Significance
Shifts of upper range limits are a key response of mountain biota to climate change. However, assessing whether species profit or suffer from the changing climate requires the simultaneous evaluation of changes in species’ lower and upper range limits, optima, and abundances. Here, we provide an integrated assessment for 183 plant species of the European Alps. We demonstrate that, over recent decades, increases in abundance were more pronounced than range shifts, suggesting an in-filling process which decreases in intensity with increasing elevation. While most species profited from recent range dynamics, a sizeable minority both decreased in abundance and experienced range contractions. These “losers” are overrepresented among species of highest elevations which represent a unique contribution to the European flora.
Keywords: elevation, range dynamic, plants, mountain, climate change
Abstract
Many studies report that mountain plant species are shifting upward in elevation. However, the majority of these reports focus on shifts of upper limits. Here, we expand the focus and simultaneously analyze changes of both range limits, optima, and abundances of 183 mountain plant species. We therefore resurveyed 1,576 vegetation plots first recorded before 1970 in the European Alps. We found that both range limits and optima shifted upward in elevation, but the most pronounced trend was a mean increase in species abundance. Despite huge species-specific variation, range dynamics showed a consistent trend along the elevational gradient: Both range limits and optima shifted upslope faster the lower they were situated historically, and species’ abundance increased more for species from lower elevations. Traits affecting the species’ dispersal and persistence capacity were not related to their range dynamics. Using indicator values to stratify species by their thermal and nutrient demands revealed that elevational ranges of thermophilic species tended to expand, while those of cold-adapted species tended to contract. Abundance increases were strongest for nutriphilous species. These results suggest that recent climate warming interacted with airborne nitrogen deposition in driving the observed dynamics. So far, the majority of species appear as “winners” of recent changes, yet “losers” are overrepresented among high-elevation, cold-adapted species with low nutrient demands. In the decades to come, high-alpine species may hence face the double pressure of climatic changes and novel, superior competitors that move up faster than they themselves can escape to even higher elevations.
The distribution of many mountain biota is shifting upslope, most probably as a result of climate warming (1–3). Research on these range shifts has so far mainly focused on the upper elevational range limit, the so-called leading edge (1, 3–6). Changes in species elevational optima or lower elevational limits, also termed rear edges, have been less frequently investigated (ref. 2, but see ref. 7). Still lower is the number of studies considering more than one of these range attributes in concert or in a combination with abundance changes (8–10). However, elevational range dynamics are complex phenomena, and optima, leading, and rear edges do not necessarily shift synchronously (8, 11), or even in the same direction (12). Therefore, only the combined evaluation of all of these range attributes allows for assessing whether species expand or retract their distributions, become more or less abundant, and are hence profiting from or rather threatened by a warming climate.
There are various possible reasons for idiosyncratic responses of range attributes to climatic change. In particular, the dynamics at rear and leading edges are determined by two different processes that may have their own rhythms (13, 14): colonization of new terrain vs. local extinction. Furthermore, environmental factors like precipitation, land use, or environmental pollution may have different impacts at low- vs. high-elevation populations of the same species (12, 15). Finally, the net effect of biotic interactions on plants is known to change along the elevational gradient, with competition predominating at lower and facilitation being more important at higher elevations (16). As a corollary, possible physiological effects of warmer temperatures on lower elevational populations may be reinforced by increased competition of species from below (17), while expansion of higher-elevation populations may be hampered by the lack of appropriate facilitators (18).
Range dynamics may not only differ at the opposed range limits and the optimum of a species, they may also vary greatly among individual species (12). While a relationship between average range shifts and climate warming has been demonstrated (1), particularly for changes at the upper range limits (19), the drivers of this variability among species have largely remained elusive. Possible reasons include variation in species-specific traits (14, 20), interference with other environmental drivers such as land use change (21) or airborne nitrogen deposition (22), buffering of species against climate warming in microrefugia (23), or cascading indirect effects of these drivers via biotic interactions (12). In addition, the elevation of the shifting range attribute may also affect the velocity of its movement. While elevation is not a driver of biological processes itself, it is correlated with many factors that directly affect organismic performance (24), especially with climatic ones such as the length of the growth period or the mean ambient temperature during this period. These climatic factors likely influence processes which are key to range dynamics. For example, increasing risk of sexual recruitment failure with colder temperatures (25) may slow down leading edge expansion, while long life cycles and increasing importance of clonal propagation at higher elevations (25) may facilitate remnant dynamics (13) and hence delay rear edge retreats. To the best of our knowledge, however, variation of climate-induced range dynamics has hardly been explored in a systematic way along the elevational gradient so far.
Here, we assess recent range dynamics of 183 vascular plant species from the European Alps (Table S1). In 2014 and 2015, we resurveyed 1,576 semipermanent plots of nonforest vegetation scattered across the Austrian, Swiss, German, Slovenian, and Italian Alps (Fig. S1). These plots were first recorded between 1911 and 1970 and span an elevational gradient of nearly 3,000 m [486 m to 3,226 m above sea level (a.s.l.)]. Based on this dataset, we calculated shifts of rear edges, optima, and leading edges, as well as abundance changes for all species that were sufficiently frequent in both the historical records and the resurvey. We then combined these attributes to a compound index that separates “winners” from “losers” of climate change. Furthermore, we tested whether and how this index, as well as shifts of individual range attributes, changes along the elevational gradient as well as along the connected gradient of ambient air temperature. Finally, we assessed whether and how species-specific differences in the observed dynamics are related to thermal and nutrient requirements as well as to persistence and dispersal related traits.
