90-minute sessions, twice a week | 90-minute lectures with structured activities and discussions on physical, mental and social well-being as well as scientific advances related to Parkinson’s Disease" given by medical students and university professors | 24 | 3 | Dance instructors who attended the Adapted Tango Workshop and with experience in working with elderly people |
| Pompeu, 2012 [37] | 14 sessions of 60 minutes, twice a week. 30 minutes of stretching, strengthening and axial mobility exercises and 30 minutes of WiiFit™ exercise game | 30 minutes of stretching, strengthening and axial mobility and 30 minutes of balance exercises without feedback / follow-up / assistance from professionals | 7 | 2 | Physiotherapists |
| Cruise, 2011 [40] | 2 sessions per week during 60 minutes. The training includes strength exercises with an increase of 5–10% RM per session. The aerobic workout consisted of 25–30 minutes of cycling, rowing or treadmill at 60–85% of maximal heart rate | Maintenance of usual routine | 12 | - | NR |
| Tanaka, 2009 [38] | Multimodal exercises (flexibility—stretching; muscular resistance -specific exercises for large muscle groups; motor coordination- rhythmic activities and balance recreational motor activities). Sixty-minute sessions were held 3 times per week. The intervention consisted of 6 phases, during which there was a progressive increase of the load | Maintenance of usual routine | 24 | - | Physical education professionals |
Source: Author’s own production
The Mini-Mental State Examination (MMSE) was the most widely used tool for cognitive screening (n = 7). [32,33,36–40] The most commonly assessed cognitive domains were: executive function (n = 6), [32,35,36,38,39,40] global cognition (n = 5), [33–35,37,39] attention (n = 3), [34,36,39] memory (n = 3), [36,38,40] language (n = 2), [36,40] and cognition (n = 2). [33,36] In order to assess cognitive function, the most used tools included the Montreal Cognitive Assessment (MoCA, n = 4), [34,35,37,39] the Trail Making Test (TMT, n = 3) [32,36,39] and the Parkinson´s Disease Questionnaire-39 (PDQ-39, n = 2). [33,36]
According to the results of mean standard deviation of the studies that showed statistically significant difference, it is generally observed that physical exercise programs promote the preservation or improvement of cognitive function in PD patients (Table 4). [33,35,37,39] Also, the effect size analysis demonstrated effects of small magnitude in global cognitive function, [35,37] processing speed, sustained attention, cognitive flexibility, [39] and cognition. [33] An effect of large magnitude was found in cognition, [33] as shown in Table 4. Furthermore, two studies showed a small magnitude effect in global cognitive function after follow-up periods of two and three months. [35,37]
Table 4. Results of cognitive function (assessed by MoCA, Trail Making Test and PDQ-39) of the studies included in this review in mean and standard deviation and the effect size of the studies that showed statistically significant differences.
| First author, year | Experimental Intervention Group- Pre | Experimental Intervention Group- Post/ Follow-up | Intervention Control Group -Pre | Intervention Control Group- Post/ Follow-up | Effect size | Effect size Follow-up |
|---|---|---|---|---|---|---|
| MoCA—Global Cognitive Function | ||||||
| Mckee, 2013 [35] | 24.9 (3.3) | 26.1 (3.3)/ 26.3 (3.9) | 26 (2.8) | 26.3 (2.3)/ 27.4 (2.5) | 0.07 | 0.33 |
| Pompeu, 2012 [37] | 20.6 (4.5) | 22.2 (4.5)/ 21.8 (4.5) | 21.7 (4.6) | 23.1 (4.6)/ 23.3 (3.4) | 0.19 | 0.37 |
| Trail Making Test (A)—Processing speed and sustained attention | ||||||
| Picelli, 2016 [39] | 141.00 (113.99) | 120.67 (104.59) | 123.50 (101.27) | 124.75 (108.55) | 0.03 | - |
| Trail Making Test (B)—Cognitive flexibility | ||||||
| Picelli, 2016 [39] | 200.00 (80.19) | 149.56 (69.33) | 195.25 (93.92) | 181.13 (78.96) | 0.42 | - |
| PDQ-39—Cognition | ||||||
| Nadeau, 2014 [33] | Speed TT: 31.3 (12.5) Mixed TT: 22.7 (14.3) |
Speed TT: 40.1 (12.3) 35.4 (13.9) Mixed TT: 26.7 (14.0) 19.3 (14.1) |
22.2 (16.4) | 21.0 (16.6) 23.9 (17.6) |
Speed TT: 1.30 Mixed TT: 0.37 |
- |
Source: Author’s own production
Quality assessment
Table 5 summarizes the quality of the included studies. The total scores for methodological quality ranged from 3 to 9 points: four studies had poor methodological quality [32,35,38,40] and five studies had good methodological quality. [33,34,36,37,39]
Table 5. PEDro scale of quality for eligible randomized controlled trials.