Results
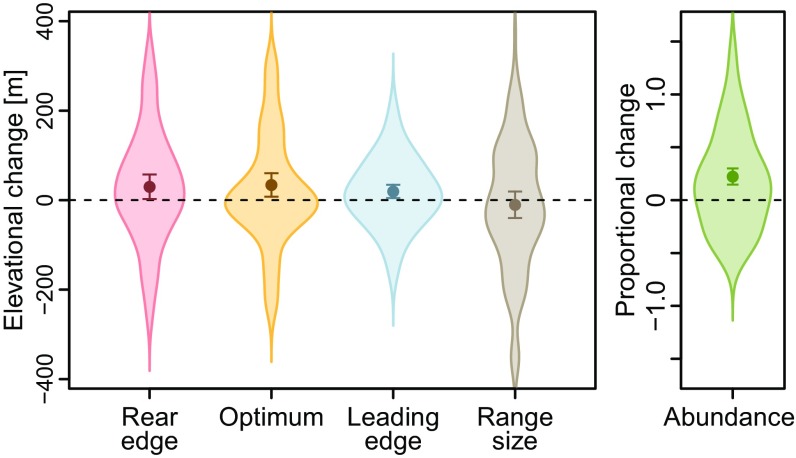
On average, the 183 plant species shifted their rear edges, optima, and leading edges upslope (rear edge: 30 m ± 14 m, df = 182, t = 2.15, P = 0.033; optimum: 34 m ± 13 m, df = 182, t = 2.55, P = 0.012; leading edge: 20 m ± 8 m, df = 182, t = 2.59, P = 0.010; Fig. 1). Albeit these mean upslope shifts, nearly half of the species shifted at least one of their range attributes downhill (rear edge: 41%, optimum: 47%, leading edge: 42%; Fig. 1). Furthermore, given an increase of 0.8 K (df = 1,548, t = 218.60, P < 0.001; Temperature Data) in the mean annual temperature between the average year of the historical records and our resurvey, and assuming an elevational lapse rate of 0.6 K per 100 m (25), all of these shifts are significantly lagging behind climate warming (rear edge: df = 182, t = 7.45, P < 0.001; optimum: df = 182, t =7.46; P < 0.001; leading edge: df = 182, t = 15.15, P < 0.001). Mean rates of shift did not differ significantly among the three range attributes, and we detected no “lean” type of range dynamics (i.e., optima shifting at a different pace than one or both of the limits; Correlations and Skewness of Elevational Shifts and ref. 10). Nonetheless, as a consequence of the numerically stronger rear edge shift, species’ mean elevational range sizes contracted, even if this contraction was not statistically significant (−10 m ± 15 m, df = 182, t = −0.69, P = 0.492; Fig. 1). By contrast, the average abundance of the 183 species increased significantly, by 22% (SE = 4%, df = 182, t = 5.75, P < 0.001; Fig. 1).
Fig. 1.
Changes of range attributes for 183 mountain plant species of the European Alps. Distributions of changes are depicted as shaded areas, with outliers removed to improve clarity. Average changes are depicted as dots with 0.95 confidence intervals as whiskers, the latter derived from intercept-only linear models.
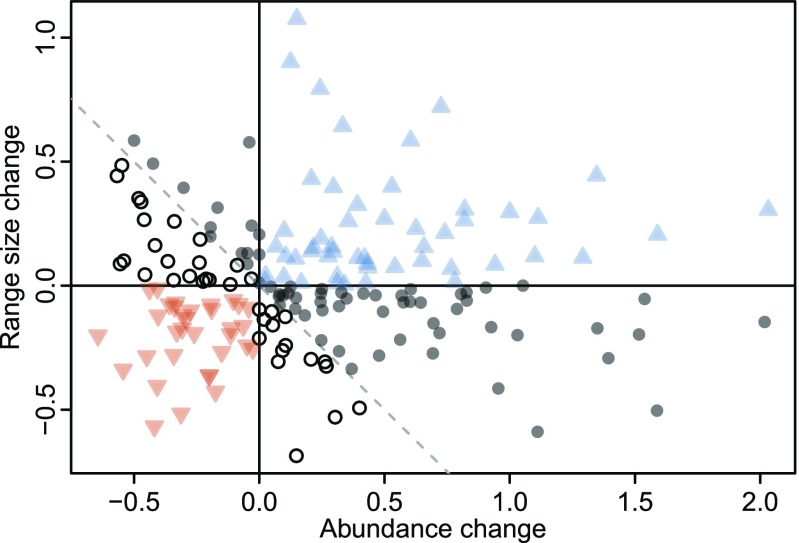
Fifty-one species expanded their range size and increased their abundance, and hence appear as winners of recent range dynamics (Fig. 2). By contrast, a sizable minority (n = 33) both contracted their range sizes and became less abundant [representing a “crash” type of range dynamic in the typology of Lenoir and Svenning (10)]. The largest group of species (n = 99) included those that combine loss in elevational range size with increased abundance, or vice versa, and cannot hence unequivocally be qualified as winners or losers. However, changes, and especially increases, were more pronounced for abundance than for range size (compare x and y axes in Fig. 2). If these two attributes are considered equally important and their proportional changes are combined without weighting (i.e., summed up), the number of net winners (n = 114) is significantly higher than the number of net losers (n = 69; χ2 = 11.1, df = 1, P < 0.001; compare species right and left of the diagonal, respectively, in Fig. 2).
Fig. 2.
Range size vs. abundance change for 183 mountain plant species of the European Alps. Changes are proportional to the respective historical values. Blue pyramids depict winners (i.e., species with increased elevational range sizes and increased abundances), red inverted pyramids depict losers (i.e., those with decreases in both of these range attributes), and dots symbolize species which combine loss in one attribute with gain in the other one. Closed darker dots to the right of the diagonal dashed line can be considered as net winners (gain in one attribute > loss in the other one), and open dots to the left of this line can be considered as net losers (gain in one attribute < loss in the other one).
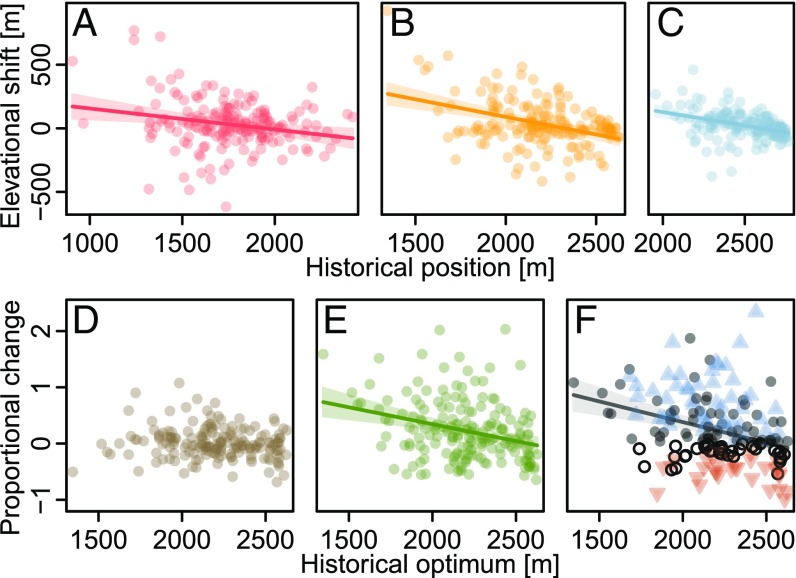
Historical elevational positions of range attributes explained 1 to 16% of the variation in their recent dynamics (Fig. 3 and Table S2). All rear edges, optima, and leading edges shifted more upslope the lower these range attributes were located in the historical surveys. In addition, abundances increased more strongly for species with historical optima at lower elevations. As a corollary, winners and losers tend to separate along the elevational gradient as well (Fig. 3 and Table S2). Due to the close relation between elevation and ambient temperature, using historical mean annual temperature of range attributes as a predictor of their dynamics provided qualitatively identical, and quantitatively very similar, results (Fig. S2 and Table S2): Both range limits and optima shifted more upslope the warmer they were situated historically, and species from warmer habitats increased their abundances more pronouncedly.
Fig. 3.
Relationships between changes of range attributes and the historical elevation of these attributes for 183 mountain plant species of the European Alps. (A) Rear edges, (B) optima, (C) leading edges, (D) elevational range size, (E) abundance, and (F) sum of proportional elevational range size and abundance changes. Lines and their shades represent significant linear regression models (Table S2) with their confidence intervals. In F, blue pyramids depict winners (i.e., species with increased elevational range sizes and increased abundances), red inverted pyramids depict losers (i.e., those with decreases in both of these range attributes), and dots symbolize species which combine loss in one attribute with gain in the other one. Closed darker dots can be considered as net winners (gain in one attribute > loss in the other one), and open dots can be considered as net losers (gain in one attribute < loss in the other one). Panels differ in size due to differing elevational ranges of the respective historical positions.
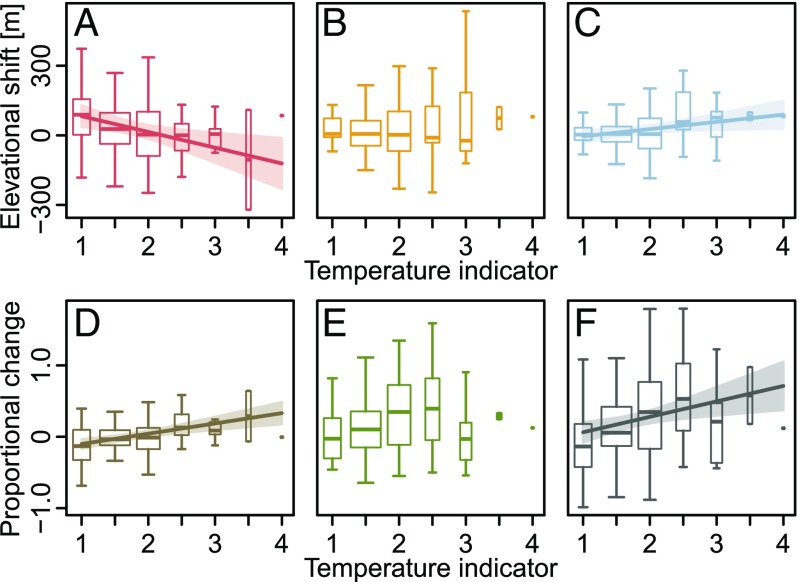
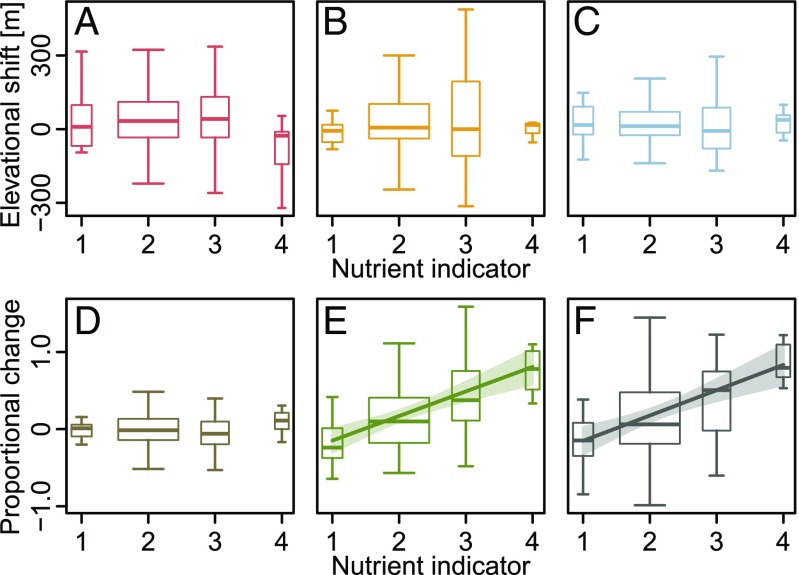
Species-specific information on thermal and nutritional demands is available in terms of so-called indicator values that have been assigned to a wide range of species of the Alpine flora (26). We related changes of range attributes to these indicator values and found that thermophilic species are winners of recent range dynamics mainly due to their stronger increase in elevational range size (Fig. 4 and Table S2). Vice versa, cold-adapted species tend to be losers because they experienced both (stronger) rear edge retreats and less pronounced leading edge expansions. We could not detect a relation between species’ thermal indicator values and changes in their optima or abundances. On the contrary, nutrient indicator values were significantly related to species’ abundance increases, highlighting nutriphilous species as winners of recent range dynamics, while elevational shifts were unaffected by nutrient requirements (Fig. 5 and Table S2).