| First Author, year | Eligibility criteria* | Random allocation | Concealed allocation | Similar at baseline | Blind subjects | Blind therapist | Blind assessors | < 15% dropout | Intention-to-treat analysis | Between-group comparison | Point measures and variability data | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Picelli, 2016 [39] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 | 1 | 1 | 9 |
| Rios Romenets, 2015 [34] | 1 | 1 | 1 | 1 | - | - | - | - | 1 | 1 | 1 | 9 |
| Duchesne, 2015 [32] | 1 | - | - | 1 | - | - | - | - | — | 1 | 1 | 3 |
| Nadeau, 2014 [33] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | - | 1 | 1 | 8 |
| Nocera, 2013 [36] | 1 | 1 | - | 1 | - | - | 1 | 1 | - | 1 | 1 | 6 |
| McKee, 2013 [35] | 1 | - | - | 1 | - | - | 1 | - | - | 1 | 1 | 4 |
| Pompeu, 2012 [37] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | - | 1 | 1 | 8 |
| Cruise, 2011 [40] | 1 | - | - | 1 | - | - | - | 1 | - | 1 | 1 | 4 |
| Tanaka, 2009 [38] | 1 | - | - | 1 | - | - | - | - | - | 1 | 1 | 3 |
Note:
* Criterion 1 is not considered for the final score because it is an item that assesses the external validity. [41]
Source: Author’s own production
Discussion
The primary objective of this study was to assess the effects of physical exercise programs on cognitive function in PD patients, compared to the control group, through a systematic review of randomized controlled trials (RCTs) in the last 10 years. Nine RCTs were analyzed including the effect of various types of physical exercise programs on cognitive function in PD patients in the mild to moderate stage (Hoehn and Yahr) and with disease time around 6 years.
In general, studies have shown that physical exercise programs promote the preservation or improvement of cognitive function in PD patients. The positive and significant effects of physical exercises on cognitive function in PD patients occurred specifically in the following interventions: adapted tango for PD patients, [35] cognitive training (Wii Fit™) combined with motor training (stretching, strengthening and axial mobility exercises), [37] and treadmill training. [33,39]
Some studies involving PD patients and elderly individuals suggest that some domains of cognitive function may improve with dance. Longer dance interventions may be needed to affect cognitive abilities that were not improved with short-term interventions, such as executive function, visual-spatial memory, or fluid intelligence. [35,42] The effects of mixed dance styles on executive function, assessed using the Frontal Assessment Battery (FAB), as well as greater benefits could be observed with dance, compared to exercise or non-intervention. Moreover, participants in mixed dance styles improved response time for a task of mental rotation. [43]
According to McNeely, Duncan and Earhart, [44] in PD, tango is the most frequently chosen dance style for interventions. [34, 35,45–47] The motor and cognitive benefits promoted by tango on motor and non-motor symptoms in PD can be explained by the sequences of movements and gestures demanded by the dance, including rhythmic back-and-forth walk, rotation, changing speeding tempo, as well as increasing self-esteem and socialization. In the study conducted by McKee and Hackney, [35] the adapted tango dancing for PD patients promoted significant improvement in global cognitive function (MoCA) after 24 weeks and after 3 months of follow-up, when compared to the control group, who attended lectures only. The cognitive benefit may also be related to the effect of aerobic exercise on cognition. [48]
Studies have reported improvement in cognitive functions in PD patients through cognitive training associated with motor training. [49] In the study conducted by Pompeu et al., [37] both groups (intervention and control) showed a significant effect on the cognitive test (MoCA) after training (14 sessions of 60 minutes, twice a week). The intervention group performed cognitive training (Wii Fit™) combined with motor training (stretching, strengthening and axial mobility exercises), and the control group, only motor training. These motor and cognitive improvements may be related to the physical and cognitive capacities stimulated through the gameplay. [50] According to Petzinger et al., [49] incorporating aerobic exercise specifically into cognitive training may potentiate neuroplasticity as well as improve cognitive function and motor skills.