Fig. 4.
Relationships between changes of range attributes and temperature indicator values for 178 mountain plant species of the European Alps. (A) Rear edges, (B) optima, (C) leading edges, (D) elevational range size, (E) abundance, and (F) sum of proportional elevational range size and abundance changes. Lines and their shades represent significant linear regression models (Table S2) with their confidence intervals. The width of the boxplots is proportional to the number of species with the respective indicator value. Outliers have been removed to improve clarity. Indicator values were taken from Landolt et al. (26): 1, alpine to nival; 1.5, lower alpine to upper subalpine; 2, subalpine; 2.5, lower subalpine to upper montane; 3, montane; 3.5, lower montane to upper colline; and 4, colline.
Fig. 5.
Relationships between changes of range attributes and nutrient indicator values for 181 mountain plant species of the European Alps. (A) Rear edges, (B) optima, (C) leading edges, (D) elevational range size, (E) abundance, and (F) sum of proportional elevational range size and abundance changes. Lines and their shades represent significant linear regression models (Table S2) with their confidence intervals. The width of the boxplots is proportional to the number of species with the respective indicator value. Outliers have been removed to improve clarity. Indicator values were taken from Landolt et al. (26): 1, very nutrient-poor; 2, nutrient-poor; 3, moderately nutrient-poor to moderately nutrient-rich; and 4, nutrient-rich.
Finally, we related elevational shifts of rear edges and optima to species traits presumably affecting their persistence ability, as well as leading edge and optima shifts with traits related to species’ dispersal ability (Species-Specific Traits). However, we detected no significant relationships (Table S2).
Discussion
Like many other studies, our data demonstrate that the ranges of mountain biota shifted upslope during recent decades. However, in contrast to studies that focus on only one attribute of species ranges, our comprehensive assessment allows a more detailed and complete characterization of recent plant range dynamics in the European Alps. We emphasize that, even if range limits and optima shifted upslope on average, the mean increase in species abundance was the most pronounced change observed. Our results hence suggest that a process of in-filling, i.e., a proliferation within the existing elevational range limits, currently prevails over a shift of these limits (cf. ref. 9) which is corroborated by an overall increase of community richness (total species number per plot; 6 ± 0.3, df = 1,548, t = 18.75, P < 0.001; Community Density, Community Richness, and Turnover of Cooccurring Species). Furthermore, both elevational shifts and abundance increases showed an elevational trend, with stronger changes at lower elevations.
The abiotic drivers of the observed dynamics cannot be unequivocally inferred from an observational study. However, both the average abundance increase and the mean upslope shift of all range attributes are consistent with the warming climate. Moreover, the importance of climate change is also consistent with the fact that thermophilic species tend to be winners and cold-adapted species tend to be losers of the observed dynamics. Nevertheless, detected shifts were less pronounced than expected from elevational lapse rates, and also slower than those reported for tree species in the Swiss Alps (27). Possible reasons for this delay include persistence of remnant populations at unsuitable sites as well as dispersal limitations (28). In addition, topographically driven microclimatic variation is particularly pronounced in alpine terrain (29). Appropriate microsites may buffer plant species from ambient climatic conditions, thus reducing the need for elevational shifts for keeping climatic growth conditions within the species’ requirements (23).
Two additional changes to the regional environment likely interfered with the effects of a warming climate on species ranges. First, 35% of the area used as pastures or hay meadows in the 1950s has been abandoned since then (Pasture Data). Subalpine grassland usage has historically allowed many alpine species to expand their ranges to lower elevations (30). It is therefore plausible that recent pasture abandonment, and associated regrowth of competitively superior subalpine species, has additionally contributed to shifting the rear edges of (some) alpine species upslope. It is unclear, however, how abandonment or less intensive use of grasslands could have triggered abundance increases and leading edge shifts of many of those species which are known to be adapted to moderate grazing and to have profited from summer pasturing (31). Second, the (more) pronounced abundance increase of nutriphilous species suggests an additional impact of airborne nitrogen deposition on the observed dynamics, possibly in combination with increased nutrient availability under a warming climate (32). Indeed, high-mountain ecosystems are often strongly nitrogen-limited (22) and the pronounced nitrogen accumulation in many parts of the European Alps during the 20th century (33) already caused imprints on the species composition of mountain plant communities (34). Nonetheless, nutritional demands of species did not affect changes in elevational position of range limits and optima. We hence argue that land use change and nitrogen deposition have most probably contributed to the observed dynamics, but that the warming climate remains the most parsimonious explanation for the overall upslope shift of range optima and both range limits.