Another intervention that showed benefits was treadmill training. Picelli et al. [39] observed a significant improvement in processing speed and sustained attention (Trail Making Test A), as well as cognitive flexibility (Trail Making Test B) after 4 weeks, compared to the control group, who maintained their usual activities. Additionally, the effect size analysis showed a large magnitude effect of treadmill training on cognition in PD patients in the mild stage (Hoehn and Yahr mean of 1.92), performing 3 sessions of 60 minutes per week, between 80 and 100% of the preferred speed, with an increase in speed of 0.2 km/h, when the participants perceived physical effort as moderate (4 on the Modified Borg Scale) and when heart rate was below 75% HRmax. [33]
A meta-analysis of RCTs (29 studies, 2,049 participants), which aimed to assess the effects of aerobic training on neurocognitive performance, showed that aerobic exercise produced modest improvements in attention, speed, executive function and memory among adults without dementia. Exercise programs ranged from 6 weeks to 18 months during 2.5 to 4 months, 3 times a week and with an intensity of 70% of maximum oxygen consumption (VO2max). The training included walking and/or running compared to the control group (waiting list or other conditions of comparison, such as stretching and strengthening, health education or relaxation). Trials that used longer interventions were associated with improved attention and processing speeds, whereas trials conducted among individuals with mild cognitive impairment (MCI) tended to show more memory improvement compared to non-dementia samples. [48]
Although it is well-established that physical exercises affect brain plasticity, different types of exercises have been found to selectively affect various regions of the brain. [51] Specifically, aerobic training has demonstrated greater benefit in the superior temporal and parietal prefrontal cortex, and the anterior and transverse tracts between the frontal and parietal lobes, i.e., in the areas involved in cognition and daily life functioning. [52]
In a large sample of adults (≥ 50 yo) with subjective memory impairment, moderate-intensity aerobic exercise performed at least during three 50-minute sessions per week promoted modest improvement in cognitive memory, language and praxis tests (Alzheimer’s Disease Assessment Scale—ADAS-Cog) after a training period of six months and a follow-up period of 18 months. The participants in the exercise group also improved delayed recall of a word list. [53]
Aerobic exercise may particularly affect executive function in PD. [49] Moderate-intensity aerobic exercise performed 2–3 times per week produced promising effects on executive function in the mild to moderate stages of PD. [54] Small preliminary case studies showed that 8 weeks of aerobic exercise, conducted 3 times a week during 20–40 minutes, produced improvements in executive function, verbal fluency and working memory in three PD patients: one with high cognitive performance (66 yo) and two with cognitive deficits (61–72 yo). [55,56]
In the systematic review conducted by Murray et al., [28] eight clinical trials in humans demonstrate that various modalities and levels of exercise intensity have also improved cognitive ability in PD, including aerobic exercise, strengthening, cognitive training (Wii Fit™) combined with motor and dance training. In the systematic review conducted by Cusso et al., [51] the authors assessed the effectiveness of physical activity, including physiotherapy and occupational therapy as an intervention in the non-motor symptoms of PD, namely cognition, among others. All studies used an active intervention, namely, aerobic training, treadmill training, walking, strength training, balance training, Tai-Chi, Qigong, physiotherapy, occupational therapy, Argentinean tango, Nordic walking, among others.
Two studies performed follow-up of periods of 2 [35] and 3 months. [37] The small-sized magnitude effect of adapted tango for PD patients [37] and cognitive training (Wii Fit™) combined with motor training (stretching, strengthening and axial mobility exercises) [35] on global cognitive function was maintained after the follow-up period. Although the improvements were maintained, it can be observed that there was not enough data to evaluate the follow-up effect, which is extremely important for clinical decision.
The Mini-Mental State Examination (MMSE) was the most widely used tool for cognitive screening. MMSE is a general screening tool for cognitive impairment. [57] It is a screening test commonly used for dementia; however, its dependence on age and educational level makes it difficult to use a rigid cut score. [58] MMSE demonstrates floor effects in individuals with severe cognitive impairment and ceiling effects in individuals with mild cognitive impairment. [59] Thus, MMSE is considered less sensitive to mild cognitive impairment in PD. [60] Its reliability for the diagnosis of dementia in Alzheimer’s disease (AD) and other dementias is not established and patients with PD and cognitive impairment may exceed the normal range of the scale. [61]
In a study involving 873 PD patients (age 70.5 ± 8.6 years, educational level 9.7 ± 2.4 years, disease duration 6.7 ± 5 years, Hoehn & Yahr 2.7 ± 1.0, MMSE score 27.3 ± 3.4), a cut-off score ≤ 24 on MMSE revealed low sensitivity for the diagnosis of Parkinson’s disease dementia (PDD). [62]
No data regarding the validation of MMSE in populations with PD has been reported. [63] Nonetheless, screening tools, such as MMSE, serve as evaluation indicators in order to provide quantitative information about global cognitive function.
The most evaluated cognitive domains were executive function, global cognitive function, attention, memory, language and cognition. It is well established that PD affects cognition. [64,65] The term "cognition" describes multiple mental processes, including executive function. Executive function includes, but is not limited to, judgment, planning, initiation, abstraction, problem solving, sequencing, and mental flexibility. [66] Executive function and visual-spatial domains may be affected after the onset of PD, [67,68] and changes in executive functions may predict the onset of dementia. [22]
According to Hanagasi, Tufekcioglu, and Emre, [69] besides the deficit in executive and visual-spatial functions, the cognitive profile of PD patients may also be characterized by attention and memory deficits. In a 5-year cohort study, approximately two-thirds of the patients showed cognitive decline. The greatest decline was observed in the areas of processing speed and memory. Elderly patients at the onset of the disease and with memory impairment at an early stage of the disease are more at risk of further cognitive decline. [7]
The most commonly used tools to assess cognitive function were the Montreal Cognitive Assessment (MoCA), the Trail Making Test A and B, and the Parkinson´s Disease Questionnaire-39 (PDQ-39). The Movement Disorder Society (MDS) diagnostic criteria for mild cognitive impairment recommend that a detailed assessment of cognitive function requires at least two tests per cognitive domain. However, MDS also stated that excessive testing per cognitive domain might cause bias. [70]
In the review conducted by Cusso et al., [51] several tests were used to assess cognition in PD patients, such as the Mini-Mental State Examination (MMSE), the Montreal Cognitive Assessment (MoCA), the subsections of the Cognitive Assessment Battery (CAB), the Stroop Test, and the Brief Test of Attention. Kalron and Zeilig [71] reviews included three clinical trials involving PD patients and the tools used to assess cognitive function were: Wisconsin Card Sorting Task, Verbal Fluency (F,A,S), Spatial Working Memory (SWM), Stockings of Cambridge (SOC), Spatial Recognition Memory (SRM), Pattern Recognition Memory (PRM), Semantic Fluency for Animals and Montreal Cognitive Assessment (MoCA).
The Montreal Cognitive Assessment (MoCA) was the most used tool in this review and it was developed as a screening tool for mild cognitive impairment (MIC). [72] MoCA is a small cognitive screening tool that is similar to the widely used MMSE, but is more sensitive to identifying MIC in the general population than MMSE. The test requires about 10 minutes to be administered in the general population and can be freely used for clinical purposes. MoCA includes tests of cognitive domains of executive and visual-spatial functions, memory, language and attention. It has adequate psychometric properties for a brief assessment of overall cognition in PD. [73]
The Trail Making Test (TMT) is another commonly used tool. It consists of two parts: A and B. In both parts, the subject is required to draw a line in the shortest possible time and without taking the pencil out of the paper. In part A, the subject is instructed to draw a line connecting the numbers 1 to 25 in ascending order. In part B, there is a greater cognitive demand on the subject´s attention, since (s)he should draw a line alternating between numbers 1 and 13 and letters A to L, i.e., 1-A, 2-B, 3-C and so on. The test assesses the aspects of sustained attention, alternating attention, mental flexibility, visual processing speed and motor function. It also evaluates visual scanning ability [74–76] It is widely used in clinical practice and research. [74] The time needed to complete the TMT is affected by age and educational level [76] and should therefore be considered in the stratification of normative samples. [74] The Trail Making Test is sensitive to the cognitive changes of Parkinson’s disease. [75]
The Parkinson´s Disease Questionnaire-39 (PDQ-39) was used in two studies. [33,36] This tool assesses the quality of life and its Brazilian version is a reliable and valid measure for PD patients in Brazil. [77] The PDQ-39 is a self-administered questionnaire with 39 items and divided into eight domains, one of which is cognition. [78]
Most eligible studies showed good methodological quality based on the PEDro scale, which suggests that the data presented are reliable. However, these results should be carefully analyzed. The diversity of cognitive tests used to assess cognitive function and the high heterogeneity identified between the physical exercise programs in the included studies made it impossible to reach a consensus on the best type of physical exercise, frequency or intensity of an intervention that may be more effective for the cognitive function of PD patients.
Conclusion
Through this systematic review of RCTs, we found that physical exercise programs promote positive and significant effects on global cognitive function, processing speed, sustained attention and mental flexibility in Parkinson’s disease patients, at a mild to moderate stage for patients with a 6-year clinical diagnosis of PD, such as tango, cognitive training associated with motor training, and treadmill training. However, treadmill training (80–100% of the preferred walking speed) performed 3 times a week for about 60 minutes and for a period of 24 weeks produced larger improvements in cognition.