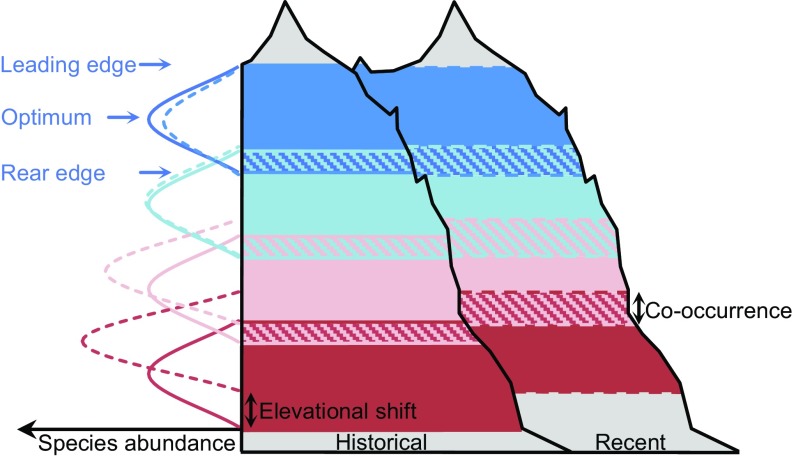
The consistent relation of elevation, and thus ambient temperature, to the dynamics of all range attributes suggests that available thermal energy affects the velocity of species’ range shifts to a certain extent, probably by a generic deceleration of vital rates and hence population processes under lower temperatures. In addition, biotic interactions likely contributed to the observed elevational trend. We suggest that the elevational gradient of abundance increases translated into a parallel gradient of increasing competitive intensity which likely codetermined the elevational gradient of rear edge (and maybe also of optima) shifts (Fig. 6). Support for this interpretation comes from circumstantial evidence: First, community density (total plant cover per plot) increased only at lower elevations, community richness increased more strongly, and turnover of cooccurring species was more pronounced at lower than at higher elevations (Fig. S3, Table S3, and Community Density, Community Richness, and Turnover of Cooccurring Species). Second, nutriphilous species—which are better capable of exploiting high nutrient levels and usually grow faster than species with low nutrient demands (35)—increased their abundance the most. Third, cold-adapted, stress-tolerant species, which often have low competitive ability, experienced the most-pronounced rear edge retreat. With respect to leading edges, expansion may have become increasingly difficult with elevation because the lack of soil substrates or facilitators often hampers the establishment of species at higher elevations, even if climatic conditions (already) satisfy their requirements (36).
Fig. 6.
Schematic illustration of elevational range dynamics of four exemplary mountain plant species. Solid and dashed curves on the left represent historical and recent species distributions, respectively. Overlapping areas of two species indicate areas of cooccurrence. Note that elevational shifts of rear edges, optima, and leading edges as well as abundance changes decrease with elevation and that areas of cooccurrence increased at all elevations.
However, elevational trends still explained only a minor part of the huge variation in range attribute shifts among species. Neutral population fluctuations and the unavoidable sampling noise in such a resurvey study certainly contributed to this variability, together with the already discussed interference of different drivers. In addition, part of the variation is probably due to the already mentioned microclimatic variability of mountainous terrain. As a consequence of this heterogeneity, species may coexist at the same elevation despite their strongly differing thermal requirements (23, 29). For example, alpine species can occasionally be found at scattered cold microsites far below the treeline (37). These extreme outposts may be particularly sensitive to a warming climate (23). Indeed, we found rear edge retreats to be most likely for species with the lowest thermal requirements (i.e., for alpine species), even if rear edges were generally less dynamic at higher and thus colder elevations (compare Fig. S2A and Fig. 4A). This apparent contradiction obviously results from a disproportional loss of cold-adapted species from their particularly low-lying outposts.
We could not detect relationships between the dynamics of range attributes and species’ persistence- and dispersal-related traits. However, the only two available metaanalyses on the issue demonstrated that such relationships are often weak or completely lacking (14, 20). Hence, it seems that a trait-based explanation probably underestimates the complexity of elevational range shifts (20) as well as the stochasticity of key processes like seed dispersal (38). In addition, intraspecific trait variability can be pronounced (39), but could not be taken into account in our study.
Whatever the reason, the observed variability impedes simple generalizations of how recent climatic change, or a changing climate in combination with nitrogen deposition and land use change, has affected mountain plant species of the European Alps. Overall, it seems that the majority of species analyzed here has so far profited rather than suffered from the changes of the recent decades. We note, however, that our analyses exclude rare species and those of highest elevations, because their (upper) range limits were not sufficiently covered by our data (Materials and Methods). We additionally underline that the dynamics observed so far may represent a transitional disequilibrium state and that the net balance of winners and losers may differ once extinction debts and invasion credits have been paid off (36).
Finally, we highlight that, independent of the preponderance of winners in our dataset, a sizeable minority of species seems to undergo a crash type of development (10) with decreasing abundance within their (contracting) range. The likelihood of such crash dynamics increases for species of higher elevations. These observations are in line with model predictions that forecast particularly pronounced range loss for alpine species (40). However, while these predictions are mainly derived from mountain topography (i.e., conical mountain shapes and limited summit heights), our data indicate that elevational differences in the pace of range dynamics might put alpine species under the additional pressure of competitors that encroach from below faster than they themselves can escape to even higher elevations. The competitive superiority of such “overtaking” migrators (17) may effectively reduce alpine plant populations even before these species reach the (topographical) end. Even if our data hence indicate that winners are more frequent than losers among the species analyzed, they nevertheless corroborate or even reinforce concern about those which are adapted to the highest elevations (4, 19) and which represent a unique and particularly rich contribution to the European flora (41).