Recommendations
It is important to consider the impact of impaired cognition on managing motor deficits associated with PD and the important contribution to the general well-being of the individual, since PD patients show an increased risk of dementia compared to the healthy population. [79,80] With cognitive impairment beginning in the early stages of PD, [81] there is a corresponding need for early cognitive strategies. [82] Medications have some effect on Parkinson´s disease dementia, but there is no convincing evidence that the progression from mild cognitive impairment (MCI) to dementia can be delayed or prevented. [83]
In this sense, this review provides a current overview of the main tools used for cognitive screening as well as the evaluation/evolution of cognitive impairment with a physical exercise program. The presence of non-motor symptoms in PD patients should be systematically researched, such as cognitive impairment, in order to contribute significantly to the structuring of physical exercise programs. Professionals who are directly involved in rehabilitation programs should understand the importance of the role of cognition in learning and motor skills, as this will assist in rehabilitation planning and management. Emerging researches suggest that aerobic exercise may improve cognition in PD. However, comprehensive and well-controlled studies should be conducted on the effects of physical exercise on cognitive function in PD patients. [82]
Supporting information
(DOC)
Acknowledgments
The authors would like thank the Coordination of Improvement of Higher Education Personnel of the Ministry of Education of Brazil and the University of Brasilia (Brasilia, Brazil).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors declare that the work has not received funding.
References
- 1.Van Uem JM, Walgaard S, Ainsworth E, Hasmann SE, Heger T, Nussbaum S, et al. Quantitative Timed-Up-and-Go Parameters in Relation to Cognitive Parameters and Health-Related Quality of Life in Mild-to-Moderate Parkinson’s Disease. PLoS One. 2016;11(4):e0151997 doi: 10.1371/journal.pone.0151997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider JS, Elm JJ, Parashos SA, Ravina BM, Galpern WR, Investigators N-P. Predictors of cognitive outcomes in early Parkinson disease patients: The National Institutes of Health Exploratory Trials in Parkinson Disease (NET-PD) experience. Parkinsonism Relat Disord. 2010;16(8):507–12. doi: 10.1016/j.parkreldis.2010.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8(5):464–74. doi: 10.1016/S1474-4422(09)70068-7 [DOI] [PubMed] [Google Scholar]
- 4.Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson’s disease and are we missing them? Mov Disord. 2010;25(15):2493–500. doi: 10.1002/mds.23394 [DOI] [PubMed] [Google Scholar]
- 5.Meireles J, Massano J. Cognitive impairment and dementia in Parkinson’s disease: clinical features, diagnosis, and management. Front Neurol. 2012;3:88 doi: 10.3389/fneur.2012.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Y, Ge J, Liu F, Wu P, Guo S, Liu Z, et al. Cerebral Metabolic Differences Associated with Cognitive Impairment in Parkinson’s Disease. PLoS One. 2016;11(4):e0152716 doi: 10.1371/journal.pone.0152716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broeders M, Velseboer DC, de Bie R, Speelman JD, Muslimovic D, Post B, et al. Cognitive change in newly-diagnosed patients with Parkinson’s disease: a 5-year follow-up study. J Int Neuropsychol Soc. 2013;19(6):695–708. doi: 10.1017/S1355617713000295 [DOI] [PubMed] [Google Scholar]
- 8.Muslimović D, Post B, Speelman JD, De Haan RJ, Schmand B. Cognitive decline in Parkinson’s disease: a prospective longitudinal study. J Int Neuropsychol Soc. 2009;15(3):426–37. doi: 10.1017/S1355617709090614 [DOI] [PubMed] [Google Scholar]
- 9.Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, et al. MDS Task Force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov Disord. 2011;26(10):1814–24. doi: 10.1002/mds.23823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol. 2013;70(5):580–6. doi: 10.1001/jamaneurol.2013.2110 [DOI] [PubMed] [Google Scholar]
- 11.Barbosa ER. Non-motor symptoms in Parkinson’s disease. Arq Neuropsiquiatr. 2013;71(4):203–4. [DOI] [PubMed] [Google Scholar]
- 12.Aarsland D. Cognitive impairment in Parkinson’s disease and dementia with Lewy bodies. Parkinsonism Relat Disord. 2016;22 Suppl 1:S144–8. [DOI] [PubMed] [Google Scholar]
- 13.Lee A, Gilbert RM. Epidemiology of Parkinson Disease. Neurol Clin. 2016;34(4):955–65. doi: 10.1016/j.ncl.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 14.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–44. doi: 10.1002/mds.21956 [DOI] [PubMed] [Google Scholar]
- 15.McKeith IG et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005. December 27;65(12):1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1 [DOI] [PubMed] [Google Scholar]
- 16.De Lau LML et al. Prognosis of Parkinson Disease Risk of Dementia and Mortality: The Rotterdam Study. Arch Neurol. 2005;62(8):1265–1269. doi: 10.1001/archneur.62.8.1265 [DOI] [PubMed] [Google Scholar]
- 17.Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord. 2005;20(10):1255–63. doi: 10.1002/mds.20527 [DOI] [PubMed] [Google Scholar]
- 18.Melo LM, Barbosa ER, Caramelli P. Declínio cognitivo e demência associados à doença de Parkinson: características clínicas e tratamento. Revista de Psiquiatria Clínica [Internet]. 2007; 34:[176–83 pp.]. [Google Scholar]
- 19.Romann AJ, Dornelles SD, Maineri NdL, Rieder CRdM, Olchik MR. Cognitive assessment instruments in Parkinson’s disease patients undergoing deep brain stimulation. Dement Neuropsychol [Internet]. 2012; 6:[2–11 pp.]. doi: 10.1590/S1980-57642012DN06010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lischka AR, Mendelsohn M, Overend T, Forbes D. A systematic review of screening tools for predicting the development of dementia. Can J Aging. 2012;31(3):295–311. doi: 10.1017/S0714980812000220 [DOI] [PubMed] [Google Scholar]
- 21.Paris AP, Saleta HG, de la Cruz Crespo Maraver M, Silvestre E, Freixa MG, Torrellas CP, et al. Blind randomized controlled study of the efficacy of cognitive training in Parkinson’s disease. Mov Disord. 2011;26(7):1251–8. doi: 10.1002/mds.23688 [DOI] [PubMed] [Google Scholar]
- 22.McKinlay A, Grace RC, Dalrymple-Alford JC, Roger D. Characteristics of executive function impairment in Parkinson’s disease patients without dementia. J Int Neuropsychol Soc. 2010;16(2):268–77. doi: 10.1017/S1355617709991299 [DOI] [PubMed] [Google Scholar]
- 23.Biundo R, Weis L, Facchini S, Formento-Dojot P, Vallelunga A, Pilleri M, et al. Cognitive profiling of Parkinson disease patients with mild cognitive impairment and dementia. Parkinsonism Relat Disord. 2014;20(4):394–9. doi: 10.1016/j.parkreldis.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 24.Caballol N, Martí MJ, Tolosa E. Cognitive dysfunction and dementia in Parkinson disease. Mov Disord. 2007;22 Suppl 17:S358–66. [DOI] [PubMed] [Google Scholar]
- 25.Olde Dubbelink KT, Hillebrand A, Twisk JW, Deijen JB, Stoffers D, Schmand BA, et al. Predicting dementia in Parkinson disease by combining neurophysiologic and cognitive markers. Neurology. 2014;82(3):263–70. doi: 10.1212/WNL.0000000000000034 [DOI] [PubMed] [Google Scholar]
- 26.Yarnall AJ, Breen DP, Duncan GW, Khoo TK, Coleman SY, Firbank MJ, et al. Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology. 2014;82(4):308–16. doi: 10.1212/WNL.0000000000000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hindle JV, Petrelli A, Clare L, Kalbe E. Nonpharmacological enhancement of cognitive function in Parkinson’s disease: a systematic review. Mov Disord. 2013;28(8):1034–49. doi: 10.1002/mds.25377 [DOI] [PubMed] [Google Scholar]
- 28.Murray DK, Sacheli MA, Eng JJ, Stoessl AJ. The effects of exercise on cognition in Parkinson’s disease: a systematic review. Transl Neurodegener. 2014;3(1):5 doi: 10.1186/2047-9158-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, W64 [DOI] [PubMed] [Google Scholar]
- 30.Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. Int J Epidemiol. 2002;31(1):150–3. [DOI] [PubMed] [Google Scholar]
- 31.Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–41. [DOI] [PubMed] [Google Scholar]
- 32.Duchesne C, Lungu O, Nadeau A, Robillard ME, Bore A, Bobeuf F, et al. Enhancing both motor and cognitive functioning in Parkinson’s disease: Aerobic exercise as a rehabilitative intervention. Brain Cogn. 2015;99:68–77. doi: 10.1016/j.bandc.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 33.Nadeau A, Pourcher E, Corbeil P. Effects of 24 wk of treadmill training on gait performance in Parkinson’s disease. Med Sci Sports Exerc. 2014;46(4):645–55. doi: 10.1249/MSS.0000000000000144 [DOI] [PubMed] [Google Scholar]
- 34.Rios Romenets S, Anang J, Fereshtehnejad SM, Pelletier A, Postuma R. Tango for treatment of motor and non-motor manifestations in Parkinson’s disease: a randomized control study. Complement Ther Med. 2015;23(2):175–84. doi: 10.1016/j.ctim.2015.01.015 [DOI] [PubMed] [Google Scholar]