Materials and Methods
Sampling Design and Field Data.
Following an intensive literature research, we digitized 3,507 historical relevés (i.e., plot data with complete lists of vascular plant species and their cover-abundance values, hereinafter referred to as “plots”) of nonforest vegetation in the European Alps. We only included plots that were recorded before 1970, since temperature anomalies accelerated after that time (42). The primary data sources usually provide a description of the locality, including elevation (meters a.s.l.), slope inclination (degrees), aspect (16 compass directions), plot size (square meters), total vascular plant cover (percent), plant community type, bedrock type, and survey date. If the survey year of a plot was not mentioned, we estimated it as publication year minus 2. Since historical plots had no geographical coordinates, we applied a standardized methodology to relocate them based on this metadata (Fig. S4): We used the description of the locality to delineate a polygon within which the plot was situated (e.g., a particular mountain). This polygon was intersected with a digital elevation model with a resolution of 25 × 25 m to delimit those areas matching the topographic specifications of the plot (tolerance ranges: elevation ± 50 m, slope ± 20°, aspect ± 40°). Bedrock information from the original publications was reclassified as calcareous, siliceous, or intermediate. If no information was given, we used the phytosociological classification of the plot in the original publication to assign it to one of these three classes. Subsequently, we further restricted the topographically matching areas by overlay with a substrate layer of the European Alps (cf. ref. 43). From the remaining candidate areas, we selected those within 200 m of trails because authors of the original publications reported using trails to reach their study sites. The network of trails was digitized using aerial images provided by Google Earth. Finally, we defined the coordinates for the resurvey as the centroid of the largest contiguous area remaining after consecutive application of all of these criteria. In cases where no trail was within 200 m of any candidate area, we selected the centroid of the largest contiguous area irrespective of trail distance. Since not all digitized historical plots had full topographic specifications, this sampling design resulted in the relocation of 2011 historical plots (Fig. S1).
In the vegetation periods of 2014 and 2015, we resurveyed 1,516 of these relocated plots. The Swiss National Park provided another 60 resurveyed historical plots, yielding, in total, 1,576 plots, spanning an elevational gradient from 485 m to 3,226 m a.s.l. with time intervals ranging from 45 y to 104 y (Fig. S1).
The resolution of the digital elevation model (25 × 25 m) is coarser than the maximum size of the historical plots (100 m2). Precise plot relocation within the cells was selected based on available additional topographic information (e.g., ridge situation) and coarse vegetation type (rock/scree, snow bed, meadow/heath, tall herbs, bog/swamp). Historical species lists were not passed on to the observers (i.e., blind resurvey). Furthermore, we took care to localize resurvey plots in ± homogeneous vegetation (i.e., avoiding obvious vegetation boundaries) because the intention of historical sampling was to document distinct plant community types. During field work, we recorded the exact geographical coordinates and all topographic parameters of each plot in the field. These data were used in all subsequent analyses for both historical and recent plots when needed.
Species within plots were identified to the most precise taxonomic level possible (i.e., subspecies where applicable). Vouchers were taken to the laboratory if a species could not be identified in the field. Species cover-abundance values were estimated using the scale of Braun−Blanquet (levels +, 1, 2, 3, 4, and 5), and total vascular plant cover per plot was estimated in percent. Following the field campaigns, subspecies or species not distinguished historically, and species notoriously difficult to distinguish (in particular several species of the genus Festuca), were grouped into species and aggregates, respectively. Species from the genera Alchemilla and Taraxacum were excluded from analyses, because these apomictic taxa have undergone extensive taxonomic revisions. Moreover, all observers working in the resurvey campaign had to self-evaluate all species with respect to the certainty with which they could identify them in the field. Species that were recorded “uncertainly” by any observer without a voucher for postdetermination taken were combined with those with which they are potentially confounded in aggregates. Finally, the dataset comprised 1,070 vascular plant taxa that we, for simplicity, call “species” hereinafter.
Statistical Analyses.
All analyses were conducted using the programming environment R (44).
We excluded all tree species from these analyses, because they were only represented by seedlings or saplings in our resurvey. For all 306 species with at least 20 occurrences in both the historical and the recent survey, we calculated a density function spanning the whole elevational gradient, separately for the historical and the recent field survey, using the function density with the default band with setting (i.e., Silverman’s rule of thumb), Gaussian kernel smoothing, and weighting by the species’ cover-abundance values. Since historical plots were recorded using different cover-abundance scales, we transformed all scales into percentage cover, i.e., we replaced all ordinal values with an approximation of the corresponding average percentage cover values (45).
To compare species abundances in the whole dataset, we used frequencies corrected for extreme outliers. We therefore multiplied the density functions by the total number of occurrences in the historical and the resurveyed plots, respectively, and removed the extreme tails of these density functions (i.e., lower than one-thousandth of the maximal density). Abundance was then defined as the integral of this modified density function. Proportional change of abundance was calculated as the difference between the recent and the historical value, divided by the historical value. Rear and leading edges were defined as the 5% and 95% quantiles of the adapted density distributions’ integral, the optimum was defined as the peak of the density distribution, and the elevational range size was defined as the difference between the leading and rear edge positions (Fig. S5).