- 35.McKee KE, Hackney ME. The effects of adapted tango on spatial cognition and disease severity in Parkinson’s disease. J Mot Behav. 2013;45(6):519–29. doi: 10.1080/00222895.2013.834288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nocera JR, Amano S, Vallabhajosula S, Hass CJ. Tai Chi Exercise to Improve Non-Motor Symptoms of Parkinson’s Disease. J Yoga Phys Ther. 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pompeu JE, Mendes FA, Silva KG, Lobo AM, Oliveira TeP, Zomignani AP, et al. Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: a randomised clinical trial. Physiotherapy. 2012;98(3):196–204. doi: 10.1016/j.physio.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 38.Tanaka K, Quadros AC, Santos RF, Stella F, Gobbi LT, Gobbi S. Benefits of physical exercise on executive functions in older people with Parkinson’s disease. Brain Cogn. 2009;69(2):435–41. doi: 10.1016/j.bandc.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 39.Picelli A, Varalta V, Melotti C, Zatezalo V, Fonte C, Amato S, et al. Effects of treadmill training on cognitive and motor features of patients with mild to moderate Parkinson’s disease: a pilot, single-blind, randomized controlled trial. Funct Neurol. 2016;31(1):25–31. doi: 10.11138/FNeur/2016.31.1.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cruise KE, Bucks RS, Loftus AM, Newton RU, Pegoraro R, Thomas MG. Exercise and Parkinson’s: benefits for cognition and quality of life. Acta Neurol Scand. 123 Denmark: 2010 The Authors. Journal compilation 2010 Blackwell Munksgaard.; 2011. p. 13–9. doi: 10.1111/j.1600-0404.2010.01338.x [DOI] [PubMed] [Google Scholar]
- 41.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–21. [PubMed] [Google Scholar]
- 42.kattenstroth JC et al. Six months of dance intervention enhances postural, sensorimotor, and cognitive performance in elderly without affecting cardio-respiratory functions. Front. Aging Neurosci. 2013;5(3): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto H. et al. Effects of dance on motor functions, cognitive functions, and mental symptoms of Parkinson’s disease: a quasi-randomized pilot trial. Complement Ther Med. 2015. April;23(2):210–9. doi: 10.1016/j.ctim.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 44.McNeely ME et al. Differential Effects of Tango Versus Dance for PD in Parkinson Disease. Front Aging Neurosci. 2015; 7: 239 doi: 10.3389/fnagi.2015.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012. February;26(2):132–43. doi: 10.1177/1545968311421614 [DOI] [PubMed] [Google Scholar]
- 46.Foster ER et al. Community-based Argentine tango dance program is associated with increased activity participation among individuals with Parkinson’s disease. Arch Phys Med Rehabil. 2013. February;94(2):240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hackney ME, Earhart GM. Effects of dance on movement control in Parkinson’s disease: a comparison of Argentine tango and American ballroom. J Rehabil Med. 2009. May;41(6):475–81. doi: 10.2340/16501977-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–52. doi: 10.1097/PSY.0b013e3181d14633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013;12(7):716–26. doi: 10.1016/S1474-4422(13)70123-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ekker MS, Janssen S, Nonnekes J, Bloem BR, de Vries NM. Neurorehabilitation for Parkinson’s disease: Future perspectives for behavioural adaptation. Parkinsonism Relat Disord. 2016;22 Suppl 1:S73–7. [DOI] [PubMed] [Google Scholar]
- 51.Cusso ME, Donald KJ, Khoo TK. The Impact of Physical Activity on Non-Motor Symptoms in Parkinson’s Disease: A Systematic Review. Front Med (Lausanne). 2016;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58(2):176–80. [DOI] [PubMed] [Google Scholar]
- 53.Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–37. doi: 10.1001/jama.300.9.1027 [DOI] [PubMed] [Google Scholar]
- 54.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–30. doi: 10.1111/1467-9280.t01-1-01430 [DOI] [PubMed] [Google Scholar]
- 55.Nocera JR, Altmann LJ, Sapienza C, Okun MS, Hass CJ. Can exercise improve language and cognition in Parkinson’s disease? A case report. Neurocase. 2010;16(4):301–6. doi: 10.1080/13554790903559663 [DOI] [PubMed] [Google Scholar]
- 56.Tabak R, Aquije G, Fisher BE. Aerobic exercise to improve executive function in Parkinson disease: a case series. J Neurol Phys Ther. 2013;37(2):58–64. [DOI] [PubMed] [Google Scholar]
- 57.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 58.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–91. [PubMed] [Google Scholar]
- 59.Wind AW, Schellevis FG, Van Staveren G, Scholten RP, Jonker C, Van Eijk JT. Limitations of the Mini-Mental State Examination in diagnosing dementia in general practice. Int J Geriatr Psychiatry. 1997;12(1):101–8. [DOI] [PubMed] [Google Scholar]
- 60.Hoops S et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009. November 24;73(21):1738–45. doi: 10.1212/WNL.0b013e3181c34b47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zadikoff C, Fox SH, Tang-Wai DF, Thomsen T, de Bie RM, Wadia P, et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson’s disease. Mov Disord. 2008;23(2):297–9. doi: 10.1002/mds.21837 [DOI] [PubMed] [Google Scholar]
- 62.Riedel O, Klotsche J, Spottke A, Deuschl G, Förstl H, Henn F, et al. Cognitive impairment in 873 patients with idiopathic Parkinson’s disease. Results from the German Study on Epidemiology of Parkinson’s Disease with Dementia (GEPAD). J Neurol. 2008;255(2):255–64. doi: 10.1007/s00415-008-0720-2 [DOI] [PubMed] [Google Scholar]
- 63.Kulisevsky J, Pagonabarraga J. Cognitive impairment in Parkinson’s disease: tools for diagnosis and assessment. Mov Disord. 2009;24(8):1103–10. doi: 10.1002/mds.22506 [DOI] [PubMed] [Google Scholar]
- 64.Gotham AM, Brown RG, Marsden CD. ‘Frontal’cognitive function in patients with Parkinson’s disease ‘on’and ‘off’levodopa. Brain. 1988;111,299–321. [DOI] [PubMed] [Google Scholar]
- 65.Owen AM et al. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain. 1992;115, 1727–1751. [DOI] [PubMed] [Google Scholar]
- 66.Tabak RPT et al. Aerobic Exercise to Improve Executive Function in Parkinson Disease: A Case Series. Journal of Neurologic Physical Therapy. 2013;37(2):58–64. [DOI] [PubMed] [Google Scholar]
- 67.Mahieux F. Neuropsychological prediction of dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998. February; 64(2): 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muslimovic D. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005. October 25;65(8):1239–45. doi: 10.1212/01.wnl.0000180516.69442.95 [DOI] [PubMed] [Google Scholar]
- 69.Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27(3):349–56. doi: 10.1002/mds.24893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanagasi HA, Tufekcioglu Z, Emre M. Dementia in Parkinson’s disease. J Neurol Sci. 2017;374:26–31. doi: 10.1016/j.jns.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 71.Kalron A, Zeilig G. Efficacy of exercise intervention programs on cognition in people suffering from multiple sclerosis, stroke and Parkinson’s disease: A systematic review and meta-analysis of current evidence. NeuroRehabilitation. 2015;37(2):273–89. doi: 10.3233/NRE-151260 [DOI] [PubMed] [Google Scholar]
- 72.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 73.Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov Disord. 2008;23(7):1043–6. doi: 10.1002/mds.22017 [DOI] [PubMed] [Google Scholar]
- 74.Campanholo KR, Romão MA, Machado MdAR, Serrao VT, Coutinho DGC, Benute GRG, et al. Performance of an adult Brazilian sample on the Trail Making Test and Stroop Test. Dementia & Neuropsychologia [Internet]. 2014; 8:[26–31 pp.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314–24. doi: 10.1002/mds.21844 [DOI] [PubMed] [Google Scholar]
- 76.Hamdan AC, Hamdan EMLR. Effects of age and education level on the Trail Making Test in a healthy Brazilian sample. Psychology & Neuroscience [Internet]. 2009; 2:[199–203 pp.]. [Google Scholar]
- 77.Carod-Artal FJ, Martinez-Martin P, Vargas AP. Independent validation of SCOPA-psychosocial and metric properties of the PDQ-39 Brazilian version. Mov Disord. 2007;22(1):91–8. doi: 10.1002/mds.21216 [DOI] [PubMed] [Google Scholar]
- 78.Souza RG, Borges V, Silva SM, Ferraz HB. Quality of life scale in Parkinson’s disease PDQ-39—(Brazilian Portuguese version) to assess patients with and without levodopa motor fluctuation. Arq Neuropsiquiatr. 2007;65(3B):787–91. [DOI] [PubMed] [Google Scholar]
- 79.Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology. 2001;56(6):730–6. [DOI] [PubMed] [Google Scholar]
- 80.Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson’s disease in the United Kingdom. Mov Disord. 2004;19(9):1043–9. doi: 10.1002/mds.20216 [DOI] [PubMed] [Google Scholar]
- 81.Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson’s disease: diagnosis, biomarkers, and treatment. Lancet Neurol. 2012;11(8):697–707. doi: 10.1016/S1474-4422(12)70152-7 [DOI] [PubMed] [Google Scholar]
- 82.Reynolds GO, Otto MW, Ellis TD, Cronin-Golomb A. The Therapeutic Potential of Exercise to Improve Mood, Cognition, and Sleep in Parkinson’s Disease. Mov Disord. 2016;31(1):23–38. doi: 10.1002/mds.26484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.