Density functions do not converge to zero at the lower and/or upper tail within the sampled elevational gradient if the elevational range of the species was not recorded completely. Since the calculation of all range attributes was based on the density distributions’ integral, such nonconvergence at one limit also affects all other range attributes. Thus, species were excluded completely from analyses if any range limit was not covered, either historically or recently. Furthermore, the precision of range limits situated at the lowermost and uppermost surveyed elevations is potentially confounded by lower sampling effort. Therefore, we calculated a density function of the plots (i.e., of sampling intensity) over the elevational gradient and subsequently removed from the analyses all species for which one of the range limits fell into the first or last 1% quantile of this sampling density distribution, either historically or recently. To be conservative, we also excluded all plots that fell into these 1% quantiles from analyses of changes of temperature, pastures and community density, community richness, and turnover of cooccurring species (Temperature Data, Pasture Data, and Community Density, Community Richness, and Turnover of Cooccurring Species), yielding, in total, 1,549 plots for these analyses (Fig. S1).
Shifts of range limits or optima were computed by subtracting the historical from the recent elevational positions. After this procedure, elevational shifts of rear edges, optima, and leading edges as well as changes in proportional abundance were available for 183 species (Table S1). Whether these changes were statistically significant (i.e., different from zero) was tested with intercept-only linear regression models, separately for each attribute.
Elevational shifts as expected from climate warming were calculated assuming a lapse rate of 0.6 K per 100 m of elevation (25), and distance lags were calculated by subtracting the observed from the expected values. Whether these distance lags were statistically different from zero was tested with intercept-only linear regression models, separately for rear edges, optima, and leading edges.
The relations between elevational shifts of rear edges, optima, and leading edges and their respective historical positions as well as between proportional changes of elevational range size, abundances, their sum, and the historical optima of the species were determined with linear regression models, using each range attribute separately as response variable and using elevation as predictor. Survey years and elevation of the historical plots were unrelated (df = 1,547, t = 1.47, P = 0.141).
Temperature and nutrient indicator values were derived from Landolt et al. (26). These two sets of indicator values represent the optimal thermal and nutrient requirements of each species at an ordinal scale, ranging from warm- to cold-adapted species and from species associated with nutrient-poor to species associated with nutrient-rich habitats. Temperature indicators were available for 178 and nutrient indicators were available for 181 of the analyzed species. Relations between these indicator values and changes of range attributes were tested with linear regression models.
Data Availability.
Data are available online in the Phaidra database at https://phaidra.univie.ac.at/view/o:630655.
Supplementary Material
Acknowledgments
We thank M. Kuttner for his technical support; the Swiss National Park and the National Park Hohe Tauern for their cooperation; C. Kuehs, S. Ertl, A. Dellinger, M. Sonnleitner, N. Helm, S. Stifter, E. Kucs, N. Sauberer, and C. Gilli for their help in the field; B. Kramer for the use of the velocimeter; and S. Venn, K. Steinbauer, G. Gimpl, M. Stehlik, and the Tundra Trait Team for the contribution of trait data. This study was funded by the Austrian Climate Research Program through the project DISEQU-ALP (B368575).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data are available online in the Phaidra database at https://phaidra.univie.ac.at/view/o:630655.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1713936115/-/DCSupplemental.
References
- 1.Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- 2.Lenoir J, Gégout JC, Marquet PA, de Ruffray P, Brisse H. A significant upward shift in plant species optimum elevation during the 20th century. Science. 2008;320:1768–1771. doi: 10.1126/science.1156831. [DOI] [PubMed] [Google Scholar]
- 3.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 4.Pauli H, et al. Recent plant diversity changes on Europe’s mountain summits. Science. 2012;336:353–355. doi: 10.1126/science.1219033. [DOI] [PubMed] [Google Scholar]
- 5.Wipf S, Stöckli V, Herz K, Rixen C. The oldest monitoring site of the Alps revisited: Accelerated increase in plant species richness on Piz Linard summit since 1835. Plant Ecol Divers. 2013;6:447–455. [Google Scholar]
- 6.Stöckli V, Wipf S, Nilsson C, Rixen C. Using historical plant surveys to track biodiversity on mountain summits. Plant Ecol Divers. 2011;4:415–425. [Google Scholar]
- 7.Wiens JJ. Climate-related local extinctions are already widespread among plant and animal species. PLoS Biol. 2016;14:e2001104. doi: 10.1371/journal.pbio.2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly AE, Goulden ML. Rapid shifts in plant distribution with recent climate change. Proc Natl Acad Sci USA. 2008;105:11823–11826. doi: 10.1073/pnas.0802891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannone N, Pignatti S. Ecological responses of plant species and communities to climate warming: Upward shift or range filling processes? Clim Change. 2014;123:201–214. [Google Scholar]
- 10.Lenoir J, Svenning JC. Climate-related range shifts–A global multidimensional synthesis and new research directions. Ecography. 2015;38:15–28. [Google Scholar]
- 11.Morueta-Holme N, et al. Strong upslope shifts in Chimborazo’s vegetation over two centuries since Humboldt. Proc Natl Acad Sci USA. 2015;112:12741–12745. doi: 10.1073/pnas.1509938112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenoir J, et al. Going against the flow: Potential mechanisms for unexpected downslope range shifts in a warming climate. Ecography. 2010;33:295–303. [Google Scholar]
- 13.Eriksson O. Functional roles of remnant plant populations in communities and ecosystems. Glob Ecol Biogeogr. 2000;9:443–449. [Google Scholar]
- 14.Angert AL, et al. Do species’ traits predict recent shifts at expanding range edges? Ecol Lett. 2011;14:677–689. doi: 10.1111/j.1461-0248.2011.01620.x. [DOI] [PubMed] [Google Scholar]
- 15.Crimmins SM, Dobrowski SZ, Greenberg JA, Abatzoglou JT, Mynsberge AR. Changes in climatic water balance drive downhill shifts in plant species’ optimum elevations. Science. 2011;331:324–327. doi: 10.1126/science.1199040. [DOI] [PubMed] [Google Scholar]
- 16.Callaway RM, et al. Positive interactions among alpine plants increase with stress. Nature. 2002;417:844–848. doi: 10.1038/nature00812. [DOI] [PubMed] [Google Scholar]
- 17.Alexander JM, Diez JM, Levine JM. Novel competitors shape species’ responses to climate change. Nature. 2015;525:515–518. doi: 10.1038/nature14952. [DOI] [PubMed] [Google Scholar]
- 18.Cavieres LA, et al. Facilitative plant interactions and climate simultaneously drive alpine plant diversity. Ecol Lett. 2014;17:193–202. doi: 10.1111/ele.12217. [DOI] [PubMed] [Google Scholar]
- 19.Gottfried M, et al. Continent-wide response of mountain vegetation to climate change. Nat Clim Change. 2012;2:111–115. [Google Scholar]
- 20.MacLean SA, Beissinger SR. Species’ traits as predictors of range shifts under contemporary climate change: A review and meta-analysis. Glob Change Biol. 2017;23:4094–4105. doi: 10.1111/gcb.13736. [DOI] [PubMed] [Google Scholar]
- 21.Gehrig-Fasel J, Guisan A, Zimmermann NE. Tree line shifts in the Swiss Alps: Climate change or land abandonment? J Veg Sci. 2007;18:571–582. [Google Scholar]
- 22.Greaver TL, et al. Key ecological responses to nitrogen are altered by climate change. Nat Clim Change. 2016;6:836–843. [Google Scholar]
- 23.Scherrer D, Körner C. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J Biogeogr. 2011;38:406–416. [Google Scholar]
- 24.Körner C. The use of ‘altitude’ in ecological research. Trends Ecol Evol. 2007;22:569–574. doi: 10.1016/j.tree.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Körner C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. 2nd Ed Springer; Heidelberg: 2003. [Google Scholar]
- 26.Landolt E, et al. Flora Indicativa: Ecological Indicator Values and Biological Attributes of the Flora of Switzerland and the Alps. 2nd Ed Haupt; Bern, Switzerland: 2010. [Google Scholar]
- 27.Küchler M, Küchler H, Bedolla A, Wohlgemuth T. Response of Swiss forests to management and climate change in the last 60 years. Ann For Sci. 2015;72:311–320. [Google Scholar]
- 28.Dullinger S, et al. Post-glacial migration lag restricts range filling of plants in the European Alps. Glob Ecol Biogeogr. 2012;21:829–840. [Google Scholar]
- 29.Scherrer D, Körner C. Infra-red thermometry of alpine landscapes challenges climatic warming projections. Glob Change Biol. 2010;16:2602–2613. [Google Scholar]
- 30.Dullinger S, Dirnböck T, Greimler J, Grabherr G. A resampling approach for evaluating effects of pasture abandonment on subalpine plant species diversity. J Veg Sci. 2003;14:243–252. [Google Scholar]
- 31.Grabherr G, Mucina L. Natürliche Waldfreie Vegetation. Gustav Fischer; Jena, Germany: 1993. [Google Scholar]
- 32.Chapin FS, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA. Responses of arctic tundra to experimental and observed changes in climate. Ecology. 1995;76:694–711. [Google Scholar]
- 33.Rogora M, et al. An overview of atmospheric deposition chemistry over the Alps: Present status and long-term trends. Hydrobiologia. 2006;562:17–40. [Google Scholar]
- 34.Bobbink R, et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol Appl. 2010;20:30–59. doi: 10.1890/08-1140.1. [DOI] [PubMed] [Google Scholar]
- 35.Grime JP. Plant Strategies, Vegetation Processes, and Ecosystem Properties. Wiley; Chichester, UK: 2001. [Google Scholar]
- 36.Svenning J-C, Sandel B. Disequilibrium vegetation dynamics under future climate change. Am J Bot. 2013;100:1266–1286. doi: 10.3732/ajb.1200469. [DOI] [PubMed] [Google Scholar]
- 37.Patsiou TS, Conti E, Theodoridis S, Randin CF. The contribution of cold air pooling to the distribution of a rare and endemic plant of the Alps. Plant Ecol Divers. 2017;10:29–42. [Google Scholar]
- 38.Clark JS, Lewis M, McLachlan JS, HilleRisLambers J. Estimating population spread: What can we forecast and how well? Ecology. 2003;84:1979–1988. [Google Scholar]
- 39.Siefert A, et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol Lett. 2015;18:1406–1419. doi: 10.1111/ele.12508. [DOI] [PubMed] [Google Scholar]
- 40.Dirnböck T, Essl F, Rabitsch W. Disproportional risk for habitat loss of high-altitude endemic species under climate change. Glob Change Biol. 2011;17:990–996. [Google Scholar]
- 41.Väre H, Lampinen R, Humphries C, Williams P. Taxonomic diversity of vascular plants in the European alpine areas. In: Nagy L, Grabherr G, Körner C, Thompson DBA, editors. Alpine Biodiversity in Europe. Springer; Berlin: 2003. pp. 133–148. [Google Scholar]
- 42.Hartmann DL, et al. Observations: Atmosphere and surface. In: Stocker TF, et al., editors. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Intergov Panel Clim Change; Cambridge, UK: 2013. [Google Scholar]
- 43.Dullinger S, et al. Extinction debt of high-mountain plants under twenty-first-century climate change. Nat Clim Chang. 2012;2:619–622. [Google Scholar]
- 44.R Core Team . R: A Language and Environment for Statistical Computing. R Found Stat Comput; Vienna: 2015. [Google Scholar]
- 45.van der Maabel E. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio. 1979;39:97–114. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available online in the Phaidra database at https://phaidra.univie.ac.at/view/o:630655.